Abstract
Triclosan (TCS) is an antimicrobial agent that was effectively banned by the FDA from hand soaps in 2016, hospital soaps in 2017, and hand sanitizers in 2019; however, TCS can still be found in a few products. At consumer-relevant, non-cytotoxic doses, TCS inhibits the functions of both mitochondria and mast cells, a ubiquitous cell type. Via the store-operated Ca2+ entry mechanism utilized by many immune cells, mast cells undergo antigen-stimulated Ca2+ influx into the cytosol, for proper function. Previous work showed that TCS inhibits Ca2+ dynamics in mast cells, and here we show that TCS also inhibits Ca2+ mobilization in human Jurkat T cells. However, the biochemical mechanism behind the Ca2+ dampening has yet to be elucidated. Three-dimensional super-resolution microscopy reveals that TCS induces mitochondrial swelling, in line with and extending the previous finding of TCS inhibition of mitochondrial membrane potential via its proton ionophoric activity. Inhibition of plasma membrane potential (PMP) by the canonical depolarizer gramicidin can inhibit mast cell function. However, use of the genetically encoded voltage indicators (GEVIs) ArcLight (pH-sensitive) and ASAP2 (pH-insensitive), indicates that TCS does not disrupt PMP. In conjunction with data from a plasma membrane-localized, pH-sensitive reporter, these results indicate that TCS, instead, induces cytosolic acidification in mast cells and T cells. Acidification of the cytosol likely inhibits Ca2+ influx by uncoupling the STIM1/ORAI1 interaction that is required for opening of plasma membrane Ca2+ channels. These results provide a mechanistic explanation of TCS disruption of Ca2+ influx and, thus, of immune cell function.
Keywords: triclosan, store-operated calcium entry, mast cell, T cell, acidification, genetically encoded voltage indicator, super-resolution microscopy
Introduction
Triclosan (TCS) is a formerly widespread antimicrobial agent: it was estimated that 75% of the US population in 2008 was exposed to TCS (Calafat et al., 2008). Recently, the U.S. Food and Drug Administration effectively banned TCS from hand soaps in 2016 (Kux, 2016), from hospital products in 2017 (Kux, 2017), and from over-the-counter hand sanitizers in 2019 (Gottlieb, 2019). TCS was also removed by the Colgate-Palmolive company from its top-selling toothpaste product (Kary, 2019) in 2019. Despite these stoppages, TCS remains in some antibacterial household products that are not regulated by the FDA and in a few remaining personal care products (www.ewg.org). TCS is readily absorbed into the skin (Queckenberg et al., 2010) and oral mucosa (Gilbert, 1987) where it remains for a significant time prior to metabolism and clearance (Moss et al., 2000), thus allowing for a constant chronic exposure upon periodic re-exposure. In this study, non-cytotoxic, micromolar TCS doses, that model human exposure levels to TCS following personal care product application (Weatherly and Gosse, 2017; Weatherly et al., 2018), are utilized. Added to consumer products for its antimicrobial properties, TCS, ironically, inhibits the functioning of mammalian immune cells whose physiological purpose is to fight microbial infections (Udoji et al., 2010; Palmer et al., 2012; Hurd-Brown et al., 2013).
Clinically, TCS is used for its antimicrobial (Daoud et al., 2014) and anti-gingivitis properties (Rover and Leu-Wai-See, 2014). Additionally, previous studies provided evidence that TCS could potentially be used to treat atopic dermatitis (Sporik and Kemp, 1997; Tan et al., 2010). Despite these positive clinical effects, within the past few years a panoply of triclosan epidemiology studies have emerged, reporting various adverse TCS health effects (Weatherly and Gosse, 2017). Adverse TCS-linked effects on the reproductive system include increased spontaneous abortion rate (Wang et al., 2015), abnormal sperm morphology (Jurewicz et al., 2018; Zamkowska et al., 2018), and decreased fecundity (Vélez et al., 2015; Zhu et al., 2019). Additionally, newborn infants’ weight, length, and head circumference decreased due to TCS exposure (Etzel et al., 2017). Cognitive effects have also been observed; TCS has been linked to lower cognitive test scores in children (Jackson-Browne et al., 2018) and to higher behavior problem scores in 8-year old boys (Jackson-Browne et al., 2019). TCS causes metabolic effects including decreased BMI (Li et al., 2015), increased risk of gestational diabetes (Ouyang et al., 2018), changes in thyroid hormone levels in blood (Koeppe et al., 2013; Wang et al., 2017) and increased risk of type 2 diabetes in women (Xie et al., 2020). Triclosan exposure is also associated with decreased bone mass density and increased osteoporosis (Cai et al., 2019). Additionally, allergic rhinitis has been associated with TCS exposure (Kim and Kim, 2019). However, the cellular and molecular mechanisms underlying these epidemiological observations are not fully understood. Triclosan inhibition of mast cells (Palmer et al., 2012; Weatherly et al., 2013) and mitochondria (Ajao et al., 2015; Shim et al., 2016; Weatherly et al., 2016; Weatherly et al., 2018; Weatherly et al., 2020), in human cells (Weatherly et al., 2016) and in other species, may be related to these human health effects.
One of the underlying mechanisms of adverse human health effects caused by TCS may be its mitochondrial toxicity. An uncoupler due to its ionizable proton (Ajao et al., 2015; Weatherly et al., 2016), triclosan inhibits adenosine triphosphate (ATP) production and increases oxygen consumption rate in multiple cell types (Weatherly et al., 2016) and in live zebrafish (Shim et al., 2016). Additionally, TCS deflates mitochondrial membrane potential (MMP), thwarts mitochondrial translocation and Ca2+ dynamics, and induces mitochondrial fission and deformation (as assessed in two-dimensional [2D], super-resolution, live-cell microscopy (Weatherly et al., 2018). These TCS effects on MMP and mitochondrial morphology have been recapitulated in an in vivo mouse study (Weatherly et al., 2020). Critically, mitochondrial toxicity by TCS has been directly connected to its instigation of inflammation and immunotoxicity: TCS increases Drp1 and decreases Opa1 expression levels, effects which both increase mitochondrial fission and activate the NLRP3 inflammasome (Weatherly et al., 2020).
Separate from its mitochondrial toxicity, TCS disrupts other cellular signal transduction processes, such as Ca2+ mobilization and cytoskeletal remodeling, which are shared by numerous cell types (Weatherly et al., 2018). For example, TCS disrupts the functioning of the immunological/neurological cell type mast cells. TCS mitotoxicity explains an estimated ~10% this inhibition (Weatherly et al., 2018); the complete biochemical mechanism underlying TCS inhibition of mast cell function remains unknown and is explored further in this manuscript. Mast cells are found in a large variety of tissues (Dvorak, 1986; Theoharides and Sant, 1991; Farrell et al., 1995; Blank et al., 2007) and are involved in defense against parasites (Metcalfe et al., 1997), bacteria (Johnzon et al., 2016), and cancer (Hempel et al., 2017). Mast cell dysfunction is associated with neurological diseases (Silver and Curley, 2013; Girolamo et al., 2017) such as multiple sclerosis (Elieh-Ali-Komi and Cao, 2017). Of course, mast cells are also major effector cells of allergy and asthma (Galli et al., 2005), and previous clinical reports of atopic disorder alleviation by TCS, noted above (Sporik and Kemp, 1997; Tan et al., 2010), align with our findings of mast cell inhibition by TCS (Palmer et al., 2012).
Mast cells undergo degranulation, the release of bioactive mediators such as histamine and serotonin, in a process that begins with multivalent antigen (Ag) mediated cross-linking of immunoglobulin E (IgE)-primed FcεRI receptors on the cell surface. This binding initiates a phosphorylation cascade in which phospholipase C gamma (PLCγ) is activated (Kinet, 1999). PLCγ subsequently cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and phosphatidylinositol 4,5-bisphosphate (IP3). The latter is then able to bind to its receptor on the endoplasmic reticulum (ER) membrane and initiate the release of ER Ca2+ stores into the cytosol (Berridge, 1993). Reduced ER Ca2+ concentration results in altered conformation of ER protein STIM1, causing it to bind to and change the conformation of the ORAI1 subunit of the Ca2+ release-activated Ca2+ (CRAC) channel, opening it to a flood of external Ca2+ flowing into the cytosol (Vig et al., 2006; Hogan et al., 2010); this process is termed store-operated Ca2+ entry, or SOCE (Putney, 1986). SOCE is required for mast cell degranulation (as reviewed in (Holowka et al., 2012) because increased cytosolic Ca2+ levels allow for events required for the movement of granules to the plasma membrane for exocytosis, such as protein kinase C (PKC) activation (Ozawa et al., 1993), phospholipase D (PLD) activation (Chahdi et al., 2002), and microtubule polymerization (Guo et al., 1998; Smith et al., 2003).
These signaling processes are important in a variety of cell types, including in the immune system and brain. SOCE mechanisms are largely conserved across cell types (Prakriya and Lewis, 2015). For example, T-cell signaling (Marano et al., 1993; Trebak and Kinet, 2019) also begins with T-cell receptor (TCR) cross-linking, as with FcεRI. In T-cells (part of the adaptive immune system) in vivo, this crosslinking is primarily done by an antigen presenting cell (APCs; innate immune cells such as macrophage or a dendritic cells), presenting antigen on the major histocompatibility complex (Punt et al., 2019). The TCR/APC interaction can be emulated in vitro through the use of an anti-TCR antibody (Marano et al., 1993). Such cross-linking leads to PLCγ activation and subsequent generation of IP3 and SOCE, via the same process outlined above for mast cells. The subsequent increase in cytosolic Ca2+ concentration allows for calmodulin/calcineurin binding and subsequent activation of the nuclear factor of activated T-cells (Punt et al., 2019; Trebak and Kinet, 2019), an important Ca2+-dependent transcription factor. Thus, signaling upstream of SOCE is very similar between mast cells and T-cells; whereas signaling downstream of SOCE is largely different between mast cells and T-cells. While TCS is known to interfere with bodily distribution of T cells (Anderson et al., 2016), cytokine production (Barros et al., 2010; Marshall et al., 2015; Anderson et al., 2016; Marshall et al., 2017), and expression of calcium-binding biomarkers of inflammation (Marshall et al., 2017), triclosan effects on intracellular signal transduction, functional outcomes, and SOCE within T cells have not yet been examined.
Triclosan disrupts mast cell function by strongly inhibiting Ag-stimulated Ca2+ influx into the cytosol via the CRAC channels (Weatherly et al., 2018). This depressed cytosolic Ca2+ concentration leads, as expected, to inhibition of downstream events, such as reduced PLD activity (Shim et al., 2019) and microtubule polymerization (Weatherly et al., 2018). Thus, inhibition of SOCE by TCS explains its inhibition of mast cell function. The question remains: how does TCS inhibit Ca2+ entry through CRAC channels?
One explanation could be TCS disruption of any of the upstream signaling events leading to SOCE: FcεRI crosslinking, any link in the phosphorylation cascade, PIP2 signaling function, or IP3 binding to its ER membrane receptor. However, TCS does not hinder Ca2+ efflux from the ER (Weatherly et al., 2018) and does not seriously interfere with PIP2 (Shim et al., 2019)— evidence that TCS does not inhibit any of these events upstream of ER Ca2+ release.
Because TCS does not hamper the signaling events culminating in ER Ca2+ release, an alternative mechanism underlies TCS inhibition of CRAC channel Ca2+ entry. Chemical depolarization of plasma membrane potential (PMP) reduces Ca2+ entry into the cytosol of mast cells (Mohr and Fewtrell, 1987a) and also of T cells (Sarkadi et al., 1990). Integrity of PMP is important for SOCE due to its contribution to the free energy, ΔGtransport, available to import Ca2+ down its electrochemical gradient into the cytoplasm. In this study, we hypothesized that TCS, as a proton ionophore (Weatherly et al., 2016), inhibits Ca2+ influx by depolarizing PMP similarly to how TCS deflates membrane potential of mitochondria (Weatherly et al., 2018) and of artificial bilayers (Popova et al., 2018)—by acting as a “Trojan horse” to provide safe passage for protons through lipid membranes.
The driving force for Ca2+ influx through the CRAC channel is calculated as (Nelson and Cox, 2017)
Using physiological temperature of 310K, the gas and Faraday’s constants, the number of charges on the transported ion (n=2), the sign of the charge of the transported ion (Z=+), resting PMP ΔΨ of −82.5 mV (Lindau and Fernandez, 1986; Wischmeyer et al., 1995), C1 = extracellular Ca2+ concentration in the buffer (1.8 mM), and C2 = average cytosolic Ca2+ concentration in an Ag-stimulated mast cell (1 μM) (Millard et al., 1988; Chandra et al., 1994), the driving force (Gibbs free energy) for Ca2+ influx through open CRAC channels is the highly exergonic value −35 kJ/mol. If TCS were to completely depolarize the mast cell PMP, the term nZFΔΨ would collapse to zero, the driving force behind SOCE would be reduced ~45%, to −19 kJ/mol, and, hence, 45% less Ca2+ would flood into the cell. In fact, this PMP-knockout-predicted ~45% reduction in integrated Ca2+ influx was observed in mast cells following 20 μM TCS exposure (Weatherly et al., 2018).
Thus, in this study, the first aim was to measure TCS effects on PMP in mast cells. The mast cell model rat basophilic leukemia, clone 2H3 (RBL), which are functionally similar to mature human mast cells, rodent mucosal mast cells, and basophils (Fewtrell et al., 1979; Metzger et al., 1982; Seldin et al., 1985; Metcalfe et al., 1997; Abramson and Pecht, 2007; Lee et al., 2012), was used. RBL cells are effective for toxicological studies due to their ability to respond to exogenous agents in a similar fashion as primary bone-marrow derived mast cells (Zaitsu et al., 2007; Thrasher et al., 2013; Alsaleh et al., 2016).
Conventionally, PMP is measured through the use of patch clamping or of voltage-sensitive fluorescent organic dyes. However, traditional patch clamping is a low-throughout process. Also, TCS is a chemical quencher of many unprotected fluorescent chromophores (such as those found in organic voltage sensitive dyes) (Weatherly, 2017). Thus, genetically encoded voltage indicators (GEVIs), reporter protein constructs with a β-barrel protein structure that protects the internal fluorophore from TCS fluorescence interference (Weatherly, 2017; Weatherly et al., 2018) were used. Targeted to the plasma membrane, GEVIs communicate changes in PMP through changes in fluorescence intensity. To our knowledge, this study represents the first use of a GEVI in an immune cell model or in the field of toxicology.
An additional novel use of a biophysical technique in toxicology, three-dimensional (3D) Fluorescence Photoactivation Localization Microscopy (FPALM) super-resolution microscopy was also employed to detail TCS effects, via membrane depolarization, on mitochondrial morphology in mast cells (Parent and Hess, 2019). Conventional light microscopy, including widefield and confocal imaging, cannot resolve objects that are less than ~250 nm apart, due to the diffraction limit, but FPALM breaks this diffraction limit (Hess et al., 2006). These 3D studies augment, by providing information on mitochondrial volume and surface area, previous 2D work showing that TCS disrupts mitochondrial nanostructure, causing “donut” shapes or fragmentation (Weatherly et al., 2018).
In this study, we test the hypothesis that TCS inhibits mast cell function by depolarizing the plasma membrane. However, careful experimentation with two GEVI constructs, which function via disparate molecular machinery, indicate a wholly different mechanism of TCS action: cytoplasmic acidification. Furthermore, TCS replicates this mechanism of action and abrogation of function in another immune cell type, T cells.
Methods
Chemicals and Reagents
Triclosan (TCS; 99% purity; Sigma-Aldrich) was prepared in BT (Tyrodes buffer containing bovine serum albumin [BSA]) without use of organic solvent (Weatherly et al., 2013) and diluted to deliver non-cytotoxic concentrations (Palmer et al., 2012) checked by UV-Vis spectrophotometry (Weatherly et al., 2013). Gramicidin (Sigma- Aldrich) was dissolved in 100% DMSO (Sigma-Aldrich) and diluted with BT.
Cell Culture
RBL-2H3 mast cells were cultured as in (Hutchinson et al., 2011).
Human Jurkat T cells, clone E6–1, were obtained from ATCC and maintained in suspension in phenol red-containing RPMI-1640 medium (ATCC) supplemented with 10% fetal bovine serum (Atlanta Biologics) and 100 U/ml penicillin-100 μg/ml streptomycin (Sigma-Aldrich). Cells were passaged once a week with thorough trituration technique to break up clumps, seeded at 35,000 cells/mL (for 6–7 days until harvest) to 125,000 cells/mL (for 3 days), and grown at 37°C and 5% CO2. Supplement fresh media was added after 3 days in culture. These culturing conditions resulted in maximal cell density of 1–2 million cells/mL.
Fluorescence Photoactivation Localization Microscopy (FPALM) Imaging and Processing
Three-dimensional FPALM mitochondrial imaging was performed as in (Parent and Hess, 2019). RBL cells were transfected with an expression vector for Dendra2-Tom20 (Weatherly et al., 2018) using an Amaxa transfection kit (Lonza), then plated in μ-Slide 8-well plates with polymer coverslip (ibidi) at 100,000 cells/well in 200 F06DL/well phenol red-free RBL media. The next day, cells were exposed to 20 μM TCS or BT for 1 hour and fixed with 4% paraformaldehyde (Sigma Aldrich) before imaging. Imaging was performed using a 558 nm laser (Crystalaser) for Dendra2-Tom20 excitation, and fluorescence was captured using an Olympus IX-71 microscope with 60X 1.45NA oil lens, 2X telescope, and an EMCCD camera (Andor iXon DU-897 #BV). Custom MATLAB analysis software was used to obtain localized data points (Hess et al., 2006; Gudheti et al., 2013; Curthoys et al., 2019); details of microscopy are in Supplemental Methods.
FPALM Mitochondrial Analysis
In MATLAB, raw data was localized first by fitting each Point Spread Function (PSF) to a two-dimensional (2D) Gaussian, which was then drift corrected. After drift correction, following the methods in (Huang et al., 2008; Parent and Hess, 2019), the z-coordinate of each localized point was obtained from the measured calibration curve connecting the x- and y- widths of the PSF as a function of z- position. After obtaining the z-position for each localized point, the data set was processed through another custom MATLAB script for cluster identification, which compares the distances between each localization and all nearby localizations: localizations that lie within dmax (75 nm) of each other are considered to be in the same cluster. Clusters with a minimum number of 50 localizations were analyzed further. The nearest-neighbor single linkage cluster analysis (SLCA) method (Sneath, 1957; Gudheti et al., 2013) used here has been extended to three dimensions (Parent and Hess, 2019). Each cluster is then analyzed using the MATLAB convex hull and alpha shape functions, in order to quantify the local mean curvature for all localizations within an individual mitochondrion. Histograms and averages of the curvature, area, and volume of the convex and alpha hulls are then determined.
Degranulation Assay
Degranulation assays were performed as in (Weatherly et al., 2013), adapted for use with gramicidin in 0.003% DMSO vehicle. This fluorescence-based assay, performed in the 96-well format in a microplate reader, detects substrate cleavage by granule marker enzyme β-hexosaminidase in the supernatant from degranulating mast cells.
DiSC3(5) Fluorescence Interference Assay
Triclosan effects on DiSC3(5) (TCI America) organic voltage sensitive dye fluorescence were assayed. The dye was dissolved in 100% DMSO. In a 96 well black bottom plate (Greiner), DisC35 dye and TCS were mixed to achieve a final dye concentration of 1.02 μM in 0.01% final DMSO percentage, with various TCS concentrations (1–15 μM) in BT. The mixture was allowed to equilibrate for 10 min at 37°C. Fluorescence measurements were taken using a microplate reader (Synergy 2, Biotek) with 530 ± 20 nm excitation and 645 ± 15 nm emission.
Plasma Membrane Potential and Cytosolic pH Assays in RBL-2H3 Mast Cells
In order to measure changes in plasma membrane potential, RBL cells were transfected with genetically-encoded voltage indicator (GEVI) protein construct ArcLight-A242 (a gift from Vincent Pieribone; Addgene plasmid # 36857; http://n2t.net/addgene:36857; RRID:Addgene_36857) (Jin et al., 2012) or ASAP2 (pcDNA3.1/Puro-CAG-ASAP2s was a gift from Francois St-Pierre; Addgene plasmid # 101274; http://n2t.net/addgene:101274; RRID:Addgene_101274) (Chamberland et al., 2017). To investigate TCS effects on cytosolic pH, RBL cells were transfected with plasma membrane-targeted Lyn-tailed mCherry-SuperEcliptic (SE) pHluorin protein construct (Lyn-tailed mCherry-SEpHlourin was a gift from Sergio Grinstein; Addgene plasmid # 32002; http://n2t.net/addgene:32002; RRID:Addgene_32002) (Koivusalo et al., 2010). RBL cells were transiently transfected with ArcLight A242, ASAP2, or Lyn-tailed mCherry-SuperEcliptic (SE) pHluorin through electroporation using RBL-specific Amaxa Nucleofector transfection kit T (Lonza), then plated in 8-well plates (ibidi) at ~150,000 cells/well in 200 μL/well phenol red-free RBL media. Cells were grown for 16–24 hrs at 37°C/ 5% CO2. Before imaging, cells were washed with BT and the media was replaced with 200 μL BT. After the initial, untreated, image was taken, cells were treated with 200 μL of either BT (for control) or various 2X concentrations of TCS or gramicidin, depending on the experiment. See “Confocal Microscopy” below for imaging details.
Plasma Membrane Potential Assay in Jurkat T Cells
Ibidi 8-well plates were coated with 150 μL of human fibronectin (VWR) prepared at 166 μg/ml in phosphate buffered saline (PBS) (Lonza) (Wang et al., 2019) overnight in the tissue culture incubator (37°C/5% CO2); these wells were washed the next day with 200 μL PBS/well before use. Jurkat cells were transiently transfected with ArcLight-A242 through electroporation using Jurkat-specific Amaxa Nucleofector transfection kit T (Lonza), then plated in the fibronectin pre-coated plates at ~1million cells/well in 300 μL phenol red-free Jurkat media. Cells were grown for 16–24 hours at 37°C/ 5% CO2. Next day, cell media was removed carefully with a transfer pipette to avoid cell detachment and replaced with 200 μL control BT before imaging. After the initial, untreated, image was taken, cells were treated with 200 μL of either additional BT (for control) or various 2X concentrations of TCS. See “Confocal Microscopy” below for imaging details.
Confocal Microcopy
For plasma membrane potential and cytosolic pH assays, an Olympus FV-1000 confocal microscope, with an Olympus IX-81 inverted microscope and a 30 milliwatt multi-argon laser, was used to collect images. ArcLight-A242 and ASAP2 plasmid-transfected cells were imaged using an oil immersion 100x objective with NA 1.4 and 488 nm excitation, 505–605 nm band pass emission filter. Lyn tailed mCherry-SEpHluorin transfected cells were imaged using oil immersion 60x objective with NA 1.4 and 488 nm excitation, 505–525 nm emission filter. All images were taken at 37°C using ibidi plate heating system.
Manual Image Analysis
All image-analysis figures in this paper were generated with this manual method except for Figure 8 and Supplement Figure 3, which were analyzed with the automated image analysis procedure noted below. Confocal microscopy images of RBL and Jurkat cells transfected with ArcLight and ASAP2 were analyzed manually using Fiji ImageJ software (NIH). Well-transfected cells (i.e. visible expression of reporter and well-targeted to the plasma membrane) were identified and a Region of Interest (ROI) was drawn on the plasma membrane using the free-hand tool. The area of the ROI (in units of square pixels), integrated density (= sum of fluorescence intensity values of all pixels in the ROI), mean intensity (= integrated density divided by area of the ROI), and length of the ROI were subsequently measured at various specified time points before and after treatment.
Figure 8.
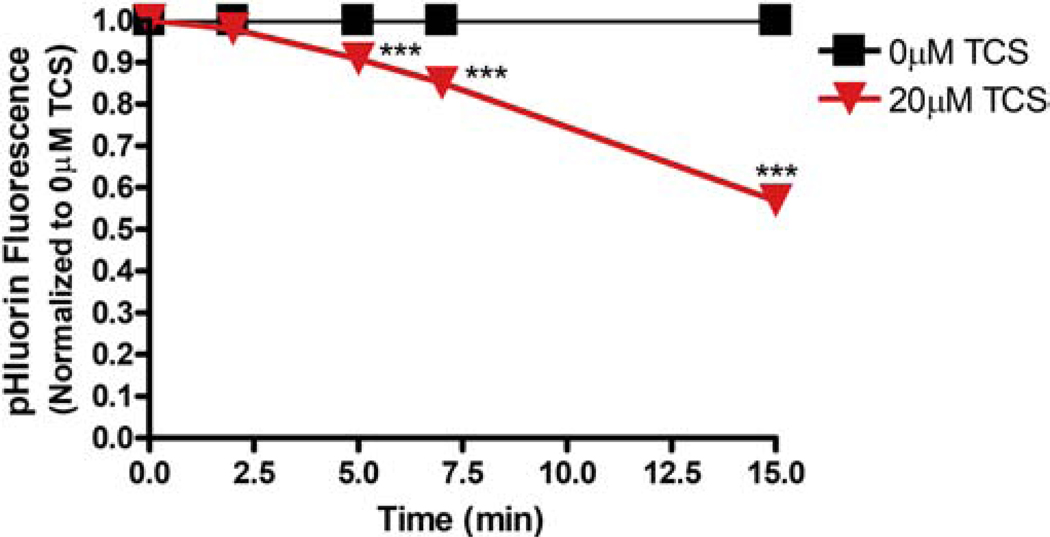
Triclosan effects on fluorescence of Lyn-tailed mCherry-SuperEcliptic (SE) pHluorin in RBL mast cells. RBL cells were transiently transfected with Lyn-tailed mCherry-SE pHluorin, washed with BT, and exposed to BT (N=35) or 20 μM TCS (N=52) for 15 minutes. At each time point, the average fluorescence of each plasma membrane was measured, background-subtracted, and normalized to the 0 min timepoint, as described in Image J Automated Methods. Values presented are means ± SEM of 3 independent days of experiments per treatment. Statistically significant results at each timepoint, as compared to the appropriate control (0 μM TCS or 0 μM gramicidin + DMSO vehicle), are represented by ***p < 0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test.
Mean background fluorescence intensity (=integrated density of ROI divided by area of ROI) for a field of view, was obtained by either drawing a square shape ROI in the cytoplasmic region of transfected cells or by drawing an ROI around the plasma membrane of untransfected cells. Background mean fluorescence intensity obtained through either of these methods was subtracted from mean ArcLight fluorescence intensity of cells present in that field of view, and the resulting value at each time point was then normalized to (divided by) its own 0 min timepoint value of mean intensity. Both of these background subtraction methods resulted in similar ArcLight fluorescence changes due to 20 μM TCS: data for 7 cells selected randomly at the 15 min with 20 μM TCS, the cytosolic square shape method of background subtraction results in a calculated decrease of 36% ± 7% SEM in Arclight fluorescence while, for the same cells, the background subtraction method using a plasma membrane trace of untransfected cells results in a calculated decrease of 36% ± 8% SEM.
Photobleaching or drifting effect during imaging was calculated from BT-treated (control) cells by calculating a change in fluorescence values from 0 min. This change value was added/subtracted back to the treatment groups to account for these non-treatment effects and thus allowing measure of the true effect of a drug. Normalized values from multiple cells were averaged and used to generate line plots.
Automated Image Analysis
In FIJI image J, individual images at different time points (0, 2, 5, 7, and 15 min) were converted to 8-bit stack. Background fluorescence was subtracted from the stack using pseudo flatfield correction. Next, by applying threshold, binary masks of the entire cell and only cytosol were created. Both of these masks were applied to transfected cells to obtain area of the ROI, integrated density, and mean intensity values. To find the area of the ROI, mean intensity and integrated density of plasma membrane, values of cytosol were subtracted from the area of the ROI and integrated density of whole cell. The calculated integrated density was divided by the calculated area of ROI. This will give mean fluorescence intensity of the plasma membrane. Mean intensity at different time points of an individual cell was normalized to (divided by) its own 0 min time point value of mean intensity. Fluorescence was adjusted for drifting or photobleaching as described in “Manual Image Analysis” Methods above. This automated method was utilized to generate Figure 8 and Supplement Figure 3.
Analysis of Triclosan Effects on ArcLight-A242 Fluorescence Intensity as a Function of Initial ArcLight-A242 Expression Level
Initial (0 min) mean plasma membrane fluorescence intensity of ArcLight for each individual cell was noted. Next, for each individual cell, the percentage drop in ArcLight fluorescence mean intensity incurred by 15 min exposure to 20 μM triclosan (or to control BT) was then plotted as a function of that cell’s initial mean intensity of ArcLight (its expression level of the construct). Linear regression analysis was performed, and the slope of the best-fit line and R2 value was determined for each plot.
Apparent Plasma Membrane Potential Percentage Decrease Calculation
Apparent PMP percentage decrease was calculated for the treatments, cell types, and GEVIs utilized in this study. Each normalized and photobleaching-/drift-corrected GEVI fluorescence value was subtracted from 1 to determine change in fluorescence due to treatment. This result was subsequently multiplied by its respective reporter’s PMP to fluorescence change ratio to calculate mV change as reported by each construct. For ArcLight-A242, this ratio is 100 mV for every 35% decrease in fluorescence (100 mV/0.35) (Jin et al., 2012). For ASAP2, this ratio is 100 mV for every 39% decrease in fluorescence (100 mV/0.39) (Chamberland et al., 2017). This change, in units of mV, was then divided by the resting PMP of the corresponding cell type, RBL mast cell (−82.5 mV is the average value from two patch-clamp studies (Lindau and Fernandez, 1986; Wischmeyer et al., 1995)) or T cell (−60 mV)(Sarkadi et al., 1990) to determine the (unitless) change in total resting cell PMP. Finally, this result was subsequently subtracted from 1 and multiplied by 100 to obtain the apparent PMP percentage.
TCS Cytotoxicity on Jurkat Cells
Trypan blue exclusion and lactate dehydrogenase (LDH) cytotoxicity assays were used to assess TCS cytotoxicity on Jurkat cells. In the trypan blue exclusion assay, 1 million cells were plated into each of 3 wells of a 24-well, flat-bottom cell culture plate (Costar) for TCS (Sigma) treatment and 3 wells for the control. Immediately after plating, BT and 2X TCS doses were added to respective wells. Next, cells were incubated for 30 minutes at 37°C/5% CO2. After the incubation, a small sample was taken from each well and mixed 1:1 with trypan blue dye (0.4%, Lonza) evenly. Cells were counted using a hemocytometer for viability and TCS-treated cells were normalized to the control. LDH cytotoxicity methods were those of Hutchinson et al., 2011 using a cytotoxicity detection kit (Roche). However, the lysis solution was added to the “high control” in the final 15 minutes of the 1-hour TCS exposure instead of an additional 15 minutes after the 1-hour TCS exposure (Palmer et al., 2012).
Cytosolic Ca2+ assay
Prior to performing Ca2+ assays, widefield fluorescence microscopy was used to assess transfection efficiency of GCaMP6 Ca2+ reporter construct in Jurkat T cells, and Ca2+ assays were performed only on samples of highly-transfected cells. First, 8-well ibidi plates were coated with 150 μL/well fibronectin (166 μg/ml) prepared in PBS and incubated overnight in tissue culture incubator. The next day, Jurkat T cells were transfected with pGP-CMV-GCaMP6f (a gift from Douglas Kim & GENIE Project; Addgene # 40755; http://n2t.net/addgene:40755; RRID: Addgene_40755) (Chen et al., 2013) using Jurkat specific Amaxa Nucleofector transfection kit T (Lonza). The electroporated cells were plated in phenol red-free Jurkat cell media at ~1 million cells/well with phenol red-free media in the fibronectin pre-coated 8-well ibidi plates. Cells were grown for 16–24 hours at 37°C/ 5% CO2. The next day, cell media was removed carefully with a transfer pipette to avoid cell detachment and replaced with 200 μL BT, and images were taken with a wide-field IX83 (Olympus, Waltham MA) microscope with a Prime 95B CMOS Camera (Photometrics) and HLD117 stage (Prior Scientific) controlled by a Proscan III. Fluorescence excitation was provided by an Xcite 120 LEDBoost (Excelitas). Images were taken at 60x (Olympus-APON-60X-TIRF objective) using standard excitation and emission filters for GFP (Semrock). The microscope is controlled by CellSens software v1.18 (Olympus).
For assessment of Jurkat T cell cytosolic Ca2+ levels following anti-T cell receptor (anti-TCR) stimulation, a plate reader assay was performed similarly to that in (Weatherly et al., 2018), with the following modifications. First, a 96-well, black-walled, clear-bottom plate (Grenier) was coated overnight with 50 μL/well fibronectin (166 μg/ml) prepared in PBS (Lonza). The next day, cells were transfected with the GCaMP6 construct as noted above and plated in 200 μL/well phenol red-free media in the fibronectin pre-coated 96-well plate at 330,000 cells/well. Cells were cultured overnight at 37°C and in 5% CO2. Next day, media was carefully aspirated from wells with a micropipette to avoid cell detachment, and cells were exposed to 100 μL/well 0.2 μg/ml (Holowka et al., 2018) Anti-TCR OKT3 monoclonal antibody (Thermo Fisher Scientific) in combination with control BT or TCS treatments for 1 hour. Fluorescence was measured with 485 ± 10 nm excitation and 528 ± 10 nm emission during this 1 hour. Area under the curve was determined as per methods in Weatherly et al., 2018.
Statistical Analyses
All analyses were performed in Graphpad Prism. In most figures, raw values for treatment groups were normalized to its appropriate untreated control. Biological replicates from at least three independent experiments were averaged, and standard error of the mean (SEM) was calculated across those independent experiments. Raw (non-normalized) values were analyzed for mitochondria swelling and LDH release experiments (Figures 1, 6D). One-way ANOVA followed by Tukey’s post-hoc test (α=0.05) was used to determine the significance level of most experiments (Figures 2, 3B, 5, 6, 7B, 8, Supplement Figures 2–6). The significance level of the FPALM mitochondrial volume and surface area were assessed using an unpaired one-tailed t-test (Figure 1). The significance level of the cytosolic Ca2+ level area under the curve (AUC) of Jurkat cells was assessed via paired t-test (Figure 9B). Significance is represented by ***p<0.001, **p<0.01, and *p<0.05.
Figure 1.
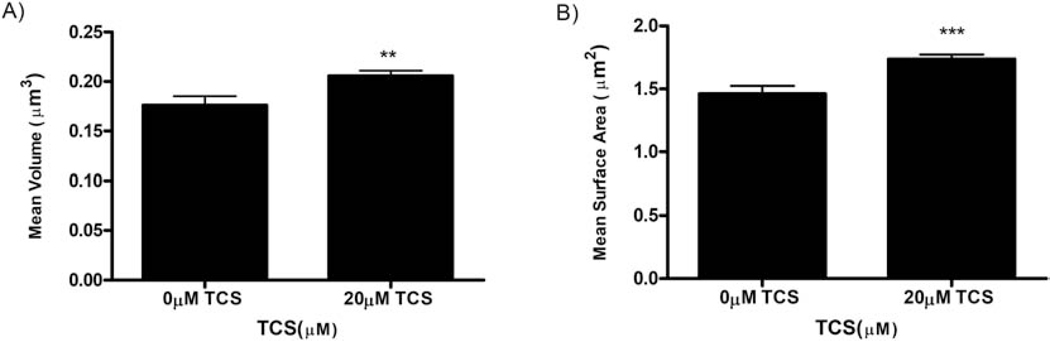
Triclosan effects on mitochondrial volume and surface area in RBL mast cells. Super-resolution FPALM 3D images of Dendra2-TOM20, which labels outer mitochondrial membranes, were processed through custom-built MATLAB code in which an algorithm identifies individual mitochondria. As detailed in the Methods, (A) average mitochondrial volume was calculated by the convex hull method, and (B) average mitochondrial surface area was calculated by the alpha shape method. Values presented are mean SEM for total of 62 cells in three independent experiments, where each independent experiment had 8–11 cells for each condition (0 vs. 20 μM triclosan). Statistically significant results are represented by **p<0.01 and ***p<0.001 as compared to control (0 μM TCS) for volume and surface area, respectively, as determined by unpaired t-test.
Figure 6.
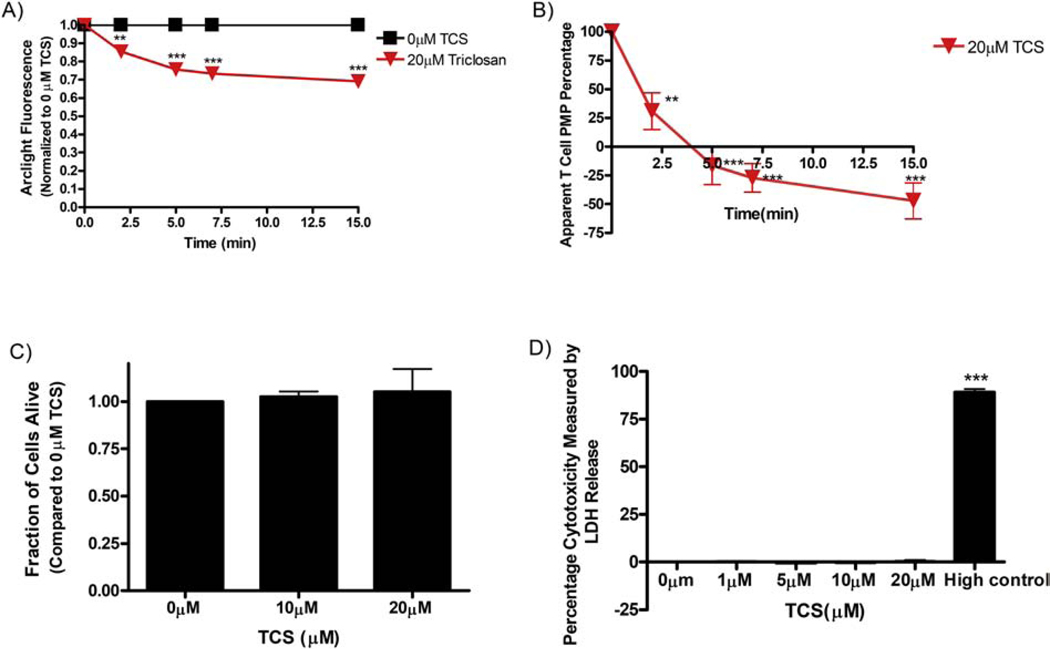
Triclosan effects on fluorescence of ArcLight-A242 in human Jurkat T cells, triclosan apparent effects on Jurkat plasma membrane potential (PMP), and cytotoxicity determination. (A) Jurkat cells were transiently transfected with ArcLight-A242, washed with BT, exposed to BT (0 μM TCS) (N=20) or 20 μM TCS (N=17) for 15 minutes. At each time point, the average fluorescence of each plasma membrane was measured, background-subtracted, and normalized to the 0 min timepoint, as described in Methods. (B) Data from Figure 6A were utilized to calculate percentage of PMP (with each 0 min timepoint defined as 100%) as a function of time (min), as described in Methods. (C) Jurkat T cell cytotoxicity to TCS was assessed by trypan-blue exclusion assay and by (D) lactate dehydrogenase (LDH) detection kit from Roche. “High control” is a sample treated with lysis solution provided by the kit. Values presented are mean ± SEM of at least three independent experiments. Statistically significant results, as compared to appropriate control, are represented by **p<0.01, ***p < 0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test.
Figure 2.
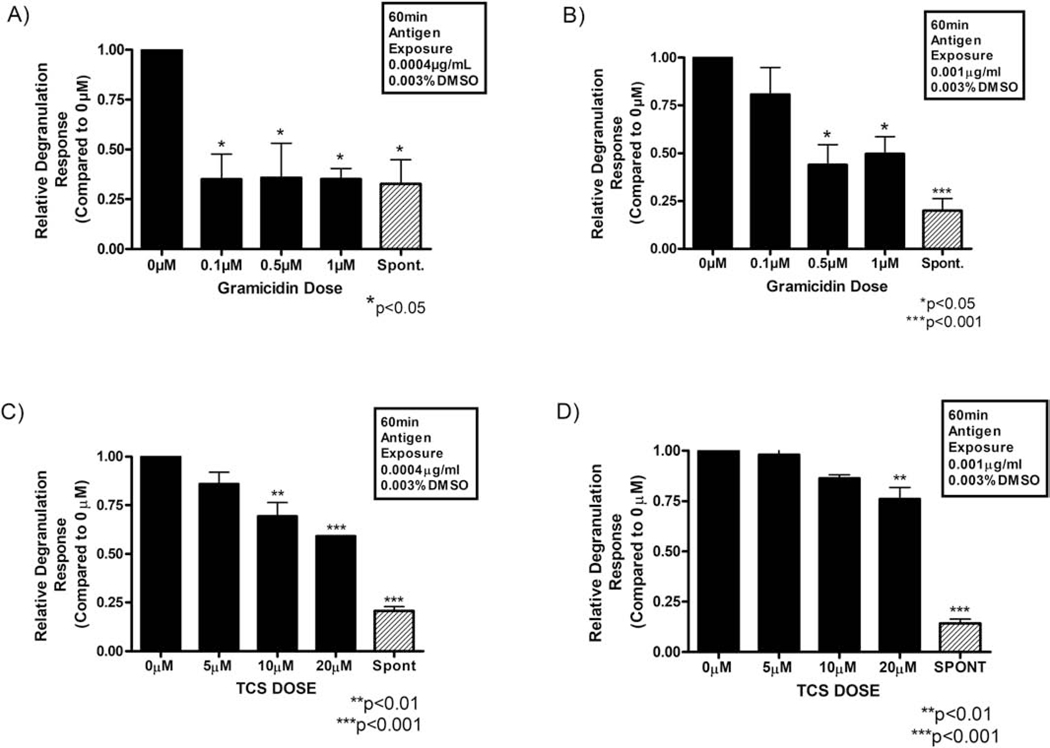
Relative degranulation response of antigen-stimulated RBL mast cells exposed to micromolar doses of the canonical plasma membrane depolarizer gramicidin (A and B) or to triclosan (C and D). IgE-sensitized cells were stimulated for 1 h with either 0.0004 μg mL−1 Ag (A and C) or 0.001 μg mL−1 Ag (B and D). All samples (A - D) contained 0.003% DMSO, which was the vehicle required for gramicidin dissolution. For spontaneous release (“Spont” on x-axes), cells were incubated for 1 h in BT with 0.003% DMSO (with no IgE, Ag, TCS, or gramicidin). Values represent the mean ± SEM of 3 independent experiments; three replicates per treatment type were used each experimental day. Statistically significant results, as compared to the appropriate control (0 μM TCS + DMSO vehicle) or (0 μM gramicidin + DMSO vehicle), are represented by *p < 0.05, **p < 0.01, ***p<0.001 as determined by one-way ANOVA followed by Tukey’s post-hoc test.
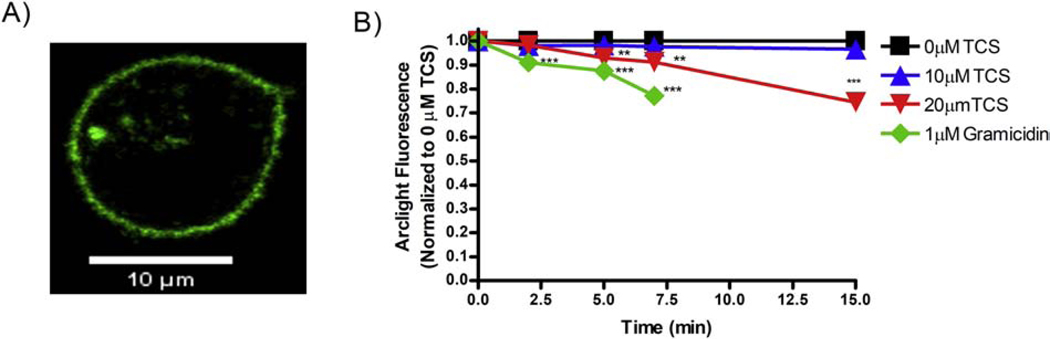
Figure 3.
Triclosan and gramicidin effects on fluorescence of ArcLight-A242 in RBL mast cells. (A) One representative live-cell confocal microscopy image of an RBL mast cell transiently transfected with ArcLight construct, prior to gramicidin or TCS treatment. Scale bar, 10 μm. (B) RBL cells were transiently transfected with ArcLight, washed with BT, exposed to control (BT) (N = 52), 10 μM TCS (N = 22), 20 μM TCS (N = 28), or 1 μM gramicidin (N = 24) for 15 minutes. At each time point, the average fluorescence of each plasma membrane was measured, background-subtracted, and normalized to the 0 min timepoint, as described in Methods. Values presented are means ± SEM of at least 3 independent days of experiments per treatment. Statistically significant results at each timepoint, as compared to the appropriate control (0 μM TCS or 0 μM gramicidin + DMSO vehicle), are represented by **p < 0.01, ***p < 0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test.
Figure 5.
Triclosan and gramicidin apparent effects on plasma membrane potential (PMP) of RBL mast cells. Data from Figure 3B were utilized to calculate percentage of PMP (with each 0 min timepoint defined as 100%) as a function of time (min), as described in Methods. Values presented are means ± SEM of at least 3 independent days of experiments per treatment. Statistically significant results at each timepoint, as compared to the appropriate control (0 μM TCS or 0 μM gramicidin + DMSO vehicle), are represented by ***p < 0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test.
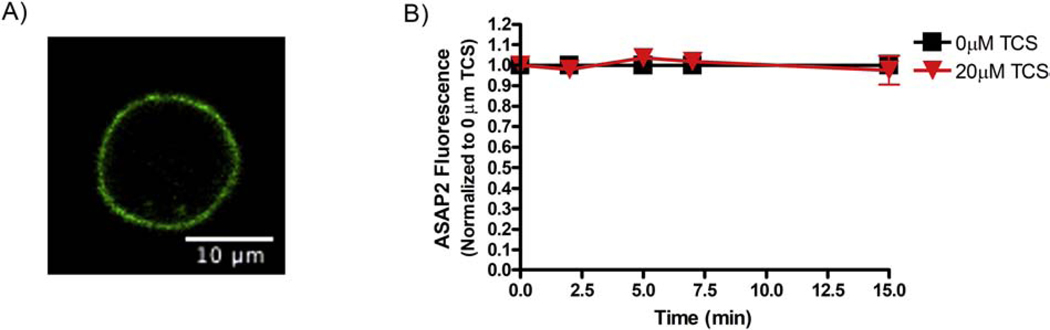
Figure 7.
Triclosan effects on fluorescence of ASAP2 in RBL mast cells. (A) One representative live-cell confocal microscopy image of an RBL mast cell transiently transfected with ASAP2 construct, prior to TCS treatment. Scale bar, 10 μm. (B) RBL cells were transiently transfected with ASAP2, washed with BT, exposed to control (BT) (N=18) or 20 μM TCS (N=21) for 15 minutes. At each time point, the average fluorescence of each plasma membrane was measured, background-subtracted, and normalized to the 0 min timepoint, as described in Methods. Values presented are means ± SEM of at least 6 independent experiments per treatment. Analysis by one-way ANOVA followed by Tukey’s post-hoc test found no statistical significance.
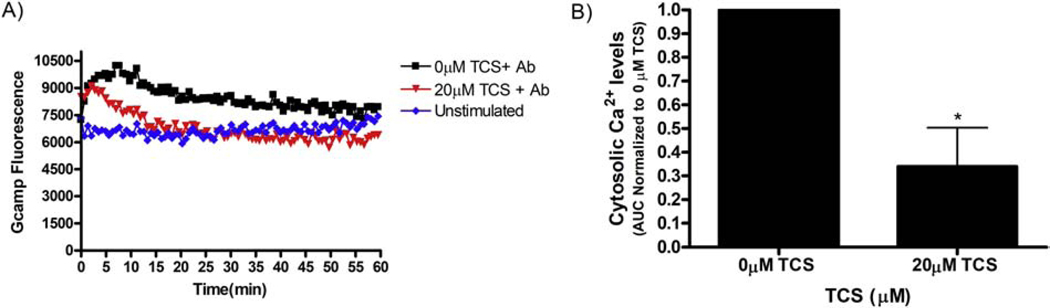
Figure 9.
Triclosan effects on cytosolic Ca2 + levels as activated by anti-T Cell Receptor (TCR) antibody in Jurkat T Cells. Jurkat cells were transiently transfected with cytosolic Gcamp6 overnight, then exposed in BT Control or 20 μM TCS, each with 0.2 μg/ml anti-TCR (“Ab”) for 1 hour. “Unstimulated” contained no TCS and no anti-TCR. Raw fluorescence was measured using a microplate reader as described in Methods. (A) Representative figure (no error bars) shows fluorescence obtained following background fluorescence subtraction (mock-transfected cells’ fluorescence) from treatment groups at each time point. (B) Area under the curve (AUC) was obtained from the fluorescence curves and values were normalized to the control of each day for 3 replicates per treatment group per day. AUC values presented are means ± SEM of 3 independent days of experiments. Statistically significant results, as compared to the appropriate control (0μM TCS), are represented by *p < 0.05, as determined by unpaired t-test.
Results
Triclosan increases mitochondrial volume and surface area in RBL-2H3 mast cells: Indicators of inhibited mitochondrial membrane potential
Previously, an organic dye fluorescence method (without imaging) revealed that TCS inhibits MMP (Weatherly et al., 2018). To test this finding at the nanoscale, three-dimensional super-resolution FPALM imaging (Huang et al., 2008; Parent and Hess, 2019) was employed to assess TCS effects on 3D mitochondrial morphology, changes in which are linked to inhibition of MMP (Guillery et al., 2008; Giedt et al., 2012; Weatherly et al., 2018). RBL cells were transiently transfected with Dendra2-TOM20 (Weatherly et al., 2018), a construct used to label the outer membrane of mitochondria. The next day, cells were exposed with TCS or BT control for 1 hour, then fixed using paraformaldehyde. While the TCS effects on mitochondrial volume and surface area were not dramatic visually (a no-TCS control figure is shown in Supplement Figure 1), they were statistically significant when analyzed quantitatively. Triclosan statistically significantly increases the mean volume, as calculated by the convex hull method, by 17% compared to control (Figure 1A). Also, triclosan increases the mean mitochondrial surface area, as calculated by the alpha shape method, by 19% (Figure 1B). Thus, TCS modulates the surface area and volume of individual mitochondria, as assessed with 3D FPALM super-resolution microscopy.
Plasma membrane potential depolarizer gramicidin potently inhibits the degranulation of RBL-2H3 mast cells
To determine the effects of the canonical plasma membrane depolarizer, gramicidin, on RBL mast cell degranulation, an adapted fluorescence-based β-hexosaminidase release assay (Weatherly et al., 2013) was employed. A multivalent DNP (dinitrophenol)-BSA Ag was used to laterally crosslink IgE-bound FcεRI receptors, thus initiating an allergic/pro-inflammatory signal transduction that ends with the release of granules containing bioactive substances including β-hexosaminidase, which is monitored fluorometrically. Gramicidin depolarizes cells by forming an ion channel at the plasma membrane, allowing for free passage monovalent ions such as H+, NH4+, K+, Na+, and Li+ down their concentration and electrochemical gradients, and, thus, depolarizing the plasma membrane (Myers and Haydon, 1972; Mohr and Fewtrell, 1987b).
Figure 2A presents the results for IgE-sensitized RBL cells incubated for 1 h in Tyrodes—BSA (BT) buffer containing a 0.0004 μg/ml Ag dose, with gramicidin or DMSO vehicle. All tested gramicidin doses, as low as 0.1 μM, inhibit degranulation, reducing the response down to the same level as the spontaneous group (unstimulated with Ag). These data indicate a potent, plasma membrane depolarization-mediated inhibition of degranulation. A similar, but slightly less potent, gramicidin-induced depression of degranulation can be seen in the group treated with the higher Ag dose, 0.001 μg/ml Ag (Figure 2B). In this case, statistically significant inhibition of degranulation does not begin until 0.5 μM gramicidin and appears to proceed in a dose-responsive fashion. Such data coincide with the previously reported observation that pharmacologically induced inhibition of degranulation occurs at lower toxicant doses when cells are stimulated at a low level (0.0004 μg/ml Ag) than when stimulated at a high level (0.001 μg/ml Ag) (Palmer et al., 2012).
A dose comparison study for gramicidin and TCS was performed as part of testing the hypothesis that TCS inhibits degranulation via PMP inhibition, by repeating the above experiments with TCS in place of gramicidin at equivalent antigen and DMSO concentrations. While DMSO is not needed/used here to dissolve the TCS, it was included to match the conditions of the gramicidin experiments. Statistically significant, dose-responsive inhibition of mast cell degranulation by TCS is reported in Figures 2C and 2D, beginning at 10 μM (Figure 2C). This observation recapitulates previously observed TCS-mediated inhibition of degranulation (Palmer et al., 2012), but the presence of DMSO reduces its potency: 10 μM TCS inhibits 0.0004 μg/ml Ag-stimulated degranulation by about one-half in the absence of DMSO (Weatherly et al., 2013) and only by about one-third in the presence of 0.003% DMSO (Figure 2C). Also, the TCS inhibitory effect lessens in the presence of higher Ag dose (0.001 μg/ml Ag; Figure 2D), as noted with gramicidin Figure 2B. Overall, depolarizer-mediated inhibition of degranulation (Figure 2A and 2B) proceeds similarly as that of TCS, though gramicidin is more potent than TCS, especially in the presence of DMSO. The result strengthens the connection between PMP inhibition and degranulation inhibition.
Gramicidin and triclosan depress ArcLight-A242 fluorescence in RBL-2H3 mast cells.
Gramicidin, a known depolarizer of PMP, strongly inhibits degranulation (Figs. 2A and 2B), and TCS also reduces degranulation (Palmer et al., 2012). Also, TCS depolarizes the MMP (Weatherly et al., 2018) (Figure 1). Thus, we hypothesized that TCS also inhibits the PMP, as its underlying mechanism of degranulation inhibition. To do so, an organic voltage-sensitive fluorescent dye, DiSC3(5) was employed. However, when TCS (micromolar levels) and DiSC3(5) (1 μM) (Te Winkel et al., 2016) are mixed together in the absence of cells, TCS chemically quenches the fluorescence of DiSC3(5) in a dose-response manner (Supplement Figure 2); thus, DiSC3(5) cannot be used to accurately measure TCS effects on PMP. Triclosan quenching of unprotected fluorophores (such as those found in various voltage-sensitive, fluorescent organic dyes) has previously been observed (Weatherly, 2017; Weatherly et al., 2018). Thus, to measure TCS effects on PMP, genetically encoded voltage indicators (GEVIs), which contain β-barrel protein structures which may protect their fluorophores from chemical quenching or aggregation effects (Chalfie and Kain, 2005), were used. Triclosan does not interfere with the fluorescence of the fluorescent protein within the reporter construct GCaMP (Weatherly et al., 2018).
To measure PMP of cells using a GEVI, RBL cells were transiently transfected with ArcLight-A242 (ArcLight) (Jin et al., 2012) (Figure 3A). The next day, confocal images were collected for each cell at different time points, up to 15 minutes, before (Figure 3A) and after (Figure 3B) addition of control (BT), TCS, or gramicidin. Fluorescence obtained was quantified using FIJI image J (Manual Image Analysis Methods) and normalized to the 0 min time point for each condition. Gramicidin, a known PMP depolarizer, statistically significantly reduces ArcLight fluorescence within 2 min of exposure and by 23% (± 2% SEM) at the end of 7 minutes. TCS, at 20 μM, statistically significantly lowers ArcLight fluorescence within 5 min of exposure and by 25% (± 3% SEM) at the end of 15 min. This result was further confirmed by an automated image analysis method (Supplement Figure 3). The 10 μM TCS data suggest an inhibition of ArcLight fluorescence by 15 min but were not statistically significant. The decrease in ArcLight fluorescence suggests that both gramicidin and TCS inhibit mast cell PMP.
Since gramicidin treatment contained DMSO vehicle (0.003%), it was important to determine whether gramicidin’s effects on ArcLight fluorescence are modulated by the presence of DMSO. Thus, a control experiment was performed by repeating the gramicidin-ArcLight experiments in the presence of varying DMSO concentrations. Gramicidin’s ability to inhibit ArcLight fluorescence is unaffected by increasing DMSO concentrations, ranging from 0.003% to 0.01% (Supplement Figures 4 and 5).
After experiments with transiently-transfected cells were successfully conducted, ArcLight stably-transfected cell lines were created, with the goal of enabling high-throughput testing of numerous concentrations of triclosan and other toxicants, as well as additional time points. However, unfortunately, clones of RBL cells stably transfected with ArcLight display construct aggregation and poor plasma membrane localization (Supplement Figure 6).
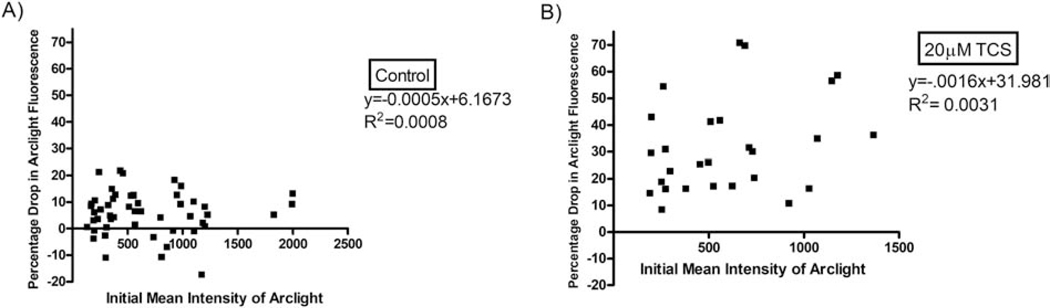
To check whether plasmid expression level affects the change in GEVI fluorescence in response to triclosan, the initial, 0 min (pre-triclosan-exposure), background-subtracted mean fluorescence intensity of each individual cell from Figure 3’s data was analyzed. The percentage drop in ArcLight fluorescence by 15 minutes time of exposure (control buffer-treated in 4A, TCS-treated in 4B), for each individual cell, was plotted as a function of that particular cell’s initial mean intensity of ArcLight. There is no correlation between plasmid expression level and the magnitude of TCS inhibition of ArcLight fluorescence (Figure 4B), as analyzed by linear regression. Thus, TCS effects on GEVI fluorescence are unaffected by the “brightness” (GEVI expression level) of the cell.
Figure 4.
Effect of cellular expression level of ArcLight-A242 on TCS inhibition of ArcLight-A242 fluorescence. Data from Figure 3B, ArcLight-A242 transfected cells analyzed by confocal imaging and image analysis comparing control (0μM TCS) and 20μM TCS treatments, were plotted as individual cells’ data. The percentage drop in fluorescence by 15 minutes time of exposure, for each individual cell, is plotted as a function of that cell’s initial mean intensity of ArcLight-A242. Statistical results per plot are represented by equation of linear regression and R2 values.
Plasma membrane depolarization causes ArcLight fluorescence intensity to decrease, in a linearly proportional fashion (Jin et al., 2012). PMP percentage decline can be calculated from the measured change in ArcLight fluorescence, as outlined in Methods. This apparent mast cell PMP, as a percentage of initial, 0 min, pre-triclosan-exposure value, is plotted as a function of time following TCS or gramicidin exposure (Figure 5). Within 7 min of exposure to 1 μM gramicidin, RBL mast cell PMP appears to decrease by 79% (± 7% SEM) of initial resting PMP. Similarly, within 15 min of exposure to 20 μM TCS, RBL cell PMP appears to decrease by 90% (± 11% SEM). There appears to be a modest, but not statistically significant, decline in PMP within 15 min exposure to 10 μM TCS.
Triclosan inhibits ArcLight fluorescence, and, thus, apparently inhibits RBL cell PMP. Thus, the next experiments tested whether these results are extendable to another immune cell type which is also dependent on PMP and SOCE for its function, T-cells.
Non-cytotoxic doses of triclosan depress ArcLight-A242 fluorescence in Jurkat T cells.
To measure triclosan effects on PMP of T cells, human Jurkat T cells were transiently transfected with ArcLight. The next day, confocal images were collected for each cell at different time points, up to 15 minutes, before and after addition of control (BT) or TCS (Figure 6A). Fluorescence obtained was quantified using FIJI image J (Manual Image Analysis Methods) and normalized to the 0 min time point for each condition. TCS, at 20 μM, lowers ArcLight fluorescence within 2 min of exposure and by 31% (± 3% SEM) at the end of 15 min (Figure 6A). The decrease in ArcLight fluorescence suggests that TCS inhibits T cell PMP.
This apparent T cell PMP was calculated (see Methods) as a percentage of initial, 0 min, pre-triclosan-exposure value and is plotted as a function of time following TCS exposure (Figure 6B). Within 15 min of exposure to 20 μM TCS, T cell PMP appears to decrease to −150% (± 15% SEM) of initial resting PMP, apparently a more-than-complete dampening of T cell PMP.
In order to determine if the results obtained above were truly functional changes or due to cytotoxicity from TCS, the effect of micromolar doses of TCS (0 μM, 10 μM, and 20 μM) on Jurkat T cell viability were assessed using a trypan blue exclusion assay. Results indicate that TCS does not cause a decrease in cell viability within a 30 min exposure (Figure 6C). In order to confirm these cytotoxicity results, a more sensitive microplate reader-based assay, LDH assay was also performed. LDH release was measured in response to varying doses of TCS for 1 hour. There is no significant release of LDH from TCS-treated cells as compared to control; the “high control” sample is a positive control detecting LDH release from lysed cells (Figure 6D). These data from cytotoxicity experiments indicate that TCS dosage timing and concentration used in this study do not cause Jurkat T cell cytotoxicity.
Triclosan does not depress the fluorescence of the genetically encoded voltage indicator ASAP2 in RBL-2H3 mast cells.
To check the results obtained using ArcLight, an alternative GEVI, called ASAP2, which utilizes a different voltage-sensing mechanism from that of ArcLight (Jin et al., 2012; Chamberland et al., 2017) was used. RBL mast cells were transiently transfected with ASAP2 (Figure 7A). The next day, confocal images were collected for each cell at different time points, up to 15 minutes, before (Figure 7A) and after (Figure 7B) addition of control (BT) or TCS. Fluorescence obtained was quantified using FIJI image J (Manual Image Analysis Methods) and normalized to 0 min time point for each condition. Triclosan (20 μM, up to 15 min) does not alter fluorescence of ASAP2 when compared to the 0 μM TCS control (Figure 7B). In stark contrast to the clear triclosan dampening of fluorescence observed with the ArcLight reporter (Figure 3), these ASAP2 results (Figure 7B) indicate that TCS does not change the PMP of RBL mast cells.
However, TCS inhibits the fluorescence of ArcLight, which contains a pH-sensitive super ecliptic pHlourin on the cytoplasmic side of the plasma membrane. Thus, the next investigation centered on whether TCS-induced depression of ArcLight fluorescence is due to a change in cytosolic pH instead of a change in PMP.
Triclosan depresses fluorescence of a plasma membrane-targeted pHlourin in RBL mast cells, indicating triclosan reduction of cytosolic pH.
To identify whether TCS affects cytosolic pH, plasma membrane-targeted fluorescence reporter called Lyn-tailed mCherry-SEpHlourin (Koivusalo et al., 2010), which measures sub-plasma membrane cytosolic pH of a cell, was employed. The transfection and imaging procedures used for the GEVI experiments were repeated, along with an automated image analysis technique (described in Methods). Triclosan, at 20 μM, decreases mCherry-SEpHlourin fluorescence intensity within 5 min of exposure and by 43% (± 2% SEM) at the end of 15 min (Figure 8). This result is similar to the magnitude of triclosan’s effect on ArcLight fluorescence (Figure 3), suggestive of a pH change in response to TCS rather than a PMP change.
TCS inhibits cytosolic Ca2+ response to Anti-T Cell Receptor stimulation in Jurkat T cells
TCS affects Ca2+ dynamics and inhibits SOCE in mast cells (Weatherly et al., 2018). Due to the similar triclosan depression of ArcLight fluorescence in Jurkat T cells (Figure 6A) as in RBL mast cells (Figure 3B), we hypothesized that a similar calcium effect also occurs in Jurkat T cells. To test this hypothesis, Jurkat T cells were transfected with Ca2+ reporter construct GCaMP6 (Chen et al., 2013). The next day, cells were stimulated with 0.2 μg/ml Anti-T Cell Receptor (TCR) OKT3 ± TCS (Holowka et al., 2018), and cytosolic Ca2+ was measured throughout the duration of 1 hour. Cells were transfected at a highly efficient rate (Supplement Figure 7). Following anti-TCR stimulation, an initial rise in Ca2+ is seen within the first few minutes, followed by a plateau Ca2+ level above unstimulated basal level (compare “0 μM TCS + Ab” to “Unstimulated” curves in Figure 9A), as expected (Holowka et al., 2018). TCS, at 20 μM, inhibits Ca2+ levels in comparison to the control in anti-TCR activated cells, in particular by flattening the plateau region (Figure 9A). Triclosan minimally affects the initial Ca2+ rise but heavily dampens calcium in the plateau region, similar to its effects in RBL mast cells (Weatherly et al., 2018). AUC analysis reveals an average decrease of 70% (± 17%) integrated Ca2+ levels in anti-TCR activated cells with TCS treatment compared to that of activated control (Figure 9B).These data indicate that TCS inhibits cytosolic Ca2+ signaling in T cells.
Discussion
Triclosan disrupts mast cell function, rapidly (within tens of minutes) and at non-cytotoxic concentrations relevant to consumer product exposure (Palmer et al., 2012; Shim et al., 2019), but the underlying biochemical mechanism was unknown. In this study, we deduced the mode of action of TCS in mast cells and replicated these TCS effects in another immune cell type, T cells.
The process of deciphering the mechanism began with two observations: TCS is a mitochondrial uncoupler (Weatherly et al., 2016; Weatherly et al., 2018) and also a disruptor of the central signal for mast cell degranulation—cytosolic Ca2+ rise (Weatherly et al., 2018). As an uncoupler, TCS depresses the MMP, which results in mitochondrial fragmentation, observed in live cells with super-resolution microscopy (Weatherly et al., 2018). Mitochondrial toxicity and deformation are important markers of TCS toxicity in numerous cell types and species (Shim et al., 2016; Weatherly et al., 2018; Weatherly et al., 2020). Disruption of mitochondrial shape plays a role in diseases such as cognitive decline (Hara et al., 2014), Parkinson’s disease (Cui et al., 2010; Bhandari et al., 2014), insulin resistance (Jheng et al., 2012), immune dysfunction (Weatherly et al., 2020), inflammation (Compan et al., 2012), and disrupted embryonic development (Chen et al., 2003). In this study (Figure 1), we have augmented these findings by utilizing 3D super-resolution FPALM to reveal TCS enhancement of mitochondrial surface area and volume, indications of mitochondrial swelling and MMP disruption (Guillery et al., 2008; Giedt et al., 2012). This increase in surface area and volume has also been observed in neuronal mitochondria due to exposure to carbonyl cyanide-4 (trifluoromethoxy)phenylhydrazone (FCCP), a known proton ionophore and MMP depolarizer (Safiulina et al., 2006). The connections between mitochondrial deformation and disrupted MMP are extended to PMP depolarization, inhibited store-operated calcium entry (SOCE), and, subsequently, dampened mast cell degranulation via studies with a similar proton ionophore mitochondrial uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP), which also inhibits MMP, PMP, SOCE, and mast cell degranulation (Mohr and Fewtrell, 1987b). Triclosan’s enhancement of mitochondrial surface area and volume led to a PMP collapse hypothesis as the mode of action of immune cell dysfunction.
Thus, we hypothesized that TCS inhibits not just MMP but also PMP. Direct evidence for this hypothesis was provided by reports that TCS induces an electrical current (evidence of its proton ionophore nature) across artificial membranes (Popova et al., 2018) and depolarizes neuronal PMP (Arias-Cavieres et al., 2018; Popova et al., 2018) at micromolar doses within tens of minutes. As calculated in the Introduction, a complete depolarization of PMP in mast cells would lead to a ~45% reduction in the driving force for cytosolic Ca2+ influx into the cell through activated CRAC channels; indeed, Weatherly et al., 2018 reported ~45% decrease in cytosolic calcium levels after TCS exposure in RBL mast cells. This study also reports (Figure 9) a robust decrease in cytosolic calcium levels after TCS exposure in Jurkat T cells. In turn, the decrease in cytosolic Ca2+ inhibits degranulation (Holowka et al., 2012). Additionally, the known PMP depolarizer gramicidin inhibits mast cell degranulation in parallel conditions to TCS mast cell disruption (Figure 2), lending further support for the PMP collapse hypothesis for the TCS mode of action.
To measure TCS effects on PMP, two genetically encoded voltage indicator (GEVI) reporter constructs, ArcLight-A242 (ArcLight) (Jin et al., 2012) and ASAP2 (Chamberland et al., 2017), were utilized. ArcLight senses changes in PMP due to its two specialized subunits: a transmembrane voltage-sensing domain which, upon changes in PMP, undergoes conformational changes that cause conformational changes in the attached green fluorescent protein (GFP)-derived, super ecliptic pHlourin fluorescent domain which is localized intracellularly, in the cytosol. ASAP2 also contains a transmembrane voltage-sensing domain but, in contrast to ArcLight, ASAP2 contains an extracellularly-located, circularly-permuted (non-pHlourin-based) fluorescent GFP (cpGFP) domain. For both of these GEVIs, their fluorescence intensity decreases upon PMP depolarization. These experiments yielded drastically different results: Triclosan inhibits ArcLight fluorescence in RBL mast cells (Figure 3) and in Jurkat T cells (Figure 6), suggesting TCS-induced collapse of PMP in these cell types (Figures 5 and 6). Note that the effect was not statistically significant with 10 μM TCS but did suggest a modest inhibition at that dose/timeframe. In contrast, TCS had no effect on ASAP2 fluorescence (Figure 7). While both ArcLight and ASAP2 are plasma membrane voltage-sensitive, there is a crucial difference in their structures: the location and pH sensitivity of their fluorescent protein domains. ArcLight’s fluorescent domain is a pHlourin, which is strongly pH-sensitive in the physiological pH range (Jin et al., 2012) (Supplement Figure 2B from this article shows that a pH reduction from 7.5 to 6.5 would drastically reduce this pHlourin’s intensity, by ~50% or more). It is located in the cytosol, so it reports changes in cytosolic pH, with acidification leading to fluorescence intensity decline. ASAP2 cannot report cytosolic pH changes because its fluorescent domain is located outside the cell, unless those changes affect the PMP (personal communication, Dr. Francois St-Pierre). Interpretation of the ASAP2 data is somewhat confounded by the fact that its cpGFP fluorescent domain is also somewhat pH sensitive (Miyawaki and Niino, 2015; Kostyuk et al., 2019). While TCS mitochondrial toxicity could lead to extracellular acidification which could affect ASAP2 fluorescence, TCS-induced extracellular acidification likely occurs on a longer timescale (Ajao et al., 2015) than the 15 min exposure assessed in the current study. Also, if TCS were acidifying the extracellular space around the RBL mast cells and Jurkat T cells within the experimental exposure time (15 min), overcoming the buffering of the BT solution, a decrease in ASAP2 fluorescence would have occurred (Stepanenko et al., 2008). Together, these data suggest that TCS does not affect mast cell or T cell PMP but, instead TCS lowers cytosolic pH.
Indeed, TCS acidifies the cytosol, as measured with lyn-tailed mCherry-SEpHluorin, a pH-sensitive, PMP-insensitive reporter construct (Figure 8). ArcLight and lyn-tailed mCherry-SEpHluorin share the same pH-sensitive fluorophore, the super ecliptic pHlourin (Koivusalo et al., 2010; Jin et al., 2012). A decrease in lyn-tailed mCherry-SEpHluorin’s fluorescence is equivalent to a cytosolic pH decrease (Koivusalo et al., 2010). This intracellular pH effect may either be localized, or be most drastic, near the plasma membrane itself as both of these pH-sensitive reporters are plasma membrane-localized (Koivusalo et al., 2010; Jin et al., 2012). As noted in Methods, a 35% decrease in the fluorescence intensity of ArcLight corresponds with a 100 mV PMP decrease (Jin et al., 2012); thus, a 29% decrease in fluorescence intensity would correspond with a complete depolarization of the RBL cell PMP (~82.5 mV) (Lindau and Fernandez, 1986; Wischmeyer et al., 1995). Thus, if the ArcLight response were measuring PMP changes in response to TCS, the maximum response of any cell should have been ~29%. On the contrary, nearly half of cells responded to TCS exposure with a greater-than-29% drop in ArcLight fluorescence (Figure 4). Some of this variation is likely due to cell-to-cell variation in signaling, even in this clonal cell line (Millard et al., 1988).
However, because ArcLight’s fluorescence can also communicate changes in pH and can be depressed nearly 100% within a 1 pH unit acidification starting at normal physiological pH (Jin et al., 2012), the cell-to-cell variation of TCS response in Figure 4 provides further evidence for TCS acidification of the cytosol.
Taking into account the pH sensitivity of its fluorophore (Jin et al., 2012), the ArcLight data (Figure 3) can be re-interpreted as a 20 μM TCS-induced pH decrease of −0.23 pH units (Table 1). The pH sensitivity of the fluorescence intensity of ArcLight’s super ecliptic A227D pHlourin (plotted in the Supplement of (Jin et al., 2012)) is approximately linear between pH 6.5 to 7.5. This relationship can be approximated using the following: F = 0.6pH – 3.8 where F is the fraction of maximal fluorescence, and the equation is valid over a range of ± 0.5 pH units. Thus, at a pH of 7.2, F = 0.52, meaning that ArcLight will exhibit 52% of its maximal fluorescence intensity at the starting, physiological pH. The 26% ± 3% (SEM) decline in ArcLight fluorescence due to 15 min of 20 μM TCS exposure (Figure 3) translates to a 26% reduction in this 0.52 value, bringing the final, normalized, ArcLight fluorescence intensity to 0.38 ± 0.02; solving for the corresponding pH value returns a final pH value of 6.97 ± 0.02 following this TCS exposure. This yields the −0.23 pH unit decrease estimate from the ArcLight data (Table 1). Additionally, the same data interpretation can be employed with lyn-tailed mCherry-SEpHluorin, resulting in a pH decrease to 6.83 ± 0.02, yielding the −0.37 pH unit decrease estimate (Table 1). These pHlourin-measured decreases in pH are similar in magnitude, providing corroborating evidence that TCS acidifies the cytosol.
Table 1.
Summary of estimated changes in cytosolic pH following TCS exposure.
| Method | Magnitude of pH Change | Citation |
|---|---|---|
| Calculated estimate | −0.3 | Discussion |
| ArcLight experiments | −0.23 | Figure 3 |
| pHlourin experiments | −0.37 | Figure 8 |
| Average | −0.3 | Table 1 |
These measured pH changes were confirmed with theoretical calculations of TCS acid-base chemistry. Taking into account the 400 μL volume of 20 μM TCS in each ibidi well containing ~400,000 cells on each experimental day (see Methods), along with an estimated 10% absorption rate (Moss et al., 2000; Weatherly and Gosse, 2017), each cell received ~5.3 × 10−15 moles, or 3.2 × 109 molecules of TCS. Considering its pKa of 7.9 (PubChem), a resting cell cytosolic pH of 7.2 (Johnson et al., 1980; Lodish et al., 2000; Beck et al., 2014), and the Henderson-Hasselbalch equation, 16.6% of TCS is deprotonated when it encounters the pH 7.2 resting cytosol. This 16.6% value equates to 5.3 × 108 deprotonated TCS molecules per cell, and hence, 5.3 × 108 excess H+ delivered to each cell as this monoprotic weak acid dissociates. While this excess proton dose, from TCS, far exceeds the proton concentration of the cell, it rapidly encounters the cellular buffering system. Carbonate (CO2/HCO3−, pKa 6.1) buffering (Nelson and Cox, 2017) accounts for approximately two-thirds of a cell’s total buffering power (Boron, 2004), and average mammalian cells contain 12 mM of HCO3− (Lodish et al., 2000), the conjugate base of this buffering system (A−); these values and the Henderson-Hasselbalch equation can be used to calculate the concentration of the weak acid (HA) in the untreated cell: 0.953 mM. The average mammalian cell has a volume of 1766 μm3 (Barrandon and Green, 1985), and the cytosol itself accounts for 70% cellular volume (Luby-Phelps, 2000). Thus, the volume of the cytosol is 1236 μm3 or 1.236 ×10−12 L. Multiplying this volume by the HA and A− concentrations noted above and by Avogadro’s number yields the number of HA and A− molecules found within the cytosol of an untreated cell: 7.1 × 108 HA molecules and 8.9 × 109 A− molecules. The 5.3 × 108 excess H+ delivered to the cell by TCS exposure interact 1:1 with these A− molecules, thereby producing 5.3 × 108 new HA molecules added to the original pool of HA molecules and subtracted from the original pool of A− molecules: resulting in 1.24 × 109 molecules of HA and 8.4 × 109 A− molecules. Plugging these values into the Henderson-Hasselbalch equation leads to a cytosolic pH of TCS-treated cells of 6.9: a −0.3 pH depression caused by TCS exposure (Table 1). In fact, the average of the pH depressions captured in the ArcLight and lyn-tailed mCherry-SEpHluorin measurements is −0.3, in agreement with this calculated value.
Acidification of the cytosol has been shown to decrease Ca2+ release-activated Ca2+ current (ICRAC), the final step in SOCE, in several cell types including RBL and Jurkat T cells expressing stromal interaction molecule 1 (STIM1) and ORAI1 (Beck et al., 2014). In the Beck study, the cytosol of each assayed cell was acidified via direct introduction of NaOH or HCl via pipette. In the current study, the cytosol was acidified by exposure to TCS (a weak acid, pKa 7.9) via the extracellular buffer (Table 1), rather than by direct injection of strong acid or base (Beck et al., 2014). A stepwise effect, reduction of ICRAC, occurred upon decreasing the pH from 7 to 6 (Beck et al., 2014). Similarly, TCS reduces Ca2+ flux into the cytosol of RBL and Jurkat cells (Weatherly et al., 2018) (Figure 9). Triclosan-mediated acidification of the cytosol may thus explain the previously observed inhibition of Ca2+ dynamics (Weatherly et al., 2018) and, thus, of degranulation (Holowka et al., 2012).
Another proton ionophore mitochondrial uncoupler, CCCP, also inhibits Ca2+ influx into RBL mast cells (Mohr and Fewtrell, 1987b). While this effect is partly caused by CCCP’s depolarization of PMP (Mohr and Fewtrell, 1987b), it can also be reversed by increasing the pH (i.e. alkalization) of the surroundings (Mohr and Fewtrell, 1987b) and, hence, of the cytosol because H+ becomes plasma membrane-permeant when CCCP is incorporated into the membrane (McLaughlin and Dilger, 1980). These findings suggest that CCCP inhibition of SOCE is partly caused by its pH modulation. Additionally, experiments on isolated neurons have shown that another proton ionophore mitochondrial uncoupler, FCCP, also acidifies the cytosol (Tretter et al., 1998). Therefore, TCS is acting as expected, from its proton ionophore mitotoxicant nature, in acidifying the immune cell cytosol and, then, inhibiting SOCE.
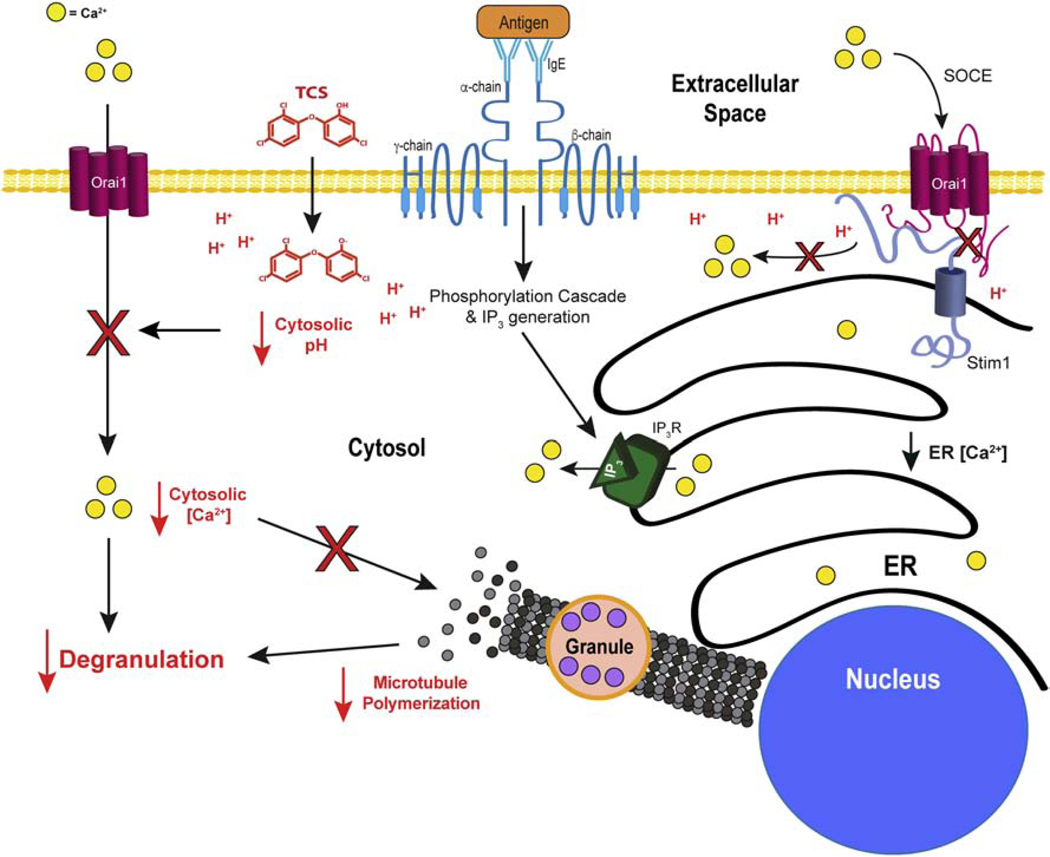
While under some conditions acidification of the cytoplasm can inhibit SOCE by blocking the binding of IP3 to its receptor on the ER (Tsukioka et al., 1994), release of Ca2+ from the ER is actually enhanced in TCS-treated mast cells (Weatherly et al., 2018). Following anti-TCR activation of SOCE in T cells, the initial rise of Ca2+ release from the ER is largely unaffected by TCS whereas the plateau region representing SOCE via CRAC channels is heavily reduced by TCS (Figure 9), further evidence that IP3 receptor interference is not the key mechanism of TCS inhibition in T cells, as well. Instead, it is likely that TCS-induced cytosol acidification blocks the proper interaction of the STIM and ORAI1 machinery that is required for CRAC channel opening (Thompson et al., 2009; Mancarella et al., 2011). Histidine 155, found between transmembrane domains (TM) 2 and 3 of ORAI1, plays an important role in sensing intracellular pH (Tsujikawa et al., 2015). Upon cytoplasmic acidification, histidine 155 becomes protonated and affects intermolecular interaction of other components in the loop between TM2 and TM3 of the ORAI1 that may result in CRAC channel closing (Tsujikawa et al., 2015). Based upon the Henderson-Hasselbalch equation and the pKa of histidine (6.0; Nelson and Cox 2017), 5.9% of these histidines are protonated at pH 7.2, whereas 11.2% are protonated at pH 6.9. (Note: if the protein environment surrounding histidine 155 changes its pKa, there could be a greater change in its charge upon acidification). Thus, the level of acidification induced by TCS (−0.3 pH unit, Table 1) likely induces a doubling of the concentration of ORAI1 proteins containing positively-charged histidines in this key cytoplasmic motif. Regardless of the mechanism of acidification (direct injection of strong acid as in Beck et al., 2014, or exposure to weak acid TCS dissolved in the extracellular buffer as in this study), the resulting acidification will cause this critical histidine to be more likely positively charged. This histidine is conserved in rat (as in RBL cells) and human (as in the Jurkat T cells), according to an NCBI Blast multiple amino acid sequence alignment. Overall, protonation and closing of CRAC channels is likely the mechanism of TCS-induced reduction of Ca2+ influx into mast cells (Weatherly et al., 2018) and T cells (Figure 9), leading to inhibition of mast cell function (Palmer et al., 2012). Future experiments utilizing the fluorescence resonance energy transfer techniques of Mancarella et al., 2011 or other approaches should be able to directly test the hypothesis that TCS disrupts the STIM1/ORAI1 interaction.
Future research will examine inhibition of T cell functions downstream of SOCE, such as release of essential cytokines (Punt et al., 2019). In addition to the known TCS inhibition of mast cell degranulation and other functions (Palmer et al., 2012), TCS also inhibits the lytic function of natural killer cells (Udoji et al., 2010; Hurd-Brown et al., 2013), which are important defenders against cancer and viral infections.
In addition to acidifying the cytosol by its direct proton ionophore mechanism of providing a pathway for charged protons to flow into the cell, TCS may also be acidifying the cell contents via other mechanisms. TCS increases the production of reactive oxygen species (ROS) in mast cells (Weatherly et al., 2018) and in other cell types (Binelli et al., 2009; Riva et al., 2012; Tamura et al., 2012; Yueh et al., 2014; Lv et al., 2016; Weatherly et al., 2018), which impair the Na/H+ exchanger, leading to reduced intracellular pH (Kaufman et al., 1993; Nakamura et al., 2006). TCS also causes mitochondrial fission/fragmentation (Weatherly et al., 2018), processes associated with reduced intracellular pH as a result of lactic acid buildup attributed to increased glycolysis (Johnson and Nehrke, 2010; Schurr, 2014). While the timeframes in which these processes acidify the cell are likely longer than the rapid (within 15 min) acidification reported in this study, these mechanisms suggest that TCS will continue to acidify the cell over longer exposure times and possibly at lower doses; ROS stimulation and mitochondrial dysfunction occur in primary human keratinocytes, mast cells, and other cell types at lower doses (starting ~1 μM) than the 10–20 μM used in the current study.
If this TCS acidification occurs in the mitochondria to a similar degree (~0.3 pH unit acidification of the matrix), the driving force (Gibbs free energy) available for producing ATP on ATP synthase in the inner mitochondrial membrane will be also reduced, as previously observed (Weatherly et al., 2016). This effect can be estimated by calculating the driving force both in the presence and absence of 20 μM TCS, via the equation
(Nelson and Cox, 2017). Using the 37°C temperature used experimentally, the gas and Faraday’s constants, pH = −0.75, and ΔΨ = −0.2 V (Nelson and Cox, 2017), the free energy released by protons flowing through ATP synthase into the matrix is a robust ~−24 kJ/mol in a healthy cell. Taking into account a 0.3 pH unit acidification of the matrix (such that ΔpH = −0.45) and a 40% reduction in ΔΨ (Weatherly et al., 2018) due to 20 μM TCS exposure, the free energy released by protons flowing through ATP synthase into the matrix is a weaker ~−14 kJ/mol in a TCS-treated cell. This result implies a ~40% reduction in the free energy available for making ATP. While imperfect, this value is a reasonable match to the >50% reduction in ATP production caused by 20 μM TCS in RBL cells (Weatherly et al., 2016).
TCS inhibits MMP (Weatherly et al., 2018); however, TCS does not inhibit PMP. This apparent contradiction can be explained by the inherent cellular mechanisms in charge of creating and then maintaining either the MMP or the PMP. The MMP is primarily generated by the action of the electron transport chain (ETC) proton pumps (Nelson and Cox, 2017); MMP is entirely reliant on this segregation of protons. TCS is a proton ionophore mitochondrial uncoupler, thus, acting in direct opposition to the proton pumps of the ETC (Weatherly et al., 2016). Such direct opposition, with no alternative means of MMP maintenance, serves to explain why TCS can significantly depress the MMP. In contrast to the MMP’s generation by proton pumping, PMP is primarily generated by the action of the Na+/K+ ATPase, (Nelson and Cox, 2017), which is located nearly exclusively on the plasma membrane (Bertorello et al., 2003) and is not present on the mitochondrial membrane. In RBL mast cells, the Na+/K+ ATPase contributes to PMP (Bronner et al., 1989) and resides on the plasma membrane of RBL cells, and its inhibition leads to dampening of Ag-stimulated degranulation (Gentile and Skoner, 1996). Thus, TCS modulation of proton concentrations could affect cytosolic pH without altering PMP. TCS does cause PMP depolarization of artificial membranes which do not contain the Na+/K+ ATPase (Popova et al., 2018). TCS-mediated changes in proton concentrations across these membranes would therefore not be counteracted by the Na+/K+ ATPase. TCS also causes PMP depolarization of neuronal models (Arias-Cavieres et al., 2018; Popova et al., 2018) at micromolar doses within tens of minutes. Neurons heavily rely on the PMP for their function and, while the Na+/K+ ATPase is still the primary source of this voltage, neurons possess additional PMP regulation mechanisms (Bean, 2007), which may explain triclosan’s differential effects on PMP of immune cells vs. neuronal cells. Interestingly, TCS inhibits Na+/K+ ATPase activity in Labeo rohita gills (Hemalatha et al., 2019), a hint that, over longer exposure periods than those used in the current study, TCS may interfere with cellular PMP.
In conclusion, we report the mechanism of TCS inhibition of mast cells and T cells (Figure 10). Three-dimensional super-resolution microscopy shows that TCS causes mitochondrial swelling in mast cells, further evidence for its depolarization of the mitochondrial membrane. However, TCS does not inhibit immune cells by dampening their plasma membrane potential. Using genetically encoded voltage indicators coupled with pH-indicating reporters, we have identified that TCS acidifies the cytosol but does not affect PMP in immune cells. Cytosolic acidification by TCS likely disrupts the Stim1-ORAI1 interaction, causing CRAC channel closing and collapse of Ca2+ influx into mast cells and T cells. TCS-induced reduction of Ag-stimulated cytosolic Ca2+ influx results in inhibited enzymatic activity and decreased microtubule polymerization, leading to suppression of mast cell degranulation. Collapse of SOCE in T cells also likely leads to inhibition of T cell function. Any cell type that depends on Ca2+ signaling or mitochondrial function is susceptible to TCS toxicity (Feske, 2007; Hill-Eubanks et al., 2011). In summary, TCS, a mitochondrial toxicant, is also an immunotoxicant via its modulation of immune cell signal transduction.
Figure 10.
Effects of triclosan on mast cell signaling. Antigen crosslinking of IgE receptors, early phosphorylation events, and calcium release from the ER are uninhibited by TCS. However, TCS brings protons into the cytosol, decreasing cytosolic pH, thereby inhibiting CRAC channel activation, cytosolic calcium levels, microtubule polymerization, and, subsequently, degranulation.
Supplementary Material
Antimicrobial triclosan inhibits mast cell and T cell signal transduction
Three-dimensional super-resolution microscopy reveals mitochondrial swelling
Triclosan inhibits crosslinker-stimulated Ca2+ influx into mast cells and T cells
Triclosan does not affect plasma membrane potential in mast cells and T cells
Triclosan acidifies the cytosol, thus impairing plasma membrane Ca2+ channels
Acknowledgments
We thank Dr. Robert Wheeler, Siham Hattab, and Bailey Blair for use of and help with the ibidi heating system and confocal; William Simke and Andrew Hart for help with image analysis; Dr. Matthew Parent for help with 3D imaging and analysis; Dr. Lili Wang for helpful discussions regarding imaging Jurkat T cells; and Dr. Jaime de Juan Sanz and Dr. Francois St-Pierre for helpful discussions regarding genetically encoded voltage indicators.
Funding
This research was supported by the National Institutes of Health: National Institute of Environmental Health Sciences award number R15ES24593 and National Institute of General Medical Sciences awards R15GM116002 and P20GM103423 (an Institutional Development Award) and the Maine Technological Asset Fund (MTAF 1106 and 2061). University of Maine funding that additionally supported this work includes a UMaine Medicine Seed Grant, a Charlie Slavin Research Grant, Frederick Radke Undergraduate Research Fellowships, Maine Top Scholar research supply funds, and the Center for Undergraduate Research.
Abbreviations:
- Ag
antigen
- APC
antigen presenting cell
- ATP
adenosine triphosphate
- AUC
area under the curve
- BSA
bovine serum albumin
- BT
Tyrodes-bovine serum albumin
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CRAC
Ca2+ release-activated Ca2+
- DMSO
dimethyl sulfoxide
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- FPALM
fluorescence photoactivation localization microscopy
- GEVI
genetically encoded voltage indicator
- IgE
immunoglobulin E
- IP3
inositol 1,4,5-triphosphate
- LDH
lactate dehydrogenase
- MMP
mitochondrial membrane potential
- PBS
phosphate buffered saline
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLCγ
phospholipase C gamma
- PLD
phospholipase D
- PMP
plasma membrane potential
- PSF
point spread function
- RBL
rat basophilic leukemia cells, clone 2H3
- ROI
region of interest
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SOCE
store-operated Ca2+ entry
- STIM1
stromal interaction molecule 1
- TCR
T-cell receptor
- TCS
triclosan
- TM
transmembrane domain
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson J, Pecht I, 2007. Regulation of the mast cell response to the type 1 Fc epsilon receptor. Immunol Rev 217, 231–254. 10.1111/j.1600-065X.2007.00518.x [DOI] [PubMed] [Google Scholar]
- Ajao C, Andersson MA, Teplova VV, Nagy S, Gahmberg CG, Andersson LC, Hautaniemi M, Kakasi B, Roivainen M, Salkinoja-Salonen M, 2015. Mitochondrial toxicity of triclosan on mammalian cells. Toxicology reports 2, 624–637. 10.1016/j.toxrep.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaleh NB, Persaud I, Brown JM, 2016. Silver Nanoparticle-Directed Mast Cell Degranulation Is Mediated through Calcium and PI3K Signaling Independent of the High Affinity IgE Receptor. PloS one 11, e0167366. 10.1371/journal.pone.0167366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ, Long CM, Lukomska E, Marshall NB, 2016. Investigations of immunotoxicity and allergic potential induced by topical application of triclosan in mice. Journal of immunotoxicology 13, 165–172. 10.3109/1547691x.2015.1029146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Cavieres A, More J, Vicente JM, Adasme T, Hidalgo J, Valdes JL, Humeres A, Valdes-Undurraga I, Sanchez G, Hidalgo C, Barrientos G, 2018. Triclosan Impairs Hippocampal Synaptic Plasticity and Spatial Memory in Male Rats. Front Mol Neurosci 11, 429. 10.3389/fnmol.2018.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H, 1985. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci U S A 82, 5390–5394. 10.1073/pnas.82.16.5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros SP, Wirojchanasak S, Barrow DA, Panagakos FS, Devizio W, Offenbacher S, 2010. Triclosan inhibition of acute and chronic inflammatory gene pathways. Journal of clinical periodontology 37, 412418. 10.1111/j.1600-051X.2010.01548.x [DOI] [PubMed] [Google Scholar]
- Bean BP, 2007. The action potential in mammalian central neurons. Nature reviews. Neuroscience 8, 451465. 10.1038/nrn2148 [DOI] [PubMed] [Google Scholar]
- Beck A, Fleig A, Penner R, Peinelt C, 2014. Regulation of endogenous and heterologous Ca²⁺ release-activated Ca²⁺ currents by pH. Cell calcium 56, 235–243. 10.1016/j.ceca.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, 1993. Inositol trisphosphate and calcium signalling. Nature 361, 315–325. 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Bertorello AM, Komarova Y, Smith K, Leibiger IB, Efendiev R, Pedemonte CH, Borisy G, Sznajder JI, 2003. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell 14, 1149–1157. 10.1091/mbc.e02-06-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW 2nd, 2014. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circulation research 114, 257–265. 10.1161/circresaha.114.302734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli A, Cogni D, Parolini M, Riva C, Provini A, 2009. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquatic toxicology (Amsterdam, Netherlands) 91, 238–244. 10.1016/j.aquatox.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y, 2007. Mast cells and inflammatory kidney disease. Immunol Rev 217, 79–95. 10.1111/j.1600-065X.2007.00503.x [DOI] [PubMed] [Google Scholar]
- Boron WF, 2004. Regulation of intracellular pH. Advances in physiology education 28, 160–179. 10.1152/advan.00045.2004 [DOI] [PubMed] [Google Scholar]
- Bronner C, Mousli M, Eleno N, Landry Y, 1989. Resting plasma membrane potential of rat peritoneal mast cells is set predominantly by the sodium pump. FEBS letters 255, 401–404. 10.1016/00145793(89)81132-9 [DOI] [PubMed] [Google Scholar]
- Cai S, Zhu J, Sun L, Fan C, Zhong Y, Shen Q, Li Y, 2019. Association Between Urinary Triclosan With Bone Mass Density and Osteoporosis in US Adult Women, 2005‒2010. The Journal of clinical endocrinology and metabolism 104, 4531–4538. 10.1210/jc.2019-00576 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environmental health perspectives 116, 303–307. 10.1289/ehp.10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahdi A, Choi WS, Kim YM, Fraundorfer PF, Beaven MA, 2002. Serine/threonine protein kinases synergistically regulate phospholipase D1 and 2 and secretion in RBL-2H3 mast cells. Mol Immunol 38, 1269–1276. 10.1016/s0161-5890(02)00074-3 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Kain S, 2005. Green Fluorescent Protein: Properties, Applications and Protocols. John Wiley & Sons. [Google Scholar]
- Chamberland S, Yang HH, Pan MM, Evans SW, Guan S, Chavarha M, Yang Y, Salesse C, Wu H, Wu JC, Clandinin TR, Toth K, Lin MZ, St-Pierre F, 2017. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. Elife 6, e25690. 10.7554/eLife.25690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fewtrell C, Millard PJ, Sandison DR, Webb WW, Morrison GH, 1994. Imaging of total intracellular calcium and calcium influx and efflux in individual resting and stimulated tumor mast cells using ion microscopy. J Biol Chem 269, 15186–15194 [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC, 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, 189–200. 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Baroja-Mazo A, López-Castejón G, Gomez AI, Martínez CM, Angosto D, Montero MT, Herranz AS, Bazán E, Reimers D, Mulero V, Pelegrín P, 2012. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500. 10.1016/j.immuni.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K, 2010. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 285, 11740–11752. 10.1074/jbc.M109.066662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys NM, Mlodzianoski MJ, Parent M, Butler MB, Raut P, Wallace J, Lilieholm J, Mehmood K, Maginnis MS, Waters H, Busse B, Zimmerberg J, Hess ST, 2019. Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering. Biophys J 116, 893909. 10.1016/j.bpj.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud FC, Edmiston CE Jr., Leaper D, 2014. Meta-analysis of prevention of surgical site infections following incision closure with triclosan-coated sutures: robustness to new evidence. Surgical infections 15, 165–181. 10.1089/sur.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, 1986. Mast-cell degranulation in human hearts. The New England journal of medicine 315, 969–970. 10.1056/nejm198610093151515 [DOI] [PubMed] [Google Scholar]
- Elieh-Ali-Komi D, Cao Y, 2017. Role of Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Clinical reviews in allergy & immunology 52, 436–445. 10.1007/s12016-016-8595-y [DOI] [PubMed] [Google Scholar]
- Etzel TM, Calafat AM, Ye X, Chen A, Lanphear BP, Savitz DA, Yolton K, Braun JM, 2017. Urinary triclosan concentrations during pregnancy and birth outcomes. Environmental research 156, 505–511. 10.1016/j.envres.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DJ, Hines JE, Walls AF, Kelly PJ, Bennett MK, Burt AD, 1995. Intrahepatic mast cells in chronic liver diseases. Hepatology (Baltimore, Md.) 22, 1175–1181. 10.1016/0270-9139(95)90627-4 [DOI] [PubMed] [Google Scholar]
- Feske S, 2007. Calcium signalling in lymphocyte activation and disease. Nature reviews. Immunology 7, 690–702. 10.1038/nri2152 [DOI] [PubMed] [Google Scholar]
- Fewtrell C, Geier M, Goetze A, Holowka D, Isenman DE, Jones JF, Metzger H, Navia M, Sieckmann D, Silverton E, Stein K, 1979. Mediation of effector functions by antibodies: report of a workshop. Mol Immunol 16, 741–754. 10.1016/0161-5890(79)90152-4 [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M, 2005. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annual review of immunology 23, 749–786. 10.1146/annurev.immunol.21.120601.141025 [DOI] [PubMed] [Google Scholar]
- Gentile DA, Skoner DP, 1996. A role for the sodium, potassium adenosine triphosphatase (Na+,K+ ATPase) enzyme in degranulation of rat basophilic leukaemia cells. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 26, 1449–1460 [PubMed] [Google Scholar]
- Giedt RJ, Pfeiffer DR, Matzavinos A, Kao CY, Alevriadou BR, 2012. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Annals of biomedical engineering 40, 1903–1916. 10.1007/s10439-012-0568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, 1987. The oral clearance of zinc and triclosan after delivery from a dentifrice. J Pharm Pharmacol 39, 480–483. 10.1111/j.2042-7158.1987.tb03425.x [DOI] [PubMed] [Google Scholar]
- Girolamo F, Coppola C, Ribatti D, 2017. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain, behavior, and immunity 65, 68–89. 10.1016/j.bbi.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Gottlieb S, 2019Federal register. Vol.84 (71): https://www.govinfo.gov/content/pkg/FR-2019-04-12/pdf/2019-06791.pdf [Google Scholar]
- Gudheti MV, Curthoys NM, Gould TJ, Kim D, Gunewardene MS, Gabor KA, Gosse JA, Kim CH, Zimmerberg J, Hess ST, 2013. Actin mediates the nanoscale membrane organization of the clustered membrane protein influenza hemagglutinin. Biophys J 104, 2182–2192. 10.1016/j.bpj.2013.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery O, Malka F, Frachon P, Milea D, Rojo M, Lombès A, 2008. Modulation of mitochondrial morphology by bioenergetics defects in primary human fibroblasts. Neuromuscular disorders : NMD 18, 319–330. 10.1016/j.nmd.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D, 1998. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell 94, 537–548. 10.1016/s0092-8674(00)81594-9 [DOI] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH, 2014. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A 111, 486–491. 10.1073/pnas.1311310110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha D, Nataraj B, Rangasamy B, Shobana C, Ramesh M, 2019. DNA damage and physiological responses in an Indian major carp Labeo rohita exposed to an antimicrobial agent triclosan. Fish physiology and biochemistry 45, 1463–1484. 10.1007/s10695-019-00661-2 [DOI] [PubMed] [Google Scholar]
- Hempel HA, Cuka NS, Kulac I, Barber JR, Cornish TC, Platz EA, De Marzo AM, Sfanos KS, 2017. Low Intratumoral Mast Cells Are Associated With a Higher Risk of Prostate Cancer Recurrence. The Prostate 77, 412–424. 10.1002/pros.23280 [DOI] [PubMed] [Google Scholar]
- Hess ST, Girirajan TP, Mason MD, 2006. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J 91, 4258–4272. 10.1529/biophysj.106.091116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT, 2011. Calcium signaling in smooth muscle. Cold Spring Harbor perspectives in biology 3, a004549. 10.1101/cshperspect.a004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A, 2010. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual review of immunology 28, 491–533. 10.1146/annurev.immunol.021908.132550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D, Calloway N, Cohen R, Gadi D, Lee J, Smith NL, Baird B, 2012. Roles for ca(2+) mobilization and its regulation in mast cell functions. Front Immunol 3, 104. 10.3389/fimmu.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D, Thanapuasuwan K, Baird B, 2018. Short chain ceramides disrupt immunoreceptor signaling by inhibiting segregation of Lo from Ld Plasma membrane components. Biol Open 7 10.1242/bio.034702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang W, Bates M, Zhuang X, 2008. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813. 10.1126/science.1153529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd-Brown T, Udoji F, Martin T, Whalen MM, 2013. Effects of DDT and triclosan on tumor-cell binding capacity and cell-surface protein expression of human natural killer cells. J Appl Toxicol 33, 495–502. 10.1002/jat.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson LM, Trinh BM, Palmer RK, Preziosi CA, Pelletier JH, Nelson HM, Gosse JA, 2011. Inorganic arsenite inhibits IgE receptor-mediated degranulation of mast cells. J Appl Toxicol 31, 231–241. 10.1002/jat.1585 [DOI] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, Braun JM, 2018. Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environmental health perspectives 126, 057001. 10.1289/ehp2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Yolton K, Lanphear BP, Braun JM, 2019. Early-life triclosan exposure and parent-reported behavior problems in 8-year-old children. Environment international 128, 446–456. 10.1016/j.envint.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS, 2012. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Molecular and cellular biology 32, 309–319. 10.1128/mcb.05603-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA, 2012. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 75, 779–785. 10.1016/j.neuron.2012.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Nehrke K, 2010. Mitochondrial fragmentation leads to intracellular acidification in Caenorhabditis elegans and mammalian cells. Mol Biol Cell 21, 2191–2201. 10.1091/mbc.e09-10-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RG, Carty SE, Fingerhood BJ, Scarpa A, 1980. The internal pH of mast cell granules. FEBS letters 120, 75–79. 10.1016/0014-5793(80)81050-7 [DOI] [PubMed] [Google Scholar]
- Johnzon CF, Rönnberg E, Pejler G, 2016. The Role of Mast Cells in Bacterial Infection. The American journal of pathology 186, 4–14. 10.1016/j.ajpath.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Wielgomas B, Kałużny P, Klimowska A, Radwan P, Hanke W, 2018. Environmental levels of triclosan and male fertility. Environmental science and pollution research international 25, 5484–5490. 10.1007/s11356-017-0866-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kary T, 2019. Colgate Total Toothpaste to Relaunch Without Controversial Chemical. Bloomberg; June 19, 2020,https://www.bloomberg.com/news/articles/2019-01-15/colgate-total-toothpaste-to-relaunch-this-time-sans-triclosan [Google Scholar]
- Kaufman DS, Goligorsky MS, Nord EP, Graber ML, 1993. Perturbation of cell pH regulation by H2O2 in renal epithelial cells. Archives of biochemistry and biophysics 302, 245–254. 10.1006/abbi.1993.1206 [DOI] [PubMed] [Google Scholar]
- Kim J, Kim K, 2019. Association of antimicrobial household exposure with development of allergic rhinitis in Korea. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 30, 569–571. 10.1111/pai.13052 [DOI] [PubMed] [Google Scholar]
- Kinet JP, 1999. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annual review of immunology 17, 931–972. 10.1146/annurev.immunol.17.1.931 [DOI] [PubMed] [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD, 2013. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. The Science of the total environment 445–446, 299–305. 10.1016/j.scitotenv.2012.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S, 2010. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188, 547–563. 10.1083/jcb.200908086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk AI, Demidovich AD, Kotova DA, Belousov VV, Bilan DS, 2019. Circularly Permuted Fluorescent Protein-Based Indicators: History, Principles, and Classification. International journal of molecular sciences 20, 4200. 10.3390/ijms20174200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kux L, 2016Federal register. Vol.81 (126): https://www.govinfo.gov/content/pkg/FR-2016-06-30/pdf/2016-15410.pdf [Google Scholar]
- Kux L, 2017Federal register. Vol.82 (243): https://www.govinfo.gov/content/pkg/FR-2017-12-20/pdf/2017-27317.pdf [Google Scholar]
- Lee J, Veatch SL, Baird B, Holowka D, 2012. Molecular mechanisms of spontaneous and directed mast cell motility. J Leukoc Biol 92, 1029–1041. 10.1189/jlb.0212091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Fernandez JM, 1986. A patch-clamp study of histamine-secreting cells. J Gen Physiol 88, 349–368. 10.1085/jgp.88.3.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J, 2000. Molecular Cell Biology, 4th ed W.H. Freeman. [Google Scholar]
- Luby-Phelps K, 2000. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. International review of cytology 192, 189–221. 10.1016/s0074-7696(08)60527-6 [DOI] [PubMed] [Google Scholar]
- Lv Y, Rui C, Dai Y, Pang Q, Li Y, Fan R, Lu S, 2016. Exposure of children to BPA through dust and the association of urinary BPA and triclosan with oxidative stress in Guangzhou, China. Environmental science. Processes & impacts 18, 1492–1499. 10.1039/c6em00472e [DOI] [PubMed] [Google Scholar]
- Mancarella S, Wang Y, Deng X, Landesberg G, Scalia R, Panettieri RA, Mallilankaraman K, Tang XD, Madesh M, Gill DL, 2011. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J Biol Chem 286, 44788–44798. 10.1074/jbc.M111.303081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano N, Liotta MA, Slattery JP, Holowka D, Baird B, 1993. Fc epsilon RI and the T cell receptor for antigen activate similar signalling pathways in T cell-RBL cell hybrids. Cell Signal 5, 155–167. 10.1016/0898-6568(93)90067-v [DOI] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Long CM, Kashon ML, Sharpnack DD, Nayak AP, Anderson KL, Jean Meade B, Anderson SE, 2015. Triclosan Induces Thymic Stromal Lymphopoietin in Skin Promoting Th2 Allergic Responses. Toxicol Sci 147, 127–139. 10.1093/toxsci/kfv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Nayak AP, Long CM, Hettick JM, Anderson SE, 2017. Topical application of the anti-microbial chemical triclosan induces immunomodulatory responses through the S100A8/A9-TLR4 pathway. Journal of immunotoxicology 14, 50–59. 10.1080/1547691x.2016.1258094 [DOI] [PubMed] [Google Scholar]
- McLaughlin SG, Dilger JP, 1980. Transport of protons across membranes by weak acids. Physiological reviews 60, 825–863. 10.1152/physrev.1980.60.3.825 [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA, 1997. Mast cells. Physiological reviews 77, 1033–1079. 10.1152/physrev.1997.77.4.1033 [DOI] [PubMed] [Google Scholar]
- Metzger H, Goetze A, Kanellopoulos J, Holowka D, Fewtrell C, 1982. Structure of the high-affinity mast cell receptor for IgE. Fed Proc 41, 8–11 [PubMed] [Google Scholar]
- Millard PJ, Gross D, Webb WW, Fewtrell C, 1988. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A 85, 1854–1858. 10.1073/pnas.85.6.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Niino Y, 2015. Molecular spies for bioimaging--fluorescent protein-based probes. Molecular cell 58, 632–643. 10.1016/j.molcel.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Mohr FC, Fewtrell C, 1987a. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol 104, 783–792. 10.1083/jcb.104.3.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr FC, Fewtrell C, 1987b. The relative contributions of extracellular and intracellular calcium to secretion from tumor mast cells. Multiple effects of the proton ionophore carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem 262, 10638–10643 [PubMed] [Google Scholar]
- Moss T, Howes D, Williams FM, 2000. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4’-trichloro-2’-hydroxydiphenyl ether). Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 38, 361–370. 10.1016/s0278-6915(99)00164-7 [DOI] [PubMed] [Google Scholar]
- Myers VB, Haydon DA, 1972. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta 274, 313–322. 10.1016/0005-2736(72)90179-4 [DOI] [PubMed] [Google Scholar]
- Nakamura U, Iwase M, Uchizono Y, Sonoki K, Sasaki N, Imoto H, Goto D, Iida M, 2006. Rapid intracellular acidification and cell death by H2O2 and alloxan in pancreatic beta cells. Free radical biology & medicine 40, 2047–2055. 10.1016/j.freeradbiomed.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM, 2017. Lehninger Principles of Biochemistry, 7th ed W.H. Freeman. [Google Scholar]
- Ouyang F, Tang N, Zhang HJ, Wang X, Zhao S, Wang W, Zhang J, Cheng W, 2018. Maternal urinary triclosan level, gestational diabetes mellitus and birth weight in Chinese women. The Science of the total environment 626, 451–457. 10.1016/j.scitotenv.2018.01.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Yamada K, Kazanietz MG, Blumberg PM, Beaven MA, 1993. Different isozymes of protein kinase C mediate feedback inhibition of phospholipase C and stimulatory signals for exocytosis in rat RBL-2H3 cells. J Biol Chem 268, 2280–2283 [PubMed] [Google Scholar]
- Palmer RK, Hutchinson LM, Burpee BT, Tupper EJ, Pelletier JH, Kormendy Z, Hopke AR, Malay ET, Evans BL, Velez A, Gosse JA, 2012. Antibacterial agent triclosan suppresses RBL-2H3 mast cell function. Toxicol Appl Pharmacol 258, 99–108. 10.1016/j.taap.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Parent M, Hess ST, 2019. Quantification of Mitochondrial Membrane Curvature by Three-Dimensional Localization Microscopy. iScience Notes 410.22580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova LB, Nosikova ES, Kotova EA, Tarasova EO, Nazarov PA, Khailova LS, Balezina OP, Antonenko YN, 2018. Protonophoric action of triclosan causes calcium efflux from mitochondria, plasma membrane depolarization and bursts of miniature end-plate potentials. Biochim Biophys Acta Biomembr 1860, 1000–1007. 10.1016/j.bbamem.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS, 2015. Store-Operated Calcium Channels. Physiological reviews 95, 1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt J, Stranford S, Jones P, Owen J, 2019. Kuby Immunology, 8th ed W.H. Freeman. [Google Scholar]
- Putney JW Jr., 1986. A model for receptor-regulated calcium entry. Cell calcium 7, 1–12. 10.1016/0143-4160(86)90026-6 [DOI] [PubMed] [Google Scholar]
- Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, Bastian B, Abdel-Tawab M, Fuhr U, 2010. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother 54, 570–572. 10.1128/aac.00615-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva C, Cristoni S, Binelli A, 2012. Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aquatic toxicology (Amsterdam, Netherlands) 118–119, 62–71. 10.1016/j.aquatox.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Rover JA, Leu-Wai-See P, 2014. Role of Colgate Total toothpaste in helping control plaque and gingivitis. American journal of dentistry 27, 167–170 [PubMed] [Google Scholar]
- Safiulina D, Veksler V, Zharkovsky A, Kaasik A, 2006. Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurones. J Cell Physiol 206, 347–353. 10.1002/jcp.20476 [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Tordai A, Gardos G, 1990. Membrane depolarization selectively inhibits receptor-operated calcium channels in human T (Jurkat) lymphoblasts. Biochim Biophys Acta 1027, 130–140. 10.1016/0005-2736(90)90076-z [DOI] [PubMed] [Google Scholar]
- Schurr A, 2014. Cerebral glycolysis: a century of persistent misunderstanding and misconception. Frontiers in neuroscience 8, 360. 10.3389/fnins.2014.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, Woodbury RG, 1985. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A 82, 3871–3875. 10.1073/pnas.82.11.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Weatherly LM, Luc RH, Dorman MT, Neilson A, Ng R, Kim CH, Millard PJ, Gosse JA, 2016. Triclosan is a mitochondrial uncoupler in live zebrafish. J Appl Toxicol 36, 1662–1667. 10.1002/jat.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Caron MA, Weatherly LM, Gerchman LB, Sangroula S, Hattab S, Baez AY, Briana TJ, Gosse JA, 2019. Antimicrobial agent triclosan suppresses mast cell signaling via phospholipase D inhibition. J Appl Toxicol 39, 1672–1690. 10.1002/jat.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Curley JP, 2013. Mast cells on the mind: new insights and opportunities. Trends in neurosciences 36, 513–521. 10.1016/j.tins.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Pfeiffer JR, Zhang J, Martinez AM, Griffiths GM, Wilson BS, 2003. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic 4, 302–312. 10.1034/j.1600-0854.2003.00084.x [DOI] [PubMed] [Google Scholar]
- Sneath PH, 1957. The application of computers to taxonomy. Journal of general microbiology 17, 201–226. 10.1099/00221287-17-1-201 [DOI] [PubMed] [Google Scholar]
- Sporik R, Kemp AS, 1997. Topical triclosan treatment of atopic dermatitis. The Journal of allergy and clinical immunology 99, 861. 10.1016/s0091-6749(97)80029-2 [DOI] [PubMed] [Google Scholar]
- Stepanenko OV, Verkhusha VV, Kuznetsova IM, Uversky VN, Turoverov KK, 2008. Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes. Current protein & peptide science 9, 338–369. 10.2174/138920308785132668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura I, Kanbara Y, Saito M, Horimoto K, Satoh M, Yamamoto H, Oyama Y, 2012. Triclosan, an antibacterial agent, increases intracellular Zn(2+) concentration in rat thymocytes: its relation to oxidative stress. Chemosphere 86, 70–75. 10.1016/j.chemosphere.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT, 2010. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clinical and experimental dermatology 35, e109–112. 10.1111/j.1365-2230.2009.03719.x [DOI] [PubMed] [Google Scholar]
- Te Winkel JD, Gray DA, Seistrup KH, Hamoen LW, Strahl H, 2016. Analysis of Antimicrobial-Triggered Membrane Depolarization Using Voltage Sensitive Dyes. Front Cell Dev Biol 4, 29. 10.3389/fcell.2016.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR, 1991. Bladder mast cell activation in interstitial cystitis. Seminars in urology 9, 74–87 [PubMed] [Google Scholar]
- Thompson MA, Pabelick CM, Prakash YS, 2009. Role of STIM1 in regulation of store-operated Ca2+ influx in pheochromocytoma cells. Cellular and molecular neurobiology 29, 193–202. 10.1007/s10571-008-9311-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher SM, Scalfone LK, Holowka D, Appleton JA, 2013. In vitro modelling of rat mucosal mast cell function in Trichinella spiralis infection. Parasite Immunol 35, 21–31. 10.1111/pim.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Kinet JP, 2019. Calcium signalling in T cells. Nature reviews. Immunology 19, 154–169. 10.1038/s41577-018-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Chinopoulos C, Adam-Vizi V, 1998. Plasma membrane depolarization and disturbed Na+ homeostasis induced by the protonophore carbonyl cyanide-p-trifluoromethoxyphenyl-hydrazon in isolated nerve terminals. Mol Pharmacol 53, 734–741. 10.1124/mol.53.4.734 [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Yu AS, Xie J, Yue Z, Yang W, He Y, Yue L, 2015. Identification of key amino acid residues responsible for internal and external pH sensitivity of Orai1/STIM1 channels. Sci Rep 5, 16747. 10.1038/srep16747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukioka M, Iino M, Endo M, 1994. pH dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized smooth muscle cells of the guinea-pig. The Journal of physiology 475, 369–375. 10.1113/jphysiol.1994.sp020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoji F, Martin T, Etherton R, Whalen MM, 2010. Immunosuppressive effects of triclosan, nonylphenol, and DDT on human natural killer cells in vitro. Journal of immunotoxicology 7, 205–212. 10.3109/15476911003667470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, Fraser WD, 2015. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertility and sterility 103, 1011–1020.e1012. 10.1016/j.fertnstert.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R, 2006. CRACM1 multimers form the ion-selective pore of the CRAC channel. Current biology : CB 16, 2073–2079. 10.1016/j.cub.2006.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Izadmehr S, Kamau E, Kong XP, Chen BK, 2019. Sequential trafficking of Env and Gag to HIV-1 T cell virological synapses revealed by live imaging. Retrovirology 16, 2. 10.1186/s12977-019-0464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Chen L, 2015. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci Rep 5, 18252. 10.1038/srep18252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J, 2017. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels: A Prospective Study. Environmental health perspectives 125, 067017. 10.1289/ehp500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM Molecular Mechanisms Underlying Effects of Antibacterial Agent Triclosan on Cellular Signal Transduction and Mitochondrial Function(2017). Electronic Theses and Dissertations https://digitalcommons.library.umaine.edu/etd/2727/ [Google Scholar]
- Weatherly LM, Gosse JA, 2017. Triclosan exposure, transformation, and human health effects. Journal of toxicology and environmental health. Part B, Critical reviews 20, 447–469. 10.1080/10937404.2017.1399306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Kennedy RH, Shim J, Gosse JA, 2013. A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: effects of triclosan without use of an organic solvent. Journal of visualized experiments : JoVE, e50671. 10.3791/50671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Nelson AJ, Shim J, Riitano AM, Gerson ED, Hart AJ, de Juan-Sanz J, Ryan TA, Sher R, Hess ST, Gosse JA, 2018. Antimicrobial agent triclosan disrupts mitochondrial structure, revealed by super-resolution microscopy, and inhibits mast cell signaling via calcium modulation. Toxicol Appl Pharmacol 349, 39–54. 10.1016/j.taap.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shane HL, Friend SA, Lukomska E, Baur R, Anderson SE, 2020. Topical application of the antimicrobial agent triclosan induces NLRP3 inflammasome activation and mitochondrial dysfunction. Toxicol Sci. 10.1093/toxsci/kfaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shim J, Hashmi HN, Kennedy RH, Hess ST, Gosse JA, 2016. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol 36, 777–789. 10.1002/jat.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischmeyer E, Lentes KU, Karschin A, 1995. Physiological and molecular characterization of an IRK-type inward rectifier K+ channel in a tumour mast cell line. Pflugers Arch 429, 809–819 [DOI] [PubMed] [Google Scholar]
- Xie X, Lu C, Wu M, Liang J, Ying Y, Liu K, Huang X, Zheng S, Du X, Liu D, Wen Z, Hao G, Yang G, Feng L, Jing C, 2020. Association between triclocarban and triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environment international 136, 105445. 10.1016/j.envint.2019.105445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Taniguchi K, Chen S, Evans RM, Hammock BD, Karin M, Tukey RH, 2014. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc Natl Acad Sci U S A 111, 17200–17205. 10.1073/pnas.1419119111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T, 2007. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol 44, 1977–1985. 10.1016/j.molimm.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkowska D, Karwacka A, Jurewicz J, Radwan M, 2018. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. International journal of occupational medicine and environmental health 31, 377–414. 10.13075/ijomeh.1896.01195 [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhou W, Huo X, Zhao S, Gan Y, Wang B, Cheng W, Ouyang F, Wang W, Tian Y, Zhang J, 2019. Triclosan and Female Reproductive Health: A Preconceptional Cohort Study. Epidemiology (Cambridge, Mass.) 30 Suppl 1, S24–s31. 10.1097/ede.0000000000001011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.