Abstract
Adipose tissue is one of the largest organs, playing important roles in physiology and pathologies of multiple diseases. However, research related to adeno-associated virus (AAV) targeting adipose tissue has been left far behind studies carried out in the liver, brain, heart, and muscle. Despite initial reports indicating poor performance, AAV-mediated gene delivery to adipose tissue has continued to rise during the past two decades. AAV8 and a novel engineered hybrid serotype, Rec2, have been shown to transduce adipose tissue more efficiently than other serotypes so far tested and have been applied in most of the in vivo studies. The Rec2 serotype displays high efficacy of gene transfer to both brown and white fat via local and systemic administration. This review summarizes the advances in developing AAV vectors with enhanced adipose tropism and restricting off-target transgene expression. We discuss the challenges and strategies to search for and generate novel serotypes with tropism tailoring for adipose tissue and develop AAV vector systems to improve adipose transgene expression for basic research and translational studies.
Keywords: AAV, adipose, gene therapy, tissue tropism, capsid engineering
Graphical Abstract
AAV8 and a novel hybrid serotype, Rec2, show higher efficacy in transducing adipose tissue than do other serotypes. This review summarizes the advances in developing AAV vectors with enhanced adipose tropism and restricting off-target transgene expression. The challenges and strategies to develop AAV vector systems tailoring for adipose tissue are discussed.
Main Text
Adeno-associated virus (AAV) vectors play an important role in gene therapy and biomedical research as a safe and effective method for gene delivery. AAV is a non-pathogenic parvovirus and one of the smallest viruses with a non-enveloped capsid size of 25 nm.1 AAVs depend on a helper virus such as adenovirus for a productive infection cycle. The AAV genome consists of a linear single-stranded DNA (ssDNA) genome of approximately 4.7 or 4.9 kb with two 145-nt palindromic inverted terminal repeats (ITRs), which flank viral genes, such as Rep (replication), Cap (capsid), and the assembly-activating protein (AAP), which is important for some, but not all AAV, serotypes.2, 3, 4, 5, 6 The Rep gene encodes four regulatory proteins through the use of two promoters and alternative splicing. These regulatory proteins are Rep78, Rep68, Rep52, and Rep40, which are involved in AAV genome replication. The Cap gene generates three capsid proteins, i.e., VP1 (virion protein 1), VP2 (virion protein 2), and VP3 (virion protein 3), through the use of an alternative splice acceptor site. VP1, VP2, and VP3 make up an AAV capsid with each subunit at a ratio of 1:1:10 to form an icosahedron structure.7,8 Capsid proteins are currently being heavily researched in order to gain insights into cell binding and entry.
Naturally occurring AAV serotypes have been identified and characterized with a variety of tissue tropisms. Currently there are 13 AAV serotypes identified and at least 11 serotypes commonly available in the laboratory setting.9 Serotypes 1, 4, and 7–11 were discovered in non-human primates, while serotypes 2, 3, 5, and 6 were isolated from humans.10, 11, 12 In addition to these mostly applied serotypes, there are more than 100 capsid variants that have been identified from human and nonhuman primates.11 One study showed that the human population is serologically positive to AAV, suggesting that antibodies existed against AAV in humans,13 which could pose a challenge for human-derived capsids in gene therapy. Thus, it is suggested that isolation of capsids from non-human sources may help overcome pre-existing immunity.14 Few among the commonly available AAV serotypes in the laboratory setting have been shown to efficiently transduce adipose tissue. Thus, it is necessary to obtain variants with specific tropisms, and variants evading pre-existing immunity.
Because ITRs are the sole requirement for packaging DNA into AAV capsids, recombinant AAVs (rAAVs) can be produced by exchanging wild-type AAV genome with any gene of interest and regulatory elements up to 4.7–4.9 kb. Pseudo-serotyping has been widely adopted in the development of AAV vectors by cloning each serotype capsid-coding domain into a common vector backbone containing the AAV serotype 2 Rep gene.15 However, reports suggest that pseudo-serotyping may confer distinct properties on AAV vectors, such as receptor binding, intracellular trafficking, transcytosis, and uncoating vector genome in nucleus,16, 17, 18 and the vast majority of research using pseudo-serotyping focuses on vector tropism, including the naturally occurring and engineered serotype vectors targeting adipose tissues that are discussed hereafter. Typically, generation of a pseudo-serotype AAV vector requires the use of three plasmids, including a cis plasmid with ITRs from AAV2 flanking an expression cassette (promoter, transgene, and optionally a post-transcriptional regulatory element), a trans plasmid containing Rep from AAV2 (most commonly) and Cap from another AAV serotype, and the addition of adeno helper to promote AAV replication.
AAV2, the best characterized AAV, is known to enter the cell by using membrane-associated heparan sulfate proteoglycan (HSPG) as its primary receptor.19 Other AAV serotypes require binding to cell surface glycans for infection19, 20, 21, 22, 23, 24. Other candidates, such as AAVR, αVβ5 integrin, and fibroblast growth factor receptor 1 (FGFR1), have also been reported to participate in AAV attachment.25, 26, 27, 28 Recent studies have shed new light on molecular mechanisms of engagement between the AAV receptor and AAV2 and other serotypes.25,28 However, the mechanisms of AAV attachment and entry into adipocytes are unknown.
Adipose Tissue
Adipose tissue is traditionally classified into two types, i.e., white adipose tissue (WAT) and brown adipose tissue (BAT), with distinct developmental origin, morphology, and function. WAT consists of white adipocytes with a single uniocular droplet in the cytoplasm and making up a majority of adipose tissue. BAT, alternatively, is composed of brown adipocytes with multiocular droplets in the cytoplasm and abundant uncoupling protein-1 (UCP1) in mitochondria. BAT is located in the interscapular and perirenal regions of rodents. Brown adipocytes as a cluster are distributed over the neck and shoulder areas in humans.29, 30, 31, 32 BAT is highly innervated and vascularized.33 WAT acts as an energy storage site while BAT dissipates stored energy in the form of heat. In addition, WAT and BAT also serve as an endocrine organ to secrete numerous adipokines, cytokines, growth factors, and circulating exosomal microRNAs (miRNAs) to exert systemic effects.34,35
Adipose tissue is composed of adipocytes and a stromal vascular fraction (or cells), which includes preadipocytes, fibroblasts, mesenchymal stem cells (MSCs), endothelial and smooth muscle cells, macrophages, and immune cells. In rodents, a cluster of UCP1-positive and brown-like cells, termed as beige or brite adipocytes, is found among various fat pad depots, and their appearance can be dramatically increased upon cold exposure or sympathetic stimulation.29,36 Adipose tissue expansion or adipogenesis is achieved through hypertrophy (increasing size of cells) and hyperplasia (increasing number of cells). Upon over-nutrition, adipose tissue expands first by hypertrophy. In humans, it appears that the number of adipocytes, but not size, is stable during the state of overfeeding or obesity. Even weight loss is associated with a reduction in adipocyte volume, but not overall number.2 In rodents however, up to 80% of epididymal adipocytes and 3% of inguinal adipocytes died after a few months of high-fat feeding, indicating depot-specific proliferation and differentiation.37 Thus, obesity, at least for rodents, is associated with hypertrophy and hyperplasia. Of note, over-nutrition or reduced energy expenditure is the primary driver of obesity, but not adipogenesis per se.29
Adipose tissue is distributed in various locations, including visceral and subcutaneous fat depots. There appears to be a distinction among fat depots.29 Unique innervation, vascularization, and cell-autonomous mechanisms in each fat depot may contribute to distinctive functions among various fat depots.38,39 The visceral fat depot, but not the subcutaneous fat depot, is associated with metabolic disease risk.40 Excessive adiposity is a well-recognized risk factor for type II diabetes, metabolic syndrome, and certain types of cancer.41, 42, 43, 44 Thus, gain- and loss-of function studies by genetically manipulating adipose tissue are essential to study adipose physiology, its role in disease, and for the development of therapeutics.
The deletion or overexpression of genes from the germline may sometimes interfere with adipocyte differentiation or development.45, 46, 47 The inducible transgenic model is one alternative that can avoid unintended consequences of germline deletion or overexpression of the gene of interest. However, the generation of transgenic models requires costly and time-consuming selection and maintenance of rodent lines. Moreover, genetic manipulation of adipocytes at a whole-body level may not necessarily reveal cross-talk or interactions between specific fat depots and other tissues such as the hypothalamus, and individual fat depot distinction and function may not be noticed. Thus, viral vectors become an attractive alternative that can avoid unintended effects on adipose development and restrict transgene expression to specific fat depots at any given age. The turnaround is faster and the expense is less than that of transgenic models. This review focuses specifically on AAV-mediated adipose tissue transduction and challenges to targeting adipose tissue with naturally occurring serotypes and engineered serotypes. We discuss the potential of AAV8 and a novel engineered hybrid serotype, Rec2, as a gene delivery platform to transduce adipose tissue and yield therapeutic efficacy.
Challenges to Target Adipose Tissue with Viral Vectors
Targeting adipose tissues or adipocytes in culture with adenovirus, retrovirus, and lentivirus have been reported with various degrees of transduction efficiencies.47,48 These viruses are immunogenic while γ-retroviruses and lentiviruses are able to integrate in the host genome,49,50 leading to unintended consequences and carcinogenic risk.51 Thus, many efforts have been made for safer lentivirus vectors. Recent clinical data suggest that the newer generation of lentivirus vectors strongly reduce the risk of insertional mutagenesis.52 In contrast, AAVs allow for long-term transgene expression while circumventing the problem of frequent genome insertion by the retrovirus family. rAAV vectors primarily remain episomal in the nucleus of transduced cells, and random integration of AAV vectors into host DNA are low-frequency events,1 although the integrations and subsequent toxicity have been observed in mouse models.53 AAVs have low immunogenicity and can transduce both dividing and non-dividing tissues.50,54,55 They have exhibited sustained transgene expression in small animal models,55 large animal models,56 and humans.57 In animal models, liver, heart, skeletal muscle, eyes, and the central nervous system have been safely and successfully targeted for gene transfer.1,14 However, AAV-mediated gene delivery to adipose tissue has been a challenge largely due to relatively low transduction efficiency and tropism with naturally occurring serotype vectors.
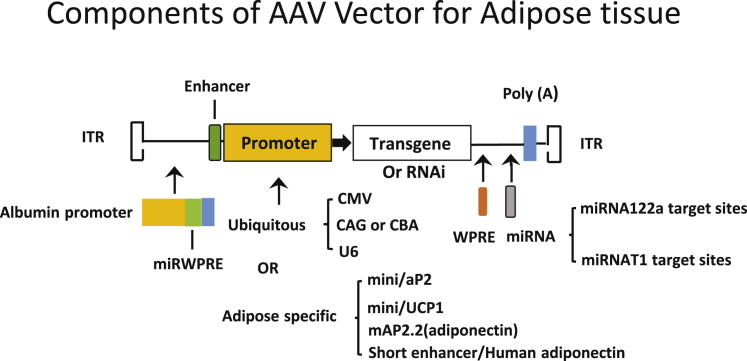
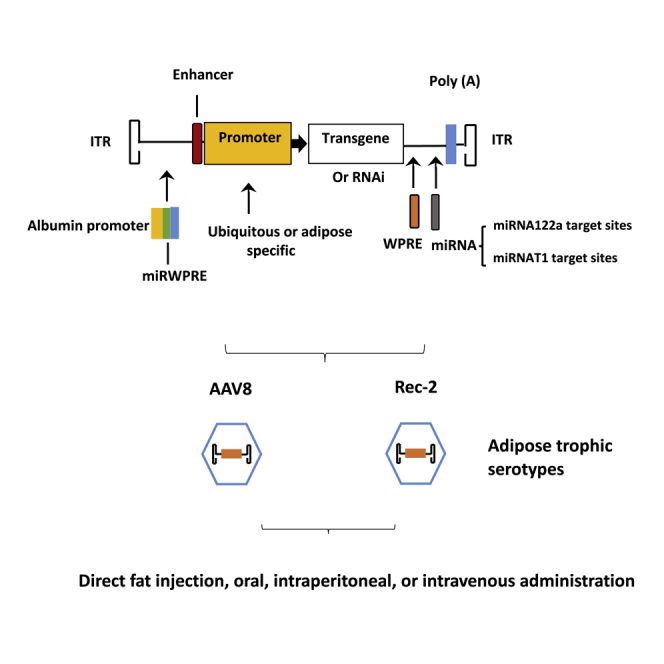
Therefore, when choosing rAAV platforms for adipose tissue gene transfer, serotype tropism for adipose tissue, transgene expression efficacy, and specificity are the three most influential factors for consideration. As most of AAV serotypes pose a broad-spectrum affinity for tissues such as liver, heart, and muscle, off-target effects are a hurdle for targeting adipose tissue. However, this issue can be mitigated by delivery route, use of an adipose-specific promoter, and incorporation of miRNA target sites in the 3′ UTR of transgene. Modifications to the AAV genome to target adipose tissue more effectively are illustrated in Figure 1. Delivering AAVs locally helps mitigate off-target effects because of a lower dose and fewer leaks in the circulation. However, injecting multiple fat depots is time-consuming, invasive, and difficult for visceral fat depots. Systemic delivery, alternatively, could distribute AAVs in more fat depots, but it requires a higher dose than that of local delivery and may provoke a greater immune response against the capsid58 and generate off-target effects. The type of promoter used for transgene expression can dictate the dosage, as an adipose-specific promoter is much less potent than that of a ubiquitous promoter such as hybrid cytomegalovirus enhancer/chicken β-actin (CBA or CAG) or cytomegalovirus (CMV). As a result, systemic delivery usually utilizes an adipose-specific promoter with a higher dose, while local delivery can be achieved by using a CBA or CMV promoter with a lower dosage. Of note, adipose tissue-specific promoters that are used in AAV vectors contain only basic or core components due to limited capacity to accommodate the DNA sequence in the AAV vector. Furthermore, liver- and heart-specific abundant miRNAs could be mobilized to de-target or suppress transgene expression in liver and heart if those miRNA target sequences are embedded in the vectors. Regulatory elements, such as the woodchuck hepatitis post-transcriptional regulatory element (WPRE), are built into some rAAV vectors to enhance transgene expression, which can serve as a target for restricting off-target transgene expression. We summarize the literature regarding rAAV vectors and dosage for gene delivery to adipose tissue in this review. Of note, comparisons of AAV vectors in adipose tissue based on previous reports, particularly among different laboratories, come with a caveat due to the difficulties in vector packaging and quantification. The following AAV serotypes have been shown to effectively transduce adipose tissue in vivo.
Figure 1.
Components of the AAV Vector Targeting Adipose Tissue
AAV1
Mizukami et al.59 first reported that AAV1 can transduce adipose tissue better than do other AAV serotypes (1–5) tested. In the study, 6 × 1010 viral genomes (vg) of AAVs were locally injected into an inguinal WAT (iWAT) for comparison of serotypes with β-galactosidase (LacZ) as a reporter gene driven by the CMV promoter. However, a dose of 2 × 1011 vg of AAV1 vector carrying the therapeutic gene erythropoietin (Epo) was needed to achieve an elevated Epo level in the circulation. Pluronic F88, a nonionic surfactant, was added to aid transduction. The authors did not observe any damage in adipose tissue despite the high dose. The same group also reported that adipose transduction required a dose of 2 × 1011 vg/mouse to achieve a similar plasma Epo level as that obtained by muscle- or liver-mediated gene transfer (6 × 1010 vg/mouse), indicating low transduction efficiency of AAV1 to adipose tissue even with the addition of Pluronic F88.59,60 Later, another nonionic surfactant, celastrol, was shown to enhance transgene expression in adipose tissue by inhibiting proteasome activity of AAV1.61 However, these nonionic surfactants have limited efficacy, and searching for new candidates of natural serotypes or engineered serotypes would help facilitate efficient transduction of adipose tissue. Between 2013 and 2014, four research laboratories reported that AAV8 and Rec2, an engineered serotype, possess a capacity to efficiently transduce adipose tissue.62, 63, 64, 65 Since then, most research was reported by using either AAV8 (Table 1) or Rec2 serotype in targeting adipose tissue (Table 2).
Table 1.
Examples of AAV Serotypes 1, 8, and 9 Used to Transduce Adipose Tissue in Functional Studies
| References | Serotype | Animal Model (Model, Sex, Age) | Transgene Cassette | Administration Route | Dose (vg) | Duration of Experiment | Adipose Transduction |
|---|---|---|---|---|---|---|---|
| Jimenez et al.62 | AAV1 | C57BL/6/J, male, 9–13 weeks | CMV-GFP or RFP | intra-BAT | 1 × 1010 | 2 weeks | ineffective |
| C57BL/6/J, male, 9–13 weeks | CMV-GFP or RFP | intra-eWAT | 2 × 1011 | 2 weeks | ineffective | ||
| Zhang et al.61 | AAV1 | Kunming | LacZ | intra-iWAT | 1 × 1010 | 4 weeks | effective |
| Mizukami et al.59 | AAV1 | C57BL/6/J, 10–12 weeks | CMV-Epo or LacZ | direct subcutaneous fat | 6 × 1010 | 2 weeks | effective |
| db/db, 10–12 weeks | CMV-Epo | direct subcutaneous fat | 6 × 1010 | 2 weeks | effective | ||
| db/db, 10–12 weeks | CMV-Epo | direct subcutaneous fat | 2 × 1011 | 4 weeks | effective | ||
| Liu et al.65 | AAV1 | C57BL/6, 6 weeks | CBA-GFP-WPRE | intra-iWAT | 1 × 1010 | 4 weeks | ineffective |
| C57BL/6, 6 weeks | CBA-GFP-WPRE | intra-BAT | 1 × 109 per pad | 4 weeks | ineffective | ||
| Uhrig-Schmidt et al.64 | AAV1 | C57BL/6 | CMV-EGFP | intra-eWAT | 4.2 × 1010 | 4 weeks | ineffective |
| Jimenez et al.62 | AAV8 | C57BL/6/J, male, 9–13 weeks | AAV8-mini/UCP1-HK2 | intra-BAT | 7 × 1010 | 2 weeks | effective |
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-GFP | intra-BAT | 2 × 109 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-mini/UCP1-GFP | intra-BAT | 2 × 1011 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8-mini/UCP1-HK2 | intra-BAT | 7 × 1010 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks; ICR, male, 8–12 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 5 × 1012 | 2 weeks | effective | ||
| ob/ob and db/db, male, 8 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 3 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 3 × 1012 | 1 month | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-null | intravenous | 2 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | CAG or CMV-GFP | intra-iWAT, intra-eWAT | 2 × 1011 | 2 weeks | effective | ||
| O’Neill et al.63 | AAV2/8 | C57BL/6/J, 8–12 weeks | CMV-EGFP and luciferase | intravenous | 1 × 1012 | 2 weeks | effective |
| C57BL/6/J, 8–12 weeks | AAV2/AAV8-Adipo-EGFP | intravenous | 1 x 1012 | 2 weeks | effective | ||
| ob/ob, male, 9–10 weeks | AAV2/AAV8-Adipo-EGFP-miR122(8) | intravenous | 1 × 1012 | 2 weeks | effective | ||
| ob/ob, male, 6 weeks | AAV2/AAV8-Adipo-leptin-miR122(8) | intravenous | 1 × 1012 | 8 weeks | effective | ||
| Uhrig-Schmidt et al.65 | AAV8 | C57BL/6, male, 8 weeks | CMV-EGFP | intra-eWAT | 4.2 × 1010 per pad | 4 weeks | effective |
| C57BL/6, female, 8 weeks | CMV-EGFP | intravenous | 1 × 1012 | 3 weeks | effective | ||
| C57BL/6, female, 8 weeks | mAP2.2-EGFP | intravenous | 1 × 1012 | 3 weeks | effective | ||
| C57BL/6, female, 8 weeks | mAP2.2-PlinA | intravenous | 1 × 1012 | 3 weeks | effective | ||
| Jimenez et al.66 | AAV8 | ob/ob, male, 11 weeks | CAG-FGF21-miRT122a(4) and miRT1(4) | intra-eWAT | 1 × 1010 | 14 weeks | effective |
| ob/ob, male, 11 weeks | CAG-FGF21-miRT122a(4) and miRT1(4) | intra-eWAT | 5 × 1010 | 14 weeks | effective | ||
| ob/ob, male, 11 weeks | CAG-FGF21-miRT122a(4) and miRT1(4) | intra-eWAT | 2 × 1011 | 14 weeks | effective | ||
| ob/ob, male, 11 weeks | CAG-FGF21-miRT122a(4) and miRT1(4) | intra-eWAT | 1 × 1012 | 14 weeks | effective | ||
| Lagarrigue et al.67 | AAV8 | Cdk4flox/flox, 20 weeks | mini/aP2-Cre | intravenous | 4 × 1012 | 3 weeks | effective |
| Albert et al.68 | AAV8 | AdRiKO, male, 10–14 weeks | CAG-RFP, CAG-human Akt | intra-BAT | 2 × 1011 | 2 weeks | effective |
| AAV9 | AdRiKO, male, 10–14 weeks | CMV-HK2 | intra-BAT | 2 × 1011 | 2 weeks | effective | |
| Jimenez et al.62 | AAV9 | C57BL/6/J, male, 9–13 weeks | AAV9-mini/aP2-null or HK2 | intra-eWAT | 1.4 × 1012 | 2 weeks | effective |
| C57BL/6/J, male, 9–13 weeks | AAV9-mini/aP2-SeAP | intra-eWAT | 2 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-GFP | intra-BAT | 2 × 109 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV9-mini/UCP1-VEGF164 | intra-BAT | 2 × 1011 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks; ICR, male, 8–12 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 5 × 1012 | 2 weeks | effective | ||
| ob/ob and db/db, male, 8 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 3 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-GFP | intravenous | 3 × 1012 | 1 month | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV8- or AAV9-CAG-null | intravenous | 2 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV9-mini/aP2 or mini/UCP1-GFP | intravenous | 2 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV9-mini/UCP1-VEGF164 | intravenous | 8 × 1012 | 1 month | effective | ||
| C57BL/6/J, male, 9–13 weeks | AAV9-CAG-GFP-miR122a or miRT1 or double miRT1 | intravenous | 1 × 1012 | 2 weeks | effective | ||
| C57BL/6/J, male, 9–13 weeks | CAG or CMV-GFP | intra-iWAT, intra-eWAT | 2 × 1011 | 2 weeks | effective |
AAV, adeno-associated virus; CMV, cytomegalovirus; GFP, green fluorescent protein; RFP, red fluorescent protein; BAT, brown adipose tissue; eWAT, epididymal white adipose tissue; LacZ, gene that encodes for β-galactosidase; iWAT, inguinal white adipose tissue; Epo, erythropoietin; CBA, chicken β-actin; WRPE, woodchuck hepatitis virus post-transcriptional element; EGFP, enhanced green fluorescent protein; UCP1, uncoupling protein 1; HK2, hexokinase II; CAG, promoter with cytomegalovirus/chicken β-actin elements; Adipo, adiponectin promoter; miR, microRNA; mAP2.2, murine adiponectin promoter; PlinA, perilipin A; FGF21, fibroblast growth factor 21; aP2, adipocyte protein 2; SeAP, placental-derived secreted alkaline phosphatase; VEGF, vascular endothelial growth factor.
Table 2.
Examples of AAV Rec2 Serotype Used to Transduce Adipose Tissue in Functional Studies
| References | Serotype | Animal Model (Model, Sex, Age) | Transgene Cassette | Administration Route | Dose (vg) | Duration of Experiment | Adipose Transduction |
|---|---|---|---|---|---|---|---|
| Liu et al.65 | Rec2 | C57BL/6, 6 weeks | CBA-GFP | intra-iWAT | 1 × 1010 | 4 weeks | effective |
| C57BL/6, 6 weeks | CBA-GFP | direct intraperitoneal WAT injection | 1 × 1010 | 10 days | effective | ||
| IRflox, male, 6 weeks | CBA-Cre | intra-BAT | 1 × 109 | 8 weeks | effective | ||
| IRflox, female, 6 weeks | CBA-Cre | intra-iWAT | 1 × 1010 | 8 weeks | effective | ||
| Huang et al.69 | Rec2 | C57BL/6, 6 weeks | CBA-GFP | oral | 5 × 109 | 2 weeks | effective (BAT) |
| C57BL/6, 6 weeks | CBA-GFP | intra-BAT | 1 × 109 | 2 weeks | effective | ||
| C57BL/6, 6 weeks | CBA-GFP | intravenous | 2 × 1010 | 2 weeks | ineffective | ||
| C57BL/6, 6 weeks | CBA-GFP | oral | 2 × 1010 | 2 weeks | effective (BAT) | ||
| C57BL/6, 6 weeks | CBA-GFP-miR122(4)-WPRE | oral | 2 × 1010 | 2 weeks | effective (BAT) | ||
| VEGF (loxP) | CBA-Cre | oral | 2 × 1010 | 6 weeks | effective (BAT) | ||
| Zhu et al.70 | Rec2 | TRE-Cx43 | CBA-Cx43 | intra-iWAT | 1 × 109 | 4 weeks | effective |
| Xie et al.71 | Rec2 | C57BL/6 DIO | U6-shSWELL1-CMV-mCherry, male, 8 weeks | retro-orbital | 1 × 1011 | 17 weeks | effective |
| Ng et al.72 | Rec2 | C57BL/6, male, 8–10 weeks | CBA-miR32-WPRE | intra-BAT | 1 × 108 | not available | effective |
| C57BL/6, male, 8–10 weeks | CBA-miR32-WPRE | intra-iWAT | 1 × 109 | not available | effective | ||
| Zhang et al.73 | Rec2 | C57BL/6, male, 6 weeks | U6-shSWELL1-CMV-mCherry | intra-iWAT | 1 × 1010 | 16 weeks | effective |
| C57BL/6, male, 6 weeks | U6-shSWELL1-CMV-mCherry | intra-iWAT | 1 × 109 | 16 weeks | effective | ||
| C57BL/6, male, 6 weeks (HFD) | U6-shSWELL1-CMV-mCherry | intra-iWAT | 2 × 109 | 16 weeks | effective | ||
| Huang et al.74 | Rec2 | BALB/c, male | CBA-GFP | intraperitoneal | 2 × 1010 (per mouse) | 2 weeks | effective |
| BALB/c, male | CBA-GFP | intraperitoneal | 5 × 108 (per mouse) | 2 weeks | ineffective | ||
| BALB/c, male | CBA-GFP | intraperitoneal | 2 × 109 (per mouse) | 2 weeks | ineffective | ||
| BALB/c, male | CBA-GFP | intraperitoneal | 1 × 1010 | 2 weeks | effective | ||
| C57BL/6, 8–12 weeks | CBA-dual cassette-albumin-miR-WPRE | intraperitoneal | 1–4 × 1010 | 2 weeks | effective | ||
| ob/ob, male, 6 weeks | CBA-dual cassette-leptin-albumin-miR-WPRE | intraperitoneal | 4 × 1010 (per mouse) | 9 weeks | effective | ||
| Xiao et al.75 | Rec2 | C57BL/6, male, 6 weeks | CBA-dual cassette-IL-15/IL-15a-albumin-miR-WPRE | intraperitoneal | 2 × 1010 | 5 weeks | effective |
| Huang et al.76 | Rec2 | C57BL/6 PTENflox, male, 9 weeks | CBA-Cre-albumin-miR-WPRE | intra-iWAT | 2 × 1010 | 7 weeks | effective |
| C57BL/6 PTENflox, female, 9 weeks | CBA-Cre-albumin-miR-WPRE | intraperitoneal | 2 × 1010 | 7 weeks | effective |
CBA, chicken β-actin; GFP, green fluorescent protein; iWAT, inguinal white adipose tissue; WAT, white adipose tissue; BAT, brown adipose tissue; miR, microRNA; WRPE, woodchuck hepatitis virus post-transcriptional element; VEGF, vascular endothelial growth factor; Cx43, connexin 43; U6, type III RNA polymerase III promoter; SWELL, protein product of the gene LRRC8a (leucine-rich repeat containing proteins); CMV, cytomegalovirus; PTEN, phosphatase and tensin homolog.
AAV8 and AAV9
In the search for AAV serotypes with higher tropism to adipose tissue, Bosch and colleagues62 made another screening attempt to include the then newly reported serotypes AAV7, AAV8, and AAV9. Promoter selection and incorporation of tissue-specific miRNA target sites in the 3′ UTR of transgene were deployed for transgene expression in WAT and BAT based on the route of delivery.
Direct Adipose Injection
The ubiquitous promoters such as CMV early enhancer/CBA (CAG) and CMV promoters are used with reporter genes such as enhanced green fluorescent protein (EGFP) and red fluorescent protein (RFP). The dosage for epididymal WAT (eWAT) and iWAT of adult mice is at 2.0 × 1011 vg (EGFP) or for BAT at 2 × 109 vg (EGFP) or 1 × 1010 vg (RFP). The group observed that AAV8 and AAV9 outperformed other serotypes (AAV1, AAV2, AAV3, AAV5, AAV6, and AAV7) tested in eWAT and iWAT, while AAV7, AAV8, and AAV9 showed superior transduction in BAT over other serotypes (AAV1, AAV2, and AAV5) tested.62
Intravenous (i.v.) Delivery
With tail vain injection of AAV8-CAG-GFP or AAV9-CAG-GFP at an extremely high dose of 5 × 1012 vg in lean mice, GFP expression was mainly distributed in eWAT and BAT for male ICR (albino) adult mice, while no difference was observed between AAV8 and AAV9 in terms of transduction. However, for male adult C57BL/6 mice with a tail vein injection of AAV9-CAG-GFP, GFP expression was distributed throughout the five fat pad depots tested.62 The highest GFP expression was observed in mesenteric WAT (mWAT) and BAT, followed by iWAT and retroperitoneal WAT (rWAT). eWAT had the least GFP expression. Lower doses (5 × 1010 vg and 5 × 1011 vg) also yielded GFP expression, but at greatly reduced efficiency. No sex difference for C57BL/6 mice was observed in AAV9-mediated transduction of various adipose depots except for eWAT. With i.v. delivery of AAV8-CAG-GFP or AAV9-CAG-GFP at a high dose of 3 × 1012 vg in obese mice, GFP expression was distributed throughout five fat pad depots in male adult ob/ob mice. iWAT had the most GFP from AAV8, while iWAT and eWAT had the most GFP from AAV9. Other fat pad depots had substantial GFP expression from both AAV8 and AAV9. However, male adult db/db mice showed a different landscape. GFP was mostly expressed in iWAT from both AAV8 and AAV9. Thus, systemic administration of AAV8 and AAV9 led to uneven transgene expression at different fat pad depots among C57BL/6, male ICR, ob/ob, and db/db mice. This interesting observation suggests that the extent of adiposity associated with insulin resistance and mouse strain background may influence AAV vector-mediated gene transfer in specific fat pad depots. The previous report showed that the mouse genetic background affects the ability of AAV vectors to transduce the brain.77 Thus, mouse strain and metabolic status as well as administration routes are factors to be considered when choosing AAV vectors for gene delivery to desired adipose depots. Of note, systemic administration of AAV8 or AAV9 did not provoke immune cell infiltration in WAT, BAT, or liver while widespread transduction in these tissues occurred.62
Mitigating Off-Target Effects
As AAV8 and AVV9 were shown to transduce liver and heart by local delivery in eWAT and BAT in this study,62 other studies also previously indicated that AAV8 and AAV9 are capable of transducing the liver and heart.78,79 Therefore, Bosch and colleagues62 used two approaches to mitigate off-target effects. First, they added the target sequence of miRNA (miR)122a and miRT1 to the 3′ UTR of the AAV vector, so that abundant miR122 and miRT1 in the liver and heart, respectively, can act on their target sequences and thereby suppress transgene expression.80 Second, they used an adipose tissue-specific promoter to avoid off-target transgene expression. Due to the size limitation of AAV vectors, the short version (so-called mini-promoters) were used, for example, adipocyte protein 2 (mini/aP2) for WAT, and uncoupling protein 1 (mini/UCP1) for BAT. The second approach required a higher dose (5- to 20-fold) because adipose-specific mini-promoters are significantly less potent than ubiquitous promoters. The local delivery of AAV8 or AAV9-mini/aP2-GFP at a high dose of 1 x 1012 vg showed GFP expression in WAT without detectable GFP in liver, heart, and BAT. Meanwhile, local delivery of AAV8 or AAV9-mini/UCP1-GFP at a dose of 2 × 1011 vg achieved efficient transduction of brown adipocytes with a marginal expression of GFP in liver and no detectable GFP in the heart. The i.v. delivery of AAV8- and AAV9-mini/aP2 or -mini/UCP1 at a dose of 2 × 1012 vg also achieved highly adipose-specific GFP expression in WAT and BAT with insignificant or no detectable GFP in liver and heart, respectively.
Transgene Functional Readout
Hexokinase 2 (HK2) is key enzyme in glucose metabolism. Bosch and colleagues62 were able to show that overexpression of HK2 in adipocytes isolated from transduced eWAT by AAV9-CMV-HK2 (2 × 1011 vg) enhanced insulin-stimulated glucose uptake. Furthermore, in vivo experiments demonstrated that eWAT treated by local delivery of AAV9-mini/aP2-HK2 at a dose of 1.4 × 1012 vg exhibited about a 3-fold increase in glucose uptake compared with the eWAT from AAV9-mini/aP2 null, while the BAT and heart of both groups showed no difference in glucose update. Local delivery of AAV8-mini/UCP1-HK2 at a dose of 7 × 1010 vg also exhibited increased glucose uptake in BAT, but not the eWAT and heart. Similarly, intra-BAT injection of AAV9-mini/UCP1-vascular endothelial growth factor (VEGF) isoform 164 (VEGF164) at a dose 2 × 1011 cg resulted in murine VEGF164 overexpression in BAT with increased vasculature density.62 In addition, the AAV9-mini/aP2 vector can also transduce eWAT and generate secretory protein and maintain its presence in circulation for at least 140 days, as indicated by placental-derived secreted alkaline phosphatase (SeAP) serum level in male adult mice after 2 × 1012 vg of AAV9-mini/aP2-SeAP was introduced into eWAT by local delivery. As mini/aP2 or mini/UCP1 promoters are much less potent than CAG promoters, it is not surprising that systemic administration of AAV9-min/UCP1-VEGF164 requires a dose as high as 8 × 1012 vg to achieve VEGF164 overexpression with increased vasculature density in BAT.62
In collaboration with the Bosch group, one team showed that systemic delivery of AAV8-mini/aP2-Cre at a dose of 4 × 1012 vg in Cdk4flox/flox adult male mice significantly decreased Cdk4 mRNA in eWAT and subcutaneous adipose tissue, resulting in reduction of fat mass and adipocyte size without affecting lean mass.67 Another team applied AAV9-CMV-HK2 and AAV8-CAG-human AktS473D to adult AdRiKO mice by local delivery to BAT at a dose of 2 × 1011 vg.68 AdRiKO mice are defective in mTORC2 signaling and thereby thermogenesis.68,81 Overexpression of HK2 or constitutively active Akt2 (AktS474D) mitigated the thermogenic defect in AdRiKO mice. The data indicate that mTORC2-Akt signaling regulated BAT glucose uptake and thereby thermogenesis.
Transgene Therapeutic Efficacy
One study in 2012 used AAV9 for correcting very long-chain acyl-CoA (coenzyme A) dehydrogenase (VLCAD) deficiency. As the focus of the study was on the therapeutic effect, the AAV9 vector with the CBA promoter was used to deliver transgene-VLCAD via i.v. administration at a dose of 1 × 1012 vg. As a result, VLCAD overexpression was found mostly in liver and muscle, then followed by BAT, gonadal WAT (gWAT), and other tissues.82 To target adipose tissue for a translational study, Bosch and colleagues62 used the same strategy from their earlier study to introduce FGF21 in eWAT of ob/ob mice with a dosage of 1 × 1010, 5 × 1010, 2 × 1011, and 1 × 1012 vg per mouse by local delivery. miR122a and miRT1 target sequence were added to the 3′ UTR of the FGF21 expression cassette (AAV8-CAG-FGF21-dmiRT). AAV8-CAG-FGF21-dmiRT transduced eWAT with a clear dose-dependent modulation of weight gain, and achieved substantial FGF21 levels in circulation, thereby alleviating the obesity phenotype.66 The therapeutic effect persisted about 14 weeks. Their study demonstrates that AAV8-mediated transgene overexpression in eWAT is sufficient enough to achieve a systemic and therapeutic effect.
In addition to the studies by Bosch and colleagues, Reilly and colleagues63 reported that AAV8 exhibited adipose tissue transduction better than that of AAV2 and AAV7 by examining reporter expression (CMV-luciferase or EGFP) via i.v. delivery at a dose of 1 × 1012 vg. Substantial reporter signals in the liver and heart were observed, consistent with previous reports.62,66,83 A similar strategy to that of Bosch’s group was applied to include the human adiponectin distal enhancer, proximal promoter, and miR122 target sequence of three or eight repeats in the expression cassette to mitigate off-target effects. Indeed, i.v. delivery of AAV2/8-Adipo (adiponectin promoter)-EGFPmiR122(8x) resulted in EGFP expression selectively in adipose tissue of male adult C57BL/6 mice. EGFP expression in different fat depots by AAV8 in this study has a similar distribution pattern as AAV9.62 As proof of efficacy, AAV2/8-Adipo-leptin miR122(8x) was systemically administered into male adult ob/ob mice at a dose of 1 × 1012 vg per mouse. Gene therapy led to circulating leptin levels at approximately 7% of that observed in age-matched wild-type mice throughout duration of the study coupled with a steady loss of food intake and body weight. Therapeutic effect was achieved and maintained for 50 days after AAV injection.
At same time, Kreuz and colleagues64 also found that AAV8-CMV-EGFP transduced adipose tissue more efficiently than did other AAV serotypes (AAV1, AAV2, AAV5, and AAV6) by i.v. delivery at a dose of 1 × 1012 vg. By using a 2.2-kb fragment of murine adiponectin promoter (mAP2.2), AAV8-mAP2.2-EGFP transduced subcutaneous and visceral fat depots while significantly limiting off-target transduction. When AAV8-mAP2.2-mediated perilipin A (a lipid droplet-associated protein) was tested in adult male C57BL/6 mice, the treatment resulted in robust perilipin A expression in subcutaneous and visceral fat depots, and significant changes in metabolic parameters (serum-free fatty acids, blood glucose, and the respiratory exchange ratio).
Taken together, AAV8 and AAV9 outperform other naturally occurring serotypes with regard to transducing adipose tissues. However, a relatively high dose on the order of 1011 or 1012 vg is required for systemic administration or even local delivery to mice to achieve a therapeutic effect. Moreover, AAV8 is a preferable serotype for liver gene delivery,14,84 and AAV9 for heart, muscle, or the central nervous system,14,85, 86, 87 indicating the challenge of avoiding off-target liver, heart, or muscle tissue transduction with AAV8 and AAV9 vectors. One possible solution is to engineer capsids to improve adipose tissue tropism.
Capsid Engineering
AAV capsid engineering can be divided into two categories: rational design and directed evolution.14 The structure of AAV2 has been determined to 3-Å resolution by X-ray crystallography.88 The most prominent features of the structure are groups of 3-fold-related peaks, each an intimate association of loops from two neighboring subunits. Mutations affecting cell entry and receptor binding are clustered near the positively charged side of each peak, implicating the region in attachment to the cellular receptor. Thus, capsid engineering by rational design is based on guidance of the structure information of parent serotype, including genetic mutation of the AAV capsid that optimizes intracellular processing and insertion of high-affinity peptides into the common VP3 region while disrupting native cellular binding motifs.89 Most recently, a new method is being developed to reveal the capsid whole landscape picture by generating all single-codon mutants of the AAV2 Cap gene.90 It will further guide rational design for capsid tailoring cellular targeting. Alternatively, directed evolution strategies utilize natural evolution of AAV serotype diversity for tissue tropism and rely on random processes, including capsid gene fragments shuffling among characterized serotypes, random peptide insertion into a known site of the AAV capsid (AAV peptide display), and error-prone (EP) PCR (or PCR-based mutagenesis) to produce the large number of randomly generated capsid mutants of a library, followed by screening in targeting cell lines.91 To simplify the screening process, a new method, i.e., barcoded rational AAV evolution, was recently generated by the combination of rational design and directed evolution. This approach enables efficient selection of engineered capsid structures on a large scale using a single screening round in vivo.92 However, few efforts have been reported with regard of capsid engineering targeting adipose tissues.
Rec2
Six engineered serotypes (Rec1–Rec6) were generated through capsid domain exchange or shuffling among AAV8, cy5 (cynomolgus macaque-variant 5), rh20 (rhesus macaque-variant 20), and rh39 (rhesus macaque-variant 39) in an attempt to create a novel hybrid to target the retina more efficiently by the During and colleagues.93 Mouse and primate studies indicated that the novel serotypes transduced retina tissue no better than AAV2 or AAV5.93 The studies described below show that out of the engineered serotypes, Rec2 was found to effectively transduce adipose tissue and has since been used in several gene therapy studies in adipose tissue. However, the mechanism of Rec2 tropism to adipose tissue is still unknown. Although Rec2 capsid is currently not widely distributed, its full amino acid sequence is published.93
Direct Adipose Injection
In searching for a tool to genetically manipulate adult adipose tissue, we assessed several hybrid capsids among this family (Rec1, Rec2, Rec3, and Rec4) by direct injection to WAT and BAT, and compared them with AAV1, AAV8, and AAV9. The cis plasmid consists of a CBA (hybrid CMV-CBA) promoter, transgene, WPRE, and bovine growth hormone polyadenylation signal flanked by an AAV2 ITR. The Rec2 serotype exhibited widespread transduction in both WAT and BAT, with the highest efficiency among the seven serotypes tested.65 After a single dose of 1 × 1010 vg directly to one iWAT depot in adult C57BL/6 mice, the Rec2 vector resulted in GFP expression more than 2-fold higher than that of AAV8,65 whereas it resulted in much lower GFP expression in the liver compared to mice injected with AAV1, AAV8, and AAV9. Similarly, Rec2 transduced BAT more efficiently than did AAV1, AAV8, and AAV9 at a dose of 1 × 109 vg bilaterally. Our data suggest that the Rec2 serotype, based on domain shuffling between AAV8 and rh20, is an improvement of AAV8 with respect to adipose transduction.
To assess the functional efficacy of AAV-mediated adipose gene transfer, Rec2 vector harboring Cre recombinase under the control of the CBA promoter was injected to the adipose tissue of insulin receptor floxed (IRlox) mice. Injection of Rec2-Cre at a dose of 1 × 1010 vg per fat pad to iWAT of adult female IRlox mice and BAT of adult male IRlox mice at a dose of 2 × 109 vg led to a 50% decrease of insulin receptor protein level and the ensuing loss of fat mass, representing molecular and morphological changes consistent with impaired adipose function.65 In addition, direct adipose injection of Rec2-Cre at these low doses showed minimal effect on insulin receptor in liver and muscle. In collaboration with our group, other laboratories have applied Rec2 vectors to genetically manipulate adipose tissues.70, 71, 72, 73
i.v. Injection
Rec2 displays interesting tissue tropism dependent on administration route. i.v. injection of Rec2 vector (2 × 1010 vg per mouse) to the tail vein led to primarily liver transduction in adult female C57BL6 mice, adult male diet-induced obesity (DIO) mice, and adult female db/db mice in a C57BL/6 background.94
Oral Administration
A surprising finding is that oral administration of Rec2 results in preferential transduction of BAT with absence of transduction in the gastrointestinal track. Among the six natural and engineered serotypes being compared (Rec1–Rec4, AAV1, AAV8) with a dose of 2 × 1010 vg per mouse via oral gavage, Rec2 achieved the highest GFP reporter level in BAT of adult C57BL/6 mice.69 Decreasing the dose highly favored transduction of BAT than liver. Oral administration of Rec2-GFP at the dose of 5 × 109 vg per mouse resulted in GFP content in BAT at least 10-fold higher than that in the liver. To further restrict transgene expression in the liver, we generated Rec2 vector with four repeats of the miR-122 target sequence. When orally administered at a high dose (2 × 1010 vg per mouse), the transgene mRNA level in the BAT was 100- to 500-fold higher than that in the liver of the same mouse. Given the exceptionally high efficiency of the Rec2 vector to transduce BAT via direct injection, we compared the gene delivery efficiency of the Rec2 vector between oral administration and direct fat injection with Rec2-luciferase. Interestingly, oral administration of the Rec2 vector at the dose of 5 × 109 vg per mouse led to a 3-fold higher transduction than did direct BAT injection at the dose of 5 × 108 vg per mouse. To assess whether oral administration of the Rec2 vector could genetically manipulate BAT functions, we targeted VEGF in BAT. A loss-of-function study via Cre-LoxP knockdown and a gain-of-function study via overexpressing VEGF at the dose of 2 × 1010 vg per mouse showed robust transgene expression in BAT, and significantly affected BAT mass and functions. This study was the first to show that an engineered AAV serotype is highly efficient to transduce distant tissues from gastrointestinal track via oral administration. Moreover, the dose of oral administration to achieve the high level expression of transgene in mice appears comparable to direct injection to the BAT in the range of 109 vg per mouse, which is approximately 2–3 orders lower than most reported doses of i.v. injection.62,63 Another interesting finding is that oral gavage and tail vein injection showed a distinctive profile of kinetic tissue distribution of viral vector DNA after Rec2 vector administration. Oral administration of Rec2 led to a very limited vector presence in the blood. In contrast, the viral vector was detected in thymus earlier than in the blood. Furthermore, as early as 10 min after oral gavage, high levels of viral vectors were found in the mesenteric fat that is rich in lymph nodes, but the levels declined over time. Thus, we hypothesize that oral administration of Rec2 vector leads to BAT transduction through the lymphatic system. The Rec2 vector could drain into the lymphatic system through transcytosis from the small intestine to lymph nodes in mesenteric fat, which requires future investigations. It is also important to investigate whether sex influences the efficacy of oral gene therapy.
Intraperitoneal (i.p.) Injection
Visceral adipose tissues are of particular interest to studies on obesity and related metabolic disorders, but they are less accessible to direct injection of viral vectors. Thus, we attempted i.p. injection and found that the Rec2 vector at the dose ranging from 1 × 1010 to 2 × 1010 vg per mouse resulted in high-level transgene expression (luciferase and GFP as reporters) in multiple visceral fat depots, including gonadal, retroperitoneal, and mesenteric fat depots,74 whereas no transgene expression were detected in small intestine, large intestine, spleen, kidney, and testis. However, heart and additional tissues have not been examined. Of note, mesenteric adipose tissue is difficult to surgically access. Thus, i.p. injection of the Rec2 vector could serve as a particularly useful tool to genetically manipulate this fat depot. In addition to visceral fats, liver was also transduced considerably. To mitigate liver transduction, we developed a novel AAV expression plasmid harboring two expression cassettes, one using the non-selective CBA promoter to drive transgene expression, and the other using the liver-specific proximal albumin promoter to drive a miRNA-targeting WPRE sequence that only exists in this AAV vector.74 This dual-cassette vector achieved highly selective transduction of visceral fats while severely restricting off-target transduction of liver at a dose of 2 × 1010 vg/mouse via i.p. injection.74
In a proof-of-concept study using a mouse model of a human genetic disease, i.e., congenital leptin deficiencies, i.p. administration of the dual-cassette Rec2 vector delivering murine leptin gene to visceral fat of ob/ob mice corrected leptin deficiency and related obesity and the metabolic syndrome.74 Although caution should be taken when comparing results from different laboratories, the new system showed remarkable therapeutic efficacy compared to a previous report using the same animal model of ob/ob mice (i.v. injection of an AAV8 vector expressing leptin controlled an by adipose-specific promoter and the miR122 target sequence:64 the dose of i.p. Rec2 vector was at least 25-fold lower [4 × 1010 versus 1 × 1012 vg per mouse]); our system normalized a circulating leptin level close to the wild-type level versus ∼7% of the wild-type level achieved by reported i.v.. injection; our AAV treatment completely reversed ob/ob abnormalities versus partial alleviation by reported i.v. treatment.74
More recently, the dual-cassette Rec2 vector enabled us to reveal an “adipose phosphatase and tensin homolog (PTEN)-leptin-sympathetic nervous system” feedback loop that regulates adipose distribution, which could not be discovered by currently available transgenic mice approaches.76 In the study, we applied the dual-cassette Rec2 vector to deliver transgene-Cre at a dose of 2 × 1010 vg to adult male and female Ptenlox/lox mice (C57BL/6 background) through i.p. administration.
To explore the additional therapeutic potential, we used the dual-cassette Rec2 vector to deliver an interleukin (IL)-15/IL-15Rα complex to the visceral fat of adult male C57BL/6 mice by i.p. injection. A single dose of the Rec2-IL-15/IL-15Rα complex (2 × 1010 vg per mouse) led to the expansion of NK cells in the adipose tissue and spleen in normal mice without a notable side effect. This gene therapy significantly suppressed the growth of subcutaneously implanted Lewis lung carcinoma by 50% and extended the median survival by approximately 40% in a B16-F10 melanoma metastasis model. The potent antitumor effect and lack of demonstrable toxicity in mouse models of cancer validate the concept of targeting adipocytes for cancer gene therapy.75
In summary, this novel rAAV (dual-cassette vector) system combines several elements, including the adipocyte tropism of an engineered serotype Rec2, the easy access of i.p. administration, and a new build-in mechanism preventing off-target transgene expression in liver, to achieve highly selective gene delivery to multiple visceral fat depots whose dysfunction is strongly associated with adverse metabolic outcomes. This new rAAV vector system can provide a powerful tool to genetically manipulate visceral fat for both basic research and potential therapeutic applications possibly beyond adipose-related disorders. However, limitations remain and additional properties of the Rec2 serotype require further characterization.
Future Directions
To date, Rec2 vectors are tested only in mouse models, and their transduction efficiency in other species are unknown. Moreover, immune profiles of Rec2-based AAV vectors have not been investigated, which is necessary to evaluate the advantage or disadvantage of adipose tissue in comparison to liver and muscle as targeting tissue for gene therapy. Data on the duration of transgene expression in adipose tissues are limited. We and collaborators observed sustained transgene expression with the Rec2 vector at least up to 5 months in mice.73 To our knowledge, the long-term (at least 1 year) efficacy of AAV vectors for adipose tissue has not been documented, though AAV8 or AAV9 vectors have been evaluated for long-term studies in liver or muscle tissues.1,14,95 As adipose tissue is a dynamic organ and subjected to size and mass dramatic changes in response to nutritional demands and environmental cues in addition to the episomal cellular location of AAV, AAV vectors may be lost during the adipose tissue renewal process. A previous study indicated that up to 80% of epididymal adipocytes and 3% of inguinal adipocytes died after a few months of high-fat feeding in rodents, indicating that renewal of adipocytes is fat depot-specific.37 Thus, depending on which fat depot is to be targeted by the AAV vector, long-term evaluation for AAV vector efficacy in adipose tissue should be addressed for translational purposes in the future.
Another limitation is selective transduction of adipose tissue with Rec2 and AAV8 or AAV9 vectors because these serotypes transduce the liver efficiently. The dual-cassette design described earlier is unable to suppress transgene expression in the liver when miRNA and short hairpin RNA (shRNA) are delivered. An alternative strategy using a Tet-On/Tet-Off system is discussed later. The size limitation of the dual-cassette vector excludes its use for CRISPR-Cas9 genome editing. Thus, more efforts are needed to generate novel adipo-tropic serotypes. Of note, more than 100 naturally occurring capsid variants have been identified from human and nonhuman primates.11 Thus, we need to step up screening and testing of those capsid variants for adipose transduction or attempt to isolate natural AAV variants from fat. Alternatively, recent studies on liver, brain, and muscle have shown the successful re-design of capsids based on a well-adopted “rational design and directed evolution” approach. New information obtained from a recent comprehensive AAV capsid fitness landscape study may provide guidance for capsid modification.90,91 AAV8 and Rec2 may serve as the parent templates to generate capsids possessing higher affinity for adipose tissue than liver.
One approach to targeting tissues more efficiently is to make particular point mutations on the viral capsid to avoid proteasome-mediated destruction through ubiquitination.96 Mutating tyrosine to phenylalanine on the AAV2 capsid has been shown to increase transduction in hepatocytes and other tissues.97 Given that each serotype possesses a unique tropism spectrum, it is unclear whether this approach works for AAV8 or Rec2. One study found that site-directed mutagenesis of capsid surface-exposed lysine residues improved transduction of AAV2, but not AAV8, in the liver.97 Further research into capsid structure and transduction efficiencies will help explain this finding.
In addition to capsid engineering, modifications in the AAV expression cassette could also improve tissue transduction efficiency and specificity. Recent advances in AAV vector improvement have been extensively reviewed in several publications.1,84,98 We now discuss options for possible application of modifications in the AAV vector for adipose tissue transduction.
Because of the limited genetic size capacity for AAV vectors, basic or core elements of adipose tissue-specific promoters, and the short form of enhancers such as mini/aP2, mini/UCP1, and mAP2.2 have been deployed in place of ubiquitous ones to achieve transcriptional specificity.62,64 However, those promoters are less potent, and a higher dosage is required with such mini-promoters. Thus, synthetic short enhancer or cis-regulatory modules (CRMs) need to be searched and exploited. Identifying adipose tissue-specific CRMs can potentially enhance adipose tissue-specific transduction. For example, a muscle-specific promoter containing clusters of transcription factor binding sites resulted in a 400-fold increase in muscle-specific gene transcription, better than the ubiquitous promoter CMV.99 Future studies can test the strategy of incorporating adipose-specific CRMs100 to enhance transgene expression in adipose tissue.
One of the issues related to the efficacy of AAV vectors for adipose tissue is that the vectors rely on host cells, such as adipocytes, for the synthesis of the second DNA strand. By using self-complementary AAV (scAAV) vectors, the single-stranded AAV (ssAAV) genome can rapidly self-anneal in the transduced cells to generate a double-stranded functional DNA. Thus, scAAV vectors tend to express the transgene faster and more potently than do the conventional ssAAV counterparts.98 scAAV9 vectors have been applied in gene therapy and achieved effective transduction in neuronal and non-neuronal tissues.101, 102, 103 Therefore, it is worth trying scAAV8, scAAV9, or scRec2 vectors in adipose transduction. Of note, the packaging capacity of scAAV vectors is about 2.5 kb, which clearly limits the choice for transgene.
Codon optimization and inclusion of a Kozak (or modified) sequence upstream of the initiation codon could be adopted for transgene expression enhancement (augment mRNA production and translation) in adipose tissue, as they have been successfully tested in rAAV gene therapy to increase gene expression levels in liver and brain.104, 105, 106, 107, 108
RNA interference (RNAi) has become an essential research tool to study gene function and is being exploited for therapeutic purposes. In addition to the earlier described strategy of de-targeting AAV vector-mediated transgene expression with miRNA target sites, AAV-mediated gene delivery of small RNA molecules, such as shRNAs and artificial miRNAs for gene knockdown, has also been applied in translational studies.109 AAV8 is one of the serotypes used in those studies. Rec2 was used in basic research studies.73 Commonly adopted RNA polymerase III (RNA Pol III) promoters such as human or murine U6 and human H1 are often used in the applications.109 For adipose tissue, shRNAs and artificial miRNAs can be deployed to modulate adipocyte gene expression of any age of animal for functional studies. However, in some cases, AAV-mediated RNAi therapeutics may cause toxicity in target organs because potent shRNAs could oversaturate the endogenous miRNA biogenesis pathway.14,109, 110, 111 Because of safety concerns over off-target silencing, local and systemic immune or inflammatory responses should be evaluated when AAV-mediated RNAi is applied to adipose tissue.
To overcome the limited capacity of the AAV vector transgene size, some teams created a two-vector delivery system, where the large transgene is split into two parts, each packed into separate AAV particles, which are then delivered to the target tissue. Two parts of the transgene product fuse together in transduced cells.112,113 The use of inducible synthetic promoters is another way to modulate AAV-mediated transgene expression. The details on inducible synthetic promoters have been specifically and thoroughly reviewed in the past, including in a recent publication.98 The tetracycline (Tet)-dependent system is most often applied in vivo to study neuronal tissue by viral vectors, including AAV vectors.114, 115, 116, 117 In addition to using the Rec2 dual cassette to deliver a transgene while limiting liver expression, another strategy to limit off-target transgene expression is to use a Tet repressor (TetR) mutant model. A U6/TO, H1/TO, or CMV/TO promoter can be used to drive miRNA or shRNA expression combined with the Alb promoter to drive TetR expression. In this way, liver-specific expression of albumin will suppress transgene expression. The switch-on and switch-off regulation of genes of interest could help define the role of genes in adipose tissue remodeling in the course of growth and aging of model animals.
In addition to facilitating basic research on adipose biology, AAV vectors targeting adipose tissue may provide an avenue to develop gene therapies for genetic disorders or disease conditions involving adipose tissue. The pathology of adipose tissue is most often associated with obesity or diabetes in humans. Underlying mechanisms of obesity and diabetes involve multiple pathways, gene networks, and other organs or tissues such as the hypothalamus, pancreas, and liver. Six forms of human monogenic obesity are primarily due to inactivating mutations in leptin-melanocortin signaling. Congenital leptin deficiency is a monogenic defect found in adipose tissue.118 This rare case in humans is equivalent to the ob/ob model in rodents. Leptin protein is an approved drug for this genetic disease but requires frequent injection for life. The previously mentioned two preclinical studies demonstrate sustained efficacy of AAV-mediated leptin gene delivery to adipose tissue in ob/ob mice,63,74 albeit much remains to be learned to assess the translational potential. Moreover, adipokines that exert metabolic benefits are therapeutic genes for adipose tissue gene therapy, such as FGF21.66 Adiponectin and adipsin, two major adipokines whose levels are decreased in obesity, could be targets for AAV-mediated gene transfer in obese or diabetic models, as they improve glycemic control and enhance insulin sensitivity or insulin release.29,119
In contrast to overexpansion of adipose tissue in obesity, lipodystrophy syndromes are the pathological state of adipose tissue deficiency associated with either genetic defects or various acquired conditions that may prevent pre-adipocyte differentiation or promote immune cell-associated cytotoxicity for adipocytes.120 Lipodystrophies include many different conditions characterized by a lack of and/or dysfunctional WAT121. While lipodystrophic patients may be lean, lipodystrophies often have the same symptoms of metabolic syndrome in obesity, including ectopically deposition of lipids in liver and other organs, deficiency of adipokines (leptin and adiponectin), insulin resistance, hyperglycemia, and dyslipidemia.121 Most cases of inherited lipodystrophy in humans are caused by mutations in genes such as encoding 1-acylglycerol-3-phosphate O-acyltransferase2 (AGPAT2), seipin (BSCL), caveolin, lamins, PPARγ, and AKT2 while the acquired lipodystrophy is not well defined in the underlying mechanism, and it includes HIV patients who received antiviral therapy.120,122,123 Current treatments for lipodystrophies are still very limited and do not always address the underlying cause. Adipose-oriented gene therapies are worthy of investigation for hereditary lipodystrophies. Other examples of adipose disorders with known hereditary components in the pathogenesis include disorders of distribution of fat, lipoedema and fibroadipose hyperplasia, tumors, and inflammation.124 Whether gene therapy might be applicable remains to be seen.
Adipose tissue as a target tissue for gene therapy is much less studied compared to muscle or liver. Until now, data on long-term transgene expression, immune response to the AAV vector and transgene, genome insertion risk, and other side effects are not available with adipose-oriented gene transfer to assess the advantages or disadvantages in comparison with muscle or liver gene therapy. The development of AAV vectors with improved efficacy and tropism is expected to stimulate investigations on adipose tissue, one of the largest organs, as a potential target for gene therapy.
In summary, the past decade has seen the development of AAV vectors that transduce the adipose tissue in basic research and of preclinical models of various diseases. AAV8 and an engineered serotype, Rec2, are the most efficient serotypes and are commonly used AAV vectors to target adipose tissue with the aid of strategies to enhance adipose gene expression while limiting off-target transgene expression in the liver. The Rec2 serotype displays high efficacy of adipose gene transfer via local and systemic administration superior to naturally occurring serotypes tested. More efforts are needed to address the many limitations and challenges in order to develop optimal AAV vectors tailored to adipose tissue.
Author Contributions
R.B., W.H., and L.C. wrote and revised the manuscript.
Conflicts of Interest
L.C. and W.H. are inventors of a provisional patent application related to the liver-restricting AAV vector. R.B. declares no competing interests.
Acknowledgments
This work was supported by NIH grants AG041250, CA166590, and CA163640, as well as internal funding from The Ohio State University Comprehensive Cancer Center.
References
- 1.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonntag F., Köther K., Schmidt K., Weghofer M., Raupp C., Nieto K., Kuck A., Gerlach B., Böttcher B., Müller O.J. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J. Virol. 2011;85:12686–12697. doi: 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earley L.F., Powers J.M., Adachi K., Baumgart J.T., Meyer N.L., Xie Q., Chapman M.S., Nakai H. Adeno-associated virus (AAV) assembly-activating protein is not an essential requirement for capsid assembly of AAV serotypes 4, 5, and 11. J. Virol. 2017;91 doi: 10.1128/JVI.01980-16. e01980-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chejanovsky N., Carter B.J. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989;173:120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 5.King J.A., Dubielzig R., Grimm D., Kleinschmidt J.A. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 2001;20:3282–3291. doi: 10.1093/emboj/20.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surosky R.T., Urabe M., Godwin S.G., McQuiston S.A., Kurtzman G.J., Ozawa K., Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J. Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warrington K.H., Jr., Gorbatyuk O.S., Harrison J.K., Opie S.R., Zolotukhin S., Muzyczka N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J. Virol. 2004;78:6595–6609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayaprolu V., Kruse S., Kant R., Venkatakrishnan B., Movahed N., Brooke D., Lins B., Bennett A., Potter T., McKenna R. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013;87:13150–13160. doi: 10.1128/JVI.01415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pipe S., Leebeek F.W.G., Ferreira V., Sawyer E.K., Pasi J. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol. Ther. Methods Clin. Dev. 2019;15:170–178. doi: 10.1016/j.omtm.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao G.-P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzyczka N., Berns K.I. Parvoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., Griffen D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Fields Virology. Volume 2. Lippincott Williams & Wilkins; 2001. pp. 2327–2359. [Google Scholar]
- 13.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowitz J.E., Rolling F., Li C., Conrath H., Xiao W., Xiao X., Samulski R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dane A.P., Wowro S.J., Cunningham S.C., Alexander I.E. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther. 2013;20:460–464. doi: 10.1038/gt.2012.67. [DOI] [PubMed] [Google Scholar]
- 17.Di Pasquale G., Chiorini J.A. AAV transcytosis through barrier epithelia and endothelium. Mol. Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C.E., Storm T.A., Huang Z., Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerford C., Samulski R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handa A., Muramatsu S.-I., Qiu J., Mizukami H., Brown K.E. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with AAV-2-based vectors. J. Gen. Virol. 2000;81:2077–2084. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- 21.Kaludov N., Brown K.E., Walters R.W., Zabner J., Chiorini J.A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt M., Govindasamy L., Afione S., Kaludov N., Agbandje-McKenna M., Chiorini J.A. Molecular characterization of the heparin-dependent transduction domain on the capsid of a novel adeno-associated virus isolate, AAV(VR-942) J. Virol. 2008;82:8911–8916. doi: 10.1128/JVI.00672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell C.L., Vandenberghe L.H., Bell P., Limberis M.P., Gao G.-P., Van Vliet K., Agbandje-McKenna M., Wilson J.M. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 2011;121:2427–2435. doi: 10.1172/JCI57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., Miller E., Agbandje-McKenna M., Samulski R.J. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillay S., Meyer N.L., Puschnik A.S., Davulcu O., Diep J., Ishikawa Y., Jae L.T., Wosen J.E., Nagamine C.M., Chapman M.S., Carette J.E. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qing K., Mah C., Hansen J., Zhou S., Dwarki V., Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 27.Summerford C., Bartlett J.S., Samulski R.J. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R., Xu G., Cao L., Sun Z., He Y., Cui M., Sun Y., Li S., Li H., Qin L. Divergent engagements between adeno-associated viruses with their cellular receptor AAVR. Nat. Commun. 2019;10:3760. doi: 10.1038/s41467-019-11668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.H., Doria A. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M.A.F.L., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 32.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.J., Enerbäck S., Nuutila P. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 33.Bartness T.J., Vaughan C.H., Song C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomou T., Mori M.A., Dreyfuss J.M., Konishi M., Sakaguchi M., Wolfrum C., Rao T.N., Winnay J.N., Garcia-Martin R., Grinspoon S.K. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Xue B., Rim J.-S., Hogan J.C., Coulter A.A., Koza R.A., Kozak L.P. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Strissel K.J., Stancheva Z., Miyoshi H., Perfield J.W., 2nd, DeFuria J., Jick Z., Greenberg A.S., Obin M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 38.Macotela Y., Emanuelli B., Mori M.A., Gesta S., Schulz T.J., Tseng Y.-H., Kahn C.R. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tchkonia T., Thomou T., Zhu Y., Karagiannides I., Pothoulakis C., Jensen M.D., Kirkland J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M.-J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genkinger J.M., Kitahara C.M., Bernstein L., Berrington de Gonzalez A., Brotzman M., Elena J.W., Giles G.G., Hartge P., Singh P.N., Stolzenberg-Solomon R.Z. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann. Oncol. 2015;26:2257–2266. doi: 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidayat K., Yang C.-M., Shi B.-M. Body fatness at an early age and risk of colorectal cancer. Int. J. Cancer. 2018;142:729–740. doi: 10.1002/ijc.31100. [DOI] [PubMed] [Google Scholar]
- 43.García-Jiménez C., Gutiérrez-Salmerón M., Chocarro-Calvo A., García-Martinez J.M., Castaño A., De la Vieja A. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br. J. Cancer. 2016;114:716–722. doi: 10.1038/bjc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson-Stuttard J., Zhou B., Kontis V., Bentham J., Gunter M.J., Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15. doi: 10.1016/S2213-8587(18)30150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullican S.E., Tomaru T., Gaddis C.A., Peed L.C., Sundaram A., Lazar M.A. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol. Endocrinol. 2013;27:127–134. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urs S., Harrington A., Liaw L., Small D. Selective expression of an aP2/fatty acid binding protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 47.Morizono K., De Ugarte D.A., Zhu M., Zuk P., Elbarbary A., Ashjian P., Benhaim P., Chen I.S., Hedrick M.H. Multilineage cells from adipose tissue as gene delivery vehicles. Hum. Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 48.Nagamatsu S., Nakamichi Y., Ohara-Imaizumi M., Ozawa S., Katahira H., Watanabe T., Ishida H. Adenovirus-mediated preproinsulin gene transfer into adipose tissues ameliorates hyperglycemia in obese diabetic KKAy mice. FEBS Lett. 2001;509:106–110. doi: 10.1016/s0014-5793(01)03146-5. [DOI] [PubMed] [Google Scholar]
- 49.Shirley J.L., de Jong Y.P., Terhorst C., Herzog R.W. Immune responses to viral gene therapy vectors. Mol. Ther. 2020;28:709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kvaratskhelia M., Sharma A., Larue R.C., Serrao E., Engelman A. Molecular mechanisms of retroviral integration site selection. Nucleic Acids Res. 2014;42:10209–10225. doi: 10.1093/nar/gku769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlimgen R., Howard J., Wooley D., Thompson M., Baden L.R., Yang O.O., Christiani D.C., Mostoslavsky G., Diamond D.V., Duane E.G. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 2016;58:1159–1166. doi: 10.1097/JOM.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milone M.C., O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandler R.J., LaFave M.C., Varshney G.K., Trivedi N.S., Carrillo-Carrasco N., Senac J.S., Wu W., Hoffmann V., Elkahloun A.G., Burgess S.M., Venditti C.P. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Invest. 2015;125:870–880. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flotte T.R., Afione S.A., Zeitlin P.L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am. J. Respir. Cell Mol. Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 55.Rizzuto G., Gorgoni B., Cappelletti M., Lazzaro D., Gloaguen I., Poli V., Sgura A., Cimini D., Ciliberto G., Cortese R. Development of animal models for adeno-associated virus site-specific integration. J. Virol. 1999;73:2517–2526. doi: 10.1128/jvi.73.3.2517-2526.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guilbaud M., Devaux M., Couzinié C., Le Duff J., Toromanoff A., Vandamme C., Jaulin N., Gernoux G., Larcher T., Moullier P. Five years of successful inducible transgene expression following locoregional adeno-associated virus delivery in nonhuman primates with no detectable immunity. Hum. Gene Ther. 2019;30:802–813. doi: 10.1089/hum.2018.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizukami H., Mimuro J., Ogura T., Okada T., Urabe M., Kume A., Sakata Y., Ozawa K. Adipose tissue as a novel target for in vivo gene transfer by adeno-associated viral vectors. Hum. Gene Ther. 2006;17:921–928. doi: 10.1089/hum.2006.17.921. [DOI] [PubMed] [Google Scholar]
- 60.Mochizuki S., Mizukami H., Kume A., Muramatsu S., Takeuchi K., Matsushita T., Okada T., Kobayashi E., Hoshika A., Ozawa K. Adeno-associated virus (AAV) vector-mediated liver- and muscle-directed transgene expression using various kinds of promoters and serotypes. Gene Ther. Mol. Biol. 2004;8:9–18. [Google Scholar]
- 61.Zhang F.-L., Jia S.-Q., Zheng S.-P., Ding W. Celastrol enhances AAV1-mediated gene expression in mice adipose tissues. Gene Ther. 2011;18:128–134. doi: 10.1038/gt.2010.120. [DOI] [PubMed] [Google Scholar]
- 62.Jimenez V., Muñoz S., Casana E., Mallol C., Elias I., Jambrina C., Ribera A., Ferre T., Franckhauser S., Bosch F. In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes. 2013;62:4012–4022. doi: 10.2337/db13-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Neill S.M., Hinkle C., Chen S.-J., Sandhu A., Hovhannisyan R., Stephan S., Lagor W.R., Ahima R.S., Johnston J.C., Reilly M.P. Targeting adipose tissue via systemic gene therapy. Gene Ther. 2014;21:653–661. doi: 10.1038/gt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhrig-Schmidt S., Geiger M., Luippold G., Birk G., Mennerich D., Neubauer H., Grimm D., Wolfrum C., Kreuz S. Gene delivery to adipose tissue using transcriptionally targeted rAAV8 vectors. PLoS ONE. 2014;9:e116288. doi: 10.1371/journal.pone.0116288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X., Magee D., Wang C., McMurphy T., Slater A., During M., Cao L. Adipose tissue insulin receptor knockdown via a new primate-derived hybrid recombinant AAV serotype. Mol. Ther. Methods Clin. Dev. 2014;1:8. doi: 10.1038/mtm.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jimenez V., Jambrina C., Casana E., Sacristan V., Muñoz S., Darriba S., Rodó J., Mallol C., Garcia M., León X. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018;10:e8791. doi: 10.15252/emmm.201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lagarrigue S., Lopez-Mejia I.C., Denechaud P.-D., Escoté X., Castillo-Armengol J., Jimenez V., Chavey C., Giralt A., Lai Q., Zhang L. CDK4 is an essential insulin effector in adipocytes. J. Clin. Invest. 2016;126:335–348. doi: 10.1172/JCI81480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Albert V., Svensson K., Shimobayashi M., Colombi M., Muñoz S., Jimenez V., Handschin C., Bosch F., Hall M.N. mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Mol. Med. 2016;8:232–246. doi: 10.15252/emmm.201505610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang W., McMurphy T., Liu X., Wang C., Cao L. Genetic manipulation of brown fat via oral administration of an engineered recombinant adeno-associated viral serotype vector. Mol. Ther. 2016;24:1062–1069. doi: 10.1038/mt.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y., Gao Y., Tao C., Shao M., Zhao S., Huang W., Yao T., Johnson J.A., Liu T., Cypess A.M. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 2016;24:420–433. doi: 10.1016/j.cmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie L., Zhang Y., Gunasekar S.K., Mishra A., Cao L., Sah R. Induction of adipose and hepatic SWELL1 expression is required for maintaining systemic insulin-sensitivity in obesity. Channels (Austin) 2017;11:673–677. doi: 10.1080/19336950.2017.1373225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng R., Hussain N.A., Zhang Q., Chang C., Li H., Fu Y., Cao L., Han W., Stunkel W., Xu F. miRNA-32 drives brown fat thermogenesis and trans-activates subcutaneous white fat browning in mice. Cell Rep. 2017;19:1229–1246. doi: 10.1016/j.celrep.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Xie L., Gunasekar S.K., Tong D., Mishra A., Gibson W.J., Wang C., Fidler T., Marthaler B., Klingelhutz A. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat. Cell Biol. 2017;19:504–517. doi: 10.1038/ncb3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W., Liu X., Queen N.J., Cao L. Targeting visceral fat by intraperitoneal delivery of novel AAV serotype vector restricting off-target transduction in liver. Mol. Ther. Methods Clin. Dev. 2017;6:68–78. doi: 10.1016/j.omtm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao R., Mansour A.G., Huang W., Chrislip L.A., Wilkins R.K., Queen N.J., Youssef Y., Mao H.C., Caligiuri M.A., Cao L. Adipocytes: a novel target for IL-15/IL-15Rα cancer gene therapy. Mol. Ther. 2019;27:922–932. doi: 10.1016/j.ymthe.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang W., Queen N.J., McMurphy T.B., Ali S., Cao L. Adipose PTEN regulates adult adipose tissue homeostasis and redistribution via a PTEN-leptin-sympathetic loop. Mol. Metab. 2019;30:48–60. doi: 10.1016/j.molmet.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He T., Itano M.S., Earley L.F., Hall N.E., Riddick N., Samulski R.J., Li C. The influence of murine genetic background in adeno-associated virus transduction of the mouse brain. Hum. Gene Ther. Clin. Dev. 2019;30:169–181. doi: 10.1089/humc.2019.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inagaki K., Fuess S., Storm T.A., Gibson G.A., Mctiernan C.F., Kay M.A., Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sands M.S. AAV-mediated liver-directed gene therapy. Methods Mol. Biol. 2011;807:141–157. doi: 10.1007/978-1-61779-370-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiao C., Yuan Z., Li J., He B., Zheng H., Mayer C., Li J., Xiao X. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Ther. 2011;18:403–410. doi: 10.1038/gt.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cybulski N., Polak P., Auwerx J., Rüegg M.A., Hall M.N. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc. Natl. Acad. Sci. USA. 2009;106:9902–9907. doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keeler A.M., Conlon T., Walter G., Zeng H., Shaffer S.A., Dungtao F., Erger K., Cossette T., Tang Q., Mueller C., Flotte T.R. Long-term correction of very long-chain acyl-coA dehydrogenase deficiency in mice using AAV9 gene therapy. Mol. Ther. 2012;20:1131–1138. doi: 10.1038/mt.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davidoff A.M., Gray J.T., Ng C.Y.C., Zhang Y., Zhou J., Spence Y., Bakar Y., Nathwani A.C. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 85.Pattali R., Mou Y., Li X.-J. AAV9 vector: a novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019;26:287–295. doi: 10.1038/s41434-019-0085-4. [DOI] [PubMed] [Google Scholar]
- 86.Katz M.G., Fargnoli A.S., Weber T., Hajjar R.J., Bridges C.R. Use of adeno-associated virus vector for cardiac gene delivery in large-animal surgical models of heart failure. Hum. Gene Ther. Clin. Dev. 2017;28:157–164. doi: 10.1089/humc.2017.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ambrosi C.M., Sadananda G., Han J.L., Entcheva E. Adeno-associated virus mediated gene delivery: implications for scalable in vitro and in vivo cardiac optogenetic models. Front. Physiol. 2019;10:168. doi: 10.3389/fphys.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie Q., Bu W., Bhatia S., Hare J., Somasundaram T., Azzi A., Chapman M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Büning H., Srivastava A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol. Ther. Methods Clin. Dev. 2019;12:248–265. doi: 10.1016/j.omtm.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogden P.J., Kelsic E.D., Sinai S., Church G.M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 2019;366:1139–1143. doi: 10.1126/science.aaw2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Büning H., Huber A., Zhang L., Meumann N., Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr. Opin. Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 92.Davidsson M., Wang G., Aldrin-Kirk P., Cardoso T., Nolbrant S., Hartnor M., Mudannayake J., Parmar M., Björklund T. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proc. Natl. Acad. Sci. USA. 2019;116:27053–27062. doi: 10.1073/pnas.1910061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charbel Issa P., De Silva S.R., Lipinski D.M., Singh M.S., Mouravlev A., You Q., Barnard A.R., Hankins M.W., During M.J., Maclaren R.E. Assessment of tropism and effectiveness of new primate-derived hybrid recombinant AAV serotypes in the mouse and primate retina. PLoS ONE. 2013;8:e60361. doi: 10.1371/journal.pone.0060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McMurphy T.B., Huang W., Xiao R., Liu X., Dhurandhar N.V., Cao L. Hepatic expression of adenovirus 36 E4ORF1 improves glycemic control and promotes glucose metabolism through akt activation. Diabetes. 2017;66:358–371. doi: 10.2337/db16-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samulski R.J., Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 96.Zhong L., Li B., Mah C.S., Govindasamy L., Agbandje-McKenna M., Cooper M., Herzog R.W., Zolotukhin I., Warrington K.H., Jr., Weigel-Van Aken K.A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li B., Ma W., Ling C., Van Vliet K., Huang L.-Y., Agbandje-McKenna M., Srivastava A., Aslanidi G.V. Site-directed mutagenesis of surface-exposed lysine residues leads to improved transduction by AAV2, but not AAV8, vectors in murine hepatocytes in vivo. Hum. Gene Ther. Methods. 2015;26:211–220. doi: 10.1089/hgtb.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domenger C., Grimm D. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum. Mol. Genet. 2019;28(R1):R3–R14. doi: 10.1093/hmg/ddz148. [DOI] [PubMed] [Google Scholar]
- 99.Sarcar S., Tulalamba W., Rincon M.Y., Tipanee J., Pham H.Q., Evens H., Boon D., Samara-Kuko E., Keyaerts M., Loperfido M. Next-generation muscle-directed gene therapy by in silico vector design. Nat. Commun. 2019;10:492. doi: 10.1038/s41467-018-08283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y., Schwalie P.C., Bast-Habersbrunner A., Mocek S., Russeil J., Fromme T., Deplancke B., Klingenspor M. Systems-genetics-based inference of a core regulatory network underlying white fat browning. Cell Rep. 2019;29:4099–4113.e5. doi: 10.1016/j.celrep.2019.11.053. [DOI] [PubMed] [Google Scholar]
- 101.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.-M., Fyfe J., Moullier P., Colle M.A., Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foust K.D., Wang X., McGovern V.L., Braun L., Bevan A.K., Haidet A.M., Le T.T., Morales P.R., Rich M.M., Burghes A.H.M., Kaspar B.K. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L., Wang H., Bell P., McMenamin D., Wilson J.M. Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector. Hum. Gene Ther. 2012;23:533–539. doi: 10.1089/hum.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nathwani A.C., Gray J.T., Ng C.Y.C., Zhou J., Spence Y., Waddington S.N., Tuddenham E.G.D., Kemball-Cook G., McIntosh J., Boon-Spijker M. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ronzitti G., Bortolussi G., van Dijk R., Collaud F., Charles S., Leborgne C., Vidal P., Martin S., Gjata B., Sola M.S. A translationally optimized AAV-UGT1A1 vector drives safe and long-lasting correction of Crigler-Najjar syndrome. Mol. Ther. Methods Clin. Dev. 2016;3:16049. doi: 10.1038/mtm.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bennicelli J., Wright J.F., Komaromy A., Jacobs J.B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T.S. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gessler D.J., Li D., Xu H., Su Q., Sanmiguel J., Tuncer S., Moore C., King J., Matalon R., Gao G. Redirecting N-acetylaspartate metabolism in the central nervous system normalizes myelination and rescues Canavan disease. JCI Insight. 2017;2:e90807. doi: 10.1172/jci.insight.90807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borel F., Kay M.A., Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol. Ther. 2014;22:692–701. doi: 10.1038/mt.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grimm D., Wang L., Lee J.S., Schürmann N., Gu S., Börner K., Storm T.A., Kay M.A. Argonaute proteins are key determinants of RNAi efficacy, toxicity, and persistence in the adult mouse liver. J. Clin. Invest. 2010;120:3106–3119. doi: 10.1172/JCI43565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun C.-P., Wu T.-H., Chen C.-C., Wu P.-Y., Shih Y.-M., Tsuneyama K., Tao M.H. Studies of efficacy and liver toxicity related to adeno-associated virus-mediated RNA interference. Hum. Gene Ther. 2013;24:739–750. doi: 10.1089/hum.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Byrne B.J. Innovative vector design: cross-packaged, self-complementary and now trans-splicing AAV vectors. Hum. Gene Ther. 2009;20:1224–1225. doi: 10.1089/hum.2009.1020. [DOI] [PubMed] [Google Scholar]
- 113.Schmelas C., Grimm D. Split Cas9, not hairs—advancing the therapeutic index of CRISPR technology. Biotechnol. J. 2018;13:e1700432. doi: 10.1002/biot.201700432. [DOI] [PubMed] [Google Scholar]
- 114.Das A.T., Tenenbaum L., Berkhout B. Tet-On systems for doxycycline-inducible gene expression. Curr. Gene Ther. 2016;16:156–167. doi: 10.2174/1566523216666160524144041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sohn J., Takahashi M., Okamoto S., Ishida Y., Furuta T., Hioki H. A single vector platform for high-level gene transduction of central neurons: adeno-associated virus vector equipped with the Tet-Off system. PLoS ONE. 2017;12:e0169611. doi: 10.1371/journal.pone.0169611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Guiner C., Stieger K., Toromanoff A., Guilbaud M., Mendes-Madeira A., Devaux M., Guigand L., Cherel Y., Moullier P., Rolling F., Adjali O. Transgene regulation using the tetracycline-inducible TetR-KRAB system after AAV-mediated gene transfer in rodents and nonhuman primates. PLoS ONE. 2014;9:e102538. doi: 10.1371/journal.pone.0102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Solis C.A., Ho A., Holehonnur R., Ploski J.E. The development of a viral mediated CRISPR/Cas9 system with doxycycline dependent gRNA expression for inducible in vitro and in vivo genome editing. Front. Mol. Neurosci. 2016;9:70. doi: 10.3389/fnmol.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J., Sewter C.P., Digby J.E., Mohammed S.N., Hurst J.A. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 119.Gómez-Banoy N., Guseh J.S., Li G., Rubio-Navarro A., Chen T., Poirier B., Putzel G., Rosselot C., Pabón M.A., Camporez J.P. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat. Med. 2019;25:1739–1747. doi: 10.1038/s41591-019-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rochford J.J. Mouse models of lipodystrophy and their significance in understanding fat regulation. Curr. Top. Dev. Biol. 2014;109:53–96. doi: 10.1016/B978-0-12-397920-9.00005-6. [DOI] [PubMed] [Google Scholar]
- 121.Mann J.P., Savage D.B. What lipodystrophies teach us about the metabolic syndrome. J. Clin. Invest. 2019;129:4009–4021. doi: 10.1172/JCI129190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agarwal A.K., Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Annu. Rev. Genomics Hum. Genet. 2006;7:175–199. doi: 10.1146/annurev.genom.7.080505.115715. [DOI] [PubMed] [Google Scholar]
- 123.Garg A. Lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Millington G.W.M. Rook’s Textbook of Dermatology. Ninth Edition. American Cancer Society; 2016. Genetic disorders of adipose tissue; pp. 1–11. [Google Scholar]