Abstract
Mediated by the nuclear vitamin D receptor (VDR), the hormonally active vitamin D metabolite, 1,25-dihydroxyvitamin D3 (1,25D), is known to regulate expression of genes impacting calcium and phosphorus metabolism, the immune system, and behavior. Urolithin A, a nutrient metabolite derived from pomegranate, possibly acting through AMP kinase (AMPK) signaling, supports respiratory muscle health in rodents and longevity in C. elegans by inducing oxidative damage-reversing genes and mitophagy. We show herein that urolithin A enhances transcriptional actions of 1,25D driven by co-transfected vitamin D responsive elements (VDREs), and dissection of this genomic effect in cell culture reveals: 1) urolithin A concentration-dependency, 2) occurrence with isolated natural VDREs, 3) nuclear receptor selectivity for VDR over ER, LXR and RXR, and 4) significant 3- to 13-fold urolithin A-augmentation of 1,25D-dependent mRNA encoding the widely expressed 1,25D-detoxification enzyme, CYP24A1, a benchmark vitamin D target gene. Relevant to potential behavioral effects of vitamin D, urolithin A elicits enhancement of 1,25D-dependent mRNA encoding tryptophan hydroxylase-2 (TPH2), the serotonergic neuron-expressed initial enzyme in tryptophan metabolism to serotonin. Employing quantitative real time-PCR, we demonstrate that TPH2 mRNA is induced 1.9-fold by 10 nM 1,25D treatment in culture of differentiated rat serotonergic raphe (RN46A-B14) cells, an effect magnified 2.5-fold via supplementation with 10 μM urolithin A. This potentiation of 1,25D-induced TPH2 mRNA by urolithin A is followed by a 3.1- to 3.7-fold increase in serotonin concentration in culture medium from the pertinent neuronal cell line, RN46A-B14. These results are consistent with the concept that two natural nutrient metabolites, urolithin A from pomegranate and 1,25D from sunlight/vitamin D, likely acting via AMPK and VDR, respectively, cooperate mechanistically to effect VDRE-mediated regulation of gene expression in neuroendocrine cells. Finally, gedunin, a neuroprotective natural product from Indian neem tree that impacts the brain derived neurotropic factor pathway, similarly potentiates 1,25D/VDR-action.
Keywords: Vitamin D, Gut microbiota, Ellagitannins, Nuclear VDR, Gene expression, Serotonergic neuronal cell line
Highlights
-
•
Hormonal 1,25-dihydroxyvitamin D acts in brain to induce tryptophan hydroxylase-2.
-
•
Urolithin A derived from ellagitannins in pomegranates curbs neuroinflammation.
-
•
Urolithin A enhances the transcriptional actions of 1,25-dihydroxyvitamin D.
-
•
Urolithin A raises 1,25-dihydroxyvitamin D-induced tryptophan hydroxylase-2 mRNA.
-
•
Serotonin rises in raphe cells exposed to urolithin A and 1,25-dihydroxyvitamin D.
1. Introduction
Vitamin D is an essential fat-soluble nutrient endowed with the unique characteristic that it is alternatively acquired via sunlight exposure of skin. In animals, vitamin D3 can be photolytically (non-enzymatically) generated from endogenous 7-dehydrocholesterol by irradiation of skin with ultraviolet light, e.g., from natural sunlight. In a sense, vitamin D acquisition still depends on the environment, but it is technically not required in the diet provided adequate sunlight exposure occurs. Similar to its nutritional counterpart, fat-soluble vitamin A, vitamin D is metabolized in the body to active, hormone-like derivatives that signal a variety of critical biological events [1]. The hormonal metabolite of vitamin D, 1,25-dihydroxyvitamin D3 (1,25D), is produced predominantly in the kidney, and acts in a variety of end-organs via the nuclear vitamin D receptor (VDR) to trigger an ensemble of molecular events that orchestrate bone mineral homeostasis, immune system regulation, and central nervous system modulation [2,3]. VDR is a classic member of the nuclear receptor superfamily, specifically the subfamily of ligand-controlled transcription factors that obligatorily heterodimerize with a retinoid X receptor (RXR) to bind cognate hormone responsive elements in DNA [4]. Interestingly, both unliganded and liganded VDR-RXR target vitamin D responsive elements (VDREs) in the vicinity of vitamin D-regulated genes [5]. When docked with high affinity on VDREs, 1,25D-liganded VDR-RXR recruits transcriptional cofactors that epigenetically modify and loop-out chromatin to govern gene expression [6]. Circulating 1,25D ligand concentrations are controlled by parathyroid hormone, an inducer of renal d-hormone biosynthesis catalyzed by CYP27B1 that functions as the key player in a calcemic endocrine loop, and fibroblast growth factor 23 (FGF23), a repressor of the CYP27B1 renal enzyme that completes a novel hypophosphatemic endocrine loop [7]. Finally, both 1,25D and FGF23 induce the primary vitamin D metabolite-degradation enzyme, CYP24A1, in all tissues to autoregulate 1,25D concentrations.
Vitamin D interacts functionally with several nutritional principles beyond the obvious calcium and phosphorus elements that it marshals to ensure a well-mineralized skeleton. We have observed that beneficial nutrients such as curcumin [8], docosahexaenoic acid (DHA) [9], delphinidin [10] and cyanidin [11] all function in part as VDR agonists by virtue of their ability to serve as relatively low-affinity VDR ligands compared to the hormonal 1,25D ligand. Thus, these “nutraceuticals” appear to act at least in part by weak association with VDR to effect 1,25D-related bio-responses, consistent with the fact that these natural agents require micromolar concentrations instead of the nano- to pico-molar concentrations necessary for sterol hormone action. Another plant-derived compound we have studied in relation to vitamin D action is resveratrol, the reputed anti-aging agent found in red grapes. Unlike curcumin, DHA and the anthocyanins, resveratrol does not compete with 1,25D for binding to VDR. In fact, bio-effective resveratrol concentrations (μM) accentuate radio-labelled 1,25D association with VDR, suggesting that resveratrol affects the VDR protein in a fundamentally different manner than low-affinity ligands [12]. We have shown that resveratrol appears to act allosterically on VDR, rather than by residing in the 1,25D-binding lipophilic pocket. Considering that resveratrol is a well-known activator of the deacetylase function of SIRT1, we have established that resveratrol potentiates the deacetylation of VDR catalyzed by SIRT1 [13]. Therefore, it has been demonstrated that resveratrol mimics and augments 1,25D action to regulate gene expression indirectly, accomplished through covalent modification of VDR. Indeed, acetylation/deacetylation of human VDR at lys-91 has recently been revealed as a mechanism for gene targeting by 1,25D/VDR through the switching of the co-modulator ensemble recruited to gene networks [14].
In the present study, we report an investigation of the potential interaction of 1,25D/VDR with an intriguing new nutraceutical, namely urolithin A, a dibenzo-α-pyrone. Urolithin A is a metabolic product of the ellagitannins prevalent in pomegranate, red and black berries, as well as other pigmented fruits. Ellagitannins are metabolized by intestinal microorganisms to ellagic acid, which is the immediate precursor of the family of urolithins. Once formed in the gut, urolithins are absorbed and enter the systemic circulation, depending on the profile of intestinal microbiota in the individual organism.
Beginning in 2010, publications appeared reporting anti-inflammatory actions of urolithin A in various experimental systems, for example, in the colitis rat model [15] and in human colonic fibroblasts treated with NFκB-signaling inflammatory agents [16]. Thus, urolithin A has emerged as a novel anti-inflammatory and antioxidant nutrient in the past decade [17]. Moreover, the power of urolithin A to curb neuroinflammation and improve cognitive function in pre-clinical models for Alzheimer's disease has also been explored. A recent study of urolithin A in a mouse model of Alzheimer's, APP/PS1, indicates that urolithin A activates cerebral adenosine monophosphate-activated protein kinase (AMPK) to attenuate Alzheimer's disease pathology and memory impairment not only via dampening neuroinflammation, but also by reducing reactive gliosis, β-amyloid plaque formation, and apoptosis [18]. Major insight into the biological effects of urolithin A has been provided by a landmark study published in 2016 that uncovered potentially significant actions of urolithin A: 1) extension of lifespan and improved fitness in C. elegans, 2) enhancement of mitochondrial function to prolong lifespan, and 3) stimulation of mitophagy in mammalian cells and elevation of muscle function in rodents [19]. Intrinsic to these actions of urolithin A is the augmentation of gene expression in muscle, including increased expression of autophagy (Atg8l, Becn1, LAMP2, Pik3c3, p62, and Ulk1) and mitophagy (Park2) transcripts. Urolithin A also induced the phosphorylation of AMPK in muscle tissue [19], which is one of the hallmarks of urolithin A signaling. Recently, Andreux and colleagues reported [20] the first-in-human clinical study of urolithin A-administration in sedentary individuals, revealing that following consumption of urolithin A, recipients experienced improved skeletal muscle mitochondrial and cellular health akin to that of control subjects who exercised regularly.
To-date, the influence of urolithin A on the ligand-activated nuclear receptor superfamily has not been examined, even though urolithin A is chemically similar to several of the nuclear receptor ligands. Equally important is the fact that many of the actions of nuclear receptors resemble those of urolithin A, e.g., beneficial modulation of lipid and carbohydrate metabolism. In particular, 1,25D/VDR induces tolerance in human dendritic cells by activation of intracellular metabolic pathways of glucose metabolism such as glucose availability and glycolysis, controlled by the PI3K/Akt/mTOR pathway [21]. Notably, 1,25D induces AMPK in these immune cells [21], raising the possibility that urolithin A and 1,25D could operate cooperatively by boosting both the activity and quantity of AMPK in order to influence the PI3K/Akt/mTOR pathway. In the present study, we probed the effect of co-treatment with urolithin A on transcriptional activation by VDR, reasoning that like urolithin A, 1,25D is known to modulate immunity, regulate behavior, suppress cancer cell proliferation, and has been intimated as a possible extender of health-span [22]. The results reported herein suggest that nutrient-derived metabolites, specifically urolithin A and 1,25D, interact functionally in intact cells, with urolithin A potentiating the transcriptional action of 1,25D/VDR.
2. Materials and Methods
2.1. Luciferase plasmid constructs
The DNA elements cloned upstream of the luciferase reporter vector, pLUC-MCS (Stratagene Corp., La Jolla, CA), included the vitamin D responsive elements termed XDR3, PER6, and CYP24A1, as well as the estrogen responsive element (ERE), liver X receptor-activated responsive element (LXRE), and retinoid X receptor-activated responsive element (RXRE) [12].
2.2. Mammalian cell culture
Embryonic rat medullary raphe (RN46A-B14) and human embryonic kidney (HEK293) cells were obtained, cultured, and treated as described previously [23].
2.3. Transient transfection of cultured cells and luciferase assay
Methodology for transient transfection of cells with DNA plasmids/luciferase-containing vectors is detailed elsewhere [13]. Twenty-two to 24 h post-transfection, the cells were treated with either an ethanol (EtOH) or dimethyl sulfoxide (DMSO) vehicle control or the ligand for the nuclear receptor (usually 1,25D), along with urolithin A (Sigma-Aldrich, St. Louis, MO) and/or resveratrol (Enzo Life Sciences, Farmingdale, NY) added as a nutraceutical adjuvant in select experiments as detailed in the figure legends. After incubation, cells were lysed and the lysates were then analyzed sequentially for Firefly and Renilla luciferase, followed by computation of transcriptional activity as described previously [13].
2.4. Total RNA isolation, cDNA synthesis, and mRNA expression analysis via quantitative real-time PCR
HEK293 and RN46A-B14 cells were plated at 500,000–900,000 cells per well in a 6-well plate, transfected with 50 ng pSG5-VDR (expression plasmid for human VDR), and treated for 24 h with either an ethanol (EtOH) or dimethyl sulfoxide (DMSO) vehicle control or 1,25D, along with urolithin A (Sigma-Aldrich, St. Louis, MO) and/or resveratrol (Enzo Life Sciences, Farmingdale, NY) addition as a nutraceutical adjuvant in select experiments as detailed in the figure legends. Total RNA was isolated, reverse transcribed, and real-time PCR performed, plus data analyzed to quantitate gene expression, as reported elsewhere [23]. Primer sets for the PCR were as follows:
Rat Gapdh (forward 5′-AGGTCGGTGTGAACGGATTTG-3′,reverse 5′-CATTCTCAGCCTTGACTGTGC-3′);
Rat Cyp24a1 (forward 5′-AACGAAGCCTACGGGTTGATG-3′, reverse 5′-AGAAAGTCAGCCAAGACCTCA-3′);
Rat Tph2 [24] (forward 5′-CTCCAAGCTTCGCATCACAG-3′, reverse 5′-AGCACTTCAGGAAGCGTACC-3′).
Human GAPDH (forward 5′-ACAACTTTGGTATCGTGGAAGGAC-3′, reverse 5′-CAGGGATGATGTTCTGGAGAGC-3′); or
Human CYP24A1 (forward 5′-CAGCGAACTGAACAAATGGTCG-3′, reverse 5′-TCTCTTCTCATACAACACGAGGCAG-3′);
2.5. Serotonin ELISA
Serotonin (5-HT) concentrations were assessed in aliquots of media/culture supernatant via colorimetric competitive 5-HT enzyme-linked immunosorbent assay (ELISA) as detailed previously [23]. Cells were treated with either ethanol or DMSO vehicle control or 1,25D, along with urolithin A (Sigma-Aldrich, St. Louis, MO) and/or resveratrol (Enzo Life Sciences, Farmingdale, NY) addition as a nutraceutical adjuvant in select experiments as detailed in the figure legends.
2.6. Statistical analysis
Because the design of the luciferase experiments was a simple 2 × 2 motif in which the ability of urolithin A to enhance the effectiveness of hormone-induced transcription was assessed, the Welch's two-sample t-test was applied to the log transformed fold change to ascertain which specific experimental results differed from their controls. Bonferroni method was used to correct for multiple comparisons in each experiment with the p-value significance threshold equal to 0.05 divided by the number of comparisons. For real-time PCR results, data are expressed as means ± SD. All data are presented as fold-effects, with transcription of the gene in question (i.e., mRNA level) in the absence of 1,25D (i.e., ethanol vehicle alone) set at 1.0-fold. Because the design of the experiments was a simple motif in which the fold-ability of 1,25D to enhance transcription over basal (i.e., ethanol vehicle) was assessed, statistical differences between two groups were determined by a two-tailed Student's t-test. However, because variance was high in several experiments, further statistical analyses were performed using GraphPad Prism 7 to generate ANOVA data, followed by a post hoc Dunnett test that compares every mean to a control mean and takes into account the scatter of all the groups.
3. Results
We hypothesized that VDR may be one of the potential mediators of select urolithin A bioactions, or at least that urolithin A is capable of modulating the transcriptional effects of nuclear receptors, specifically VDR. In the first experiment designed to test this hypothesis, we carried out transcription assays employing a VDRE-firefly luciferase reporter plasmid in cultured human embryonic kidney cells (HEK293). The isolated XDR3 element employed to generate the data pictured in Fig. 1 is comprised of two copies of the proximal VDRE in the human CYP3A4 gene, a direct-repeat of hexanucleotides separated by 3 nucleotides (DR3) [25]. To distinguish between two possible mechanisms for urolithin A-elicited influence on VDR-mediated transcriptional activation, namely that urolithin A either acts as a VDR agonist ligand itself, or that urolithin A affects VDR function only when VDR is liganded with 1,25D, we performed the first experiment testing the activity of urolithin A alone and in combination with four concentrations of 1,25D.
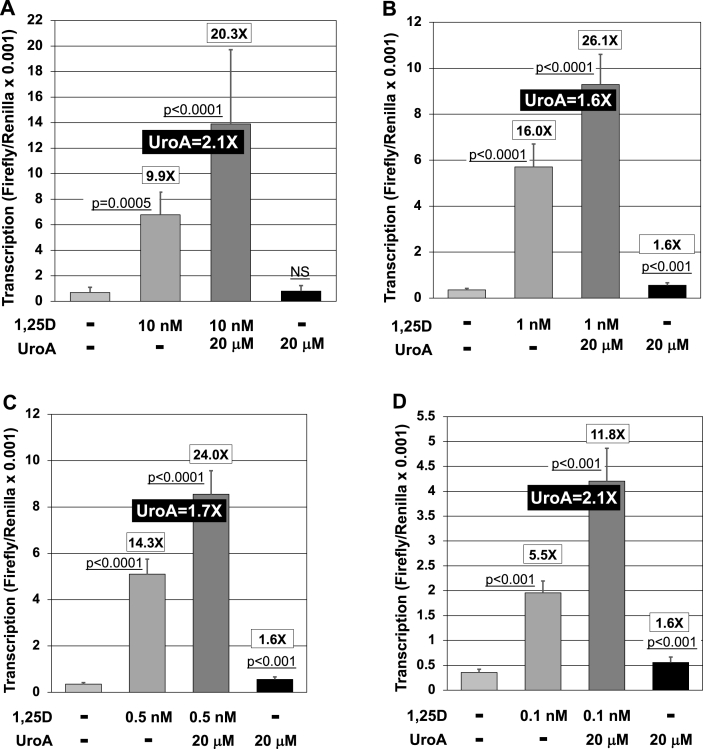
Fig. 1.
Urolithin A (UroA) augments 1,25D-triggered transcriptional activation mediated by a range (0.1 nM–10 nM) of concentrations of 1,25D. For all four panels, fold-effect of 1,25D or 1,25D + UroA above vehicle control is listed at the top of the appropriate bar. The UroA fold-potentiation of the 1,25D-effect is depicted in white text on a black background. Values are the average of nine biological replicates (n = 9) ± Std. Dev. (A) Effect of UroA on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 10 nM 1,25D. Cells were exposed in culture to either EtOH/DMSO vehicle or a final concentration of 10 nM 1,25D (alone), 20 μM UroA (alone), or both 1,25D and UroA for 24 h and transcription assessed by luciferase activity as described in Methods. (B) Effect of UroA on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to either EtOH/DMSO vehicle or a final concentration of 1 nM 1,25D (alone), 20 μM UroA (alone), or both 1,25D and UroA for 24 h and transcription assessed by luciferase activity as described in Methods. (C) Effect of UroA on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to either EtOH/DMSO vehicle or a final concentration of 0.5 nM 1,25D (alone), 20 μM UroA (alone), or both 1,25D and UroA for 24 h and transcription assessed by luciferase activity as described in Methods. (D) Effect of UroA on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to either EtOH/DMSO vehicle or a final concentration of 0.1 nM 1,25D (alone), 20 μM UroA (alone), or both 1,25D and UroA for 24 h and transcription assessed by luciferase activity as described in Methods.
The data in Fig. 1A reveal that 20 μM urolithin A alone does not activate transcription driven by the XDR3 VDRE, whereas 10 nM 1,25D elevates transcription by 9.9-fold in this experiment. But 20 μM urolithin A did stimulate 1,25D-triggered transcription to 20.3-fold above basal, or a 2.1-fold effect of urolithin A in the presence of 1,25D. This result points to a vitamin D-dependent mechanism for urolithin A action instead of an independent effect of urolithin A on nuclear receptor action. Next we titrated the concentration of 1,25D down from 10 nM to 0.1 nM to evaluate the influence of vitamin D-hormone levels on the urolithin A-effect. Shown in Fig. 2B, 2C, and 2D are experiments in which the concentration of 1,25D was lowered to 1, 0.5 and 0.1 nM, respectively. In all three cases with progressively lower concentrations of 1,25D, urolithin A exerts a statistically significant amplification of 1,25D/VDR-stimulated transcription, with a relatively consistent boost of 1.6- to 2.1-fold.
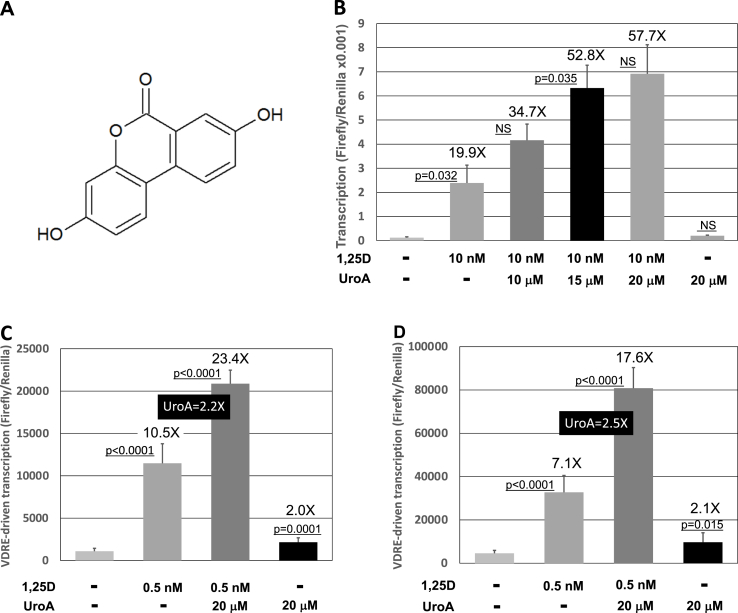
Fig. 2.
UroA augments 1,25D-triggered transcriptional activation mediated by three different VDREs in cultured cells. (A) Chemical structure of urolithin A. (B) Effect of UroA on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to 10 nM 1,25D (added in EtOH vehicle) and either 10 μM, 15μM or 20 μM UroA (added in DMSO vehicle) for 24 h and transcription assessed by luciferase activity as described in Methods. Fold-effect over EtOH/DMSO vehicle control is listed at the top of each bar. Values are the average of three biological replicates (n = 3) ± Std. Dev. (C) Effect of UroA on transcriptional activation driven by the PER6 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to 0.5 nM 1,25D (added in EtOH vehicle) and 20 μM UroA (added in DMSO vehicle) for 24 h and transcription assessed by luciferase activity as described in Methods. Fold-effect of UroA over 1,25D control is listed at the top of the central pair of bars. Values are the average of six biological replicates (n = 6) ± Std. Dev. (D) Effect of UroA on transcriptional activation driven by the human CYP24A1 VDRE in HEK293 cells treated with 1,25D. The transfected VDRE-reporter was a plasmid consisting of a 5.5 kb natural promoter fragment of the human CYP24A1 gene fused to the luciferase reporter vector. Cells were exposed in culture 0.5 nM 1,25D (added in EtOH vehicle) and 20 μM UroA (added in DMSO vehicle) for 24 h and transcription assessed by luciferase activity as described in Methods. Fold-effect of UroA over 1,25D control is listed at the top of the central pair of bars. Values are the average of six biological replicates (n = 6) ± Std. Dev.
Thus, the urolithin A effect to augment 1,25D/VDR-driven transcription is relatively independent of 1,25D ligand levels and urolithin A alone displays only a very small (1.6X) but statistically significant activity (in Fig. 1B–D, only) in evoking VDRE-driven basal transcription. This minimal influence of urolithin A on basal transcription in the ultrasensitive luciferase assay (Fig. 1B–D) is approximately 12% of the magnitude of the impact of the 1,25D VDR ligand, indicating that urolithin A is not likely to constitute a biologically relevant VDR agonist. Chemically, urolithin A is a dibenzo-α-pyrone, and its structure is depicted in Fig. 2A. In a second set of experiments, we evaluated three concentrations of urolithin A, namely 10, 15 and 20 μM, to examine the dose-dependency of the response in HEK293 cells transfected with the XDR3 VDRE-reporter construct (Fig. 2). Treatment with 10 nM 1,25D resulted in a statistically significant, 19.9-fold increase in luciferase activity over the EtOH/DMSO vehicles (Fig. 2B). Inclusion of 10 μM urolithin A boosted this 1,25D-effect to 34.7-fold, although this enhancement in transcription was not statistically significant despite the positive trend. However, as depicted in Fig. 2B, inclusion of 15 μM and 20 μM urolithin A produced statistically significant potentiation of 1,25D action to 52.8-fold and 57.7-fold over the EtOH/DMSO vehicle, respectively.
As illustrated in the last bar on the right in Fig. 2B, this 20 μM concentration of urolithin A did not exert a statistically significant influence on basal transcription driven by the XDR3 VDRE, essentially repeating the findings of Fig. 1A. As shown in Fig. 2C, when an alternative isolated VDRE is utilized, namely the perfect everted-repeat of hexanucleotides spaced by six nucleotides (PER6) which lies 5′-distal in the human CYP3A4 gene [25], to evaluate the generality of potentiation of VDR-driven transcription by urolithin A, 20 μM urolithin A elicits a significant 2.2-fold (p < 0.0001) stimulation of the 1,25D-effect on transcription. Next, we examined the influence of urolithin A on VDR-mediated transcriptional activation employing a third VDRE, specifically the CYP24A1 VDRE in the context of a natural 5.5 kb fragment of the promoter region of the human gene [12]. The human CYP24A1 gene possesses two antisense DR3 VDREs, AGGTGAGCGAGGGCG and AGTTCACCGGGTGTG (hexanucleotide repeats underlined), in the proximal promoter. In a transfection experiment, this duplex VDRE with a natural spacer of approximately 100 bp, would presumably exist in a nucleosomal environment consistent with the structure achievable for a gene fragment of the 5.5 kb-size. The results depicted in Fig. 2D reveal that the CYP24A1 VDREs produce transcriptional activation by 1,25D that is statistically significantly enhanced 2.5-fold (p < 0.0001) by 20 μM urolithin A treatment of the HEK293 cells. Thus, the VDR-enhancing capacity of urolithin A is maintained for VDREs in their DNA sequence context within transfected cells. The magnitude of 1,25D stimulation of transcription (7.6X) is slightly dampened in the instance of the CYP24A1 promoter fragment, possibly a consequence of restricted chromatin architecture with reasonably sized promoter fragments. Taken together (Fig. 2B–D), the ability of urolithin A to boost 1,25D/VDRE-mediated transcription with three different responsive elements lies in the range of 220–290%.
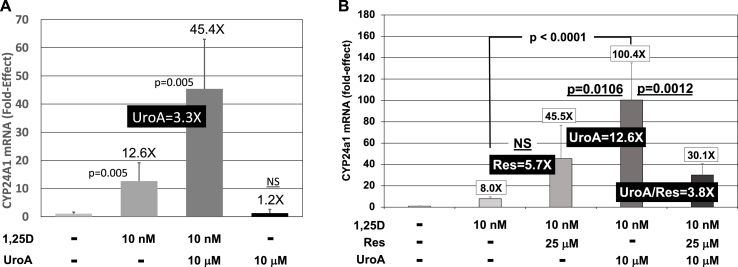
In order to determine if urolithin A is capable of amplifying 1,25D/VDR-stimulated transcription of a VDRE-containing gene in the setting of the native genome, we probed two cell lines and monitored the induction of CYP24A1 mRNA via qPCR. Fig. 3A illustrates the results from experiments in which HEK293 cells were treated with 1,25D and urolithin A followed by an assessment of CYP24A1 mRNA levels. In this experiment, 1,25D induced CYP24A1 mRNA concentrations to 12.6-fold over control. Addition of 10 μM urolithin A further elevated CYP24A1 mRNA concentrations to 3.3-fold (p = 0.005) over 1,25D-treatment alone (Fig. 3A), indicating that urolithin A is functional at boosting 1,25D-enhanced, VDR-mediated transcription in the natural chromatin setting of the CYP24A1 gene. 10 μM urolithin A alone did not statistically significantly augment cyp24a1 mRNA concentrations (Fig. 3A), indicating that urolithin A is not a low-affinity ligand for VDR. The second cell line utilized was differentiated rat serotonergic raphe (RN46A-B14) and the gene that was to be investigated was that encoding the initial enzyme in tryptophan metabolism to serotonin, tryptophan hydroxylase-2 (TPH2). However, for the purpose of comparing the action of urolithin A in HEK293 cells (Fig. 3A) with that in RN46A-B14 cells (Fig. 3B), we first evaluated the rat cyp24a1 gene for the ability of urolithin A to potentiate 1,25D-responsiveness.
Fig. 3.
Effect of 1,25D and UroA and/or Res on CYP24A1 mRNA concentrations in cultured cells. (A) Expression of CYP24A1 in human HEK293 cells treated with 1,25D (10 nM) and UroA (10 μM). Each mRNA value is the average of five biological replicates (n = 5) ± Std. Dev. (B) Expression of Cyp24a1 in serotonergic rat raphe RN46A-B14 cells treated with 1,25D (10 nM) and UroA (10 μM) and/or Res (25 μM). Each mRNA value is the average of five biological replicates (n = 5) ± Std. Dev.
As shown in Fig. 3B, 1,25D induces cyp24a1 8-fold in RN46A-B14 cells and this effect is magnified 12.6-fold by urolithin A to achieve an overall 100-fold induction of cyp24a1 with the combination of nutrient metabolites. Resveratrol was also included in this experiment as an alternative nutraceutical to compare its actions to those of urolithin A. As revealed in Fig. 3B, resveratrol appears to boost 1,25D/VDR-mediated transcription, but the potentiation is not statistically significant. Therefore, resveratrol is unable to mimic urolithin A and, in fact, it significantly attenuates urolithin A action when both nutraceuticals are added simultaneously (Fig. 3B, far right treatment group). The conclusion, at least with respect to CYP24A1, is that urolithin A uniquely and significantly potentiates VDR-driven gene induction within the context of native chromatin structure in intact cells, and does so at the half-maximal concentration of 10 μM (one-half the urolithin A concentration utilized in the transcription experiments that employed a VDRE-firefly luciferase reporter plasmid).
TPH2 is known to constitute the isoform of the rate-limiting enzyme in the pathway that catalyzes brain serotonin synthesis [26], and serotonergic neurons express predominantly the TPH2 isoenzyme [27]. Thus, RN46A-B14 is a serotonergic neuronal cell line model for investigating the modulation of serotonin synthesis in the CNS, and we have demonstrated that 1,25D induces TPH2 mRNA synthesis in this cell line [3].
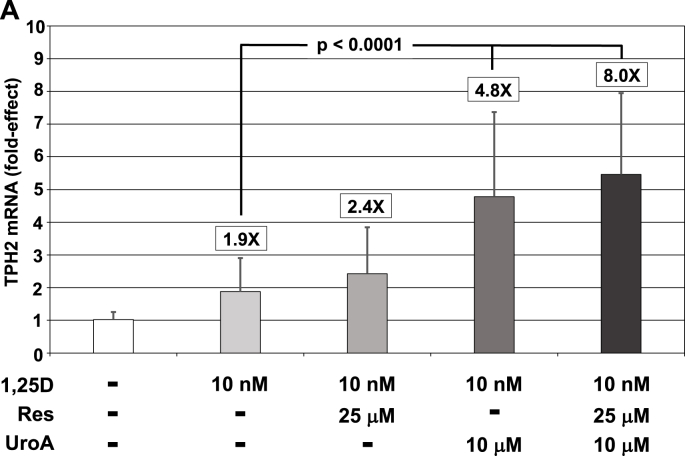
Because of our interest in the behavioral influences of vitamin D and other micronutrients, we next examined the effect of urolithin A on TPH2 induction by 1,25D. Employing the RN46A-B14 serotonergic cell line, 10 μM urolithin A was able to statistically significantly augment the 1,25D-response of TPH2 mRNA two and one-half times over 1,25D alone, achieving an overall 4.8-fold enhancement of TPH2 mRNA compared to control (Fig. 4A). The pattern of TPH2 mRNA-responsiveness in RN46A-B14 cells resembles that of cyp24a1 (Fig. 3B) in that 1,25D elicits a non-statistically significant, 1.9-fold increase in TPH2 mRNA and resveratrol is unable to mimic urolithin A in boosting 1,25D/VDR-mediated transcription. However, in the case of TPH2 mRNA in RN46A-B14 cells (Fig. 4A), resveratrol does not attenuate urolithin A-potentiated expression as it did for cyp24a1 (Fig. 3B). Therefore, based on the data pictured in Fig. 4A, only urolithin A is capable of significantly augmenting 1,25D/VDR-mediated induction of TPH2 mRNA in serotonergic neuronal cells.
Fig. 4.
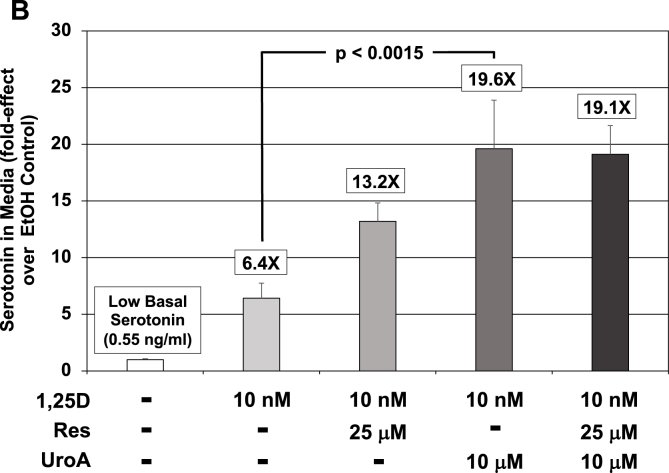
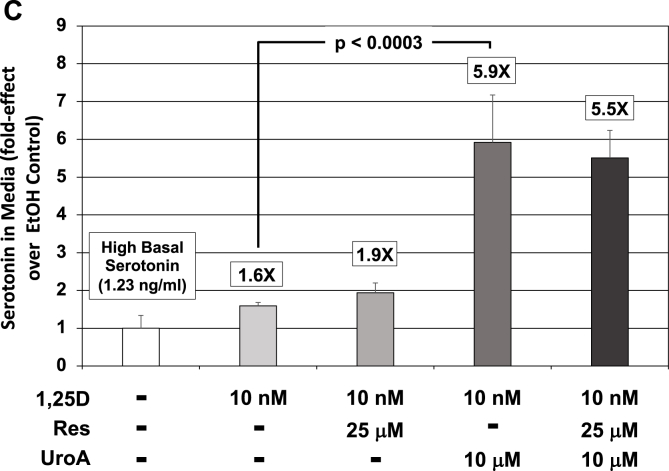
Effect of 1,25D and UroA and/or Res on TPH2 mRNA concentrations in serotonergic rat raphe RN46A-B14 cells as well as on total serotonin (5-HT) levels in the neuronal cell culture medium. (A) Expression of TPH2 in serotonergic rat raphe RN46A-B14 cells treated with 1,25D (10 nM) and UroA (10 μM) and/or Res (25 μM). Each mRNA value is the average of 19 biological replicates ± Std. Dev. Fold-effect over EtOH/vehicle control is listed at the top of each bar. Only UroA and UroA + Res are capable of statistically significantly elevating TPH2 mRNA level above that achieved by 1,25D treatment alone. (B) Serotonin (5-HT) levels in low basal serotonin (0.55 ng/ml) RN46A-B14 cell culture medium in response to treatment with 1,25D (10 nM) and UroA (10 μM) and/or Res (25 μM). A 200 μL aliquot of medium was removed from each plate after 72 h of treatment and 5-HT quantitated by ELISA as detailed in Methods. Values are the average of three biological replicates (n = 3) ± Std. Dev., with duplicate or triplicate assay samples averaged in each group. Fold-effect over EtOH/vehicle control is listed at the top of each bar. Employing ANOVA, only UroA is capable of statistically significantly (p = 0.0015) elevating 5-HT level above that achieved by 1,25D treatment alone; all other values are not statistically significantly different from their respective controls. (C) Serotonin (5-HT) levels in high basal serotonin (1.23 ng/ml) RN46A-B14 cell culture medium in response to treatment with 1,25D (10 nM) and UroA (10 μM) and/or Res (25 μM). A 200 μL aliquot of medium was removed from each plate after 72 h of treatment and 5-HT quantitated by ELISA as detailed in Methods. Values are the average of three biological replicates (n = 3) ± Std. Dev., with duplicate or triplicate assay samples averaged in each group. Fold-effect over EtOH/vehicle control is listed at the top of each bar. Employing ANOVA, only UroA is capable of statistically significantly (p = 0.0003) elevating 5-HT level above that achieved by 1,25D treatment alone; all other values are not statistically significantly different from their respective controls.
To determine if the modulation of TPH2 mRNA in RN46A-B14 cells (Fig. 4A) is reflected in the absolute levels of serotonin present in the cell media, we next employed ELISA to quantitate culture media serotonin concentrations in experiments identical to those documented in Fig. 4A. The results of two experiments appear in Fig. 4B and C. In the first experiment, wherein basal serotonin levels were relatively low (0.55 ng/ml) in the media, 1,25D stimulated the neurotransmitter 6.4-fold over the control, and urolithin A statistically significantly magnified this effect of 1,25D to 19.6-fold over control, or a 3.1-fold potentiation by urolithin A (Fig. 4B). This boost in serotonin accomplished by urolithin A is quite comparable to the 2.5-fold elevation in TPH2 mRNA, indicating that the serotonin levels do indeed reflect the increment in TPH2 mRNA achieved by urolithin A (Fig. 4A), consistent with the major action of 1,25D/urolithin A occurring via transcription at the rate-limiting enzyme in the serotonin pathway.
Moreover, as with the TPH2 mRNA data for resveratrol (Fig. 4A), resveratrol is unable to mimic urolithin A in boosting serotonin concentrations in a statistically significant manner, and resveratrol neither attenuates nor amplifies urolithin A-potentiated serotonin (Fig. 4B). This observation indicates that urolithin A stands-out as a novel nutraceutical in support of vitamin D action in neuronal cell lines. Interestingly, in the second experiment wherein basal serotonin levels were relatively high (1.23 ng/ml) in the media (Fig. 4C), 1,25D stimulated the neurotransmitter only 1.6-fold over the control, but urolithin A still statistically significantly magnified this effect of 1,25D to 5.9-fold over control, or a 3.7-fold potentiation (Fig. 4C). Clearly, the urolithin A potentiation effect is independent of the prevailing serotonin production rate, although the capacity of 1,25D to induce serotonin synthesis appears to be dampened under conditions of high TPH2 expression, as we have observed a wide range of magnitudes for the 1,25D effect of inducing TPH2 in RN46A-B14 cells [3,23]. Finally, as with low basal serotonin, resveratrol is unable to mimic urolithin A in statistically significantly boosting serotonin concentration and resveratrol neither attenuates nor amplifies urolithin A-potentiated serotonin concentration (Fig. 4C).
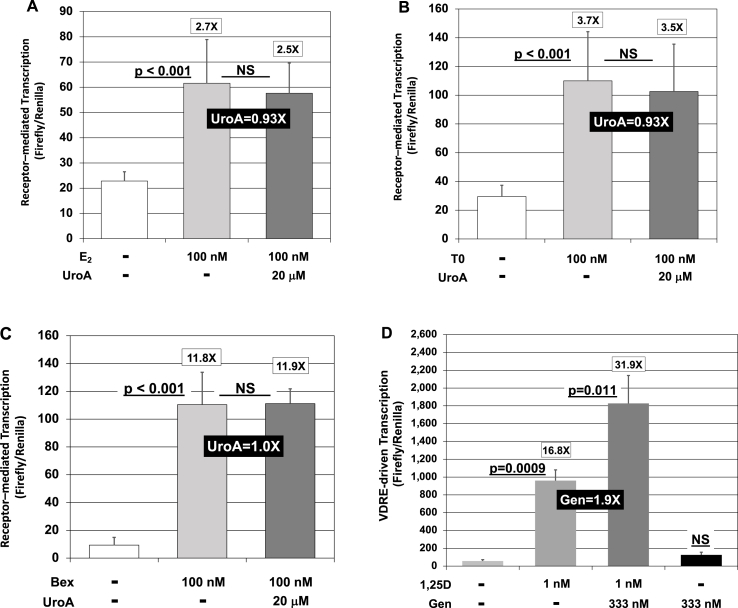
Finally, we performed several experiments to evaluate further the newly observed action of urolithin A on 1,25D/VDR-induced transcription. The possible influence of urolithin A co-treatment on the action of other nuclear receptors was examined as depicted in Fig. 5A-C, in particular for the estrogen receptor (ER), liver X receptor (LXR) and retinoid X receptor (RXR), respectively. As can be seen in the results presented in Fig. 5A–C, none of these selected other nuclear receptors are affected in their ligand-dependent stimulation of transcription by co-administration of urolithin A, and urolithin A does not function as a surrogate ligand for any of the three selected receptors (data not shown). Thus, VDR remains the only nuclear receptor tested to date for which activation is potentiated, suggesting at least some specificity.
Fig. 5.
UroA has no impact on ligand-triggered transcriptional activation mediated by ER/ERE, LXR/LXRE and RXR/RXRE, but gedunin mimics UroA in potentiating transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 10 nM 1,25D. (A) Influence of UroA on HEK293 cells transiently transfected with an ERE-reporter construct and exposed in culture to either EtOH/DMSO vehicle or a final concentration of 100 nM estradiol-17β (Ε2) or 20 μM UroA + E2 for 24 h. Transcription was assessed by luciferase activity as described in Methods. Values are the average of nine biological replicates (n = 9) ± Std. Dev. (B) Influence of UroA on HEK293 cells transiently transfected with an LXRE-reporter construct and exposed in culture to either EtOH/DMSO vehicle or a final concentration of 100 nM synthetic LXR agonist T0901317 (Τ0), a non-steroidal synthetic ligand composed of a tertiary sulfonamide and a bistrifluoromethyl carbinol or 20 μM UroA + T0 for 24 h. Transcription was assessed by luciferase activity as described in Methods. Values are the average of nine biological replicates (n = 9) ± Std. Dev. (C) Influence of UroA on HEK293 cells transiently transfected with an RXRE-reporter construct and exposed in culture to either EtOH/DMSO vehicle or a final concentration of 100 nM bexarotene (Bex, synthetic RXR ligand) or 20 μM UroA + Bex for 24 h. Transcription was assessed by luciferase activity as described in Methods. Values are the average of eleven biological replicates (n = 11) ± Std. Dev. (D) Effect of the tetranortriterpenoid, gedunin (Gen; purchased from Santa Cruz Biotechnology, Santa Cruz, CA), on transcriptional activation driven by the XDR3 VDRE in HEK293 cells treated with 1,25D. Cells were exposed in culture to either EtOH/DMSO vehicle or a final concentration of 1 nM 1,25D, 333 nM Gen (alone), or both 1,25D and Gen for 24 h and transcription assessed by luciferase activity as described in Methods. Values are the average of three biological replicates (n = 3) ± Std. Dev., and the experiment was repeated once with essentially identical results (data not shown).
However, specificity for urolithin A with respect to the adjuvant nutraceutical in question is not maintained in the case of VDR, as several alternative polyphenol nutrients are also reported to boost VDR action. In Fig. 5D, we report that gedunin, a proposed neuroprotective nutrient isolated from Indian neem tree (Azadirachta Indica) that functions as an agonist for TrkB [28], a receptor for brain derived neurotropic factor (BDNF), appears to be analogous to urolithin A in potentiating 1,25D action, likely via the alternative mechanism of impacting the BDNF pathway. This finding is relevant to the present urolithin A story in that we have highlighted the apparent neural significance of the potentiation of 1,25D/VDR action on serotonin levels, another aspect of the effects of 1,25D/VDR on the CNS.
4. Discussion
It has been proposed that there exists a significant relationship between vitamin D and the tryptophan-derived neurotransmitter, serotonin [3,23]. Herein we report data on the influence of several natural compounds on the ability of the vitamin D hormonal metabolite, 1,25D, to induce the serotonergic neuron-expressed initial enzyme in tryptophan metabolism to serotonin, namely TPH2. In the process, we uncovered two novel natural products, urolithin A and gedunin, that function as potentiators of the action of 1,25D to stimulate gene expression via VDR/VDREs. More specifically, we targeted urolithin A for an in-depth analysis of its ability to interact with the vitamin D neuroendocrine system. There are many instances of nutrient interactions significant to biology and disease prevention, with the vitamin B12-folate connection being an exemplar of two essential nutrients cooperating to ensure CNS development, health, and protection against anemia and the ravages of homocysteine excess associated with deranged methylation [29]. The specific discovery reported herein is that both urolithin A and gedunin potentiate transactivation of gene expression by 1,25D, and may do so by allosterically enhancing the activity of VDR, the nuclear receptor for the 1,25D-ligand. Therefore, we propose that urolithin A and gedunin join the group of phytochemicals including polyphenols, terpenoids, etc., that were reviewed recently [30] and cited as beneficial mimics of caloric restriction and exercise in delaying aging and preventing chronic diseases. Indeed, we have put forth the thesis that vitamin D also displays actions that appear to increase health-span and delay chronic diseases [7].
The data herein reveal that urolithin A is likely not a low-affinity ligand for the VDR (Fig. 1A–D), but that the effect of urolithin A on 1,25D/VDR-directed gene induction is urolithin A concentration-dependent (Fig. 2B), occurs with multiple different bona fide VDREs (Fig. 2, Fig. 3A), is selective for a VDR-VDRE over other nuclear receptor-responsive element pairs such as ER-ERE (Fig. 5A), LXR-LXRE (Fig. 5B) and RXR-RXRE (Fig. 5C), and takes place in intact cells in culture to yield augmentation of the appropriate mRNA (Fig. 3AB, 4A). The magnitude of the urolithin A-triggered boost in 1,25D action ranges from 1.6-fold to 2.9-fold with an average of 2.2-fold as quantitated with an isolated VDRE through transactivation studies, in vitro. This urolithin A-elicited boost in 1,25D action rises to a range from 2.5-fold to 12.6-fold with an average of 7.8-fold when measured as mRNA concentrations in intact cells. It should be noted that the 2.2-fold potentiation of transcription attained by adding urolithin A is relatively small in comparison with the 10- to 20-fold primary induction produced by 1,25D hormone (alone) treatment, although a modifier would be expected to have a smaller effect than the primary hormonal ligand. Thus, a 2.2-fold boost by urolithin A is reasonable considering its modulatory role. In the case of TPH2-augmentation by urolithin A, it is important to examine the relevant neurotransmitter end product, serotonin. Notably, as exhibited in Fig. 4A (TPH2 mRNA levels) versus Fig. 4BC (serotonin concentrations), there exists a corresponding profile of urolithin A effects on TPH2 mRNA and on absolute serotonin concentrations. This strikingly similar pattern is indicative of the significance of TPH2 transcription in determining serotonin levels, and suggests that a combination of 1,25D and urolithin A is an attractive candidate to raise serotonin in the CNS and perhaps improve mood.
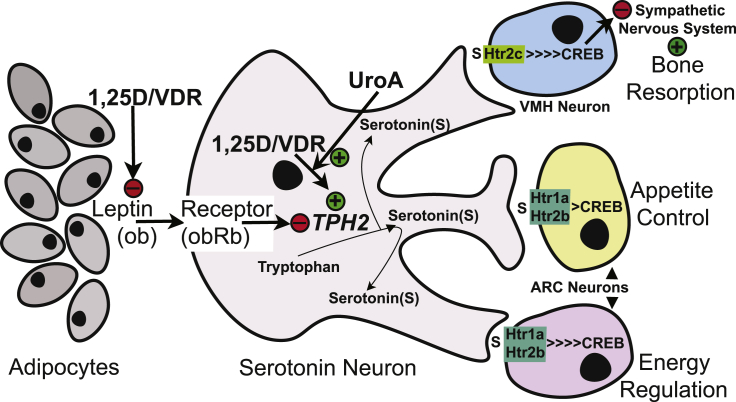
In a previous publication [3], we reported that 1,25D induces TPH2 gene expression in differentiated serotonergic rat raphe cells, a result that was supported by in vivo experiments [31] demonstrating an increase of TPH2 mRNA in rat prefrontal cortex during long-term 1,25D dosing. Moreover, in our 2015 publication [3], we reported that 1,25D acted in mouse adipocytes to suppress leptin expression. This observation is relevant as leptin is a well-recognized satiety and energy metabolism hormone whose endocrine functions overlap several serotonin actions, such as regulating appetite and energy expenditure. In fact, there exists a serotonin-dependent neuronal relay through which leptin regulates bone mass, appetite, and energy expenditure [32]. Fig. 6 illustrates a model that places urolithin A-fueled 1,25D actions into the context of human neurophysiology/neuropharmacology as executed by the serotonin-dependent neuronal relay that mediates leptin regulation of bone mass, appetite and energy expenditure. Leptin inhibits synthesis and release of brain-derived serotonin, favoring bone mass accrual, appetite, and energy metabolism [32]. As depicted in Fig. 6 in schematic form, 1,25D represses adipocyte leptin and induces TPH2 to potentiate serotonin relay signaling [3] via serotonergic nerve transmission at synapses in the cerebral cortex. Analogous to the many pathways of leptin activity, serotonin exerts far-reaching actions beyond those of the neuronal relay that governs appetite, bone mass, and energy expenditure. Serotonin functions include governing: executive behavior, sensory gating, prosocial demeanor, etc., and serotonin concentrations are frequently depressed in dysfunctions of these actions. The present results reiterate the potential role of 1,25D to induce TPH2 mRNA and for the novel nutrient metabolite, urolithin A, to further enhance TPH2 gene expression in cultured neuronal cells. If these nutrient effects maintain serotonin output by serotonergic neurons in the intact CNS, then urolithin A and/or 1,25D become candidates as new pharmacologic agents to positively affect behavior. However, neither urolithin A nor 1,25D have been tested for their ability to alter serotonin in humans, although it was recently reported that a low vitamin D diet reduces cerebral serotonin concentration in mature female mice, in vivo [33].
Fig. 6.
A model for the actions of 1,25D/VDR and its modulation by UroA on the serotonin-dependent neuronal relay that mediates leptin regulation of bone mass, appetite and energy expenditure. Leptin normally inhibits synthesis and release of brain-derived serotonin, favoring bone mass accrual, appetite, and energy metabolism. 1,25D represses adipocyte leptin and induces TPH2 to potentiate serotonin relay signaling via serotonergic nerve transmission at synapses in the cerebral cortex. Serotonin action is spurred by 1,25D and amplified by UroA at both presynaptic and postsynaptic sites through Type 1A and 2A serotonin receptors, respectively. VMH, ventromedial hypothalamus; ARC, arcuate nucleus.
Nevertheless, at least within the confines of a select cell culture model system, the current results reinforce the vitamin D-serotonin connection and extend it to the level of pharmacological intervention to enhance the concentration of beneficial nutrients, specifically urolithin A. The fact that when the generation of serotonin in neuronal cell culture was monitored after treatment with 10 nM 1,25D, serotonin concentrations were escalated (1.6- to 6.4-fold) in the medium (Fig. 4BC), and total serotonin was amplified statistically significantly to 5.9- to 19.6-fold over baseline when the cells were exposed to 10 μMurolithin A along with 10 nM 1,25D (Fig. 4BC), compels us to propose that the combination of 1,25D and urolithin A may be able to boost serotonin in the central nervous system. Therefore, we propose that the present hypothesis be tested through extensive behavioral and metabolic experiments, in vivo. Indeed, urolithin A actions have recently been reported [20] in a clinical study of sedentary human subjects, revealing that following consumption of urolithin A, recipients experienced improved skeletal muscle mitochondrial and cellular health akin to that of control subjects who exercised regularly. It is not known at present if urolithin A levels consistent with disease prevention and aging suppression can be achieved by ingesting rich sources of ellagitannins such as pomegranate, red and black berries, as well as certain nuts (e.g., pecans, walnuts, etc.). Thus, the best approach to raising ambient urolithin A concentrations in animals and humans most likely is direct consumption of purified urolithin A, as was done safely by Ryu and colleagues [19] and Andreux and associates [20], respectively. In the human investigation, circulating levels of urolithin A that were in the majority (69%) of subjects undetectable, rose to 2–6 nM in the 24-h period after consumption of 250–1000 mg purified urolithin A and then decayed to pretreatment concentrations by 72–96 h. These values suggest that the concentrations of urolithin A employed in cell culture experiments reported herein were approximately three orders of magnitude greater than those achievable, in vivo, but such a ratio (1000:1) is typical for cell culture-based investigations. In conclusion, the current investigation is presented not as a biological/nutritional endeavor but rather as a pharmacological investigation of the potential impact of urolithin A on vitamin D receptor function.
Follow-up studies should probe the underlying molecular mechanism(s) by which urolithin A enhances the transcriptional activity of 1,25D/VDR. The general mechanism whereby 1,25D/VDR induces gene expression is well-understood, as liganded VDR heterodimerizes with RXR in order to bind cognate VDREs in the vicinity of vitamin D-regulated genes, subsequently attracting transcriptional coactivators that catalytically modify and loop-out chromatin to stimulate gene expression [6]. However, little is known about the specific molecular events whereby 1,25D transcriptionally regulates TPH2, particularly whether liganded VDR-RXR functions via a primary (TPH2 VDRE-mediated) or secondary (induction of an intermediary protein that actually governs TPH2 mRNA expression) molecular mechanism. To date, putative VDREs in human TPH2 [3] have not been shown to be occupied by VDR-RXR through definitive (in vivo) chromatin immunoprecipitation experiments, leaving open the possibility of a secondary mechanism. With respect to TPH2, it is established that the primary inducer, in vivo, is the stress response and resulting glucocorticoid release [34]. A transcriptional element has been characterized in the TPH2 promoter [35] that contains a binding site for a silencer of transcription to restrict expression of non-neuronal genes. Therefore, it is also conceivable that 1,25D/VDR either supports glucocorticoid/GR function or is able to interrupt the silencing mechanism that is normally in effect during the neuronal development program. Regardless of these details, the real question is how does urolithin A function molecularly to potentiate TPH2 induction by 1,25D/VDR?
No receptor has been identified for urolithin A, although its most prominent signal transduction pathway is that of AMPK-activation [36], and urolithin A appears to turn-on AMPK either by binding directly to a subunit of the enzyme complex or by stimulating two upstream protein kinases, LKB1 and Ca2+/calmodulin-dependent protein kinase β (CaMKKβ), which have been shown to phosphorylate Thr-172 of the AMPKα subunit [36]. Alternatively, urolithin A may indirectly activate AMPK in a fashion analogous to that of other phytochemicals such as curcumin and resveratrol, which bind to and inhibit the F1F0–ATPase/ATP synthase complex in mitochondria, resulting in enhanced AMP/ATP ratio and binding of AMP to the AMPKγ2 subunit [36]. Regardless of the exact mechanism of AMPK activation, as demonstrated by Ryu et al. [19], life extension by urolithin A is heavily dependent on AMPK, because only a minimal increase in lifespan occurs in AMPK homolog mutant (aak-2) C. elegans treated with urolithin A. Urolithin A also enhances muscle function in mice, primarily by causing mitophagy and inducing mitochondrial biogenesis to improve the quality of muscle cells rather than their quantity, resulting in increased muscle strength and endurance without elevating lean muscle mass [19]. Interestingly, vitamin D and VDR are believed to be required for optimal muscle strength [37], unveiling one possible common action of urolithin A and vitamin D wherein the amplification of 1,25D function by urolithin A may be relevant to human health. In addition, vitamin D has recently been shown to regulate steroidogenesis in granulosa cells through AMPK activation in a mouse model of polycystic ovary syndrome [38]. This finding establishes a mechanism whereby urolithin A could potentiate 1,25D signaling, namely by boosting active AMPK, a signal transduction intermediate common to both pathways.
Following its illumination as a central regulator of cellular bioenergetics, AMPK has become known as the biochemical orchestrator of a variety of physiological actions such as autophagy, inflammation, and metabolic reprogramming during mycobacterial infection [39]. Indeed, the immune system represents yet another nexus wherein 1,25D-action and AMPK signaling (triggered by urolithin A) cooperate for biological defense against cellular dysfunction, chronic disease and limited lifespan. Therefore, a pattern is emerging whereby exercise, intermittent fasting, and phytochemicals that mimic such behaviors, exert their effects through the central mediator, AMPK [30]. Moreover, sirtuin-1 (SIRT1) appears to function as a partner to AMPK in executing biological protection from cellular dysfunction and the diseases of aging [30]. Pertinent to the involvement of vitamin D in the marshaling of bioprotective mechanisms, SIRT1 has recently been implicated as a potentiator of 1,25D/VDR action [13]. Furthermore, as proposed by Martel et al. [30], combined AMPK and SIRT1 signaling achieves four desirable cellular defense objectives: 1) activating FOXO for stress resistance, 2) prompting Nrf2 to generate antioxidant fortifications, 3) inducing PGC-1α to promote mitochondrial biogenesis, and 4) opposing mTOR to elicit autophagy. 1,25D/VDR dove-tails nicely with these four avenues of cellular protection through AMPK/SIRT1 cooperation. As one example, 1,25D-bound VDR induces FOXO3 [7,40], a human longevity gene, the product of which controls cellular metabolism and resists oxidative stress. As a second example of VDR actions to oppose oxidative damage, 1,25D has been reported to induce the expression of nuclear factor-erythroid-2-related factor 2 (Nrf2) [13,41].
Finally, although there exists a plethora of signaling avenues whereby the potentiation of 1,25D/VDR by urolithin A could come into play, we still do not know exactly how this potentiation is accomplished at the molecular level. Assuming that AMPK is the central mediator of this action, then the most logical hypothesis is that AMPK directly phosphorylates VDR to boost its transcriptional potency. Indeed, there are six consensus phosphorylation sites for AMPK catalytic action that can be identified bioinformatically in human VDR. These include Ser-9 (the strongest consensus AMPK site), which may be in a relevant position because we have previously shown that the N-terminal amino acids in human VDR are responsible for recruiting TFIIB and thereby enhancing the transcriptional potency of the receptor [42]. A more negative charge imparted on the N-terminus of VDR likely renders it a superior trans-activator by virtue of a newly acquired “acid-blob” domain [43]. The other five amino acid residues in human VDR that qualify as AMPK sites are: Ser-177, Ser-179, Ser-183, Thr-192 and Ser-193, all positioned in close proximity within the “hinge” region of VDR. Located just C-terminal of this stretch of putative VDR phosphorylation sites lies Ser-208, the most prominent phosphorylated residue in human VDR [44]. Phosphorylation of Ser-208 by casein kinase II enhances the trans-activation capacity of VDR [45] by increasing its interaction with coactivators [46,47]. This fact lends credence to the possibility that AMPK-catalyzed phosphorylation of one or more of the consensus residues between Ser-177 and Ser-193 could embody an analogous mechanism through which urolithin A signaling via AMPK potentiates VDR trans-activation. However, a separate publication reporting that PKA-catalyzed phosphorylation of Ser-182 in hVDR attenuates transcriptional activation by VDR [48] tends to support the notion that the more likely AMPK phosphorylation site in human VDR is Ser-9. Additional studies are required to elucidate just how AMPK cross-talks to VDR. Finally, AMPK may influence downstream effectors of VDR function, such as Nrf2. AMPK is reported to phosphorylate Nrf2 to drive its nuclear accumulation and facilitate antioxidant signaling [49], creating yet another mechanism by which urolithin A and 1,25D may cooperate, given that 1,25D is an established inducer of Nrf2 [13,41].
Author statement
SL initiated the study design, and carried through the data collection, data analysis/interpretation, and developed a preliminary version of the manuscript in poster format. SM, DAL, MSS, ZLS, HP, and SN contributed to the data collection/analysis. CAH contributed to experimental insight as well as to the independent revision of the article. MRH, the corresponding author, contributed via conception/design of the work and wrote the initial version, as well as the final revisions of the manuscript. PWJ, the senior investigator directing the study, contributed via conception/design of work, data interpretation, and critical revision of the article. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers: DK033351 (to MRH) and CA140285 (to PWJ)].
References
- 1.Haussler M.R., Whitfield G.K., Kaneko I., Haussler C.A., Hsieh D., Hsieh J.C., Jurutka P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 2.Jurutka P.W., Whitfield G.K., Forster R., Batie S., Lee J., Haussler M.R. Vitamin D: a fountain of youth in gene regulation. In: Gombart A.F., editor. Vitamin D: Oxidative Stress, Immunity, and Aging. CRC Press; Boca Raton: 2013. pp. 3–35. [Google Scholar]
- 3.Kaneko I., Sabir M.S., Dussik C.M., Whitfield G.K., Karrys A., Hsieh J.C., Haussler M.R., Meyer M.B., Pike J.W., Jurutka P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. Faseb. J. 2015;29:4023–4035. doi: 10.1096/fj.14-269811. [DOI] [PubMed] [Google Scholar]
- 4.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)vitamin D: genomic and non-genomic mechanisms. Best practice & research. Clinical endocrinology & metabolism. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.M., Riley E.M., Meyer M.B., Benkusky N.A., Plum L.A., DeLuca H.F., Pike J.W. 1,25-Dihydroxyvitamin D3 controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for calcium-regulating components. J. Biol. Chem. 2015;290:18199–18215. doi: 10.1074/jbc.M115.665794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.-C., Jurutka P.W. In: Nuclear vitamin D receptor: natural ligands, molecular structure-function, and transcriptional control of vital genes. 3 Ed. Vitamin D., Feldman D., Pike J.W., Adams J., editors. Academic Press; San Diego: 2011. pp. 137–170. [Google Scholar]
- 7.Haussler M.R., Whitfield G.K., Haussler C.A., Sabir M.S., Khan Z., Sandoval R., Jurutka P.W. 1,25-Dihydroxyvitamin D and klotho: a tale of two renal hormones coming of age. Vitam. Horm. 2016;100:165–230. doi: 10.1016/bs.vh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Bartik L., Whitfield G.K., Kaczmarska M., Lowmiller C.L., Moffet E.W., Furmick J.K., Hernandez Z., Haussler C.A., Haussler M.R., Jurutka P.W. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. JNB (J. Nutr. Biochem.) 2010;21:1153–1161. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurutka P.W., Bartik L., Whitfield G.K., Mathern D.R., Barthel T.K., Gurevich M., Hsieh J.C., Kaczmarska M., Haussler C.A., Haussler M.R. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J. Bone Miner. Res. 2007;22(Suppl 2):V2–V10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- 10.Hoss E., Austin H.R., Batie S.F., Jurutka P.W., Haussler M.R., Whitfield G.K. Control of late cornified envelope genes relevant to psoriasis risk: upregulation by 1,25-dihydroxyvitamin D and plant-derived delphinidin. Arch. Dermatol. Res. 2013;305:867–878. doi: 10.1007/s00403-013-1390-1. [DOI] [PubMed] [Google Scholar]
- 11.Austin H.R., Hoss E., Batie S.F., Moffet E.W., Jurutka P.W., Haussler M.R., Whitfield G.K. Regulation of late cornified envelope genes relevant to psoriasis risk by plant-derived cyanidin. Biochem. Biophys. Res. Commun. 2014;443:1275–1279. doi: 10.1016/j.bbrc.2013.12.128. [DOI] [PubMed] [Google Scholar]
- 12.Dampf-Stone A., Batie S., Sabir M., Jacobs E.T., Lee J.H., Whitfield G.K., Haussler M.R., Jurutka P.W. Resveratrol potentiates vitamin D and nuclear receptor signaling. J. Cell. Biochem. 2014;116:1130–1143. doi: 10.1002/jcb.25070. [DOI] [PubMed] [Google Scholar]
- 13.Sabir M.S., Khan Z., Hu C., Galligan M.A., Dussik C.M., Mallick S., Stone A.D., Batie S.F., Jacobs E.T., Whitfield G.K., Haussler M.R., Heck M.C., Jurutka P.W. SIRT1 enzymatically potentiates 1,25-dihydroxyvitamin D3 signaling via vitamin D receptor deacetylation. J. Steroid Biochem. Mol. Biol. 2017;172:117–129. doi: 10.1016/j.jsbmb.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Z., Yoshihara E., He N., Hah N., Fan W., Pinto A.F.M., Huddy T., Wang Y., Ross B., Estepa G., Dai Y., Ding N., Sherman M.H., Fang S., Zhao X., Liddle C., Atkins A.R., Yu R.T., Downes M., Evans R.M. Vitamin D switches BAF complexes to protect beta cells. Cell. 2018;173:1135–1149. doi: 10.1016/j.cell.2018.04.013. e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larrosa M., Gonzalez-Sarrias A., Yanez-Gascon M.J., Selma M.V., Azorin-Ortuno M., Toti S., Tomas-Barberan F., Dolara P., Espin J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Sarrias A., Larrosa M., Tomas-Barberan F.A., Dolara P., Espin J.C. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010;104:503–512. doi: 10.1017/S0007114510000826. [DOI] [PubMed] [Google Scholar]
- 17.Ishimoto H., Shibata M., Myojin Y., Ito H., Sugimoto Y., Tai A., Hatano T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011;21:5901–5904. doi: 10.1016/j.bmcl.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 18.Gong Z., Huang J., Xu B., Ou Z., Zhang L., Lin X., Ye X., Kong X., Long D., Sun X., He X., Xu L., Li Q., Xuan A. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflammation. 2019;16:62. doi: 10.1186/s12974-019-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Felix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., Aebischer P., Sandi C., Rinsch C., Auwerx J. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 20.Andreux P.A., Blanco-Bose W., Ryu D., Burdet F., Ibberson M., Aebischer P., Auwerx J., Singh A., Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nature Metabolism. 2019;1:595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira G.B., Vanherwegen A.S., Eelen G., Gutierrez A.C.F., Van Lommel L., Marchal K., Verlinden L., Verstuyf A., Nogueira T., Georgiadou M., Schuit F., Eizirik D.L., Gysemans C., Carmeliet P., Overbergh L., Mathieu C. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Rep. 2015;10:711–725. doi: 10.1016/j.celrep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Haussler M.R., Haussler C.A., Whitfield G.K., Hsieh J.C., Thompson P.D., Barthel T.K., Bartik L., Egan J.B., Wu Y., Kubicek J.L., Lowmiller C.L., Moffet E.W., Forster R.E., Jurutka P.W. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the "Fountain of Youth" to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010;121:88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabir M.S., Haussler M.R., Mallick S., Kaneko I., Lucas D.A., Haussler C.A., Whitfield G.K., Jurutka P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018;13:19. doi: 10.1186/s12263-018-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro B., Sanchez P., Torres J.M., Ortega E. Effects of adult exposure to bisphenol a on genes involved in the physiopathology of rat prefrontal cortex. PloS One. 2013;8 doi: 10.1371/journal.pone.0073584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson P.D., Jurutka P.W., Whitfield G.K., Myskowski S.M., Eichhorst K.R., Dominguez C.E., Haussler C.A., Haussler M.R. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem. Biophys. Res. Commun. 2002;299:730–738. doi: 10.1016/s0006-291x(02)02742-0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Beaulieu J.M., Sotnikova T.D., Gainetdinov R.R., Caron M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 27.Gutknecht L., Kriegebaum C., Waider J., Schmitt A., Lesch K.P. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur. Neuropsychopharmacol. 2009;19:266–282. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 28.English A.W., Liu K., Nicolini J.M., Mulligan A.M., Ye K. Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16217–16222. doi: 10.1073/pnas.1303646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D'Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 30.Martel J., Ojcius D.M., Ko Y.F., Ke P.Y., Wu C.Y., Peng H.H., Young J.D. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol. Metabol. 2019;30:335–346. doi: 10.1016/j.tem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Jiang P., Zhang L.H., Cai H.L., Li H.D., Liu Y.P., Tang M.M., Dang R.L., Zhu W.Y., Xue Y., He X. Neurochemical effects of chronic administration of calcitriol in rats. Nutrients. 2014;6:6048–6059. doi: 10.3390/nu6126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metabol. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Miller J.W., Bello N.T., Shapses S.A. Low vitamin D diet lowers cerebral serotonin concentration in mature female mice. Nutr. Res. 2020;81:71–80. doi: 10.1016/j.nutres.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Chen G.L., Miller G.M. Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin-stress interaction and clinical implications. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:152–171. doi: 10.1002/ajmg.b.32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel P.D., Bochar D.A., Turner D.L., Meng F., Mueller H.M., Pontrello C.G. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron restrictive silencing factor (REST/NRSF) binding motif. J. Biol. Chem. 2007;282:26717–26724. doi: 10.1074/jbc.M705120200. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Yang G., Kim Y., Kim J., Ha J. AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med. 2016;48:e224. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girgis C.M., Cha K.M., Houweling P.J., Rao R., Mokbel N., Lin M., Clifton-Bligh R.J., Gunton J.E. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif. Tissue Int. 2015;97:602–610. doi: 10.1007/s00223-015-0054-x. [DOI] [PubMed] [Google Scholar]
- 38.Bakhshalizadeh S., Amidi F., Shirazi R., Shabani Nashtaei M. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase (AMPK) activation in a mouse model of polycystic ovary syndrome. Cell Biochem. Funct. 2018;36:183–193. doi: 10.1002/cbf.3330. [DOI] [PubMed] [Google Scholar]
- 39.Jo E.K., Silwal P., Yuk J.M. AMPK-targeted effector networks in mycobacterial infection. Front. Microbiol. 2019;10:520. doi: 10.3389/fmicb.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eelen G., Verlinden L., Meyer M.B., Gijsbers R., Pike J.W., Bouillon R., Verstuyf A. 1,25-Dihydroxyvitamin D3 and the aging-related forkhead box O and sestrin proteins in osteoblasts. J. Steroid Biochem. Mol. Biol. 2013;136:112–119. doi: 10.1016/j.jsbmb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Nakai K., Fujii H., Kono K., Goto S., Kitazawa R., Kitazawa S., Hirata M., Shinohara M., Fukagawa M., Nishi S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014;27:586–595. doi: 10.1093/ajh/hpt160. [DOI] [PubMed] [Google Scholar]
- 42.Jurutka P.W., Remus L.S., Whitfield G.K., Thompson P.D., Hsieh J.-C., Zitzer H., Tavakkoli P., Galligan M.A., Dang H.T., Haussler C.A., Haussler M.R. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 2000;14:401–420. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 43.Sigler P.B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988;333:210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- 44.Jurutka P.W., Hsieh J.-C., MacDonald P.N., Terpening C.M., Haussler C.A., Haussler M.R., Whitfield G.K. Phosphorylation of serine 208 in the human vitamin D receptor: the predominant amino acid phosphorylated by casein kinase II, in vitro, and identification as a significant phosphorylation site in intact cells. J. Biol. Chem. 1993;268:6791–6799. [PubMed] [Google Scholar]
- 45.Jurutka P.W., Hsieh J.-C., Nakajima S., Haussler C.A., Whitfield G.K., Haussler M.R. Human vitamin D receptor phosphorylation by casein kinase II at ser-208 potentiates transcriptional activation. Proceedings of the National Academy of Sciences U.S.A. 1996;93:3519–3524. doi: 10.1073/pnas.93.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barletta F., Freedman L.P., Christakos S. Enhancement of VDR-mediated transcription by phosphorylation: correlation with increased interaction between the VDR and DRIP205, a subunit of the VDR-interacting protein coactivator complex. Mol. Endocrinol. 2002;16:301–314. doi: 10.1210/mend.16.2.0764. [DOI] [PubMed] [Google Scholar]
- 47.Arriagada G., Paredes R., Olate J., van Wijnen A., Lian J.B., Stein G.S., Stein J.L., Onate S., Montecino M. Phosphorylation at serine 208 of the 1alpha,25-dihydroxy Vitamin D3 receptor modulates the interaction with transcriptional coactivators. J. Steroid Biochem. Mol. Biol. 2007;103:425–429. doi: 10.1016/j.jsbmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh J.C., Dang H.T., Galligan M.A., Whitfield G.K., Haussler C.A., Jurutka P.W., Haussler M.R. Phosphorylation of human vitamin D receptor serine-182 by PKA suppresses 1,25(OH)(2)D(3)-dependent transactivation. Biochem. Biophys. Res. Commun. 2004;324:801–809. doi: 10.1016/j.bbrc.2004.09.139. [DOI] [PubMed] [Google Scholar]
- 49.Joo M.S., Kim W.D., Lee K.Y., Kim J.H., Koo J.H., Kim S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]