ABSTRACT
Phagocytosis is a complex process by which cells within most organ systems remove pathogens and cell debris. Phagocytosis is usually followed by inflammatory pathway activation, which promotes pathogen elimination and inhibits pathogen growth. Delayed pathogen elimination is the first step in sepsis development and a key factor in sepsis resolution. Phagocytosis thus has an important role during sepsis and likely contributes to all of its clinical stages. However, only a few studies have specifically explored and characterized phagocytic activity during sepsis. Here, we describe the phagocytic processes that occur as part of the immune response preceding sepsis onset and identify the elements of phagocytosis that might constitute a predictive marker of sepsis outcomes. First, we detail the key features of phagocytosis, including the main receptors and signaling hallmarks associated with different phagocytic processes. We then discuss how the initial events of phagosome formation and cytoskeletal remodeling might be associated with known sepsis features, such as a cytokine-driven hyperinflammatory response and immunosuppression. Finally, we highlight the unresolved mechanisms of sepsis development and progression and the need for cross-disciplinary approaches to link the clinical complexity of the disease with basic cellular and molecular mechanisms.
Keywords: Monocyte, phagocytosis, sepsis, signaling
INTRODUCTION
Sepsis is a serious life-threatening condition that affects ∼49 million people and contributes to 11 million deaths worldwide every year (1). Patients with sepsis experience an excessive systemic inflammatory reaction to microbial infection, perhaps due to insufficient phagocytosis and cellular clearnace of pathogen. Sepsis is characterized by an initial hyperinflammatory phase and a later immunosuppressive phase (2, 3). During the initial phase, a broad spectrum of cytokines, chemotactic mediators, and other effector molecules is secreted which influences phagocytic events and the downstream signaling processes. The later immunosuppressive phase is characterized by reduced activity of phagocytes, metabolic rewiring of monocytes, reduced functionality of antigen presenting cells associated with decreased MHC-II expression, expanded myeloid-derived suppressor cells, and depleted effector cells (3, 4) (Fig. 1).
Fig. 1.
Dynamic changes in the immune system during sepsis progression.
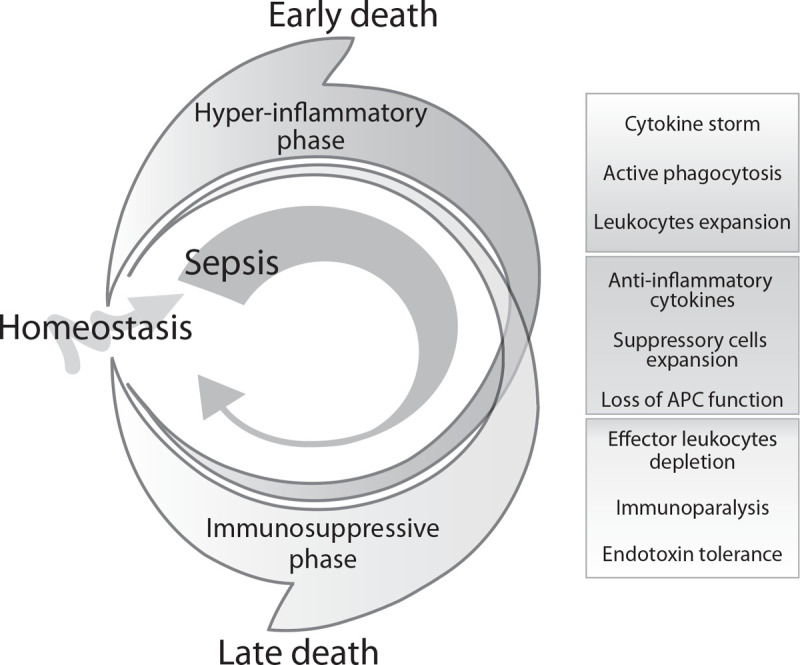
During initial phase of sepsis, the hyperinflammatory phase develops and is associated with cytokine storm, active removing of pathogens by phagocytosis and expansion of different leukocyte types. Too excessive hyperinflammatory phase can lead to early death of septic patients. During progression of sepsis, production of anti-inflammatory cytokines is progressively augmented leading to disbalance of pro- and anti-inflammatory cytokines levels. This fact also contributes to the expansion of suppressory cells and loss of antigen presenting function. Subsequently, when the immunosuppressive phase completely prevails, the depletion of effectory cells contributes to immunoparalysis of immune system. The disproportionate levels during immunosuppressive phase can cause late death of patients during progression of sepsis. The immunoparalysis as well as endotoxin tolerance can persis also when the homeostasis is restored.
Current sepsis treatment includes initial antibiotic administration but can further escalate to organ supporting therapies (such as artificial ventilation, circulatory support, and renal replacement therapy) if the patient fails to respond. Therapies that specifically target pathophysiological mechanisms are lacking but urgently needed, as the number of sepsis cases are likely to increase due to the growing proportion of antibiotic resistant pathogens, immune-compromised individuals, and the aged population (5, 6). One such pathophysiological mechanism underlying sepsis might be phagocytosis.
Phagocytosis is a crucial cellular mechanism devoted to eliminating pathogens and damaged or infected cells, promoting tissue regeneration, and ensuring homeostasis. Through this process, particles >0.5 μm are recognized via phagocyte transmembrane surface receptors and ingested into membrane-derived vesicles, known as phagosomes. These phagosomes later fuse with lysosomes for complete cargo digestion (7, 8). Here, we focus on the effects that sepsis and its pathophysiology might have on phagocytosis and vice versa. First, we outline the major events of the phagocytic process. Then, we highlight how intense cytokine expression and release during phagocytosis contributes to the “cytokine storm” that occurs in the initial phase of sepsis. We also identify how the molecular control of phagocytosis in the latter sepsis phase might affect the pathology outcome. Furthermore, we link some of the events in phagocytosis–inflammation crosstalk with therapeutic interventions currently used to control sepsis. We conclude by proposing new connections among phagocytic processes, sepsis mechanisms, and treatment solutions.
RECOGNITION RECEPTORS AND PHAGOCYTOSIS CONTROL IN SEPSIS
Phagocytosis progresses through four main steps: activation; chemotaxis; attachment; and ingestion (9). These steps revolve around the energetically demanding process of actin filament polymerization and depolymerization that facilitates phagocytic receptor mobility, pathogen detection, and engulfment (10). These four steps rely on a tightly tuned signaling network driven by various phagocytic receptors (9, 11–13) categorized as opsonic, nonopsonic, and integrins (Table 1 and Fig. 2). Opsonic receptors, mainly Fc receptors (FcR), bind antibodies coating the pathogen surface (14). Nonopsonic receptors directly recognize the pathogen-associated molecular patterns (PAMPs) or acts as scavenger receptors such as MARCO, CD36, SR-BI/II (15–18). Finally, the integrins have an important role in activating immune pathways and initiating cytoskeletal rearrangements (19). Orchestrated receptor signaling in sepsis is thus crucial for the timely detection and elimination of localized pathogens and the subsequent prevention of a systemic infection and endotoxin circulation.
Table 1.
Phagocytic receptors that recognize opsonized and nonopsonized targets
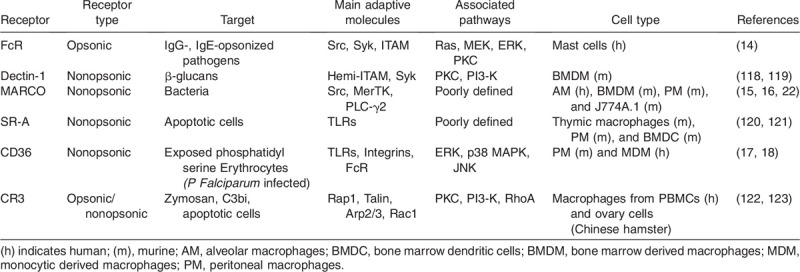
Fig. 2.
Phagocytosis: the main receptors and down-stream signaling outcomes.
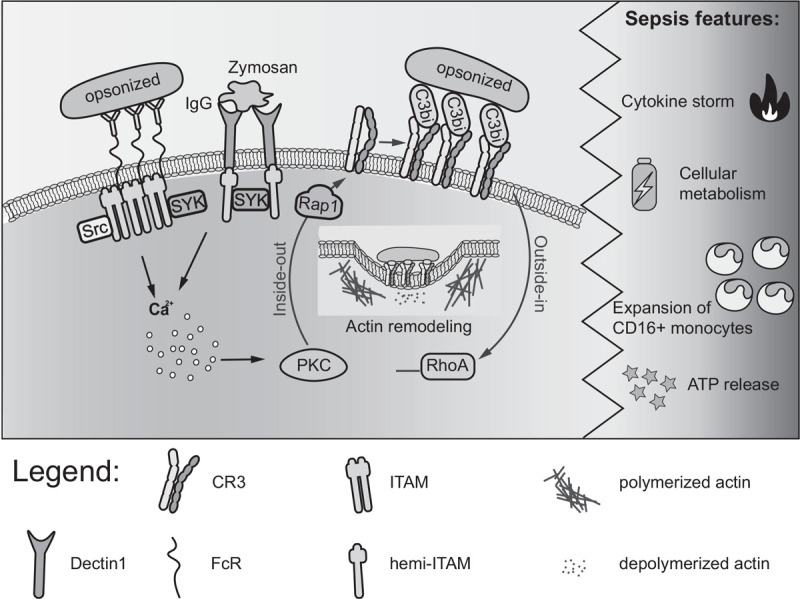
Recognition of IgG-coated particles leads to FcR clustering on the cell membrane, followed by Src kinase activation and phosphorylation of the ITAM motif present in the cytoplasmic domain. Once phosphorylated, ITAMs expose a docking site for Syk (124). The Src–ITAM–Syk complex triggers calcium signaling and eventually guides phagosome formation and invagination (125). Dectin-1 is a C-type lectin receptor: particulate β-glucan recognition leads to Dectin-1 clustering in the phagocytic area. The recognition of the target initiates signal transduction through an ITAM-like domain, which is phosphorylated by Src kinases and allows the recruitment of Syk kinases to drive downstream signaling (126). CR3 recognizes targets opsonized by C3bi (127). In this case, “inside-out” signaling increases the affinity of CR3 for its ligand C3bi, an opsonin coating the surface of phagocytic preys. The binding of this C3bi-opsonized particle to the integrin receptor CR3 triggers “outside-in” signaling, which is responsible for Rho-dependent actin polymerization (128). During the sepsis, the overall outcomes of phagocytosis are influenced by systemic consequences of the inflammatory process (right panel).
The recognition of soluble versus particulate pathogens is important for activating the appropriate inflammatory signaling pathways (20, 21). Indeed, in the context of sepsis, several pathogen evasion strategies have been reported which are based on blocking phagocytic processes (22–24). Despite these clear links between phagocytosis and sepsis risk, only a few studies have focused on the impact of phagocytosis during sepsis progression to date. In a typical sepsis mouse model based on cecal ligation and puncture (CLP) (25), Liu et al. (26) showed that knock-out of CD11b, a component of the CR3 integrin receptor, was associated with poorer outcomes, higher bacterial load, systemic inflammation, and splenic apoptosis compared with wild-type control mice. Consistently, impaired integrin signaling and in particular CR3, potentiates bacterial sepsis in mice (26, 27). However, the use of a monoclonal antibody capable of blocking the interaction between CR3 and its pro-inflammatory ligand CD40L elicited only a mild increase in the phagocytic capabilities of isolated murine macrophages (28). Leelahavanichkul et al. (29) improved the sepsis response in a mouse model by blocking phagocytic scavenger receptors. Specifically, CLP knock-out mice for either CD36 or CR-BI/II, two nonopsonic receptors belonging to the family of class B scavenger receptors (SR-B), exhibited increased survival, limited bacterial proliferation, and reduced systemic inflammation compared with control mice (29). The authors suggested that while SR-B receptors are responsible for both pathogen-induced signaling and pathogen-triggered phagocytosis, under conditions of incomplete phagocytosis or lysosomal bacterial escape, they can also contribute to intracellular bacterial growth. These conclusions remain controversial as opposing findings were reported by Guo et al. (30). Interestingly, de Tymowski et al. recently showed that the IgA FcR CD89 can directly bind to bacteria even in the absence of its cognate ligands (IgA and CRP). Furthermore, CD89 transgenic mice were protected against CLP and preumonia-induced polymicrobial sepsis, highlighting the essential role of the FcR during the early phase of infection before specific antibodies are produced (31).
These examples pinpoint the need to investigate phagocytic receptors in the context of sepsis. Targeting such receptors has thus far led to promising results in murine models but validation in clinical studies is still lacking. Nonetheless, the nature of the receptors, their parallel roles in eliciting detrimental high-grade inflammation, and their efficiency in recognizing and clearing bacteria seem to be important parameters that should be carefully addressed in a therapeutic context.
SEPSIS-ASSOCIATED CHANGES TO PHAGOCYTE SUBSETS
Phagocytic activity
The different phagocytes share receptor profiles, pathogen engulfing ability, and also some intracellular processes (such as phagolysosome formation or respiratory burst) maintain subset-specific roles (32, 33). During sepsis, the presence of pathogens and high levels of inflammatory cytokines results in a dynamic change in the proportion of leukocytes in the blood (34). Neutrophils are the most common phagocytic cells in the blood, constituting 60% to 80% of blood leukocyte counts; their production and release from the bone marrow can increase up to 10-fold upon infection (35, 36). Studies using CLP models, and observations of humans suffering from sepsis, have shown that the bacterial endotoxins and pro-inflammatory cytokines released in response to infection induce substantial rigidity to the neutrophil membrane and reduce neutrophil adhesion, margination, and rolling along the blood vessel endothelium, resulting in impaired migration to the inflammed tissue (37, 38). Interestingly, this phenomenon seems to only occur during lethal sepsis and is linked to an early downregulation of CD18 (a key component of ICAM-1-binding integrins) on the polymorphonuclear cell (PMN) surface (39). Consequently, increased neutrophil membrane rigidity is associated with sepsis severity (38), although the molecular mechanism remains unknown. Others have shown that the phagocytic activity of neutrophils and monocytes has a direct influence on severe sepsis outcomes (40). Specifically, Danikas et al. (40) showed that a worse prognosis and survival in affected patients correlated with decreased expression of CD64 (a Fc receptor associated with neutrophil activation and phagocytosis of antibody-opsonized particles) and with impaired neutrophil phagocytic activity. Based on these results, the researchers proposed that PMN phagocytic activity might serve as a prognostic indicator for sepsis. As the patients were uniformly recruited 24 h after ICU admission, it remains possible that the differences observed in phagocytic activity might be a consequence of a different phase of pathology progression. Another study performed on a septic cohort reported sepsis-associated expansion of circulating immature neutrophils with low phagocytic activity (41).
CD16+ monocyte expansion
Human monocytes represent 2% to 8% of blood leukocyte counts and can be categorized into three major subpopulations: classical (CD14++CD16−), intermediate (CD14++CD16+), and patrolling (CD14+CD16++) monocytes (42). Sepsis leads to the expansion of CD14++CD16+ monocytes (43–45) but the clinical relevance of this expansion is unclear. Others have observed that an increased level of intermediate and patrolling monocytes in the peripheral blood correlates with a better sepsis prognosis (46, 47). In healthy individuals, classical monocytes are highly phagocytic compared with the other two subsets (44, 48, 49). Differences in phagocytic ability between monocyte populations are also observed in sepsis (45). A recent study showed an expansion of CD16++ patrolling monocytes in early phase septic patients followed by gradual reduction in 7 and 14 days after ICU admission (50). During the late immunosuppressive phase of sepsis, the phagocytic activity of monocytes and pro-inflammatory cytokine expression is notably reduced in septic patients (51), undoubtedly contributing to functional decline and immune paralysis.
Monocyte endotoxin tolerance
Monocytes that are acutely exposed to endotoxin develop a transient unresponsive state toward subsequent LPS challenges, a paradigm known as endotoxin tolerance (ET) (52). Although the underlying mechanisms of ET are debated, this phenotype seems to be characterized by altered cytokine release and monocyte effector functions due to complex rewiring of metabolic pathways (which, in turn, control specific epigenetic signatures), molecular signaling pathways, and transcription factor activation (52–56). Although ET monocytes produce less pro-inflammatory cytokines (as TNF-α and IL-6), they increase their phagocytic activity, as evidenced by the upregulation of genes involved in phagocytosis (e.g., Fc receptors CD64 and CD23, MARCO), reactive oxygen species (ROS) production and wound-healing capacity, while showing impaired antigen presentation (57–59). Even though the classic dichotomy of M1/M2 macrophage subsets does not reflect the complexity of tissue macrophages, especially in clinical setups, this phenotype closely resembles the one of M2-like macrophages (57).
Interestingly, in a model of experimental peritonitis in baboons, a more pronounced expansion of M2-like circulating monocytes was found in surviving animals compared with non-survivors. Interestingly, the non-survivors showed mostly M1-like monocyte expansion (60). A recent study using a human in vivo model of endotoxemia, however, showed that ET monocytes cannot induce an oxidative response to Escherichia coli or Candida albicans challenge. This effect was due to a failure of ET monocytes to modify their metabolism during ex vivo LPS restimulation, resulting in reduced Candida killing capacity (54). However, as most of these studies used different protocols to induce ET, both in vivo and ex vivo, the unambiguous interpretation of the process remains challenging.
Monocytes isolated from patients with sepsis exhibit several features reminiscent of ET monocytes, including reduced cytokine production after LPS exposure and increased phagocytic ability (61–63). Two recent studies performed on the same cohort demonstrated that circulating monocytes also show a shift toward an M2-like phenotype, where the levels of CD86, HLA-DR, PD-1, and PD-L1 positively correlated with patient survival (50). These same monocytes showed no difference in phagocytosis ability compared with healthy volunteers, but increased production of ROS and nitric oxide (NO) (63). Consistent with the first study, high CD64 levels in patients during sepsis correlated with increased PMN phagocytosis and better clinical outcomes, while HLA-DR down-regulation correlated with worse clinical outcomes (40, 64). Another study on a septic cohort showed that neutrophil and monocyte functions, including those related to phagocytosis, progressively diminished as sepsis persisted (65). This phenomenon correlated with increased PD-L1 expression on neutrophils and monocytes; consequently, the condition was reversed upon incubating these cells with PD-1 or PD-L1 blocking antibodies, which helped to restore phagocyte function (65).
Taken together, these data suggest that phagocytosis has a key role during sepsis resolution and the mechanisms underlying phenotypic shifts in monocytes and neutrophils might be crucial for designing novel therapeutic approaches. The limitations arising from simplicistic phenotypeing of myeloid subsets (e.g., M1/M2) must now be overcome using new techniques such as single-cell sequencing of tissue macrophages or circulating monocytes, to provide detailed pathology-specific results. Despite the remaining controversies among clinical studies, a further detailed assessment of phagocytic process might help identify novel markers of sepsis severity.
CYTOKINE-MEDIATED REGULATION OF PHAGOCYTOSIS IN SEPSIS
As discussed, microbe internalization by phagocytes is accompanied by an inflammatory cascade and cytokine secretion. These processes are mediated mainly via pattern recognition receptors (PRRs), thus constituting a functional link between phagocytosis and PRR-driven inflammation (21). Together with phagocytic efficacy, cytokine expression levels are among the most significantly divergent parameters between patients with sepsis, and thus can be used to guide patient stratification (66).
The acute phase of sepsis is associated with a deregulated auto-amplifying secretion of pro-inflammatory cytokines, which transmits the “danger signal” of invading pathogens and alerts the host defense. Pro-inflammatory cytokines are secreted in high levels during the hyperinflammatory phase of sepsis, suggesting that cytokines might be able to modulate phagocytosis itself. Indeed, IL-1β, IL-8, TNF-α, and IFN-γ can enhance the phagocytic function of human neutrophils but they are not involved in the bactericidal functions of these cells (67). A similar effect was also shown in a murine macrophage cell line, where IL-18 boosted phagocytosis efficacy (68). IL-6, however, improves the bactericidal effects of neutrophils without any impact on phagocytosis (67).
High IL-10 levels serve as a marker of the immunosuppressive phase of sepsis associated with a poor patient prognosis (69). Non-surviving patients affected by severe sepsis exhibit high IL-10 serum levels and low HLA-DR expression on circulating monocytes compared with surviving patients (64). Conversely, surviving patients exhibit higher TGF-β1 serum levels than non-survival patients; however, the underlying mechanism of this effect is unclear (64). Interestingly, IL-10 can interfere with phagocytosis by blocking phagosome maturation (70) and inhibiting the expression of MHC II and other costimulatory molecules in monocytes and macrophages (71). This mechanism might, therefore, underlie the differential patient outcomes reported based on IL-10 expression levels.
Given the growing body of evidence on the role of cytokines in sepsis, many researchers have attempted to develop specific anti-cytokine-based therapies (72–74). Efforts have also been made to develop and test various blood purification methods to remove endotoxins and/or cytokines from patients, providing partially promising results, such as early circulatory stabilization and prevention of organ failure (74). However, patients affected by sepsis can also profit from some cytokine-based therapies. GM-CSF, a myelopoietic growth factor, has been proposed for the treatment of sepsis (75, 76) as GM-CSF is a strong activator of phagocytosis and promotes phagocyte infiltration and expansion in inflamed tissues (77).
Interestingly, the cytokine storm typically observed during sepsis is not exclusively induced by microbial infection. Many other life-threatening, noninfectious causes such as shock states, trauma or ischemia–reperfusion injury are associated with systemic inflammatory response syndrome (SIRS) (78). Although the etiology of these pathologies is different, human SIRS and sepsis share similar patterns of cytokine expression (79). Like in sepsis, this robust inflammatory storm is also associated with the extensive formation of cell debris arising from apoptosis (80) and necroptosis (81) of many different cell types, which needs to be cleared by phagocytes. During these processes, several damage-associated molecular patterns (DAMPs) (such as S100A8 and S100A9) are released into the bloodstream. These DAMPs act as endogenous TLR4 ligands and are able to induce a state of “tolerance” in monocytes, similar to that observed after LPS challenge (82). Moreover, SIRS can induce profound changes in gastro-intersticial (GI) permeability (83, 84), which leads to the translocation of symbiotic bacteria from the GI tract to the blood stream, finally resulting in multiple amplification of inflammatory responses and switching “sterile” SIRS into “nonsterile” with bacterial (PAMPs) immune-stimulation.
IMBALANCED ENERGY METABOLISM AND PHAGOCYTOSIS: A POSSIBLE CROSSTALK IN SEPSIS?
A key characteristic of sepsis is imbalanced cellular energy metabolism (85, 86), which contributes to immune paralysis and vulnerability to secondary infections (87). Although the involvement of ROS and NO during sepsis is debated, as boosting and inhibiting their activity has opposing effects (88, 89), they also have an indirect role in phagocyte metabolic switch during the sepsis (90). The initial phase of sepsis is characterized by elevated mitochondrial respiration and ATP production (91). When the overproduction of inflammatory molecules such as ROS and NO disrupts mitochondrial respiration by the oxidation of respiratory enzymes, the main source for ATP generation is substituted by enhanced glycolysis (92, 93). The possible effects of the energy source on phagocytosis can be demonstrated on M1-like and M2-like macrophages: while M2-like macrophages produce ATP through mitochondrial respiration and show a high level of phagocytosis, the less phagocytic M1-like macrophages mostly rely on glycolysis (94). M1-like and M2-like macrophages also differ in phagosome metabolic activity. Whereas M1-like macrophages rely mostly on the generation of superoxides to eliminate pathogens and acidify their phagosome quite slowly, M2-like macrophages promptly ensure phagosome acidification to quickly and effectively clear apoptotic cells (95). Nevertheless, M2-like macrophages are able to produce IL-10, which can inhibit phagosome maturation (70). Finally, because M2-like macrophages are mostly involved in clearing apoptotic cells rather then bacteria and can release immunosuppressive chemokines, it is likely that these cells participate in the immunosuppressive phase of the pathology (50, 63, 96, 97).
Damaged cells and tissues release large quantities of ATP into the extracellular space. This ATP acts as a DAMP, signaling through PRRs as a paracrine and autocrine modulator of cellular responses (98). Zumerle et al. (99) demonstrated that ATP released by immune cells rapidly transmits danger signals between cells and boosts phagocytosis through purinergic signaling and calcium release. Consistently, blocking the purinergic receptors P2X4 and P2X7 on macrophages reduces their phagocytic activity (99).
To sustain the high-energy demands during acute catabolic responses in sepsis, proper therapeutic nutrition support is generally needed. Interestingly, increased catabolism can persist for up to 2 years after sepsis (100). The high demand of essential nutrients is also highlighted by the obesity paradox: obese and severely obese individuals have a better prognosis during the first year after sepsis compared with nonobese patients (101).
We believe that such severe metabolic defects reported in septic patients might be considered as new therapeutic targets. For example, IFNγ administration to septic patients can restore energy metabolism and reverse immunoparalysis (85). Consistent with the impairment of mTOR pathways described in monocytes isolated from septic patients, inhibiting mTOR-dependent metabolic processes with metformin in a murine model of fungal sepsis resulted in decreased cytokine production and higher mortality (85). We speculate that the decrease of phagocytic activity in late phase of sepsis might thus be linked to dynamic changes in phagocytes’ energy metabolism.
ROUTINE THERAPEUTIC APPROACHES INFLUENCING PHAGOCYTIC PROCESSES
Our knowledge of the molecular control of phagocytosis has, to date, had little impact on therapeutic approaches in sepsis. As mentioned above, specific phagocytosis-targeted therapy is not in routine use but some routine approaches influence phagocytosis as an off-target effect. Phagocytosis is affected by many physico-chemical factors, many of which are readily influenced in critically ill patients. For example, an elevation in phagocytic capacity has been reported in conditions such as hyperthermia (102), hypoxia (103), and high insulin levels (104). All of these conditions can be a component of early sepsis syndrome and we suggest that they might be part of an evolutionary adaptation to systemic infection. Conversely, diminished phagocytosis has been reported in hypercapnia (105), hypothermia and hyperoxia (106, 107). As a result, organ-supporting strategies in sepsis treatment could be potentially harmful, at least in the context of phagocytosis. For example, ventilator support and efforts to increase oxygen delivery to hypoxic peripheral tissues during septic shock may lead to long-lasting hyperoxia in other tissues, especially in the lungs. The phagocytic activity of pulmonary macrophages is impaired in hyperoxic conditions (106) and long-term hyperoxia is associated with a high incidence of complicating pneumonia (107).
Similarly, some routinely used drugs may affect the activity of immune cells or their phagocytic capabilities. Antibiotics, which a crucial in sepsis therapy, have been intensively studied for their antibacterial effects but they also have rich immunomodulatory potential (108). The beneficial immunomodulatory effects of macrolides have been intensively discussed (108); these have been shown to reduce the mortality of community-acquired pneumonia when used as a part of combination therapy (109). Clindamycin, erythromycin, and chloramphenicol have all been described to increase phagocyte functions, whereas tetracycline, gentamicin, and ciprofloxacin seem to have the opposite effect (110, 111). Catecholamines, epinephrine, and norepinephrine promote M2-like macrophage polarization and enhance macrophage phagocytotic activity via β2-adrenergic receptors in vitro(112, 113). Similarly, another routinely used vasoactive drug, vasopressin, can enhance monocyte and neutrophil functions — namely migration and chemotaxis (114).
Although recommended by current Surviving Sepsis Campaign guidelines (115), the safety and efficacy of corticosteroids in septic shock is also controversial. The primary goal of corticoid therapy is to substitute for sepsis-induced adrenal insufficiency, but clearly it may also have an immunomodulatory effect. In the context of phagocytosis, preliminary data suggest that corticoid treatment might amplify neutrophil-mediated phagocytosis in the early phases of septic shock (116, 117).
CONCLUDING REMARKS
Most of the key events in sepsis, including the dynamic changes in metabolism, cytokine expression, and immune signaling, are associated with the initiation and control of phagocytic processes. These findings imply that intervening on monocytes and neutrophils to enhance phagocytosis during the course of sepsis (both in the early and late phases) could have clinical benefits. To date, most therapeutic approaches tackling sepsis do impact on phagocytic processes. Nevertheless, the detailed mechanistic link between the therapy and phagocytosis is elusive, meaning that this key point of potential intervention thus far has been overlooked. We believe that a specific approach to enhance and prolong the phagocytic capacity of cells would promote sepsis resolution. Unfortunately, most of the data linking sepsis pathobiology and phagocytosis have been generated indirectly or reported in isolated studies, frequently addressing sepsis progression markers than the potential treatment strategies. Going forward, future research should aim to address phagocytosis directly in larger clinical studies, this might suggest new therapeutic targets enhancing the phagocytic process and clearance of pathogens.
Acknowledgments
The authors thank Insight Editing London for reviewing and editing the manuscript prior to submission. All rights reserved.
Footnotes
The authors were supported by European Social Fund and European Regional Development Fund—Project MAGNET (No. CZ.02.1.01/0.0/0.0/15_003/ 0000492) and ENOCH (CZ.02.1.01/0.0/0.0/16_019/0000868), by LQ1605 from the National Program of Sustainability II (MEYS CR), and by Ministry of Health of the Czech Republic, grant nr. NV18-06-00529.
The authors report no conflicts of interest.
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav H, Cartin-Ceba R. Balance between hyperinflammation and immunosuppression in sepsis. Semin Respir Crit Care Med 37:42–50, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis 10:782, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J, Vincent JL, Adhikari NKJ, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 15:581–614, 2015. [DOI] [PubMed] [Google Scholar]
- 6.De La Rica AS, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Ann Transl Med 4:325, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20:825–852, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol 14:136–145, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262:193–215, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Salvi AM, DeMali KA. Mechanisms linking mechanotransduction and cell metabolism. Curr Opin Cell Biol 54:114–120, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S. Phagocytosis: an immunobiologic process. Immunity 44:463–475, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, FcγRI, on human mast cells: up-regulation by IFN-γ. J Immunol 164:4332–4339, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Arredouani MS, Palecanda A, Koziel H, Huang Y-C, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol 175:6058–6064, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Killpack TL, Ballesteros M, Bunnell SC, Bedugnis A, Kobzik L, Hu LT, Petnicki-Ocwieja T. Phagocytic receptors activate Syk and Src signaling during Borrelia burgdorferi phagocytosis. Infect Immun 85:1–11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J Immunol 161:6250–6257, 1998. [PubMed] [Google Scholar]
- 18.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203:2613–2625, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman SA, Goyette J, Furuya W, Woods EC, Bertozzi CR, Bergmeier W, Hinz B, van der Merwe PA, Das R, Grinstein S. Integrins form an expanding diffusional barrier that coordinates phagocytosis. Cell 164:128–140, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fric J, Zelante T, Ricciardi-Castagnoli P. Phagocytosis of particulate antigens—all roads lead to calcineurin/NFAT signaling pathway. Front Immunol 4:513, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect 6:1368–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Da Silva FP, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, Chiamolera M, Verbeek JS, Launay P, Monteiro RC. CD16 promotes Escherichia coli sepsis through an FcRγ inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med 13:1368–1374, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Van Avondt K, van Sorge NM, Meyaard L. Bacterial immune evasion through manipulation of host inhibitory immune signaling. PLoS Pathog 11:e1004644, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beppler J, Mkaddem Ben, Michaloski J, Honorato RV, Velasco IT, de Oliveira PSL, Giordano RJ, Monteiro RC, Pinheiro da Silva F. Negative regulation of bacterial killing and inflammation by two novel CD16 ligands. Eur J Immunol 46:1926–1935, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94:331–335, 1983. [PubMed] [Google Scholar]
- 26.Liu JR, Han X, Soriano SG, Yuki K. The role of macrophage 1 antigen in polymicrobial sepsis. Shock 42:532–539, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Jawhara S, Pluskota E, Cao W, Plow EF, Soloviev DA. Distinct effects of integrins αXβ2 and αMβ2 on leukocyte subpopulations during inflammation and antimicrobial responses. Infect Immun 85:1–17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf D, Anto-Michel N, Blankenbach H, Wiedemann A, Buscher K, Hohmann JD, Lim B, Bäuml M, Marki A, Mauler M, et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat Commun 9:525, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leelahavanichkul A, Bocharov AV, Kurlander R, Baranova IN, Vishnyakova TG, Souza ACP, Hu X, Doi K, Vaisman B, Amar M, et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol 188 (6):2749–2758, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo C, Yi H, Yu X, Hu F, Zuo D, Subjeck JR, Wang XY. Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunol Cell Biol 90:101–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Tymowski C, Heming N, Correia MDT, Abbad L, Chavarot N, Le Stang MB, Flament H, Bex J, Boedec E, Bounaix C, et al. CD89 is a potent innate receptor for bacteria and mediates host protection from sepsis. Cell Rep 27:762.e5–775.e5, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Davies LC, Rice CM, McVicar DW, Weiss JM. Diversity and environmental adaptation of phagocytic cell metabolism. J Leukoc Biol 105:37–48, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimmelé T, Payen D, Cantaluppi V, Marshall J, Gomez H, Gomez A, Murray P, Kellum JA. Immune cell phenotype and function in sepsis. Shock 45:282–291, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavaillon J-M, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res 11:311–320, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 56:672–686, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Stiel L, Meziani F, Helms J. Neutrophil activation during septic shock. Shock 49:371–384, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun 70:3602–3610, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skoutelis AT, Kaleridis V, Athanassiou GM, Kokkinis KI, Missirlis YF, Bassaris HP. Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Crit Care Med 28:2355–2359, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Lindbom L, Xie X, Raud J, Hedqvist P. Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol Scand 146:415–421, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 154:87–97, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taneja R, Sharma AP, Hallett MB, Findlay GP, Morris MR. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock 30:618–622, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Front Immunol 6:423, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HWL. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 82:3170–3176, 1993. [PubMed] [Google Scholar]
- 44.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-Classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep 5:13886, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Döring M, Cabanillas Stanchi KM, Erbacher A, Haufe S, Schwarze CP, Handgretinger R, Hofbeck M, Kerst G. Phagocytic activity of monocytes, their subpopulations and granulocytes during post-transplant adverse events after hematopoietic stem cell transplantation. Immunobiology 220:605–613, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Gainaru G, Papadopoulos A, Tsangaris I, Lada M, Giamarellos-Bourboulis EJ, Pistiki A. Increases in inflammatory and CD14dim/CD16pos/CD45pos patrolling monocytes in sepsis: Correlation with final outcome. Crit Care 22:56, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung H, Lee JH, Jo YH, Hwang JE, Kim J. Circulating monocyte counts and its impact on outcomes in patients with severe sepsis including septic shock. SHOCK 51:423–429, 2019. [DOI] [PubMed] [Google Scholar]
- 48.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2:1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira da Mota NV, Brunialti MKC, Santos SS, Machado FR, Assuncao M, Azevedo LCP, Salomao R. Immunophenotyping of monocytes during human sepsis shows impairment in antigen presentation. Shock 50:293–300, 2018. [DOI] [PubMed] [Google Scholar]
- 51.Xu P, Lou J-S, Ren Y, Miao C, Deng X. Gene expression profiling reveals the defining features of monocytes from septic patients with compensatory anti-inflammatory response syndrome. J Infect 65:380–391, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30:475–487, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972–978, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Grondman I, Arts RJW, Koch RM, Leijte GP, Gerretsen J, Bruse N, Kempkes RWM, Ter Horst R, Kox M, Pickkers P, et al. Frontline science: endotoxin-induced immunotolerance is associated with loss of monocyte metabolic plasticity and reduction of oxidative burst. J Leukoc Biol 106:11–25, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, DI Conza G, Cheng WC, Chou CH, Vavakova M, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 18:985–994, 2017. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 335:936–941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock REW. Endotoxin tolerance represents a distinctive state of alternative polarization (m2) in human mononuclear cells. J Immunol 186:7243–7254, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, Toledano V, Cubillos-Zapata C, Gómez-Campelo P, Varela-Serrano A, Casas-Martin J, Llanos-González E, Alvarez E, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α. J Infect Dis 217:393–404, 2018. [DOI] [PubMed] [Google Scholar]
- 59.del Fresno C, García-Rio F, Gómez-Piña V, Soares-Schanoski A, Fernández-Ruíz I, Jurado T, Kajiji T, Shu C, Marín E, Gutierrez del Arroyo A, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 182:6494–6507, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Mehta A, Brewington R, Chatterji M, Zoubine M, Kinasewitz GT, Peer GT, Chang ACK, Taylor FB, Shnyra A. Infection-induced modulation of m1 and m2 phenotypes in circulating monocytes: role in immune monitoring and early prognosis of sepsis. Shock 22:423–430, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Wiersinga WJ, Veer CVT, Van Den Pangaart PS, Dondorp AM, Day NP, Peacock SJ, Van Der Poll T. Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in gram-negative sepsis (melioidosis). Crit Care Med 37:569–576, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Escoll P, del Fresno C, García L, Vallés G, Lendínez MJ, Arnalich F, López-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun 311:465–472, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Santos SS, Carmo AM, Brunialti MKC, Machado FR, Azevedo LC, Assunção M, Trevelin SC, Cunha FQ, Salomao R. Modulation of monocytes in septic patients: preserved phagocytic activity, increased ROS and NO generation, and decreased production of inflammatory cytokines. Intensive Care Med Exp 4:5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos CA. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Vaccine Immunol 11:161–167, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol 100:1239–1254, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL, Nürnberg P, Schultz MJ, Horn J, Cremer OL, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med 5:816–826, 2017. [DOI] [PubMed] [Google Scholar]
- 67.Pechkovsky DV, Potapnev MP, Zalutskaya OM. Different patterns of cytokine regulation of phagocytosis and bacterial killing by human neutrophils. Int J Antimicrob Agents 7:33–40, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Henan X, Toyota N, Yanjiang X, Fujita Y, Zhijun H, Touma M, Qiong W, Sugimoto K. Enhancement of phagocytosis and cytotoxicity in macrophages by tumor-derived IL-18 stimulation. BMB Rep 47:286–291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monneret G, Finck M-E, Venet F, Debard A-L, Bohé J, Bienvenu J, Lepape A. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95:193–198, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Leemans JC, Juffermans NP, Florquin S, van Rooijen N, Vervoordeldonk MJ, Verbon A, van Deventer SJH, van der Poll T. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol 166:4604–4611, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Honore PM, Hoste E, Molnár Z, Jacobs R, Joannes-Boyau O, Malbrain MLNG, Forni LG. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care 9:56, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS One 14:e0220444, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif 47:2–15, 2019. [DOI] [PubMed] [Google Scholar]
- 75.Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL. A review of GM-CSF therapy in sepsis. Medicine (Baltimore) 94:e2044, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chousterman BG, Arnaud M. Is there a role for hematopoietic growth factors during sepsis? Front Immunol 9:1015, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spath S, Komuczki J, Hermann M, Pelczar P, Mair F, Schreiner B, Becher B. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity 46:245–260, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Pierce A, Pittet JF. Inflammatory response to trauma: Implications for coagulation and resuscitation. Curr Opin Anaesthesiol 27:246–252, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flores-Mejía LA, Cabrera-Rivera GL, Ferat-Osorio E, Mancilla-Herrera I, Torres-Rosas R, Boscó-Garate IB, López-Macías C, Isibasi A, Cérbulo-Vazquez A, Arriaga-Pizano LA. Function is dissociated from activation-related immunophenotype on phagocytes from patients with SIRS/Sepsis Syndrome. Shock 52:E68–E75, 2019. [DOI] [PubMed] [Google Scholar]
- 80.Papathanassoglou EDE, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit Care Med 28:537–549, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Cui YL, Qiu LH, Zhou SY, Li LF, Qian ZZ, Liu XM, Zhang HL, Ren XB, Wang YQ. Necroptosis as a potential therapeutic target in multiple organ dysfunction syndrome. Oncotarget 8:56980–56990, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freise N, Burghard A, Ortkras T, Daber N, Chasan AI, Jauch SL, Fehler O, Hillebrand J, Schakaki M, Rojas J, et al. Signaling mechanisms inducing hyporesponsiveness of phagocytes during systemic inflammation. Blood 134:134–146, 2019. [DOI] [PubMed] [Google Scholar]
- 83.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28:384–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care 7:17, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng S-C, Scicluna BP, Arts RJW, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JAL, Cremer OL, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17:406–413, 2016. [DOI] [PubMed] [Google Scholar]
- 86.Dreschers S, Ohl K, Lehrke M, Möllmann J, Denecke B, Costa I, Vogl T, Viemann D, Roth J, Orlikowsky T, et al. Impaired cellular energy metabolism in cord blood macrophages contributes to abortive response toward inflammatory threats. Nat Commun 10:1685, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamers L, Kox M, Pickkers P. Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva Anestesiol 81:426–439, 2015. [PubMed] [Google Scholar]
- 88.Amatullah H, Shan Y, Beauchamp BL, Gali PL, Gupta S, Maron-Gutierrez T, Speck ER, Fox-Robichaud AE, Tsang JLY, Mei SHJ, et al. DJ-1/PARK7 Impairs bacterial clearance in sepsis. Am J Respir Crit Care Med 195:889–905, 2017. [DOI] [PubMed] [Google Scholar]
- 89.Gu J, Luo L, Wang Q, Yan S, Lin J, Li D, Cao B, Mei H, Ying B, Bin L, et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab Investig 98:715–733, 2018. [DOI] [PubMed] [Google Scholar]
- 90.Victor V, Esplugues J, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets 9:376–389, 2012. [DOI] [PubMed] [Google Scholar]
- 91.Lewis AJ, Billiar TR, Rosengart MR. Biology and metabolism of sepsis: innate immunity, bioenergetics, and autophagy. Surg Infect (Larchmt) 17:286–293, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venter G, Oerlemans FTJJ, Wijers M, Willemse M, Fransen JAM, Wieringa B. Glucose controls morphodynamics of LPS-stimulated macrophages. PLoS One 9:e96786, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong SY, Guerdoud LM, Cantin A, Speert DP. Glucose stimulates phagocytosis of unopsonized Pseudomonas aeruginosa by cultivated human alveolar macrophages. Infect Immun 67:16–21, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galván-Peña S, O’Neill LAJ. Metabolic reprograming in macrophage polarization. Front Immunol 5:420, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canton J, Khezri R, Glogauer M, Grinstein S. Contrasting phagosome pH regulation and maturation in human M1 and M2 macrophages. Mol Biol Cell 25:3330–3341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci 10:520–529, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun 8:1–14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509:310–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zumerle S, Calì B, Munari F, Angioni R, Di Virgilio F, Molon B, Viola A. Intercellular calcium signaling induced by ATP potentiates macrophage phagocytosis. Cell Rep 27:1.e4–10.e4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wischmeyer PE. Nutrition Therapy in Sepsis. Crit Care Clin 34:107–125, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prescott HC, Chang VW, O’Brien JM, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med 42:1766–1774, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 15:335–349, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiers HD, Scheffer G-J, van der Hoeven JG, Eltzschig HK, Pickkers P, Kox M. Immunologic consequences of hypoxia during critical illness. Anesthesiology 125:237–249, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walrand S, Guillet C, Boirie Y, Vasson M-P. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J Leukoc Biol 76:1104–1110, 2004. [DOI] [PubMed] [Google Scholar]
- 105.Wang N, Gates KL, Trejo H, Favoreto S, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PHS. Elevated CO 2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J 24:2178–2190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patel VS, Sampat V, Espey MG, Sitapara R, Wang H, Yang X, Ashby CR, Thomas DD, Mantell LL. Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection. Am J Respir Cell Mol Biol 55:511–520, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaffal K, Six S, Zerimech F, Nseir S. Relationship between hyperoxemia and ventilator associated pneumonia. Ann Transl Med 5:453, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:139–148, 2008. [DOI] [PubMed] [Google Scholar]
- 109.Emmet O’Brien M, Restrepo MI, Martin-Loeches I. Update on the combination effect of macrolide antibiotics in community-acquired pneumonia. Respir Investig 53:201–209, 2015. [DOI] [PubMed] [Google Scholar]
- 110.Labro MT. Interference of antibacterial agents with phagocyte functions: Immunomodulation or “Immuno-Fairy Tales”? Clin Microbiol Rev 13:615–650, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang JH, Bhargava P, McCloskey D, Mao N, Palsson BO, Collins JJ. Antibiotic-induced changes to the host metabolic environment inhibit drug efficacy and alter immune function. Cell Host Microbe 22:757.e3–765.e3, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6:607–618, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou J, Yan J, Liang H, Jiang J. Epinephrine enhances the response of macrophages under LPS stimulation. Biomed Res Int 2014:1–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiedermann FJ, Watzinger K, Stichlberger M, Joannidis M, Kaehler C, Lederer W. Effects of arginine vasopressin on migration and respiratory burst activity in human leukocytes. Open Med (Wars) 13:122–129, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377, 2017. [DOI] [PubMed] [Google Scholar]
- 116.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev 2015:CD002243, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk H-D, Doecke W-D, Falke KJ, Gerlach H. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock. Am J Respir Crit Care Med 167:512–520, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 mediates the biological effects of β-Glucans. J Exp Med 197:1119–1124, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herre J, Marshall ASJ, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104:4038–4045, 2004. [DOI] [PubMed] [Google Scholar]
- 120.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci 93:12456–12460, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B. Pivotal Advance: toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J Leukoc Biol 85:595–605, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med 188:2313–2320, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Le Cabec V, Carréno S, Moisand A, Bordier C, Maridonneau-Parini I. Complement receptor 3 (CD11b/CD18) mediates type i and type ii phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J Immunol 169:2003–2009, 2002. [DOI] [PubMed] [Google Scholar]
- 124.García-García E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol 72:1092–1108, 2002. [PubMed] [Google Scholar]
- 125.Tohyama Y, Yamamura H. Protein tyrosine kinase, syk: a key player in phagocytic cells. J Biochem 145:267–273, 2009. [DOI] [PubMed] [Google Scholar]
- 126.Goodridge HS, Underhill DM, Touret N. Mechanisms of Fc receptor and Dectin-1 activation for phagocytosis. Traffic 13:1062–1071, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Lukácsi S, Nagy-Baló Z, Erdei A, Sándor N, Bajtay Z. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol Lett 189:64–72, 2017. [DOI] [PubMed] [Google Scholar]
- 128.Dupuy AG, Caron E. Integrin-dependent phagocytosis—spreading from microadhesion to new concepts. J Cell Sci 121:1773–1783, 2008. [DOI] [PubMed] [Google Scholar]


