Abstract
Background
A growing body of literature addresses the possible long-term cognitive effects of anaesthetics, but no study has delineated the normal trajectory of neural recovery attributable to anaesthesia alone in adults. We obtained resting-state functional MRI scans on 72 healthy human volunteers between ages 40 and 80 (median: 59) yr before, during, and after general anaesthesia with sevoflurane, in the absence of surgery, as part of a larger study on cognitive function postanaesthesia.
Methods
Region-of-interest analysis, independent component analysis, and seed-to-voxel analysis were used to characterise resting-state functional connectivity and to differentiate between correlated and anticorrelated connectivity before, during, and after general anaesthesia.
Results
Whilst positively correlated functional connectivity remained essentially unchanged across these perianaesthetic states, anticorrelated functional connectivity decreased globally by 35% 1 h after emergence from general anaesthesia compared with baseline, as seen by the region-of-interest analysis. This decrease corresponded to a consistent reduction in expression of canonical resting-state networks, as seen by independent component analysis. All measures returned to baseline 1 day later.
Conclusions
The normal perianaesthesia trajectory of resting-state connectivity in healthy adults is characterised by a transient global reduction in anticorrelated activity shortly after emergence from anaesthesia that returns to baseline by the following day.
Clinical trial registration
Keywords: consciousness, functional connectivity, functional MRI, general anaesthesia, MRI, recovery
Editor's key points.
-
•
Despite possible long-term cognitive effects of anaesthetics, the normal trajectory of neural recovery attributable to anaesthesia alone has not been defined.
-
•
Resting-state functional MRI of 72 healthy human adults was performed before, during, and after general anaesthesia with sevoflurane in the absence of surgery.
-
•
There was a global reduction in anticorrelated functional connectivity in the first hour after emergence from general anaesthesia, and reductions in expression of canonical resting-state networks that all recovered to baseline by the next day.
-
•
These changes in neural dynamics help define a normal trajectory of neural recovery after general anaesthesia in healthy adults that may underlie early postoperative cognitive impairments.
The opportunity to image the adult brain during anaesthesia in the absence of surgery enables deciphering the effects of anaesthetics on neural function independently of surgery, illness, or other factors. The full range of brain functional dynamics can be accessed through resting-state functional MRI (rs-fMRI),1, 2, 3 thus enabling study of functional connectivity under anaesthesia and comparison with awake states.4, 5, 6, 7, 8 This modality therefore offers the possibility of uncovering new patterns in resting-state functional connectivity unique to anaesthesia itself and discovery of imaging-based biomarkers predictive of individual or age-based variation that may be related to normal cognitive recovery or pathological states.
Such a mechanistic understanding of neural recovery from general anaesthesia is vital to understanding the role, if any, of anaesthetic agents in the pathogenesis of perioperative neurocognitive disorders (PNDs).9, 10, 11, 12, 13 Around 60 000 patients undergo general anaesthesia and surgery every day in the USA alone,14 and it is currently unclear why some patients develop prolonged emergence, delirium, and delayed neurocognitive recovery lasting weeks to months. There is a preponderance of evidence suggesting that PND is not attributable to the pharmacodynamic properties of the anaesthetics themselves, but is more likely related to underlying medical conditions15 coupled with the persistent stress/inflammatory response elicited by surgery.16,17 Ultimately, interpretation of studies focusing on PND requires an understanding of the normal, expected trajectory of neural recovery in healthy adults, an essential yet missing piece of information.
Here, we describe normal functional connectivity changes along the perianaesthetic trajectory, as derived from analysis of the fMRI imaging acquired on 72 healthy adults tested as part of the Trajectory of Recovery in the Elderly (TORIE)18 protocol. This prospective cohort study is designed to delineate the normal trajectory of cognitive recovery after general anaesthesia in the absence of surgery in healthy adults (40–80 yr of age). In the TORIE neuroimaging protocol, rs-fMRI scans were obtained shortly before propofol induction, during a 2 h general anaesthetic at a depth of one age-adjusted minimum alveolar concentration (MAC) of sevoflurane anaesthesia, 1 h after emergence, and the following day.
In addition to delineating a normal trajectory of perianaesthetic functional connectivity changes, we focus particularly on the pattern of changes in anticorrelated functional connectivity. Recent fMRI studies of pathological states of impaired attention, cognition, and consciousness have demonstrated a reduction in anticorrelated functional connectivity that is thought to be a functional neural correlate of the associated derangements. Generally, the observed signature is seen when focusing on the interaction of the default mode network (DMN), the predominant task-negative resting-state network (RSN) that is active during internal reflection and mind wandering, with associated task-positive networks associated with executive functioning. Specific examples of reduction of anticorrelated connectivity include sleep deprivation,19 adolescents with attention deficit hyperactivity disorder (ADHD),20 ageing,21, 22, 23 mild cognitive impairment,24 and acute delirium.25
Methods
Participant selection
Results were from 72 healthy human volunteers who successfully completed the neuroimaging protocols of the TORIE project through the first day postanaesthesia (Fig. 1). This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (New York, NY, USA) and registered at ClinicalTrials.gov (NCT02275026; principal investigators: Joshua Mincer, Mark Baxter, and Mary Sano; registered on October 23, 2014).
Fig 1.
Consolidated Standards of Reporting Trials diagram.
Full details of participant enrolment, exclusion criteria, anaesthesia and monitoring protocols, and experimental design can be found in the original TORIE protocol paper.18 Briefly, all 72 volunteers were healthy (ASA physical status 1 or 2) adults between the ages of 40 and 80 yr, and had no underlying cognitive dysfunction as determined by baseline cognitive function testing (within the week before anaesthesia exposure). Anatomical scans obtained before anaesthesia induction were read by an on-site certificate of added qualification-credentialed neuroradiologist for evidence of intracranial pathology. Exclusion criteria were selected to ensure safety during anaesthesia and MRI, the ability to complete testing at longer-term follow-up, and the absence of pathophysiology that could predispose to postoperative cognitive dysfunction (such as inflammatory conditions or cerebral microvascular disease). Exposure to general anaesthesia within the last year was not a specific exclusion criterion. The characteristics of the 72 subjects are found in Table 1.
Table 1.
Subject characteristics. Data shown as median [inter-quartile range].
| Subject characteristics | Age group, N (%) |
||
|---|---|---|---|
| Youngest 20 | Oldest 20 | All participants | |
| Total enrolled | 20 | 20 | 72 (58) |
| Gender | |||
| Male | 10 (50) | 11 (55) | 42 (42) |
| Female | 10 (50) | 9 (45) | 30 (41.6) |
| Ethnicity | |||
| Hispanic or Latino | 4 (20) | 1 (5) | 9 (12.5) |
| Not Hispanic or Latino | 16 (80) | 19 (95) | 63 (87.5) |
| Unknown or not reported | 0 | 0 | 0 |
| Race | |||
| American Indian/Alaska native | 0 | 0 | 0 |
| Asian | 2 (10) | 0 | 3 |
| Black or African American | 11 (55) | 4 (20) | 31 (43) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 |
| White | 4 (20) | 16 (80) | 34 (47) |
| More than one race | 3 (15) | 0 | 3 (4.1) |
| Unknown or not reported | 0 | 0 | 1 (1.3) |
| Age (yr) | 45 [43, 47] | 71.5 [70, 75] | 59 [48.5, 69.5] |
| Minimum | 40.1 | 69.5 | 40.1 |
| Maximum | 49.9 | 80.7 | 80.7 |
| BMI (kg m−2) | 26.7 [23.1, 28.5] | 25 [22.8, 27.6] | 26.5 [24.3, 28.4] |
| ASA physical status | |||
| 1 | 15 | 13 | 57 |
| 2 | 5 | 7 | 15 |
| Disease history | |||
| Hypertension | 2 | 0 | 3 |
| Diabetes mellitus | 0 | 0 | 0 |
| Asthma | 1 | 0 | 1 |
| Malignancy (any) | 0 | 4 | 7 |
| Number of medications on initial assessment | |||
| 0 | 18 | 14 | 61 |
| 1 | 1 | 3 | 4 |
| 2 | 1 | 3 | 6 |
| 3+ | 0 | 0 | 1 |
General anaesthesia and monitoring protocols
The participants arrived early in the morning on the day they were to have general anaesthesia and neuroimaging solely for the purpose of the study. (They did not receive general anaesthesia to facilitate otherwise necessary clinical neuroimaging.) General anaesthesia was induced following acquisition of the baseline TORIE neuroimaging battery, which included rs-fMRI and other modalities and anatomical scans reviewed by the study neuroradiologist.
A baseline rs-fMRI scan was acquired before anaesthesia induction. Following the awake scan, in preparation for induction of general anaesthesia, the participant was positioned supine on the gurney in the MRI suite, and a 22-gauge i.v. catheter was placed. Standard monitors were applied, and the participant was preoxygenated. Anaesthesia was induced with propofol 2 mg kg−1 i.v., after which a laryngeal mask airway (LMA) was placed. If the LMA could not be seated properly, then the procedure would have been aborted, although this was not an issue with any of the volunteers.
Anaesthesia was maintained with inhaled sevoflurane at an age-adjusted depth of 1 MAC. A bispectral index (BIS)26 of 40–60 was obtained after LMA placement to aid in assessment of anaesthetic depth during equilibration of inhaled sevoflurane and washout of propofol. Subsequently, BIS was removed, and the participant was returned to the MRI bore for scanning. End-tidal sevoflurane concentration was used to measure anaesthetic depth during scanning, along with physiological measures. Controlled ventilation was maintained to achieve a target end-tidal CO2 of 4–4.6 kPa (30–35 mm Hg). Whilst this represents mild hyperventilation, it is consistent with anaesthesia practice at our institution. Such mild hypoventilation helps to avoid spontaneous ventilation and thereby avoid imaging artifacts and greater variability in end-tidal CO2 (with consequent variability in brain perfusion) that could affect interpretation of imaging results. Anaesthesia was maintained for the next 2 h, during which three rs-fMRI scans (and other modalities used in the TORIE neuroimaging protocol) were obtained at ∼1 h after induction, ensuring that they were obtained at a steady state of anaesthesia. Bolus administration of a pressor, such as ephedrine (5 mg i.v. or 25 mg i.m.) or phenylephrine (100 μg i.v.), was occasionally administered by the anaesthesiologist to maintain mean arterial blood pressure within 20% of baseline.
Following completion of the 2 h anaesthetic and scanning protocols, the participant was removed from the MRI bore and emerged from anaesthesia. The LMA was removed when the participant awakened. Ondansetron (4 mg i.v.) was given before emergence for antiemetic prophylaxis. No opioids, benzodiazepines, steroids, or neuromuscular blocking agents were administered. The participant was then allowed to more fully awaken, all the time being monitored by the anaesthesiologist.
The participant was returned to the MRI bore approximately 1 h after anaesthesia emergence for rs-fMRI scanning. By this point, the participant exhibited normal and adequate spontaneous ventilation and as such would be normocapnic. Following this, the participant was transported to the PACU and further monitored until discharge. The participant returned the next morning (∼24 h after anaesthesia induction) for rs-fMRI acquisition.
RS-fMRI acquisition and preprocessing
RS-fMRI scans were obtained before propofol induction, during a 2 h anaesthetic at a depth of 1 age-adjusted MAC of sevoflurane anaesthesia, 1 h after emergence, and the following day (∼24 h after anaesthesia induction). For awake scans, the participants were instructed to keep their eyes open and to let their thoughts wander.
RS-fMRI data were acquired with a multiband (MB) accelerated gradient multi-echo echo-planar imaging sequence (field of view [FOV] 224×224 mm; matrix 64×64; slice thickness 3.6 mm; 40 [32-channel] slices for whole-brain coverage; TR/TE=1500/[10.8, 28.68, 46.56] ms; MB Factor 2; blipped controlled aliasing in parallel imaging results in higher acceleration (CAPIRINHA) phase-encoding shift=FOV/3; bandwidth ∼1600 Hz/pixel; echo spacing ∼0.5 ms; total acquisition time ∼10 min [∼680 frames]).
Preprocessing of the rs-fMRI data used the SAPIENT pipeline at the Icahn School of Medicine at Mount Sinai, which combines tools from the Analysis of Functional NeuroImages (AFNI) software suite27 in an automated fashion, including slice-timing correction, motion correction, and standard space normalisation to a Montreal Neurological Institute template.27, 28, 29, 30 The tools were applied in a manner appropriate for processing of the multiecho functional MRI data. For example, motion correction parameters were estimated from the first echo image and applied to images of the other echoes. Framewise displacement (FD) traces were computed for each fMRI data set. Time points corresponding to FD >2 mm were censored from analyses. Degrees of freedom lost in censoring were accounted for in subsequent analysis. Additional nuisance regressors were generated as time courses computed from averaging fMRI signals of the white matter and CSF. These nuisance time courses were used as baseline regressors for subject-level analyses.
Data analysis to determine components of blood-oxygen-level-dependent (BOLD) signal was done using the meica.py Python software for analysis of multiecho fMRI data, implementing a pipeline involving preprocessing, high-dimensional independent components analysis, artifact component detection, and denoising by artifact removal. This software performs high-dimensional multiecho independent components analysis (ME-ICA) and automatically separates BOLD and non-BOLD signals based on information on these respective signal types germane to multiecho fMRI.31, 32, 33 Notably, the ME-ICA approach does not require the application of full-width/half-maximum spatial filters or temporal high-pass filtering to attenuate noise in preprocessing, which is all handled in the main data analysis step involving separating BOLD from non-BOLD signals. Multiecho independent components analysis denoising was performed on SAPIENT also.
The ME-ICA denoised BOLD time series for each rs-fMRI scan (i.e. each subject at each time point along the perianaesthetic trajectory) was further denoised using CompCor34 to fully remove the remaining non-BOLD respiratory artifact.35 Unlike other methods used for global signal removal (e.g. global signal regression [GSR]), CompCor does not introduce artifactual anticorrelations,36 a characteristic of particular importance when quantifying anticorrelated functional connectivity. CompCor denoising was carried out with the CONN functional connectivity toolbox. Out of 288 total scans (72 subjects×4 time points), 281 remained after preprocessing.
RS-fMRI analysis
CONN37 was used to analyse rs-fMRI data. CONN is an open-source MATLAB/statistical parametric mapping-based cross-platform software that enables computation, display, and analysis of functional connectivity fMRI data. Age as a linear covariate is included in all analyses (excluding Supplementary Figs S1c, S3, and S4); difference from mean age for every subject is entered as a second-level analysis covariate. Independent component analysis (ICA)38 was performed within CONN to obtain a set of ICA maps for all subjects at all time points: baseline before anaesthesia (PRE), during anaesthesia (ANA), 1 h after emergence (POST), and 1 day later (D1). An ICA map incorporates voxels with correlated BOLD time series. The ICA components were matched to known RSNs (salience [SAL], visual, dorsal attention [DA], language, DMN, somatosensory [SM], and frontoparietal [FP]), which were subsequently visualised and further analysed. In particular, the RSN expression map at each time point was quantified as the volume of the ICA component(s) matching each RSN. Volume was measured as the number of voxels counted in the corresponding ICA component (P<0.001, false discovery rate [FDR] corrected; cluster size P<0.05, FDR corrected).39 This allowed for comparison of each RSN expression across time points and mean expression for all RSNs.
For seed-driven functional connectivity (region of interest [ROI] to ROI and seed-to-voxel), Pearson's correlation coefficients were calculated between the seed time course and the time course of all other voxels, after which correlation coefficients were converted to normally distributed scores using Fisher's transform to allow for second-level general linear model analysis. ROI analysis was also performed with CONN, with a bivariate correlation utilising 106 cortical and subcortical ROIs predefined in CONN (Supplementary Table S1; CONN default anatomical atlas40, 41, 42 and FMRIB Software Library Harvard–Oxford atlas, excluding the cerebellar ROIs). The ROI-to-ROI analysis calculated the functional connectivity between pairs of ROIs, defined as the Fisher-transformed bivariate correlation coefficients between the BOLD time series of each ROI. The ROI time series was computed by averaging voxel time series across all voxels within each ROI. Correlations above a determined significance threshold (P<0.001 multiple testing correction utilising FDR at the network level) constituted edges between ROIs. These were further divided into correlated and anticorrelated edges, in which the sign of the correlation was either positive or negative, respectively. Correlated and anticorrelated connectivities across all ROIs were compared at the four time points. Seed-to-voxel analysis was performed within the CONN suite. For the DMN, four preset seeds were selected (medial prefrontal cortex [MPFC], posterior cingulate cortex [PCC], and left and right parietal cortex) for combined main effect ([0.25 0.25 0.25 0.25] vector).
Statistical analysis
Statistical analysis was performed within the CONN37 functional connectivity suite. For ROI-to-ROI analysis, the threshold for statistical significance was set at P<0.001, FDR corrected for multiple comparisons (i.e. 106 ROIs). For ICA and seed-to-voxel analyses, the threshold was set at P<0.001. The FDR-corrected and cluster size threshold was set to P<0.05, FDR corrected. Visualisation of data used images from CONN. Excel™ (Microsoft Corp., Redmond, WA, USA) was also used for basic data analysis of quantities from CONN: means, standard deviation, and errors. For ROI-to-ROI analysis, two-sided paired t-tests were used on the 106 ROIs.
Results
Functional connectivity along the perianaesthetic trajectory
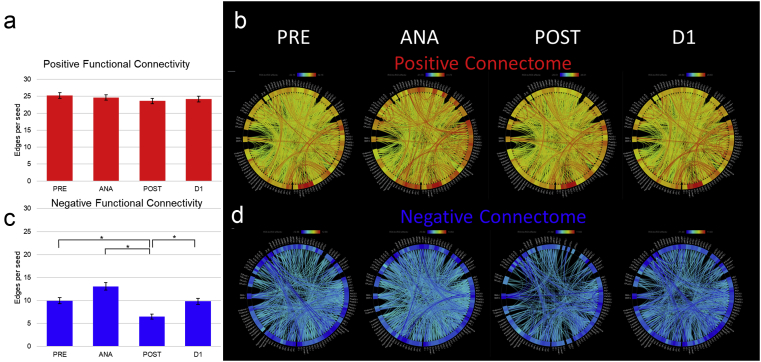
As a first step in assessing functional connectivity changes, we performed a global ROI-to-ROI analysis on all subjects (subject characteristics are summarised in Table 1) using 106 cortical and subcortical ROIs (predefined within the CONN Functional Connectivity Toolbox,37 as listed in Supplementary Table S1) across the four perianaesthesia time points: at baseline (PRE), under anaesthesia (ANA), 1 h after emergence from general anaesthesia (POST), and the following day (D1) (see Supplementary Fig. S1c for distribution of correlation coefficients at these different time points). Statistically significant connections (edges) between ROI pairs (P<0.001, FDR corrected) were counted, divided into correlated (positive) and anticorrelated (negative) edges, and averaged (Fig. 2) as the number of edges (either correlated or anticorrelated) per ROI for a given time point (total number of edges is counted in Supplementary Fig. S1a and b). Correlated (positive) functional connectivity was roughly the same across the perianaesthesia trajectory (Fig. 2a and b). In contrast, anticorrelated connectivity changed significantly across the perianaesthesia trajectory (Fig. 2c and d). Specifically, averaged across all ROIs, the number of anticorrelated edges per ROI increased by 31% at ANA (P=2×10−6) and decreased by 35% (P=5.28×10−14) in POST compared with PRE (9.96 at PRE, 13.07 at ANA, and 6.47 at POST) with return to baseline at D1 (see Supplementary Table S2 for edges per ROI). These changes in edge number are not caused by signal dropout, as the average T-statistic of all ROIs was essentially unchanged across conditions (Supplementary Table S3).
Fig 2.
Global ROI-to-ROI analysis. ROI-to-ROI analysis was performed using 106 cortical and subcortical ROIs (predefined in CONN). Functional connectivity was characterised as the number of statistically significant edges connecting ROIs and further divided into correlated and anticorrelated, represented by positive and negative edges, respectively (P<0.001, false discovery rate corrected for multiple comparisons). (a) Quantification of correlated (positive) functional connectivity as average edges per ROI is shown schematically for each time point along the perianaesthesia trajectory. (b) Connectome map of correlated functional connectivity. (c) and (d) Anticorrelated (negative) edges are similarly characterised. Correlated functional connectivity does not change significantly across the perianaesthesia trajectory. Changes in anticorrelated functional connectivity were significant: average anticorrelated edges per ROI increased under anaesthesia and decreased postanaesthesia compared with baseline (PRE) (∗P<E−10; paired t-test). ANA, during anaesthesia; D1, 1 day later; POST, 1 h after emergence; PRE, baseline before anaesthesia; ROI, region of interest.
The return to baseline at D1 is not an average phenomenon, but rather represents each ROI essentially returning to its baseline connectivity value. This is shown in Fig. 3, where the connectivity value at D1 is plotted against baseline for each ROI (correlated connectivity in Fig. 3a and anticorrelated connectivity in Fig. 3b). The best-fit line for each curve is plotted as well, with intercept set at zero. For positive correlations, the slope was 0.96 (R2=0.88), whereas for anticorrelations the slope was 0.93 (R2=0.68). The nearness of these slopes to 1 illustrates that the regression line is close to the line D1=baseline (PRE).
Fig 3.
Return to baseline connectivity at day 1. For each ROI, connectivity is plotted for D1 (y-axis) vs baseline (x-axis) values. (a) Correlated connectivity and (b) anticorrelated connectivity. The best-fit regression line is plotted as well (with intercept set to 0). Slope is 0.96 (R2=0.87) for correlated connectivity and 0.93 (R2=0.65) for anticorrelated connectivity. D1, 1 day later; PRE, baseline before anaesthesia; ROI, region of interest.
RSN expression along the perianaesthesia trajectory
Complementing the ROI-to-ROI analysis, we characterised RSN activity using group ICA applied to all subjects at all four time points. Resulting components were aligned to canonical RSNs, specifically the DMN, DA, FP, SM, and SAL. For each component, we quantified volume at each time point corresponding to the number of statistically significant voxels (P<0.001, FDR corrected; cluster size P<0.05, FDR corrected).43,44
The expression map of the DMN ICA component is shown in Fig. 4a and b. The DMN volume of both the positive and negative components was reduced in POST compared with PRE, returning to baseline levels at D1. As a control for the adequacy of the DMN ICA component adequacy, we performed a seed-to-voxel analysis of the DMN (Fig. 4c and d, where the DMN is represented by MPFC, PCC, and parietal cortices ROIs from CONN). Overall, the ICA and seed-to-voxel analyses were similar. Note that in Fig. 4a and c, the component volumes were quantified as the count of statistically significant voxels at each time point.
Fig 4.
Resting-state network expression along the perianaesthesia trajectory. (a) Quantification of volume (statistically significant voxels) of the ICA component matching the DMN (correlated volume in red; anticorrelated volume in blue), with (b) multiple slices illustrated. Seed-to-voxel analysis of the DMN is presented as a control for the adequacy of the ICA component, with (c) quantification of statistically significant voxels and (d) multiple slices illustrated. For seed voxel, the DMN is represented by medial prefrontal cortex, posterior cingulate cortex, and parietal cortex ROIs. Statistical threshold was set at P<0.001, false discovery rate (FDR) corrected. ICA analysis: normalised (e) positive and (f) negative components of various resting state networks at each time point (with voxel counts normalised to baseline value for each RSN). (g) Average volume across RSNs (red, positive; blue, negative). Statistically significant changes (paired t-test) are shown (∗P<0.05; ∗∗P<0.01). ANA, during anaesthesia; D1, 1 day later; DA, dorsal attention; DMN, default mode network; FP, frontoparietal; ICA, independent component analysis; POST, 1 h after emergence; PRE, baseline before anaesthesia; ROI, region of interest; RSN, resting-state network; SAL, salience; SM, sensory motor.
Similar quantification of other RSN volumes is shown in Fig. 4e and f. To facilitate comparison between networks, expression at each time point was normalised to each network's baseline (Fig. 4g). Volumes were divided into positively correlated (Fig. 4e) and negatively correlated (Fig. 4f) constituent voxels for each RSN. A consistent decrease in RSN expression was observed postanaesthesia in both the positive and negative constituents of each RSN. This is further captured by the mean normalised expression across networks (Fig. 4g), illustrating an average 40% decrease for POST (P<0.005). Average RSN expression under ANA did not change compared with baseline, although individual RSNs showed either an increase or decrease. For each RSN, expression returned to baseline at D1.
Effect of subject age
Age was entered as a continuous linear covariate in all of the analyses. Still, to investigate the potential effects of age in our data set, we conducted additional analyses: Supplementary Fig. S2 features global ROI-to-ROI analysis at baseline (PRE) with and without age as a linear covariate, which were essentially identical. Next, we conducted a global ROI-to-ROI analysis with age as the primary modifier, rather than a covariate. Using the conventional significance threshold in our analysis (P<0.001, FDR), we found a single edge significantly affected by age (data not shown). Utilising a more liberal threshold (P<0.05 FDR) revealed a maximum change of 12 edges, as seen in Supplementary Fig. S3 (POST). Comparing this with the total number of edges (Supplementary Fig. S1; about 2500 positive edges and 1000 negative edges) revealed this change to be on the order of 0.1%.
We also conducted a subgroup analysis focusing on extremes of age in the overall cohort (i.e. the 20 youngest [range: 40–50; mean: 45.6 yr] and oldest subjects [range: 69–80; mean: 73.1 yr]). Supplementary Fig. S4 shows the similar distributions of correlation coefficients of young and old subgroups at baseline. Global ROI-to-ROI analysis (Supplementary Fig. S5a and c) showed total edges over the four time points. Overall, there were ∼15% more positively correlated edges in the younger subgroup compared with the older (1246 vs 1034, respectively). Otherwise, the trends in each group were similar as were the DMN volumetric maps resulting from seed-to-voxel analysis (Supplementary Fig. S5b and d). Whilst we observed the previously shown reduction in anticorrelated activity in the younger group at the POST time point (Supplementary Fig. S5a), the ability to identify anticorrelated edges was reduced in both subgroups (Supplementary Fig. S5a and c). Fragmentation of the overall cohort into subgroups results in a loss of statistically significant signal as compared with the overall cohort analysis. Quantitatively, this is illustrated by the ROI-to-ROI analysis, where at each time point, each subgroup has less than half the number of statistically significant edges seen in the overall cohort (Supplementary Fig. S1a and b). Notably, the return to PRE levels at D1 is evident in both subgroups.
Discussion
We determined the normal perianaesthetic trajectory of functional connectivity as seen through analysis of 72 healthy adults from the TORIE study.18 Whilst functional connectivity returned to baseline by 1 day after anaesthesia exposure, the degree of anticorrelated functional connectivity increased globally under anaesthesia and was reduced in early recovery as measured 1 h after emergence from sevoflurane anaesthesia without surgery.
These changes in anticorrelated activity were not reciprocated by a change in positively correlated activity, as the latter was essentially unchanged along the perianaesthetic trajectory. This does not reflect a decrease in statistical power of the anticorrelated connections (edges). Both of these observations run counter to the notion that the observed reduction in anticorrelations in early recovery is artifactual, a possibility we take seriously given concerns raised about the validity and meaning of anticorrelated connectivity since the introduction of the concept by Fox and colleagues.45 We also note that concerns about the introduction of artifactual anticorrelations in fMRI analysis are thought to be limited to preprocessing methods that use GSR,46,47 which we avoided. Our denoising methods involving ME-ICA and CompCor do not introduce artifactual anticorrelations.34, 35, 36, 37
Our finding of reduced anticorrelations in early recovery after anaesthesia fits into an emerging picture that links a reduction in anticorrelated connectivity to attentional states generally48,49 and to pathological brain states in particular. The reduced anticorrelations signature has been observed (although perhaps more locally than what we have observed globally in early recovery) in states characterised by impairments in attention, cognition, and consciousness, such as sleep deprivation,19 mild cognitive impairment,24 ADHD,20 and acute delirium.25 Anticorrelated connectivity may maintain segregation between network activity and therefore maintain the boundaries and integrity of RSNs associated with distinct neural processes. As such, perturbations in anticorrelated connectivity might perturb the boundaries between distinct RSNs, with consequences for information processing relevant to cognition and for maintenance of levels of attention and consciousness. Whilst we found some reduced anticorrelations with increased age at baseline, as suggested by Spreng and colleagues,21 we did not find a global reduction in anticorrelations at baseline.
The analogy between early recovery and pathological states of attention and cognition fostered by a common signature of reduced anticorrelations may be informative. From a clinical perspective, patients in early recovery after anaesthesia show transient cognitive and attentional deficits that normally resolve with time. We propose that the globally reduced anticorrelations seen in early recovery may be a functional neural correlate of the observed cognitive deficits seen in early recovery. Seen in this light, the global increase in anticorrelations that we observe under anaesthesia may correlate with anaesthetic-induced loss of consciousness in so much as tightly bounded neural networks are unable to support consciousness. To complete the picture, the awake baseline state represents a balance between network integrity and the need for information transfer and integration between networks.
The notion of network integrity is supported by the ICA analysis that focuses on individual RSNs. The ICA component analysis differs profoundly from the ROI-to-ROI analysis; yet, the recapitulation of a similar trend supports our main finding. In particular, a similar signature was seen across RSNs at the early recovery time point (i.e. an overall reduction in RSN volume as measured by ICA component volume). This reduction in RSN volume may be interpretable in a similar fashion as the reduction in anticorrelations: namely, a brain characterised by mild dysfunction in attention and cognition. We propose that early recovery from general anaesthesia with sevoflurane is accompanied by a transient reduction in functional segregation, and that this is reflected by a decrease in anticorrelated functional activity as measured by rs-fMRI. Because we saw little variation in the pattern of change in anticorrelated activity with age, this pattern may be permissive for postoperative neurocognitive dysfunction, but not directly causative.
We recognise several potential limitations of our study. First, age-adjusted MAC is not a perfect parameter, as it excludes normal variability between subjects in their sensitivity to volatile anaesthetics. However, in the absence of a truly reliable, fMRI-compatible depth-of-anaesthesia monitor, it is the best possible. Second, only sevoflurane was used in this cohort, and generalisation to other anaesthetic agents should be done cautiously. Third, generalisation of insights attained from neuroimaging data is best supported by some physiological or performance data. We plan to report analysis of the TORIE cognitive testing data in a future study. Fourth, our analytical approach of global ROI-to-ROI analysis, whilst highly significant and robust, may conceivably not be reproduced with partial subsets, which may deviate from the general pattern described previously. To that concern, we note that the signal of interest here is the global reduction in anticorrelations, and as such is better captured by a more inclusive ROI set rather than a more limited subset. Fifth, we note that by necessity the four time points correspond to fMRI acquisition time windows that are not on the same fMRI sequence. This necessary shortcoming has prevented us from performing direct contrast subtraction between time points, and consequently all analyses are comparisons of finalised analyses within each scan. Sixth, the subjects' eyes were closed under general anaesthesia and open at other time points, conceivably affecting comparison between ANA and other time points, although this would most likely be limited to visual networks. This should not detract from the main findings in early recovery as the PRE, POST, and D1 time points all featured eyes open. Seventh, end-tidal CO2 levels differed between ANA and the other time windows as mild hyperventilation was used under ANA to reduce respiratory drive and minimise movements. This was deemed safer than the use of neuromuscular blocking agents (and tracheal intubation) in this cohort of healthy volunteers. Eighth, although the overall TORIE study was designed to look at impact of ageing on trajectory of recovery of cognitive function after general anaesthesia, we did not prospectively power the study to look at patterns of anticorrelated activity specifically. Finally, we note that whilst we have looked in detail at potential effects of age on our results, future study focusing on this specific aspect is necessary to more definitively address this potential factor.
In summary, our results indicate a global reduction in anticorrelated functional connectivity in the first hour after emergence from general anaesthesia across a wide range of ages in healthy adults. In addition, there were expected reductions in expression of canonical RSNs that all recovered to baseline by the next day. These changes in neural dynamics help define a normal trajectory of neural recovery after general anaesthesia in healthy adults. This provides a key comparison for investigation of neural dynamics after recovery from anaesthesia and surgery, and insight into network activity states that may be permissive for the development of postoperative neurocognitive dysfunction.
Authors' contributions
Research design: JSM, MGB, SGD, PJM, MS, CYT, BND
Conducting of research: AES, JWB, JSM, PJM, HA
Analytical tools contribution: PK, K-HH
Data analysis: TN, YJ
Writing of paper: JSM, TN
Acknowledgements
The authors would like to thank their colleagues James Leader, Jacqueline Crittendon, Mohammed Ismail, Matthew Hartnett, Jessica Jong Kim, Carolyn Fan, Kirklyn Escondo, Rachelle Jacoby, Shanice Dumay, Johnny Ng, and Victoria Wang, who all assisted in the research. The authors gratefully acknowledge Jeffrey Silverstein, who conceived and implemented this study and served as principal investigator until his death. JSM acknowledges Sahrena London for helpful conversations.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.06.058.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
US National Institutes of Health’s National Institute on Aging (R01AG046634) to MB, JM, MS; National Cancer Institute Support Grant (P30 CA008748) to PJM, JWB, and JSM.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Smith S.M., Fox P.T., Miller K.L. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M.H., Smyser C.D., Shimony J.S. Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol. 2013;34:1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boveroux P., Vanhaudenhuyse A., Bruno M.A. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 5.Liu X., Lauer K.K., Ward B.D., Li S.J., Hudetz A.G. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akeju O., Loggia M.L., Catana C. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife. 2014;3 doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonhomme V., Vanhaudenhuyse A., Demertzi A. Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology. 2016;125:873–888. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 8.Maier K.L., McKinstry-Wu A.R., Palanca B.J.A. Protocol for the reconstructing consciousness and cognition (ReCCognition) study. Front Hum Neurosci. 2017;11:284. doi: 10.3389/fnhum.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jevtovic-Todorovic V., Absalom A.R., Blomgren K. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleason L.J., Schmitt E.M., Kosar C.M. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150:1134–1140. doi: 10.1001/jamasurg.2015.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleisher L.A. Brain Health Initiative: a new ASA patient safety initiative. ASA Monit. 2016;80:10–11. [Google Scholar]
- 12.Inouye S.K., Marcantonio E.R., Kosar C.M. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer's Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evered L., Silbert B., Knopman D.S. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown E.N., Lydic R., Schiff N.D. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dokkedal U., Hansen T.G., Rasmussen L.S., Mengel-From J., Christensen K. Cognitive functioning after surgery in middle-aged and elderly Danish twins. Anesthesiology. 2016;124:312–321. doi: 10.1097/ALN.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacas S., Degos V., Feng X., Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckenhoff R.G., Laudansky K.F. Anesthesia, surgery, illness and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:162–166. doi: 10.1016/j.pnpbp.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mincer J.S., Baxter M.G., Brallier J.W., Sano M., Schwartz A.E., Deiner S.G. Delineating the trajectory of cognitive recovery from general anesthesia in older adults: design and rationale of the TORIE (Trajectory of Recovery in the Elderly) project. Anesth Analg. 2018;126:1675–1683. doi: 10.1213/ANE.0000000000002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Havas J.A., Parimal S., Soon C.S., Chee M.W.L. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Mills B.D., Miranda-Dominguez O., Mills K.L. ADHD and attentional control: impaired segregation of task positive and task negative brain networks. Netw Neurosci. 2018;2:200–217. doi: 10.1162/netn_a_00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzmeier N., Buerger K., Teipel S. Neurobiology of aging cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol Aging. 2017;50:152–162. doi: 10.1016/j.neurobiolaging.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Esposito R., Cieri F., Chiacchiaretta P. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018;12:127–141. doi: 10.1007/s11682-017-9686-y. [DOI] [PubMed] [Google Scholar]
- 25.Choi S.H., Lee H., Chung T.S. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169:498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 26.Sigl J.C., Chamoun N.G. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit. 1994;10:392–404. doi: 10.1007/BF01618421. [DOI] [PubMed] [Google Scholar]
- 27.Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Avants B., Tustison N., Song G. Advanced normalization tools (ANTs) Insight J. 2009:1–35. [Google Scholar]
- 29.Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kundu P., Inati S.J., Evans J.W., Luh W.M., Bandettini P.A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60:1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundu P., Brenowitz N.D., Voon V. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U S A. 2013;110:16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundu P., Voon V., Balchandani P., Lombardo M.V., Poser B.A., Bandettini P. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage. 2017;154:59–80. doi: 10.1016/j.neuroimage.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power J.D., Plitt M., Gotts S.J. Ridding fMRI data of motion-related influences: removal of signals with distinct spatial and physical bases in multiecho data. Proc Natl Acad Sci U S A. 2018;115:201720985. doi: 10.1073/pnas.1720985115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai X.J., Castañán A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 38.Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobromyslin V.I., Salat D.H., Fortier C.B. Distinct functional networks within the cerebellum and their relation to cortical systems assessed with independent component analysis. Neuroimage. 2012;60:2073–2085. doi: 10.1016/j.neuroimage.2012.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desikan R.S., Ségonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein J.M., Seidman L.J., Makris N. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Frazier J.A., Chiu S., Breeze J.L. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 43.Case M., Zhang H., Mundahl J. Characterization of functional brain activity and connectivity using EEG and fMRI in patients with sickle cell disease. Neuroimage Clin. 2017;14:1–17. doi: 10.1016/j.nicl.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noirhomme Q., Soddu A., Lehembre R. Brain connectivity in pathological and pharmacological coma. Front Syst Neurosci. 2010;4:1–6. doi: 10.3389/fnsys.2010.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Clare Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Popa D., Popescu A.T., Paré D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.