Abstract
Background
Interleukin-6 (IL-6) is a pleiotropic cytokine that controls numerous physiological processes both in basal and neuroinflammatory conditions, including the inflammatory response to experimental autoimmune encephalomyelitis (EAE). IL-6 is produced by multiple peripheral and central cells, and until now, the putative roles of IL-6 from different cell types have been evaluated through conditional cell-specific IL-6 knockout mice. Nevertheless, these mice probably undergo compensatory responses of IL-6 from other cells, which makes it difficult to assess the role of each source of IL-6.
Methods
To give some insight into this problem, we have produced a novel mouse model: a conditional reversible IL-6 KO mouse (IL6-DIO-KO). By using double-inverted, open-reading-frame (DIO) technology, we created a mouse line with the loss of Il6 expression in all cells that can be restored by the action of Cre recombinase. Since microglia are one of the most important sources and targets of IL-6 into the central nervous system, we have recovered microglial Il6 expression in IL6-DIO-KO mice through breeding to Cx3cr1-CreER mice and subsequent injection of tamoxifen (TAM) when mice were 10–16 weeks old. Then, they were immunized with myelin oligodendrocyte glycoprotein 35-55 peptide (MOG35-55) 7 weeks after TAM treatment to induce EAE. Clinical symptoms and demyelination, CD3 infiltration, and gliosis in the spinal cord were evaluated.
Results
IL6-DIO-KO mice were resistant to EAE, validating the new model. Restoration of microglial Il6 was sufficient to develop a mild version of EAE-related clinical symptoms and neuropathology.
Conclusions
IL6-DIO-KO mouse is an excellent model to understand in detail the role of specific cellular sources of IL-6 within a recovery-of-function paradigm in EAE.
Keywords: DIO technology, Conditional reversible IL-6 knockout, Experimental autoimmune encephalomyelitis, Microglial IL-6
Introduction
Since their inception in the early 80s, genetically engineered mice have been a very powerful tool in biomedical research [1]. With time, the technology has been refined to allow for conditional knock-out of genes in specific cell populations [2] or in a timed manner [3]. However, the reversibility of these knockouts to allow for recovery of function experiments has been limited and somewhat flawed. Therefore, in this paper, we introduce a conditional (Cre-dependent) and reversible knockout for interleukin-6 (IL-6) using the double-inverted open reading frame (DIO) paradigm [4]. Our gene of choice for this proof-of-concept knockout was IL-6 since we had already successfully created a floxed mouse to achieve a conditional knockout [5]. The new mouse (IL6-DIO-KO) lacks IL-6 production in all cells, which can be turned back on by the action of Cre recombinase. In this paper, we demonstrate the knockout nature of this mouse in its initial form and test its applicability to specific research questions. We show the effect of selective recovery of IL-6 expression in microglia by crossing IL6-DIO-KO with Cx3cr1-CreER mice [6, 7] in the context of experimental autoimmune encephalomyelitis (EAE), the most popular mouse model of multiple sclerosis (MS).
IL-6 is a pleiotropic and multifunctional cytokine that mediates numerous physiological processes and is classically involved in acute and chronic inflammatory conditions [8]. Indeed, its overproduction has been reported in many pathological situations, including MS [9]. In the nervous parenchyma of patients with MS, IL-6 is predominantly linked to the active CNS plaques and located within resident glial cells concentrated in demyelinated areas [10]. IL-6 is also essential in the pathogenesis of EAE, as evidenced by IL-6 KO mice being resistant to it [11–14] and displaying altered markers of the disease such as reduced infiltration of T lymphocytes and monocytes into the central nervous system (CNS), reduced expression of adhesion molecules in endothelial cells, as well as defective differentiation of Th17 lymphocytes, which have been proposed as key factors leading to EAE resistance [11, 15]. In this context, and knowing that IL-6 regulates EAE in a source-specific way, given the varied results with conditional cell-specific IL-6 KO mice [16–19], we chose microglia as the cell population to test our reversible KO. Microglial cells have been described as one of the most important IL-6-producing brain cells in neuroinflammatory conditions and are clearly implicated in MS and EAE pathogenesis [20–23].
Our results show that this new IL6-DIO-KO mouse model is a useful tool to easily investigate the specific functions of IL-6 from each cellular source in EAE pathology. In particular, in the case of recovery of microglial IL-6, this was sufficient to initiate mild paralyzing symptoms related to EAE and slightly regulate the inflammatory cascade of EAE.
Material and methods
Animals
Mice were kept under constant temperature and a 12-h light:12-h dark with food and water available ad libitum. All experiments were approved by the Ethics Committee on Animal Experiments of the Universitat Autònoma de Barcelona and the Generalitat de Catalunya (Refs. 3782 and 9684, respectively).
Generation of IL6-DIO-KO mice
The conditional reversible IL-6 KO (IL6-DIO-KO) mice were developed using double-inverted open reading frame (DIO) technology [4]. Exon 2 of the Il6 gene (Il6DIOex2) was inverted and flanked by two pairs of mutually incompatible loxP sites (lox2272 and loxP) in opposite orientations resulting in an inactive Il6 allele. The action of Cre recombinase inverts exon 2 thereby restoring normal Il6 expression.
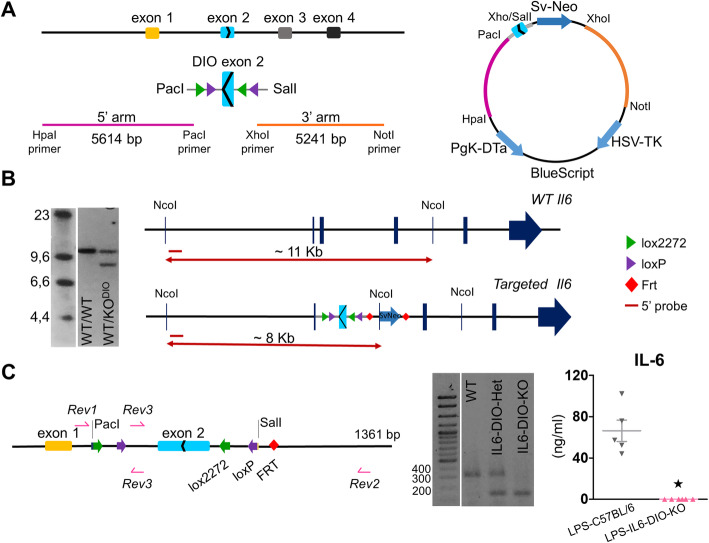
This mouse was generated as follows: a BAC clone from a C57Bl/6 J mouse containing the Il6 sequence was obtained from Invitrogen (RP23-121 M2). A 5614 bp fragment with engineered HpaI and PacI sites upstream of exon 2 and a 5241 bp fragment with engineered XhoI and NotI sites downstream of exon 2 of the Il6 gene were synthesized by PCR (using Q5 polymerase, NE Biolabs) (Fig. 1a, left). Exon 2 flanked by double lox sites with PacI and SalI sites at the ends was synthesized by Genscript and inserted into a targeting vector in the reverse orientation. The three fragments were sequentially cloned into a targeting vector containing Pgk-DTa and HSV-TK genes for negative selection and frt-flanked SvNeoR for positive selection. The final construct was purified from a CsCl gradient, linearized with AscI and electroporated into G4 hybrid (C57Bl/6 × Sv129) embryonic stem (ES) cells (Fig. 1a, right). Correctly targeted clones were identified by Southern blot; approximately 5 μg of DNA from ES cells were digested with NcoI, electrophoresed on a 0.7% agarose gel and transferred to a nylon membrane. Next, the membrane was hybridized to a unique probe of 621 bp located outside the 5’ arm (synthesized using the primers Fw: 5’-CAGCATCTCATCTGAGTTCCG-3’ and Rv: 5’-CTCACTGTTCACAAAGCACAGG-3’) (Additional file 1) that would give a ~11 kb band for the wild-type allele and a ~8 kb band for the targeted allele (Fig. 1b). Clones with the two bands were reassessed using Neo as a probe. Four positive clones (out of 81 analyzed) were correctly targeted and had a single NeoR gene insert. Three of those were injected into C57Bl/6 blastocysts, which were implanted in receptive females. Those mice with a high percentage of chimerism were then crossed with Gt(ROSA)26Sor-FLP recombinase to remove the Sv-NeoR gene [24]. Mice heterozygous for the Il6DIOex2 gene were backcrossed >10 times to C57BL/6OlaHsd mice, and non-littermate heterozygous mice for Il6DIOex2 gene from the tenth crossing were crossed to each other to obtain three possible genotypes: Il6DIOex2/DIOex2 (IL6-DIO-KO mice), Il6WT/WT (WT mice) and Il6DIOex2/WT (IL6-DIO-Het mice, with one functional Il6 allele).
Fig. 1.
Generation of a conditional reversible KO mouse for IL-6. a Outline of the targeting strategy. Left: 5614 bp HpaI-PacI (pink; upstream) and a 5241 bp XhoI-NotI (orange; downstream) flanking Il6DIOex2 fragments. Right: the final product that consists of a PacI-SalI fragment with inverted exon 2 flanked by double lox sites, and three fragments containing Pgk-DTa and HSV-TK genes for negative selection and frt-flanked SvNeo gene for positive selection. b Southern blot for Il6 gene of the positive ES clones using the probe (left) revealed a ~11 kb band on the wild type allele and a ~8 kb band on the targeted allele, as expected (right); those clones with two bands were selected. c Left: The Il6 gene-modified sequence from IL6-DIO-KO mouse was sequenced by the Sanger method. The resulting sequence had 1361 bp and exon 1 (yellow), exon 2 (blue), lox2272 cassettes (green), loxP cassettes (purple), and FRT (red) were genetic structures observed in that sequence. Middle: PCR products for Il6 gene of WT, IL6-DIO-Het, and IL6-DIO-KO mice yielded the expected bands. Right: IL-6 protein in serum was undetectable by ELISA in IL6-DIO-KO mice after LPS administration. All results are represented as mean ± SEM; ★p ≤ 0.05 vs. LPS-C57BL/6 mice
Reactivation of microglia-derived Il6 expression
IL6-DIO-KO mice were crossed with heterozygous Cx3cr1-CreER mice, produced by Drs. Jung and Prinz and obtained from the European Mouse Mutant Archive (EMMA) repository, where were backcrossed with C57BL/6 mice more than 10 generations [7]. Then, the offspring Cre-positive animals from the first crossing were selected and crossed again with homozygous IL6-DIO-KO mice to obtain mice homozygous for IL6-DIO-KO and carrying Cx3cr1-CreER allele; these mice are designated IL6-DIO-ONCx3cr1 and should have Il6 expression only where Cx3cr1 gene is expressed, including microglia.
Genotyping
Mice were genotyped by PCR analysis of tail and liver DNA, extracted previously by boiling in 100 μl of 50 mM sodium hydroxide for 7 min, using three primers (Rev1: 5’-GAGACTGTGAGAGAGGAGTGTG-3’; Rev2: 5’-CATCTTATCTGGGCTGACCCTAG-3’; Rev3: 5’-TCTCTGCTGGGATCTAGGGCC-3’) and the following reaction conditions: 95 °C 2 min, followed by 30 cycles of 95 °C 30 s, 52 °C 30 s, and 72 °C 30 s, and 72 °C 5 min. Finally, Cre primers and PCR conditions were adapted from Sanz et al. [25].
Sanger DNA sequencing
The modified fragment of Il6 gene from IL6-DIO-KO mouse was sequenced by the Sanger method. Two PCR products were amplified separately using two pairs of primers. The first pair of primers amplified from the middle of the first exon to the end of the third intron (1F: 5’-CCCACCAAGAACGATAGTCA-3’ and ΔR: 5’-ATGCCCAGCCTAATCTAGGT-3’ [5] while the second pair amplified from the first intron to the end of the second intron (fw: 5’-CGATGCTAAACGACGTCACA-3’and Rev3 primer). Then, the PCR fragments were purified by ExoSAP-IT Express PCR Cleanup Reagent (ThermoFisher, 75001). The enzymatic cleanup method and sequencing were performed by Servei de Genòmica i Bioinformàtica (IBB-UAB). The data were imported firstly into Snapgene Viewer 4.3.4. Software (from Insightful Science; available at snapgene.com), in which both sequences were overlapped creating a unique sequence with 1361 bp.
Tamoxifen treatment
Cx3cr1-CreER mouse is a tamoxifen (TAM)-inducible model [7], then the IL6-DIO-ONCx3cr1 mice and their littermates were injected for five or eleven consecutive days with TAM. Their littermates were also administered with TAM to control the effects of TAM in the analyzed parameters. TAM (Sigma 5648) was dissolved in ethanol at a concentration of 10 mg/100 μl by shaking and heating at 37 °C [26]; next, the solution was diluted with sunflower oil to 10 mg/ml and one daily dose of 100 μl (1 mg) was injected intraperitoneally to each mouse.
Lipopolysaccharide injection
Mice were injected intraperitoneally with 0.5 mg/Kg lipopolysaccharide (LPS O55:B5, Sigma L2880) and blood was drawn from the tail 90 min later; it was centrifuged at 9.500xg for 10 min at 4 °C and serum was stored at −80 °C before testing.
Microglia isolation
IL6-DIO-KO and IL6-DIO-ONCx3cr1 mice were euthanized 1 month after 11 days of TAM treatment and brains (excluding cerebellum) were quickly dissected and chopped in small pieces. Then, the tissue was dissociated both enzymatically and mechanically using the adult brain dissociation kit (Miltenyi 130-107-677) and placing it on a gentleMACS Octo Dissociator for 30 min at 37 °C. After dissociation, debris and erythrocytes were removed and microglia were isolated using magnetic microbeads against Cluster of differentiation molecule 11b (CD11b; Miltenyi 130-093-634) following the manufacturer’s instructions. The purity of microglia in CD11b+ cell population was previously checked in [26]. The CD11b-negative flow-through was named “remaining sample”. DNA from cells was extracted as described above.
Induction of EAE and clinical evaluation
The induction of EAE was carried out using adult mice. All animals were immunized under isoflurane anesthesia (oxygen flowmeter to 0.8 L/min and isoflurane vaporizer to 4 and 1.5% during induction and maintenance, respectively) as described [19]. On day 0, mice were injected subcutaneously into the hind flanks with an emulsion of 100 μL MOG35–55 (3 mg/ml; sequence: MEVGWYRSPFSRVVHLYRNGK-carboxyl. Purity: >98%. Peptide synthetized upon request in the Peptide Synthesis Facility at the Universitat Pompeu Fabra) and 100 μL of complete Freund’s adjuvant (CFA; Sigma-Aldrich F5881) supplemented with 3 mg/ml Mycobacterium tuberculosis H37RA (BD Difco 231141). Control mice were immunized with an emulsion containing bovine serum albumin (BSA; 3 mg/ml; Sigma A9647) instead MOG35–55 peptide. All mice received an intraperitoneal injection of 500 ng Bordetella pertussis toxin (Native Antigen Company PT-TNL-50) on day 0 following MOG35-55/BSA injection and on day 2 post-immunization. Bordetella pertussis toxin is widely used to facilitate the induction of EAE since it destabilizes the blood-brain barrier and facilitates the migration of T cells into the CNS.
After immunization, body weight and clinical score were monitored daily. The progression of the disease was assessed in each animal with a numerical scale following these criteria: 0 = no clinical signs, 0.25 = slight loss of tail tonus, 0.5 = partial loss of tail tonus, 1 = paralyzed tail, 2 = moderate hind limb paraparesis, 2.5 = severe hind limb paraparesis, 3 = partial hind limb paralysis, 3.5 = hind limb paralysis, 4 = tetraplegia, and 5 = death. When mice lost more than 10% weight, they were injected intraperitoneally with 200 μl of saline buffer supplemented with 3.6% glucose. We applied the following endpoint criteria: (i) self-mutilation; (ii) weight loss greater than 25%; or (iii) tetraplegia for more than 2 days. The time to disease onset and time to peak disease (days to peak score) were defined by a clinical score ≥1. The peak score (highest score) and median score were also calculated individually.
Mice were euthanized by decapitation and their spine was cut between the ninth and tenth thoracic vertebrae. The upper part was processed for histological analysis (see “Histology” section) and the lower part was cut open to extract the spinal cord (segments T12-Co3), which was snap-frozen in liquid nitrogen and stored at −80 °C for molecular analysis of the inflammatory response. Blood was collected from the trunk and the serum was obtained as mentioned before and stored at −80 °C.
IL-6 enzyme-linked immunosorbent assays (ELISA)
IL-6 levels in serum were measured using IL-6 ELISA following manufacturer’s instructions (Bionova 860020192).
Real-time polymerase chain reaction (qPCR)
RNA was extracted using Promega Maxwell RSC simplyRNA kit following the manufacturer’s instructions. Then, 2 μg of RNA of each sample were treated with an extra DNase I step (Qiagen). After removing DNA from the RNA samples, a two-step qPCR protocol was performed using iScript cDNA synthesis kit (BioRad) and iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories 1725124). Fold change expression was calculated with an adaptation of delta-delta-Ct method using Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) as the reference gene and the EAE IL6-DIO-Het group as calibrator.
Histology
The upper part of the vertebral column with the spinal cord from sham and MOG35-55-immunized mice was fixed with 4% PFA for 24 h. Afterwards, they were carefully dissected, being the spinal cord post-fixed for another 24 h in 4% PFA, subsequently embedded in paraffin and cut sagittally into 8-μm-thick sections. Two non-consecutive slices from each animal were used for each histological analysis. For myelin evaluation, samples were stained with 0.1% Luxol Fast Blue (LFB) overnight at 56 °C. The following day, the tissue was differentiated with 0.05% lithium carbonate (Fluka, 62 K70) and 70% ethanol, and counterstained with hematoxylin solution (Sigma MHS16). For IHC analysis, microglia/macrophages, astrocytes and lymphocytes cluster of differentiation 3+ (CD3+) were identified by antibodies against ionized calcium-binding adaptor molecule-1 (IBA-1; Wako 019-19741, 1/1500), Glial fibrillary acidic protein (GFAP; Dako Z0334, 1/900), and CD3+ (Dako A0452, 1/100), respectively.
Antigen retrieval was necessary in IBA-1 and CD3 IHC. For the former, samples were treated with 10 mM sodium citrate 0.05% tween for 20 min at 96 °C, whereas for the latter, they were incubated with 1 g/l protease type XIV (Sigma, P5147) for 8 min at 37 °C. Then, samples were incubated with endogenous peroxidase blocking buffer (70% methanol and 3% hydrogen peroxide (H2O2)) for 15 min and then blocked in 1% BSA in 0.05 M Tris-buffered saline and 0.5% triton-X100 for 1 h. The incubation with primary antibodies was overnight at 4 °C. The following day, tissues were incubated with a biotin-conjugated secondary antibody Atom BA-1000 (1/300) for 1 h and then, with horseradish peroxidase-coupled streptavidin (Vector SA-5004, 1/500-600). Immunoreactivity was visualized by adding 50 mg/ml 3,3-diaminobenzidine-tetrahydrochloride and 0.033% H2O2 for 1–5 min, depending on the primary antibody. Samples for CD3 were counterstained with hematoxylin solution (Sigma MHS16-500 ml).
Serial images of the tissues from duplicates of each animal were taken at ×10 using a Nikon Eclipse 90i microscope and Nikon Act-1 software. Quantification was performed with ImageJ software [27], using a color deconvolution plugin [28] in CD3 IHC counterstained with hematoxylin and LFB/hematoxylin staining to separate both colors and quantify only CD3+ cells and myelin, respectively. In LFB staining, the percentage of demyelination was evaluated in the white matter (WM). For CD3 IHC, we quantified the integrated density (sum of all the pixel intensities in the region of interest) of CD3 per infiltrate. In IBA-1 immunostaining, manual count of IBA-1+ cells in the white and gray (GM) matter of the spinal cord was carried out in the first EAE experiment whereas the quantification of the integrated density of white and gray matter, as well as the manual count of IBA-1+ cells in the white matter, were performed in the second EAE experiment. In addition, we characterized IBA-1+ cells in terms of their morphology. IBA-1+ cells with long and thin processes (ramified morphology) were classified as resting IBA-1+ cells whereas those with an enlarged body and small process were considered reactive IBA-1+ cells. IBA-1+ cells with a round body were classified as fully activated cells. In GFAP immunostaining, manual count of GFAP + cells were performed in the GM in the first experiment; however, this was not possible in the white matter given its background was too high. The quantification of the integrated density of white and gray matter, as well as manual count of GFAP + cells in the GM gray matter, were carried out in the second EAE experiment. Furthermore, those cells with thin and reduced processes (ramified morphology) were classified as resting GFAP+ cells whereas those with thicker hypertrophic processes (star-shaped morphology) were recorded as reactive GFAP+ cells. All results were normalized to the measured area.
Statistics
Data is shown as mean ± SEM, plus individual data points (except for clinical score), except for EAE summary tables, where median and interquartile range (IQR) are used. Statistical calculations were carried out using the Statistical Package for Social Sciences software (SPSS, version 19) unless otherwise stated. The clinical score was analyzed with a generalized linear model (GzLM) using the area under the curve (AUC), which had been previously calculated using GraphPad Prism. Body weight gain and incidence were analyzed by generalized estimated equations (GEE) and Fisher’s exact test, respectively. The day of onset (with score ≥1) and time to peak (with score ≥1) were examined using Kaplan–Meier survival analysis plus log-rang test, and the post-hoc pairwise comparisons were performed using the Holm-Bonferroni correction (R packages “survival” and “survminer”; RStudio (version 1.2.5033, with R version 3.6.3)). Peak score and median score were analyzed by Mann–Whitney U test to compare two groups or Kruskal–Wallis rank test and Dunn’s test (post hoc) to compare three groups. Histological and gene expression results were analyzed by GzLM using genotype as the main factor and a post hoc sequential Bonferroni test was carried out when the main factor was significant. In the case of comparisons between two groups, the Student t test was used. Outlier detection was carried out using the interquartile range (IQR) rule computed from Tukey’s hinges, and values more than 3 IQR’s from the end of the box were considered potential outliers and removed from the statistical analysis of histological and gene expression results. Heatmaps to show IL6 levels (or gene expression) alongside AUC were created with the R package “pheatmap” with no clustering and color breaks set with quantiles. Statistical significance was set at p ≤ 0.05 for all analyses.
Results
Generation and characterization of IL6-DIO-KO mice
We created a new murine model with an inactive Il6 allele (see “Generation of IL6-DIO-KO mice” section) that can be specifically reactivated by the action of Cre recombinase (Fig. 1), using a strategy based on DIO technology [4, 29–31]. In the absence of Cre recombinase, homozygous mice are null for Il6 expression (exon 2 is inverted) and designated as IL6-DIO-KO. The action of Cre recombinase flips exon 2 so that it can be spliced normally thereby restoring Il6 expression; these mice are referred to as IL6-DIO-ON with a superscript indicating what Cre-driver line was used.
We sequenced the modified Il6DIOex2 gene of IL6-DIO-KO mice by the Sanger method. By using two pairs of primers, we obtained two sequences that were overlapped to create a single genetic map of 1361 bp (Fig. 1c, left and Additional file 2). As expected, 2 pairs of lox cassettes are flanking exon 2 of the Il6 gene on each side (LoxP and Lox2272), two heterologous loxP sites that cannot recombine with each other. In addition, one residual Frt (FLPase recognition target [32];) site is also present. The result of the sequencing showed the inverted exon 2 resulting in an exon that cannot be spliced due to lack of splice acceptor and donor sites.
Three primers were designed for the routine genotyping of the normal and inverted exon 2 (see “Genotyping” section). By using Rev3 and Rev2, a product of 358 bp was amplified for the WT allele of WT and IL6-DIO-Het mice, whereas using Rev3 and Rev1 primers, a 193 bp band was obtained for the KO allele of IL6-DIO-Het and IL6-DIO-KO mice (Fig. 1c, middle). Analysis of IL-6 production in IL6-DIO-KO and non-littermate control mice was carried out in serum collected 90 min after a 0.5 mg/kg LPS injection. IL-6 protein levels were dramatically increased by LPS in C57BL/6OlaHsd mice, but they remained absent in LPS-IL6-DIO-KO mice (Fig. 1c, right).
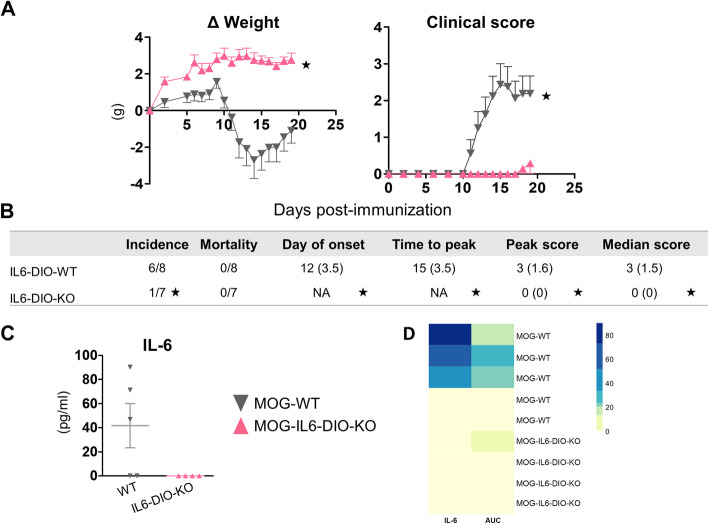
IL6-DIO-KO mice and littermate WT controls were immunized with the MOG33-55 peptide, and the clinical course and body weight were monitored for 19 days post-immunization (dpi). MOG-WT mice showed the prototypical course of ascending paralysis starting at 12 dpi (IQR 3.5) and peaking approximately at 15 dpi (IQR 3.5) with a clinical score of 3 (IQR 1.6) (Fig. 2a, right and 2b). They also showed a prominent body weight loss in parallel with the appearance of clinical signs (Fig. 2a, left). MOG-IL6-DIO-KO mice were resistant to EAE since only 14% showed minor paralyzing symptoms. The day of disease onset, peak score, time to peak, and median score were all statistically significant between genotypes (Fig. 2b and Additional file 3). As expected, IL-6 protein was undetectable in serum samples from MOG-IL6-DIO-KO mice, but present in their controls (Fig. 2c). Furthermore, a heatmap of IL-6 levels and area under the curve was performed, reinforcing that the idea that mice with more severe EAE-related symptoms also show more serum IL-6 levels (Fig. 2d).
Fig. 2.
IL6-DIO-KO mice are resistant to EAE. a Left: Weight loss of WT and IL6-DIO-KO mice after being immunized with MOG35–55 peptide. Right: Clinical evaluation of MOG35-55-immunized WT and IL6-DIO-KO mice. IL6-DIO-KO mice did not develop EAE-related symptoms. b Clinical evaluation of EAE. A significant effect on the disease onset, time to peak disease, peak score, and median score between WT and IL6-DIO-KO mice was observed. The incidence, disease onset, and time to peak were calculated using a clinical score ≥1. c Serum IL-6 protein levels were not detected in IL6-DIO-KO mice at 19 dpi. d Heatmap of IL-6 ELISA levels and the respective AUC for each animal in the experiment. Mice with higher disease scores also showed higher IL-6 levels. It is also clear that the two WT mice that did not develop clinical symptoms also did not have increased IL-6 levels. All results are represented as mean ± SEM; ★p ≤ 0.05 vs. MOG-WT mice
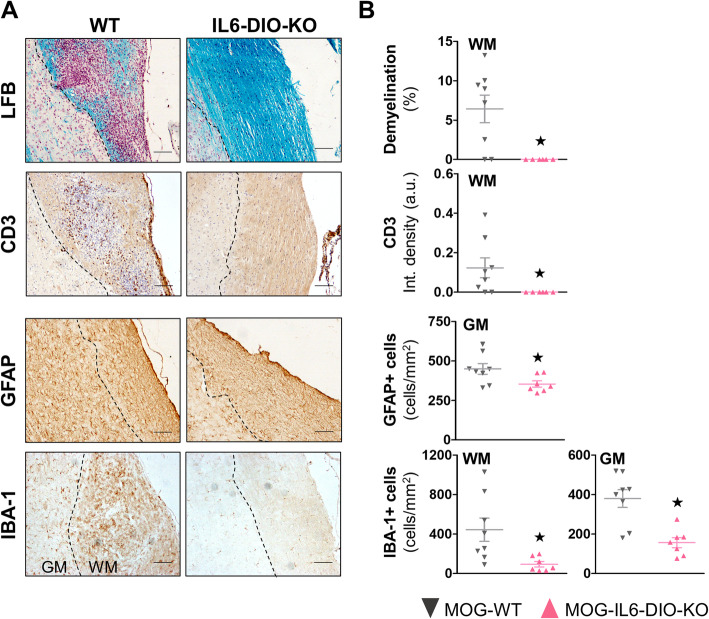
The upper part of the spinal cord (segments C1–T11) from MOG-WT mice, but not those from MOG-IL6-DIO-KO mice, showed clear signs of focal demyelination at 19 dpi, in agreement with clinical signs. Infiltration of CD3+ T cells was also prominent in the white matter of MOG-WT mice whereas it was practically absent in MOG-IL6-DIO-KO mice (Fig. 3a and b, top). We also counted both the number of GFAP+ and IBA-1+ cells along the spinal cord since astrocytes (GFAP+ cells) and microglia /macrophages (IBA-1+ cells) since they play an important role in EAE pathogenesis mediating the inflammatory response. A more prominent accumulation of GFAP+ cells in the gray matter and IBA-1+ cells both in the white and gray matter were seen in spinal cords from MOG-WT mice, and to a lesser extent in those from MOG-IL6-DIO-KO mice (Fig. 3a and b, bottom).
Fig. 3.
MOG35-55-immunized IL6-DIO-KO mice showed an absence of demyelination and CD3 infiltrates, and limited gliosis. a Representative images of LFB staining and CD3 infiltrates (top), astrogliosis and microgliosis (bottom) of the spinal cord from MOG-WT and MOG-IL6-DIO-KO mice. The contrast of the representative images was enhanced. The lack of IL-6 prevented the infiltration of inflammatory cells. The discontinuous line delimits white matter (WM) from gray matter (GM). Scale bar: 100 μm. b From top to bottom: Quantification of demyelination by LFB staining and CD3 immunostaining levels (top) and the total number of GFAP+ and IBA-1+ cells (bottom) in the spinal cord from MOG-WT and MOG-IL6-DIO-KO mice. All results are represented as mean ± SEM; ★p ≤ 0.05 vs. MOG-WT mice
IL6-DIO-ONCx3cr1 mice showed genetic rearrangement of Il6 specifically in microglia 1 month after TAM injections
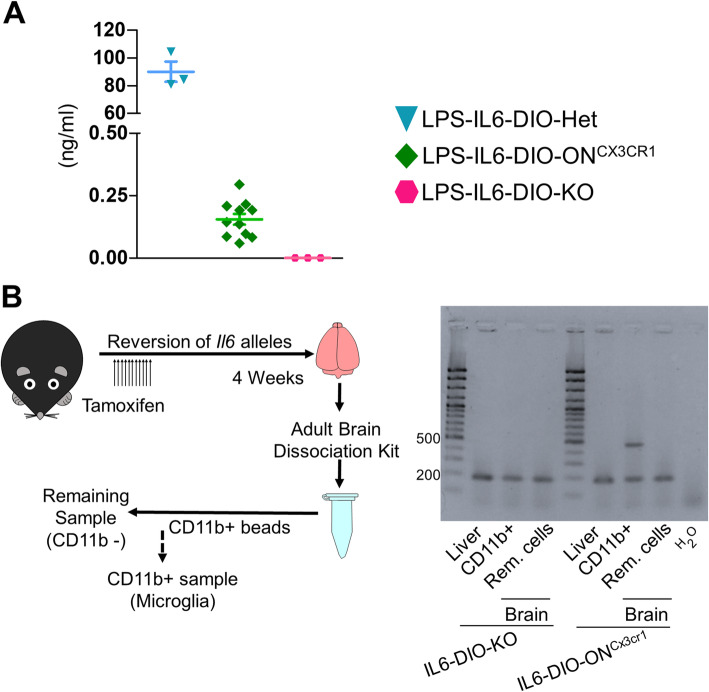
We evaluated the ability to reactivate Il6 expression selectively in Cx3cr1-expressing cells and defined the duration of TAM exposure for optimal inversion of exon 2 specifically in Cx3cr1-expressing cells. First, we treated 13- to 17-week-old mice with TAM for 5 days (1 mg/day) and 2 weeks afterwards injected them with LPS. We detected IL-6 in the serum of none of LPS-IL6-DIO-KO mice, all of LPS-IL6-DIO-Hz animals, but only in two out of six LPS-IL6-DIO-ONCx3cr1 animals, showing that recombination was insufficient (data not shown). Since exon 2 inversion did not work with 5 days of TAM treatment, 10- to 16-week-old mice were treated with TAM for 11 days, and with LPS 1 day after the TAM treatment ended. Serum IL-6 now increased modestly in all LPS-injected IL6-DIO-ONCx3cr1 mice while all LPS-treated IL6-DIO-KO controls remained undetectable. LPS-treated IL6-DIO-Het mice produced high levels of IL-6, as expected (Fig. 4a). This protocol was therefore established for subsequent use.
Fig. 4.
Reactivation of microglia-derived Il6 expression in IL6-DIO-ONCx3cr1 mice. a Serum IL-6 protein levels of LPS-injected IL6-DIO-Het, IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice 1 day following 11 days of TAM treatment. b Left: IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice were euthanized 1 month after TAM treatment and the brains were homogenized and incubated with CD11b+ antibody. Besides, the positive cell population was selected using columns with ferromagnetic spheres. Right: The reversion band (463 bp) was only seen in the brain CD11b+ fraction of IL6-DIO-ONCx3cr1 mice. All results are represented as mean ± SEM
To test the specificity of recombination in microglial cells, we reactivated Il6 expression in Cx3cr1-expressing cells by treating with TAM for 11 days and, 4 weeks later, extracted DNA from the liver (as a control) and the brain, separating its cells in microglia (CD11b+ sample) and the remainder (CD11b- sample) (Fig. 4b, left). This control is necessary because in Cx3Cr1-CreER mice, CreER is driven by the Cx3cr1 promoter, which is expressed in all mononuclear phagocytic cells, which include macrophages [7]. Also, DNA inversion should be present longer in microglia than in Cx3cr1-expressing peripheral cells given microglia’s longevity. Therefore, we showed a partial reversion of Il6 in IL6-DIO-ONCx3cr1 mice by PCR detection of the reversed band (463 bp) in microglia (CD11b+ sample) but not in a liver sample (where presumably there are circulating Kupffer cells and/or mononuclear cells). As expected, the inverted exon 2 band was observed in both tissues from IL6-DIO-KO mice (Fig. 4b, right).
Reactivation of Il6 expression in microglia partially rescues EAE pathology
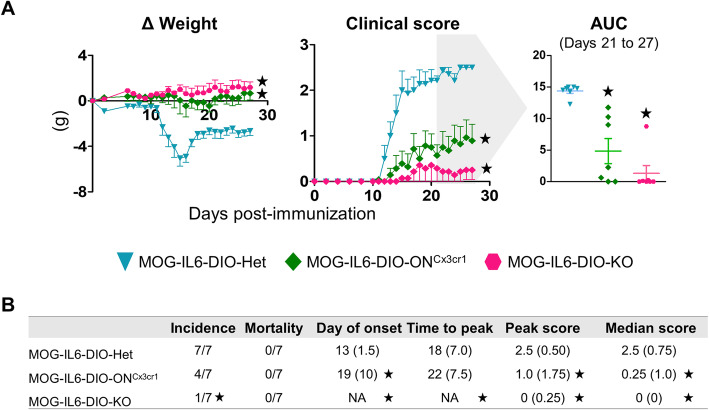
IL6-DIO-Het, IL6-DIO-ONCx3cr1, and IL6-DIO-KO females were used to physiologically confirm Il6 reversion in Cx3cr1-expressing cells. These mice were immunized with MOG35-55 peptide 7 weeks after being injected with a total of 11 mg of TAM. A group of BSA-injected female and male IL6-DIO-Het mice (but not injected previously with TAM either LPS) was also included as a control for illustrating the neuropathology of EAE.
Following MOG35-55 immunization, MOG-IL6-DIO-Het mice showed the typical ascending paralysis course of EAE, starting at 13 dpi (IQR 1.5) and peaking at 18 dpi (IQR 7) with a clinical score of 2.5 (IQR 0.5) (Fig. 5a, middle and Fig. 5b). Moreover, a prominent body weight loss occurred in these mice in parallel with the clinical signs (Fig. 5a, left). Consistent with our initial results, MOG-IL6-DIO-KO mice were almost completely resistant to EAE (Fig. 5a, b). In contrast, mice with the recovery of Il6 only in microglia (MOG-IL6-DIO-ONCx3cr1) showed a delayed progression of the clinical course, with paralyzed tail at 19 dpi (IQR 10) and peaking at 22 dpi (IQR 7.5), with a peak score of 1 (IQR 1.75) (Fig. 5a and b). As shown in Fig. 5b, the incidence of disease symptoms (clinical score ≥1) was 100% in MOG-IL6-DIO-Het and 57% in MOG-IL6-DIO-ONCx3cr1 mice. No mice died during the experiment (27 days) (Fig. 5b).
Fig. 5.
IL6-DIO-ONCx3cr1 showed mild EAE-related symptoms. a Clinical course of MOG-IL6-DIO-Het, MOG-IL6-DIO-ONCx3cr1 and MOG-IL6-DIO-KO mice. Body weight (left) and clinical signs (middle) changes were observed in IL6-DIO-Het and IL6-DIO-ONCx3cr1 mice whereas IL6-DIO-KO mice were almost completely resistant to EAE. Area under the curve (AUC; right) was calculated from EAE clinical course for each mouse between days 21 and 27. b Clinical evaluation of EAE. IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice were significantly less affected than IL6-DIO-Het mice. The incidence, disease onset, and time to peak were calculated using a clinical score ≥1. Results in (a) are represented as mean ± SEM, while (b) shows median and IQR (except for incidence and mortality). NA (not applicable) shows the impossibility to calculate the median (given the criterion for disease). ★p ≤ 0.05 vs. MOG-IL6-DIO-Het mice
Significant differences regarding EAE symptomatology were observed between MOG-IL6-DIO-Het and MOG-IL6-DIO-KO mice, and between MOG-IL6-DIO-Het and MOG-IL6-DIO-ONCx3cr1 mice. Furthermore, the severity of the disease was also analyzed by the evaluation of the AUC for each mouse from 21 to 27 dpi. We observed differences between MOG-IL6-DIO-Het and MOG-IL6-DIO-ONCx3cr1 mice (p < 0.001), and MOG-IL6-DIO-Het and MOG-IL6-DIO-KO mice (p < 0.001). Regarding the day of disease onset, peak score, and median score, MOG-IL6-DIO-Het mice were significantly different from both MOG-IL6-DIO-ONCx3cr1 and MOG-IL6-DIO-KO mice (Fig. 5b and Additional file 4). Immunization with BSA did not cause any paralyzing symptoms in IL6-DIO-Het mice. Furthermore, they maintained body weight for all days analyzed, as expected (data not shown).
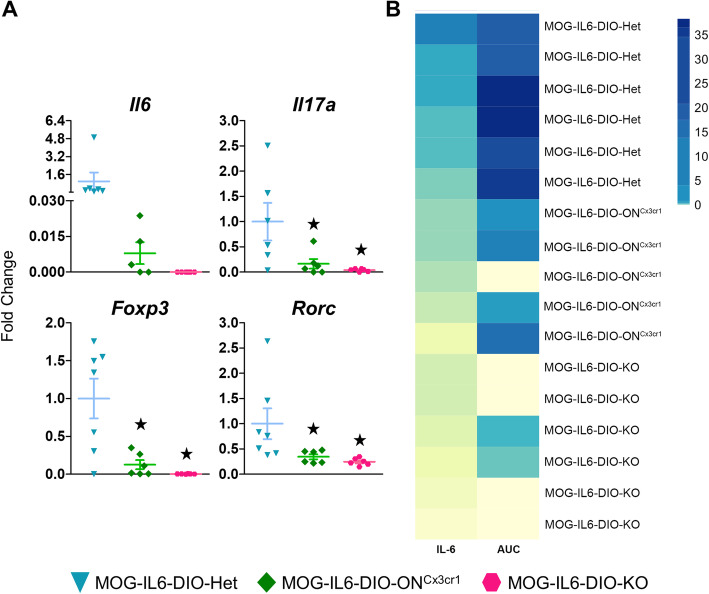
Il6 mRNA levels were higher in the lower part of the spinal cord (segments T12-Co3) from MOG-IL6-DIO-Het than MOG-IL6-DIO-ONCx3cr1 mice and were undetectable in MOG-IL6-DIO-KO mice at 27 dpi, even though no differences were significant (Fig 6a, top left). However, Il6 levels were in line with the disease extent (AUC) (Fig. 6b). In addition, to dissect the putative role of microglial IL-6 in EAE dynamics, we also analyzed the expression of several genes related to different subsets of T cells (Rorc, which encodes RORyt protein, and Foxp3) and Il17a expression. The expression of all these genes was higher in MOG-IL6-DIO-Het mice than MOG-IL6-DIO-KO and MOG-IL6-DIO-ONCx3cr1 mice, but no differences were seen between MOG-IL6-DIO-KO and MOG-IL6-DIO-ONCx3cr1 mice (Fig. 6a).
Fig. 6.
Inflammation-related genes are down-regulated accordingly to disease severity. The changes of inflammation-related genes expression were studied using the upper part of the spinal cord (segments C1–T11) of three groups of mice euthanized at 27 dpi. a Gene expression of Il6, Il17a, Foxp3, and Rorc was lower in MOG-IL6-DIO-ONCx3cr1 and MOG-IL6-DIO-KO mice than in MOG-IL6-DIO-Het control mice. b Heatmap of Il6 expression and AUC of disease score. Il6 was only increased in mice with higher AUC. Results in (a) are represented as mean ± SEM; ★p ≤ 0.05 vs. MOG-IL6-DIO-Het mice
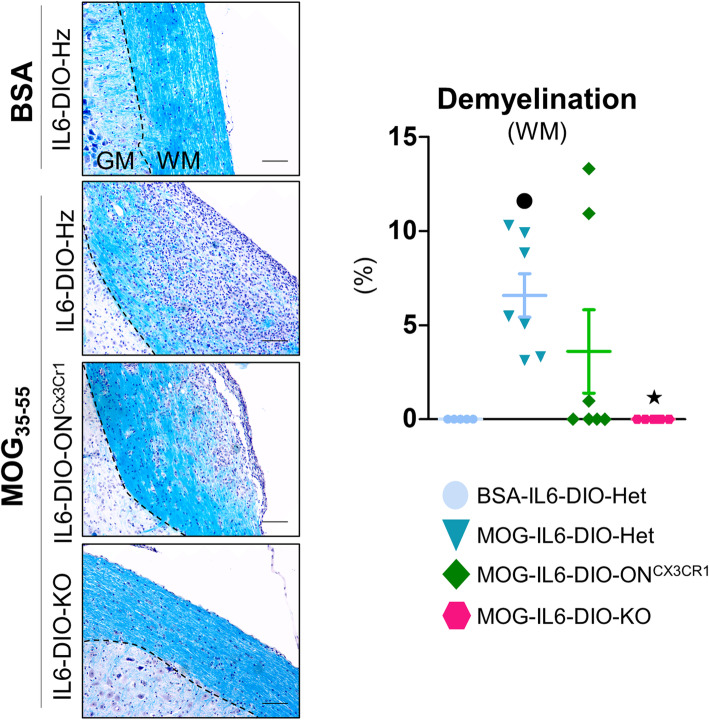
In accordance with the clinical signs, the upper part of the spinal cord (segments C1–T11) from MOG-immunized IL6-DIO-Het mice, but not those immunized with BSA, showed demyelination of the white matter. Moreover, a significant effect of IL-6 was observed, since MOG-immunized IL6-DIO-KO mice were practically equal to those of BSA-immunized IL6-DIO-Het mice, while recovery of microglial IL-6 in MOG-IL6-DIO-ONCx3cr1 mice, reverted the demyelination (seemingly partially, but it was not significantly different from MOG-immunized IL6-DIO-Het mice) (Fig. 7).
Fig. 7.
Demyelination was mildly affected by the recovery of microglial IL-6 at 27 dpi. Representative images of LFB/H&E staining and percentage of demyelination in the white matter of the spinal cord from BSA-immunized IL6-DIO-Het and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1, and IL6-DIO-KO mice. A prominent accumulation of cells was shown in MOG-IL6-DIO-Het mice, and to a lesser extent in MOG-IL6-DIO-ONCx3cr1 mice. The contrast of the representative images was enhanced. The discontinuous line delimits white matter (WM) from gray matter (GM). Scale bar: 100 μm. Results were normalized to the total area and are represented as mean ± SEM; ●p ≤ 0.05 vs. BSA-IL6-DIO-Het mice; ★p ≤ 0.05 vs. MOG-IL6-DIO-Het mice
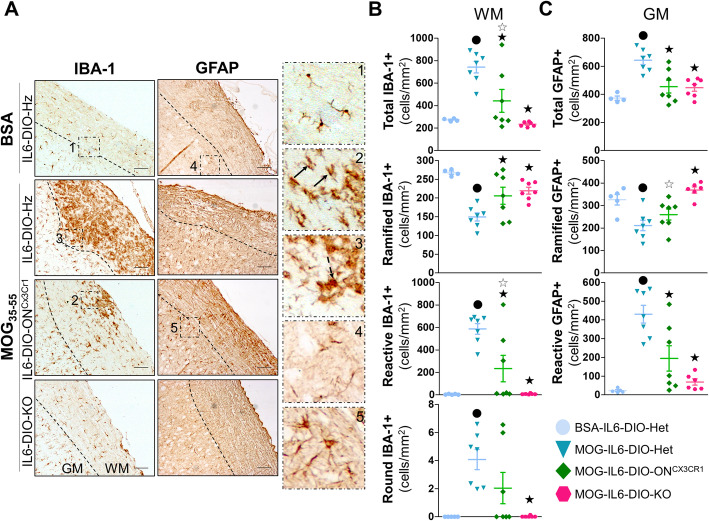
MOG35-55-immunization also caused an increase of microgliosis (analyzed both by counting IBA-1+ cells and quantitating IBA-1 immunostaining levels) in the white and gray matter of the upper part of the spinal cord (segments C1–T11) from IL6-DIO-Het mice compared with those immunized with BSA. In the white matter, MOG-IL6-DIO-Het showed more microgliosis than MOG-IL6-DIO-KO and MOG-IL6-DIO-ONCx3cr1 mice (Fig. 8a and b, and Additional file 5, left top). Although the IBA-1 immunostaining levels in the white matter of MOG-IL6-DIO-ONCx3cr1 and MOG-IL6-DIO-KO mice were not different, more IBA-1+ cells were counted in MOG-IL6-DIO-ONCx3cr1 than MOG-IL6-DIO-KO mice. In the gray matter, MOG-IL6-DIO-Het showed more IBA-1 immunostaining levels than MOG-IL6-DIO-KO mice, but not compared with MOG-IL6-DIO-ONCx3cr1 mice (Fig. 8a, left, and Additional file 5, left bottom). These results were similar to those for demyelination and suggesting that recovery of microglial IL-6 in MOG-immunized IL6-DIO-ONCx3cr1 mice might partially revert the microgliosis in this spinal cord.
Fig. 8.
Microglial IL-6 plays a minor role in the regulation of microgliosis and astrogliosis. a Representative images of IBA-1 (left) and GFAP (middle) immunostaining of BSA-immunized IL6-DIO-Het and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice. High magnification (right) of (a) showing the different morphologies of IBA-1+ and GFAP+ cells: (1) ramified, resting IBA-1+ cells; (2) reactive IBA-1+ cells, with shorter and thicker cell processes (black arrows); (3) round IBA-1+ cells (dashed black arrow); (4) Ramified, resting GFAP+ cells; (5) reactive, hypertrophic GFAP+ cells. The contrast of the representative images was enhanced. The discontinuous line delimits white matter (WM) from gray matter (GM). Scale bar: 100 μm. b The total number of IBA-1+ cells and the number of IBA-1+ cells showing a resting phenotype (ramified), partially activated (reactive), and fully activated (round) counted in the white matter of the spinal cord from BSA-immunized WT and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1, and IL6-DIO-KO mice. c The total number of GFAP+ cells and the number of GFAP+ cells showing a resting phenotype (ramified) and activated (reactive) counted in the gray matter of the spinal cord from BSA-immunized WT and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1, and IL6-DIO-KO mice. All results were relativized per total area and are represented as mean ± SEM; ●p ≤ 0.05 vs. BSA-IL6-DIO-Het mice; ★p ≤ 0.05 vs. IL6-DIO-Het mice. ☆p ≤ 0.05 vs. IL6-DIO-KO mice
A detailed study about the morphology of IBA-1+ cells (microglia/macrophages) in the white matter was also carried out (Fig. 8a, right, and 8b, differentiating three kinds of cells: ramified cells (resting; Fig. 8a, right, panel 1), reactive cells (activated; Fig. 8a, right, panel 2), and round cells (fully activated; Fig. 8a, right, panel 3). The 98.2 ± 0.72 % of IBA-1+ cells from negative control (BSA-injected) IL6-DIO-Het mice displayed a ramified morphology. Similar results were seen in MOG-IL6-DIO-KO since 96.7 ± 0.70% of IBA-1 cells displayed a ramified morphology whereas 3.3 ± 0.7% seemed to take reactive morphology. Among all cell types, the reactive IBA-1+ cells were the largest population (78.9 ± 1.70%) present in MOG-IL6-DIO-Het mice. The IL6-DIO-ONCx3cr1 mice, however, displayed 64.9 ± 14.7% and 34.9 ± 14.5% of ramified and reactive IBA-1+ cells, respectively. A treatment-dependent effect was observed in all three groups of IBA-1 + cells. Regarding MOG-immunized mice, MOG-IL6-DIO-Het mice showed more ramified and reactive IBA-1+ cells compared with MOG-IL6-DIO-KO and IL6-DIO-ONCx3cr1 mice, and IL6-DIO-ONCx3cr1 mice showed more reactive, but not ramified IBA-1+ cells compared with MOG-IL6-DIO-KO mice. Concerning round IBA-1+ cells, a significant increase was appreciated in MOG-IL6-DIO-Het compared with MOG-IL6-DIO-KO mice whereas a trend (p = 0.098) was shown in MOG-IL6-DIO-Het as to IL6-DIO-ONCx3cr1 mice. The same trend was observed among MOG-IL6-DIO-KO and IL6-DIO-ONCx3cr1 mice (p = 0.098).
In addition, we analyzed the astrogliosis by counting GFAP+ cells and studying GFAP immunostaining levels in the upper part of the spinal cord (segments C1–T11). In particular, no differences between treatments and genotypes were appreciated concerning GFAP immunostaining levels in the white matter, perhaps because of the myelin tracts also seemed to be immunostained, thus increasing background levels (Fig. 8a, middle and Additional file 5, right top). Regarding the gray matter, MOG35-55 immunization showed a trend to increase the GFAP immunostaining levels in IL6-DIO-Het compared with those with BSA immunization (p = 0.067), being this difference statistically significant when we counted the number of GFAP+ cells. Both GFAP immunostaining levels and the number of GFAP+ cells were different among MOG-IL6-DIO-Het and MOG-IL6-DIO-KO mice (Fig. 8c and Additional file 5, right bottom). Although GFAP immunostaining levels in the gray matter were not statistically significant between MOG-IL6-DIO-Het and MOG-IL6-DIO-ONCx3cr1 mice, we counted a higher number of GFAP+ cells in MOG-IL6-DIO-Het than MOG-IL6-DIO-ONCx3cr1 mice in this matter (Fig. 8c and Additional file 5, right bottom).
We also classified the number of astrocytes in the gray matter of the spinal cord according to their ramified (Fig. 8a, right, panel 4) or reactive (Fig. 8a, right, panel 5) morphology. In IL6-DIO-Het mice immunized with BSA, 93.5 ± 1.15 % of GFAP+ cells displayed a resting ramified morphology, while in those immunized with MOG35-55, 33.9 ± 4.76 % had a ramified morphology and 66.1 ± 4.76 % GFAP+ cells seemed to take a star-shaped morphology. In contrast, 85.0 ± 2.93% and 63.2 ± 10.30% of GFAP+ cells displayed a resting morphology in the gray matter of MOG-IL6-DIO-KO and IL6-DIO-ONCx3cr1 mice, respectively. MOG35-55-immunization decreased ramified GFAP+ cells, but increased reactive GFAP+ cells in IL6-DIO-Het mice compared with those with BSA-immunization. The number of ramified GFAP+ cells were lower in MOG-IL6-DIO-Het and MOG-IL6-DIO-ONCx3cr1 than MOG-IL6-DIO-KO mice, and a trend to increase the number of ramified GFAP+ cells was also appreciated in MOG-IL6-DIO-ONCx3cr1 compared to MOG-IL6-DIO-Het mice (p = 0.081). Genotypic differences were also observed regarding the number of GFAP+ cells with a reactive morphology, which was higher in MOG-IL6-DIO-Het than MOG-IL6-DIO-KO and MOG-IL6-DIO-ONCx3cr1 mice. The recovery of microglial IL-6 in MOG-IL6-DIO-ONCx3cr1 mice tended to increase the number of reactive GFAP+ cells in comparison with MOG-IL6-DIO-KO mice (p = 0.063; Fig. 8b, right).
Discussion
Traditional methods for creating reversible KO mice are based on strategies that require numerous crossings or the continuous administration of drugs. The main methods are (1) cassette stop/trap flanked by loxP sites [33–35], (2) Tet-off and tet-on [36–38], and (3) LOFT: LoxP-flippase (FLP) recognition target (FRT) Trap [39].
The stop-cassette strategy is based on creating a knockout by introducing transcription termination elements that prevent the complete production of the target protein. Reversibility is achieved by driving Cre expression with tissue-specific promoters fused with the estrogen receptor (ER) paired with tamoxifen injection for temporal and spatial specificity. Alternatively, injection of a Cre-expressing virus can rescue in a similarly time- and spatial-specific manner. However, stop cassettes can be leaky and trace expression must be assessed empirically.
Tet-off and Tet-on use Escherichia coli’s tetO operon to induce expression of the adjacent gene. In the “off” system, the mouse becomes a KO upon (and only during) administration of tetracycline (or, in practice, its derivative doxycycline), which then binds to a tetracycline-controlled transactivator (tTA) that has been engineered to be constitutively expressed, and prevents the transcription of the gene downstream of tetO. In the “on” system, the transactivator is modified to have the reverse phenotype (rtTA), i.e., it only binds to tetO in the presence of doxycycline. In this case, the animal is initially a KO, and it will only produce the target protein upon drug administration. As downsides, both strategies depend on continuous administration of the drug to maintain the animal’s genotype; the use of a stop cassette with loxP sites in order to obtain tissue specificity has the problem mentioned above; and furthermore, they require extensive breeding and animals that are transgenic for multiple genes.
The LOFT system uses two modified alleles for the gene of interest, one of them floxed and the other with an frt-flanked gene-trap cassette (that also has Neo and GFP) inserted in an intron. The latter will produce by default an inactive fusion protein with the N-terminal domain of the target protein and Neo. When Cre is expressed, the animal will become a KO. If there is additionally flippase activity, the phenotype will be reversed. This method requires a considerable number of crossings to incorporate the two alleles and recombinases making it labor- and cost-intensive. In addition, given that the allele with the gene-trap is null by default, the gene must be haplosufficient.
To overcome these pitfalls, we have generated a conditional and reversible KO mouse using the widely used in opto- and chemogenetics double-inverted, open-reading-frame paradigm [4, 29–31]. This technique consists of flanking exons of interest by pairs of incompatible loxP sites such that Cre recombinase flips the exons and then locks them into the flipped orientation [4, 29–31]. Exon 2 of the Il6 gene was inverted in IL6-DIO-KO mice causing a depletion of IL-6 protein (undetectable after inflammation challenges such as LPS and EAE), which is equivalent to results with the total IL-6 KO mice [12, 40]. In our EAE paradigm, almost all IL6-DIO-KO mice were resistant to MOG35-55-inducible EAE, except for one, which presented EAE-related symptoms even without showing serum IL-6 levels, as previously observed with other IL-6 KO models [11, 12, 41].
The EAE-related symptoms of IL6-DIO-KO mice were similar to those of WT mice immunized with MOG35-55 peptide and administered intraperitoneally with anti-IL-6 receptor monoclonal antibody (clone MR16-1) at the same day of EAE induction, but different to those of WT mice immunized with MOG35-55 peptide and treated with clone MR16-1 after disease onset [42]. In addition, WT mice immunized with MOG35-55 peptide and orally treated during ongoing EAE with tocilizumab, a humanized monoclonal antibody that inhibits IL-6R-mediated signaling, showed a drastic reduction of EAE-related symptoms [43]. It is therefore clear that IL-6 signaling is involved in the pathogenesis of EAE. However, given the functional complexity of IL-6, the priority of many groups during the last decades has been to study the multiple cellular sources of IL-6 to better understand the contribution of each one to the EAE pathogenesis. Until now, the roles of IL-6 in a specific context have been described through the use of mice lacking IL-6 throughout the body or in specific cells (total and conditional IL-6 KO mice, respectively). Nevertheless, the interpretation of the results using the latter model is difficult because the data could be the result of a compensatory response from IL-6 of other cells. With our new conditional reversible IL-6 KO mouse, we now have a tool that allows for the reactivation of Il6 expression in cells expressing Cre recombinase. Thus, we were able to recover microglial IL-6 in the reversible total IL-6 KO mouse through breeding to Cx3cr1-CreER mice and subsequent TAM administration. Our results suggest that recovery of microglial IL-6 gives rise to a mild version of EAE, albeit with a limited role in the regulation of the inflammatory cascade. In conclusion, we have verified that the new reversible total IL-6 KO is an excellent tool to study the role of specific cellular sources of IL-6 within a recovery-of-function paradigm.
Although EAE is considered a peripheral disease, microglia, besides CD4+ cells, have been described as responsible for EAE initiation [23, 44]. In fact, in MOG35-55-immunized C57BL/6 mice, CD4+ T cells infiltrated the brain before mice developed EAE-related symptoms, coinciding with activation of CD11b+ microglia and production of IL-1β, TNF-α, and IL-6 [45]. Furthermore, we have recently observed that microglia are one of the main brain sources of IL-6 during EAE pathogenesis and microglial Il6-deficient females seemed to be less affected by MOG35-55 immunization than their controls [19]. In this paper, we have focused on studying the effect of selective recovery of microglial IL-6 expression by crossing IL6-DIO-KO with Cx3cr1-CreER mice to show the applicability of this new system and to gain insight into the putative role(s) of microglial IL-6 in the EAE disease.
In Cx3Cr1CreER mice, Cre is driven by the Cx3cr1 promoter, which is broadly expressed in the mononuclear phagocytic cells and it is only expressed in microglia in the adult brain [7, 46, 47]. Furthermore, Cre recombinase protein of these mice is fused to the estrogen ligand-binding domain (CreER), thereby requiring TAM for its activation [6, 7]. Then, IL6-DIO-ONCx3cr1 mice should revert Il6 expression in Cx3cr1-expressing cells, including macrophages and microglia, after TAM treatment. Since IL-6 protein levels in serum are practically undetected in basal conditions, mice were also injected with LPS following TAM treatment to increase IL-6 production. The recovery of IL-6 occurred in IL6-DIO-ONCx3cr1 mice administrated with TAM for 11 days and not for 5 days. However, IL-6 production was lower in LPS-IL6-DIO-ONCx3cr1 than LPS-IL6-DIO-Het mice, probably due to the fact that only CX3CR1+ cells in IL6-DIO-ONCx3cr1 mice would be responsible for IL-6 production following LPS administration while all cells would produce IL-6 protein in IL6-DIO-Het mice (these mice have one WT allele and one DIO allele; in addition, TAM administration would cause the Cre activation and subsequent Il6 reversion of the DIO allele in CX3CR1+ cells in these mice). We did not attempt longer TAM administrations given that our current dose is already at the higher end of the reported spectrum [48, 49] and the drug is a known immunosuppressant which could interfere with EAE evaluation [50].
The longevity and limited self-renewal are the main features of microglia which distinguishes them from other mononuclear phagocytic cells [51, 52]. In fact, Goldmann and colleagues observed that inducible Cx3cr1CreER:R26-YFP mice, obtained by crossing Cx3Cr1CreER mice with R26-YFP mice, displayed YFP-labeled microglia at all times after TAM treatment whereas other mononuclear phagocytic cells lost the YFP-label for the first 4 weeks after treatment [6]. We also observed similar results using our inducible microglial IL-6 KO mice, obtained by crossing Cx3Cr1CreER mice with Il6lox/lox mice, since microglia of these mice, but not other brain cells or liver (assuming this tissue as representative of macrophages/monocytes), kept the genomic modification 4 weeks following TAM administration [26]. Therefore, we also performed the validation of IL6-DIO-ONCx3cr1 mice at the same time as above mentioned. As expected, IL6-DIO-ONCx3cr1 mice kept the reversion of the DIO allele in microglia, but not in other brain cells or liver, 4 weeks after being treated with TAM for 11 days. These data confirm that the contribution of mononuclear phagocytic cells to results obtained with IL6-DIO-ONCx3cr1 mice from 4 weeks following TAM-treatment will be limited.
Reactivation of microglial Il6 expression in MOG35-55-immunized IL6-DIO-ONCx3cr1 females developed some of the prototypical paralyzing symptoms showing that despite the fact that EAE is considered essentially a peripheral disease, microglial Il6 was sufficient to trigger a mild version of EAE-related symptoms. Results are in line with females lacking IL-6 in microglia, which tended to reduce EAE score during the acute phase of the disease in comparison with their controls [19]. Clinical signs of IL6-DIO-ONCx3cr1 mice were lower than those of IL6-DIO-Het animals, reinforcing the idea that IL-6 derived from other cellular sources is also involved in the EAE-related symptomatology. Of note, scores (AUC) within the IL6-DIO-ONCx3cr1 group have a bimodal distribution, which also does not fully correlate with Il6 expression. This could be due to only partial recovery of microglial Il6 or to this source not being as relevant to disease progression and just indicating a stochastic process. Indeed, T-cell- and astrocyte-derived Il6-deficient mice showed ameliorated symptoms compared with their controls after being immunized with MOG35-55 [17–19]. However, deficiency of dendritic cell-derived Il6 caused that naïve T cells could not differentiate into Th17 cells blocking EAE score in mice [17]. In contrast, Il6 deficiency in other cells caused no (in neurons), contradictory (in B cells), or aggravated (macrophage) effects in the regulation of EAE-related symptoms [16, 17, 19].
The expression of mRNA encoding Il6 was higher in the lower part of the spinal cord (segments T12-Co3) from MOG-IL6-DIO-Het than MOG-IL6-DIO-ONCx3cr1 mice and it was absent in MOG-IL6-DIO-KO mice at 27 dpi, but high variability precluded any statistically significant effect between genotypes. Moreover, we cannot rule out that differences would exist at other time points. EAE is a complex disease in which IL-6 undoubtedly plays an important role since it suppresses the differentiation of TGF-β-induced Foxp3+ Treg, and with TGF-β promotes the differentiation of pathogenic TH17 cells from naive T cells [15]. In this paper, IL6-DIO-KO mice showed downregulation of Rorc expression, the crucial transcription factor of Th17, suggesting a reduction of Th17 differentiation, and a similar trend regarding Il17 mRNA levels in the spinal cord compared to IL6-DIO-Het. Indeed, less percentage of Th17 cells infiltrating the CNS was previously reported using MOG35-55-immunized IL-6 KO mice [15]. Surprisingly, the recovery of microglial IL-6 did not modify Il17 expression in comparison with the lack of total IL-6 in mice at 27 dpi, as it was observed in IL-17 immunostaining levels in microglial Il6 deficient mice at 27–29 dpi [19]. Besides, the expression of mRNA encoding Foxp3, the master transcription factor of Treg, was also downregulated by lack of total IL-6, in agreement with IL-6 KO mice, whose cerebellum showed less percentage of Foxp3 cells per infiltrate than those of MOG35–55-immunized WT mice [12]. Interestingly, another work reported that IL-6 KO mice (crossed previously with the Foxp3gfp.KI mice to monitor Treg cells) showed more Foxp3+ Treg cells in the draining lymph nodes than their controls [53], which suggests the necessity of further studies to determine these discrepancies. Foxp3 and Rorc expression levels were practically equal among MOG-IL6-DIO-ONCx3cr1 and MOG-IL6-DIO-KO mice at 27 dpi, but distinct to MOG-IL6-DIO-Het, suggesting that other cellular sources of IL6, and not microglia, might regulate these transcription factors at this time point. IL-6 has also been described as a regulatory protein of some inflammation-related genes at 15-20 dpi [12, 54]. In particular, microglial Il6 deficiency caused changes in Gfap and Cd206 (alternatively activated macrophages/microglia (M2) phenotypic marker) expression in the lower part of the spinal cord (segments T12-Co3) from mice at 15 dpi [19]. Future studies are needed to determine what occurs regarding inflammation-related markers in the MOG-IL6-DIO-ONCx3cr1 mice at peak disease.
The histological analysis of the upper part of the spinal cord (segments C1–T11) from MOG-IL6-DIO-KO mice revealed a decrease of demyelination and limited invasion of CD3+ in the white matter, as previously observed using other IL-6 KO models immunized with MOG35-55 [11, 12], and highlighting once again a critical role of IL-6 in the prototypical autoimmune disorder. Interestingly, the recovery of microglial IL-6 in MOG-IL6-DIO-ONCx3cr1 females seemed to revert partially the demyelination, in agreement with results obtained with microglial Il6-deficient females at 29 dpi, which showed a trend to decrease the demyelination [19], pointing to in both cases that IL-6 derived from microglia might regulate the demyelination. As stated, another key pathological feature of EAE is the inflammatory response of astrocytes and microglia/macrophages, therefore we quantified the GFAP and IBA-1 immunostaining levels and classify IBA-1 and GFAP+ cells according to their morphology. In general, the lack of IL-6 reduced the microgliosis and astrogliosis in the spinal cord of mice, displaying most of IBA-1+ and GFAP+ cells a resting morphology, in line with IL-6 KO [12]. The recovery of microglial IL-6, in agreement with the mild clinical signs of MOG-IL6-DIO-ONCx3cr1 mice, increased the number of reactive IBA-1+ cells in the white matter and a similar trend was observed regarding IBA-1 immunostaining levels in the gray matter. Somewhat surprisingly, considering the relevance of IL-6 in inflammatory processes of EAE, is that the recovery of microglial IL-6 in MOG-IL6-DIO-ONCx3cr1 mice did not modify the total number of GFAP+ cells in the gray matter, although it reduced the ramified GFAP+ cells and tended to increase the reactive GFAP+ cells. Taking into account the results obtained with microglial Il6-deficient females at 29 dpi, in which deficiency of microglial Il6 tended to reduce IBA-1 immunostaining levels in the white matter, but it did not play a relevant role in the regulation and GFAP immunostaining levels [19], it seems that microglial Il6 may regulate the microgliosis, but it would have a limited function in the regulation of astrogliosis during the inflammatory response to EAE.
Conclusions
In summary, we have presented and validated a novel reversible total IL-6 KO mouse, whose Il6 expression was recovered specifically in microglia. The recovery of microglial Il6 was sufficient to develop a mild version of EAE-related symptoms in females, although it seemed to have a minor role in the regulation of the inflammatory cascade. Furthermore, the reversion of Il6 expression in different cells using this IL6-DIO-KO mouse model, combined with cell-specific Cre transgenic lines, would be key to elucidate the specific functions of IL-6 from each cellular source and study cell-specific IL-6 targeting strategies in EAE and a wide range of autoimmune and neuroinflammatory diseases. Indeed, our group is currently analyzing the role of IL-6 in a mouse model of Alzheimer’s disease and in a mouse model of progressive encephalopathy resembling Leigh syndrome using this novel strategy. Furthermore, the IL6-DIO-KO mouse model will be available for the scientific community and will be distributed worldwide following material transfer agreements with Universitat Autònoma de Barcelona. Finally, we also encourage other investigators to take up this reversible and conditional knock-out strategy to carry out rescue experiments in other genetic models.
Supplementary information
Additional file 1. Probe sequence. Primers Fw:5’-CAGCATCTCATCTGAGTTCCG-3’ and Rv:5’-CTCACTGTTCACAAAGCACAGG-3’ were used to design a unique probe of 621 bp.
Additional file 2. DNA sequencing of the modified Il6 gene of the IL6-DIO-KO mice. The resulting sequence had 1361 bp and the localization, size and color code of the genetic structures (exons and protein binding zones) detected in this sequence are explained in the figure legend. The loxP and lox2272 cassettes (purple and green, respectively) are in the opposite orientation, then sequence between them (including the exon 2) will be reverted after Cre action.
Additional file 3. Kaplan-Meier analysis in the EAE experiment with IL6-DIO-KO and WT mice. Mice were assigned status “sick” on the first day their score was ≥1 and the proportion of sick mice was represented for each day. Mice that never showed scores above the threshold were censored. The time-course of disease for IL6-DIO-KO mice is significantly delayed, with only one mouse fulfilling the disease criterion (no median). Table shows number of mice, sick mice, median day and 95% confidence interval. NA (not applicable) indicates impossibility to calculate value.
Additional file 4. Kaplan-Meier analysis in the EAE experiment with IL6-DIO-ONCx3cr1, IL6-DIO-KO and IL6-DIO-Het mice. Mice were assigned status “sick” on the first day their score was ≥1 and the proportion of sick mice was represented for each day. The time-course of disease for IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice is significantly delayed. IL6-DIO-KO again showed almost complete resistance to EAE, with only one mouse actually developing the disease (no median). Tables show number of mice, sick mice, median day and 95% confidence interval; as well as post-hoc pair-wise comparisons. NA (not applicable) indicates impossibility to calculate value.
Additional file 5. Quantification of IBA-1 and GFAP immunostaining levels shown in Fig. 8. Quantification was carried out in both white and gray matter of BSA-immunized IL6-DIO-Het and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice. All results were relativized per total area and are represented as mean ± SEM; ●p ≤ 0.05 vs. BSA-IL6-DIO-Het mice; ★p ≤ 0.05 vs. IL6-DIO-Het mice.
Acknowledgements
We thank Profs. Marco Prinz and Steffan Jung for providing Cx3cr1-CreER mice.
Abbreviations
- AUC
Area under the curve
- BSA
Bovine serum albumin
- CD11b
Cluster of differentiation molecule 11b
- CNS
Central nervous system
- DIO
Double-inverted open reading frame
- EAE
Experimental autoimmune encephalomyelitis
- GFAP
Glial fibrillary acidic protein
- GM
Gray matter
- IBA-1
Ionized calcium-binding adaptor molecule-1
- IL-6
Interleukin-6
- LFB
Luxol Fast Blue
- LOFT
LoxP-flippase (FLP) recognition target (FRT) Trap
- LPS
Lipopolysaccharide
- MOG35-55
Myelin oligodendrocyte glycoprotein 35-55 peptide
- MS
Multiple sclerosis
- TAM
Tamoxifen
- WM
White matter
- YFP
Yellow fluorescent protein
Authors’ contributions
Conceptualization: JH and RDP; methodology: PS, OFG, GC, KA, AE, MG; formal analysis and investigation: PS, OFG and JH; writing - —original draft preparation: PS and OFG; writing - —review and editing: JH and RDP; funding acquisition: JH. All authors read and approved the final manuscript.
Funding
PS, OFG, KA, and AE, acknowledge the support of BES-2015-071959, FPU2012-00365, FPU17/02065 and La Marató de TV3 20142210, fellowships respectively. This study was supported by Ministerio de Economía y Competitividad y Fondo Europeo de Desarrollo Regional SAF2014-56546-R and RTI2018-101105-B-I00 to J.H.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee on Animal Experiments of the Universitat Autònoma de Barcelona and the Generalitat de Catalunya (Refs. 3782 and 9684, respectively).
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paula Sanchis and Olaya Fernández-Gayol contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12974-020-01969-0.
References
- 1.Palmiter RD, Brinster R. Transgenic mice. Cell. 1985;41:343–345. doi: 10.1016/S0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination I. recombination between loxP sites. J Mol Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 3.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintana A, Erta M, Ferrer B, Comes G, Giralt M, Hidalgo J. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav Immun. 2013;27:162–173. doi: 10.1016/j.bbi.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Goldmann T, Wieghofer P, Müller PF, Wolf Y, Varol D, Yona S, et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 7.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frei K, Fredrikson S, Fontana A, Link H. Interleukin-6 is elevated in plasma in multiple sclerosis. J Neuroimmunol. 1991;31:147–153. doi: 10.1016/0165-5728(91)90020-8. [DOI] [PubMed] [Google Scholar]
- 10.Maimone D, Guazzi GC, Annunziata P. IL-6 detection in multiple sclerosis brain. J Neurol Sci. 1997;146:59–65. doi: 10.1016/S0022-510X(96)00283-3. [DOI] [PubMed] [Google Scholar]
- 11.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Giralt M, Ramos R, Quintana A, Ferrer B, Erta M, Castro-Freire M, et al. Induction of atypical EAE mediated by transgenic production of IL-6 in astrocytes in the absence of systemic IL-6. Glia. 2013;61:587–600. doi: 10.1002/glia.22457. [DOI] [PubMed] [Google Scholar]
- 13.Okuda Y, Sakoda S, Bernard CCA, Fujimura H, Saeki Y, Kishimoto T, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 14.Samoilova EB, Horton JL, Hilliard B, Liu TT, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactiveT cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, et al. MHC class II–dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol. 2017;18:74–85. doi: 10.1038/ni.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erta M, Giralt M, Jiménez S, Molinero A, Comes G, Hidalgo J. Astrocytic IL-6 influences the clinical symptoms of EAE in mice. Brain Sci. 2016;6:E15. doi: 10.3390/brainsci6020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchis P, Fernández-gayol O, Comes G, Escrig A, Giralt M, Palmiter RD, et al. Interleukin-6 derived from the central nervous system may influence the pathogenesis of experimental autoimmune encephalomyelitis in a cell-dependent manner. Cells. 2020;9. [DOI] [PMC free article] [PubMed]
- 20.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356. [DOI] [PMC free article] [PubMed]
- 21.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 22.Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1150. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 24.Farley F, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. doi: 10.1002/1526-968X(200011/12)28:3/4<106::AID-GENE30>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchis P, Fernández-Gayol O, Vizueta J, Comes G, Canal C, Escrig A, et al. Microglial cell-derived interleukin-6 influences behavior and inflammatory response in the brain following traumatic brain injury. Glia. 2020;68:999–1016. doi: 10.1002/glia.23758. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH image of ImageJ: 25 years of Umage analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 29.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turan S, Bode J. Site-specific recombinases: from tag-and-target- to tag-and-exchange-based genomic modifications. FASEB J. 2011;25:4088–4107. doi: 10.1096/fj.11-186940. [DOI] [PubMed] [Google Scholar]
- 33.Samokhvalov IM, Thomson AM, Lalancette C, Liakhovitskaia A, Ure J, Medvinsky A. Multifunctional reversible knockout/reporter system enabling fully functional reconstitution of the AML1/Runx1 locus and rescue of hematopoiesis. Genesis. 2006;44:115–121. doi: 10.1002/gene.20190. [DOI] [PubMed] [Google Scholar]
- 34.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Vink M, Klaver B, Berkhout B, Das AT. Optimization of the Tet-on system for regulated gene expression through viral evolution. Gene Ther. 2006;13:1382–1390. doi: 10.1038/sj.gt.3302780. [DOI] [PubMed] [Google Scholar]
- 37.Heyer J, Kwong LN, Lowe SW, Chin L. Non-germline genetically engineered mouse models for translational cancer research. Nat Rev Cancer. 2010;10:470–480. doi: 10.1038/nrc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu E, Tang Y-P, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 39.Chaiyachati BH, Kaundal R, Zhao J, Wu J, Flavell R, Chi T. LoxP-FRT trap (LOFT): a simple and flexible system for conventional and reversible gene targeting. BMC Biol. 2012;10:96. doi: 10.1186/1741-7007-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopf M, Baumannt H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 41.Mendel I, Katz A, Kozak N, Ben-Nun A, Revel M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur J Immunol. 1998;28:1727–1737. doi: 10.1002/(SICI)1521-4141(199805)28:05<1727::AID-IMMU1727>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brod SA, Bauer VL. Ingested (oral) tocilizumab inhibits EAE. Cytokine. 2014;68:86–93. doi: 10.1016/j.cyto.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 45.Murphy ÁC, Lalor SJ, Lynch MA, Mills KHG. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of Fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 48.Valny M, Honsa P, Kirdajova D, Kamenik Z, Anderova M. Tamoxifen in the mouse brain: implications for fate-mapping studies using the tamoxifen-inducible cre-loxP system. Front Cell Neurosci. 2016;10:1–12. doi: 10.3389/fncel.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellisor D, Zervas M. Tamoxifen dose response and conditional cell marking: is there control? Mol Cell Neurosci. 2010;45:132–138. doi: 10.1016/j.mcn.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Bebo BF, Dehghani B, Foster S, Kurniawan A, Lopez FJ, Sherman LS. Treatment with selective estrogen receptor modulators regulates myelin specific T-cells and suppresses experimental autoimmune encephalomyelitis. Glia. 2009;57:777–790. doi: 10.1002/glia.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 52.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;701:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T H17 cells. Nature. 2007;448:484–488. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2009;183:2079–2088. doi: 10.4049/jimmunol.0900242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Probe sequence. Primers Fw:5’-CAGCATCTCATCTGAGTTCCG-3’ and Rv:5’-CTCACTGTTCACAAAGCACAGG-3’ were used to design a unique probe of 621 bp.
Additional file 2. DNA sequencing of the modified Il6 gene of the IL6-DIO-KO mice. The resulting sequence had 1361 bp and the localization, size and color code of the genetic structures (exons and protein binding zones) detected in this sequence are explained in the figure legend. The loxP and lox2272 cassettes (purple and green, respectively) are in the opposite orientation, then sequence between them (including the exon 2) will be reverted after Cre action.
Additional file 3. Kaplan-Meier analysis in the EAE experiment with IL6-DIO-KO and WT mice. Mice were assigned status “sick” on the first day their score was ≥1 and the proportion of sick mice was represented for each day. Mice that never showed scores above the threshold were censored. The time-course of disease for IL6-DIO-KO mice is significantly delayed, with only one mouse fulfilling the disease criterion (no median). Table shows number of mice, sick mice, median day and 95% confidence interval. NA (not applicable) indicates impossibility to calculate value.
Additional file 4. Kaplan-Meier analysis in the EAE experiment with IL6-DIO-ONCx3cr1, IL6-DIO-KO and IL6-DIO-Het mice. Mice were assigned status “sick” on the first day their score was ≥1 and the proportion of sick mice was represented for each day. The time-course of disease for IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice is significantly delayed. IL6-DIO-KO again showed almost complete resistance to EAE, with only one mouse actually developing the disease (no median). Tables show number of mice, sick mice, median day and 95% confidence interval; as well as post-hoc pair-wise comparisons. NA (not applicable) indicates impossibility to calculate value.
Additional file 5. Quantification of IBA-1 and GFAP immunostaining levels shown in Fig. 8. Quantification was carried out in both white and gray matter of BSA-immunized IL6-DIO-Het and MOG35-55-immunized IL6-DIO-Het, IL6-DIO-ONCx3cr1 and IL6-DIO-KO mice. All results were relativized per total area and are represented as mean ± SEM; ●p ≤ 0.05 vs. BSA-IL6-DIO-Het mice; ★p ≤ 0.05 vs. IL6-DIO-Het mice.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.