Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, catheter ablation, coronary sinus, pulmonary vein, tachycardia
Background:
A novel stochastic trajectory analysis of ranked signals (STAR) mapping approach to guide atrial fibrillation (AF) ablation using basket catheters recently showed high rates of AF termination and subsequent freedom from AF.
Methods:
This study aimed to determine whether STAR mapping using sequential recordings from conventional pulmonary vein mapping catheters could achieve similar results. Patients with persistent AF<2 years were included. Following pulmonary vein isolation AF drivers (AFDs) were identified on sequential STAR maps created with PentaRay, IntellaMap Orion, or Advisor HD Grid catheters. Patients had a minimum of 10 multipolar recordings of 30 seconds each. These were processed in real-time and AFDs were targeted with ablation. An ablation response was defined as AF termination or cycle length slowing ≥30 ms.
Results:
Thirty patients were included (62.4±7.8 years old, AF duration 14.1±4.3 months) of which 3 had AF terminated on pulmonary vein isolation, leaving 27 patients that underwent STAR-guided AFD ablation. Eighty-three potential AFDs were identified (3.1±1.1 per patient) of which 70 were targeted with ablation (2.6±1.2 per patient). An ablation response was seen at 54 AFDs (77.1% of AFDs; 21 AF termination and 33 cycle length slowing) and occurred in all 27 patients. No complications occurred. At 17.3±10.1 months, 22 out of 27 (81.5%) patients undergoing STAR-guided ablation were free from AF/atrial tachycardia off antiarrhythmic drugs.
Conclusions:
STAR-guided AFD ablation through sequential mapping with a multipolar catheter effectively achieved an ablation response in all patients. AF terminated in a majority of patients, with a high freedom from AF/atrial tachycardia off antiarrhythmic drugs at long-term follow-up.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02950844.
WHAT IS KNOWN?
Success rates of catheter ablation in persistent atrial fibrillation (AF) remains limited.
A novel stochastic trajectory analysis of ranked signals (STAR) mapping approach to guide AF ablation using basket catheters recently showed high rates of AF termination and subsequent freedom from AF.
However, sequential mapping of AF drivers has not been used prospectively to guide ablation.
WHAT THE STUDY ADDS?
This study has shown that AF drivers can effectively be identified using sequential mapping with the STAR mapping method.
STAR mapping guided ablation was associated with a high freedom from AF/atrial tachycardia off antiarrhythmic drugs at long-term follow-up.
This study has demonstrated the clinical utility of the novel STAR mapping method in targeting AF drivers.
Several studies have demonstrated the presence of intermittent focal and rotational activations in atrial fibrillation (AF). Ablation at the sites of these phenomena have resulted in cycle length (CL) slowing or AF termination, supporting the notion that they may be localized AF drivers (AFDs).1–5 These have mostly been identified with panoramic mapping of the atria, but recently sequential mapping using conventional multipolar mapping catheters has shown potential for mapping sites identified using simultaneous mapping with basket catheters.6,7 However, sequential mapping of AFDs has not been used prospectively to guide ablation. Sequential mapping has several advantages, most notably that it allows the use of a catheter that can also be used to create the geometry and guide isolation of the pulmonary veins (PVs; avoiding the need for basket catheters) which reduces the cost and complexity of the procedure. Sequential mapping may also allow higher density acquisition of data to identify AFDs where limited contact and electrode coverage are achieved with the basket catheter.7,8
A novel stochastic trajectory analysis of ranked signals (STAR) mapping approach to guide AF ablation using basket catheters recently showed high rates of AF termination and subsequent freedom from AF.9 The aim of this study was to determine whether ablation prospectively guided by the STAR mapping method utilizing sequential recordings from a PV mapping catheter could achieve similar results. Different mapping catheters and mapping systems were used to investigate the feasibility of using different datasets. The rates of AF termination and CL slowing were examined using this sequential approach. To investigate long-term success in terms of freedom from AF/atrial tachycardia (AT) with STAR-guided ablation generally, outcomes were examined in a larger cohort including those guided by basket mapping and those guided by sequential mapping.
Methods
Patients with persistent AF (<24 months and no previous AF ablation) undergoing catheter ablation were included. Procedures were performed under either general anesthetic or local anesthetic and sedation with diamorphine and midazolam. Anticoagulation therapy was uninterrupted, and heparin intravenous boluses were administered to maintain an activated clotting time >300 s. Patients that were on antiarrhythmic therapy preablation had this continued preablation and postablation. Patients provided informed consent for their study involvement. The study was approved by the UK National Research Ethics Service (16/LO/1379) and prospectively registered (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02950844). This was a single-center study and all recruitment occurred at Barts Heart Centre between the periods of 2018 to 2020. The data, methods used in the analysis, and materials used to conduct the research will not be made available to any research for the purposes of reproducing the results or replication of the procedure. This is on the grounds that the mapping method is currently undergoing a patent and CE marking application process.
STAR Mapping Method
The STAR mapping method has been described in detail previously. In brief, the method uses data obtained from multiple individual wavefront trajectories and creates a statistical picture from which it determines the predominant direction of wavefront propagation which allows it to identify atrial regions that most often precede activation of neighboring sites.9,10 For these regions to be classified as an AFD it was required to lead for ≥75% of wavefronts during a recording period.
Unipolar recordings were obtained with the multipolar catheters. The export data obtained from each mapping system which consisted of electrogram data, geometry data, and electrode coordinates were processed by the STAR mapping method in a custom-written Matlab script. STAR maps were displayed on a replica geometry created in Matlab. The same electrodes identifying an AFD on the STAR maps were manually tagged on the identical geometry on the 3-dimensional mapping system which was used to guide ablation.
Activation times were compared across all the electrodes of the multipolar catheter used at each position. Importantly, the activation times were compared within each recording taken rather than across all the recordings due to the sequential nature of the recordings. Each sequential catheter recording was then superimposed on the same geometry to produce an amalgamation map of separate recordings.
The STAR mapping method has been effectively validated in AT using both basket catheter mapping and sequential mapping when comparing to conventional local activation mapping.7 The same previously validated geodesic distances to pair electrodes10 were used across both mapping methods without the need for any modification. The STAR mapping algorithm used for this study is consistent with that used with the basket catheter. Besides the modifications made to the algorithm to take into account the differences in format of the export data obtained from each mapping system, no other changes were made when applied to the different mapping systems. No changes were made to the algorithm to take into account the differences in electrode spacing.
Electrophysiology Procedure
The procedures were performed using either CARTO (Biosense Webster, CA), Rhythmia (Boston Scientific, MA), or EnSite Precision system (Abbott, CA). Right atrial (RA) and left atrial (LA) geometries were created in all patients. All patients underwent a minimum of 10 separate 30-second sequential recordings using a multipolar catheter (PentaRay with CARTO, IntellaMap Orion with Rhythmia, and Advisor HD Grid with EnSite Precision System) to achieve optimal LA coverage. Sequential multipolar catheter recordings were performed pre- and post-PV isolation (PVI) in all patients. The aim of the study was to map the LA. However, if activation was thought suggestive of an RA AFD as indicated by coronary sinus activation being predominantly proximal to distal and LA AF CL being fastest around the septum, then the RA was also mapped.
When using the CARTO mapping system, the unipolar electrograms were recorded through Bard (Labsystem pro Electrophysiology system). A decapolar catheter (Biosense Webster, CA) positioned in the IVC was used as the indifferent catheter and electrograms were filtered between 0.5 and 500 Hz. With the Rhythmia and EnSite Precision mapping system raw unfiltered unipolar electrograms were obtained from the mapping system using Wilson Central Terminal as the indifferent electrode. Electrode coordinates and anatomic data were obtained from each mapping system. The unipolar electrograms, electrode coordinates, and anatomic data were imported into Matlab (Matlab 2017b, MathWorks MA) and using a custom-written script, the sequential STAR maps were created.
Ablation Strategy
All patients underwent PVI. Following this, a 20-minute waiting period was observed so that no delayed effect of ablation could affect rhythm or CL during AFD ablation. During this waiting period, the sequential mapping data was obtained, and the data exported to create the STAR maps. The post-PVI amalgamation sequential map was used to guide ablation of AFDs. The ablation strategy has previously been described in detail.9 Ablation was performed at the identified AFDs with further consolidating lesions surrounding this site intentionally avoiding line formation. All AFDs on a STAR map were targeted in order of ranked priority whereby sites that were leading 100% of the time being targeted first followed by 90%, etc.
Ablation at an AFD site was stopped if (1) 5 minutes of ablation had been performed, (2) no signal remained at the ablation site, or (3) a study-defined ablation response had been achieved. Ablation responses were defined as termination of AF (into AT or sinus rhythm), or CL slowing of ≥30 ms. Our group has previously applied this definition.5,9 AF CL was measured over 30 beats from the multipolar catheter positioned in the LA appendage. AF CL pre- and post-AFD ablation was used to assess the ablation response. If AF terminated before some AFDs had been ablated, these sites were not empirically targeted. No ablation beyond targeting AFDs was allowed in AF. If AF organized into an AT, this was mapped and ablated. DC cardioversion was performed at the end of the procedure if AF did not terminate following ablation of all identified AFDs.
Follow-Up
Patients were followed-up at 3, 6, 9, and 12 months, and 6 monthly thereafter. Forty-eight–hour Holter monitoring was performed at 6 and 12 months. Documented AF or AT lasting for >30 seconds or the use of antiarrhythmic drugs after the 3-month blanking period was defined as recurrence of AF/AT after a single procedure as per current guidelines.11 Follow-up to date is reported for the sequential STAR-guided ablation cohort. To assess long-term outcomes in a larger cohort undergoing STAR-guided ablation, including those guided by basket catheters and sequential recordings, 1-year outcomes are reported to allow comparison between groups (since the procedures utilizing basket guided ablation were performed before the sequential cohort and follow-up to date is therefore longer).
Statistical Analysis
Statistical analyses were performed using SPSS (IBM SPSS Statistics, Version 24 IBM Corp, NY). Continuous variables are displayed as mean±SD (SD) or median (interquartile range). Categorical variables are presented as a number and percentage. Fisher exact test was used for the comparison of nominal variables. The Student t test, or its nonparametric equivalent, Mann-Whitney U test when appropriate was used for comparison of continuous variables. P value of <0.05 was deemed significant.
Results
Procedural Data
A total of 30 patients were included in the study. Baseline characteristics are shown in Table 1. The results are summarized in Figure 1. A majority of the patients underwent ablation under local anesthetic and sedation (26/30, 86.7%). Out of the 30 patients, 2 patients terminated to sinus rhythm and one terminated to cavotricuspid isthmus–dependent flutter on PVI leaving 27 patients who underwent ablation guided by STAR mapping. The mean procedure duration was 259.4±62.1 minutes, and the total ablation duration post-PVI was 10.3±3.5 minutes (including ablation for AFDs and AT if AF terminated to AT during the ablation of AFDs). The average ablation duration at an AFD identified using the STAR mapping method was 2.7±0.5 minutes. No complications were encountered in any of the patients.
Table 1.
Baseline Characteristics
Figure 1.
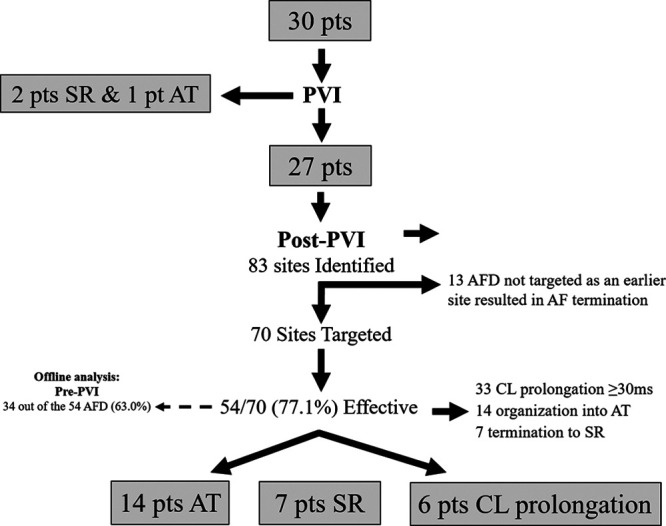
Flow diagram summarizing the study findings. AF indicates atrial fibrillation; AFD, AF driver; AT, atrial tachycardia; CL, cycle length; pts, patients; PVI, pulmonary vein isolation; and SR, sinus rhythm.
AFDs Ablated
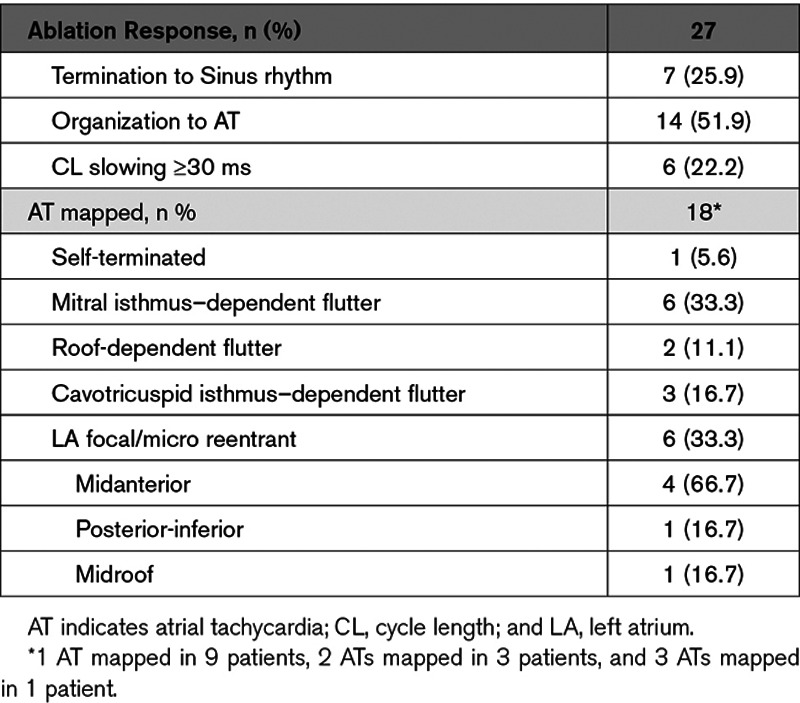
After PVI, a mean of 14.4±3.5 sequential acquisitions was made per patient, of the LA which were exported to generate the STAR map in AF. In the 27 patients remaining in AF post-PVI, 83 potential AFDs were identified (3.1±1.1 per patient) of which 70 (84.3%; 2.6±1.2 per patient) were targeted with ablation. An ablation response was achieved with 54 out of 70 AFDs (77.1%) which included AF termination with 21 AFDs and CL slowing ≥30 ms in 33 AFDs (Table I in the Data Supplement). This meant that 13 AFDs were not targeted because the patient cardioverted before all of their AFDs were ablated.
An ablation response was achieved in all 27 patients (Figure 2A through 2C, Figure 3A through 3C, Figure 4A through 4F, Figure 5A through 5C, and Figure 6A through 6D). On a per-patient basis, AF termination was achieved with 21 patients (7 sinus rhythm and 14 AT) and CL slowing ≥30 ms in the 6 remaining patients (Table 2). Out of the 14 patients in whom AF organized into an AT, this was mapped and successfully ablated to sinus rhythm in 12 patients, whereas the remaining 2 patients underwent DC cardioversion. In one of these patients, mitral isthmus block was not achieved, whereas the other patient had 3 ATs mapped with alternating AT CL. The 6 patients that had CL slowing achieved at the end of their procedure underwent DC cardioversion to achieve sinus rhythm. Sequential RA mapping was performed post-PVI in one of the 27 patients. However, no AFD was identified in the RA and therefore no ablation was performed in the RA in any of the patients.
Figure 2.
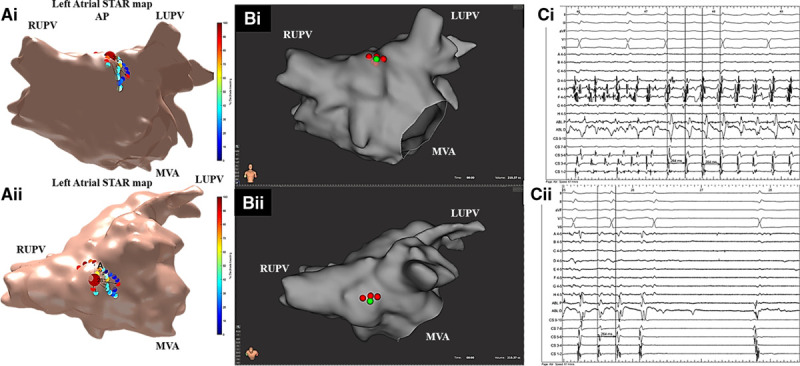
Patient ID 16. A, i and ii, Stochastic trajectory analysis of ranked signals (STAR) map in (A, i) an anterior-posterior (AP) view and (A, ii) tilted roof view demonstrating an atrial fibrillation driver (AFD) mapped to the high anterior/roof. On this STAR map, the recording segment that demonstrated the AFD that was targeted with ablation is only displayed. B, i and ii, Rhythmia map in (B, i) an AP view and (B, ii) tilted roof view demonstrating ablation at the AFD. C, Bard signals including surface ECG, coronary sinus (CS), ablation (ABL), and selected IntellaMap Orion catheter electrograms demonstrating (i) AF termination to atrial tachycardia (AT) on ablation at the AFD and (ii) with further consolidating lesions at AFD resulting in AT termination to sinus rhythm. LUPV indicates left upper pulmonary vein; MVA, mitral valve annulus; and RUPV, right upper pulmonary vein.
Figure 3.
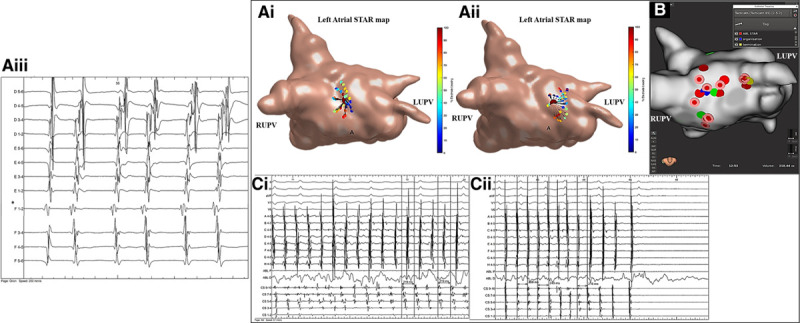
Patient ID 18. A, i and ii, Stochastic trajectory analysis of ranked signals (STAR) map in a tilted roof view demonstrating (A, i) an atrial fibrillation driver (AFD) mapped to the midroof/right upper pulmonary vein (RUPV) and (A, ii) roof/left atrial appendage (LAA). A, iii, Demonstrates the electrograms obtained from the mapping catheter at the site of the AFD. The asterisk highlights the electrode that is leading the greatest proportion of time compared to its neighbors. On this STAR map the recording segments that demonstrated the AFD with ablation response are only displayed. B, Rhythmia map in a roof view demonstrating ablation at both AFDs (the blue tag highlights the site of intermittent organization and yellow tag highlights site of termination to sinus rhythm). Ablation at an AFD mapped to the high anterior wall did not result in a study-defined ablation response. C, i and ii, Bard signals including surface ECG, coronary sinus (CS), ablation (ABL), and selected IntellaMap Orion catheter electrograms demonstrating (C, i and ii) intermittent AF organization into atrial tachycardia during ablation at the AFD mapped to the midroof/RUPV and cycle length slowing and (C, ii) termination to sinus rhythm on ablation at the AFD mapped to the roof/LAA. LUPV indicates left upper pulmonary vein.
Figure 4.
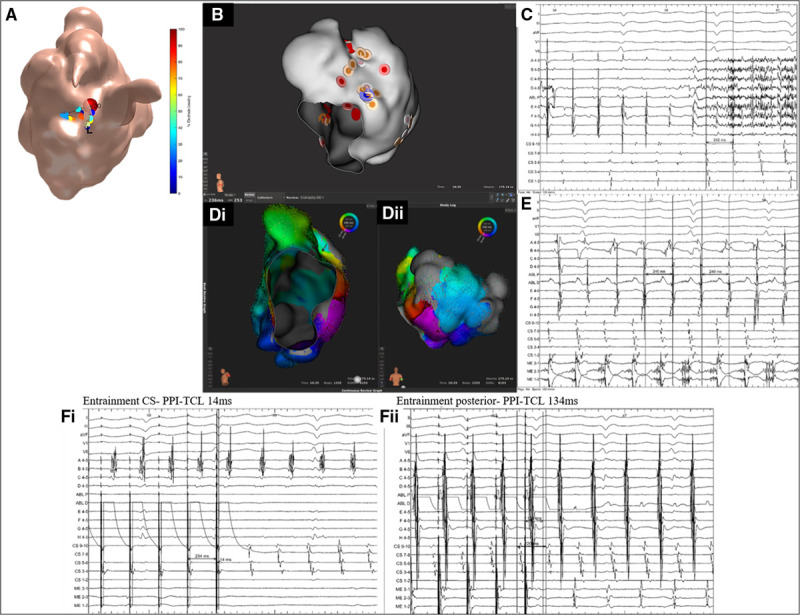
Patient ID 20. A, Stochastic trajectory analysis of ranked signals (STAR) map in a tilted lateral view demonstrating an atrial fibrillation driver (AFD) mapped to the midlateral wall. B, Rhythmia map in a titled lateral view demonstrating ablation at the AFD. C, Bard signals including surface ECG, coronary sinus (CS), ablation, and selected IntellaMap Orion catheter electrograms demonstrating AF termination to atrial tachycardia (AT) on ablation at the AFD. D, i and ii, Rhythmia LAT map in a (D, i) lateral valve view and (D, ii) posterior-anterior view demonstrating a mitral isthmus dependent tachycardia. E, Bard signals including surface ECG, CS, ablation (ABL), and selected IntellaMap Orion catheter electrograms (ME) demonstrating AT with a cycle length of 240 ms. F, i and ii, Bard electrograms demonstrating entrainment at (F, i) CS with a postpacing interval–tachycardia cycle length (PPI-TCL) of 14 ms and (F, ii) posterior wall with a PPI-TCL of 134 ms. LAA indicates left atrial appendage; LUPV, left upper pulmonary vein; and MVA, mitral valve annulus.
Figure 5.
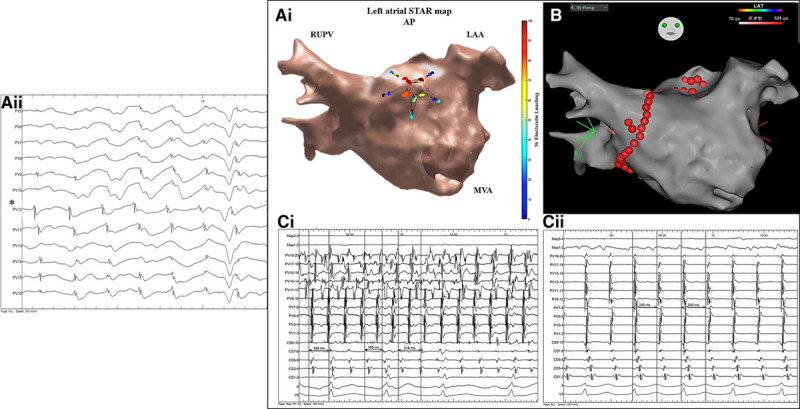
Patient ID 23. A, i, Demonstrates a stochastic trajectory analysis of ranked signals (STAR) left atrial (LA) map in an anterior-posterior view with an atrial fibrillation driver (AFD) identified midanterior wall of the LA. A, ii, Demonstrates the electrograms obtained from the mapping catheter at the site of the AFD. The asterisk highlights the electrode that is leading the greatest proportion of time compared to its neighbors. B, This AFD was targeted with ablation as shown on the CARTO LA map in an anterior-posterior view. On this STAR map, the recording segment that demonstrated this AFD is only displayed. C, i and ii, Ablation here resulted in AF termination to atrial tachycardia (AT) as shown on the Bard electrograms. The AT was mapped to a focal/micro-reentry AT in the close vicinity to the ablation lesions that organized the AF into AT. Further cluster ablation lesions at this site terminated the AT to sinus rhythm. The Bard electrograms include surface ECG, pulmonary vein (PV), Map, and coronary sinus (CS). LAA indicates left atrial appendage; MVA, mitral valve annulus; and RUPV, right upper pulmonary vein.
Figure 6.
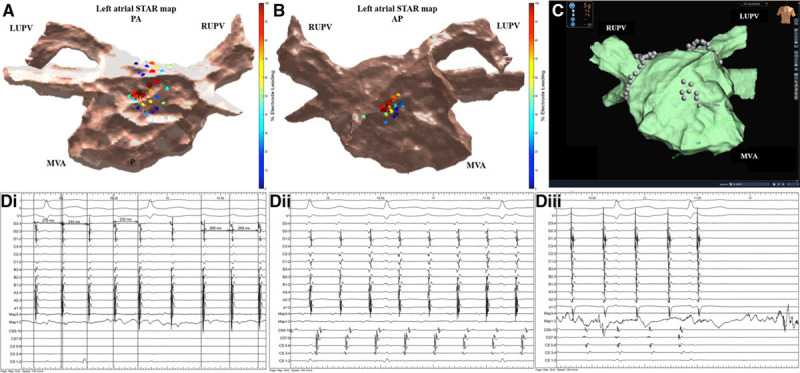
Patient ID 21. A, Demonstrates a stochastic trajectory analysis of ranked signals (STAR) left atrial (LA) map in a posterior-anterior view that highlights 2 atrial fibrillation drivers (AFDs) at the posterior wall in close vicinity to each other with the arrows demonstrating that the wavefront is spreading to away from each AFD in the opposite direction suggesting that a potential AF driver is present from which radial spread of activation occurs. This site was targeted with ablation that slowed the AF from ≥30 ms and intermittently organized it. On this STAR map, the two recording segments that demonstrated this wavefront activation pattern is only displayed. B, STAR LA map in an anterior-posterior view that highlights an AFD at the midanterior wall. On this STAR map, the recording segment that demonstrated this AFD is only displayed. C, Ablation was performed at this site as shown on the EnSite Precision system LA map in an anterior-posterior view. D, i and ii, Ablation here resulted in AF termination to an atrial tachycardia (AT) as shown on the Bard electrograms. D, iii, The AT was mapped to roof-dependent flutter and a roofline was ablated with a 10 ms slowing in AT cycle length. Remap was suggestive of mitral isthmus–dependent AT which was successfully ablated with a mitral line resulting in sinus rhythm as shown on the Bard electrograms. The Bard electrograms include surface ECG, pulmonary vein, ablation, and coronary sinus (CS). LUPV indicates left upper pulmonary vein; MVA, mitral valve annulus; and RUPV, right upper pulmonary vein.
Table 2.
Summarizes the Ablation Response Seen in the Cohort of Patients That Underwent Ablation Guided by STAR Mapping and the Mechanism of the ATs Mapped
AFD and Pre-PVI Recordings
For comparison, STAR maps were made of the LA pre-PVI using a mean of 13.2±2.5 sequential recordings and analyzed offline. Out of the 83 AFDs identified post-PVI, 42 (50.6%) of these AFD were also identified on the pre-PVI maps. All patients had at least one consistent AFD identified on both the pre- and post-PVI maps (1.6±0.7 AFDs per patient).
Out of the 42 AFDs identified on the pre-PVI maps, 40 were targeted on the post-PVI maps. An ablation response was seen with 33 out of 40 (82.5%) of these drivers. Out of the 54 AFDs targeted post-PVI that were associated with an ablation response, 34 out of 54 (63.0%) were also identified on the pre-PVI maps. All of the 21 AFDs that were associated with AF termination on ablation were identified pre-PVI.
On the pre-PVI maps, 2 additional AFDs were identified that were not seen on the post-PVI maps. This was in the 2 patients in whom AF termination occurred during PVI whereby AFDs were identified at the PV ostia in both patients on the pre-PVI maps.
Drivers targeted with ablation and identified pre- and post-PVI were more likely to be associated with AF termination than drivers only identified post-PVI (21/40 versus 0/30; P<0.001).
Mapping System
A majority of the procedures were performed using CARTO (n=24, 80.0%). Out of the 24 patients that had mapping performed with CARTO, 2 terminated to sinus rhythm and one to cavotricuspid isthmus–dependent flutter with PVI. In the remaining 21 patients that had ablation guided by STAR mapping, AF termination was achieved in 16 out of 21 patients (76.2%). Using the Rhythmia and EnSite Precision mapping system, AF termination was achieved in 3 out of the 4 patients (75.0%; P=1.00) and 1 out of 2 (50%) patients, respectively.
Clinical Outcomes in Sequential STAR Mapping Cohort
During an average follow-up of 17.3±10.1 months, 25 out of the 30 patients (83.3%) remained free from AF/AT off antiarrhythmic drugs after a single procedure. When only assessing the 27 patients that underwent STAR mapping guided ablation (so excluding the patients in whom AF terminated with PVI) 22 out of 27 patients (81.5%) remained free from AF/AF during follow-up off antiarrhythmic therapy after a single procedure. Of the 5 patients with recurrent arrhythmia, 3 had recurrent AF, and 2 had AT. Both patients with recurrent AT underwent repeat ablation and were found to have mitral isthmus–dependent flutter in one patient and roof-dependent flutter in the other.
Electrophysiological and Clinical Outcomes in Larger Cohort Guided by STAR Mapping: Comparison of Sequential and Basket Mapping
Electrophysiological outcomes were examined in the 35 patients who underwent STAR-guided ablation utilizing basket catheters (reported previously in Honarbakhsh et al9) and the 30 patients comprising the sequential cohort (reported here). The proportion of AFDs ablated that resulted in a study-defined ablation response was 127 out of 153 (83.0%). This was comparable between these 2 groups (54/70, 77.1% sequential versus 73/83, 88.0% basket; P=0.09). The proportion of AFDs whereby ablation resulted in AF termination was 45 out of 127 (35.4%). This was again comparable between groups (21/54, 38.9% sequential versus 24/73, 32.9% basket; P=0.57). The proportion of patients that had AF termination on ablation was 45 out of 64 (70.3%) and was again similar between groups (21/30, 70.0% sequential versus 24/35, 68.6% basket; P=0.79).
For examination of clinical outcomes, follow-up has been truncated at 1 year (since follow-up in the basket group was longer). In the sequential group, 23 patients had reached 1-year follow-up. Therefore, the 1-year freedom from AF/AT after a single procedure off antiarrhythmic drugs was 46 out of 58 (79.3%). The outcome was similar in the 2 cohorts with 18 out of 23 (78.3%) remaining free from arrhythmia in the sequential group compared with 28 out of 35 (80.0%) in the basket group (P=1.00).
Discussion
This study has demonstrated that ablation of persistent AF guided by STAR mapping utilizing sequential recordings is associated with an acute response in all patients, with AF termination in the majority (78%). Rates of freedom from AF/AT during follow-up were high (83%). The acute response to ablation and 1-year clinical outcomes with STAR-guided ablation were confirmed across a larger cohort including patients mapped with basket catheters and those mapped sequentially, with no apparent difference between modalities. These data also demonstrate that the principles underlying STAR mapping can be consistently applied regardless of the technology used to implement them, and hence this approach was feasible with a range of different catheters and mapping systems.
STAR Mapping
The STAR mapping method is a novel mapping method that utilizes multiple wavefront trajectories to determine the directionality and patterns of wavefront propagation and thereby identifying originating sites that were defined as AFDs. This mapping method has been validated both in vitro and in vivo.9,10 Our group has previously demonstrated that AFDs identified on STAR mapping with basket catheters are frequently identified on sequential mapping with a high sensitivity and specificity.6,7 This is the first study that has evaluated AF ablation prospectively guided by sequential STAR mapping.
Our group has previously demonstrated that global mapping with basket catheters using the STAR mapping method is associated with high rates of electrophysiological end points (AF termination or CL slowing, as in the current study) and freedom from AF/AT during follow-up.9 In those patients, a high proportion of the AFDs (84%) identified on global STAR maps using basket catheters were also identified on sequential STAR maps recorded during the same procedures using a PentaRay catheter but created offline.7 The AFDs that were identified on both global and sequential STAR maps were more likely to be associated with AF termination on ablation rather than CL slowing compared to those drivers that were identified by global mapping with a basket catheter alone.7 In the current study, AF termination was achieved in the majority of patients with ablation prospectively guided by sequential STAR mapping (78%), and again all patients reached the study-defined ablation response. Ablation guided by sequential STAR mapping was associated with a high rate of freedom from AF/AT, off antiarrhythmic drugs, at long-term follow-up (82% at 17 months). A direct comparison between ablation guided by STAR mapping utilizing sequential and basket catheter mapping showed that the outcomes are comparable in terms of electrophysiological end points and freedom from AF/AT during follow-up.
The chaotic nature of AF and variable CL has meant that mapping AF using multipolar catheters has not been widely attempted. Mapping rotors or reentrant activity using sequential mapping is dependent on mapping the whole circuit to allow distinction with passive activations and may be further hindered by rotors meandering over small areas. Because the STAR mapping method does not attempt to distinguish AFD mechanism and simply aims to identify organized activity through mapping wavefront propagation away from a source, this does not appear to be a limitation for STAR mapping and the whole circuit does not necessarily need to be mapped. A driver moving over a small area would still demonstrate wavefronts emanating from that area, possibly with >1 electrode leading intermittently (depending on resolution). Theoretically, STAR mapping could be performed using any catheter with >1 electrode with any resolution, although it is unclear at present how many electrodes over what area is required to apply this technique. These data show that the multipolar catheters used with different numbers of electrodes and resolutions yielded similarly useful information regarding the mapped area.
It has been shown that although AFDs demonstrate temporal periodicity, they are recurrent and spatially relatively conserved.1,5,12–14 During a 30-second recording an AFD recurs ≈8×5,12 and, therefore, would be expected to be identifiable using sequential mapping during this time period. Although AFDs have been shown to be intermittent, the STAR mapping method takes this driver characteristic into account. As the STAR mapping method compares data from many wavefront trajectories that occur during the recording duration and then uses the data to generate a statistical picture of the proportion of time an electrode is leading its neighboring electrodes it is not dependent on the driver being stable but only repetitive. This is a significant advantage of this mapping method.
In contrast to Topera and ECGI mapping systems used to map AFDs, the STAR mapping method allows both global and sequential mapping. There are several advantages to using sequential mapping over global mapping with basket catheters. Sequential mapping is less expensive because the same catheter used to assess for PVI is also used for the unipolar electrogram recordings, and therefore, there is no need for additional equipment. Sequential mapping also avoids the need for additional operator experience with catheters they may not use routinely for PVI or AT ablation.
Although the proportion of patients that underwent mapping using the Rhythmia and EnSite Precision mapping systems was small the aim of the study was to demonstrate feasibility in applying the STAR mapping method using a sequential mapping approach. The multipolar catheters used with these mapping systems are very different both in the number of electrodes (20 with CARTO, 16 with EnSite Precision and 64 with Rhythmia) and shape. Therefore, the STAR mapping method can be implemented effectively using different multipolar catheters. Further to this, the geometry created with both of the mapping systems could effectively be used to generate the STAR AF maps. The patient numbers are too small to infer anything meaningful about long-term clinical outcomes between the different mapping systems, but there was no significant difference in the AF termination rates on ablation between the mapping systems, and a study-defined ablation response was achieved in all patients. In theory, the STAR mapping method can be applied to any mapping system that allows export data to be obtained that includes unipolar electrograms, electrode location, and geometry data.
This study has shown that AFDs identified pre- and post-PVI are more likely to be associated with an ablation response particularly AF termination. This is consistent with the findings from our previous studies.5,7,9,12 This suggests that these AFDs are mechanistically more important. On a practical level, this raises the question as to whether STAR mapping should be performed pre- or post-PVI. AFDs identified pre-PVI may be more important, but more AFDs will be identified on mapping post-PVI. Mapping could be performed at both time points to prioritize targets and apply a hierarchical approach to AFD ablation, but this would be time-consuming. Further testing of different ablation protocols are necessary. We would still advocate that patients undergo PVI as a baseline procedure because to date this has been shown to be the most effective strategy for treatment of both persistent and paroxysmal AF.
More recent studies have shown promising results with other novel mapping technologies used to guide AF ablation.15,16 However, the success rates reported in the de-novo persistent ablation patients in regards to freedom from AF/AT off antiarrhythmic drugs following a single procedure were much lower than those seen with the STAR mapping method (58% in RADAR versus 86% in STAR). UNCOVER trial13 evaluates the use of noncontact charge density mapping which uses CT reconstruction of the cardiac chamber whilst the STAR mapping method utilizes a replica of the geometry created in the 3-dimensional mapping system making it more accurate when tagging AFDs for ablation.
Limitations
One of the study limitations is the small patient numbers. Outcome data for a cohort of this size, although very encouraging should be viewed as pilot/feasibility data. The patient numbers are compatible with that of other proof of concept studies evaluating novel methods and technologies.9,17–19
To determine the mechanistic significance of potential AFDs, we necessarily focused on electrophysiological end points; since there is arguably no other way to verify that a driver has been mapped. Although termination of AF is clear, the importance of CL prolongation is less certain. Others have used termination of AF or CL prolongation as a surrogate for the interruption of mechanisms important for the maintenance of AF.9,12,20–23 In a patient who may have multiple potential drivers it would seem important to note any marked response to ablation. In defining a significant change in AF CL as ≥30 ms we have used the most stringent criteria of any published study reporting AF CL. Nevertheless, even if we only confirmed as drivers those phenomena where AF terminated altogether, the results of this study would be very similar albeit with fewer confirmed drivers.
In this study, the inability to induce AF was not used as an end point due to the lack of clinical evidence of its importance in regard to clinical outcomes. However, this is something that would be interesting to evaluate in future studies.
Conclusions
Ablation guided by sequential STAR mapping was associated with AF termination in a majority of patients and produced an ablation response in all patients with short radiofrequency ablation times. This study lends further weight to the driver hypothesis for maintenance of AF and details a novel consecutive mapping approach to identification of AFDs. The STAR mapping method can be applied using either global mapping with basket catheters or consecutive mapping with conventional multipolar mapping catheters; each may have advantages, but the results appear similar. The principles underlying STAR mapping consistently identified AFDs when different mapping systems and catheters were utilized, and the most effective technique remains to be defined. Prospective randomized studies powered to assess long-term outcomes are needed to evaluate the clinical impact.
Sources of Funding
Supported by Project Grant from the British Heart Foundation (grant number: PG/16/10/32016).
Disclosures
Prof Schilling has received speaker and travel grants from Biosense Webster and research grants from Biosense Webster and Boston Scientific. Prof Hunter has received research grants from Medtronic and educational grants from Biosense Webster. Dr Honarbakhsh, Prof Schilling, Dr Finlay, and Prof Hunter are shareholders in Rhythm AI Ltd. The other author reports no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AFD
- AF driver
- AT
- atrial tachycardia
- CL
- cycle length
- LA
- left atrium
- PV
- pulmonary vein
- PVI
- pulmonary vein isolation
- RA
- right atrium
- STAR
- stochastic trajectory analysis of ranked signals
For Sources of Funding and Disclosures, see page 1122.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.120.008824.
References
- 1.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014; 130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421 [DOI] [PubMed] [Google Scholar]
- 2.Knecht S, Sohal M, Deisenhofer I, Albenque JP, Arentz T, Neumann T, Cauchemez B, Duytschaever M, Ramoul K, Verbeet T, et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace. 2017; 19:1302–1309. doi: 10.1093/europace/euw168 [DOI] [PubMed] [Google Scholar]
- 3.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. 2014; 63:1761–1768. doi: 10.1016/j.jacc.2014.02.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA, Shivkumar K. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm. 2016; 13:636–641. doi: 10.1016/j.hrthm.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R, Chow A, Earley MJ, Hunter RJ. A novel mapping system for panoramic mapping of the left atrium: application to detect and characterize localized sources maintaining atrial fibrillation. JACC Clin Electrophysiol. 2018; 4:124–134. doi: 10.1016/j.jacep.2017.09.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honarbakhsh S, Schilling RJ, Providencia R, Keating E, Sporton S, Lowe M, Lambiase PD, Chow A, Earley MJ, Hunter RJ. Automated detection of repetitive focal activations in persistent atrial fibrillation: validation of a novel detection algorithm and application through panoramic and sequential mapping. J Cardiovasc Electrophysiol. 2019; 30:58–66. doi: 10.1111/jce.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honarbakhsh S, Schilling RJ, Finlay M, Keating E, Ullah W, Hunter RJ. STAR mapping method to identify driving sites in persistent atrial fibrillation: application through sequential mapping. J Cardiovasc Electrophysiol. 2019; 30:2694–2703. doi: 10.1111/jce.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honarbakhsh S, Schilling RJ, Providência R, Dhillon G, Sawhney V, Martin CA, Keating E, Finlay M, Ahsan S, Chow A, et al. Panoramic atrial mapping with basket catheters: a quantitative analysis to optimize practice, patient selection, and catheter choice. J Cardiovasc Electrophysiol. 2017; 28:1423–1432. doi: 10.1111/jce.13331 [DOI] [PubMed] [Google Scholar]
- 9.Honarbakhsh S, Hunter RJ, Ullah W, Keating E, Finlay M, Schilling RJ. Ablation in persistent atrial fibrillation using stochastic trajectory analysis of ranked signals (STAR) mapping method. JACC Clin Electrophysiol. 2019; 5:817–829. doi: 10.1016/j.jacep.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Honarbakhsh S, Hunter RJ, Finlay M, Ullah W, Keating E, Tinker A, Schilling RJ. Development, in vitro validation and human application of a novel method to identify arrhythmia mechanisms: the stochastic trajectory analysis of ranked signals mapping method. J Cardiovasc Electrophysiol. 2019; 30:691–701. doi: 10.1111/jce.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017; 14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honarbakhsh S, Schilling RJ, Providencia R, Keating E, Chow A, Sporton S, Lowe M, Earley MJ, Lambiase PD, Hunter RJ. Characterization of drivers maintaining atrial fibrillation: correlation with markers of rapidity and organization on spectral analysis. Heart Rhythm. 2018; 15:1296–1303. doi: 10.1016/j.hrthm.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 13.Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, et al. Adenosine-induced atrial fibrillation: localized reentrant drivers in lateral right atria due to heterogeneous expression of adenosine A1 receptors and GIRK4 subunits in the human heart. Circulation. 2016; 134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalewski CAB, Shenasa F, Rodrigo M, Clopton P, Meckler G, Alhusseini MI, Swerdlow MA, Joshi V, Hossainy S, Zaman JAB, et al. Interaction of localized drivers and disorganized activation in persistent atrial fibrillation: reconciling putative mechanisms using multiple mapping techniques. Circ Arrhythm Electrophysiol. 2018; 11:e005846 doi: 10.1161/CIRCEP.117.005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudry S, Mansour M, Sundaram S, Nguyen DT, Dukkipati SR, Whang W, Kessman P, Reddy VY. RADAR: a multicenter food and drug administration investigational device exemption clinical trial of persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020; 13:e007825 doi: 10.1161/CIRCEP.119.007825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willems S, Verma A, Betts TR, Murray S, Neuzil P, Ince H, Steven D, Sultan A, Heck PM, Hall MC, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF trial. Circ Arrhythm Electrophysiol. 2019; 12:e007233 doi: 10.1161/CIRCEP.119.007233 [DOI] [PubMed] [Google Scholar]
- 17.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012; 23:1277–1285. doi: 10.1111/jce.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daoud EG, Zeidan Z, Hummel JD, Weiss R, Houmsse M, Augostini R, Kalbfleisch SJ. Identification of repetitive activation patterns using novel computational analysis of multielectrode recordings during atrial fibrillation and Flutter in Humans. JACC Clin Electrophysiol. 2017; 3:207–216. doi: 10.1016/j.jacep.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 19.Calvo D, Rubin J, Perez D, Moris C. Ablation of rotor domains effectively modulates dynamics of human: long-standing persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2017; 10:e005740 doi: 10.1161/CIRCEP.117.005740 [DOI] [PubMed] [Google Scholar]
- 20.Hunter RJ, Diab I, Tayebjee M, Richmond L, Sporton S, Earley MJ, Schilling RJ. Characterization of fractionated atrial electrograms critical for maintenance of atrial fibrillation: a randomized, controlled trial of ablation strategies (the CFAE AF trial). Circ Arrhythm Electrophysiol. 2011; 4:622–629. doi: 10.1161/CIRCEP.111.962928 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, O’Neill MD, Hocini M, Dubois R, Matsuo S, Knecht S, Mahapatra S, Lim KT, Jaïs P, Jonsson A, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008; 51:1003–1010. doi: 10.1016/j.jacc.2007.10.056 [DOI] [PubMed] [Google Scholar]
- 22.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012; 60:628–636. doi: 10.1016/j.jacc.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haissaguerre M, Lim KT, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P, Virag N. Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace. 2007; 9suppl 6vi64–70. doi: 10.1093/europace/eum208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


