ABSTRACT
Regulatory guidelines mandate housing for laboratory mice at temperatures below their thermoneutral zone, creating chronic cold stress. However, increases in housing temperature could alter immune responses. We hypothesized housing mice at temperatures within their thermoneutral zone would improve sepsis survival and alter immune responses. Male C57BL/6 mice were housed at 22°C or 30°C after cecal ligation and puncture (CLP) for 10 days. Survival of mice housed at 30°C (78%) after CLP was significantly increased compared with mice housed at 22°C (40%). Experimental groups were repeated with mice euthanized at 0, 12, 24, and 48 h post-surgery to examine select immune parameters. Raising housing temperature minimally altered systemic, peritoneal, or splenic cell counts. However, IL-6 levels in plasma and peritoneal lavage fluid were significantly lower at 12 h post-surgery in mice housed at 30°C compared with 22°C. Bacterial colony counts from peritoneal lavage fluid were significantly lower in mice housed at 30°C and in vivo studies suggested this was the result of increased phagocytosis by neutrophils. As previously demonstrated, adoptive transfer of fibrocytes significantly increased sepsis survival compared with saline at 22°C. However, there was no additive effect when adoptive transfer was performed at 30°C. Overall, the results demonstrated that thermoneutral housing improves survival after CLP by increasing local phagocytic activity and technical revisions may be necessary to standardize the severity of the model across different housing temperatures. These findings stress the pronounced impact housing temperature has on the CLP model and the importance of reporting housing temperature.
Keywords: Bacterial clearance, cell therapy, CLP, innate immunity, sepsis
INTRODUCTION
In recent years, the clinical relevance of mouse models of sepsis has been heavily scrutinized due to repeated translational failures following promising preclinical studies. The most frequently used model, cecal ligation and puncture (CLP), has been discussed extensively and the need to improve the clinical relevance of that model has been emphasized (1–3). Several recommendations have been made to more closely align the CLP model with human sepsis including attention to subject-related variables (age, strain, sex); provision of supportive measures (fluids, antibiotics); and modeling with comorbid conditions (4). Standardization of the technical aspects and functional parameters of the CLP model has also been advocated to enhance experimental reproducibility and model standards have been adopted successfully in other fields of study (5). However, there may be even greater impediments to translation from mouse models due to the underlying physiologic stress induced by routine research conditions such as the ambient temperature in animal housing areas.
The ideal temperature for housing laboratory mice has been greatly debated. Current recommendations range from 20°C to 26°C (6), and rodent vivaria typically are maintained at 20°C to 23°C for the comfort of humans wearing personal protective equipment (7). However, this range falls below the established thermoneutral zone (TNZ) of mice, 26°C to 34°C (6, 8) which can have a major impact on murine thermoregulation. Within the TNZ, mice can maintain stable core body temperatures by simply changing behaviors (activity level, nesting, huddling) and adjusting peripheral blood vessel diameters (9). Housed in thermoneutral conditions, mice display mean energy expenditure rates 1.8 times higher than basal metabolism, which resembles the human situation (10). At cooler temperatures, mice must use metabolic heat production to maintain core body temperatures (8, 9), and exhibit increased energy expenditure rates up to 3.1 times basal metabolism (10) along with significant cardiovascular compensation in terms of increased blood pressure and heart rate (11). Therefore, mice live in a state of chronic cold stress at current housing temperatures, which triggers adaptive glucocorticoid production and sympathetic nervous system activation, resulting in secondary, immunosuppressive effects (12). These findings imply current housing temperatures influence the development of numerous mouse models of human disease and any proposed measures to relieve cold stress could impact those models.
Several studies have shown the housing temperature of mice influences experimental phenotypes (13), model development (12), and translation of study results (12–15). In particular, temperature-related alteration of immune responses has direct effects on mouse models of cancer (16), graft-versus-host-disease (17), LPS-induced inflammation (18, 19), and infectious disease (20, 21). In each case, increased housing temperature heightened immune responses and caused major changes in the mouse models. In some cases, the changes caused by thermoneutrality resulted in what was considered to be a better model of the human condition (10, 22). Sepsis models involve similar immune responses to the aforementioned studies, suggesting that they might also be affected by warmer housing environments.
Overall, reviews of the controversies surrounding housing temperature emphasize the importance of conducting preliminary experiments at warmer temperatures to see how the difference affects experimental outcomes (15, 23). While it has been shown that short-term, perioperative warming decreases sepsis mortality after CLP (24), the effect of chronic housing at thermoneutral temperatures remains to be ascertained. Therefore, the purpose of our study was to investigate the effects of housing temperature on a CLP model of sepsis. We hypothesized housing mice at temperatures within their thermoneutral zone would improve sepsis survival and alter immune responses. To test this, survival and select immune parameters were compared between mice housed at standard (22°C) and thermoneutral (30°C) temperatures after CLP. In addition, we repeated our previously reported preclinical study (25) to examine the effects of housing temperature on outcomes after the adoptive transfer of cultured fibrocytes.
MATERIALS AND METHODS
Ethical statement
This study followed the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan, which approved all of the animal procedures and animal care methods presented here. The IACUC is in full compliance with the eighth edition of the Guide for the Care and Use of Laboratory Animals (6).
Animals
Male (6 wk–8 wk) C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Maine) were used in all the experiments. Mice were housed on approximately 300 mL of a 50:50 blend of 1/4-in. and 1/8-in. irradiated corncob bedding (Anderson's Bed-O’Cobs, Frontier Distributing, Maumee, Ohio) with 6 g of brown crinkle paper encased in a white tea bag material (EnviroPak, Shepherd Specialty Papers, Watertown, Tenn). Prior to experimental use, mice were acclimated for at least 5 days in ventilated microisolation cages in a temperature-controlled room kept at 22.2°C ± 1.1°C and relative humidity of 60% ± 10%. After CLP, mice were housed three to five per cage in static microisolation cages in specialized climate chambers (TSE ActiMot2 303021 series) set to 22°C or 30°C. Relative humidity was set to 60%. Housing was in a SPF barrier facility with a 12-h dark/light cycle. Mice were SPF for viruses, bacteria, and parasites including mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, polyomavirus, Mycoplasma pulmonis, and pinworms. Mouse norovirus, Helicobacter spp., and other bacterial pathogens are not tested routinely at our institution. Mice had ad libitum access to food (Laboratory Rodent Diet 5001, PMI Lab Diet, St. Louis, Mo) and water.
Survival studies
Under isoflurane anesthesia (Vetone, Boise, Idaho), CLP was performed with ligation of 50% of the cecum and two punctures with a 26-gauge needle using previously described methods (26). At induction, mice received 1 mL saline and 0.05 mg/kg buprenorphine SQ. Additional analgesia (0.05 mg/kg buprenorphine in 0.1 mL saline) was given SQ every 12 for 48 h. The abdominal musculature was closed with silk sutures followed by closure of the skin with tissue glue. Mice recovered while receiving heat support before being placed back into their home cage. Postoperatively, mice were randomized to housing at either 22°C or 30°C (n = 18–20 per group) in climate controlled chambers (TSE ActiMot2 303021 series) for up to 10 days. Loss of righting reflex was used as a humane endpoint. The experimental reproducibility was assured by performing CLP in six small independent trials and then combining the results for this study.
Endpoint studies
The CLP studies were repeated and mice were euthanized at 12, 24, and 48 h (n = 7–10 per temperature group/time point) for evaluation of immune parameters. For core body temperature measurements, electronic ID transponders (IPTT-30; BioMedic Data systems, Inc, Seaford, Del) were inserted into the peritoneum of mice (n = 5/temperature group) prior to abdominal closure after CLP. Temperature readings were obtained with the corresponding handheld reader 24 h after return to housing. A group of unmanipulated mice (n = 10) were euthanized after initial acclimation to provide reference values. At specific time points, mice were deeply anesthetized with isoflurane, and approximately 500 μL blood was collected from the retroorbital sinus into 1.5-mL tubes containing 50 μL of 160 mM EDTA. Mice then were euthanized by cervical dislocation and bilateral pneumothorax. Peritoneal lavage fluid was collected by injecting 10 mL Hanks Balanced Salt Solution (Invitrogen, Grand Island, NY) containing 1:100 heparin sodium (5,000 USP U/mL; Abraxis, Schaumberg, Ill) into the abdomen and retrieving fluid.
Cell counts
A 50-μL aliquot of blood was used for automated CBC analysis (Hemavet, Drew Scientific, Miami, Fla). The remainder of the blood was centrifuged (2,000 × g, 5 min) and the plasma stored at −20°C for later cytokine analysis. The peritoneal lavage fluid was centrifuged (600 × g, 5 min), and the supernatant saved at −20°C for later cytokine analysis. The cell pellet was resuspended in 200 μL RPMI 1640 (Invitrogen) containing 0.1% heat-inactivated fetal bovine serum (Invitrogen). Cells were counted after RBC lysis (Zap-O-globin II (Beckman Coulter, Indianapolis, Ind)) using a hemocytometer (Hausser Scientific, Horsham, Pa). Experimental groups were known to the operator during initial cell counts but evaluation of differentials was blinded. Slides were loaded with 1 × 105 cells, centrifuged (109 × g, 5 min), and stained with Diff-Quick (Baxter, Detroit, Mich). Differentials (300 cells) were counted under light microscopy.
Cytokine measurements
Cytokines were measured in plasma (1:10 dilution) and peritoneal lavage fluid (1:2 dilution) using sandwich ELISA kits for IL-6, IL-10, IL-1β, and TNF-α (Mouse Uncoated ELISA Kits, ThermoFisher Scientific, Waltham, Mass), according to manufacturer's instructions. Absorbance was read at 450 nm on a plate reader (Biotek, Winooski, Vt).
T cell counts and intracellular cytokines
The spleens of mice were harvested at 0, 12, 24, and 48 h after CLP. Spleens were macerated, combined with 5 mL saline, and passed through 100 μM filters. Samples were centrifuged at 2,000 rpm for 5 min and red blood cells lysed (Red Blood Cell Lysis Buffer, eBiosciences, San Diego, Calif). Cells were centrifuged (2,000 rpm, 5 min), resuspended in 200 μL of 1× PBS, and counted using a hemocytometer (Hausser Scientific). Trypan blue (Invitrogen, Carlbad, Calif) was used to demonstrate viability of greater than or equal to 95% in suspensions. After total splenic cell counts, cells were resuspended in PBS with 0.1% sodium azide and 1.0% bovine calf serum (HyClone, Logan, Utah) at 1.0 × 107 cells/mL. Cells were then incubated with FcγII/III reagent to block Tc receptors (BD Biosciences; 0.5 ug/100 uL aliquot, 5–10 min, 4°C). The cells were stained with the appropriate antibodies against surface antigens (2.5 μg/mL, 30 min, 4°C): PerCP-Cy5.5 antimouse CD3ε (BD Biosciences); V450 antimouse CD4 (ebiosciences), pacific orange antimouse CD8a (Invitrogen), APC Cy7x antimouse CD69 (BD Biosciences) and their appropriate isotype controls. For intracellular cytokines, cells were stained with BV 786 antimouse IFN-γ (BD Biosciences) and FITC antimouse IL-4 (BD Biosciences) using an established protocol (BD Cytofix/Cytoperm Kit, BD Biosciences). For all flow cytometry, fluorescence was measured utilizing a BD LSR II flow cytometer (BD Biosciences) with a 488 nm excitation laser with peak emission ranging from 515 nm to 545 nm. Compensation was performed in all experiments utilizing Winlist 6.0 software (Topsham, Maine).
Bacterial colony counts
Aseptic samples (blood, spleen, peritoneal lavage fluid) were collected from the animals euthanized 24 h after CLP. The samples were serially diluted and plated on TSA w/ 5% sheep blood agar (ThermoFisher Scientific). Colonies were counted after 24 h at 37°C when dilutions yielded 30 to 300 colonies.
Phagocytosis study
Mice were housed in 22°C or 30°C housing after CLP. Ten hours after surgery, heat-killed (70°C for 15 min) E coli (1 × 108 CFUs in 200 μL saline) labeled with Sytox Blue (ThermoFisher Scientific), a DNA-specific fluorescent dye, per manufacturer's instructions were administered IP. Two hours later, the animals were euthanized and peritoneal lavage samples and spleens were collected for total cell counts and flow cytometry as described above. Neutrophils were identified using Anti-Mouse Ly-6G APC (Affymetrix, San Diego, Calif). Macrophages were identified with anti-mouse F4/80 PE (Affymetrix). Appropriate negative controls were used (Armenian Hamster IgG Isotype Control, APC; Armenian Hamster IgG Isotype Control, PE). For each cell type, the percentage of cells expressing fluorescent bacteria and the mean fluorescence intensity of individual cells were quantified using flow cytometry.
Fibrocyte adoptive transfer study
Fibrocytes were obtained from primary culture of male C57BL/6J mouse lungs as described in past studies (27). Minced lungs were suspended in fibrocyte culture media (DMEM, 10% FBS, 1% L-glutamine, and 1% antibiotic/antimycotic; Invitrogen) with media changes every 3 to 4 days for 2 weeks (37°C, 5% CO2), until cultures were approximately 80% congruent. Adherent cells were incubated with trypsin + EDTA in a CO2 incubator and then scraped, centrifuged, and washed with PBS. Positive separation of CD45+ cells was achieved using antibody coupled magnetic beads per the manufacturer's instructions (Miltenyi, Bergisch Gladbach, Germany). The fibrocytes were then counted on a hemocytometer (Hausser Scientific) and their viability confirmed with trypan blue (Invitrogen). The cells were resuspended at 1×106/200 μL sterile saline and injected into the tail vein of mice at the time of CLP. Controls received an equal volume of saline IV. The mice were randomized to either 22°C or 30°C housing for up to 10 days.
Statistics
All analyses were performed by using Prism (GraphPad, LaJolla, Calif). For the survival study, Kaplan–Meier survival curves were calculated for each group, and differences between groups were measured by using log-rank tests. The Shapiro–Wilk test was used to assess normality (alpha = 0.05). Treatment group means and standard errors were calculated for each parameter of interest. For major comparisons between two groups, Student t tests were used, except for core body temperature, which was evaluated by Mann–Whitney U. A P value of <0.05 was considered significant.
RESULTS
Thermoneutral housing enhances sepsis survival
When mice were housed at either 22°C or 30°C (n = 18–20/group, six independent experiments), the majority of deaths occurred between the first and fourth day post-CLP in the 22°C group (Fig. 1A). The 22°C group had a survival rate of 40% while the 30°C group had a significantly increased (P < 0.05) survival of 78%, demonstrating thermoneutral housing has an effect on the outcome of sepsis in this model.
Fig. 1.
Thermoneutral housing enhances survival after CLP and influences core body temperatures.
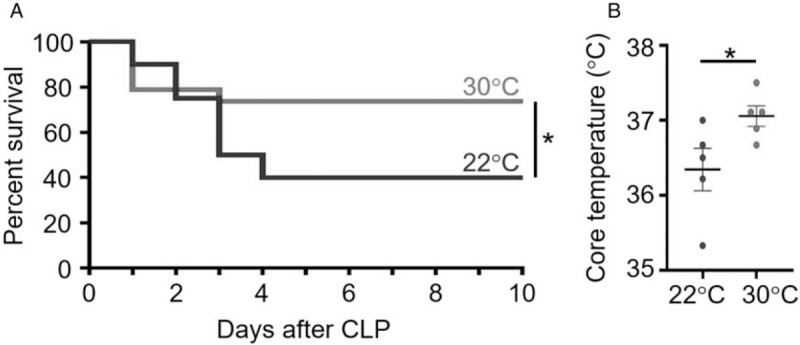
Male, C57BL/6 mice underwent CLP surgery and then were randomized to housing conditions in temperature-controlled chambers at either 22°C or 30°C. A, Animals were monitored for survival for up to 10 days. n = 20 for 22°C group; n = 19 for 30°C group. B, Core body temperatures were evaluated at 24 h post-CLP (n = 5 mice per group). ∗ = <0.05. CLP indicates cecal ligation and puncture.
Thermoneutral housing influences core body temperature.
Core body temperatures were evaluated using electronic transponders. The body temperature of mice housed at 30°C was significantly greater (P < 0.05) than the body temperature of mice housed at 22°C at 24 h after CLP (Fig. 1B).
Thermoneutral housing alters few markers of inflammation.
To examine potential mechanisms for the improved survival, select markers of inflammation were assessed at acute time points (12, 24, and 48 h after CLP) selected because they precede the time points associated with the majority of deaths in the survival studies.
Complete blood counts— Compared with reference values, septic mice had lower mean total white blood cell counts, particularly monocytes and lymphocytes. However, there were no significant differences in total WBC, monocyte, neutrophil, or lymphocyte counts between the housing temperature groups (Fig. 2, A–D) and cell counts showed similar temporal fluctuations between groups.
Fig. 2.
Thermoneutral housing alters few systemic cell counts.
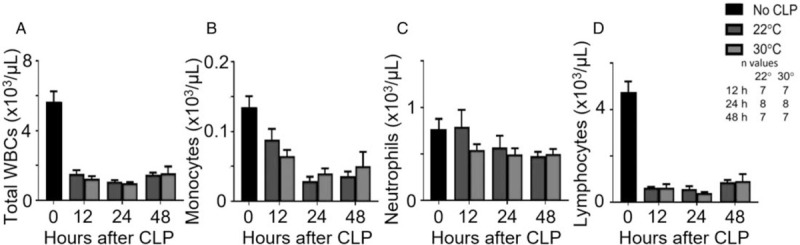
After CLP and housing at 22°C or 30°C, mice were sacrificed at 12, 24, or 48 h post-CLP. An unmanipulated group provided reference values (n = 10). Retroorbital blood was collected for cell counts and differentials including (A) total WBCs, (B) monocytes, (C) neutrophils, and (D) lymphocytes. Data are reported as mean ± SEM. CLP indicates cecal ligation and puncture.
Peritoneal cell counts— In comparison with reference values, the cell counts rose over the first 24 h. However, there were no significant differences between housing temperature groups at any time point (Fig. 3, A–C).
Fig. 3.
Thermoneutral housing alters few local cell counts.
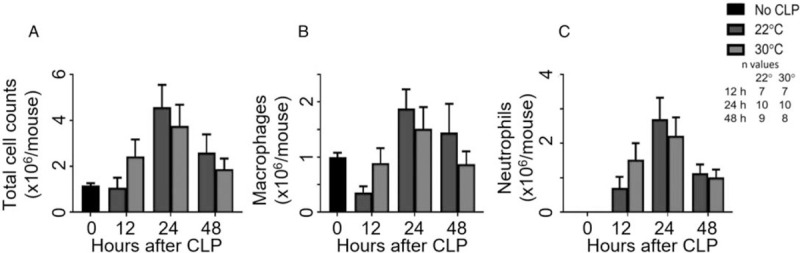
After CLP and housing at 22°C or 30°C, mice were sacrificed at 12, 24, or 48 h post-CLP. An unmanipulated group provided reference values (n = 10). Cell counts and differentials were determined in the peritoneal lavage fluid. Graphs depict (A) total cell counts, (B) macrophages, and (C) neutrophils. Data are reported as mean ± SEM. CLP indicates cecal ligation and puncture.
Plasma cytokines—None of the cytokines were detected in the plasma of the reference animals. The mean plasma IL-6 level of the 22°C group was significantly higher than the 30°C group at 12 h post-CLP (Fig. 4A). The plasma IL-1β levels (Fig. 4B) and IL-10 levels (Fig. 4C) were not significantly different between the temperature groups at any time point. The plasma TNF-α values were near or below detection limits (<9.0 pg/mL) for the assay at all of the time points in both temperature groups.
Fig. 4.
Thermoneutral housing decreases IL-6 levels.
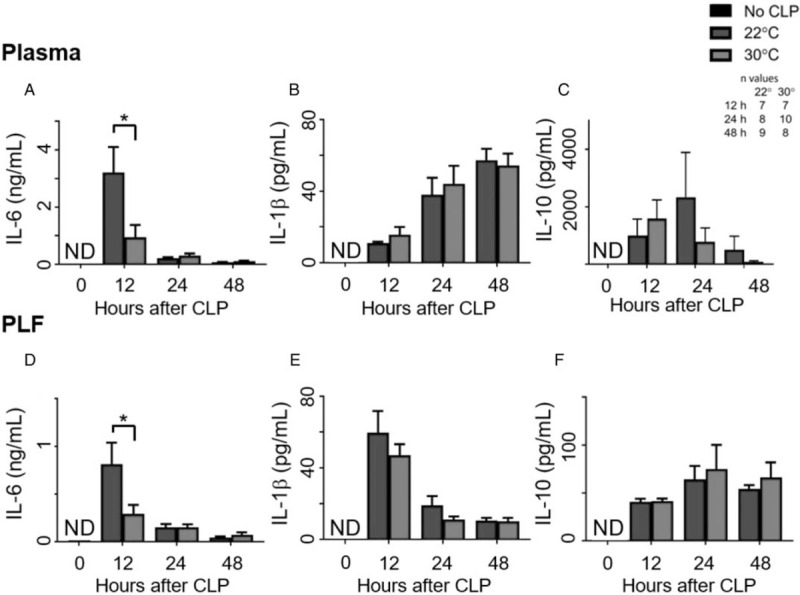
After CLP and housing at 22°C or 30°C, mice were sacrificed at 12, 24, or 48 h post-CLP. An unmanipulated group provided reference values (n = 10). Blood and peritoneal lavage fluid were analyzed for cytokine levels with ELISAs. Graphs depict plasma levels of (A) IL-6, (B) IL-1β, (C) IL-10 and peritoneal lavage fluid levels of (D) IL-6, (E) IL-1β, and (F) IL-10. PLF indicates peritoneal lavage fluid. Data are reported as mean ± SEM. ND indicates not detected, levels lower than assay limits. ∗ = P < 0.05. CLP indicates cecal ligation and puncture.
Peritoneal cytokines— None of the cytokines were detected in the peritoneal lavage fluid from the reference animals. Like the plasma IL-6, the mean peritoneal IL-6 level of the 22°C group was significantly increased (P < 0.05) in comparison with the 30°C group at 12 h post-CLP (Fig. 4D). The peritoneal IL-1β and IL-10 levels were also not different between groups (Fig. 4E and F, respectively). The peritoneal TNF-α levels were near or below detection limits (<9.0 pg/mL) for the assay at all-time points.
Splenic T cell assessments—In comparison with reference values, splenic CD4+ and CD8+ T cells declined in the septic animals while expression of CD69, a marker of activation, and intracellular cytokines increased over the first 12 h. However, splenic counts of both CD4+ and CD8+ T cells had no significant differences between housing temperature groups (Fig. 5, A and E, respectively). Likewise, the expression of CD69 was not different between groups for either cell type (Fig. 5, B and F, respectively). The numbers of CD4+ and CD8+ T cells expressing intracellular IFN-ɣ and IL-4 were not significantly different between housing temperature groups (Fig. 5C, D, G, H).
Fig. 5.
Thermoneutral housing does not alter splenic T cell counts.
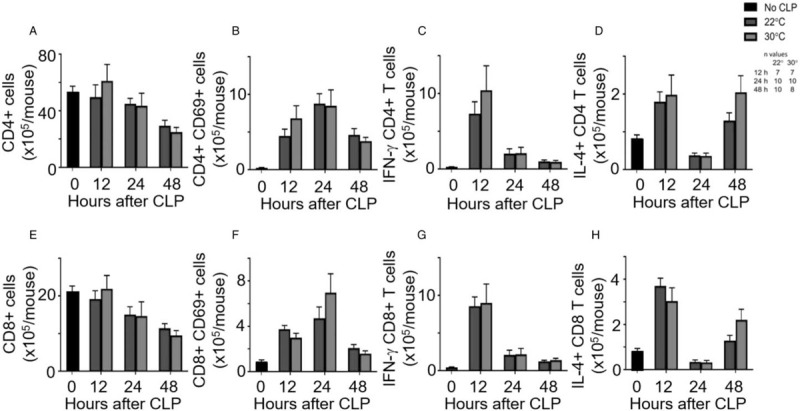
After CLP and housing at 22°C or 30°C, mice were sacrificed at 12, 24, or 48 h post-CLP. An unmanipulated group provided reference values (n = 10). Splenic T-cell populations were analyzed using flow cytometry. Graphs depict (A) total CD4+ cells, (B) activated CD4+ cells, (C) intracellular IFN-ɣ expression in CD4+ cells, (D) IL-4 expression in CD4+ cells, (E) total CD8+ cells, (F) activated CD8+ cells, (G) IFN-ɣ expression in CD8+ cells, and (H) IL-4 expression in CD8+ cells. Data are reported as mean ± SEM. CLP indicates cecal ligation and puncture.
Thermoneutral housing reduces bacterial burden
At 24 h post-CLP, the mean colony counts derived from peritoneal lavage fluid (Fig. 6A) were significantly (P < 0.05) lower in the 30°C group as compared with the 22°C group. Compared with the peritoneal compartment, spleen (Fig. 6B) and blood (Fig. 6C) had no differences between the groups.
Fig. 6.
Thermoneutral housing lowers local bacterial burden.
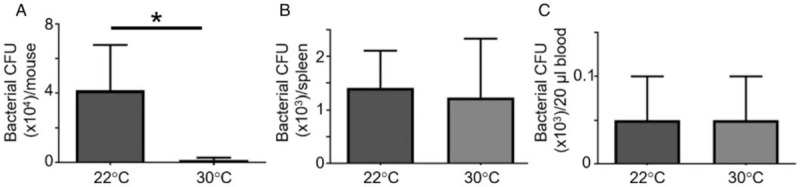
After CLP and housing at 22°C or 30°C, samples were harvested aseptically at 24 h for serial dilution and culture on blood agar plates. After 24 h of incubation at 37°C, the colony forming units were recorded from (A) peritoneal cavity, (B) spleen, and (C) blood. Data are reported as mean ± SEM. ∗ = P < 0.05 as compared with saline 22°C group. n = 10 mice (22°C), n = 6 mice (30°C), two independent experiments. CLP indicates cecal ligation and puncture.
Thermoneutral housing enhances the phagocytic activity of leukocytes
To explain differences in peritoneal bacterial load, the phagocytic activity of peritoneal cells was examined at an early time point. At 12 h post-surgery, a significantly higher (P < 0.05) percentage (Fig. 7A) and absolute number (Fig. 7B) of peritoneal neutrophils displayed phagocytosis of fluorescently labeled E coli in mice housed at 30°C compared with mice housed at 22°C. The mean fluorescence intensity between groups was also significantly higher (P < 0.05) in neutrophils from mice housed at 30°C as compared with 22°C (Fig. 7C). Although the number of macrophages demonstrating phagocytosis was not different (Fig. 7, A and B), the mean fluorescence intensity approached significance (P = 0.056) in macrophages from mice housed at 30°C (Fig. 7D) compared with 22°C.
Fig. 7.
Thermoneutral housing potentiates neutrophil phagocytosis.
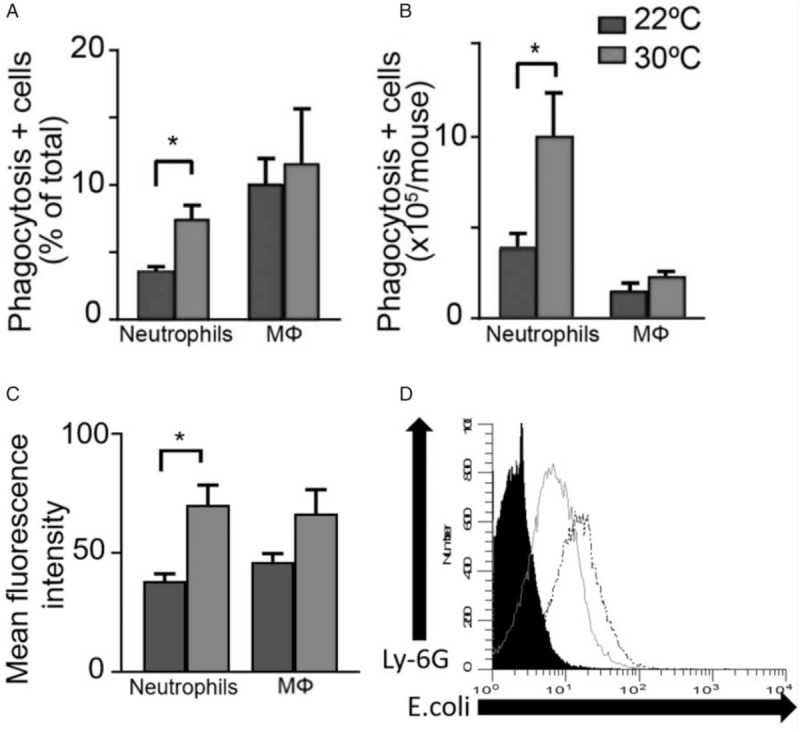
Mice underwent CLP and housing at 22°C or 30°C. Ten hours later, heat-killed Sytox blue labeled E coli were administered IP. Twelve hours after surgery, the peritoneal phagocyte cell populations (Ly-6G+ neutrophils and F4/80+ macrophages) were quantified and presence of intracellular bacteria determined with flow cytometry. Graphs indicate results at either 22°C or 30°C for (A) mean percentage of each cell type demonstrating phagocytosis, (B) mean number of each cell type demonstrating phagocytosis, and (C) the mean fluorescence intensity of each cell type. n = 5 mice/group. Data reported as mean ± SEM. ∗ = P < 0.05. Mɸ = macrophage. D, Representative histogram comparing E coli uptake by neutrophils (Ly-6G+) from mice housed at either 22°C (sold gray line) or 30°C (dotted black line). Black filled area represents fluorescence by control cells unexposed to E coli. CLP indicates cecal ligation and puncture.
Thermoneutral housing does not significantly enhance adoptive transfer
Previously, we have shown improved outcomes when IV adoptive transfer of fibrocytes is performed at the time of CLP. A repeat of this experiment at standard housing temperatures showed similar findings. The fibrocyte adoptive transfer group had an improved survival rate of 74% compared with the saline group (40%) housed at 22°C (Fig. 8A). However, when mice were housed at 30°C, the survival rate of the adoptive transfer group (80%) was not significantly different from the saline group (70%) (Fig. 8B). The major impact of the raised housing temperature appeared to be on the saline group and not the adoptive transfer group.
Fig. 8.
Thermoneutral housing does not alter survival after fibrocyte adoptive transfer.
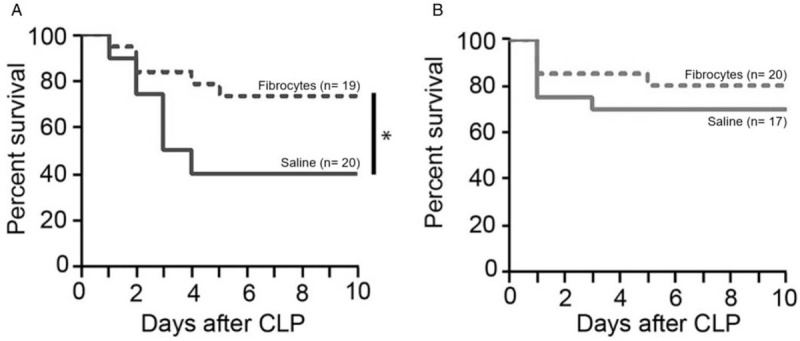
At the time of CLP, saline alone or 1 × 106 fibrocytes were transferred IV. Mice were then randomized to housing conditions in temperature-controlled chambers at either (A) 22°C or (B) 30°C. Animals were monitored for survival for up to 10 days. ∗ = P < 0.05. CLP indicates cecal ligation and puncture.
DISCUSSION
The studies presented here demonstrate that thermoneutral housing at 30°C resulted in improved survival as compared with standard housing at 22°C. Several recent reviews have described similar impact of thermoneutral housing on survival in other mouse models of human disease (12, 15, 22, 23), including those used in metabolism, cancer, and infectious disease research. For instance, housing at thermoneutrality resulted in slower tumor growth and reduced metastasis in mouse cancer models compared with housing at standard temperatures, due to enhanced antitumor immune responses (23, 28). Warmer housing environments have also been reported to have a favorable impact on survival in a number of infection models (29–31). Overall, the results of our CLP studies suggest that technical modifications would be required to produce CLP models yielding similar levels of lethality if current housing recommendations are changed. Of note, the mice in our studies were group housed and had access to nesting material to replicate the routine experimental conditions used in our laboratory. These are factors that may vary between laboratories and do influence the ability of mice to thermoregulate (32). Therefore, initiatives to standardize CLP models across multiple laboratories would be highly dependent upon standardization of housing temperatures and other related housing factors.
To further characterize temperature-related immune responses after CLP, we examined a number of select local and systemic parameters, including cell counts and cytokines. Here, the finding of lower IL-6 levels in the blood and peritoneal lavage fluid at 12 h post-CLP in mice housed at thermoneutral temperatures compared with 22°C was consistent with our survival outcomes and the known role of IL-6 as a mediator and biomarker negatively correlated with survival after CLP (33–35). Immediate postoperative warming at 35°C for 1 h followed by housing at standard temperatures has also been shown to significantly lower plasma IL-6 concentrations and improve survival (60% vs. 42%, respectively) after CLP (33). However, all of the mice in our study received immediate postoperative heat support prior to randomized housing and still demonstrated a shift in survival after CLP (78% vs. 40%, respectively), suggesting impact occurred after the immediate recovery period.
Other than IL-6, we saw few temperature-associated differences in cytokine levels, intracellular T cell cytokines, or immune cell counts, at either local or systemic sites. In contrast, thermoneutral housing conditions have been associated with suppression or delay of systemic cytokine levels (TNF-α and IFN-ɣ) in a murine model of Klebsiella pneumoniae peritonitis (30) and enhanced T-cell cytokine production in mice vaccinated with Francisella tularensis(20) and in tumor-bearing mice (28). Other studies have shown increases of peripheral blood neutrophil counts in response to brief, postoperative warming after CLP (33) and increased local levels of neutrophils and macrophages after the induction of sterile peritonitis in thermoneutral housing temperatures (36). The differences seen between our study and other reports could be due to inherent characteristics of the models, timing of observations or the parameters selected. It is also noteworthy that, even in the 22°C group, core body temperatures at 24 h were not as low (<32°C) as we have previously documented after CLP in singly housed mice (37). The group housing used in this study and the introduction of nesting materials can affect thermoregulation (32, 38) and therefore, may have impacted our findings in comparison with other studies. In addition, the mice in our study were not raised in or acclimated to thermoneutral temperatures prior to experimentation which could have additional impact. Therefore, comprehensive comparisons between studies can be difficult and are highly dependent upon complete reporting of environmental factors that may affect murine thermoregulation.
In our study, thermoneutral housing temperatures were associated with lower bacterial loads in the peritoneum 24 h after CLP, while loads within the blood and spleen were similar between the housing temperatures. These findings are comparable to those seen after intraperitoneal inoculation of Klebsiella pneumoniae that showed mice housed at 35.5°C had lower peritoneal bacterial counts but similar spleen and blood bacterial counts at 24 h when compared with mice housed at 23°C (30). That study ruled out any effects of housing temperature on bacterial proliferation, and the differences in bacterial loads were attributed to alterations in unidentified host defense mechanisms (30). Our study results suggest that enhanced activity by local phagocytes is one mechanism for the effects of thermoneutral housing. These results are in keeping with multiple preclinical sepsis studies that have found increased survival associated with increased phagocytic activity of leukocytes (39–41), and studies that have shown enhanced phagocytosis at warmer temperatures mediated through a number of extra- and intracellular pathways (42–44). Phagocytosis, an energy-dependent process, can vary with temperature due to changes in opsonization, attachment, receptor-mediated uptake, and ingestion (44). Activation of mitochondrial enzymes may also lead to enhanced phagocytosis (43), although temperatures above 39°C may cause impairment in the function of enzymes responsible for pseudopod movement (42). Of note, many of these studies investigated phagocytosis by macrophages while our studies found increased housing temperatures primarily enhanced phagocytosis by neutrophils.
Thermoneutral housing improved the outcome of our control CLP model but did not add to the impact of adoptive transfer studies. Repeatedly, we have shown a survival benefit from the adoptive transfer of fibrocytes in the CLP model (25, 45). This benefit is due, at least in part, to a reduction in bacterial load and increased phagocytic activity (45). In the current study, we once again demonstrated a significant difference from adoptive transfer in standard housing conditions; however, this difference was lost in thermoneutral housing. Since both the adoptive transfer and thermoneutral housing appear to lower bacterial load by enhancing phagocytosis, the effects of housing temperature may have maximized bacterial clearance and negated the significance of the cell therapy. Therefore, further evaluation of the adoptive transfer in a CLP model with greater severity would be warranted. This raises important questions as to which housing condition would best replicate the responses in a clinical patient and whether metabolic alterations caused by housing temperature would impact the efficacy of therapies tested in preclinical studies. There have been reports that temperature-associated changes have produced models that better recapitulate the human disease response. This is evident in allogenic hematopoietic cell transplantation where the major, clinical complications of graft versus host disease and lethality will manifest in mouse models when housed at thermoneutral temperatures but not at standard housing temperatures. Likewise, when housed at 31°C, mice may develop the hyperthermia seen in humans (19) after administration of endotoxin. Yet, at standard housing temperatures, mice develop paradoxical hypothermia in response to endotoxin (19). In cases of clinical sepsis, the majority of patients are hyperthermic (46), and recent reports suggest these patients are at a lower risk of mortality than septic patients with hypothermia (46, 47). Therefore, the inherent cold stress of standard housing may result in murine CLP models representative of the more severely affected clinical cohort. In our study, mean core body temperature was higher in the mice housed at 30°C as compared with 22°C at the 24 h time point and further, continuous temperature monitoring could determine whether transient hyperthermia develops. This suggests that comprehensive preclinical testing of sepsis therapeutics in mice might benefit from studies at a range of housing temperatures, an approach that has already been advocated in cancer research due to similar temperature dependency (23).
Overall, a broad change to thermoneutral housing of mice could present some challenges to ongoing sepsis studies employing the CLP model. The metabolic changes associated with relief of cold stress might necessitate modifications to re-establish the severity of some CLP models and/or, depending upon the underlying mechanisms, might require re-evaluation of interventions. With regard to implementation, regulation of thermoneutral housing at the room level would create an uncomfortable working environment for personnel, while cage level regulation would increase the labor and expense associated with husbandry (7, 48). On the other hand, further study could reveal advantages to a murine CLP model that more closely replicates the metabolic and immunologic responses of humans and yields a better translational link with clinical sepsis studies.
In conclusion, this study demonstrates that thermoneutral housing improves the survival outcome of the CLP model and reduces bacterial load by enhancing phagocytosis. These results reinforce the importance of reporting housing temperature and related factors such as housing density, nesting materials, and relative humidity to improve reproducibility of animal models. They also highlight that the effects of cold stress must be considered when interpreting the results of current mouse studies. Therefore, housing temperature, a very basic variable, may have further, serious implications for preclinical models and the treatment of septic patients in intensive care settings.
ACKNOWLEDGMENT
The authors thank Dr Randy Seeley (Department of Surgery, University of Michigan) for the use of specialized climate chambers.
Footnotes
This work was supported by funding from the National Institutes of General Medical Sciences of the National Institutes of Health (RO1GM112799).
The authors report no conflicts of interest.
REFERENCES
- 1.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock 26 (6):565–570, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119 (10):2868–2878, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol 98 (6):945–952, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zingarelli B, Coopersmith CM, Drechsler S, Efron P, Marshall JC, Moldawer L, Wiersinga WJ, Xiao X, Osuchowski MF, Thiemermann C. Part I: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for study design and humane modeling endpoints. Shock 51 (1):10–22, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remick DG, Ayala A, Chaudry IH, Coopersmith CM, Deutschman C, Hellman J, Moldawer L, Osuchowski MF. Premise for standardized sepsis models. Shock 51 (1):4–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal, Research, Guide for the care and use of laboratory, animals. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 7.Gaskill B N, Rohr S A, Pajor E A, Lucas F, Garner F Some like it hot: mouse temperature preferences in laboratory housing. Appl Anim Behav Sci 116 (2):279–285, 2009. [Google Scholar]
- 8.Gordon C. Temperature Regulation in Laboratory Animals. New York: Cambridge University Press; 1993. [Google Scholar]
- 9.Gordon CJ. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol Behav 179:55–66, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab 7:161–170, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287 (2):R391–R396, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209 (6):1069–1074, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 34: suppl 2: S53–S58, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29 (6):413–420, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Hankenson FC, Marx JO, Gordon CJ, David JM. Effects of rodent thermoregulation on animal models in the research environment. Comp Med 68 (6):425–438, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messmer MN, Kokolus KM, Eng JWL, Abrams SI, Repasky EA. Mild cold-stress depresses immune responses: implications for cancer models involving laboratory mice. BioEssays 36 (9):884–891, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, et al. Housing temperature-induced stress is suppressing murine graft-versus-host disease through beta2-adrenergic receptor signaling. J Immunol 195 (10):5045–5054, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu E, Lewis K, Al-Saffar H, Krall CM, Singh A, Kulchitsky VA, Corrigan JJ, Simons CT, Petersen SR, Musteata FM, et al. Naturally occurring hypothermia is more advantageous than fever in severe forms of lipopolysaccharide- and Escherichia coli-induced systemic inflammation. Am J Physiol Regul Integr Comp Physiol 302 (12):R1372–R1383, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289 (5):R1244–R1252, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Rubin RL. Mice housed at elevated vivarium temperatures display enhanced T-cell response and survival to Francisella tularensis. Comp Med 67 (6):491–497, 2017. [PMC free article] [PubMed] [Google Scholar]
- 21.Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun 21 (7):975–987, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 13 (8):458–465, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hylander BL, Repasky EA. Thermoneutrality, mice, and cancer: a heated opinion. Trends Cancer 2 (4):166–175, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H, Remick DG. Correction of perioperative hypothermia decreases experimental sepsis mortality by modulating the inflammatory response. Crit Care Med 33 (1):161–167, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Nemzek JA, Fry C, Moore BB. Adoptive transfer of fibrocytes enhances splenic T-cell numbers and survival in septic peritonitis. Shock 40 (2):106–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugunin KM, Fry C, Shuster K, Nemzek JA. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34 (3):250–260, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166 (3):675–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A 110 (50):20176–20181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underwood GE, Baker CA, Weed SD. Protective effect of elevated temperature on mice infected with Coe virus. J Immunol 96 (6):1006–1012, 1966. [PubMed] [Google Scholar]
- 30.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 68 (3):1265–1270, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Won WD, Ross H. Relationship of low temperature to mouse resistance to infection with Klebsiella pneumoniae. Aerosp Med 42 (6):642–645, 1971. [PubMed] [Google Scholar]
- 32.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110-111:87–95, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun 73 (5):2751–2757, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17 (6):463–467, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol 170 (1):503–507, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, Chawla A. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 23 (1):165–178, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. Humane endpoints in shock research. Shock 21 (1):17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Speakman JR. Measuring energy metabolism in the mouse—theoretical, practical, and analytical considerations. Front Physiol 4:34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu X-J, Chen J, Yu C-H, Shi Y-H, He Y-Q, Zhang R-C, Huang Z-A, Lv J-N, Zhang S, Xu L. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med 210 (1):5–13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redlich S, Ribes S, Schutze S, Nau R. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 by macrophages and increases the resistance of mice against infections. J Neuroinflammation 11:108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, Nakahira K, Ryter SW, Choi AM. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal 20 (3):432–442, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pramanik T, Thapa M, Saikia TC. Effect of temperature on phagocytic activity of neutrophils. Nepal Med Coll J 6 (1):39–40, 2004. [PubMed] [Google Scholar]
- 43.Djaldetti M, Bessler H. High temperature affects the phagocytic activity of human peripheral blood mononuclear cells. Scand J Clin Lab Invest 75 (6):482–486, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Holtzman E. Lysosomes. 1989; New York: Plenum Press, 39–40. [Google Scholar]
- 45.Collins D, Fry C, Moore BB, Nemzek JA. Phagocytosis by fibrocytes as a mechanism to decrease bacterial burden and increase survival in sepsis. Shock 51 (4):464–471, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, Araki T, Ikeda H, Kotani J, Miki Y, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care 17 (6):R271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rumbus Z, Matics R, Hegyi P, Zsiboras C, Szabo I, Illes A, Petervari E, Balasko M, Marta K, Miko A, et al. Fever is associated with reduced, hypothermia with increased mortality in septic patients: a meta-analysis of clinical trials. PLoS One 12 (1):e0170152, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon CJ, Puckett ET, Repasky ES, Johnstone AF. A device that allows rodents to behaviorally thermoregulate when housed in vivariums. J Am Assoc Lab Anim Sci 56 (2):173–176, 2017. [PMC free article] [PubMed] [Google Scholar]


