Pseudomonas aeruginosa is one of the most critical multidrug-resistant opportunistic pathogens in humans, able to cause both lethal acute and chronic lung infections. Thorough knowledge of the regulatory mechanisms involved in the establishment and persistence of the airways infections by P. aeruginosa remains elusive. Emerging candidates as molecular regulators of pathogenesis in P. aeruginosa are small RNAs, which act posttranscriptionally as signal transducers of host cues. Known for being involved in the regulation of biofilm formation and responsive to envelope stress response, we show that the small RNA ErsA can play regulatory roles in acute infection, stimulation of host inflammatory response, and mechanisms of acquirement of antibiotic resistance and adaptation during the chronic lung infections of cystic fibrosis patients. Elucidating the complexity of the networks regulating host-pathogen interactions is crucial to identify novel targets for future therapeutic applications.
KEYWORDS: ErsA, Pseudomonas aeruginosa, antibiotic resistance, clinical isolates, cystic fibrosis, mouse model of infection, opportunistic infections, pathogenicity, respiratory infections, small RNAs, virulence
ABSTRACT
Bacterial small RNAs play a remarkable role in the regulation of functions involved in host-pathogen interaction. ErsA is a small RNA of Pseudomonas aeruginosa that contributes to the regulation of bacterial virulence traits such as biofilm formation and motility. Shown to take part in a regulatory circuit under the control of the envelope stress response sigma factor σ22, ErsA targets posttranscriptionally the key virulence-associated gene algC. Moreover, ErsA contributes to biofilm development and motility through the posttranscriptional modulation of the transcription factor AmrZ. Intending to evaluate the regulatory relevance of ErsA in the pathogenesis of respiratory infections, we analyzed the impact of ErsA-mediated regulation on the virulence potential of P. aeruginosa and the stimulation of the inflammatory response during the infection of bronchial epithelial cells and a murine model. Furthermore, we assessed ErsA expression in a collection of P. aeruginosa clinical pulmonary isolates and investigated the link of ErsA with acquired antibiotic resistance by generating an ersA gene deletion mutant in a multidrug-resistant P. aeruginosa strain which has long been adapted in the airways of a cystic fibrosis (CF) patient. Our results show that the ErsA-mediated regulation is relevant for the P. aeruginosa pathogenicity during acute infection and contributes to the stimulation of the host inflammatory response. Besides, ErsA was able to be subjected to selective pressure for P. aeruginosa pathoadaptation and acquirement of resistance to antibiotics commonly used in clinical practice during chronic CF infections. Our findings establish the role of ErsA as an important regulatory element in the host-pathogen interaction.
IMPORTANCE Pseudomonas aeruginosa is one of the most critical multidrug-resistant opportunistic pathogens in humans, able to cause both lethal acute and chronic lung infections. Thorough knowledge of the regulatory mechanisms involved in the establishment and persistence of the airways infections by P. aeruginosa remains elusive. Emerging candidates as molecular regulators of pathogenesis in P. aeruginosa are small RNAs, which act posttranscriptionally as signal transducers of host cues. Known for being involved in the regulation of biofilm formation and responsive to envelope stress response, we show that the small RNA ErsA can play regulatory roles in acute infection, stimulation of host inflammatory response, and mechanisms of acquirement of antibiotic resistance and adaptation during the chronic lung infections of cystic fibrosis patients. Elucidating the complexity of the networks regulating host-pathogen interactions is crucial to identify novel targets for future therapeutic applications.
INTRODUCTION
The bacterium Pseudomonas aeruginosa is a common pathogen associated with respiratory tract infections in patients with diverse diseases (1–3). P. aeruginosa causes fatal acute lung infections in critically ill individuals who are, for instance, hospitalized, intubated in an intensive care unit, or immunocompromised (e.g., transplant recipients and patients with burns, cancer, and neutropenia or infected with HIV). In acute pneumonia, P. aeruginosa causes necrosis of the lung epithelium and disseminates into circulation, resulting in septic shock and multiple organ failure. P. aeruginosa is also a major cause of chronic lung infections in individuals with cystic fibrosis (CF), non-CF bronchiectasis, and chronic obstructive pulmonary disease (COPD). It was shown that long-term P. aeruginosa persistence in CF airways triggers tissue remodeling that finally leads to lung function decline and ultimately results in respiratory failure.
Biofilm formation is a well-known essential requisite for P. aeruginosa during chronic airway infections (4). However, the relevant role of biofilm aggregation of P. aeruginosa on the apical surface of polarized epithelial cells at early time points of acute lung infections has also been pinpointed (5–7), challenging the classical notion that acute infections are associated only with the planktonic lifestyle. Indeed, P. aeruginosa initiates most acute infections with a transition from planktonic bacteria to host cell-attached aggregates (7). Initial binding of individual sentinel bacteria at the mucosal barrier through two major adhesins, flagella, and retractile type IV pili (8) leads in few minutes to the recruitment of free-swimming bacteria, with the resultant formation of antibiotic-resistant biofilm-like bacterial aggregates of 10 to hundreds of bacteria embedded in an exopolysaccharide (EPS) and extracellular DNA (eDNA) matrix and localized in spots on the host cell surface (5, 6). Surface-bound bacterial aggregates, and not individual bacteria, trigger a dramatic remodeling of the apical membrane, namely, the formation of protrusions (6). Apical membrane remodeling is linked with localized nuclear translocation of NF-κB underneath aggregates but not beneath single bacteria (9). This indicates the activation of the innate immune response to bacterial aggregates (9). However, aggregate-induced protrusion formation is necessary, but not sufficient, for activation of the innate immune response (9). Indeed, NF-κB activation and the subsequent production of proinflammatory cytokines require both pathogen-induced membrane protrusions and the recognition of pathogen-associated molecular patterns (PAMPs) such as flagellin or lipopolysaccharide (LPS) via the cognate Toll-like receptors (TLRs) (10). Once mucosal colonization is established, P. aeruginosa delivers a large battery of virulence factors to cause disease, for instance, through the type III secretion system (T3SS) that is also required for the bacterial aggregate-mediated induction of membrane protrusions (6). At this stage of acute infection, all virulence factors participate, at different levels, in the cytotoxicity of P. aeruginosa that leads to bypassing the epithelial barrier and then to invasion and systemic dissemination (1). Most of the P. aeruginosa invasive functions characteristic of acute infection are selected against in CF chronic infection, leading to less-virulent but more-persistent phenotypes (4, 10).
The two pathogenetic processes associated with the progression of P. aeruginosa airway infection toward either rapid and acute systemic dissemination or chronic colonization are complex and depend on the coordinate up- or downregulation of several virulence lifestyle functions that imply both short- and long-term adaptations to the host environment (4, 11, 12). For instance, in the pathogenesis of CF chronic infections, P. aeruginosa adapted variants can shape the innate immune response favoring their persistence and contribute to the emergence of CF airway hallmarks (13). P. aeruginosa adaptive response leading to pathogenesis relies on a wide, intricate, and “prone-to-remodeling” regulatory network formed both by transcription factors and posttranscriptional regulators, including small RNAs (sRNAs) (14–16). The dynamicity of this regulatory network is frequently observed during the adaptive radiation of P. aeruginosa for long-term persistence in the CF lung environment, where bacteria endure various attacks, encompassing oxidative stresses, immune responses, and prolonged antibiotic treatments. To survive these harsh conditions, initial infecting P. aeruginosa clones undergo substantial phenotypic changes that may include slow growth, auxotrophy, virulence attenuation, loss of motility, mucoid capsule, biofilm formation, hypermutability, lipopolysaccharide (LPS) modifications, and antibiotic resistance (17). Analysis of several CF clinical isolates showed that adaptive mutations in approximately 50 genes are mainly responsible for the convergent molecular evolution toward the above-mentioned phenotypes. Of these genes, common mutations occur in around 15 regulatory genes for transcription factors and are supposed to be at the base of remodeling of the infection regulatory network leading to P. aeruginosa adaptation to the CF lung (17). These regulatory pathoadaptive mutations also involve genes for alternative sigma factors such as PvdS, σ54 (RpoN), and σ22 (AlgT/U) and its repressor MucA (17, 18). P. aeruginosa σ22 is the functional homolog of Escherichia coli σE (19) that, along with several σE-regulated sRNAs (20, 21), orchestrates the envelope stress response, which in Gram-negative bacteria is critical for maintaining envelope integrity in the host environment and thus to successfully cause infection (20–22).
Generally, sRNAs are key components of the regulatory networks involved in the adaptive response to the stressful conditions that pathogenic bacteria experience during host infection (16, 20–25). Specific protein-RNA and RNA-RNA interactions in the P. aeruginosa adaptive regulatory network have been identified for approximately 16 sRNAs (16). One such P. aeruginosa sRNA is ErsA, a 132-nucleotide (nt)-long transcript that was described for the first time in a work in which 52 novel sRNAs were identified in PAO1 (26) and PA14 (27), two prototype laboratory strains in which ErsA was shown to be similarly expressed under laboratory conditions (28). Later, ErsA expression was shown to be strictly dependent on and responsive to envelope stress by σ22 (29). Other infection cues such as temperature shifts from environmental to body temperature and reduced oxygen conditions upregulate ErsA expression (29). Functional studies showed that ErsA contributes to the regulation of virulence traits such as biofilm formation and motility (29, 30). Phenotypically, the knockout ersA mutant strain forms a flat and uniform biofilm and shows enhanced swarming and twitching capability (30). ErsA influences the dynamics of exopolysaccharide production and the consequent biofilm formation via negative posttranscriptional regulation of algC mRNA (29). The algC gene encodes a key point enzyme that coordinates the alginate biosynthetic pathway and the synthesis of several P. aeruginosa polysaccharide exoproducts such as Psl, Pel, LPS, and rhamnolipids (31–33). Like that for ErsA, the expression of algC is also dependent on σ22 (34, 35), which generates an incoherent feed-forward loop to fine-tune the expression of the AlgC enzyme. Besides acting as a positive posttranscriptional regulator, ErsA also stimulates exopolysaccharide production and biofilm formation through the posttranscriptional activation of AmrZ (30), a transcription factor known to regulate alginate production and motility and indicated as a molecular switch that triggers biofilm maturation in P. aeruginosa. Moreover, ErsA regulatory activity impacts considerably the P. aeruginosa transcriptome. More than 160 genes are differentially expressed in transcriptome sequencing (RNA-seq) experiments comparing the knockout ersA mutant with the PAO1 wild type. Among these are genes for biofilm formation and motility regulation that also belong to the AmrZ regulon. Furthermore, other differentially expressed genes in the ΔersA mutant are involved in several aspects of P. aeruginosa-host interaction, such as denitrification and nitrate metabolism, nitrate transport, type VI and III secretion system effectors, energy and carbon metabolism, heat shock proteins, and pyocyanin production (30).
Overall, the ErsA ability to respond to host cues and influence the expression of several virulence-associated genes was thought to play a relevant role during host infection. Also, ErsA was implicated in other aspects of P. aeruginosa lifestyle linked to infection processes, such as niche establishment/protection in mixed populations and antibiotic resistance. Indeed, ErsA was suggested to also coordinate biofilm maturation dynamics during mixed-species biofilm growth (36). In the presence of Staphylococcus aureus, ErsA is part of that 0.3% of the P. aeruginosa genome which becomes differentially expressed. The increase of its transcription level suggests a role not only in counteracting agents produced by S. aureus but also in modulating the state of the exopolymeric matrix for typical biofilm maturation (36). ErsA was also shown to negatively regulate oprD mRNA (37), coding for the OprD porin that is the major channel for entry of the carbapenem antibiotics into the periplasm of P. aeruginosa. Coherent with these results, strains lacking ErsA were more susceptible to meropenem than the PAO1 wild-type strain (37).
This study aimed to assess the role of ErsA in the regulatory network of P. aeruginosa pathogenicity in the infection of the airways. Here, we provide evidence that the ErsA-mediated regulation is relevant during acute infection and contributes to the stimulation of the host inflammatory response. Besides, ErsA could also play a regulatory role during chronic infection, in mechanisms of adaptation and acquirement of antibiotic resistance leading to the typical resilient phenotype of P. aeruginosa in the CF airways.
RESULTS
ErsA contributes to the regulation of bacterial functions involved in cytotoxicity and stimulation of the proinflammatory response.
We started to evaluate the role of ErsA in virulence and pathogenicity of P. aeruginosa by using an in vitro infection system based on pulmonary cell lines. Infections were performed with two prototype laboratory strains, PA14 and PAO1, which are hypervirulent and moderately virulent, respectively, along with their knockout counterparts (ΔersA), or with ersA-overexpressing PAO1 strains in a wild-type genetic background. The overexpression of ErsA by the vector pGM-ersA mimics the increase in ErsA levels induced by the σ22-mediated envelope stress response, producing a 5-fold increase of the sRNA levels (29).
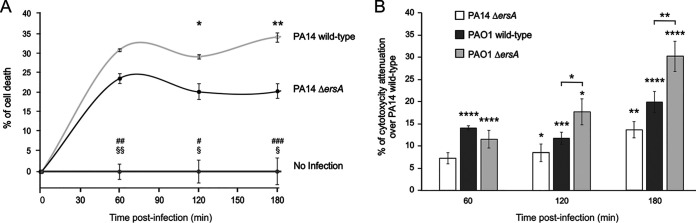
We evaluated the influence of ErsA on the cytotoxicity elicited by P. aeruginosa during the infection of proliferating CF bronchial epithelial cells, IB3-1, through the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) colorimetric assay. We exposed pulmonary IB3-1 cells to PA14 or PAO1 wild-type strains and their corresponding ΔersA mutants and followed cell viability by MTS assay during the subsequent 3 h. Nonexposed cells were used as a control of unaffected viability in the experimental time window (Fig. 1A and B). Infection of IB3-1 cells with the PA14 wild-type strain (Fig. 1A) caused significant cytotoxic effects, 30% cell death (loss of viable cells) compared to the noninfected control at the first time point postinfection (60 min). At later time points of 120 and 180 min, the percentage of dead cells was supposed to mirror the pattern of the ratio between death and proliferation rates. Under the same conditions, the PA14 ΔersA strain showed an interestingly different trend of infection-induced cytotoxicity. Following the killing effects detected at 60 min postinfection, the percentage of dead IB3-1 cells significantly and progressively decreased relative to that for the PA14 wild-type infection at time points of 120 and 180 min, indicating a cytotoxicity attenuation of the PA14 ΔersA strain (Fig. 1A and B).
FIG 1.
Deletion of ErsA results in decreased P. aeruginosa-induced cytotoxicity of pulmonary cells. (A) Time course of cell death of CF bronchial epithelial cells after bacterial infection with P. aeruginosa PA14 wild-type and ΔersA. Viability of IB3-1 cells uninfected (no infection) or infected with an MOI of 100 (PA14 wild-type and PA14 ΔersA) was analyzed by MTS assay. At each time point, results are plotted as the ratio of the average values for infected (blank subtracted) cells to that for the uninfected cells. The data are pooled from three independent experiments and are represented as means ± standard errors of the means (SEMs). Significance by one-way analysis of variance (ANOVA) with post hoc Tukey’s honestly significant difference (HSD) is indicated as follows. PA14 wild-type versus ΔersA: *, P < 0.05; **, P < 0.01. PA14 wild-type versus no infection: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. PA14 ΔersA versus no infection: §, P < 0.05; §§, P < 0.01. (B) Relative viability percentages of IB3-1 cells after bacterial infection as measured by MTS assay. Cytotoxicity attenuation (%) of PAO1 wild-type, PAO1 ΔersA, and PA14 ΔersA is shown with respect to the PA14 wild-type strain during infection of IB3-1 cells with an MOI of 100. At each time point after infection, results are plotted as the ratio of the average values for infected (blank subtracted) cells to that for the uninfected cells. Results are shown as the difference of the ratios between each strain and PA14 wild type. Data are pooled from three independent experiments and are represented as means ± SEMs. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 in the one-way ANOVA with post hoc Tukey’s HSD. Significance of each strain versus PA14 wild-type is indicated above single histograms.
The infection-induced cytotoxicity was assessed for PAO1 wild-type and PAO1 ΔersA in the same experiments described above. Figure 1B reports the results in terms of percentage of cytotoxicity attenuation compared to that in PA14 wild-type infections shown in Fig. 1A, namely, the differences, at each time point, between the percentage of dead IB3-1 cells elicited by PA14 wild-type, the most virulent strain of the panel, and those for the other strains. As expected, the PAO1 wild-type strain showed an attenuated phenotype compared to PA14 wild type. At each time point, the percentages of dead IB3-1 cells were significantly lower than during the infection with PA14 wild type (i.e., a positive percentage of attenuation) (Fig. 1B). The same was true for the PAO1 ΔersA strain but with an extremely relevant difference. Indeed, the percentage of cytotoxicity attenuation was significantly higher than that in PAO1 wild type at 120 min and further increased at 180 min postinfection. These results for PAO1 are consistent with those presented above for PA14 and strongly suggest that the loss of ErsA affects the cytotoxic potential of P. aeruginosa.
We then evaluated the impact of ErsA on the inflammatory response of the IB3-1 cells by monitoring the infection-induced secretion of the proinflammatory marker interleukin 8 (IL-8), the major chemokine associated with neutrophil extravasation from the vasculature into the lumen of the airways when respiratory epithelial cells are exposed to P. aeruginosa. To minimize negative effects that could perturb a robust evaluation of IL-8 secretion, we set out to expose the IB3-1 cells to P. aeruginosa strains at a multiplicity of infection (MOI) 103-fold lower than the infection experiments described above. However, PA14-based strains at this MOI still caused relevant cell death, suffering, and detachment from the plastic surface. Such effects were negligible following infection with PAO1-based strains that thus were chosen for the subsequent analyses as follows.
IB3-1 cells were exposed to bacteria for 2 h, washed, supplemented with amikacin to kill bacteria, and further incubated in the presence of fresh medium with amikacin. Uninfected cells treated and incubated under the same conditions were used as a control of nonstimulated IL-8 production. The amounts of IL-8 released in the supernatants by IB3-1 cells at 24 h postinfection were measured through an enzyme-linked immunosorbent assay (ELISA). Our results showed that the infection of IB3-1 cells with P. aeruginosa PAO1 ΔersA causes a significant decrease in the secretion of IL-8 of approximately 37% compared to that for the infection with PAO1 wild type (Fig. 2A). Consistently, IB3-1 cells infected with the ErsA-overexpressing PAO1 strain showed an increase of IL-8 secretion of approximately 30% compared to that in cells infected with the PAO1 strain harboring the empty vector pGM931 (Fig. 2B). Given the difference in the growth conditions of the markerless strains (PAO1 wild-type and ΔersA strains) (Fig. 2A) and the vector-harboring strains (PAO1/pGM931 and PAO1/pGM-ersA) (Fig. 2B), the absolute amounts of secreted IL-8 are not comparable in these two experimental settings. In the latter case, the presence of carbenicillin for vector maintenance and arabinose for induction of ErsA expression may influence the bacterial physiology and, as a consequence, affect the degree of the IB3-1 inflammatory response. In any case, higher ErsA levels in bacterial cells result in a significantly higher secretion of IL-8 in infected IB3-1 cells, as can be concluded from the analysis of both these experimental sets.
FIG 2.
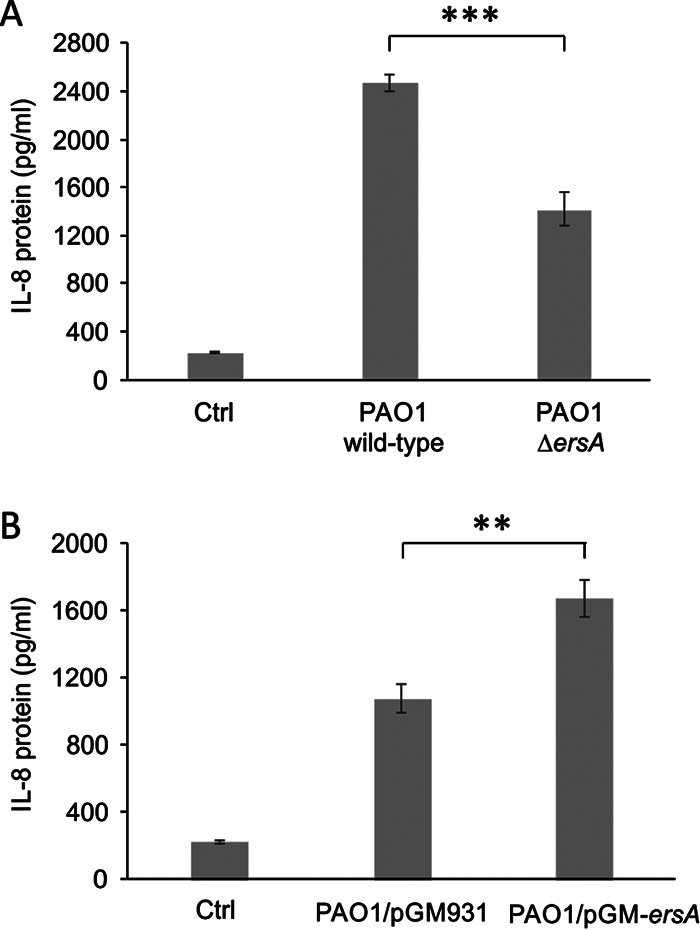
ErsA levels influence the proinflammatory response in pulmonary cells. Inflammatory response of CF bronchial epithelial cells after stimulation with P. aeruginosa PAO1 wild-type and PAO1 ΔersA deleted mutant strains (A) and P. aeruginosa PAO1 strain harboring the empty vector pGM931 or the sRNA-overexpressing vector pGM-ersA (B). IL-8 was evaluated by ELISA in supernatants of IB3-1 cells 24 h postinfection (MOI = 0.1). Uninfected IB3-1 cells were used as control (Ctrl). Data are represented as means ± SEMs. The data are pooled from three independent experiments. **, P < 0.01; ***, P < 0.001 in the Student’s t test.
Overall, these in vitro infection models indicated that the ErsA regulatory role impacts P. aeruginosa-induced cytotoxicity and contributes to the stimulation of the proinflammatory response of infected epithelial cells.
The deletion of ErsA impairs virulence and decreases the proinflammatory response in a murine model of airway infection.
To further assess the ErsA involvement in the pathogenicity of P. aeruginosa, we observed the infection outcomes in two groups of immunocompetent C57BL/6NCrlBR mice whose lungs were inoculated with either the PAO1 wild-type or PAO1 ΔersA strain. For this assessment, we followed a protocol of airway infection in which mice are inoculated with bacterial cells embedded in agar beads and monitored for 13 days. We selected this model of infection since it allows the simultaneous analysis of the effects of bacterial mutations on both acute and chronic infection rates. Indeed, agar beads provide microaerobic/anaerobic conditions that allow bacteria to experience a lung environment resembling that of CF (and COPD) patients, which is characterized by a thick mucus (13, 38–40). Under these conditions, infecting bacteria can either colonize, spread locally and persist in lung establishing a chronic infection, or undertake early systemic dissemination and eventually induce death (acute infection). Alternatively, bacterial cells can be cleared by the host. Depending on virulence, CF airway adaptation, and the dose of the inoculated bacteria, the fatality rate due to acute infection and the percentage of surviving mice with stable bacterial loads in the lung, signs of chronic infection, can differ considerably in this model of airway infection. For example, the P. aeruginosa CF-adapted strain RP73 elicits very low mortality and a high percentage of chronic infection (approximately 80%), while PAO1 causes significantly higher acute infection-induced mortality and lower chronicity rates (approximately 15% to 20%), thus showing more virulence and lower resilience to host-mediated clearance (38).
Since the PAO1 ΔersA strain was shown to be less proinflammatory than the wild type in the infection experiments in vitro (Fig. 2A), in addition to the assessment of bacterial chronic colonization versus clearance, surviving mice were also inspected for immune system activation markers, both in lung and in bronchoalveolar lavage fluid (BALF). Specifically, neutrophil and macrophage titers were measured in BALF samples while the levels of two proinflammatory mediators of the response of airway epithelial cells to P. aeruginosa infection, namely, the keratinocyte chemoattractant KC (homologous to human IL-8) and the monocyte secretory protein JE (homologous to human monocyte chemoattractant protein-1 [MCP-1]), were assessed in lung homogenates.
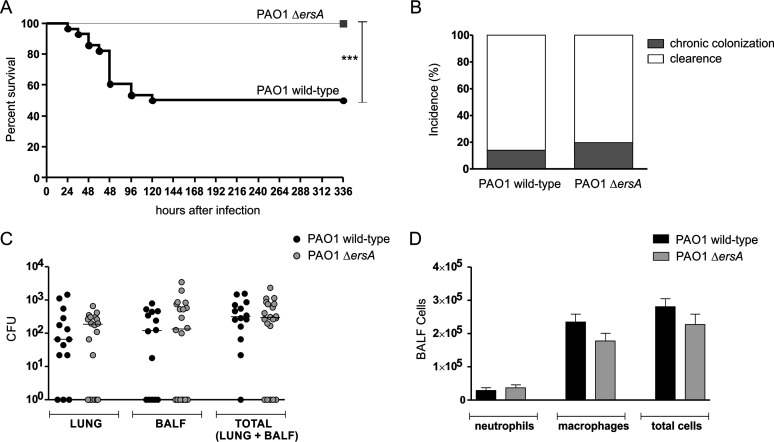
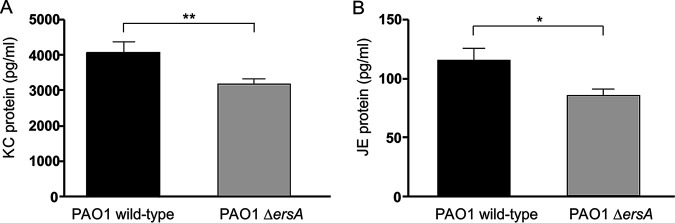
As shown in Fig. 3A, the PAO1 ΔersA mutant strain caused significantly lower mortality than the PAO1 wild-type counterpart with an infection fatality rate of 0% for ΔersA mutant versus 50% for the wild type. Hence, the loss of ErsA resulted in a strong decrease in virulence. Conversely, the incidences of chronic colonization in surviving mice at 13 days postinfection did not differ significantly between ΔersA and wild-type strains (20% versus 14% for ΔersA and wild-type strains, respectively) (Fig. 3B). Likewise, CFU counts were similar in the lungs of mice infected with ΔersA mutant and with the wild-type strain (median values of total CFU: 3.63 × 102 for the ΔersA mutant versus 3.14 × 102 for the wild type) (Fig. 3C). The inflammatory response of mice infected by PAO1 ΔersA in terms of leukocyte recruitment in the bronchoalveolar lavage fluid (BALF) was only slightly lower than in those infected by PAO1 wild type (mean values of total cells: 2.28 × 104 for the ΔersA mutant versus 2.81 × 104 for the wild type) (Fig. 3D). However, when chemokines were measured in lung homogenates, we found that PAO1 ΔersA induced significantly lower levels of both KC and JE than PAO1 wild type (mean values of KC: 3.21 × 103 pg/ml for the ΔersA mutant versus 4.07 × 103 pg/ml for the wild type; mean values of JE: 85.7 pg/ml for the ΔersA mutant versus 115.6 pg/ml for the wild type) (Fig. 4A and B).
FIG 3.
Survival, the incidence of chronic colonization, bacterial burden, and leukocyte recruitment after chronic lung infection by wild-type and ΔersA P. aeruginosa PAO1. C57BL/6NCrlBR mice were infected with 1 × 106 CFU/lung embedded in agar beads. At day 13 postinfection, mice were sacrificed, bronchoalveolar lavage fluid (BALF) was collected, and lungs were excised and homogenized. (A) Survival was evaluated on challenged mice. (B) Clearance (<1,000 CFU of P. aeruginosa from lung plus BALF cultures) and capacity to establish chronic airways infection (≥1,000 CFUs of P. aeruginosa from lung plus BALF cultures) were determined on surviving mice. (C) CFUs were evaluated in the lungs and BALF after plating onto tryptic soy agar. Dots represent values for individual mice, and horizontal lines represent median values. (D) Neutrophils, macrophages, and total cells were measured in the BALF. Values represent the means ± SEMs. The data were pooled from at least three independent experiments (n = 20 to 28). ***, P < 0.001 in the Mantel-Cox test.
FIG 4.
Chemokine levels after chronic lung infection by wild-type and ΔersA P. aeruginosa PAO1. C57BL/6NCrlBR mice were infected with 1 × 106 CFU/lung embedded in agar beads. At day 13 postinfection, mice were sacrificed and lungs were excised and homogenized. KC (A) and JE (B) levels were measured by ELISA in the supernatant fluids of lung homogenates. Values represent the means ± SEMs. The data were pooled from at least three independent experiments (n = 14 to 20). *, P < 0.05; **, P < 0.01 in the nonparametric two-tailed Mann-Whitney U test.
Overall, these results indicated that the ErsA regulatory function strongly impacts the cascade of events leading to acute infection, lethality, and the proinflammatory response. No influence of ErsA on chronic bacterial colonization of airways was visible in this experimental system.
Variable ErsA expression in P. aeruginosa isolates recovered from human airway chronic infections.
Despite the lack of influence on chronic colonization of murine airways, it could be speculated that the attenuation of virulence and a lower stimulation of the immune response potentially deriving from spontaneous deletion, point mutations, or even downregulation of the ersA gene might favor P. aeruginosa persistent lifestyle in human lungs. In PAO1 and PA14 strains, we showed that ErsA expression is strictly dependent on the envelope stress-responsive sigma factor σ22 (29). Moreover, ErsA levels can be fine-tuned in response to other environmental cues by additional transcription factors (29). Comparison of the ersA gene along with its upstream DNA region in several P. aeruginosa isolates from clinical and environmental niches indicated high and extended sequence conservation, including also the −10/−35 core promoter motifs recognized by the RNA polymerase containing σ22 (29). This preliminary observation hinted at the possibility that the ersA gene itself and its expression responsiveness might be conserved independently of the origin of the P. aeruginosa isolates. Nevertheless, it may be feasible that pathoadaptive mutations leading to ersA downregulation contributed to the chronic colonization of the human lung by P. aeruginosa.
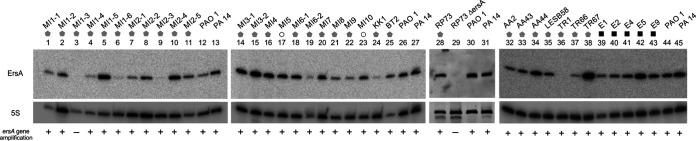
To address this issue, the presence of the ersA gene and its expression levels were assessed in a panel of 31 P. aeruginosa strains isolated in respiratory samples from CF and COPD patients collected during intermittent or chronic infections at different stages and compared with those in 5 P. aeruginosa isolates from environmental habitats (13, 41, 42), using the PAO1 strain as a reference. Detection by PCR of the ersA gene and Northern blot analyses is shown in Fig. 5 and summarized in Table S1 in the supplemental material. The ersA gene was detected in 30 of the 31 clinical isolates and in all environmental strains. In approximately 55.6% of the analyzed strains, the expression levels of ErsA were not significantly different from those detected in PAO1. This set included the 5 environmental strains and 15 clinical isolates. In another assembly of 13 CF strains, ErsA was significantly downregulated: in 10 strains from 2- to 7.5-fold, in 2 strains, MI2-3 and TR1, it was strongly downregulated 28- and 120-fold, respectively, and in one strain, MI1-3, no expression was detected because of the loss of the ersA gene as mentioned above. In only three clinical strains, ErsA was significantly upregulated from 2- to 3-fold. Hence, these results indicated that in 13 of 31 clinical strains analyzed (approximately 42%), ErsA was moderately to strongly downregulated (in one case lost) relative to that in the environmental strains that were shown to express ErsA at levels comparable to PAO1. This would suggest that ErsA expression is under selective pressure in the CF lung and that mutation(s) resulting in ErsA downregulation might contribute in some cases to P. aeruginosa pathoadaptation during CF chronic infections.
FIG 5.
Dissemination of the ersA gene and its expression levels in a collection of clinical isolates. Bacterial strains from CF (gray pentagons), COPD patients (white circles), and environmental isolates (black squares) are indicated at the top. After overnight growth at 37°C on BHI agar plates, culture samples were processed for genomic DNA extraction and total RNA purification and analysis by Northern blotting. PAO1 and PA14 were used as control strains. The presence (+) or absence (−) of the ersA gene is indicated below each Northern blot lane. The relative abundance of ErsA in each isolate was calculated to the reference strain PAO1 after normalization to 5S RNA.
Analysis of ErsA expression in a panel of clinical and environmental strains of P. aeruginosa. Download Table S1, PDF file, 0.2 MB (202.6KB, pdf) .
Copyright © 2020 Ferrara et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ErsA can contribute to P. aeruginosa adaptation to long-term antibiotic treatment.
To further investigate the potential role of ErsA in the P. aeruginosa adaption to the CF lung environment, we considered the emergence of antibiotic resistance that is observed frequently in P. aeruginosa isolates from CF patients following prolonged antibiotic treatment. To this end, we generated a knockout ersA mutant in RP73, one member of the panel of P. aeruginosa clinical isolates that we inspected for ErsA expression (Fig. 5, lanes 28 and 29). We selected RP73 because it was isolated from a CF patient 16.9 years after the onset of chronic colonization and showed acquired multidrug resistance to amikacin, gentamicin, ceftazidime, imipenem, and meropenem (43).
The RP73 ΔersA mutant was tested for the MICs of seven antibiotics commonly used in the clinical practice to which RP73 is resistant. As shown in Table 1, RP73 ΔersA was sensitive to ceftazidime (MIC from 16 in RP73 to 8 μg/ml) and cefepime (MIC from ≥64 in RP73 to 8 μg/ml) and intermediate to meropenem (MIC from ≥16 in RP73 to 4 μg/ml). Furthermore, RP73 ΔersA showed a decrease of MIC from 2 to 1 μg/ml for ciprofloxacin. These results suggest that ErsA could contribute to P. aeruginosa adaptation to long-term antibiotic treatment undergone by CF patients.
TABLE 1.
Antibiotic sensitivity of RP73 and RP73 ΔersA strains
| Antibiotic | Strain | MIC (μg/ml) | Interpretationa |
|---|---|---|---|
| Ceftazidime | RP73 | 16 | R |
| RP73 ΔersA | 8 | S | |
| Cefepime | RP73 | ≥64 | R |
| RP73 ΔersA | 8 | S | |
| Imipenem | RP73 | ≥16 | R |
| RP73 ΔersA | ≥16 | R | |
| Meropenem | RP73 | ≥16 | R |
| RP73 ΔersA | 4 | I | |
| Amikacin | RP73 | ≥64 | R |
| RP73 ΔersA | ≥64 | R | |
| Gentamicin | RP73 | ≥16 | R |
| RP73 ΔersA | ≥16 | R | |
| Ciprofloxacin | RP73 | 2 | R |
| RP73 ΔersA | 1 | R |
R, resistant; I, intermediate; S, sensitive to the indicated antibiotic according to the European Society of Clinical Microbiology and Infectious Diseases (EUCAST) guidelines.
DISCUSSION
We investigated the regulatory role of ErsA in the pathogenicity and adaptation of P. aeruginosa during the infection of the airways. Before this study, several features of ErsA suggested its involvement in the interaction with the host. ErsA regulates EPS production (29) and positively influences biofilm formation and maturation (30). Besides, ErsA responds to cues that are related to airway infection, both at early and late stages, such as a shift from room to body temperature, oxygen availability, iron concentration, and σ22-mediated envelope stress response (29), the latter strongly involved in pathogenicity regulation in Gram-negative bacteria (21). ErsA is also involved in the resistance to carbapenem antibiotics through the negative regulation of the porin OprD (37). The results presented here indicate that the regulatory function exerted by ErsA is relevant in the airways for the progression of P. aeruginosa acute infection and might also endure remodeling during the adaptive process leading to P. aeruginosa persistence in CF lungs.
Most information on ErsA regulatory function(s) in our past work (29, 30) and this study is derived from observations of variant phenotypes displayed by P. aeruginosa ΔersA mutants of the two laboratory strains PAO1 and PA14. We generated both mutant strains by targeted mutagenesis (29) using a robust method of precise genome editing for producing markerless gene replacements in Pseudomonas (44). This protocol of allelic exchange was tailored to generate targeted deletion mutants devoid of nonhomologous off-site rearrangements and also validated for not increasing the rate of random mutagenesis, which might cause secondary mutations both at the genome-wide level and at the site where the homologous exchange occurs. As verified by sequencing, we were able to produce accurately by this procedure the planned deletion of the ersA gene in both PAO1 and PA14 (29). In the present study, these independently obtained ersA-deleted strains showed similar attenuated virulence phenotypes if compared with the corresponding wild-type strain in the in vitro infection assays (Fig. 1). This observation strongly suggests that the in vitro virulence attenuation is a genuine effect of the ersA deletion and not due to secondary mutations occurring during the generation of the ersA-deleted strains. The latter case appears unlikely. It should be indeed assumed that identical patterns of random secondary mutations arose independently in the two strains.
Experiments for complementing the total loss of ErsA in the ersA deletion mutants with an uncalibrated dosage of ErsA via ectopic expression on a plasmid vector under a heterologous promoter could pose some issues, since the physiological thresholds of ErsA amounts needed for the regulation of the target genes involved in host interaction might not be reached. Supplying extra ErsA amounts, i.e., ectopically overexpressing ErsA in a wild-type background, could be a valid alternative to get insights on the role of ErsA in the regulation of host-pathogen interaction functions. Indeed, ErsA overexpression enhanced IL-8 production by the infected epithelial cells (Fig. 2B), in agreement with the behavior of the PAO1 ΔersA deleted mutant that is impaired in the stimulation activity of IL-8 secretion (Fig. 2A).
The lower in vitro cytotoxicity induced by the P. aeruginosa ΔersA mutants is consistent with the strong decrease of the fatality rate due to acute infection observed for the PAO1 ΔersA strain. Remarkably, we administered to mice the median lethal dose (LD50) of 1 × 106 CFU for PAO1, which was completely ineffective in the case of PAO1 ΔersA. Besides, there is another consistency of results between the in vitro and in vivo infection experiments: the loss of ErsA determines a lower activation of the innate immune response, measured in terms of levels of the NF-κB-dependent proinflammatory mediators IL-8 (in vitro) and KC and JE (in vivo). Hence, the regulatory role of ErsA impacts both the virulence of the acute infection and the innate immune response. To explain these results, we first speculated that ErsA, in response to the lung environment, could positively regulate invasive functions that can also act as PAMPs (e.g., flagella, LPS, T3SS, and ExoS) (10) or the expression of non-PAMP invasive functions and PAMP products. Alternatively, the significant impairment of PAO1 ΔersA in biofilm formation and maturation (30) could justify the simultaneous involvement of ErsA in acute infection and immune response activation. Previous relevant studies (5, 6, 9) have evidenced that, at initial stages of P. aeruginosa acute infection, biofilm aggregates with a canonical matrix composed of Psl, Pel, alginate, and eDNA (4) assemble on airway mucosal surfaces and trigger both a dramatic remodeling of the apical membrane (i.e., protrusions) of epithelial cells and NF-κB-dependent activation of the innate immune response. Neither protrusion formation nor NF-κB activation was observed upon binding of individual bacteria to epithelial cells. This strongly indicated that biofilm formation is a key P. aeruginosa function to initiate acute infection and, through the induced changes in epithelial cell polarity, a danger signal for host cells that warns of an incoming threat (7). On the bases of this model, we suggest that acute virulence attenuation and decreased proinflammatory stimulation of PAO1 ΔersA could be due to its defect in biofilm formation and maturation (30). This phenotype induced by the loss of ErsA was attributed to the dysregulation of the expression of AlgC (29, 33) and AmrZ (45, 46), two proteins that play important roles in posttranscriptional and transcriptional regulation, respectively, of the production of Psl, Pel, and alginate. Furthermore, transcriptomics analysis indicated that the pelCDEFG genes for Pel biosynthesis (47), the ppyR gene for an activator of the Psl operon coding for the Psl biosynthetic pathway (48), and the algD gene for alginate biosynthesis are significantly downregulated in the PAO1 ΔersA mutant (30). The significant impairment of PAO1 ΔersA in biofilm formation is complemented by the ectopic expression of ErsA (30). This indicated that biofilm impairment is not the consequence of secondary mutations within the ersA mutant background occurring potentially during the mutant generation.
Specifically, Psl and Pel (referred to as aggregative EPS) are important for initiating and maintaining cell-cell interaction in biofilms, while alginate (referred to as capsular EPS) is instrumental in biofilm maturation, structural stability, and protection from antibiotics (49). To further assess the role of ErsA in the regulation of Psl- and Pel-linked aggregation and adherence, we performed the experiments shown in Fig. S1 in the supplemental material. As a result of ErsA deletion, aggregation and adherence potentials of PAO1 are strongly reduced, while they are enhanced when ErsA is overexpressed. Overall, ErsA could participate in the regulation of biofilm formation at the early stages of acute infection. However, this scenario might be wider since (i) transcriptomics data (30) suggested that ErsA deletion can affect other aspects of P. aeruginosa interaction with its host and (ii) at the posttranscriptional level, ErsA could influence the expression of virulence-associated genes other than algC and amrZ.
ErsA influences aggregation and adherence of the P. aeruginosa PAO1 strain. P. aeruginosa PAO1 strains with wild-type, deleted, or overexpressed ErsA grown in liquid T-broth in presence of 40 μg/ml of Congo red. To observe aggregation, strains were inoculated at optical density at 600 nm (OD600) of 0.05 using glass culture tubes with 10 ml of medium and incubated overnight at 37°C in a roller drum. Carbenicillin and arabinose were added for ectopic expression of ErsA from pGM-ersA and the growth of the control culture harboring the pGM931 empty vector. Adherent biomass is noticeable on the culture tube of the PAO1 wild-type strain. Conversely, the absence of adherence is shown in the ΔersA strain tube. More abundant aggregation and biofilm are present when ErsA is overexpressed by pGM-ersA from the wild-type background than in the empty vector-harboring control strain PAO1/pGM931 grown under the same conditions. Download FIG S1, TIF file, 2.7 MB (2.6MB, tif) .
Copyright © 2020 Ferrara et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The deletion of ErsA did not influence the chronic infection rate of PAO1 in the mouse model. However, virulence attenuation and lower recognition by the immune system showed by PAO1 ΔersA are favorable traits for P. aeruginosa chronic infection of CF airways (4, 10). Therefore, we evaluated whether ErsA expression could be downregulated or even deleted in a panel of CF clinical isolates.
A significant proportion (approximately 42%) of the clinical strains analyzed showed that ErsA was moderately to strongly downregulated (in one case lost) relative to that in both environmental strains and PAO1. This suggested that the ersA gene, under selective pressure, lower its expression in a CF context. This phenomenon might contribute in some cases to P. aeruginosa pathoadaptation toward low virulence and evasion of the immune system during CF chronic infections (10). We speculate that this putative evolution of ErsA expression occurs in the frame of the remodeling process involving the infection regulatory network, in which σ22 is one main component, that leads to P. aeruginosa adaptation to CF lung (17).
Finally, we found that loss of ErsA induces sensitization to ceftazidime, cefepime, and meropenem in the multidrug-resistant clinical isolate RP73, which was in the group of CF clinical isolates analyzed for ErsA expression. It is worth noting that RP73 was demonstrated to establish long-term infection, replacing an initial isolate (RP1) and adapting within CF airways compared to its clonal ancestor RP45 (43). The adaptive microevolution has led RP73 to differentiate significantly from RP45 in terms of virulence and antibiotic resistance, with RP45 being more virulent than RP73 and sensitive to amikacin, ceftazidime, imipenem, and meropenem (43). The multiantibiotic resistance that RP73 has acquired compared to its clonal ancestor RP45 and that is lost in RP73 ΔersA reveals an interesting link between ErsA and mechanisms of adaptation to host environment during P. aeruginosa chronic infection of CF patients.
The emergence and rapid dissemination of antibiotic resistance demand the development of new antibiotics and antivirulence agents (50). These latter compounds directly target virulence factors or virulence regulators. The contributions to acute infection regulation and the acquirement of antibiotic resistance suggest that ErsA may be a candidate target for the development of novel antivirulence and coantibiotic drugs.
MATERIALS AND METHODS
Ethics statement.
The study on human P. aeruginosa isolates from Hannover was approved by the Ethics Commission of Hannover Medical School, Germany (41). The patients and parents gave oral informed consent before the sample collection. Approval for storing the biological materials was obtained by the Ethics Commission of Hannover Medical School, Germany. The study on human P. aeruginosa isolates from the Regional CF Center of Lombardia was approved by the Ethical Committees of San Raffaele Scientific Institute and Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Milan, Italy, and written informed consent was obtained from patients enrolled or their parents according to the Ethical Committees rules, under the laws of the Italian Ministero della Salute (approval no. 1874/12 and 1084/14) (42).
Animal studies strictly followed the Italian Ministry of Health guidelines for the use and care of experimental animals. This study was performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC; protocol no. 789) of the San Raffaele Scientific Institute (Milan, Italy).
Bacterial strains and culture conditions.
P. aeruginosa strains PAO1 (26), PA14 (27), and RP73 (41, 43) and the corresponding deleted mutants PAO1 ΔersA, PA14 ΔersA (29), and RP73 ΔersA were grown at 37°C in Luria-Bertani rich medium at 120 rpm. sRNA-overexpressing strains PAO1/pGM-ersA and PA14/pGM-ersA and their empty vector-harboring control strains PAO1/pGM931 and PA14/pGM931 (29) were grown with the addition of 300 μg/ml carbenicillin. For PBAD induction in vector plasmid pGM931, arabinose was added to a final concentration of 10 mM. The RP73 ΔersA mutant strain was generated from the multidrug-resistant (MDR) RP73 isolate using a method of markerless gene replacement (44) improved for P. aeruginosa using oligonucleotides and molecular techniques as described previously (29) and cloning in the tetracycline resistance-harboring plasmid pSEVA512S to allow selection of the exconjugant on 30 μg/ml tetracycline.
Bacterial isolate analysis.
Bacterial isolates (13, 41, 42) were plated on 1.5% brain heart infusion (BHI) agar plates and grown overnight at 37°C. Culture samples were taken and processed for genomic DNA and total RNA extraction as described previously (28). PAO1 and PA14 strains treated under the same conditions were used as controls.
Oligonucleotides CGAATGGCTTGAGCCCTTCGATGCT/AAAAAAAACCCCGAGCTTCGTA and TGTCGTCAGCTCGTGTCGTGA/ATCCCCACCTTCCTCCGGT were used for PCR amplification of the genomic region containing the ersA and 16S (as positive PCR control) loci, respectively. Northern blot analyses were performed as described previously (28). Briefly, DNA oligonucleotide probes were 5′-end labeled with [γ-32P]ATP (PerkinElmer, NEG502A) and T4 polynucleotide kinase (Promega, M4103) according to the manufacturer’s instructions. Oligonucleotides CCCGAGCTTCGTATGGGG and GGAGACCCCACACTACCATCGGCGATG were used to probe ersA and 5S RNA, respectively. Radioactive bands were acquired after exposure to phosphor screens using a Typhoon 8600 variable mode imager scanner (GE Healthcare BioSciences) and visualized with image-Quant software (Molecular Dynamics). The intensities of the bands were quantified using LI-COR Image Studio Lite. The signal of ersA was normalized to those of 5S RNA in the same lane. For each clinical isolate, the relative abundance of ersA was calculated in comparison to that in the reference strain PAO1.
Cytotoxicity and IL-8 secretion in human CF respiratory cells.
IB3-1 cells, an adeno-associated virus-transformed human bronchial epithelial cell line derived from a CF patient (ΔF508/W1282X) and obtained from LGC Promochem, were grown as described previously (51). Cell viability was evaluated using the MTS-based CellTiter 96 AQueous One Solution Cell Proliferation assay kit (Promega, G3582), which determines viable cell number by measuring the conversion at 490 nm of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) to formazan by the dehydrogenase enzyme of the intact mitochondria of living cells. In a 96-well plate, triplicate samples of IB3-1 cells were infected with P. aeruginosa strains at a multiplicity of infection (MOI) of 100 in a final volume of 100 μl. Immediately after infection, 20 μl of the CellTiter 96 AQueous One Solution reagent was added directly to culture and control wells. MTS was also added to noninfected cells and to wells containing only the same bacterial load in the absence of IB3-1 cells (blank/control). According to the manufacturer’s instructions, plates were incubated at 37°C with 5% CO2, read at 490 nm at different time points, and returned to the incubator for further color development. The average measurement from infected cells was subtracted from the average from the corresponding blank. The relative percentages of cell death or cell viability were calculated as the ratio between the average value in normalized infected cells (blank subtracted) and the uninfected cells. The stimulation of the host inflammatory response was evaluated by monitoring the secretion of the proinflammatory interleukin IL-8 as described previously (51). Briefly, after infection with P. aeruginosa strains at an MOI of 0.1, IB3-1 cells were incubated at 37°C with 5% CO2 for 2 h, washed with phosphate-buffered saline (PBS) supplemented with 1 mg/ml amikacin, and incubated in the presence of fresh medium supplemented with 1 mg/ml amikacin. Uninfected cells treated and incubated under the same conditions were used as a control of nonstimulated IL-8 production. Released IL-8 was determined in supernatants collected at 24 h using an ELISA kit (Biosource Europe and R&D Systems), according to the manufacturer’s instructions.
Agar bead preparation.
The agar bead mouse model was used (39, 40). An aliquot of wild-type or ΔersA P. aeruginosa PAO1 strains from glycerol stocks was streaked for isolation on Trypticase soy agar (TSA) and incubated at 37°C overnight. One colony was picked from the plate and used to inoculate 5 ml of tryptic soy broth (TSB) and placed in a shaking incubator at 37°C at 200 rpm overnight. The overnight bacterial suspension was diluted to 0.15 optical density (OD)/ml in 20 ml of TSB/flask and grown for 4 h at 37°C at 200 rpm to reach the log phase. The bacteria were pelleted by centrifugation (2,700 × g, 15 min, and 4°C) and resuspended in 1 ml PBS (pH 7.4). A starting amount of 2 × 109 CFU of P. aeruginosa was used for inclusion in the agar beads prepared according to the previously described method (39, 40, 52). Bacteria were added to 9 ml of 1.5% TSA (wt/vol) prewarmed to 50°C. This mixture was pipetted forcefully into 150 ml heavy mineral oil at 50°C and stirred rapidly with a magnetic stirring bar for 6 min at room temperature, followed by cooling at 4°C with continuous slow stirring for 35 min. The oil-agar mixture was centrifuged at 2,700 × g for 15 min to sediment the beads and washed six times in PBS. The size of the beads was verified microscopically, and only those preparations containing beads of 100 μm to 200 μm in diameter were used as inoculum for animal experiments. The number of P. aeruginosa CFU in the beads was determined by plating serial dilutions of the homogenized bacteria-bead suspension on TSA plates. The inoculum was prepared by diluting the bead suspension with PBS to 2 × 107 CFU/ml to inoculate approximately 1 × 106 CFU/50 μl. P. aeruginosa beads were prepared the day before inoculation and stored overnight at 4°C for a maximum of 2 days. The number of P. aeruginosa CFU in the beads inoculated was determined by plating serial dilutions of the homogenized bacteria-bead suspension on the day of the infection.
Mouse model of chronic P. aeruginosa lung infection.
Immunocompetent C57BL/6NCrlBR male mice (8 to 10 weeks of age) were purchased from Charles River (Calco, Italy), shipped in protective filtered containers, transported in climate-controlled trucks, and allowed to acclimatize for at least 2 days before use. Three to five mice were housed per cage and maintained in the biosafety level 3 (BSL3) facility at San Raffaele Scientific Institute (Milan, Italia) in sterile ventilated cages. Mice were fed with standard rodent autoclaved chow (VRFI, Special Diets Services, UK) and autoclaved tap water. Fluorescent lights were cycled 12 h on and 12 h off, and ambient temperature (23 ± 1°C) and relative humidity (40% to 60%) were regulated.
For infection experiments, mice were anesthetized by an intraperitoneal injection of a solution of Avertin (2,2,2-tribromethanol; 97%) in 0.9% NaCl and administered at a volume of 0.015 ml/g body weight. Mice were placed in a supine position. The trachea was directly visualized by ventral midline, exposed, and intubated with a sterile, flexible 22-gauge cannula attached to a 1-ml syringe. An inoculum of 50 μl of agar bead suspension was implanted via the cannula into the lung. After inoculation, all incisions were closed by suture.
Infections and sacrifices were all performed in the late morning. In all the experiments, mice had been subdivided according to the body weight to have similar means among all the groups of treatment.
Mice were monitored daily for coat quality, posture, attitude, ambulation, hydration status, and bodyweight. Mice that lost >20% body weight and had evidence of severe clinical diseases, such as scruffy coat, inactivity, loss of appetite, poor locomotion, or painful posture, were sacrificed before the termination of the experiments with an overdose of carbon dioxide. Gross lung pathology was checked. After 13 days postinfection, bronchoalveolar lavage fluid (BALF) was collected, and the lungs were aseptically excised.
BALF was extracted with a 22-gauge venous catheter ligated to the trachea to prevent backflow. The lungs were washed three times each with 1 ml of RPMI 1640 (Euroclone) with protease inhibitors (complete tablets; Roche Diagnostic) and pooled. Quantitative bacteriology on BALF was performed by plating serial dilutions on TSA. Total cells present in the BALF were counted using an inverted light optical microscope after diluting an aliquot of the BALF 1:2 with Tuerk solution in a disposable counting chamber. BALF cells were centrifuged at 330 × g for 8 min at 4°C. If the pellet was red, erythrocytes were lysed by resuspending the pellet in 250 to 300 μl of RBC lysis buffer diluted 1:10 in ultrapure distilled water for 3 min. Then, 2 to 3 ml PBS was added and cells were centrifuged at 330 × g for 8 min at 4°C. The pellet was resuspended in RPMI 1640 with 10% fetal bovine serum (FBS) at a concentration of 1 × 106 cells/ml, and an aliquot of 170 μl was pipetted into the appropriate wells of the cytospin and centrifuged at 300 × g for 5 min with medium brake. Slides were then stained by Diff-Quik staining using a commercial kit (Medion Diagnostics, code 726443), according to the manufacturer’s instructions. A differential cell count was performed on an inverted light optical microscope.
Lungs were excised aseptically and homogenized in 2 ml PBS with protease inhibitors using the homogenizer gentleMACS Octo Dissociator. One hundred microliters of the homogenates and 10-fold serial dilutions were spotted onto TSA. CFU was determined after overnight growth at 37°C. Recovery of ≥1,000 CFU of P. aeruginosa from lung plus BALF cultures was considered evidence of chronic infection.
Quantification of murine chemokines.
Lung homogenates were centrifuged at 16,000 × g for 30 min at 4°C, and then supernatants were collected and stored at −80°C. Murine KC and JE concentrations were determined in the lung homogenate supernatants by DuoSet ELISA Development Systems (R&D Systems), according to the manufacturer’s instructions.
MIC measurement.
MICs of antibiotics were determined according to CLSI guidelines (53), as previously described (54). The medium used for the MIC testing was cation-adjusted Mueller-Hinton broth (MH-II broth). MIC testing was run in sterile 96-well microtiter plates (polystyrene V shape) and analyzed after 20 h.
Statistics.
Statistical analyses were performed with GraphPad Prism. Survival curves and incidences of chronic colonization were compared using a Mantel-Cox test and Fisher exact test, respectively. Levels of chemokines, leukocytes, and CFU were compared using a nonparametric two-tailed Mann-Whitney U test. A P value of <0.05 was considered significant. Significance of the differences in the levels of secreted IL-8 and converted MTS by infected IB3-1 cells was determined using the Student’s t test. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank B. Tümmler (Hannover Medical School, Hannover, Germany) and L. Cariani (Cystic Fibrosis Microbiology Laboratory, Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Milan, Italy) for supplying the P. aeruginosa strains from CF and COPD patients and C. Winstanley (Institute of Infection and Global Health, University of Liverpool, Liverpool, UK) for providing the LESB58 strain.
This work was supported by the European Commission (NABATIVI-223670, EUFP7-HEALTH-2007-B) and Italian Cystic Fibrosis Research Foundation (FFC no. 13/2015) with the contributions of Gruppo di Sostegno FFC di Sassari Castelsardo and Delegazione FFC di Boschi Sant'Anna Minerbe and (FFC no. 14/2016) and Delegazione FFC di Reggio Calabria and Gruppo di Sostegno FFC di Vigevano. S.F. was the recipient of a Postdoctoral Fellowship of the Università degli Studi di Milano. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner VE, Filiatrault MJ, Picardo KF, Iglewski BH. 2008. Pseudomonas aeruginosa virulence and pathogenesis issues, p 129–158. In Cornelis P (ed), Pseudomonas genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 3.Williams BJ, Dehnbostel J, Blackwell TS. 2010. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Moradali MF, Ghods S, Rehm BH. 2017. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepanto P, Bryant DM, Rossello J, Datta A, Mostov KE, Kierbel A. 2011. Pseudomonas aeruginosa interacts with epithelial cells rapidly forming aggregates that are internalized by a Lyn-dependent mechanism. Cell Microbiol 13:1212–1222. doi: 10.1111/j.1462-5822.2011.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran CS, Rangel SM, Almblad H, Kierbel A, Givskov M, Tolker-Nielsen T, Hauser AR, Engel JN. 2014. The Pseudomonas aeruginosa type III translocon is required for biofilm formation at the epithelial barrier. PLoS Pathog 10:e1004479. doi: 10.1371/journal.ppat.1004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruch TR, Engel JN. 2017. Targeting the mucosal barrier: how pathogens modulate the cellular polarity network. Cold Spring Harb Perspect Biol 9:a027953. doi: 10.1101/cshperspect.a027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanin M, Baviskar P, Webster R, Webby R. 2016. The interaction between respiratory pathogens and mucus. Cell Host Microbe 19:159–168. doi: 10.1016/j.chom.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran CS, Eran Y, Ruch TR, Bryant DM, Datta A, Brakeman P, Kierbel A, Wittmann T, Metzger RJ, Mostov KE, Engel JN. 2014. Host cell polarity proteins participate in innate immunity to Pseudomonas aeruginosa infection. Cell Host Microbe 15:636–643. doi: 10.1016/j.chom.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cigana C, Lore NI, Bernardini ML, Bragonzi A. 2011. Dampening host sensing and avoiding recognition in Pseudomonas aeruginosa pneumonia. J Biomed Biotechnol 2011:852513. doi: 10.1155/2011/852513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonker LM, Cigana C, Hurley BP, Bragonzi A. 2015. Host-pathogen interplay in the respiratory environment of cystic fibrosis. J Cyst Fibros 14:431–439. doi: 10.1016/j.jcf.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhagirath AY, Li Y, Somayajula D, Dadashi M, Badr S, Duan K. 2016. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm Med 16:174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cigana C, Lore NI, Riva C, De Fino I, Spagnuolo L, Sipione B, Rossi G, Nonis A, Cabrini G, Bragonzi A. 2016. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep 6:21465. doi: 10.1038/srep21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasubramanian D, Schneper L, Kumari H, Mathee K. 2013. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Shao X, Xie Y, Wang T, Zhang Y, Wang X, Deng X. 2019. An integrated genomic regulatory network of virulence-related transcriptional factors in Pseudomonas aeruginosa. Nat Commun 10:2931. doi: 10.1038/s41467-019-10778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pita T, Feliciano JR, Leitao JH. 2018. Small noncoding regulatory RNAs from Pseudomonas aeruginosa and Burkholderia cepacia complex. Int J Mol Sci 19:3759. doi: 10.3390/ijms19123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winstanley C, O'Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potvin E, Sanschagrin F, Levesque RC. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev 32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Schurr MJ, Deretic V. 1995. Functional equivalence of Escherichia coli sigma E and Pseudomonas aeruginosa AlgU: E. coli rpoE restores mucoidy and reduces sensitivity to reactive oxygen intermediates in algU mutants of P. aeruginosa. J Bacteriol 177:3259–3268. doi: 10.1128/jb.177.11.3259-3268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabowicz M, Silhavy TJ. 2017. Envelope stress responses: an interconnected safety net. Trends Biochem Sci 42:232–242. doi: 10.1016/j.tibs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hews CL, Cho T, Rowley G, Raivio TL. 2019. Maintaining integrity under stress: envelope stress response regulation of pathogenesis in Gram-negative bacteria. Front Cell Infect Microbiol 9:313. doi: 10.3389/fcimb.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmqvist E, Wagner EGH. 2017. Impact of bacterial sRNAs in stress responses. Biochem Soc Trans 45:1203–1212. doi: 10.1042/BST20160363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldelari I, Chao Y, Romby P, Vogel J. 2013. RNA-mediated regulation in pathogenic bacteria. Cold Spring Harb Perspect Med 3:a010298. doi: 10.1101/cshperspect.a010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty S, Masse E. 2019. RNA-dependent regulation of virulence in pathogenic bacteria. Front Cell Infect Microbiol 9:337. doi: 10.3389/fcimb.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frohlich KS, Gottesman S. 2018. Small regulatory RNAs in the enterobacterial response to envelope damage and oxidative stress. Microbiol Spectr 6:RWR-0022-2018. doi: 10.1128/microbiolspec.RWR-0022-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 27.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara S, Brugnoli M, De Bonis A, Righetti F, Delvillani F, Deho G, Horner D, Briani F, Bertoni G. 2012. Comparative profiling of Pseudomonas aeruginosa strains reveals differential expression of novel unique and conserved small RNAs. PLoS One 7:e36553. doi: 10.1371/journal.pone.0036553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara S, Carloni S, Fulco R, Falcone M, Macchi R, Bertoni G. 2015. Post-transcriptional regulation of the virulence-associated enzyme AlgC by the σ22-dependent small RNA ErsA of Pseudomonas aeruginosa. Environ Microbiol 17:199–214. doi: 10.1111/1462-2920.12590. [DOI] [PubMed] [Google Scholar]
- 30.Falcone M, Ferrara S, Rossi E, Johansen HK, Molin S, Bertoni G. 2018. The small RNA ErsA of Pseudomonas aeruginosa contributes to biofilm development and motility through post-transcriptional modulation of AmrZ. Front Microbiol 9:238. doi: 10.3389/fmicb.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deretic V, Schurr MJ, Yu H. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol 3:351–356. doi: 10.1016/s0966-842x(00)88974-x. [DOI] [PubMed] [Google Scholar]
- 32.Olvera C, Goldberg JB, Sanchez R, Soberon-Chavez G. 1999. The Pseudomonas aeruginosa algC gene product participates in rhamnolipid biosynthesis. FEMS Microbiol Lett 179:85–90. doi: 10.1111/j.1574-6968.1999.tb08712.x. [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Wang J, Wang S, Anderson EM, Lam JS, Parsek MR, Wozniak DJ. 2012. Synthesis of multiple Pseudomonas aeruginosa biofilm matrix exopolysaccharides is post-transcriptionally regulated. Environ Microbiol 14:1995–2005. doi: 10.1111/j.1462-2920.2012.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood LF, Ohman DE. 2009. Use of cell wall stress to characterize sigma 22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Mol Microbiol 72:183–201. doi: 10.1111/j.1365-2958.2009.06635.x. [DOI] [PubMed] [Google Scholar]
- 35.Lizewski SE, Lundberg DS, Schurr MJ. 2002. The transcriptional regulator AlgR is essential for Pseudomonas aeruginosa pathogenesis. Infect Immun 70:6083–6093. doi: 10.1128/iai.70.11.6083-6093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CL, Van Laar TA, Chen T, Karna SLR, Chen P, You T, Leung KP. 2017. Global transcriptome responses including small RNAs during mixed-species interactions with methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Microbiologyopen 6:e00427. doi: 10.1002/mbo3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YF, Han K, Chandler CE, Tjaden B, Ernst RK, Lory S. 2017. Probing the sRNA regulatory landscape of P. aeruginosa: post-transcriptional control of determinants of pathogenicity and antibiotic susceptibility. Mol Microbiol 106:919–937. doi: 10.1111/mmi.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Facchini M, De Fino I, Riva C, Bragonzi A. 2014. Long term chronic Pseudomonas aeruginosa airway infection in mice. J Vis Exp 2014:51019. doi: 10.3791/51019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kukavica-Ibrulj I, Bragonzi A, Paroni M, Winstanley C, Sanschagrin F, O'Toole GA, Levesque RC. 2008. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J Bacteriol 190:2804–2813. doi: 10.1128/JB.01572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cigana C, Ranucci S, Rossi A, De Fino I, Melessike M, Bragonzi A. 2020. Antibiotic efficacy varies based on the infection model and treatment regimen for Pseudomonas aeruginosa. Eur Respir J 55:1802456. doi: 10.1183/13993003.02456-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di SC, Doring G, Tummler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara S, Falcone M, Macchi R, Bragonzi A, Girelli D, Cariani L, Cigana C, Bertoni G. 2017. The PAPI-1 pathogenicity island-encoded small RNA PesA influences Pseudomonas aeruginosa virulence and modulates pyocin S3 production. PLoS One 12:e0180386. doi: 10.1371/journal.pone.0180386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianconi I, Jeukens J, Freschi L, Alcala-Franco B, Facchini M, Boyle B, Molinaro A, Kukavica-Ibrulj I, Tummler B, Levesque RC, Bragonzi A. 2015. Comparative genomics and biological characterization of sequential Pseudomonas aeruginosa isolates from persistent airways infection. BMC Genomics 16:1105. doi: 10.1186/s12864-015-2276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Garcia E, de Lorenzo V. 2011. Engineering multiple genomic deletions in Gram-negative bacteria: analysis of the multi-resistant antibiotic profile of Pseudomonas putida KT2440. Environ Microbiol 13:2702–2716. doi: 10.1111/j.1462-2920.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 45.Waligora EA, Ramsey DM, Pryor EE, Jr, Lu H, Hollis T, Sloan GP, Deora R, Wozniak DJ. 2010. AmrZ beta-sheet residues are essential for DNA binding and transcriptional control of Pseudomonas aeruginosa virulence genes. J Bacteriol 192:5390–5401. doi: 10.1128/JB.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CJ, Newsom D, Kelly B, Irie Y, Jennings LK, Xu B, Limoli DH, Harrison JJ, Parsek MR, White P, Wozniak DJ. 2014. ChIP-Seq and RNA-Seq reveal an AmrZ-mediated mechanism for cyclic di-GMP synthesis and biofilm development by Pseudomonas aeruginosa. PLoS Pathog 10:e1003984. doi: 10.1371/journal.ppat.1003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasseur P, Vallet-Gely I, Soscia C, Genin S, Filloux A. 2005. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology (Reading) 151:985–997. doi: 10.1099/mic.0.27410-0. [DOI] [PubMed] [Google Scholar]
- 48.Byrd MS, Sadovskaya I, Vinogradov E, Lu HP, Sprinkle AB, Richardson SH, Ma LY, Ralston B, Parsek MR, Anderson EM, Lam JS, Wozniak DJ. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol 73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann EE, Wozniak DJ. 2012. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev 36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickey SW, Cheung GYC, Otto M. 2017. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16:457–471. doi: 10.1038/nrd.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cigana C, Curcuru L, Leone MR, Ierano T, Lore NI, Bianconi I, Silipo A, Cozzolino F, Lanzetta R, Molinaro A, Bernardini ML, Bragonzi A. 2009. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 4:e8439. doi: 10.1371/journal.pone.0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cigana C, Bianconi I, Baldan R, De Simone M, Riva C, Sipione B, Rossi G, Cirillo DM, Bragonzi A. 2018. Staphylococcus aureus impacts Pseudomonas aeruginosa chronic respiratory disease in murine models. J Infect Dis 217:933–942. doi: 10.1093/infdis/jix621. [DOI] [PubMed] [Google Scholar]
- 53.CSLI. 2014. CLSI M100-S24. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 54.Cigana C, Bernardini F, Facchini M, Alcala-Franco B, Riva C, De Fino I, Rossi A, Ranucci S, Misson P, Chevalier E, Brodmann M, Schmitt M, Wach A, Dale GE, Obrecht D, Bragonzi A. 2016. Efficacy of the novel antibiotic POL7001 in preclinical models of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 60:4991–5000. doi: 10.1128/AAC.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of ErsA expression in a panel of clinical and environmental strains of P. aeruginosa. Download Table S1, PDF file, 0.2 MB (202.6KB, pdf) .
Copyright © 2020 Ferrara et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ErsA influences aggregation and adherence of the P. aeruginosa PAO1 strain. P. aeruginosa PAO1 strains with wild-type, deleted, or overexpressed ErsA grown in liquid T-broth in presence of 40 μg/ml of Congo red. To observe aggregation, strains were inoculated at optical density at 600 nm (OD600) of 0.05 using glass culture tubes with 10 ml of medium and incubated overnight at 37°C in a roller drum. Carbenicillin and arabinose were added for ectopic expression of ErsA from pGM-ersA and the growth of the control culture harboring the pGM931 empty vector. Adherent biomass is noticeable on the culture tube of the PAO1 wild-type strain. Conversely, the absence of adherence is shown in the ΔersA strain tube. More abundant aggregation and biofilm are present when ErsA is overexpressed by pGM-ersA from the wild-type background than in the empty vector-harboring control strain PAO1/pGM931 grown under the same conditions. Download FIG S1, TIF file, 2.7 MB (2.6MB, tif) .
Copyright © 2020 Ferrara et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.