Abstract
Background and purpose:
Antibodies against programmed cell death protein 1 (PD-1) are standard treatments for advanced melanoma. Palliative radiation therapy (RT) is commonly administered for this disease. Safety and optimal timing for this combination for melanoma has not been established.
Materials and methods:
In this retrospective cohort study, records for melanoma patients who received anti-PD-1 therapy at Duke University or Emory University (1/1/2013–12/30/2015) were reviewed. Patients were categorized by receipt of RT and RT timing relative to anti-PD-1.
Results:
151 patients received anti-PD-1 therapy. Median follow-up was 12.9 months. Patients receiving RT (n = 85) had worse baseline prognostic factors than patients without RT (n = 66). One-year overall survival (OS) was lower for RT patients than patients without RT (66%, 95% CI: 55–77% vs 83%, 95% CI: 73–92%). One-year OS was 61% for patients receiving RT before anti-PD-1 (95% CI: 46–76%), 78% for RT during anti-PD-1 (95% CI: 60–95%), and 58% for RT after anti-PD-1 (95% CI: 26–89%). On Cox regression, OS for patients without RT did not differ significantly from patients receiving RT during anti-PD-1 (HR 1.07, 95% CI: 0.41–2.84) or RT before anti-PD-1 (HR 0.56, 95% CI: 0.21–1.45). RT and anti-PD-1 therapy administered within 6 weeks of each other was well tolerated.
Conclusion:
RT can be safely administered with anti-PD-1 therapy. Despite worse baseline prognostic characteristics for patients receiving RT, OS was similar for patients receiving concurrent RT with anti-PD-1 therapy compared to patients receiving anti-PD-1 therapy alone.
Keywords: Melanoma, Radiotherapy, Programmed cell death 1 receptor, Immunotherapy, Dose fractionation, Prognosis
Anti-PD-1 based therapies are a standard of care option for patients with unresectable melanoma and have demonstrated benefit in the adjuvant setting [1,2]. Nivolumab and pembrolizumab are monoclonal antibodies targeting the negative regulatory T-cell surface receptor programmed cell death protein 1 (PD-1). Both drugs were approved for use in advanced melanoma by the Food and Drug Administration in 2014 and significantly prolong progression-free and/or overall survival (OS) in patients with advanced melanoma [2–5]. Several preclinical studies and phase I–II clinical trials in varying tumor types indicate that radiation therapy (RT) may enhance the therapeutic effect of immune checkpoint inhibitors [6–13]. However, optimal RT timing relative to anti-PD-1 therapy has not been elucidated. We conducted a retrospective analysis of advanced melanoma patients treated with anti-PD-1 therapy with or without RT to evaluate toxicity and outcomes with this treatment approach. We hypothesized that RT and anti-PD-1 therapy would be well-tolerated and that OS would be affected by RT timing relative to anti-PD-1 administration.
Materials and methods
In this retrospective cohort study, medical records of consecutive patients with melanoma initiated on anti-PD-1 therapy at Duke University or Emory University between January 1, 2013 and December 30, 2015 were reviewed. This study received institutional review board approval with waiver of informed consent at both institutions. Baseline patient evaluation included staging studies, standard clinical laboratory testing, and performance status assessment. Data extracted included demographics, history of autoimmune disease, melanoma characteristics and prior treatments, including surgery, systemic therapy, and radiation therapy (RT). Disease characteristics included mutation status, stage at diagnosis, stage at anti-PD-1 therapy initiation (American Joint Committee on Cancer [AJCC] 7th edition), presence of brain metastases, number and location of metastases at time of anti-PD-1 therapy initiation. For patients with >10 metastases at anti-PD-1 initiation, exact number of metastases was not recorded.
RT was delivered for standard indications for unresectable and/or metastatic disease. Patients were divided into two groups based on RT receipt, excluding adjuvant RT for resected disease. Patients were further categorized into groups based on RT timing relative to anti-PD-1: no RT, RT before anti-PD-1, RT during anti-PD-1, or RT after anti-PD-1. Treatment group assignment was according to timing of RT course delivered in closest temporal proximity to anti-PD-1 administration. RT during anti-PD-1 therapy was defined as receipt of at least 1 anti-PD-1 cycle before and after RT. Ablative RT was defined as fraction size ≥5 Gy, with RT categorization (ablative versus conventional) based on RT course in closest temporal proximity to anti-PD-1.
Patients were followed regularly, including cross-sectional imaging (CT and/or 18FDG-PET/CT) and typically brain MRI. Abscopal effect was defined as patients having progressive or stable disease on anti-PD-1 therapy prior to starting RT with subsequent response observed for lesion(s) outside the radiotherapy field after RT. Adverse events (AEs) were graded by Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. To determine whether RT in close temporal proximity to anti-PD-1 therapy increases AE risk, AEs were summarized based on whether RT was administered within 6 weeks of anti-PD-1 infusion.
Statistical analysis
AEs were characterized by RT timing to anti-PD-1 therapy (no RT or RT >6 weeks from anti-PD-1 therapy versus RT within 6 weeks of anti-PD-1 therapy) and described using frequencies and percentages. Baseline characteristics of RT and no RT groups were compared using Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test for numeric variables. Progression-free survival (PFS) was defined as time from anti-PD-1 therapy initiation to date of first progression based on imaging or physical exam or death. OS was defined as time from anti-PD-1 therapy initiation to death from any cause. For PFS and OS, patients were censored at date of last follow-up or study end date. Kaplan–Meier’s method was used to estimate survival endpoints, and median follow-up was estimated using reverse Kaplan–Meier’s method. Cox proportional hazard regression was used to investigate association of variables with OS. Two models were fitted: Model 1 included patients who did not receive RT; Model 2 excluded them to compare type of RT received. Both models included RT timing relative to anti-PD-1, number of metastases at anti-PD-1 initiation, and presence of brain metastases. Additionally, Model 2 included RT type (ablative versus conventional). PFS and OS were also compared in a propensity-matched patient subset. Propensity scores were calculated for probability of receiving RT (Supplemental Methods), and patients were matched with nearest neighbors using a greedy algorithm. All statistical tests were two-sided (α = 0.05). Reported p values are adjusted for multiple comparisons. Statistical analyses were conducted with SAS version 9.4 (SAS Institute) and R 3.4.
Results
Patient characteristics
One hundred fifty-one patients with melanoma received at least one dose of anti-PD-1 therapy during the study period. Median anti-PD-1 cycle number was 8 (interquartile range [IQR], 4–15). One hundred twenty-four patients received pembrolizumab, twenty-two patients received nivolumab, and five patients received both. Ninety-nine patients had received ipilimumab prior to anti-PD-1. The date of ipilimumab completion was available for 72 of these patients, with median interval between ipilimumab and anti-PD-1 of 6.5 months (range 0.2–30.6; IQR 3–9.8 months). Six patients received concurrent ipilimumab and nivolumab, including five who had previously had received single-agent ipilimumab with a 14.3 month median interval between ipilimumab and dual immune checkpoint blockade. Median follow-up was 12.9 months (95% CI: 11.8–14.4 months). For patients receiving RT, median number of RT courses was 1 (range 1–5; IQR 1–2). Thirty-nine patients received stereotactic radiosurgery (SRS) (range 1–4). For patients alive at the end of the evaluation period, median follow-up was 12.5 months (IQR 10.1–17.4 months).
Sixty-six patients received anti-PD-1 therapy alone, and eighty-five received anti-PD-1 therapy and RT. Most baseline characteristics were not statistically different between the RT and no RT groups (Table 1; Supplemental Table 1). However, patients receiving RT were more likely to have worse performance status (p = 0.003) and brain metastases at anti-PD-1 initiation (p < 0.0001). RT patients were more heavily pre-treated with prior ipilimumab (p = 0.006) and more prior systemic therapy courses (p = 0.007). RT patients also had more metastases (p = 0.03) and organs involved with metastatic disease (p = 0.008) at anti-PD-1 initiation. Propensity-matching selected fifty no RT and forty-one RT patients.
Table 1.
Patient characteristics.
| No RT (n = 66) | RT (n = 85) | p value | |
|---|---|---|---|
| Age at PD-1 start, median (IQR) | 66.9 (54.3–74.6) | 62.3 (55.0–69.0) | 0.17 |
| Gender | 1.00 | ||
| Male | 38 (57.6) | 48 (56.5) | |
| Female | 28 (42.4) | 37 (43.5) | |
| Institution | 1.00 | ||
| Emory | 18 (27.3) | 24 (28.2) | |
| Duke | 48 (72.7) | 61 (71.8) | |
| Race | 1.00 | ||
| White | 63 (95.5) | 81 (95.3) | |
| Black | 1 (1.5) | 1 (1.2) | |
| Hispanic | 0 (0) | 1 (1.2) | |
| Asian | 1 (1.5) | 1 (1.2) | |
| Not available | 1 (1.5) | 1 (1.2) | |
| ECOG performance status | 0.003 | ||
| 0 | 54 (81.8) | 49 (57.6) | |
| 1 | 12 (18.2) | 34 (40.0) | |
| 2 | 0 (0) | 2 (2.4) | |
| Prior ipilimumab | 0.006 | ||
| No | 31 (47.0) | 21 (24.7) | |
| Yes | 35 (53.0) | 64 (75.3) | |
| Anti-PD-1 therapy | 0.31 | ||
| Nivolumab | 13 (19.7) | 9 (10.6) | |
| Pembrolizumab | 50 (75.8) | 74 (87.1) | |
| Both | 3 (4.5) | 2 (2.4) | |
| Stage at anti-PD-1 therapy initiation | 0.15 | ||
| IIB | 1 (1.5) | 0 (0) | |
| III | 7 (10.6) | 4 (4.7) | |
| IV | 58 (87.9) | 81 (95.3) | |
| Brain metastases at anti-PD-1 therapy initiation | <0.0001 | ||
| No | 64 (97.0) | 52 (61.2) | |
| Yes | 2 (3.0) | 33 (38.8) | |
| Courses of prior systemic therapy, median (IQR) | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) | 0.007 |
| # Organs with metastatic disease at anti-PD-1 therapy initiation, median (IQR) | 2.0 (1.0–3.0) | 3.0 (2.0–4.0) | 0.008 |
| # Metastases at anti-PD-1 therapy initiation, median (IQR) | 5.0 (3.0–6.0) | 6.0 (4.0–10.0) | 0.03 |
| Cycles anti-PD-1 therapy, median (IQR) | 9.0 (5.0–16.0) | 7.0 (3.0–13.0) | 0.06 |
| # RT courses, median (IQR) | 0 (0.0–0.0) | 1.0 (1.0–2.0) | N/A |
| # SRS courses, median (IQR) | 0 (0.0–0.0) | 0.0 (0.0–1.0) | N/A |
Abbreviations: RT, radiation therapy; PD-1, programmed cell death protein 1; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; SRS, stereotactic radiosurgery.
Among patients treated with anti-PD-1 therapy and RT, forty-three received RT before anti-PD-1 (50.6%), twenty-six received RT during anti-PD-1 (30.6%), and sixteen received RT after anti-PD-1 therapy (18.8%). Median time between anti-PD-1 therapy infusion and RT was 36 days (range 0–994; IQR 7–116). For the sixteen patients receiving RT after anti-PD-1 therapy, the median interval was 46 days (range 3–156; IQR 29–77.5). RT dose and/or fractionation data were not available for at least one RT course for 19 patients, including 11 patients for whom the missing RT details corresponded to the treatment course in closest proximity to anti-PD-1. An abscopal effect was observed in two patients. One patient had progressive disease after four months on pembrolizumab, then demonstrated significant response in an anterior abdominal soft tissue mass after receiving ablative RT during anti-PD-1 therapy (21 Gy/1 to brain). This response lasted at least 2 years and 11 months, through the last available imaging. The other patient with an abscopal effect received conventional RT (35 Gy/14 to pelvis) at 79 days after completing 5 cycles of pembrolizumab. This patient had progressive thoracic disease on pembrolizumab and subsequent decrease in size of mediastinal lymph nodes at one month after completing RT. No subsequent imaging was available to determine the duration of the abscopal response. Two other patients had distant responses after radiation therapy that may have been secondary to initiating combination ipilimumab/nivolumab rather than an abscopal effect.
Univariate analyses of PFS and OS
For the entire cohort, there were 90 PFS events. One-year PFS was 48% (95% CI: 40–56%), and median PFS was 8.2 months (95% CI: 5.1–16.8) (Fig. 1A). There were 42 deaths, with overall probability of 1-year OS of 73% (95% CI: 66–81%) (Fig. 1B). Patients receiving RT had significantly worse PFS compared to no RT (HR 2.01, 95% CI: 1.29–3.13, p = 0.002). For RT patients, 1-year PFS was 37% (95% CI: 27–47%) versus 62% (95% CI: 50%–73%) for patients who had not received RT (Fig. 1C). Patients receiving RT had significantly worse OS compared to no RT (HR 2.43, 95% CI: 1.22–4.84, p = 0.011). For RT patients, one-year OS was 66% (95% CI: 55–77%) versus 83% (95% CI: 73–92%; Fig. 1D) for patients without RT. In a propensity-matched subset analysis, effects were reduced but overall differences in PFS based on RT receipt remained statistically significant (p = 0.04, Supplemental Fig. 1A). OS did not differ between groups in the propensity-matched subset analysis (p = 0.73, Supplemental Fig. 1B).
Fig. 1.
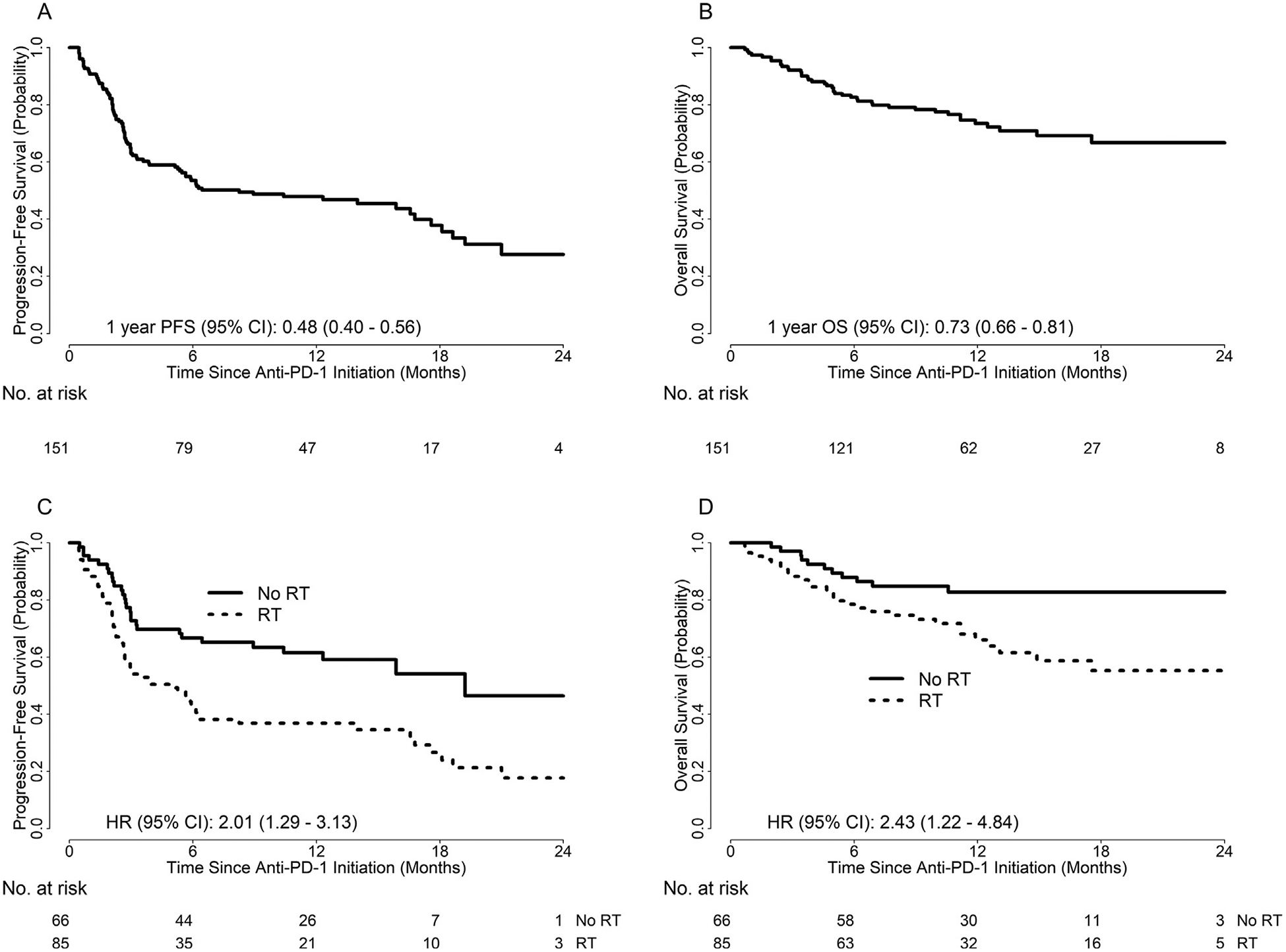
Kaplan–Meier’s curves illustrating (A) PFS of the entire cohort; (B) OS of the entire cohort; (C) PFS by receipt of RT; (D) OS by receipt of RT.
Patients without RT had significantly better PFS than patients with RT during anti-PD-1 (HR 0.38, 95% CI: 0.22–0.66; p = 0.003) or RT after anti-PD-1 therapy (HR 0.24; 95% CI: 0.12–0.45; p < 0.0001) (Fig. 2A). PFS was similar for patients without RT versus RT before anti-PD-1 therapy (HR 0.79, 95% CI: 0.45–1.38; p = 1.0). Compared to patients without RT, OS did not differ significantly for patients receiving RT during anti-PD-1 (HR 1.59, 95% CI: 0.61–4.10, p = 1.0) or after anti-PD-1 (HR 3.06, 95% CI: 1.18–7.94, p = 0.13) (Fig. 2B). However, OS was significantly worse for patients receiving RT before anti-PD-1 (HR 2.81, 95% CI: 1.31–6.02, p = 0.046). One-year OS was 83% for no RT (95% CI: 73%–92%), 61% for RT before anti-PD-1 (95% CI: 46%–76%), 78% for RT during anti-PD-1 (95% CI: 60–95%), and 58% for RT after anti-PD-1 (95% CI: 26–89%).
Fig. 2.
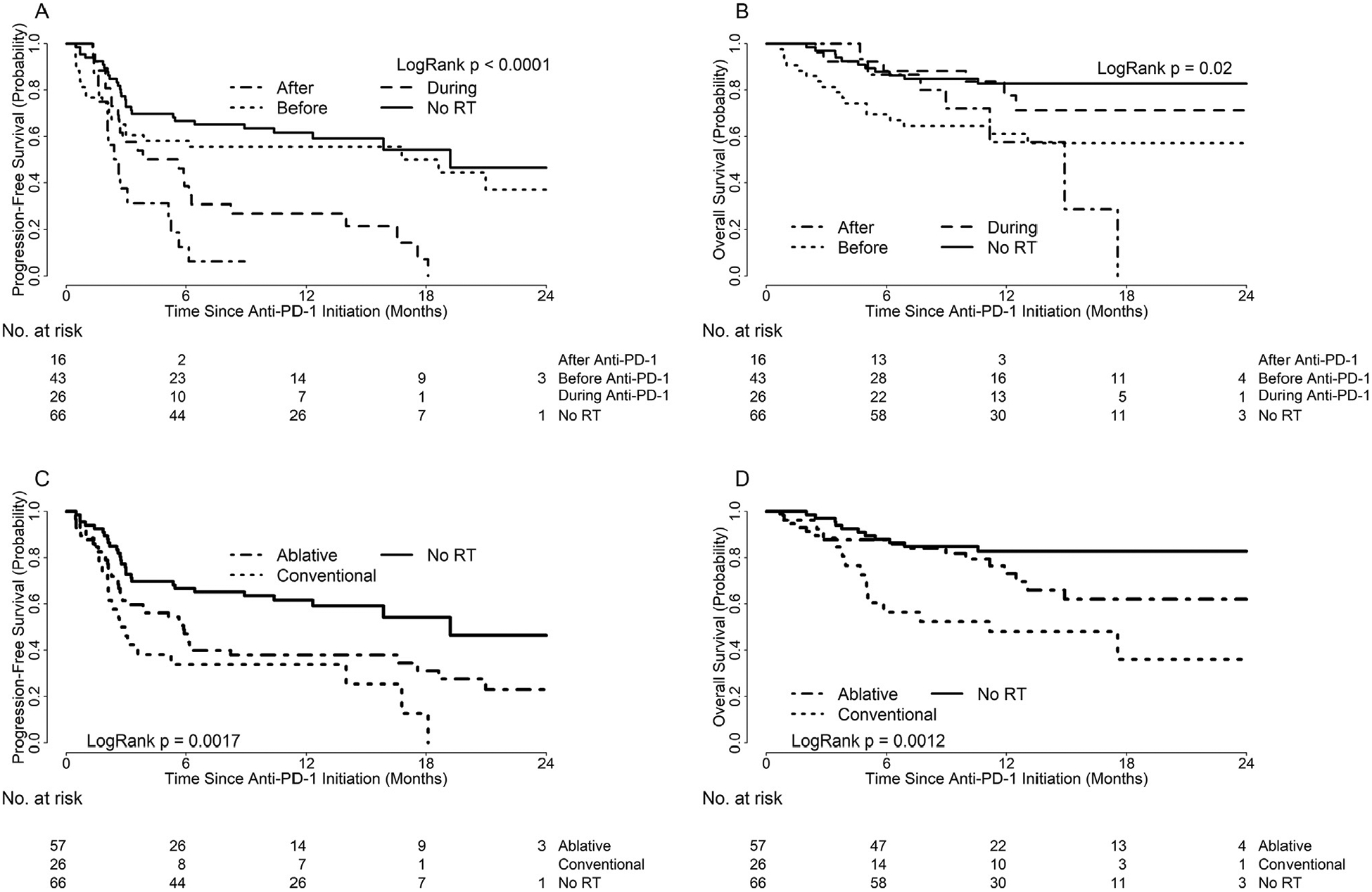
Kaplan–Meier’s curves illustrating (A) PFS by RT timing relative to anti-PD-1 therapy; (B) OS by RT timing relative to anti-PD-1 therapy; (C) PFS by RT type (ablative versus conventional); (D) OS by RT type (ablative versus conventional).
Compared to patients without RT, PFS trended worse for patients receiving ablative RT (HR 1.79, 95% CI: 1.11–2.90, p = 0.052) or conventional RT (HR 2.67, 95% CI: 1.50–4.74, p = 0.002) (Fig. 2C). PFS did not differ significantly between patients with ablative versus conventional RT (HR 0.67, 95% CI: 0.39–1.16, p = 0.46). OS did not differ significantly for patients without RT compared to ablative RT (HR 0.53, 95% CI: 0.25–1.13, p = 0.31) (Fig. 2D). Patients without RT had significantly better OS than patients receiving conventional RT (HR 0.25, 95% CI: 0.11–0.55, p = 0.002). There was no difference in OS between ablative versus conventional RT (HR 0.47, 95% CI: 0.23–0.96, p = 0.11).
Multivariate analyses of PFS and OS
On Cox regression (Table 2), CNS disease was not significantly associated with PFS or OS. OS was worse with higher number of metastases (Model 1 HR 1.34, 95% CI: 1.19–1.51). Between patients without RT and those with RT before anti-PD-1 therapy, PFS did not differ (HR 1.0, 95% CI: 0.51–1.95). However, PFS was significantly better for patients without RT compared to RT during anti-PD-1 (HR 0.46, 95% CI: 0.25–0.82) or RT after anti-PD-1 (HR 0.25, 95%: CI: 0.13–0.47). Compared to patients without RT, there was no statistical difference in OS for patients receiving RT before anti-PD-1 (HR 1.80, 95% CI: 0.69–4.70), RT during anti-PD-1 (HR 0.93, 95% CI: 0.35–2.46), or RT after anti-PD-1 therapy (HR 2.50, 95% CI: 0.97–6.48).
Table 2.
Cox Multivariable Analysis of RT Timing Including No RT Group, Model 1.
| PFS | OS | |||
|---|---|---|---|---|
| HR (95% CI) | Adj. p | HR (95% CI) | Adj. p | |
| No RT vs. RT during anti-PD-1 | 0.455 | 0.05 | 1.072 | 1.00 |
| (0.254–0.815) | (0.406–2.835) | |||
| No RT vs. RT after anti-PD-1 | 0.249 | 0.0001 | 0.399 | 0.35 |
| (0.131–0.474) | (0.154–1.034) | |||
| No RT vs. RT before anti-PD-1 | 0.996 | 1.00 | 0.556 | 1.00 |
| (0.510–1.947) | (0.213–1.455) | |||
| # Mets, continuous | 1.068 | 0.06 | 1.340 | <0.0001 |
| (0.996–1.144) | (1.189–1.509) | |||
| CNS mets vs. no CNS mets | 1.351 | 0.33 | 1.387 | 0.47 |
| (0.741–2.464) | (0.573–3.354) | |||
Abbreviations: RT, radiation therapy; PD-1, programmed cell death protein 1; HR, hazard ratio; CI, confidence interval; adj., adjusted; Mets, metastases.
Model 2 included the same variables as Model 1 with addition of RT fractionation type, thus excluding patients without RT (Supplemental Table 2). Ablative RT was not associated with any difference in PFS or OS compared to conventional RT. RT timing relative to anti-PD-1 was generally not significantly associated with PFS or OS. Number of metastases remained significantly associated with OS (HR 1.26, 95% CI: 1.09–1.46).
Toxicity
Anti-PD-1 therapy was generally well-tolerated. AE types and grades were similar between patients who received RT within six weeks of anti-PD-1 infusion versus those who never received RT or underwent RT at least six weeks before or after anti-PD-1 (Supplemental Table 3). While proportionally more toxicities were observed in patients without RT in close proximity to anti-PD-1, toxicities tended to be higher grade for patients receiving RT within six weeks of anti-PD-1. One Grade 4 toxicity (hypokalemia) occurred in a patient receiving palliative abdominal radiotherapy (40.5 Gy at 2.25 Gy/fraction) concurrently with pembrolizumab. No Grade 5 toxicities were observed.
Pneumonitis occurred in four patients (n = 3 grade 1, n = 1 grade 2), including three patients in the RT group. One patient developed grade 1 pneumonitis after thoracic RT (39 Gy at 3 Gy/fraction) administered concurrently with pembrolizumab. The other three patients with pneumonitis had not received thoracic RT (n = 1 without RT, n = 1 with RT to extremity, n = 1 with RT to abdomen).
Colitis occurred in eighteen patients (n = 7 grade 1; n = 5 grade 2; n = 6 grade 3), including six during combination ipilimumab/nivolumab (n = 1 grade 1; n = 2 grade 2; n = 3 grade 3). Three had received RT to the abdomen or pelvis prior to anti-PD-1 therapy. One patient with grade 3 colitis had received 20 Gy/5 fractions to the pelvis two weeks prior to initiating nivolumab. Another patient with grade 2 colitis had undergone pelvic RT (30 Gy/10 fractions) five months before starting pembrolizumab. The third patient had grade 3 colitis while on pembrolizumab, initiated seven months after stereotactic body radiotherapy (40 Gy/5 fractions) to a periaortic lymph node. The remaining patients developed colitis prior to any abdominal or pelvic RT or never received RT to these areas.
RT-associated toxicity was limited, with similar AE profiles for RT courses administered within six weeks of anti-PD-1 (n = 40) compared to RT delivered outside of this six week window (n = 45) (Supplemental Table 4). Fatigue occurred more frequently during RT administered within six weeks of anti-PD-1 (15.6% grade 1, 8.9% grade 2, 2.1% grade 3) compared to RT at least six weeks apart from anti-PD-1 (5% grade 1, 5% grade 2, 0% grade 3). Radionecrosis rates were not increased for SRS within six weeks of anti-PD-1. No grade 4 or 5 RT-related toxicities were observed.
Few central nervous system (CNS) toxicities were observed among the 39 patients who underwent SRS, with a total of 106 intracranial lesions treated. Three patients were diagnosed with radionecrosis, graded based on the CTCAE 4.03 scale for “central nervous system necrosis.” Two of these patients had not yet received anti-PD-1 therapy, and the other had undergone SRS between cycles of pembrolizumab. One patient had grade 3 radionecrosis, requiring surgical resection for an enlarging intracranial lesion at four months after SRS, with pathology revealing gliosis and necrosis. This patient had not yet received anti-PD-1 therapy, but had completed four cycles of ipilimumab initiated three weeks after SRS. A second patient received steroids for symptoms associated with grade 2 radionecrosis, diagnosed based on radiographic appearance on MRI at three months after SRS. This patient had received SRS with concurrent anti-PD-1 therapy at two months after initiation of pembrolizumab. The third patient was asymptomatic and diagnosed with grade 1 radionecrosis based on imaging appearance at ten months after undergoing SRS, prior to initiation of pembrolizumab. This patient had received a single cycle of ipilimumab 6 weeks prior to SRS. Three additional patients required hospitalization due to CNS toxicity after SRS. One developed symptomatic intracranial hemorrhage at 6 weeks after SRS. This patient had not yet received anti-PD-1 therapy, but had completed four cycles of ipilimumab two months prior to SRS. Another patient required steroids after developing seizures at two months after SRS and three weeks after pembrolizumab. Finally, a third patient developed seizures starting one day after SRS, which was performed six weeks after completing eight cycles of pembrolizumab. This patient required multiple hospitalizations for CNS symptoms and ultimately underwent craniotomy at three months after SRS. Four treated lesions were resected, and each demonstrated residual melanoma.
Discussion
This retrospective series reports toxicity and survival outcomes for 151 melanoma patients treated with anti-PD-1 therapy, including 85 patients who received RT. To our knowledge, this represents the largest reported series to date for melanoma patients receiving RT and anti-PD-1 therapy. Anti-PD-1 therapy administration within six weeks of RT was well-tolerated with no increase in anti-PD-1-related toxicity. A slight increase in grade 2–3 fatigue was observed in patients receiving RT within six weeks of anti-PD-1, but other RT toxicity was similar compared to patients receiving RT more than six weeks apart from anti-PD-1.
RT patients had worse prognostic features, including worse performance status, more prior systemic therapy, greater likelihood of brain metastases, and higher number of metastases. Although one-year OS unadjusted for covariates differed for patients without and with RT (83% vs 66%, respectively), selecting for patients receiving RT during anti-PD-1 therapy reduced the difference (83% vs 78%), potentially suggesting some benefit from concurrent RT administration with anti-PD-1 therapy. On Cox multivariable analysis accounting for presence of brain metastases, timing of RT, and number of metastases, OS did not differ significantly between patients without RT and patients receiving RT with different timing relative to anti-PD-1. OS also did not differ based on RT receipt on propensity-matched subset analysis.
Several analyses have examined clinical outcomes and toxicity for patients receiving RT and anti-PD-1 therapy. Ahmed et al. reported that SRS administration within 6 months of nivolumab was well-tolerated in 26 patients with melanoma brain metastases [14]. Median OS of 11.8 months from RT and 12 months from nivolumab initiation compared favorably to historical control patients receiving RT or surgery alone for brain metastases. Another series of 21 melanoma patients receiving RT for brain metastases within 4 months of pembrolizumab demonstrated no grade 4–5 toxicities and only one grade 3 toxicity with whole brain radiation therapy (WBRT) after prior SRS [15]. A retrospective Australian study described outcomes for 53 patients treated with anti-PD-1 therapy and RT for metastatic melanoma [16]. This study included patients receiving extracranial RT, WBRT, and/or SRS. There was no increase in toxicity with extracranial RT, and the observed 17% rate of symptomatic radionecrosis after SRS and anti-PD-1 therapy was similar to the expected rate of ~10% with SRS alone [17,18]. However, authors raised concerns about possible increased toxicity for WBRT with anti-PD-1 therapy due to three of twenty-one patients having significant toxicity. Additional prospective trials in other solid tumor types suggest that RT of varying fractionation can be safely administered concurrently or in close proximity to anti-PD-1/PD-L1 therapy [13,19,20]. In line with these studies, the current series demonstrates similar toxicity, regardless of temporal proximity of RT to anti-PD-1 administration.
Optimal RT timing relative to immune checkpoint blockade (ICB) remains unknown. Other studies have evaluated RT timing and sequencing for patients treated with cytotoxic T-lymphocyte associated protein-4 (CTLA-4) inhibitor ipilimumab, anti-PD-1 therapy, or both. Chen et al. observed improved OS on multivariable analysis among 79 patients with metastatic melanoma, non-small cell lung cancer (NSCLC) or renal cell carcinoma receiving SRS with concurrent ICB (anti-CTLA-4 or anti-PD-1 therapy) compared to SRS before ICB (HR 3.82, p = 0.002) or after ICB (HR 2.64, p = 0.021) [21]. Similarly, an analysis of 37 NSCLC patients treated with SRS and anti-PD-1 therapy showed longer OS with concurrent SRS and anti-PD-1 therapy compared to SRS before or after anti-PD-1 therapy (1-year OS 87.3% vs 70% vs 0%, respectively; p = 0.008) [22]. Qian et al. examined outcomes for 55 melanoma patients receiving SRS and ICB, showing a trend toward longer median OS for patients receiving concurrent (19.1 months) versus non-concurrent therapy (9 months, p = 0.0691) [23].
Preclinical data suggest that optimal RT timing varies based on immune checkpoint inhibitor [24], and RT dose fractionation may impact synergy with immunotherapy [11]. Few clinical studies have examined RT timing and fractionation with anti-PD-1 therapy without including patients treated with anti-CTLA-4 therapy. A study of SRS and anti-PD-1/PD-L1 therapy for NSCLC suggested improved 6-month distant brain recurrence-free survival for patients receiving SRS before or during ICB compared to patients receiving anti-PD-1/PD-L1 treatment before SRS [25]. In a study of anti-PD-L1 therapy and RT in syngeneic mouse tumor models, concurrent therapy improved OS in contrast to sequential therapy [26]. The current study suggests that RT administered concurrently with anti-PD-1 therapy is associated with better OS than sequential therapy. Patients receiving ablative RT (≥5 Gy/fraction) also trended toward better OS than patients treated with conventional RT, but this effect did not persist on multivariable analysis. Prospective studies are necessary to determine optimal radiation dose fractionation and sequencing relative to anti-PD-1 therapy.
The current study is limited by its retrospective nature and relatively small sample size. Due to low patient and event numbers, our multivariable models could not include all potential prognostic factors. However, more patients undergoing RT and anti-PD-1 therapy were analyzed in this series compared to other available reports. Given the non-randomized study design, additional confounding factors are likely present that were not collected and incorporated into the analysis. We also did not have access to a few RT plans administered at outside institutions. Therefore, RT dose and fractionation data were missing for some patients. Furthermore, RT-related toxicity for these patients may have been incompletely captured leading to underreporting of toxicity. Generalizability of our results to community settings is unclear given that both institutions included are large academic centers.
In conclusion, no clinically significant excess toxicity was observed with RT and anti-PD-1 therapy in patients with advanced melanoma. Although patients receiving RT had more adverse prognostic factors, OS was similar for patients treated with concurrent RT and anti-PD-1 therapy compared to patients who did not receive RT. Several ongoing prospective clinical trials of anti-PD-1 therapy and RT in melanoma and other malignancies will further elucidate how these therapies can be optimally combined.
Supplementary Material
Funding
This research was conducted without funding.
Abbreviations:
- PD-1
programmed cell death protein 1
- RT
radiation therapy
- OS
overall survival
- AJCC
American Joint Committee on Cancer
- CT
computed tomography
- 18FDG-PET
2-deoxy-2-[fluorine-18]fluoro-d-glucose positron emission tomography
- AE
adverse event
- CTCAE
Common Terminology Criteria for Adverse Events
- PFS
progression-free survival
- IQR
interquartile range
- CI
confidence interval
- SRS
stereotactic radiosurgery
- HR
hazard ratio
- WBRT
whole brain radiation therapy
- ICB
immune checkpoint blockade
- CTLA-4
cytotoxic T-lymphocyte associated protein-4
- NSCLC
non-small cell lung cancer
Footnotes
Declaration of Competing Interest
Yvonne Mowery receives research funding by Merck & Co. (United States) through a Stand Up To Cancer Catalyst Grant for an ongoing clinical trial of radiation and pembrolizumab for sarcoma. April Salama receives research funding (paid to institution) from Bristol-Myers Squibb Company (United States), Celldex Therapeutics, Inc. (United States), Dynavax Technologies (United States), Genentech, Inc. (United States), Immunocore (United Kingdom), Merck & Co., and Reata Pharmaceuticals, Inc. (United States). She has served as a consultant for Array BioPharma (United States), Bristol-Myers Squibb Company, and Merck & Co.. She has served as a speaker for Bristol-Myers Squibb Company (non-promotional). Brent Hanks has received research funding from Merck & Co., OncoMed Pharmaceuticals, Inc. (United States), AstraZeneca, (United Kingdom), Drug Discovery & Development (D3) Agency for Science, Technology And Research (A*STAR) (Singapore), Tempest Therapeutics, Inc. (United States), and Leap Therapeutics, Inc. (United States). Mohammad Khan has received research funding by Merck & Co. to conduct preclinical research and clinical trials investigating the role of radiation and pembrolizumab combinations. All other authors declare that they have no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2019.06.013.
References
- [1].Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- [2].Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- [3].Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018;36:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [5].Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390:1853–62. [DOI] [PubMed] [Google Scholar]
- [6].Hiniker SM, Reddy SA, Maecker HT, Swetter SM, Shura L, Knox SJ. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2015;93:S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Victor CT, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 2015;3(4):345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013;86:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434–41. [DOI] [PubMed] [Google Scholar]
- [15].Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud A, Chan TA, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016;5:e1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996–1001. [DOI] [PubMed] [Google Scholar]
- [19].Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016;68:156–62. [DOI] [PubMed] [Google Scholar]
- [20].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- [21].Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys 2018;100:916–25. [DOI] [PubMed] [Google Scholar]
- [22].Schapira E, Hubbeling H, Yeap BY, Mehan WA Jr, Shaw AT, Oh K, et al. Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys 2018. [DOI] [PubMed] [Google Scholar]
- [23].Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016;122:3051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahmed KA, Kim S, Arrington J, Naghavi AO, Dilling TJ, Creelan BC, et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol 2017;133:331–8. [DOI] [PubMed] [Google Scholar]
- [26].Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


