Abstract
Heart failure (HF) is a complex clinical syndrome that is increasingly prevalent among US adults and accounts for substantial burden of healthcare costs and morbidity. HF is commonly associated with prior myocardial infarction as well as prolonged exposure to hypertension, diabetes, and coronary atherosclerosis. Exercise training is becoming established in the management of HF because of its beneficial effect on both central (cardiac) and peripheral (skeletal muscle) HF mechanisms. The role of habitual physical activity in the primary prevention of HF is less clear. Recent prospective observational studies suggest there is lower risk of developing HF in adults who are more physically activity and have higher cardiorespiratory fitness compared with their less active and fit peers. This article reviews the published evidence on physical activity and HF prevention, discusses potential mechanisms for this benefit, and suggests areas where further research is needed to establish recommendations on the type, amount, and intensity of physical activity required to prevent occurrence of HF.
Keywords: exercise, heart failure, prevention, epidemiology, physical fitness
‘. . . regular physical activity is associated with reduced risks of developing hypertension, diabetes, and coronary heart disease, . . .’
Cardiovascular disease continues to impose a substantial burden on population health in the United States as the leading cause of morbidity and mortality in middle-aged and older adults.1 Much of this burden is attributed to coronary heart disease and ischemic stroke. Recent advances in the treatment of acute coronary events,2,3 and in device-based4 and pharmacologic-based5 control of clinical sequelae in those with impaired left ventricular function has greatly improved survival among coronary patients. Better primary prevention guidelines6,7 and development of new drug therapies8,9 have led to reductions in the occurrence of myocardial infarction. A consequence of enhanced primary prevention of coronary disease and treatment of acute coronary events is increasing frequency of chronic heart failure (HF).
HF imposes a large and growing public health burden in the United States.10 Based on population surveillance data in 2012, it is estimated that about 6.5 million adults 20 years and older (≈2% of the 2012 US population) are living with diagnosed HF. Prevalence of HF is projected to increase at least 45% to about 9 million by 2030.1 HF disproportionately affects older adults with 80% of cases occurring in individuals 65 years and older, for whom HF is the leading cause of hospitalization with 5-year mortality of about 50%.1,10 In 2012, the total cost for HF was $30 billion, and by 2030 this is expected to increase 127% to nearly $70 billion. The US adult population aged 65 years and older is expected to double between 2000 and 2030, older women outnumbering men, and the population burden of HF will be substantial, especially among women at advanced ages.
HF is a progressive clinical syndrome involving cardiac structural and functional alterations that give rise to impaired ventricular contractility and/or relaxation.11 Neurohormonal activation secondary to cardiac insult exerts vasoactive responses and promotes fluid retention contributing to HF progression. HF subtypes based on left ventricular ejection fraction (EF) have been defined as HF with reduced EF (HFrEF; EF ≤40%), HF with preserved EF (HFpEF; EF ≥50%), and borderline HF (EF 41%-49%).12 About 50% of patients hospitalized with HF have HFpEF, which is more common at older age and in women.1 Whereas antecedent myocardial infarction is frequent in HFrEF, this tends not to be the case in HFpEF, for which prolonged exposure to hypertension, diabetes and obesity are common underpinnings.11 Conventionally, HFrEF is associated with systolic cardiac dysfunction (poor contractility) whereas HFpEF is associated with diastolic cardiac dysfunction (impaired myocardial relaxation). The cardiac muscle dysfunction that characterizes HF involves alteration of myocardial excitation-contraction coupling and sarcomeric cross-bridge cycling (reduced myocardial force production), myocardial interstitial fibrosis, ventricular remodeling involving either, or both, ventricular dilation and hypertrophy, venous congestion and an imbalance between myocardial oxygen supply and demand. As such, prominent among the clinical signs and symptoms of HF are dyspnea at rest or on exertion and reduced physical functioning capacity. In HFrEF and HFpEF peripheral skeletal muscle dysfunction is increasingly recognized as a mechanism for exercise intolerance in addition to the more established central cardiac limitations.13 Because many of the central and peripheral mechanisms of HF respond favorably to exercise training, its role in HF management is becoming established.13-15
Less is known about the role physical inactivity has in the development of HF. Increasing physical activity levels and reducing sedentary time are 2 behavioral strategies that have promise with regard to HF prevention.16 There is clear and strong evidence that regular physical activity is associated with reduced risks of developing hypertension, diabetes, and coronary heart disease,17 which are major HF antecedents. In fact, human evolution has depended on a physically active lifestyle; hunting, gathering, fighting, fleeing, and surviving long enough to reproduce were essential elements of our ancestral past.18 A sedentary way of life, therefore, is an unnatural aberration from our evolutionary constitution and should logically be unhealthy to our species. Reduced physical activity and prolonged exposure to sedentarism impose serious consequences to cardiovascular function.19 Because of the high prevalence of sedentary lifestyles and the strong association between physical inactivity and cardiovascular disease (CVD) endpoints,17 it is not surprising that a high proportion of CVD mortality is attributed to physical inactivity, rivaling that of smoking, obesity, and other established risk factors as causes of decreased longevity.20,21
In this article, the role of physical activity in the primary prevention of HF is discussed. Available scientific evidence is briefly reviewed, and additional research needs are recommended. To begin, a brief discussion is given on terminology, assessment methods, and paradigms that pertain to physical activity and exercise.
Terminology, Assessment, and Paradigms
Physical activity refers to a behavior, specifically body movement that occurs from skeletal muscle contraction resulting in increased energy expenditure above resting metabolic rate.22 It is now accepted that activity-related energy expenditure, or the dose of physical activity, is more important for health benefits than is the specific type of activity (eg, walking, running, cycling, occupational activities).23 Sedentary behavior is a behavioral domain that is seen as not just being at the low end of the physical activity continuum, but instead, as a phenotype with related but separate physiologic consequences (and disease risks) in addition to those ascribed to low levels of physical activity.22,24 Exercise, or “exercise training,” is a subcategory of physical activity that is systematically structured toward enhancing one or more components of physical fitness.22 Physical fitness is a set of physiological attributes (eg, cardiorespiratory fitness, body composition, muscular strength and endurance, flexibility, agility, balance) that may be enhanced through exercise training or through regular participation in physical activity.22 Although it is plausible that enhancing each aspect of physical fitness may in some manner confer health benefits, the component of physical fitness that most often has been related with health outcomes is cardiorespiratory fitness (CRF). Determinants of CRF include age, sex, health status, and genetics; however, the principal modifiable determinant is habitual physical activity level. CRF responses to a standardized dose of aerobic exercise vary widely among individuals, and the observed heterogeneity is not random but aggregates in families through both genetic and environmental components.25 Nevertheless, in most individuals, and particularly among those who are sedentary, increases in physical activity result in increases in CRF, whereas, CRF declines soon after cessation of physical activity.22 Thus, CRF can be used as an objective surrogate measure of recent physical activity patterns.
Assessment Methods
Physical activity is a complex multidimensional behavior that is difficult to assess in free-living populations. A gold standard measurement does not exist at present. Several methods have been used to assess physical activity and these measurements have a broad range of accuracy, reproducibility, and feasibility.26 For example, self-administered questionnaires have relatively low cost and administrative burden, and can be used to obtain a crude categorization of activity status (eg, sedentary vs active) or to obtain more detailed descriptions of activities (eg, type, duration, frequency) and their estimated energy cost (eg, kcal/wk). Several issues, including population specificity of the questionnaire, completeness in capturing relevant activity domains of interest, and the potential for inaccurate recall must be considered when selecting a specific questionnaire and when generalizing associations between health outcomes and activity levels obtained therewith. Alternatively, direct monitoring of body movement and related energy expenditure can be performed using electronic motion sensors (eg, accelerometers, pedometers), global positioning satellite technology, heart rate monitors, portable indirect calorimeters, doubly labeled water, or some combination thereof. Lack of information on the type of activity being performed, potential changes in habitual physical activity behavior as a consequence of monitoring (eg, reactivity), calibration of device output to the targeted population’s physical activity habits and intensities, and the associated costs and administrative burden have precluded using direct monitoring in most large-scale studies. Approaches and challenges to assessing adult sedentary behavior are similar to those described for physical activity.27
CRF measurement methods have included the duration of exercise or the final estimated work rate achieved during maximal and submaximal exercise testing, performance on the 6-minute walk test and 400-m walk tests and, less frequently, indirect calorimetry measures of maximal oxygen uptake. CRF is less prone to misclassification due to response biases or behavior reactivity than self-reported or directly monitored activity habits.26 CRF, therefore, may better reflect the adverse consequences of a sedentary lifestyle than can be quantified using conventional physical activity assessments.28 This might be because CRF is a more reliable measure than is self-reported free-living PA, and because CRF may better reflect the combined effects of genetics and behavior in determining an individual’s health risk. Recent studies also have demonstrated cardiovascular benefits associated with greater reported frequency of participating in resistance exercises,17 and with greater levels of skeletal muscle strength as measured by isometric grip strength, isokinetic torque at different joints, and integrated tests of function such as the repeated chair stand and the “up and go” tests.29
Broadening the Paradigm
The 1996 US Surgeon General’s report on physical activity and health promoted a broadening of the exercise paradigm from one that focused almost exclusively on enhancing physical fitness to one that includes both health and fitness domains,30 a paradigm shift further supported by the 2008 Physical Activity Guidelines for Americans.31 Historically, under the exercise and fitness paradigm recommendations included relatively intense exercise and a more formal exercise prescription. Exercise benefits were thought to result through a threshold concept, which asserts that improvements in physical fitness and functioning can only occur after the exercise prescription level is achieved. The health and fitness paradigm derives from epidemiological data that suggested an inverse dose-response curve between physical activity, fitness, and health outcomes. The dose-response curve generally is characterized by a steep reduction in health risk across lower and intermediate physical activity or fitness categories, followed by a more gradual decline and tapering off across the highest categories. Accordingly, even moderate amounts and intensities of physical activity may confer important health benefits among those who are sedentary and have low CRF.
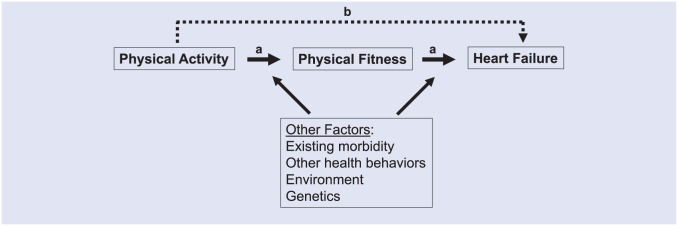
A simple schematic of possible relationships among physical activity, fitness, and health is shown in Figure 1. Factors such as genetics, the environment, and other health behaviors likely influence these relationships. There probably exists specificity of causal pathways between physical activity, fitness, and health wherein some outcomes may derive only when physical activity sufficiently enhances one or more domains of physical fitness; whereas, other outcomes may occur only if the metabolic capacity of skeletal muscle is enhanced, irrespective of changes in maximal physical performance.32 Moreover, the probability of adverse event occurrence, such as musculoskeletal injury or cardiac arrest, also tracks along the physical activity dose-response curve. At lower amounts and intensities of physical activity, risks of adverse events directly induced by the activity tends to be low. Adverse event risks increase gradually with greater amounts and intensities of physical activity, and generally are highest when engaging in high amounts and intensities of activity especially in individuals with increased susceptibility to such events because of existing medical conditions or because of deconditioning.22 Despite these potential events, the overall risk of serious adverse cardiovascular events with physical activity is extremely rare (detailed below).
Figure 1.
A schematic of possible relationships between physical activity, fitness, and heart failure. (a) Physical activity may have direct benefits on fitness, which in turn affects heart failure or (b) physical activity may benefit heart failure independent of any effect on measures of fitness. Other behavioral factors (eg, smoking, diet, alcohol intake), the environment (eg, air pollution exposure), health status (eg, prevalent morbidity), and genetics likely influence these potential relational pathways.
How Much Physical Activity Is Needed?
A critical mass of observational and experimental evidence has been marshaled into consensus recommendations on the type and amount of PA and exercise training required to achieve specific health and fitness outcomes.22,23,30,31 Generally, all adults are encouraged to minimize time spent in prolonged sedentary behaviors. Healthy adults are further encouraged to achieve a minimum of 150 minutes per week of moderate intensity (3-6 metabolic equivalents [METs]) physical activity; or at least 75 minutes per week of vigorous intensity (>6 METs) activity.22,23,30,31 The targeted minimum volume of PA is about 8 MET-hours per week (≈1000 kcal/wk) above routine activities of daily living. The targeted volume of energy expenditure can be achieved through a combination of moderate and vigorous intensity activities. Sedentary individuals should gradually increase their PA levels toward meeting the minimal recommended dose. Greater health benefits likely are conferred in individuals whose physical activity energy expenditure exceeds the recommended minimum dose. Recent clinical trial data indicate that recommended levels of moderate intensity physical activity are a sufficient stimulus to improve CRF,33,34 and that adherence is greater for moderate compared with high intensity physical activity programs.35,36
Energy expenditure for individual activities can be estimated by multiplying the frequency, duration, and absolute intensity (eg, METs) of the activity; total energy expenditure then is estimated by summing across the individual activities. Standardized energy costs in METs have been published for a variety of activity types.37 An example that fulfils the required energy expenditure for health-related benefits might be 30 minutes of brisk walking (moderate intensity) on 3 days, plus 20 minutes of jogging (vigorous intensity) on another 1 day of the week. Given that brisk walking (3.5 mph [5.6 km/h] on level ground) is a 3.8-MET activity and that jogging (5.96 mph or 10 min/mile [9.6 km/h] on level ground) is a 10-MET activity, the weekly volume of combined moderate intensity (30 minutes × 3.8 METs × 3 days = 342 MET-minutes) and vigorous intensity (20 minutes × 10 METs × 1 day = 200 MET-minutes) physical activity would be 542 MET-minutes per week, or 9 MET-hours per week.
Physical Activity, CRF, and HF Risk
When the 2008 federal Physical Activity Guidelines for Americans were developed, there was insufficient evidence to support a recommendation on HF benefit associated with reduced sedentary time or increased physical activity.17 It was unclear if the cardiovascular benefit of regular physical activity extends to HF prevention. However, accumulating findings from observational studies suggest that greater levels of aerobic physical activity and CRF are associated with lower incidence of hospitalization for acute HF.38-64 The basic design and major findings of these studies are summarized in Table 1. Of the 28 published studies in the table, there were 18 primary studies that evaluated HF risk in relation to physical activity*; 6 in relation to CRF;41,45,49,50,54,57 and 1 in relation to sedentary behavior.63 The remaining studies were a pooled analysis on physical activity and HF risk59 and 2 meta-analyses of studies on physical activity or CRF with HF risk.44,65 Cohort size ranged from 1142 participants52 to 137 303 participants55 for studies on physical activity; 1873 participants50 to 66 329 participants54 for studies on CRF; and 82 695 participants63 for the study on sedentary behavior. Follow-up intervals for incident HF cases in the 25 primary studies were 15 years or longer in 10 of the studies; 10 to 15 years in 12 studies; and <10 years in the remaining 4 studies. Numbers of HF case counts for analysis ranged from 88 (Patel et al64) to 3609 (Rahman et al61) in studies on physical activity or sedentary behavior, and 221 (Khan et al49) to 4652 (Kupsky et al54) in studies on CRF. Results for women were reported in 8 of the primary studies (6 physical activity; 2 CRF);41,46,47,54-56,60,62 and for participants from non-Caucasian race ethnic groups in 4 studies (3 physical activity; 1 CRF).40,54,58,63 Lifetime risks of HF were estimated in 2 studies,43,53 and results for HFrEF and HFpEF risks were reported in 2 primary studies.52,55
Table 1.
Prospective Observational Associations of Physical Activity or CRF With Incidence of Heart Failure.
| Study | Population and Outcome | Follow-up (HF Cases) | Exposure | Main Findingsa |
|---|---|---|---|---|
| Physical activity (PA) | ||||
| He et al (2001)46 | 5545 men and 8098 women, mean age 52.2 y (men) and 48.1 y (women), without known HF in the US NHANES follow-up study. Outcome: ≥1 hospitalizations for HF or death due to HF. |
Mean 19 y (741 men, 641 women) |
Unspecified assessment; exposure defined as low PA (vs high PA) | RR (95% CI) All: 1.23 (1.09, 1.38), P <.001 Men: 1.14 (0.94, 1.38), P = .19 Women: 1.31 (1.11, 1.54), P = .002 |
| Djousse et al (2009)43 | 20 900 men, mean age 53.6 y, without known HF in the Physician’s Health Study I. Outcome: Lifetime risk of HF between 40 and 80 y of age, defined as incident diagnosis of HF or death due to HF. |
Mean 22.4 y (1200) |
Frequency per week of vigorous exercise (no recall time given) Lifetime risk estimates adjusted for mortality during follow-up interval. |
PA level lifetime risk, % (95% CI) <5 times/wk 14.3 (13.2, 15.4) ≥5 times/wk 11.4 (9.4, 13.5) |
| Kenchaiah et al (2009)48 | 21 094 men, mean age 53 y, without known HF or CHD in the Physicians Health Study. Outcome: incident diagnosis of HF or death due to HF. |
Mean 20.5 y (1109) |
Frequency per week of vigorous exercise (no recall time given) | PA level [cases] RR (95% CI) Never/rarely [206] 1.00 (referent) 1-3 times/mo [145] 0.78 (0.63, 0.97) 1-4 times/wk [610] 0.86 (0.73, 1.01) 5-7 times/wk [148] 0.73 (0.59, 0.90) Trend, P = .016 Diabetes—no Never/rarely [183] 1.00 (referent) N≥1-3 times/mo [829] 0.80 (0.68, 0.94) Diabetes—yes Never/rarely [183] 1.00 (referent) ≥1-3 times/mo [829] 1.02 (0.63, 1.64) |
| Hu et al (2010)47 | 28 842 men and 30 366 women, ages 25-74 y, in the FINN-MONICA study. Outcome: incidence of diagnosed HF documented in the Finnish Hospital Discharge Register. |
Mean 18.4 y (3614) |
Combined occupational, commuting, and leisure-time PA during the previous year. | PA level [cases] RR (95% CI) Men: Low [240] 1.00 (referent) Moderate [601] 0.79 (0.68, 0.92) High [1080] 0.69 (0.60, 0.80) Trend, P < .001 Women: Low [346] 1.00 (referent) Moderate [597] 0.86 (0.75, 0.99) High [750] 0.68 (0.59, 0.78) Trend, P < .001 |
| Wang et al. (2010)62 | 28,334 men and 29,874 women, ages 25-74 yr, in the FINN-MONICA study. Outcome: incidence of diagnosed HF documented in the Finnish Hospital Discharge Register. |
Mean 18.4 yr (3,508) |
Walking or cycling to and from work during previous year. | Walking/cycling, min/wk RR (95% CI) Men [cases]: 0 [984] 1.00 (referent) 1-29 [499] 1.01 (0.90, 1.13) ≥30 [385] 0.99 (0.87, 1.12) Trend, P = .37 Women [cases]: 0 [959] 1.00 (referent) 1-29 [351] 0.87 (0.76, 0.99) ≥30 [330] 0.94 (0.82, 1.07) Trend, P = .11 |
| Bell et al. (2013)40 | 3,707 black and 10,018 white adults, ages 45-64 yr, without known CVD (including HF) in the ARIC study. Outcome: incident hospitalization for acute HF, or death due to HF. |
Mean ≈17 yr (1,748) |
Combined occupational, sport, and leisure PA during previous yr AHA Categories of MVPA (min/wk): Poor (0) Intermediate (1-149) Recommended (≥150) |
Blacks [cases] RR (95% CI) Poor [350]: 1.00 (referent) Intermediate [178]: 0.62 (0.51-0.75) Recommended [105]: 0.59 (0.47-0.74) Trend, P <.0001 Whites [cases] Poor [303]: 1.00 (referent) Intermediate [414]: 0.76 (0.65-0.88) Recommended [398]: 0.64 (0.54-0.75) Trend, P < .0001 |
| Kraigher-Krainer et al (2013)52 | 1142 adults, ages 67-97 y, without known HF, in the Framingham Heart Study. Outcome: incident hospitalization for HF overall, and HFrEF (LVEF ≤45%) or HFpEF (LVEF >45%). |
Mean 10 y (250 overall; 108 HFpEF, 106 HFrEF) |
Combined occupation and leisure-time PA used to compute a PA Index, analogous to MET-h/wk: Tertiles: Low (men 24.9-30; women 24.2-29.8) Middle (men 30.1-34.3; women 29.9-33.7) High (men 34.5-63.2; women 33.8-57.6) |
PA tertile [cases] RR (95% CI) Overall HF [250] Low 1.00 (referent) Middle 0.84 (0.60,1.17) High 0.65 (0.46, 0.91) Trend, P = .01 HFpEF [108] Low 1.00 (referent) Middle 0.54 (0.31, 0.92) High 0.66 (0.41, 1.07) Trend, P = .11 HFrEF [106] Low 1.00 (referent) Middle 1.07 (0.64, 1.79) High 0.69 (0.41, 1.19) Trend, P = .16 |
| Patel et al (2013)64 | 5503 adults, mean age 73 y, without known HF, in the Cardiovascular Health Study. Outcome: adjudicated, physician-diagnosis of HF. |
13 y (88) |
Leisure-time PA, previous 2 wk. Categories MET-min/wk [median]: 0 [0] 1-499 [253] 500-999 [729.5] ≥1000 [2054.5] |
PA level [cases] RR (95% CI) 0 [26] 1.00 (referent) 1-499 [23] 0.97 (0.79, 1.20) 500-999 [20] 0.86 (0.69, 1.08) ≥1000 [19] 0.79 (0.64, 0.97) Trend, P = .003 |
| Agha et al (2014)38 | 84 537 women, ages 50-79 y, without known HF in the US Women’s Health Initiative. Outcome: incident hospitalization for HF. |
Mean 11 y (1826) |
Recreational walking and physical activity (usual levels), summarized as MET-h/wk. Inactive: 0 Somewhat active: 1-149 Active: ≥150 |
PA level [cases] RR (95% CI) Inactive [386] 1.00 (referent) Somewhat [614] 0.77 (0.67, 0.87) Active [826] 0.69 (0.61, 0.79) Trend, P = .06 |
| Andersen et al (2014)39 | 39 805 adults, mean age 52.2 y, without known HF in the Swedish National March Cohort study. Outcome: incident hospitalization for HF. |
Median 13 y (1545) |
Combined occupational, household, exercise and leisure-time PA during previous year, summarized to total PA MET-h/d. PA exposure categorized in quintiles. |
Leisure PA (MET-h/d) RR (95% CI) Q1 (<1.2) 1.00 (referent) Q2 (1.2-1.9) 0.93 (0.79, 1.09) Q3 (1.9-3.0) 0.79 (0.67, 0.94) Q4 (3.0-4.7) 0.73 (0.60, 0.89) Q5 (>4.7) 0.65 (0.53, 0.81) Trend, P < .01 Total PA (MET-h/d) RR (95% CI) Q1 (<30.5) 1.00 (referent) Q2 (30.5-33.9) 0.82 (0.68, 1.00) Q3 (33.9-39.1) 0.87 (0.72, 1.04) Q4 (39.1-48.7) 0.87 (0.73, 1.05) Q5 (>48.7) 0.90 (0.76, 1.06) Trend, P = .59 |
| Rahman et al (2014)60 | 27 895 women, ages 47-83 y, without known HF, CHD, or cancer in the Swedish Mammography Cohort study. Outcome: incident hospitalization for HF or death due to HF. |
Mean 13 y (2402) |
Combined occupational, household, exercise, and walking/bicycling during the previous year; summarized into total PA MET-h/d. | Cubic spline regression resulted in a nonlinear (P < .01) dose-response across the range of total PA MET-h/d: PA level (spline) RR (95% CI): 30 (lowest) 1.70 (1.42, 2.07) 42 (median) 1.00 (referent) 58 (highest) 0.98 (0.70, 1.30) Walking/Bicycling: ≥20 vs <20 min/d 0.71 (0.64, 0.80) Exercise: ≥1 vs <1 h/wk 0.83 (0.75, 0.92) Household chores: ≥1 vs <1 h/d 0.82 (0.70, 0.97) |
| Young et al (2014)63 | 82 695 men ages 45-69 y, without known HF, in the California Men’s Health Study. Outcome: ≥1 hospitalization for HF, or ≥2 outpatient HF diagnoses as determined using the Kaiser Permanente Southern or Northern health plans. |
Mean 7.8 y (3473) |
Leisure-time MVPA during previous 3 months Tertiles (MET-min/wk): Low (≤470) Middle (471-1584) High (≥1585) Multivariable model includes adjustment for sedentary time. |
PA Tertile [cases] RR (95% CI) Low [1505] 1.52 (1.38, 1.67) Middle [1051] 1.15 (1.04, 1.26) High [829] 1.00 (referent) Trend, P <.0001 Low Middle High Whites 1.56* 1.13* 1.00 Blacks 1.29 1.12 1.00 Hispanic 1.61* 1.45* 1.00 Asian 1.45* 1.22 1.00 CHD—no 1.70* 1.23* 1.00 CHD—yes 1.32* 1.06 1.00 HTN—no 1.53* 1.15 1.00 HTN – yes 1.53* 1.18* 1.00 *P < .05 |
| Del Gobbo et al (2015)42 | 4490 adults, ≥65 y, without known HF in the Cardiovascular Health Study. Outcome: Incidence of diagnosed and treated HF. |
Maximum 21.5 y (1380) |
Leisure-time PA and walking during previous year. PA and walking exposures updated at 3 and 7 years postbaseline; analyzed using repeated measures modeling. |
PA, kcal/wk [cases] RR (95% CI) <845 [624] 1.00 (referent) ≥845 [756] 0.78 (0.69, 0.87) Walking, mph [cases] RR (95% CI) <2 [454] 1.00 (referent) ≥2 [926] 0.80 (0.71, 0.90) |
| Rahman et al (2015)61 | 33 012 men, mean age 60 y, without known HF or CHD, in the Cohort of Swedish Men Study. Outcome: incident hospitalization for HF or death due to HF. |
Mean 13 y (3609) |
Combined occupational, household, exercise, and walking/bicycling during the previous year; summarized into total PA MET-h/d. | Cubic spline regression resulted in a nonlinear (P < .001) dose-response across the range of total PA MET-h/d: PA level (spline) RR (95% CI): 30 (lowest) 1.44 (1.24, 1.68) 41 (median) 1.00 (referent) 57 (highest) 1.25 (1.03, 1.53) Walking/Bicycling: ≥20 vs <20 min/d 0.79 (0.72, 0.87) Exercise: ≥1 vs <1 h/wk 0.86 (0.79, 0.94) Household chores: ≥1 vs <1 h/d 0.95 (0.89, 1.02) |
| LaMonte et al (2016)55 | 137 303 women, ages 50-79 y, without known HF in the US Women’s Health Initiative. Outcome: incident hospitalization for HF overall, and HFpEF (LVEF ≥45%) or HFrEF (LVEF <45%). |
Mean 14 y (2523 overall, 734 HFpEF, 451 HFrEF) |
Recreational walking and physical activity (usual levels), summarized as MET-h/wk. PA exposure defined as: Inactive (0 MET-h/wk) and tertiles of PA Multivariable adjustment included time-varying CHD |
Total PA Tertile (MET-h/wk) RR (95% CI) HF overall [cases] Inactive [434] 1.00 (referent) >0-7.2 [855] 0.88 (0.79, 0.99) 7.3-17.0 [682] 0.79 (0.70, 0.90) >17.0 [522] 0.75 (0.65, 0.85) Trend, P < .001 HFpEF [cases] Inactive [145] 1.00 (referent) >0-7.2 [272] 0.93 (0.76, 1.14) 7.3-17.0 [170] 0.77 (0.61, 0.96) >17.0 [147] 0.81 (0.64, 1.03) Trend, P = .07 HFrEF [cases] Inactive [103] 1.00 (referent) >0-7.2 [160] 0.77 (0.60, 0.99) 7.3-17.0 [94] 0.58 (0.44, 0.77) >17.0 [94] 0.71 (0.54, 0.95) Trend, P = .06 |
| Larsson et al (2016)56 | 33 966 men and 30 713 women, ages 45-83 y, without known HF and CHD, in established Swedish cohort studies. | Mean 13 y (1488 men, 1096 women) |
Combined walking, bicycling, and exercise during previous year; summarized into MVPA min/wk. Inactive <150 min/wk Active ≥150 min/wk |
Men [cases] RR (95% CI) Active [73] 0.83 (0.74, 0.94) Women [cases] RR (95% CI) Active [77] 0.71 (0.63, 0.81) |
| Koo et al (2017)51 | 4066 black adults, mean age 55.0 y, without known HF, in the Jackson Heart Study. Outcome: incident hospitalization for HF. |
Mean 7 y (168) |
Combined occupational, sport, and leisure PA during past year AHA categories of MVPA (min/wk): Poor (0) Intermediate (1-149) Recommended (≥150) |
PA level [cases] RR (95% CI) Poor [114]: 1.00 (referent) Intermediate [42]: 0.74 (0.52-1.07) Recommended [12]: 0.41 (0.22-0.74) Trend, P = .003 Excluding those with interim CHD: Poor: 1.00 (referent) Intermediate: 0.83 (0.52-1.33) Recommended: 0.39 (0.17-0.91) Trend, P = .03 |
| Kubota et al (2017)53 | 5807 men and 7252 women, ages 45-64 y, without known CVD (including HF) in the ARIC study. Outcome: lifetime risk of HF between 45 and 85 years of age, defined as incident hospitalization for HF or death due to HF. |
Mean 18.9 y (not reported) |
Combined occupational, sport, and leisure PA during past year AHA categories of MVPA (min/wk): Poor (0) Intermediate (1-149) Recommended (≥150) |
Lifetime risk (%) Men Women Poor 29.8 27.3 Intermediate 23.0 22.0 Recommended 21.9 19.2 Trend, P value not reported |
| Ogunmoroti et al. (2017)58 | 6506 adults, mean age 62 y, without known HF, in the Multiethnic Study of Atherosclerosis. |
Median 12 y |
Combined occupational, sport/exercise, household, and leisure PA during a typical week. AHA categories of MVPA (min/wk): |
RR (95% CI) All: Poor: 1.00 (referent) Intermediate: 0.96 (0.66-1.39) Recommended: 0.72 (0.54-0.96) White: Poor: 1.00 (referent) Intermediate: 0.95 (0.51-1.76) Recommended: 0.76 (0.46-1.24) |
| Outcome: incident diagnosis (probable or definite) of HF. | (262) | Poor (0) Intermediate (1-149) Recommended (≥150) |
Black: Poor: 1.00 (referent) Intermediate: 1.09 (0.55-2.17) Recommended: 0.85 (0.49-1.45) Hispanic: Poor: 1.00 (referent) Intermediate: 0.79 (0.38-1.63) Recommended: 0.41 (0.22-0.74) |
|
| Pandey et al (2017)59 | 51 451 adults, mean age 63 y, without known HF, pooled together from 3 large US cohorts: Cardiovascular Health Study, Multiethnic Study of Atherosclerosis, Women’s Health Initiative. Outcome: incident hospitalization for HF overall, and HFpEF (LVEF ≥45%) or HFrEF (LVEF <45%). |
Mean 11 y (3180 overall; 1252 HFpEF; 941 HFrEF) |
Leisure-time PA recalled over variable periods, summarized as total PA MET-min/wk. PA exposure defined as: Inactive (0 MET-min/wk) and tertiles of PA |
PA tertile (MET-min/wk) RR (95% CI) HF overall Inactive 1.00 (referent) 1-499 0.94 (0.85, 1.04) 500-1000 0.89 (0.79, 0.99) >1000 0.78 (0.70, 0.87) HFpEF Inactive 1.00 (referent) 1-499 0.99 (0.86, 1.17) 500-1000 0.87 (0.72, 1.05) >1000 0.81 (0.68, 0.97) HFrEF Inactive 1.00 (referent) 1-499 0.88 (0.73, 1.06) 500-1000 0.86 (0.70, 1.07) >1000 0.86 (0.71, 1.05) |
| Sedentary behavior | ||||
| Young et al (2014)63 | 82 695 men, ages 45-69 y, without known HF, in the California Men’s Health Study. Outcome: ≥1 hospitalization for HF, or ≥2 outpatient HF diagnoses as determined using the Kaiser Permanente Southern or Northern health plans. |
Mean 7.8 y (3473) |
Sitting time during previous 3 mo Sedentary tertiles (h/d): Low (≤2) Middle (3-4) High (≥5) Multivariable model includes adjustment for physical activity. |
Sedentary tertile [cases] RR (95% CI) Low [1041] 1.00 (referent) Middle [1488] 1.09 (1.00, 1.26) High [828] 1.27 (1.15, 1.41) Trend, P < .0001 Low Middle High Whites 1.00 1.12 1.29* Blacks 1.00 1.01 1.23 Hispanic 1.00 1.19 1.78* Asian 1.00 1.22 1.10 CHD – no 1.00 1.11 1.43* CHD – yes 1.00 1.05 1.11 HTN – no 1.00 1.25* 1.32* HTN – yes 1.00 1.08 1.33* *P < .05 |
| Cardiorespiratory fitness (CRF) | ||||
| Berry et al. (2013)41 | 16,303 men and 4,339 women, mean age 49 yr at CRF assessment, who survived to receive Medicare Claims in later life. Outcome: incident hospitalization for acute HF based on Medicare Claims data. |
133,514 PY (1051) |
CRF defined as the age- and sex-standardized distribution of duration on a maximal treadmill exercise test: Low = lowest 20%, Moderate = middle 40%, High = upper 40%. |
CRF level [cases] RR (95% CI) Men: Low [331] 1.00 (referent) Mod [387] 0.60 (0.49, 0.75) High [189] 0.31 (0.24, 0.41) Trend, P < .001 Women: Low [35] 1.00 (referent) Mod [72] 0.53 (0.31, 0.93) High [37] 0.38 (0.20, 0.71) Trend, P = .01 |
| Khan et al (2014)50 | 1873 men, ages 42-61 y, without known HF or COPD in the Finnish Kuopio Heart Study. Outcome: incidence of diagnosed HF. |
Mean 20.4 y (152) |
CRF defined as measured maximal oxygen uptake (mL/kg/min) during cycle ergometry. | CRF (mL/kg/min) [cases] RR (95% CI) 6.4-25.7 [57] 1.00 (referent) 25.7-30.4 [49] 0.96 (0.64, 1.44) 30.4-35.4 [32] 0.74 (0.46, 1.20) 35.4-65.4 [13] 0.48 (0.25, 0.92) CRF Per 3.5 ml/kg/min: All 0.81 (0.69, 0.95) CVD—no 0.96 (0.92, 0.99) CVD—yes 0.97 (0.94, 1.01) HTN—no 0.97 (0.94, 1.00) HTN—yes 0.96 (0.93, 1.00) |
| Kahn et al (2017)49 | 2089 men, ages 42-61 y, without known CVD including HF in the Finnish Kuopio Heart Study. Outcome: incident nonfatal HF diagnosis. |
Mean 19.1 y (221) |
CRF defined as measured maximal oxygen uptake (mL/kg/min) during cycle ergometry. | CRF (mL/kg/min) [cases] RR (95% CI) 6.4-25.7 [88] 1.00 (referent) 25.7-30.4 [66] 0.95 (0.66, 1.37) 30.4-35.4 [40] 0.60 (0.38, 0.93) 35.4-65.4 [27] 0.50 (0.29, 0.86) CRF per 3.5 mL/kg/min: All 0.85 (0.78, 0.93) CVD—no [98] 0.87 (0.82, 0.91) CVD—yes [123] 0.82 (0.78, 0.87) Diabetes—no [200] 0.84 (0.81, 0.88) Diabetes—yes [21] 0.86 (0.76, 0.96) |
| Kupsky et al. (2017)54 | 66,329 adults, mean age 55 yr, without known HF in the Henry Ford Exercise Testing Project. Outcome: incident diagnosis of HF based on claims data within the clinic’s payer system. |
Median 6.8 yr (4,652) |
CRF defined as maximal MET level achieved during a treadmill exercise test. | CRF (ml/kg/min) RR (95% CI) <21.0 1.00 (referent) 21.0-34.9 0.60 (0.53, 0.67) 35.0-42.0 0.38 (0.33, 0.44) >42.0 0.19 (0.14, 0.29) CRF per 3.5 mL/kg/min: All 0.84 (0.82, 0.86) Men 0.84 (0.82, 0.87) Women 0.81 (0.78, 0.84) White 0.84 (0.82, 0.87) Black 0.83 (0.80, 0.86) CVD—no 0.83 (0.81, 0.86) CVD—yes 0.85 (0.82, 0.88) |
| Myers et al (2017)57 | 21 080 men, mean age 58.3 y, without known HF in the Palo Alto Veterans Exercise Testing Study. Outcome: incident diagnosis of HF based on Veterans Affairs Hospital records. |
Mean 12.3 y (1902) |
CRF defined as the age-standardized MET level achieved on a maximal treadmill exercise test. Quintiles [mean METs]: Q1 ≤20% [4.3] Q2 21%-40% [6.0] Q3 41%-60% [7.3] Q4 61%-80% [8.7] Q5 >80% [11.8] |
CRF quintile RR (95% CI) Q1 1.00 (referent) Q2 0.64 (0.57, 0.74) Q3 0.59 (0.52, 0.67) Q4 0.33 (0.29, 0.39) Q5 0.24 (0.21, 0.32) |
| Georgiopoulou et al (2017)45 | 2935 adults, mean age 73.6 y, without known HF in the Health ABC Study. Outcome: incident hospitalization for HF. |
10 y (398) |
CRF defined as (a) completion status (excluded, noncompleter, completer) of a timed long-distance corridor 400 m walk; and (b) walking speed (m/s) and heart rate (HR) recovery (HRR) among completers. | CRF level [cases] RR (95% CI) Completers [253] 1.00 (referent) Noncompleters [63] 1.37 (1.00, 1.88) Excluded [82] 1.41 (1.06, 1.89) CRF level among completers: 20m walk speed (per 1 m/s) 0.45 (0.18, 1.09) 400 m walk speed (per 1 m/s) 0.50 (0.19, 1.31) HRR (per beat/min) 0.99 (0.98, 1.01) |
| Meta-analysis on PA or CRF | ||||
| Pandey et al (2015)64 | 12 prospective cohorts; 370 460 adults (53% women); mean age 50-76 y. | 13 y (20 203) |
PA harmonized across studies into MET-min/wk, then grouped into low, moderate, and high categories for analysis. | PA pooled RR (95% CI) Low 1.00 (referent) Moderate 0.78 (0.75, 0.82) wHigh 0.70 (0.67, 0.73) High vs low PA: Men 0.75 (0.63, 0.87) Women 0.73 (0.68, 0.78) Mean age <55 y 0.71 (0.64, 0.79) Mean age ≥55y 0.69 (0.65, 0.73) |
| Echouffo-Tcheugui et al (2015)44 | PA: 282,889 adults (10 cohorts), ages 20-97 y CRF: 22,515 adults (2 cohorts), aged ≥42 y. Outcome: incident HF. |
PA: 7-30 y (14 626) CRF: 6.5-20.4 (1203) |
PA: highest vs lowest category of reported PA. CRF: per 1-MET |
RR (95% CI)PA: 0.72 (0.67, 0.79)CRF: 0.79 (0.75, 0.83)PA: HFpEF 0.60 (0.37, 0.99) HFrEF 0.69 (0.41, 1.19) |
Abbreviations: CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction, LVEF, left ventricular ejection fraction; RR, relative risk; CI, confidence interval; PA, physical activity; MVPA, moderate-to-vigorous intensity PA (≥3 METs); CRF, cardiorespiratory fitness; PY, person-years; HTN, hypertension; MET, metabolic equivalent (1 MET = 3.5 mL O2/kg/min) denotes PA intensity; MET-min/wk, MET-minutes per week of PA energy expenditure; MET-h/wk, MET-hours per week of PA energy expenditure. Poor PA = 0 min/wk of MVPA; Intermediate PA = 1-149 min/wk moderate PA or 1-74 min/wk vigorous PA; Recommended PA = ≥150 min/wk moderate PA or ≥75 min/wk vigorous PA.
In each study, the point and interval estimates of association were adjusted for age, sex (where applicable), race-ethnicity (where applicable), and several other risk predictors; the most fully adjusted associations reported in the original studies are provided in the table.
Inverse multivariable-adjusted associations between physical activity or CRF and risk of overall HF were reported in all studies shown in Table 1. In the majority of available studies leisure-time physical activity was defined as the exposure, and there was about a 30% lower risk of HF when comparing the highest with the lowest activity level. In subgroup analyses, the inverse association between physical activity and HF risk was seen in those without but not with diabetes48; whereas, an inverse association was seen in those with and without CHD or hypertension.63 Higher CRF was associated with lower HF risk in those with and without CVD or diabetes.49,54 Studies reporting results specifically for women tended to show inverse associations for HF with physical activity46,47,55,56,60 and with CRF.41,54 The apparent protection against developing HF also was observed in non-Caucasian participants with higher levels of physical activity40,58,63 and CRF.54 The one study that related sedentary behavior with HF risk in men, showed positive associations in the overall cohort as well as in non-Caucasian subgroups, in those with and without hypertension, and in those without but not with CHD.63 HFpEF and HFrEF risks were evaluated in 2 primary studies on physical activity, one study showing a nonsignificant inverse association with physical activity for both endpoints in women and men combined,52 the other study showing significant inverse associations with physical activity for both endpoints in postmenopausal women.55 Studies that specifically evaluated exposure to current guideline recommended amounts of physical activity (eg, 150 min/wk of moderate intensity activity)31 reported lower multivariable adjusted relative risks of HF of about 28% to 61% when compared with physically inactive individuals.40,51,53,58 Lifetime risks of HF between ages 40 and 85 years were inversely associated with physical activity levels in both women and men.43,53
Additional Research Needs
Evidence from observational studies supporting a potential protective association between higher levels of self-reported physical activity or measured CRF is accumulating, and provides a basis for further consideration of a role for promotion of aerobic physical activity in the primary prevention of HF. Available data demonstrate generally consistent findings for inverse associations between self-reported physical activity or measured CRF with HF incidence across observational cohort studies in diverse populations of women and men with wide range across the adult age span. These inverse associations are present after adjustments for several relevant confounders and are often observed with little variation between clinical subgroups defined by presence or absence of existing morbidity.
Additional studies are needed to expand and clarify the results from the studies summarized in Table 1. Less clear is the independent role that prolonged sedentary behavior might have in HF development, and the influence that physical activity, CRF and sedentary behavior have on HFpEF and HFrEF subtypes, given the sparse amount of available data in these areas. Although all studies attempted to control for confounding of associations with HF risk, the extent of adjustments varied across studies and it is plausible that the reported associations are biased due to residual or unmeasured confounding. While most studies dealt with prevalent CHD at baseline, either by exclusion or by analytic methods, few studies included control for CHD antecedent to HF diagnosis.55 This is important because a postbaseline interim CHD event not only increases the likelihood of HF during follow-up but also increases the likelihood of the individual reducing their physical activity level and increasing sedentary time which could lead to spurious associations. Prospective studies that include time-varying information on CHD events and on physical activity or sedentary behavior are needed to accurately evaluate this possibility. Objective measures (eg, accelerometers) of physical activity and sedentary behavior are needed in large community-living cohorts with diversity on age, sex, and race-ethnicity to better understand how much and what intensity of physical activity, how long and what pattern of sedentary behavior is associated with HF risk, and whether the association varies according to sociodemographic or clinical subgroups. The use of objective monitoring of physical activity and sedentary behavior will be particularly useful when studying older adults or other populations with limited movement patterns for which questionnaire assessments are less sensitive. The role of resistance exercise and skeletal muscle fitness also needs to be evaluated in relation to HF development. There are known benefits of resistance exercise on cardiometabolic health and CVD risk17 and there is accumulating evidence that skeletal muscle dysfunction is a peripheral mechanism through which cardiac dysfunction worsens as part of HF progression.13 Because of the potential biases and other issues that limit causal inferences based on observational study data, ultimately a randomized controlled primary prevention trial is needed to rigorously test the hypothesis that increasing levels of physical activity or CRF, and decreasing time spent in sedentary behavior, reduce the incidence of HF and its subtypes HFrEF and HFpEF. Exercise for patients with known HF is now widely accepted,13 as underscored by the recent Centers for Medicare and Medicaid Services approval for coverage for rehabilitation, which cedes the potential role physical activity has in HF prevention.
Mechanisms for Physical Activity Benefits on HF
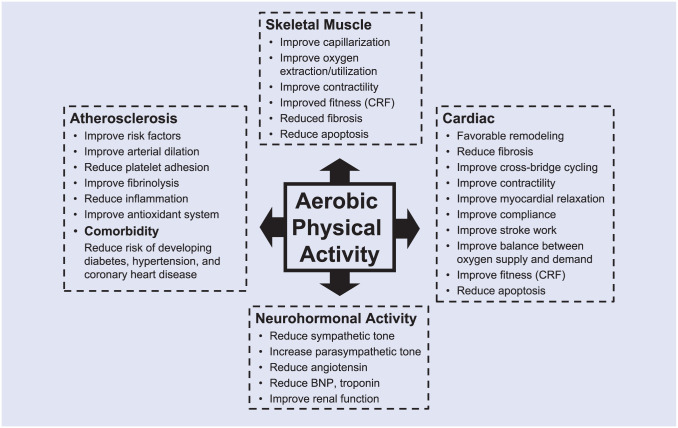
Laboratory and clinical studies using animal and human models have documented a variety of acute and long-term structural and functional responses to physical activity and exercise training that improve both physical performance and risk factors for chronic disease.17,22,66 Enhanced physical activity or CRF could influence HF development through direct or indirect pathways that ultimately effect either, or both, myocardial contractility or relaxation (Figure 2). Established biological pathways that mediate beneficial effects of physical activity or CRF relevant to atherosclerosis, myocardial ischemia, and cardiac function include improvements in blood pressure regulation, lipid and lipoprotein metabolism, insulin sensitivity and glycemic control, adiposity and fat distribution, skeletal muscle mass and function, oxidative stress and immunologic reactivity, cardiac oxygen demand and supply, and myocardial electrical stability.66,67 It also appears that physical activity may favorably influence novel biomarkers involved with intra- and intercellular signaling pathways, endothelial cell function, inflammation, thrombosis and thrombolysis, angiogenesis, cellular apoptosis, and micro-RNA expression.67-73 Direct effects on favorable cardiac remodeling, reduced ventricular stiffness, and improved systolic and diastolic function have been reported in cross-sectional and longitudinal studies on physical activity and CRF.74-78 Exercise trials have demonstrated beneficial structural adaptions in HF patients.79,80 Physical activity may lessen propensity for water retention through favorable effects on neurohormonal activity and renal function.81,82 Skeletal muscle myopathy is an emerging factor in the pathophysiology of HF83,84 that responds to exercise training.13 The human response to both acute and chronic physical activity is governed to some extent by the human genome.85 Additional research on these established and newly emerging pathways will further elucidate the biological basis of the potential benefits on HF risk conferred by physical activity and CRF.
Figure 2.
Potential mechanisms through which regular physical activity contributes to prevention of heart failure. BNP, B-type natriuretic peptide; CRF, cardiorespiratory fitness.
Hazards of Physical Activity
The net benefits of regular physical activity only can be realized after considering any risks that may result from being physically active. During recreational activity, the most common risk is for musculoskeletal injuries, such as sprained ligaments, strained muscles, and overuse injuries.22 Incidence of activity-related musculoskeletal injury is only slightly higher among adults who meet recommended physical activity levels (17.9 per 1000) compared with their sedentary peers (12.4 per 1000).86 Injury risk is higher in those with a history of previous musculoskeletal injury and appears to be positively associated with the intensity of activity. The risk of exercise-related cardiovascular complications (eg, cardiac arrest or myocardial infarction) is quite low, but is transiently increased particularly during vigorous physical activity.87 Cardiac events during exercise are most likely to occur in individuals with existing cardiovascular disease and in those who are sedentary and deconditioned. However, recent randomized trials have demonstrated that appropriately implemented and monitored physical activity programs can be safely engaged in by individuals at high risk for clinical cardiovascular events including patients with HFrEF.88,89 The hazards of physical activity and exercise can be reduced through sensible habits that include properly warming up before and cooling down after exertion, gradually increasing the volume and intensity of activity toward the dose recommended for health benefits, monitoring untoward sensations or responses during exercise, and when indicated, medical screening examinations.22,87 Overall, physical activity levels within the range recommended for health benefits have an acceptable risk-to-benefit ratio.
Summary
Physical activity is not a fad, rather it is part of our evolutionary way of living—the kind for which our body is engineered and which facilitates proper function of our anatomy, biochemistry, and physiology. Sedentary life habits result in maldaptative changes in our constitution and increase the likelihood of disease development and premature death. A substantial amount of observational and experimental research has supported development of practical physical activity recommendations directed toward adults who are sedentary and have low CRF. Engaging in at least 150 minutes per week in moderate or 75 minutes per week in vigorous physical activities to achieve a minimum weekly dose of 8 MET-hours of energy expended (≈1000 kcal/wk) is sufficient for most adults to achieve healthful levels of CRF and to lower the mortality and morbidity associated with several diseases.
It appears that another of the health benefits associated with regular physical activity and sufficient CRF extends to the development of HF. Better atherosclerotic risk factor profiles associated with higher levels of physical activity reduce the risks of hypertension, diabetes, and coronary disease which are major antecedents to HF. Improved cardiac geometry and function, and better balance between myocardial oxygen supply and demand appear to be direct mechanisms through which physical activity and CRF confer HF benefit. Further suggestion of a direct effect of physical activity on development of HF comes through prospective observational studies showing reduced risk of HF even after statistical control for major HF risk factors and showing lower risks of both HFrEF and HFpEF which suggest that physical activity benefit is not constrained only to HF of ischemic origin. While the available scientific evidence is persuasive in supporting a role for physical activity in the development of HF, considerably more work is needed to inform guideline recommendations for physical activity and prevention of overall HF as well as HFrEF and HFpEF. Future studies should further characterize the dose-response between physical activity and HF risks, better evaluate the role of physical activity intensity, particularly light-intensity activities, in preventing occurrence of HF, and determine the independent and joint effect that physical activity and sedentary behavior have on HF risks.
There are about 70 million US adults who report being sedentary. Because of this large number exposed and because of the increased relative risks for HF in physically inactive and sedentary individuals, the population burden of HF attributed to these 2 behaviors is substantial and will grow with an aging society. Increased attention to this problem should be given by those involved in healthcare, research, and public health. Critical to this effort is development of cost-effective interventions that employ efficacious and practical approaches to changing physical activity and sedentary behaviors, and efforts to evaluate and modify the role of social and environmental factors that determine community-level physical activity habits. By these means, steps can be taken to strengthen HF prevention and to control an emerging epidemic.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. [DOI] [PubMed] [Google Scholar]
- 3. Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205-2241. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Hall WJ, Cannom DS, et al. ; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338. [DOI] [PubMed] [Google Scholar]
- 5. Jaiswal A, Nguyen VQ, Carry BJ, le Jemtel TH. Pharmacologic and endovascular reversal of left ventricular remodeling. J Card Fail. 2016;22:829-839. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517-584. [DOI] [PubMed] [Google Scholar]
- 7. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45. [DOI] [PubMed] [Google Scholar]
- 8. Burnier M, Vuignier Y, Wuerzner G. State-of-the-art treatment of hypertension: established and new drugs. Eur Heart J. 2014;35:557-562. [DOI] [PubMed] [Google Scholar]
- 9. Hess CN, Low Wang CC, Hiatt WR. PCSK9 inhibitors: mechanisms of action, metabolic effects, and clinical outcomes. Annu Rev Med. 2018;69:133-145. [DOI] [PubMed] [Google Scholar]
- 10. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11:404-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007-2018. [DOI] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [DOI] [PubMed] [Google Scholar]
- 13. Fleg JL, Cooper LS, Borlaug BA, et al. ; National Heart, Lung, and Blood Institute Working Group. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535-e578. [DOI] [PubMed] [Google Scholar]
- 15. Pina IL, Apstein CS, Balady GJ, et al. ; American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210-1225. [DOI] [PubMed] [Google Scholar]
- 16. Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544-2565. [DOI] [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 18. Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med. 1988;84:739-749. [DOI] [PubMed] [Google Scholar]
- 19. Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(5 suppl):VII1-VII78. [PubMed] [Google Scholar]
- 20. Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haapanen-Niemi N, Vuori I, Pasanen M. Public health burden of coronary heart disease risk factors among middle-aged and elderly men. Prev Med. 1999;28:343-348. [DOI] [PubMed] [Google Scholar]
- 22.Garber CE, Blissmer B, Deschenes MR, et al. ; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334-1359. [DOI] [PubMed] [Google Scholar]
- 23. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423-1434. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655-2667. [DOI] [PubMed] [Google Scholar]
- 25. Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33(6 suppl):S446-S451. [DOI] [PubMed] [Google Scholar]
- 26. LaMonte MJ, Ainsworth BE, Reis JP. Measuring physical activity. In:Wood TM, Zhu W. eds. Measurement Theory and Practice in Kinesiology. Champaign, IL: Human Kinetics; 2006:237-271. [Google Scholar]
- 27. Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obes Rev. 2009;10:7-16. [DOI] [PubMed] [Google Scholar]
- 28. Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6 suppl):S379-S399. [DOI] [PubMed] [Google Scholar]
- 29. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572-584. [DOI] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services; 1996. [Google Scholar]
- 31. US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans: Fact Sheet for Health Professionals on Physical Activity Guidelines for Adults. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 32. Haskell WL. J. B. Wolffe Memorial Lecture. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc. 1994;26:649-660. [DOI] [PubMed] [Google Scholar]
- 33. Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081-2091. [DOI] [PubMed] [Google Scholar]
- 34. Duncan GE, Anton SD, Sydeman SJ, et al. Prescribing exercise at varied levels of intensity and frequency: a randomized trial. Arch Intern Med. 2005;165:2362-2369. [DOI] [PubMed] [Google Scholar]
- 35. Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1-8. [DOI] [PubMed] [Google Scholar]
- 36. Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452-458. [PubMed] [Google Scholar]
- 37. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575-1581. [DOI] [PubMed] [Google Scholar]
- 38. Agha G, Loucks EB, Tinker LF, et al. Healthy lifestyle and decreasing risk of heart failure in women: the Women’s Health Initiative observational study. J Am Coll Cardiol. 2014;64:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersen K, Mariosa D, Adami HO, et al. Dose-response relationship of total and leisure time physical activity to risk of heart failure: a prospective cohort study. Circ Heart Fail. 2014;7:701-708. [DOI] [PubMed] [Google Scholar]
- 40. Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in atherosclerosis risk in communities. Med Sci Sports Exerc. 2013;45:901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of major lifestyle risk factors for incident heart failure in older adults: the cardiovascular health study. JACC Heart Fail. 2015;3:520-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Echouffo-Tcheugui JB Butler J Yancy CW Fonarow GC.. Association of physical activity or fitness with incident heart failure: a systematic review and meta-analysis. Circ Heart Fail. 2015;8:853-861. [DOI] [PubMed] [Google Scholar]
- 45. Georgiopoulou VV, Kalogeropoulos AP, Chowdhury R, et al. ; Health ABC Study. Exercise capacity, heart failure risk, and mortality in older adults: the Health ABC Study. Am J Prev Med. 2017;52:144-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He J Ogden LG Bazzano LA Vupputuri S Loria C Whelton PK.. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996-1002. [DOI] [PubMed] [Google Scholar]
- 47. Hu G Jousilahti P Antikainen R Katzmarzyk PT Tuomilehto J.. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237-244. [DOI] [PubMed] [Google Scholar]
- 48. Kenchaiah S Sesso HD Gaziano JM.. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khan H Jaffar N Rauramaa R Kurl S Savonen K Laukkanen JA.. Cardiorespiratory fitness and nonfatal cardiovascular events: a population-based follow-up study. Am Heart J. 2017;184:55-61. [DOI] [PubMed] [Google Scholar]
- 50. Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail. 2014;16:180-188. [DOI] [PubMed] [Google Scholar]
- 51. Koo P, Gjelsvik A, Choudhary G, et al. Prospective association of physical activity and heart failure hospitalizations among black adults with normal ejection fraction: the Jackson Heart study. J Am Heart Assoc. 2017;6:e006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kraigher-Krainer E, Lyass A, Massaro JM, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail. 2013;15:742-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kubota Y Evenson KR Maclehose RF Roetker NS Joshu CE Folsom AR.. Physical activity and lifetime risk of cardiovascular disease and cancer. Med Sci Sports Exerc. 2017;49:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kupsky DF, Ahmed AM, Sakr S, et al. Cardiorespiratory fitness and incident heart failure: The Henry Ford ExercIse Testing (FIT) Project. American Heart J. 2017;185:35-42. [DOI] [PubMed] [Google Scholar]
- 55. LaMonte MJ, Larson JC, Manson JE, et al. Physical activity and heart failure incidence among postmenopausal women. Circulation. 2016;134(suppl 1):A19444. [Google Scholar]
- 56. Larsson SC Tektonidis TG Gigante B Akesson A Wolk A.. Healthy lifestyle and risk of heart failure: results from 2 prospective cohort studies. Circ Heart Fail. 2016;9:e002855. [DOI] [PubMed] [Google Scholar]
- 57. Myers J, Kokkinos P, Chan K, et al. Cardiorespiratory fitness and reclassification of risk for incidence of heart failure: the Veterans exercise testing study. Circ Heart Fail. 2017;10:e003780. [DOI] [PubMed] [Google Scholar]
- 58. Ogunmoroti O, Oni E, Michos ED, et al. Life’s Simple 7 and incident heart failure: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2017;6:e005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rahman I Bellavia A Wolk A.. Relationship between physical activity and heart failure risk in women. Circ Heart Fail. 2014;7:877-881. [DOI] [PubMed] [Google Scholar]
- 61. Rahman I Bellavia A Wolk A Orsini N.. Physical activity and heart failure risk in a prospective study of men. JACC Heart Fail. 2015;3:681-687. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Tuomilehto J, Jousilahti P, et al. Occupational, commuting, and leisure-time physical activity in relation to heart failure among Finnish men and women. J Am Coll Cardiol. 2010;56:1140-1148. [DOI] [PubMed] [Google Scholar]
- 63. Young DR, Reynolds K, Sidell M, et al. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7:21-27. [DOI] [PubMed] [Google Scholar]
- 64. Patel K, Sui X, Zhang Y, et al. Prevention of heart failure in older adults may require higher levels of physical activity than needed for other cardiovascular events. Int J Cardiol. 2013;168:1905-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132:1786-1794. [DOI] [PubMed] [Google Scholar]
- 66. Bouchard C, Shephard RJ, Stephens T. The consensus statement. In:Bouchard C, Shephard RJ, Stephens T. eds. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics; 1994:9-76. [Google Scholar]
- 67. Gielen S Schuler G Adams V.. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221-1238. [DOI] [PubMed] [Google Scholar]
- 68. Gielen S Hambrecht R.. Effects of exercise training on vascular function and myocardial perfusion. Cardiol Clin. 2001;19:357-368. [DOI] [PubMed] [Google Scholar]
- 69. McCarthy JJ.. microRNA and skeletal muscle function: novel potential roles in exercise, diseases, and aging. Front Physiol. 2014;5:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mora S Cook N Buring JE Ridker PM Lee IM.. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mora S Lee IM Buring JE Ridker PM.. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412-1419. [DOI] [PubMed] [Google Scholar]
- 72. Moyna NM Thompson PD.. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113-123. [DOI] [PubMed] [Google Scholar]
- 73. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154-1162. [DOI] [PubMed] [Google Scholar]
- 74. Andersen LJ, Hansen PR, Sogaard P, Madsen JK, Bech J, Krustrup P. Improvement of systolic and diastolic heart function after physical training in sedentary women. Scand J Med Sci Sports. 2010;20(suppl 1):50-57. [DOI] [PubMed] [Google Scholar]
- 75. Andersson C, Lyass A, Larson MG, et al. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle-aged adults. J Am Heart Assoc. 2015;4:e001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arbab-Zadeh A, Perhonen M, Howden E, et al. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bhella PS, Hastings JL, Fujimoto N, et al. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail. 2014;2:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Edelmann F, Gelbrich G, Dungen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780-1791. [DOI] [PubMed] [Google Scholar]
- 80. Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095-3101. [DOI] [PubMed] [Google Scholar]
- 81. Christensen NJ, Galbo H. Sympathetic nervous activity during exercise. Annu Rev Physiol. 1983;45:139-153. [DOI] [PubMed] [Google Scholar]
- 82. Parsons TJ, Sartini C, Ash S, et al. Objectively measured physical activity and kidney function in older men; a cross-sectional population-based study. Age Ageing. 2017;46:1010-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller MS, Vanburen P, Lewinter MM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sarzynski MA, Loos RJ, Lucia A, et al. Advances in exercise, fitness, and performance genomics in 2015. Med Sci Sports Exerc. 2016;48:1906-1916. [DOI] [PubMed] [Google Scholar]
- 86. Carlson SA, Hootman JM, Powell KE, et al. Self-reported injury and physical activity levels: United States 2000 to 2002. Ann Epidemiol. 2006;16:712-719. [DOI] [PubMed] [Google Scholar]
- 87. Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358-2368. [DOI] [PubMed] [Google Scholar]
- 88. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. O’Connor CM, Whellan DJ, Lee KL, et al. ; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]