Abstract
Background
A novel G-protein signalling-biased mu opioid peptide (MOP) receptor agonist, PZM21, was recently developed with a distinct chemical structure. It is a potent Gi/o activator with minimal β-arrestin-2 recruitment. Despite intriguing activity in rodent models, PZM21 function in non-human primates is unknown. The aim of this study was to investigate PZM21 actions after systemic or intrathecal administration in primates.
Methods
Antinociceptive, reinforcing, and pruritic effects of PZM21 were compared with those of the clinically used MOP receptor agonists oxycodone and morphine in assays of acute thermal nociception, capsaicin-induced thermal allodynia, itch scratching responses, and drug self-administration in gonadally intact, adult rhesus macaques (10 males, six females).
Results
After subcutaneous administration, PZM21 (1.0–6.0 mg kg−1) and oxycodone (0.1–0.6 mg kg−1) induced dose-dependent thermal antinociceptive effects (P<0.05); PZM21 was 10 times less potent than oxycodone. PZM21 exerted oxycodone-like reinforcing effects and strength as determined by two operant schedules of reinforcement in the intravenous drug self-administration assay. After intrathecal administration, PZM21 (0.03–0.3 mg) dose-dependently attenuated capsaicin-induced thermal allodynia (P<0.05). Although intrathecal PZM21 and morphine induced MOP receptor-mediated antiallodynic effects, both compounds induced robust, long-lasting itch scratching.
Conclusions
PZM21 induced antinociceptive, reinforcing, and pruritic effects similar to clinically used MOP receptor agonists in primates. Although structure-based discovery of PZM21 identified a novel avenue for studying G-protein signalling-biased ligands, biasing an agonist towards G-protein signalling pathways did not determine or alter reinforcing (i.e. abuse potential) or pruritic effects of MOP receptor agonists in a translationally relevant non-human primate model.
Keywords: opoid addiction, G protein, opioid, pruritus, rhesus macaque, spinal analgesics
Editor's key points.
-
•
Opioid analgesics remain mainstay therapies in acute and cancer pain management, but there are significant concerns about potential harms, such as abuse liability.
-
•
An opioid agonist without addiction potential would have significant clinical utility. Rodent models indicate that agonists biased towards G-protein signalling rather than β-arrestin-2 recruitment may meet this criterion.
-
•
In this study, non-human primates were used to explore the analgesic, addictive, and pruritic effects of a novel biased opioid agonist, and no major difference from current strong opioids was observed.
-
•
A robust approach is critical to translating preclinical findings to humans given concerns about the applicability of rodent models.
Mu opioid peptide (MOP) receptor agonists are commonly used to alleviate pain.1,2 However, the abuse potential associated with this class of drugs remains a serious public health concern,3,4 and their adverse effects limit the value of opioid analgesics for pain management.5,6 Therefore, research into non-addictive analgesics is critical. Among research strategies7,8 to mitigate MOP receptor-mediated adverse effects, the development of ‘biased’ ligands has emerged.8,9 MOP receptor activation drives at least two major different signalling pathways: G-protein signalling and β-arrestin recruitment.10,11 In β-arrestin-2 knockout mice, enhanced morphine analgesia and attenuated morphine side-effects are observed, such as constipation and respiratory suppression.12,13
Computational docking of more than 3 million molecules with the MOP receptor structure identified ligands with new chemical scaffolds (e.g. alkyl ureas) and the lead molecule, PZM21.14 PZM21 signalling is mediated primarily by the activation of G-protein Gi/o signalling but not β-arrestin-2 recruitment. In rodents, PZM21 induced antinociceptive effects in the hot plate assay but not in the tail flick assay.14,15 It did not induce rewarding effects in rodents, as measured by the conditioned place preference assay.14 However, the potential reinforcing effects (i.e. abuse potential) of PZM21 have not been studied in any animal species by using an intravenous drug self-administration assay.16,17 In addition, the intrathecal delivery of MOP receptor agonists is a standard procedure for perioperative analgesia, and effective for pain management in different clinical settings.18,19 However, itching sensation (pruritus) is a common side-effect caused by spinal MOP analgesics, and significantly compromises their value for pain management.20,21 It has not been determined whether a G protein signalling-biased MOP agonist could show reduced itching sensation.
Numerous studies have documented differences in the pharmacological properties of MOP receptor-related ligands between rodents and primates.22, 23, 24 Given the similarity of the anatomical, neurochemical, and pharmacological aspects of receptors between humans and non-human primates,25, 26, 27 in vivo pharmacological experiments using awake, behaving monkeys may provide a translational bridge to advance our understanding of the integrated outcomes of MOP receptor activation. To date, no known G-protein signalling-biased MOP receptor agonists have been investigated in non-human primates. Given that PZM21 is a novel G-protein-biased ligand,14 we compared the effects of PZM21 and clinically used MOP receptor agonists using primate assays with strong predictive validity and translational value.16,17,26,27 This study was the first to evaluate pharmacological, behavioural, and physiological factors in non-human primates to address three specific questions. (1) Did PZM21 exert antinociceptive effects in a manner similar to the prescription opioid analgesic, oxycodone? (2) Did PZM21 induce reinforcing effects with a similar strength to oxycodone? (3) Did intrathecal PZM21 induce itch scratching, similar to intrathecal morphine?
Methods
Animals
All animal care and experiments in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals by the US National Institutes of Health (Bethesda, MD, USA)28 and were approved by the Institutional Animal Care and Use Committee of Wake Forest University (Winston–Salem, NC, USA). This study is reported according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.28 Sixteen adult (10 male and six female) rhesus monkeys (Macaca mulatta), 10–18 yr of age with a body weight of 6.4–12.1 kg, were obtained from the US National Primate Centers for Biomedical Research. According to our previous experience using power and statistical analysis for quantifying behavioural and physiological responses, a sample size of four animals was considered sufficient to determine the magnitude and dose-dependency of ligand-induced effects on the study endpoints, such as tail-withdrawal latency and drug self-administration.29, 30, 31 Animals were assigned to each assay based on their training and consistent behaviour with each outcome measure. All experiments followed a within-subject design (i.e. each group of animals served as its own control and all dosing conditions were randomised by a counterbalanced design). The monkeys were housed at an indoor facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Frederick, MD, USA), individually in cages with 6–12 ft2 of floor space, with ceilings 2.7–5.4 ft high, in an environmentally controlled room (21–25°C, 40–60% relative humidity) with a 12 h light/dark cycle (lights on: 06:30–18:30). The monkeys were provided with water and diet, which comprised approximately 20–30 biscuits (Purina Monkey Chow; Ralston Purina Co., St. Louis, MO, USA) and fresh fruit ad libitum. Primate enrichment devices and small amounts of treats were provided daily. Animals were not subjected to any experiments or given opioid compounds 1 month before the experiments. All animals completed the study with no welfare issues; upon completion, they were assigned to another study.
Sensory assays
Acute thermal nociception
The thermal antinociceptive effects of PZM21 and the MOP agonists oxycodone and morphine were evaluated by using the warm water tail withdrawal assay.29,32 Using positive reinforcement techniques,33 the monkeys were trained to cooperate with pole-and-collar transfer to a primate restraint chair. In a procedure room, the lower parts of their shaved tails (∼15 cm) were immersed in water maintained at 42, 46, or 50°C. The monkeys were randomly assigned to a dosing condition. Water at 42 and 46°C was used as a non-noxious stimuli (i.e. no tail-withdrawal movement was expected), and water at 50°C was used as an acute noxious stimulus (i.e. 2–3 s tail withdrawal latency). Experimenters blinded to the dosing conditions measured tail withdrawal latencies at each temperature by using a computerised timer. If the monkey did not withdraw its tail within 20 s (the cut-off), the stimulus was terminated and a maximum time of 20 s was recorded. After the measurement of baseline latency at each temperature, subsequent tail withdrawal latencies were measured at multiple time points after subcutaneous or intrathecal administration of the test compound.
Capsaicin-induced thermal allodynia
The antiallodynic effects of PZM21 and morphine were evaluated in the capsaicin-induced thermal allodynia assay, which is a clinically relevant model to evaluate analgesics in humans.34,35 In a procedure room, capsaicin (1.2 mg ml−1 × 0.3 ml) was administered topically through a bandage attached on the terminal 3–5 cm of the tail for 15 min.36 Capsaicin-induced allodynia was defined as a shortened tail withdrawal latency in 46°C water from 20 s (maximum value) in the absence of capsaicin treatment to ∼2–3 s when treated with capsaicin. The peak of the allodynic effect 15 min after removal of the capsaicin bandage has been used previously to measure tail withdrawal latency in 46°C water to evaluate the antiallodynic effects of the test compound.36,37 To determine whether the MOP receptor mediated the antiallodynic effects of PZM21 and morphine, an MOP receptor-selective dose (0.03 mg kg−1, s.c.) of the opioid receptor antagonist naltrexone was administered 5 min before the intrathecal administration of PZM21 or morphine. The dose and pretreatment time for naltrexone were determined based on previous studies.32,37
Itch scratching responses
To assess the itching sensation caused by the test compound, scratching behaviour38 was recorded when monkeys were in their home cages. Each 15 min recording session was conducted after the intrathecal administration of PZM21 or morphine. A scratch was defined as one brief (<1 s) scraping on the skin surface of other body parts using the forepaw or hind paw. The total number of scratches was counted and summed for each 15 min period by individuals blinded to the dosing regimen.
Drug self-administration
Monkeys implanted with intravenous catheters were used to evaluate the reinforcing effect and strength of the test compound. In operant chambers situated in a procedure room, we examined whether monkeys would self-administer PZM21 using a fixed ratio (FR30) schedule of reinforcement.39,40 Next, we compared the self-administration behaviour for PZM21 and oxycodone using a progressive ratio (PR) schedule.31 In addition to determining whether or not a drug served as a reinforcer using an FR30 schedule, a PR schedule was used to compare the degree to which drugs served as reinforcers, termed the ‘reinforcing strength’ of the drugs. The PR schedule can differentiate reinforcing strengths among abused drugs, which function as positive reinforcers.41,42 Operant response was maintained using 0.3 μg kg−1 per injection of remifentanil until the response was stable (mean [three injections for three consecutive sessions]). Dose-dependent responses were determined by substituting vehicle or various doses of oxycodone (0.3–3 μg kg−1 per injection, i.v.) or PZM21 (3–30 μg kg−1 per injection, i.v.) randomly with the maintenance dose. Doses were available for at least five consecutive sessions and until the response was considered stable.
Surgical implantation
Intrathecal catheterisation was performed as previously described.37,43 Briefly, before surgery, animals were administered atropine (0.04 mg kg−1, i.m.), buprenorphine (0.01–0.03 mg kg−1, i.m.), dexamethasone (2 mg kg−1, i.v.), and cefotaxime (500 mg, i.v.) for pain relief and infection prevention. Animals were then anaesthetised with ketamine (10 mg kg−1, i.m.) and intubated; anaesthesia was maintained via isoflurane inhalation (1–2% in 1 L min−1 O2). Intraoperative monitoring was conducted to determine the depth of anaesthesia and physiological status. Monkeys were administered postoperative buprenorphine (0.003–0.02 mg kg−1, i.m.) and meloxicam (0.15 mg kg−1, s.c.) to alleviate pain and inflammation and ceftiofur (2.2 mg kg−1, i.m.) to prevent infection. Postoperative care was performed daily until veterinarians confirmed that healing was complete. All animals were monitored daily by veterinarian and laboratory staff to ensure the animals remained healthy throughout the study.
Data analysis
Data are presented as mean values (standard deviation [sd]) calculated from individual data from all studies. Comparisons were made for the same monkeys across all test sessions for the same experiments. Because of the small sample size and the correlated data structure (e.g. repeated measures over time, the same monkey received more than one treatment), rank repeated measures analysis was performed to examine the dose effect and the time effect (if available). The generalised estimating equations technique was used to construct the rank tests.44 Dose, time, and the interaction between dose and time were included in the model when longitudinal data were available. The hypothesis test for dose effect at each time point was performed using a contrast. Overall comparisons between doses across all time points (i.e. dose-dependent manner) and between times across all doses (i.e. time-dependent manner) were obtained using contrasts as well. F-statistics from the rank repeated measures were reported. When only cross-sectional data were available, dose was included in the model. The criterion for significance was set at P<0.05. Raw data, ranks of raw data for all experiments, and figures showing individual raw data points with median and inter-quartile range are presented in the Supplementary Materials.
Drugs
PZM21 was synthesised45 and provided by Yanan Zhang (RTI International, Research Triangle Park, NC, USA). PZM21 was dissolved in dimethyl sulfoxide/Tween 80/sterile water at a ratio of 1:1:8. Morphine sulfate, oxycodone HCl, remifentanil HCl, and naltrexone HCl (National Institute on Drug Abuse, Bethesda, MD, USA) were dissolved in sterile water. Capsaicin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 70% (v/v) ethanol. For systemic administration, the animals received 0.1–0.3 ml kg−1 of the drugs. For intrathecal administration,43 the test compound, in a total volume of 1 ml, was administered through the access port, followed by 0.35 ml saline to flush the dead volume of the port and catheter.
Results
Systemic PZM21 induces antinociceptive effects
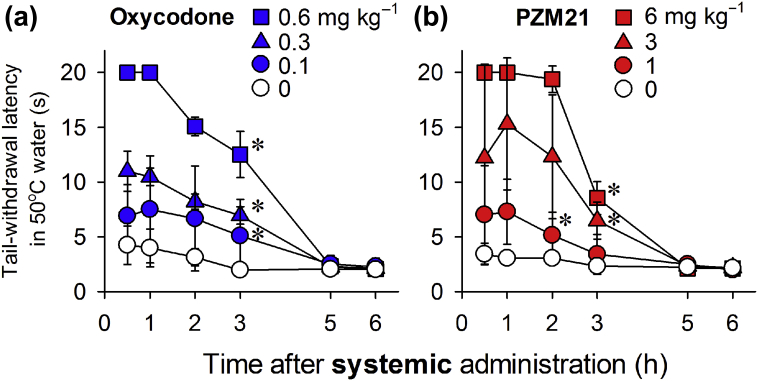
Oxycodone (0.1–0.6 mg kg−1, s.c.) exerted antinociceptive effects against 50°C water in a dose- (F3, 9=100.6; P<0.05) and time-dependent (F3, 15=73.7; P<0.05) manner (Fig. 1a). In the same group of animals, PZM21 (1–6 mg kg−1, s.c.) also exerted antinociceptive effects against the 50°C water in a dose- (F3, 9=17 628.7; P<0.05) and time-dependent (F3, 15=1556; P<0.05) manner (Fig. 1b). The minimum effective dose of PZM21 required to produce maximum antinociception was 6 mg kg−1; this was approximately 10-fold less potent than oxycodone, which induces maximal antinociception at 0.6 mg kg−1. The duration of antinociceptive action was approximately 3 h for each compound, and had abated by 5 h.
Fig 1.
Comparison of systemic oxycodone- and PZM21-induced antinociceptive effects as measured by warm water tail withdrawal latency in monkeys. Antinociception induced by oxycodone (a) and PZM21 (b) was measured against an acute noxious stimulus, 50°C water. Each data point represents the mean (standard deviation) (n=4). Both compounds were administered subcutaneously. ∗P<0.05, significantly different from vehicle condition from the first time point to the corresponding time point.
PZM21 produces reinforcing effects
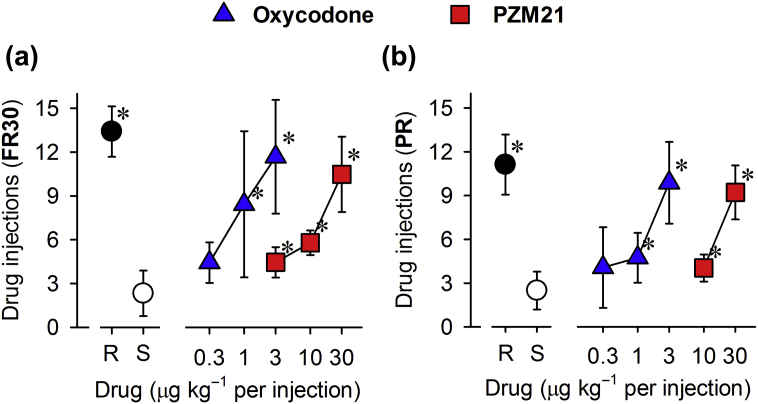
Substitution of saline between administrations of test compounds resulted in fewer reinforcers. Under the FR30 schedule, remifentanil (0.3 μg kg−1 per injection), oxycodone (3 μg kg−1 per injection), and PZM21 (30 μg kg−1 per injection) significantly induced reinforcing effects (Fig. 2a). Although PZM21 was less potent than oxycodone, both compounds showed similar reinforcing strengths over the dose range studied, as determined using the PR schedule (Fig. 2b). These data suggested that PZM21 may have similar abuse potential to the MOP analgesic oxycodone.
Fig 2.
Comparison of oxycodone- and PZM21-induced reinforcing effects as measured by intravenous drug self-administration in monkeys. Number of injections received as a function of dose in monkeys responding to remifentanil (R, 0.3 μg kg−1 per injection), saline (S, ∼0.14 ml kg−1 per injection), oxycodone (0.3–3 μg kg−1 per injection), or PZM21 (3–30 μg kg−1 per injection) under a fixed ratio (FR30) schedule (a) or a progressive ratio (PR) schedule (b) of reinforcement. Each data point represents the mean (standard deviation) (n=4–6). ∗P<0.05, significantly different from saline.
Intrathecal PZM21 produces antinociceptive effects and itch sensation
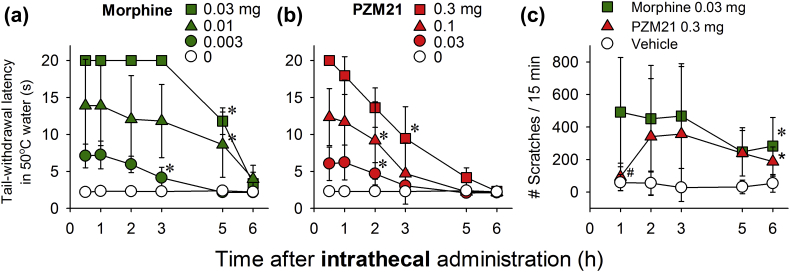
Intrathecal morphine (0.003–0.03 mg) exerted antinociceptive effects against 50°C water in a dose- (F3, 9=70; P<0.05) and time-dependent (F3, 15=1419.9; P<0.05) manner (Fig. 3a). In the same group of animals, intrathecal PZM21 (0.03–0.3 mg) also exerted antinociceptive effects against 50°C water in a dose- (F3, 9=121.3; P<0.05) and time-dependent (F3, 15=122.1; P<0.05) manner (Fig. 3b). The minimum effective dose of PZM21 for antinociception was 0.3 mg, which was approximately 10-fold less potent than the minimum effective dose for morphine (0.03 mg). The duration of antinociceptive action for PZM21 (3 h) was slightly shorter than that observed for morphine. To determine whether intrathecal PZM21 induced itching, its effects were compared with those of intrathecal morphine, which causes itch in humans21 and a robust scratching response in monkeys.38 Compared with vehicle treatment, intrathecal morphine (0.03 mg) and PZM21 (0.3 mg), at equivalent doses to induce antinociception, significantly elicited scratching responses (F2, 14=14.6; P<0.05) (Fig. 3c). Although intrathecal PZM21 had a slower onset of scratching behaviour (lack of scratching at 1 h after injection) than morphine, intrathecal PZM21 and morphine both elicited robust scratching responses that lasted for 6 h.
Fig 3.
Comparison of intrathecal morphine- and PZM21-induced antinociception and itch scratching in monkeys. Antinociception induced by intrathecal morphine (a) or PZM21 (b) against an acute noxious stimulus, 50°C water, n=4. (c) Itch scratching responses elicited by intrathecal morphine (0.03 mg) and PZM21 (0.3 mg), n=8. Each data point represents the mean (standard deviation). Both compounds were delivered intrathecally. ∗P<0.05, significantly different from the vehicle condition from the first time point to the corresponding time point. ∗P<0.05, significantly different from the vehicle condition from the second time point to the corresponding time point. #P<0.05, significantly different from morphine.
Intrathecal PZM21 induces MOP receptor-mediated antiallodynia
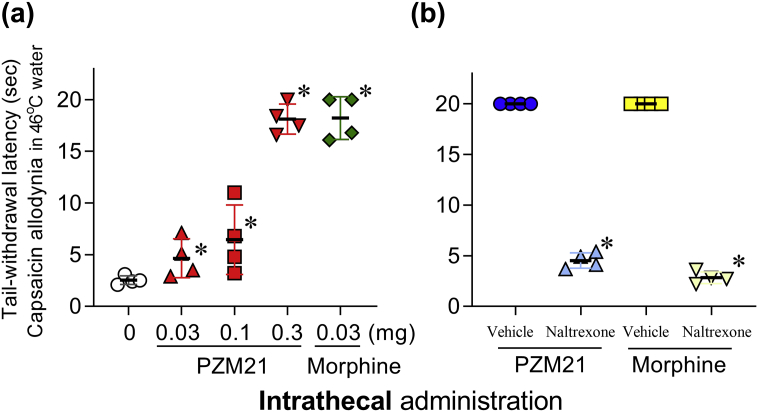
Intrathecal PZM21 (0.03–0.3 mg) alleviated capsaicin-induced thermal allodynia in 46°C water in a dose-dependent manner (F3, 9=517.2; P<0.05) (Fig. 4a). Intrathecal PZM21 (0.3 mg) and morphine (0.03 mg) exerted similar maximal antiallodynic effects. We conducted an antagonist study using a MOP receptor-selective dose (0.03 mg kg−1, s.c.) of naltrexone. Pretreatment with naltrexone completely blocked the antiallodynic effects of intrathecal PZM21 (F1, 3=115.2; P<0.05) and morphine (F1, 3=320; P<0.05) (Fig. 4b). These results demonstrated that intrathecal PZM21 and morphine both induced spinal MOP receptor-mediated antiallodynia.
Fig 4.
Comparison of intrathecal morphine- and PZM21-induced antiallodynia in monkeys. (a) Antiallodynic effects induced by PZM21 or morphine against capsaicin-induced allodynia in 46°C water. (b) Effects of the mu opioid peptide (MOP) receptor antagonist naltrexone (0.03 mg kg−1) on antiallodynia induced by intrathecal PZM21 (0.3 mg) or morphine (0.03 mg). Individual data points with the mean (standard deviation) (n=4) are presented. Vehicle and naltrexone were delivered subcutaneously. PZM21 and morphine were delivered intrathecally. ∗P<0.05, significantly different from vehicle.
Discussion
This study identified three significant findings that contributed to characterisation of the functional profile of PZM21 in primates. First, after systemic administration, PZM21 was approximately 10-fold less potent than oxycodone for inducing antinociceptive effects against an acute noxious stimulus. Second, PZM21 induced reinforcing effects and had a similar reinforcing strength as oxycodone, as determined using an intravenous drug self-administration assay. Third, after intrathecal administration, PZM21 induced MOP receptor-mediated antiallodynic effects in a dose-dependent manner. However, intrathecal PZM21 and morphine both elicited robust itch scratching responses that lasted for more than 5 h. Overall, PZM21 induced similar antinociceptive, reinforcing, and pruritic effects as the clinically used MOP receptor agonists, oxycodone and morphine, in non-human primates.
MOP receptor agonists are known to increase thermal nociceptive threshold and exert analgesia in humans.46 PZM21 produced antinociceptive effects in the hot plate assay but not in the tail flick assay, which may represent a mouse model of affective pain and reflexive pain, respectively.14 The warm water tail withdrawal assay in non-human primates has been widely used to validate the analgesic effects of opioid-related ligands and to determine their therapeutic windows.29,30,47 We found that both PZM21 and oxycodone induced full antinociceptive effects with similar durations of action, but PZM21 was 10-fold less potent than oxycodone. All clinically used MOP receptor agonists, including morphine, fentanyl, and buprenorphine, effectively increase primate tail withdrawal latency.22,30,37 Although the potency of PZM21 is lower than that of most clinically used MOP analgesics, PZM21 is expected to produce opioid-like analgesic effects in humans.
Although PZM21 did not induce conditioned place preference in rodents,14 its abuse potential can be more appropriately evaluated by the intravenous drug self-administration assay.16,17 We first used the FR30 schedule to determine whether PZM21 induced reinforcing effects, as this operant schedule of reinforcement is commonly used to determine the reinforcing effects of MOP receptor agonists in primates.39,40 Interestingly, although PZM21 was 10-fold less potent than oxycodone, both PZM21 and oxycodone induced reinforcing effects. Next, we used a PR schedule to compare the relative reinforcing strength of PZM21 with oxycodone. In evaluating abuse liability, PR schedule can distinguish between abused drugs that function as positive reinforcers.41,48 For example, oxycodone and buprenorphine exert strong and mild reinforcing strengths, respectively, in non-human primates,31,42 consistent with their abuse liabilities in humans.49 In our study, both PZM21 and oxycodone had similar reinforcing strengths over the dose ranges studied, indicating that PZM21 has oxycodone-like abuse liability in humans. Importantly, one of the first G-protein signalling-biased MOP agonists developed, TRV-130, exerted strong reinforcing strength similar to oxycodone in rats.50 These findings indicated that biasing a drug towards the G-protein signalling pathway may not determine or alter the reinforcing effects or abuse potential of MOP receptor agonists.
Spinal MOP opioids provide effective pain control as an alternative administration route.18,19 Although itching is a common adverse effect caused by spinal morphine,20,21 rodent models of spinal morphine-induced itch are limited as intrathecal morphine does not elicit robust and long-lasting scratching responses in mice or rats.51,52 In contrast, primate models of spinal morphine-induced itching established a translational bridge to validate the functional efficacy of specific ligand–receptor systems for regulating itching.38,43,53 Intrathecal PZM21 and morphine both induced antinociceptive effects. However, at antinociceptive doses, intrathecal PZM21 and morphine both elicited the same degree of robust scratching responses. Reportedly, intrathecal MOP receptor agonists with different intrinsic efficacy for activating G proteins elicit different degrees of increased scratching activities.38,54 Although PZM21 and morphine have similar intrinsic efficacy for activating spinal MOP receptors, biasing a drug towards the G-protein signalling pathway does not significantly affect itching after spinal MOP receptor activation.
Unexpectedly, intrathecal PZM21 had a slower onset of scratching responses. To the best of our knowledge, intrathecal administration of MOP receptor agonists has typically resulted in similar onsets for both antinociception and scratching.43,53,55 To determine if the early effects of intrathecal PZM21 were mediated by the MOP receptor, we conducted an antagonist study using a MOP receptor-selective dose of naltrexone.32,37 Pretreatment with naltrexone blocked the antiallodynic effects induced by either intrathecal PZM21 or morphine, indicating that both compounds induced MOP receptor-mediated antiallodynia at this early time point. Although the mechanisms underlying the different temporal patterns of intrathecal PZM21 vs morphine are unknown, future studies should examine if other biased ligands administered intrathecally produce similar effects.
Although structure-based discovery of PZM21 resulted in new avenues of study regarding G-protein signalling-biased ligands,14 the functional profile of these ligands may not be significantly different than those of clinically used MOP agonists. β-Arrestin-2 knockout mice exhibited reduced respiratory depression and constipation.13 However, a more recent mouse model, in which MOP receptors were made unable to recruit β-arrestin-2, showed morphine-induced respiratory depression, constipation, and withdrawal signs.56 In rodents, PZM21 is a low-efficacy MOP receptor agonist that can still recruit β-arrestin-2 with a lower potency than morphine, and it induces morphine-like respiratory depression.15 These findings may indicate that opioid side-effects, such as respiratory depression and constipation, are not mediated by β-arrestin-2.
The complexity of the functional role of β-arrestin-2 in opioid side-effects exists among different genetic knockout mice13,56,57 and pharmacological tools.14,15,58 Although our study focuses on the antinociceptive, reinforcing, and pruritic effects of PZM21, whether PZM2114 and other biased MOP agonists with different bias factors58 produce other side-effects, such as respiratory depression, physical dependence, and tolerance in non-human primates, warrant further investigation. PZM21 did not induce rewarding effects in rodents.14 However, PZM21 produced oxycodone-like reinforcing effect and strength as determined by the intravenous drug self-administration assay. The biased signalling towards G-protein pathways does not change the abuse potential of MOP receptor agonists. Other chemical strategies may provide promising alternatives.7,8 For example ligands with mixed agonist activities do not induce respiratory depression or reinforcing effects.31,37,42 Pharmacological studies using non-human primate models will continue to advance our understanding of receptor functions and drug effects, and provide translational relevance in the development of effective medications to treat pain and substance abuse.
Authors' contributions
Study design/planning: HD, NK, MCK
Writing of paper: HD, NK, PWC, YZ, MCK
Study conduct and data analysis: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
National Institutes of Health, National Institute on Drug Abuse (grants R01DA040693, R21DA044450, and R21DA044775).
Acknowledgements
We thank Kelsey Reynolds and Brittany Kelly for technical assistance with animal training and data collection; Heather DeLoid for performance of intrathecal catheterisation; Nicole Bacarella and Tyler Aycock, and Melinda Leary for veterinary care; and Editage for editing this manuscript.
Handling editor: Lesley Colvin
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.06.057.
Contributor Information
Yanan Zhang, Email: yzhang@rti.org.
Mei-Chuan Ko, Email: mko@wakehealth.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hewson D.W., Struys M., Hardman J.G. Opioids: refining the perioperative role of God's own medicine. Br J Anaesth. 2019;122:e93–e95. doi: 10.1016/j.bja.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Egan T.D. Are opioids indispensable for general anaesthesia? Br J Anaesth. 2019;122:e127–e135. doi: 10.1016/j.bja.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Volkow N.D., McLellan A.T. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 4.Kreek M.J., Reed B., Butelman E.R. Current status of opioid addiction treatment and related preclinical research. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins C., Smith B.H., Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122:e114–e126. doi: 10.1016/j.bja.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Algera M.H., Kamp J., van der Schrier R. Opioid-induced respiratory depression in humans: a review of pharmacokinetic–pharmacodynamic modelling of reversal. Br J Anaesth. 2019;122:e168–e179. doi: 10.1016/j.bja.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Lambert D.G. Mixed mu-nociceptin/orphanin FQ opioid receptor agonists and the search for the analgesic holy grail. Br J Anaesth. 2019;122:e95–e97. doi: 10.1016/j.bja.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Azzam A.A.H., McDonald J., Lambert D.G. Hot topics in opioid pharmacology: mixed and biased opioids. Br J Anaesth. 2019;122:e136–e145. doi: 10.1016/j.bja.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Rankovic Z., Brust T.F., Bohn L.M. Biased agonism: an emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett. 2016;26:241–250. doi: 10.1016/j.bmcl.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whistler J.L., Chuang H.H., Chu P., Jan L.Y., von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Ferguson S.S., Barak L.S. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohn L.M., Lefkowitz R.J., Gainetdinov R.R., Peppel K., Caron M.G., Lin F.T. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 13.Raehal K.M., Walker J.K., Bohn L.M. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 14.Manglik A., Lin H., Aryal D.K. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill R., Disney A., Conibear A. The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol. 2018;175:2653–2661. doi: 10.1111/bph.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ator N.A., Griffiths R.R. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 17.Mello N.K., Negus S.S. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 18.Bujedo B.M., Santos S.G., Azpiazu A.U. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8:177–192. doi: 10.5055/jom.2012.0114. [DOI] [PubMed] [Google Scholar]
- 19.Caraway D., Walker V., Becker L., Hinnenthal J. Successful discontinuation of systemic opioids after implantation of an intrathecal drug delivery system. Neuromodulation. 2015;18:508–515. doi: 10.1111/ner.12318. discussion 15–6. [DOI] [PubMed] [Google Scholar]
- 20.Ganesh A., Maxwell L.G. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333. doi: 10.2165/00003495-200767160-00003. [DOI] [PubMed] [Google Scholar]
- 21.Waxler B., Dadabhoy Z.P., Stojiljkovic L., Rabito S.F. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103:168–178. doi: 10.1097/00000542-200507000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Cremeans C.M., Gruley E., Kyle D.J., Ko M.C. Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther. 2012;343:72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko M.C., Divin M.F., Lee H., Woods J.H., Traynor J.R. Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther. 2006;316:772–779. doi: 10.1124/jpet.105.094409. [DOI] [PubMed] [Google Scholar]
- 24.Neilan C.L., Husbands S.M., Breeden S. Characterization of the complex morphinan derivative BU72 as a high efficacy, long-lasting mu-opioid receptor agonist. Eur J Pharmacol. 2004;499:107–116. doi: 10.1016/j.ejphar.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 25.Chen J., Kang D., Xu J. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin A.P., Ko M.C. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4:214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips K.A., Bales K.L., Capitanio J.P. Why primate models matter. Am J Primatol. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko M.C., Johnson M.D., Butelman E.R., Willmont K.J., Mosberg H.I., Woods J.H. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- 30.Sukhtankar D.D., Lee H., Rice K.C., Ko M.C. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 2014;231:1377–1387. doi: 10.1007/s00213-013-3341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding H., Kiguchi N., Yasuda D. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med. 2018;10:eaar3483. doi: 10.1126/scitranslmed.aar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu E., Calo G., Guerrini R., Ko M.C. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan J.L., Perlman J.E., Galvan A., Wichmann T., Bloomsmith M.A. Refining the pole-and-collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2014;53:61–68. [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenach J.C., Hood D.D., Curry R., Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Park K.M., Max M.B., Robinovitz E., Gracely R.H., Bennett G.J. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 36.Butelman E.R., Harris T.J., Kreek M.J. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- 37.Kiguchi N., Ding H., Cami-Kobeci G. BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth. 2019;122:e146–e156. doi: 10.1016/j.bja.2018.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko M.C., Song M.S., Edwards T., Lee H., Naughton N.N. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 39.Ko M.C., Woods J.H., Fantegrossi W.E., Galuska C.M., Wichmann J., Prinssen E.P. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko M.C., Terner J., Hursh S., Woods J.H., Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z., Woolverton W.L. Estimating the relative reinforcing strength of (+/–)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology (Berl) 2007;189:483–488. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- 42.Ding H., Czoty P.W., Kiguchi N. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A. 2016;113:E5511–E5518. doi: 10.1073/pnas.1605295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding H., Hayashida K., Suto T. Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol. 2015;172:3302–3312. doi: 10.1111/bph.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan C., Zhang D. Rank repeated measures analysis of covariance. Commun Stat Theory Methods. 2017;46:1158–1183. [Google Scholar]
- 45.Perrey D., Zhang D., Nguyen T., Carroll F.I., Ko M.-C., Zhang Y. Synthesis of enantiopure PZM21: a biased agonist of the mu-opioid receptor. Eur J Org Chem. 2018;2018:4006–4012. doi: 10.1002/ejoc.201800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staahl C., Christrup L.L., Andersen S.D., Arendt-Nielsen L., Drewes A.M. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123:28–36. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Kiguchi N., Ko M.C. Effects of NOP-related ligands in nonhuman primates. Handb Exp Pharmacol. 2019;254:323–343. doi: 10.1007/164_2019_211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowlett J.K. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- 49.Morley K.I., Ferris J.A., Winstock A.R., Lynskey M.T. Polysubstance use and misuse or abuse of prescription opioid analgesics: a multi-level analysis of international data. Pain. 2017;158:1138–1144. doi: 10.1097/j.pain.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 50.Austin Zamarripa C., Edwards S.R., Qureshi H.N., Yi J.N., Blough B.E., Freeman K.B. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend. 2018;192:158–162. doi: 10.1016/j.drugalcdep.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H., Naughton N.N., Woods J.H., Ko M.C. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–508. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukhtankar D.D., Ko M.C. Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H., Ko M.C. Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep. 2015;5:11676. doi: 10.1038/srep11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H., Naughton N.N., Woods J.H., Ko M.C. Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology. 2007;107:478–485. doi: 10.1097/01.anes.0000278876.20263.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko M.C., Naughton N.N. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kliewer A., Schmiedel F., Sianati S. Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 2019;10:367. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raehal K.M., Bohn L.M. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid C.L., Kennedy N.M., Ross N.C. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171:1165–1175. doi: 10.1016/j.cell.2017.10.035. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.