Abstract
The emerging fungal pathogen Batrachochytrium salamandrivorans (Bsal) is a major threat to amphibian species worldwide with potential to infect many species if it invades salamander biodiversity hotspots in the Americas. Bsal can cause the disease chytridiomycosis, and it is important to assess the risk of Bsal-induced chytridiomycosis to species in North America. We evaluated the susceptibility to Bsal of the common and widespread spotted salamander, Ambystoma maculatum, across life history stages and monitored the effect of Bsal exposure on growth rate and response of the stress hormone, corticosterone. We conclude that spotted salamanders appear resistant to Bsal because they showed no indication of disease or infection, and experienced minor effects on growth upon exposure. While we focused on a single population for this study, results were consistent across conditions of exposure including high or repeated doses of Bsal, life-stage at exposure, environmental conditions including two temperatures and two substrates, and promoting pathogen infectivity by conditioning Bsal cultures with thyroid hormone. Exposure to high levels of Bsal elicited an acute but not chronic increase in corticosterone in spotted salamanders, and reduced growth. We hypothesize that the early acute increase in corticosterone facilitated mounting an immune response to the pathogen, perhaps through immunoredistribution to the skin, but further study is needed to determine immune responses to Bsal. These results will contribute to development of appropriate Bsal management plans to conserve species at risk of emerging disease.
Keywords: Ambystoma maculatum, corticosterone, chytridiomycosis, disease resistance, Notophthalmus viridescens, Salamandra salamandra, susceptibility, thyroid
Graphical Abstract

The emerging fungal pathogen, Batrachochytrium salamandrivorans (Bsal), is a major threat to amphibian species worldwide, with the potential to invade biodiversity hotspots in the Americas. We evaluated the susceptibility of the common and widespread spotted salamander, Ambystoma maculatum, to Bsal across life history stages and environmental conditions and monitored the effect of Bsal exposure on growth rate and response of the stress hormone, corticosterone. While corticosterone increases initially upon exposure to Bsal, we found no indication of disease or persisting infection, and minor effects on growth upon exposure.
INTRODUCTION
It is increasingly apparent in a world of emerging infectious diseases (Daszak, Cunningham & Hyatt, 2000) that getting an early start on preventing and combating major epidemics is crucial for maintaining overall biodiversity and ecosystem health. One emerging infectious disease, chytridiomycosis, brought on by infection with the fungal pathogens, Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal), is causing substantial concern (Martel et al., 2013; Martel et al., 2014; Gray et al., 2015). Bsal has already spread and caused major declines in salamander populations in parts of Europe (Martel et al., 2013; Spitzen-van der Sluijs et al., 2016). Chytridiomycosis is predicted to be a severe threat to North American salamanders (Martel et al., 2014; Yap et al., 2015; Richgels et al., 2016), underscoring the importance of determining species susceptibility and identifying disease reservoirs in order to develop strategic management plans for disease mitigation (Gray et al., 2015; Grant et al., 2016).
Chytridiomycosis has led to population declines and extinctions of hundreds of amphibian species worldwide (Scheele et al., 2019). However, Bd and Bsal have distinct morphologies and differential environmental and host preferences (Martel et al., 2013; James et al., 2015; Woodhams et al., 2018). The optimal growth temperature of Bsal (10–15°C; Martel et al., 2013) is lower than that of Bd. It also appears to affect primarily salamander hosts (Martel et al., 2014); however, anurans can be infected (Stegen et al., 2017). Like Bd, Bsal has zoospores that can infect amphibian skin. These zoospores develop into sporangia with discharge tubes that can release more zoospores allowing for recurring infection. Symptoms from Bsal infection include skin ulceration, lethargy, lack of appetite, and ultimately death, mainly through septicemia (Martel et al., 2013). Preliminary testing for susceptibility to Bsal-induced chytridiomycosis in adults of over 30 salamander species from the USA and Mexico has demonstrated responses ranging from infection resistance to high infection and disease susceptibility (North American Bsal Task Force, M. Gray, pers. comm.). To date, larvae have only been tested for Bsal-susceptibility in fire salamanders, Salamandra salamandra, and they demonstrated resistance to infection (Van Rooij et al., 2015). Susceptibility of larval amphibians to infection can be extremely important for disease transmission and dynamics at the host and community ecology levels (Martel et al., 2014). Indeed, larvae may inhabit water bodies for an extended period of time and act as reservoir hosts for Bd (Woodhams and Alford 2005).
During amphibian development, metamorphic hormones facilitate transitions in immunity (Rollins-Smith 1998), which could directly or indirectly influence pathogens. Vertebrates secrete glucocorticoids to help mediate a rapid behavioral and physiological response to a stressor (Sapolsky, Romero & Munck, 2000). Corticosterone is the primary stress hormone secreted by amphibians (Jungreis, Huilbregtse & Ungar, 1970). As corticosterone is released, it may activate the immune system (Dhabhar 2002) and aid in maintaining homeostasis (McDougall-Shackleton, Bonier, Romero, & Moore, 2019), but chronic stress can be damaging by decreasing survival, reproduction, growth, and suppressing the immune system (McEwen and Wingfield, 2003; Gabor, Fisher & Bosch, 2013). Several studies using a non-invasive method to obtain an integrative measure of corticosterone release rates using water baths have demonstrated that larval amphibians naturally infected with Bd or ranavirus (Gabor et al.,2013; Gabor, Fisher & Bosch, 2015; Davis et al., 2019), or adults exposed to probiotic bacteria (Kearns et al., 2017), exhibit elevated corticosterone release rates. Integrative measures are not point responses as obtained from plasma but instead provide longer term responses to potential stressors as found in feces, urine, and saliva. These changes can be measured, for example, within hours to weeks after exposure to probiotics, chemical pollutants such as atrazine, or infection (Kearns et al., 2017; Gabor et al., 2018; Davis et al., 2019). In addition to corticosterone, thyroid hormone increases during amphibian metamorphosis (Denver 2009), and Bd exhibits chemotaxis to thyroid hormone (3, 5, 3′-triiodothyronine; TH; Thekkiniath et al., 2013). TH can also enhance serine protease gene expression in Bd allowing for increased degradation of amphibian defense peptides and perhaps increased the infectivity of Bd (Thekkiniath et al., 2013). Thus, high levels of TH may be present during critical windows of amphibian development and promote Bsal infection. While virulence factors and mechanisms of host immune suppression by chytrid fungi are described (Rollins-Smith et al., 2015), previous studies have not attempted to alter pathogen infectivity.
Most disease susceptibility trials on amphibians incorporate a set of controlled conditions. A range of pathogen exposure doses are commonly tested (e.g., Carey et al., 2006; Garner et al., 2009; Gervasi et al., 2013; Forzán et al., 2015). While temperature, humidity, and salinity are environmental conditions that can affect amphibian disease outcome (Woodhams, Alford & Marantelli, 2003; Bustamante, Livo & Carey, 2010; Raffel et al., 2015; Stockwell, Clulow & Mahoney, 2015; Brand et al., 2016), few amphibian disease studies have examined the effects of substrate. For Bsal exposure trials specifically, studies to date have used minimal sample sizes (e.g. 2–8 adults), and a single low-dose exposure (Martel et al., 2014). Increasing propagule pressure with high or repeated doses of Bsal may be an additional factor necessary for Bsal to overcome host defense thresholds and initiate pathogenesis. Alternatively, pathogen infectivity may be enhanced by aquatic factors including secreted TH (Thekkiniath et al., 2013). Host developmental stage, temperature, and housing conditions can also affect disease susceptibility, sometimes through effects on the amphibian skin microbiome (Kueneman et al., 2016).
Following Martel et al., (2014), host responses to Bsal exposure can be categorized as resistant (i.e., no infection or disease), tolerant (i.e., infection in the absence of disease and mortality), susceptible (i.e., infection resulting in clinical disease with possible recovery), and lethal (i.e., infection resulting in lethal disease). Sublethal impacts of pathogen exposure can also be detrimental, and for Bd infection, this can include loss of tadpole keratinized jaw sheaths, behavioral changes, reduction of growth, and immune suppression (Venesky, Parris & Storfer, 2009; Rollins-Smith et al., 2011; Hess et al., 2015).
Here, we describe the effects of Bsal exposure on infection and disease outcome, growth rates, and stress hormone response in a focal salamander species. We used the spotted salamander, Ambystoma maculatum, because of its wide distribution across the eastern U.S. and limited previous investigation, including unknown susceptibility of the larval and juvenile stage to Bsal infection. We validated infectivity of Bsal with a known-susceptible host, eastern red-spotted newts (Notophthalmus viridescens; Martel et al., 2014). In addition to disease and infection, we examined sublethal effects of pathogen exposure on salamander growth. We hypothesized that corticosterone (CORT) release rates of juvenile salamanders would increase with exposure to Bsal. While here we do not investigate treatment effects on skin characteristics, we instead took a broader approach comparing the overall function of skin mucosal defenses among Bsal resistant and susceptible host species.
Woodhams et al., (2014) found that antifungal function of the amphibian skin mucosome (including host and microbiome-derived defenses) predicts the prevalence of infection with Bd in natural populations. Amphibian mucosal defenses have not previously been examined for activity against Bsal, and here we examined mucosome activity of the disease susceptible, terrestrial adult stage of fire salamanders, S. salamandra, with that of spotted salamanders, here shown to resist infection and disease. Bsal thrives at an optimal pH of 6–8 in culture (Woodhams et al., 2018), and thus we also compared the pH of the skin mucosome of these species, as well as aquatic adult eastern newts. We predicted that spotted salamanders have either greater mucosome activity against Bsal than more susceptible hosts, or a skin pH outside the optimal range for Bsal.
These studies aimed to determine the factors important for susceptibility to Bsal infection and chytridiomycosis to better inform proactive conservation strategies. Evaluating both lethal and sublethal impacts of Bsal exposure will aid in strategic preparation to mediate threats in pathogen-naïve populations.
MATERIALS AND METHODS
Animal Collection and Housing
We collected egg clutches of spotted salamanders, A. maculatum, from Blue Hills Reservation, Milton, MA in March, 2016, and transferred them to tanks with sterile artificial pond water (Provosoli medium; Wyngaard and Chinnappa, 1982), and an airstone in a 15°C cold room in the laboratory. The recipe for Provosoli medium (pH 7.0, autoclave or filter-sterilize) is 2 mL of each salt solution added to 1 L deionized water: NaNO3 (6.25 g in 250 mL diH2O), MgSO4·7H2O (5.0 g in 250 mL diH2O), CaCl2·2H2O (3.3 g in 250 mL diH2O), K2HPO4 (0.75 g in 250 mL diH2O), KCl (6.25 g in 250 mL diH2O), and KH2PO4 (0.75 g in 250 mL diH2O). We kept all animals on a 12-hour day/night light schedule. Fire salamanders, S. salamandra, were raised in captivity in Germany and imported under U.S. Fish and Wildlife permit (PERMIT # MA28161C-0). We collected adult eastern red-spotted newts, N. viridescens, from a pond in Colchester, Vermont in May, 2017. Scientific collection permits were obtained from Vermont and Massachusetts Fish and Wildlife, and all experiments were conducted under an approved IACUC protocol at the University of Massachusetts Boston animal care facility. Biosecurity and disinfection procedures for Bsal were strictly enforced. A timeline of rearing spotted salamanders for experiments and the mean mass at the initiation of each experiment is provided in Figure 1. Unless otherwise noted, the terms larval and juvenile salamanders refer to our focal species, the spotted salamander, A. maculatum.
Figure 1.
Timeline of rearing and infection trials with spotted salamanders, Ambystoma maculatum, including experimental treatments and response variables. Starting mass and sample size in each experiment is indicated. All experiments consisted of individuals from three clutches. All individuals used in juvenile infection trial II were previously used in juvenile infection trial I as described in the text.
Larvae Infection Trials
Upon hatching from three egg clutches, larvae were fed brine shrimp and had their tanks cleaned daily. We randomly selected 12 larvae for each treatment and housed four per tank (2L capacity). Treatment groups included a control, exposure to Bsal (5x103 zoospores), and exposure to Bsal conditioned with TH upon harvesting zoospores (5x103 zoospores). We collected Bsal zoospores by flooding plates grown at 15°C with 3–6 ml of water or TH solution at a 50 nM concentration according to Thekkiniath (2013), filtered through a 10 micron cone filter (Chemrus) to remove sporangia, and then counted zoospores using a hemocytometer. We exposed larvae to treatments in 200 ml containers for 24 hours, and then moved them back to their original 2L tanks (polycarbonate mouse cage, 365 x 205 x 140mm). After exposure, larvae were monitored daily for survival. Larvae were swabbed weekly, 5 times each across dorsal and ventral surfaces and tail to analyze infection intensity. Mass was measured at the beginning and end of the 45 day experiment, and for each tank the proportional change in mass was calculated. Corticosterone release rates were measured for control larvae on day 45, as described below.
Juvenile Infection Trial I
Upon metamorphosis under controlled conditions described above, we transferred juvenile salamanders to terrariums (2L capacity mouse cages) for individual housing with 2.5cm soil (Atlanta Botanical Garden Mix, Josh’s Frogs), sphagnum moss, and springtails (Josh’s Frogs), and fed with crickets on a bi-weekly basis. We moistened terrariums with sterile artificial pond water as needed. Terrariums were randomly designated for one of six treatments in a bio-secure facility at 18.3°C. Treatment groups consisted of: 1) controls (n=15), 2) a stressed “agitated” control group (n=10), 3) exposure to a low dose of Bsal (n=10), 4) exposure to a high dose of Bsal (n=10), 5) repeated exposure (three times) to a high dose of Bsal (n=10), and 6) exposure to a high dose of Bsal conditioned with TH (n=10). The low dose of Bsal consisted of 5x103 zoospores (Martel et al., 2014), the high dose consisted of 1.75x106 zoospores, and the high dose repeated included three inoculations at days 0, 7, and 9. Salamanders were exposed to their respective treatments for 1 hour in 30 ml of artificial pond water in VWR 4 oz HDPE containers with lids (CS250, #89009–662). Salamanders were monitored daily for survival and signs of disease. Swabs were taken on a weekly basis in order to determine presence/absence and quantity of zoospores on the skin. Each individual was swabbed with a sterile rayon-tip Dryswab™ (Medical Wire & Equipment MW 113) a total of 30 times: 5 strokes on each side of the abdomen and 5 strokes on each leg and feet (Hyatt et al., 2007). Mass was recorded and proportional change in mass was calculated over the seven week experiment for comparison among treatments. Corticosterone release rates were measured in this experiment as described below on day 0 (for 1 hour post-exposure) and again on day 30 of the 82-day experiment.
Juvenile Infection Trial II
A portion of juvenile salamanders used in infection trial I were moved to a different bio-secure room set at a constant 15°C and were housed individually in 2L mouse cages, divided into four treatment groups, and randomized on wire shelving: 1) previously control, housed on unbleached paper towels with Bsal exposure (n=10), 2) previously exposed, housed on unbleached paper towels with Bsal exposure (n=10), 3) previously control, housed on soil (same as in Trial I) with Bsal exposure (n=7), and 4) previously control, housed on unbleached paper towels without Bsal exposure (n=7). One hypothesis was that if salamanders were more susceptible under hygienic laboratory (no soil or sphagnum moss) conditions, then they may show reduced susceptibility if previously exposed to Bsal because of potential adaptive immune responses (Dhabar, 2002). We also exposed five adult eastern newts housed with paper towels to Bsal in order to determine if our infection trials were successful on a species with known susceptibility (Martel et al., 2014). Five adult eastern newts were treated as unexposed controls. Animals were exposed to treatments (1x106 Bsal zoospores) in 10 ml of sterile pond water in 50 ml conical tubes for a period of 24 hours before being placed back into their individual terrariums. This exposure matched dose and duration of exposure with the Bsal Task Force research working group protocol (https://ag.tennessee.edu/fwf/bsalproject/) to standardize comparisons among species. Salamanders were monitored daily for survival and signs of disease. Swabs were taken weekly for infection diagnostics as described above. Proportional change in mass was calculated over the eight-week experiment.
DNA Extraction and qPCR of Swabs
DNA from weekly swabs for infection trials were extracted using 40µl of PrepMan and were frozen at −20°C for storage. DNA was analyzed through quantitative polymerase chain reaction (qPCR) using the methods from Blooi et al. (2013), with a slight modification to the protocol: we used an MGB probe with a VIC fluorophore; primer and probe sequences remained unchanged. We used gBlock® (Integrated DNA Technologies, Coralville, IA) synthetic DNA sequences for both Bd and Bsal ITS genes as standards to estimate zoospore copy numbers. Samples were run in duplicate. For the larvae experiment, weeks 1–3 were analyzed. For the juvenile infection trial I, weeks 1, 2, and 4 were analyzed. For the juvenile infection trial II, weeks 1, 2, 4, and 8 were analyzed. We used a threshold of duplicate detection of 100 ITS gene copies, our lowest standard, as a positive result. This standard indicates 1 zoospore according to calculations of ITS gene copies per zoospore as described in Rebollar et al. (2017).
Water-borne Hormone Methods and Corticosterone Release Rates
We extracted water-borne hormones from our samples following Gabor et al. (2016). The dried hormone residue was re-suspended for all salamander and control water samples in a 230 µl solution of 95% enzyme-immunoassay (EIA) buffer (Cayman Chemicals Inc., Ann Arbor, MI, USA) and 5% EtOH. CORT samples were measured in duplicate using a CORT EIA plate (Cayman Chemicals Inc.) on a spectrophotometer plate reader at 405 nm (BioTek ELX800). We multiplied the CORT concentrations (pg/ml) by the re-suspension volume to obtain total CORT (pg) within each sample. To control for different batches of artificial spring water we subtracted the amount of CORT measured in spring water from each of the three time periods (larvae, Juvenile Day 0, and Juvenile Day 30). To control for differences in CORT release rates based on size, we divided salamander CORT values by host mass, yielding a CORT release rate in units of pg/g/h. The sensitivity of the CORT EIA plates ranged from 22.5 – 1509.6 pg/ml and all samples were well above the sensitivity of the relative plates. We ran 5 EIA plates and based on our control samples intra-plate variation ranged from 0.26–3.84% and inter-plate variation was 12.65%. The use of the water-borne CORT collection method for A. maculatum was previously validated (Charbonnier et al., 2018).
Water-borne corticosterone release rates were measured for control larvae and a subset of juveniles one hour after the initial exposure to treatments (Juvenile infection trial I, Day 0) as a measure of acute stress, and 4 weeks after exposure (Juvenile infection trial I, Day 30) as a measure of chronic stress (long term effects of early exposure). Larval salamanders (n =12) were placed individually in 250 ml beakers with 80 ml of sterile artificial pond water for 1 hour. Juvenile salamanders (n= 9–14/treatment) were placed individually in plastic cups with 30 ml of sterile artificial pond water for 1 hour between 0900–1300 h to minimize the effects of circadian rhythms. As a stress response-inducing treatment “agitation” (Belden et al., 2007; Gabor et al., 2016), we placed salamanders in cups and agitated the cups by swirling for 1 min every 3 min over the 1-hour leaching period. At the end of the hour we removed the salamander and stored the water sample on ice before storing at −20°C. We then measured the mass of the salamander. We wore gloves throughout the hormone collection process and rinsed our collection equipment with 95% ethanol and DI water before use.
Statistical analyses for survival, change in mass, and corticosterone
To compare survival among treatments in each of the three experiments, survival was analyzed in R (Development Core Team, 2016) using a mixed effect Cox regression to assess survival in co-housed salamander larvae (package coxme; Therneau, 2019) and the function coxph (package survival; Therneau, 2015) for individually housed juvenile salamanders. For the larval experiment, we included tank as a random effect (or covariate) in the Cox’s hazard’s analysis.
Salamander mass through time (growth) was not a linear function, and we thus chose to compare the proportional change in mass among treatments for each experiment using a beta regression analysis with the package betareg in R (Cribari-Neto & Zeileis, 2010). This analysis is ideal for proportional data, and an overall significant effect was followed by Tukey pairwise comparisons. An overall test was conducted to examine growth differences among all three larval treatments and among all four treatments of juvenile salamanders in juvenile infection trial II. In juvenile infection trial I, we tested two hypotheses: 1) proportional change in mass differs between control salamanders and agitated salamanders, and 2) proportional change in mass differs among control and Bsal exposure treatments.
To test whether CORT release rates differed by development stage we compared control larvae and control juveniles at day 0 and 30 time points using a Kruskal Wallis test, as variances were not homogeneous. To test whether CORT release rate was affected by juvenile exposure to the treatments (early on and later) we used a repeated measures GLMM with treatment, time (Juvenile Day 0 vs. Day 30), and the interaction term as fixed effects, and used salamander ID as the random effect (to account for repeated measures). CORT data were ln-transformed for this analysis. Models were run using the nlme package in R (Pinheiro et al., 2014). We also used Pearson correlation to explore the relationship between CORT release rates on Day 0 and the proportional change in mass for salamanders in juvenile infection trail I in the control, agitated, and high dose Bsal treatments.
Mucosome Collection and Luminescence Assay Analysis
Separate from the above experiments, and to more broadly compare Bsal resistant and susceptible host species, we tested for overall function of skin mucus in resistance to Bsal with mucosome assays on Bsal zoospore viability. The assay was based on a method for testing Bd viability (Woodhams et al., 2014) and modified for Bsal. Mucosome samples were collected from captive-raised juvenile spotted salamanders (n=10) and fire salamanders (n=10) of the same age (approximately 1 year old), housed under identical conditions with moist soil and cover object. Dorsal and ventral salamander skin was gently rubbed with a microbial inoculation loop in order to collect mucosal secretions non-invasively (Pasmans et al., 2013; Smith et al., 2018). Inoculation loops were then placed in 1ml of sterile artificial pond water and then frozen at −20°C. To determine Bsal viability in the presence of these mucosome samples, a ProMega Cell-Titer Glo 2.0 kit was utilized following the manufacture’s protocol. Mucosome samples with and without Bsal were incubated for one hour, and the production of adenosine triphosphate (ATP) was measured through luminescence. Because ATP may be present within the mucosome samples, background controls were run containing only mucosome (no Bsal). Luminescence was measured on a POLARstar Omega Microplate Reader (BMG LABTECH). Luminescent values from background controls were subtracted from mucosal samples incubated with Bsal. A Wilcoxon rank sum test was performed in order to determine differences in mucosome function and background luminescence between species.
Skin pH of Salamanders
The dorsal skin pH of 10 juvenile spotted salamanders, adult eastern newts, and juvenile fire salamanders, all pathogen-unexposed controls, was determined using a flat glass probe (Mettler Toledo, pH Electrode InLab Surface). Both species of salamanders were housed terrestrially as described for mucosome assays above, and newts were housed in aquatic conditions. Differences among species were tested by ANOVA.
RESULTS
Larval Survival, Sublethal Effects, and Bsal Infection
Exposure to Bsal was hypothesized to cause infection, reduced growth, and mortality in larval salamanders. However, there were no significant differences in larval survival among treatments (𝜒22 = 1.1771, P = 0.5551). The proportional change in mass of larvae (Table 1) also did not differ among treatments throughout the experiment (𝜒22 = 1.259, P = 0.5329). There were no Bsal-positive samples detected for the larvae during the experiment (weeks 1, 2, or 3), and no lesions or other clinical indications of chytridiomycosis were observed.
Table 1.
Spotted salamander, Ambystoma maculatum, responses to treatments including proportional change in mass, mean survival, and infection with Batrachochytrium salamandrivorans.
| Infection Trial |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Larvae | Juvenile I | Juvenile II | ||||||
| mean (SD) proportion mass change* | mean survival (%) | N infected at endpoint | mean (SD) proportion mass change | mean survival (%) | N infected at endpoint | mean (SD) proportion mass change | mean survival (%) | N infected at endpoint | |
| 1 | 0.25 (0.05) | 66.7 | 0 | 0.46 (0.22) | 93.3 | 0 | 0.42 (0.10) | 100 | 0 |
| 2 | 0.21 (0.06) | 83.3 | 0 | 0.33 (0.21) | 100.0 | 0 | 0.35 (0.11) | 100 | 0 |
| 3 | 0.20 (0.17) | 91.7 | 0 | 0.31 (0.12) | 100.0 | 0 | 0.33 (0.11) | 100 | 0 |
| 4 | 0.26 (0.15) | 100.0 | 0 | 0.28 (0.16) | 100 | 0 | |||
| 5 | 0.36 (0.16) | 100.0 | 0 | ||||||
| 6 | 0.24 (0.16) | 100.0 | 0 | ||||||
Individuals housed separately except for larval experiment where data represent mean mass for groups in each treatment.
Juvenile Survival and Proportional Change in Mass
Exposure to Bsal was hypothesized to cause reduced growth, and mortality in juvenile salamanders after metamorphosis. For juvenile infection trial I, there were no differences in survival among treatments (𝜒26 = 2.933, P = 0.710), and only one mortality from the control group (Table 1). The proportional change of mass differed among control and Bsal treatments (𝜒24 = 13.769, P = 0.008). Overall, Bsal exposure (all dose and treatments combined) reduced growth by 16% on average compared to controls. Growth rate was highest for control salamanders (42% growth over 12 weeks), and significantly lower than controls for high-dose Bsal exposed salamanders (26% growth, Tukey post-hoc test, P = 0.047). Similarly, growth rate was lower than controls for high-dose Bsal conditioned with TH exposed salamanders (24% growth, Tukey post-hoc test, P = 0.003). No other pairwise comparisons differed significantly. Growth of agitated controls was not significantly reduced compared to controls (𝜒21 = 1.470, P = 0.225), but was lower than controls by 13% on average.
All salamanders in control and Bsal exposure treatments and all control newts survived juvenile infection trial II, while all Bsal exposed newts died. There was a significant difference among treatments for proportional change in salamander mass (𝜒23 = 8.181, P = 0.042). Tukey post-hoc tests indicated one pairwise difference between control (28% growth) and Bsal exposed salamanders (42% growth; P = 0.016).
Juvenile Bsal Infection
Exposure to Bsal was hypothesized to cause skin infection in juvenile salamanders after metamorphosis. For juvenile infection trial I, Bsal was detected in one juvenile (of 40 exposed to Bsal) in the High Bsal treatment at weeks 2 and 4 with 12.6 and 11.8 zoospore equivalents, respectively. All other samples were negative. Bsal was not detected in any spotted salamander samples for juvenile infection trial II (n = 27 exposed to Bsal). However, positives (measured in ITS gene copies, or copies) were detected beginning at 7 days post exposure for eastern newts (Figure 2). All five unexposed control newts remained uninfected.
Figure 2.

Log infection intensity with Batrachochytrium salamandrivorans (Bsal) for five eastern red-spotted newts, Notophthalmus viridescens, over the eight-week experiment. Five unexposed control newts remained uninfected. Note that there were approximately 196 ITS gene copies per Bsal zoospore, calculated at the time the isolate was used. Spotted salamanders, Ambystoma maculatum, in the same experiment did not become infected (Table 1).
Corticosterone Release Rates
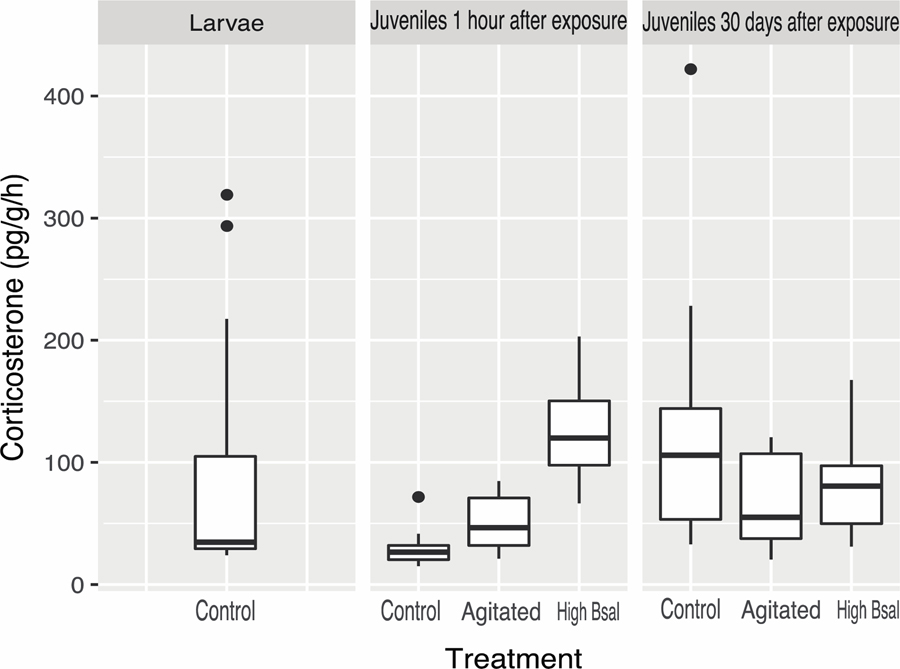
The stress hormone CORT was hypothesized to fluctuate through development, and to increase in response to agitation upon immediate exposure to infectious Bsal zoospores, and to remain persistently high in Bsal infected or exposed salamanders weeks after exposure. CORT release rates differed among control larvae and juveniles on days 0 and 30, (Kruskal-Wallis test, 𝜒22 =16.938, P < 0.001), and was lowest in juveniles shortly after metamorphosis (Figure 3). There was an interaction between treatment and time on CORT release rates of juvenile salamanders exposed to four treatments (Treatment: 𝜒2= 6.24, P = 0.044, Time: 𝜒2= 0.80, P = 0.37, Interaction: 𝜒2= 31.94, P < 0.001; Figure 3). There was a significant difference among treatments at the first time point (Treatment: F2,29 = 34.10, P < 0.001), but not at the second time point (Treatment: F2,32 = 2.34, P = 0.113). Specifically, one hour after the initial exposure to treatments, the high-dose Bsal exposed juveniles released 1.25 times more CORT compared to the agitation stress treatment, and 1.45 times more than the control treatment. Post-hoc tests revealed that all treatments differed (Control-Agitated: P = 0.017; Control-High Bsal: P = <0.001; Agitated-High Bsal: P = 0.001). However, 30 days after exposure, when no salamanders were infected, there were no significant differences among the three treatments for which we had repeated measures. Treatments which were examined only at day 30 are presented in Supplemental Materials (Figure S1), and were not significantly different. There was no significant correlation between day 0 CORT release rates in juvenile salamanders and their proportional change in mass during the experiment (n = 32, R = −0.231, P = 0.203). However, Bsal exposed salamanders had higher CORT on day 0 and lower proportional change in mass compared to control salamanders (Figure 3).
Figure 3.
Corticosterone release rates of larval and juvenile spotted salamanders, Ambystoma maculatum. Juvenile corticosterone was sampled at 1 hour or 30 days after exposure to treatments. One high value is out of range (not removed from the analysis) from the juvenile day 30 control treatment for better viewing the rest of the data. Bsal indicates Batrachochytrium salamandrivorans. Box plots indicate median, range and first and third quartiles. Dots indicate outliers. Additional treatments were examined at day 30 only, and data are presented in Supplemental Material (Fig. S1).
Mucosome Luminescence Assays
The function of skin mucus in protecting host amphibians by killing infectious zoospores was hypothesized to be greater in disease resistant species compared to disease susceptible species. Spotted salamander mucosome samples significantly reduced Bsal viability (Wilcoxon rank sum test, W = 5, P < 0.01) and had significantly higher background luminescence (W = 54, P < 0.01) compared to those of fire salamanders (Figure 4).
Figure 4.

Viability of Batrachochytrium salamandrivorans (Bsal) zoospores in the presence of mucosal components of fire salamanders (FS; Salamandra salamandra) and spotted salamanders (SS; Ambystoma maculatum). Note the reduced Bsal viability for spotted salamanders as determined by a Cell-Titer Glo 2.0 Luminescent Assay. Inset boxplot displays the observed differences in background luminescence within the mucosome sample between the two salamander species. Box plots indicate median, range and first and third quartiles. Dots indicate outliers.
Skin pH of Salamanders
Skin pH was hypothesized to differ among species and to match Bsal growth optima most closely (Woodhams et al., 2018) in disease susceptible host species. However, skin pH of three species tested did not differ significantly (ANOVA, F2,25 = 0.438, P = 0.65): spotted salamanders had an average (± SD) dorsal skin pH of 5.72 ± 0.38 while fire salamanders had pH 5.73 ± 0.30, and eastern newts had pH 5.57 ± 0.54.
DISCUSSION
During this time of extreme biodiversity loss (McCallum, 2007; Wake and Vredenburg, 2008; Ceballos et al., 2015), it is vital that we take the necessary precautions to preserve populations declining due to anthropogenic factors. Bsal is one threat to amphibians likely to spread by human actions (Yuan et al., 2018), similar to the global spread of Bd (O’Hanlon et al., 2018). This study was performed to determine the susceptibility of a common species of North American salamander to Bsal, and to explore whether host life-stage, environmental conditions, or pathogen dose may influence the previous “disease resistant” designation for this species based on five adults tested by Martel et al. (2014). We monitored the effects of Bsal exposure on corticosterone release rates to understand whether exposure to Bsal is a stressor, and measured growth rates to test for sublethal effects of pathogen exposure.
We determined that larval and juvenile spotted salamanders from one population are resistant to Bsal infection under standard laboratory conditions, and with more natural soil substrate. None of the spotted salamanders exhibited infection at the end of the experiment (Table 1) and only one salamander had a low-intensity infection on weeks 2 and 4 of the experiment after exposure to the high dose of Bsal. No clinical signs of chytridiomycosis including lesions, abnormal sloughing, discoloration, or behavioral changes were observed at any time in any of the experiments. This finding corroborates and expands previous work indicating that adult spotted salamanders were resistant to chytridiomycosis (Martel et al., 2014). Importantly, we found that eastern newts, N. viridescens, were susceptible to Bsal, confirming that a viable Bsal exposure protocol was used in this study.
We detected potential sublethal reductions of growth caused by Bsal exposure in juvenile spotted salamanders soon after metamorphosis, but not in older juveniles, which is a common finding of frogs and salamanders with chytridiomycosis (Retallick and Miera, 2004; Becker and Harris, 2010; Reeder, Pessier & Vredenburg, 2012; Woodhams et al., 2012). The mechanisms of sublethal effects including growth reduction or mass loss caused by Bd infection appear different for tadpoles which lose mouthparts affecting grazing behavior (Hanlon et al., 2015), compared to post-metamorphic frogs and salamanders which respond to infection with a stress response correlated with dysregulation of ion homeostasis, reduced appetite and body condition, increased skin shedding rates, and changes in white blood cell profiles (Peterson et al., 2013). Because of the acute CORT response shown here, resources may have been diverted toward skin immunity (Dhabhar 2002) at the expense of growth early in development.
We predicted that conditioning Bsal cultures with TH upon zoospore harvest would increase the virulence of Bsal (Thekkiniath et al., 2013), but no differences were detected among treatments in terms of susceptibility including survival, Bsal infection load, or mass change. Alternative housing conditions lacking microbes from environmental (soil and sphagnum moss) reservoirs also did not increase susceptibility. Salamanders housed with only sterile paper towels were not susceptible, and without differences in infection rates among treatments it was not possible to determine whether adaptive immune responses were at work in previously exposed salamanders. With the caveat that only one population was tested, these data suggest that spotted salamanders are not susceptible to Bsal, and Bsal may not present an immediate threat to spotted salamanders. This supports findings by Martel et al. (2014) on adult spotted salamander susceptibility. Furthermore, spotted salamanders will likely not function as a reservoir species which has important implications for community level disease dynamics as reservoir species serve as important vectors for disease spread and propagation (Woodhams et al., 2008; Reeder et al., 2012; Scheele et al., 2017; Canessa et al., 2018). Our study also suggests that standard methods for susceptibility testing (e.g., https://ag.tennessee.edu/fwf/bsalproject/) provide disease risk results that are consistent with our findings across conditions for A. maculatum. However, variation in conditions may impact studies of more susceptible species.
There are several possible explanations as to why spotted salamanders are not susceptible to Bsal chytridiomycosis. Firstly, amphibians have immune system responses including antibody production (Rollins-Smith, 2001; Ramsey et al., 2010), antimicrobial peptides (AMPs; Rollins-Smith et al., 2002; Woodhams et al., 2007; Sheafor et al., 2008), and alkaloids (Daly, Myers & Whittacker, 1987). These salamanders may be eliciting an adaptive immune response to exposure to the pathogen through antibody production (Horton et al., 1992; Rollins-Smith, 2001). African clawed frog, Xenopus laevis, skin mucus was found to have elevated levels of IgM, IgY, and IgX antibodies after exposure to Bd (Ramsey et al., 2010). Amphibians have a hypothalamic-pituitary-interrenal (HPI) axis that is similar to the stress hypothalamic-pituitary-adrenal (HPA) axis of mammals which mediates immune function (Kapcala, Chautard & Eskay, 1995). As an immune response mounts, inflammatory cytokines stimulate the HPI axis to release corticotropin-releasing factor and glucocorticoids (Warne, Crespi & Brunner, 2011). B-cells and antibody production are then stimulated (Dhabhar, 2009). Indeed, we found that exposure to Bsal significantly increased corticosterone release rates in juvenile salamanders; the rate was 1.45 times that of controls (Figure 3). This finding indicates that salamanders were responding to the pathogen in a physiological manner. Similarly, elevated CORT release rates were found when prometamorphic tadpoles were exposed to a lethal dose of ranavirus (Warne et al., 2011) and Bd (Gabor et al., 2018). Similarly, larval Western tiger salamanders (Ambystoma mavortium) had elevated CORT release rates in ranavirus infected individuals (Davis et al. 2019). Therefore, it is plausible that the initial exposure to Bsal created a stress response that helped activate the immune system; this activation dissipated after the threat dissipated, and no chronic stress was detected 4 weeks post-exposure to Bsal. Elevated CORT, however, may come at a cost because juvenile salamanders with higher CORT release rates immediately after exposure to Bsal had lower growth compared to control group salamanders 30 days post-exposure. CORT levels may naturally fluctuate in amphibians and even increase enough to inhibit the immune system. This inhibitory elevation in CORT generally occurs during the peak of metamorphosis (Kikuyama et al., 1993), during breeding season (Mendonça et al., 1985; Orchinik, Licht & Crews, 1988; Harvey et al., 1997), and under high densities (Charbonnier et al. 2018). While our data did not show long-term CORT elevation, it is important to consider that in the wild, stress from pathogen exposure can be compounded by other natural stressors (metamorphosis, breeding, competition, high densities, parasites, climate change, etc.), and immunoredistribution of resources at the expense of growth may present a significant sublethal impact. Our results contribute to the growing theory on critical disease windows and trade-offs between development and immune function (Kirschman, Crespi & Warne, 2018; Prest et al., 2018). If the salamanders were exposed continually to the pathogen, they may suffer chronic stress, which then could impair the immune system. Further investigations of the production of mucosal antibodies, shifts in microbiota, or other physiological defense responses are needed for salamanders.
Spotted salamander mucosome containing both host and microbiome components decreased viability of Bsal in luminescence assays and was significantly more effective than mucosome from disease-susceptible fire salamanders, S. salamandra that occur in similar terrestrial habitats, though in different geographical regions. Further studies are needed to describe the skin microbiome of salamanders, including testing the function of symbiotic algae (e.g., Oophila sp.; Kerney et al., 2019) in disease resistance. Skin pH did not differ among these species and therefore does not appear to contribute to the susceptibility differences among these species.
Skin defense compounds may function as elements of pathogen defense. AMPs are produced and secreted from the skin of some amphibians in response to stress or injury and can inhibit Bd and ranavirus (Rollins-Smith, 2009). Furthermore, some alkaloids have been shown to possess antimicrobial abilities (Preusser et al., 1975; Macfoy et al., 2005; Cushnie, Cushnie & Lamb, 2014). Salamandra species were found to produce steroidal alkaloids (Vences et al., 2014; Lüddecke et al., 2018) although it is unknown whether similar alkaloids, or AMPs, are produced by spotted salamanders.
Amphibians additionally harbor protective bacterial symbionts on their skin (Harris et al., 2009; Bletz et al., 2013). It is possible that the spotted salamanders we tested had a substantial amount of antifungal symbionts capable of reducing Bsal growth (Harris et al., 2006; Harris et al., 2009; Woodhams et al., 2015). For example, high background activity in spotted salamander mucosome compared to fire salamander mucosome (Figure 4 inset) may indicate that spotted salamanders have abundant microbiota. Pathogen genotype and temperature may alter the ability of microbes to inhibit Bd and Bsal (Muletz-Wolz et al., 2017). The skin microbiome is a complex system that works with the immune system in pathogen defense. A follow-up analysis of the bacterial and fungal microbial skin composition of spotted salamanders will provide insight into antifungal microbes present that are potentially fighting off Bsal (Bletz et al., 2017).
Overall, we determined that spotted salamanders do not appear susceptible to chytridiomycosis caused by Bsal, but may suffer sublethal growth reduction upon exposure to this pathogen early after metamorphosis. Bsal remains a potentially massive threat to other salamanders worldwide, including eastern newts shown here and elsewhere to be highly susceptible to disease. These data will help inform conservation management and contribute to epidemiological models by providing a nuanced understanding of sublethal impacts of exposure to an emerging pathogen, even without host infection.
Supplementary Material
Acknowledgments:
The authors thank the Bsal Task Force, and Alberto Campos, Fanghemei Zhang (Jessica), Kathleen Conroy, Diego Aparicio, Jo-Hanna Azzara, Terence Cook, Sendy Lamour, Vivian Le, Andrea Lu, Modi Maitri, Jessie Ngandjui, Bryan Peguero, Natan Pirete, Kiloni Quiles-Franco, Rayshawn Reece, Marina Teixeira, and Steven Tran for their technical assistance, and Michel Ohmer for statistical consulting. Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under Award Number R25GM076321. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided in part by the BAND foundation and Association of Fish and Wildlife Agencies, The Wildlife Without Borders-Amphibians in Decline program (F15AP00968 to DCW), the Nancy Goranson Endowment Fund, the UMass Boston Biology Department, the National Science Foundation grant DGE 1249946, Integrative Graduate Education and Research Traineeship (IGERT): Coasts and Communities—Natural and Human Systems in Urbanizing Environments, and the University of Massachusetts Sanofi-Genzyme Doctoral Fellowship.
LITERATURE CITED
- Becker MH & Harris RN (2010). Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS One 5, e10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden LK, Rubbo MJ, Wingfield JC & Kiesecker JM (2007). Searching for the physiological mechanism of density dependence: Does corticosterone regulate tadpole responses to density? Physiol. Biochem. Zool 80, 444–451. [DOI] [PubMed] [Google Scholar]
- Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KP & Harris RN (2013). Mitigating amphibian chytridiomycosis with bioaugmentation: Characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett 16, 807–820. [DOI] [PubMed] [Google Scholar]
- Bletz MC, Myers J, Woodhams DC, Rabemananjara FCE, Rakotonirina A, Weldon C, Edmonds D, Vences M & Harris RN (2017). Estimating herd immunity to amphibian chytridiomycosis in madagascar based on the defensive function of amphibian skin bacteria. Front. Microbiol 8, 1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blooi M, Pasmans F, Longcore JE, Spitzen-van der Sluijs A, Vercammen F & Martel A (2013). Duplex real-time pcr for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol 51, 4173–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Hill RD, Brenes R, Chaney JC, Wilkes RP, Grayfer L, Miller DL & Gray MJ (2016). Water temperature affects susceptibility to ranavirus. Ecohealth 13, 350–359. [DOI] [PubMed] [Google Scholar]
- Bustamante HM, Livo LJ & Carey C (2010). Effects of temperature and hydric environment on survival of the Panamanian Golden Frog infected with a pathogenic chytrid fungus. Integrat. Zool 5,143–153. [DOI] [PubMed] [Google Scholar]
- Canessa S, Bozzuto C, Campbell Grant EH, Cruickshank SS, Fisher MC, Koella JC, Lötters S, Martel A, Pasmans F & Scheele BC (2018). Decision-making for mitigating wildlife diseases: From theory to practice for an emerging fungal pathogen of amphibians. J. Appl. Ecol 55, 1987–1996. [Google Scholar]
- Carey C, Bruzgul JE, Livo LJ, Walling ML, Kuehl KA, Dixon BF, Pessier AP, Alford RA & Rodgers KB (2006). Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3:5–21. [Google Scholar]
- Ceballos G, Ehrlich PR, Barnosky AD, Garcia A, Pringle RM & Palmer TM (2015). Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv 1, e1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier J, Pearlmutter J, Vonesh J, Gabor C, Forsburg Z & Grayson K (2018). Cross-life stage effects of aquatic larval density and terrestrial moisture on growth and corticosterone in the spotted salamander. Diversity 10, 68. [Google Scholar]
- Cushnie TT, Cushnie B & Lamb AJ (2014). Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Interntl. J. Antimicrob. Agents 44, 377–386. [DOI] [PubMed] [Google Scholar]
- Daly JW, Myers CW & Whittaker N (1987). Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxicon 25, 1023–1095. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA & Hyatt AD (2000). Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 287, 443. [DOI] [PubMed] [Google Scholar]
- Davis DR, Ferguson KJ, Schwarz MS & Kerby JL (2019). Effects of agricultural pollutants on stress hormones and viral infection in larval salamanders. Wetlands, 10.1007/s13157-019-01207-1 [DOI] [Google Scholar]
- Denver RJ (2009). Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann. N. Y. Acad. Sci 1163, 1–16. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2002). A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integ. Comp. Biol 42, 556–564. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2009). Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 16, 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzán MJ, Jones KM, Vanderstichel RV, Wood J, Kibenge FS, Kuiken T Wirth W Ariel E & Daoust PY (2015). Clinical signs, pathology and dose-dependent survival of adult wood frogs, Rana sylvatica, inoculated orally with frog virus 3 (Ranavirus sp., Iridoviridae). J. Gen. Virol 96, 1138–1149. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Fisher MC & Bosch J (2013). A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLoS One 8, e56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Fisher MC & Bosch J (2015). Elevated corticosterone levels and changes in amphibian behavior are associated with Batrachochytrium dendrobatidis (Bd) infection and Bd lineage. PLoS One 10, e0122685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Knutie SA, Roznik EA & Rohr JR (2018). Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? Oecologia 186, 393–404. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Zabierek KC, Kim DS, da Barbiano LA, Mondelli MJ, Bendik NF & Davis DR (2016). A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia 104, 172. [Google Scholar]
- Garner TWJ, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA & Fisher MC (2009). Life history tradeoffs influence mortality associated with amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118, 783–791. [Google Scholar]
- Gervasi S, Gondhalekar C, Olson DH & Blaustein AR (2013). Host identity matters in the amphibian-Batrachochytrium dendrobatidis system: Fine-scale patterns of variation in responses to a multi-host pathogen. PLoS ONE 8, e54490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EHC, Muths EL, Katz RA, Canessa S, Adams MJ, Ballard JR, Berger L, Briggs CJ, Coleman J & Gray MJ (2016). Salamander chytrid fungus (Batrachochytrium salamandrivorans) in the united states—developing research, monitoring, and management strategies.): US Geological Survey Open-File Report 2015–1233, 16 p., 10.3133/ofr20151233. [DOI] [Google Scholar]
- Gray MJ, Lewis JP, Nanjappa P, Klocke B, Pasmans F, Martel A, Stephen C, Parra Olea G, Smith SA, Sacerdote-Velat A, Christman MR, Williams JM & Olson DH (2015). Batrachochytrium salamandrivorans: The north american response and a call for action. Plos Pathog 11, e1005251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SM, Lynch KJ, Kerby J & Parris MJ (2015). Batrachochytrium dendrobatidis exposure effects on foraging efficiencies and body size in anuran tadpoles. Dis. Aquat. Org 112, 237–242. [DOI] [PubMed] [Google Scholar]
- Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT & Minbiole KP (2009). Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3, 818–824. [DOI] [PubMed] [Google Scholar]
- Harris RN, James TY, Lauer A, Simon MA & Patel A (2006). Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3, 53. [Google Scholar]
- Harvey LA, Propper CR, Woodley SK & Moore MC (1997). Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. Gen. Comp. Endocrinol 105, 102–113. [DOI] [PubMed] [Google Scholar]
- Hess A, McAllister C, DeMarchi J, Zidek M, Murone J & Venesky MD (2015). Salamanders increase their feeding activity when infected with the pathogenic chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org 116, 205–212. [DOI] [PubMed] [Google Scholar]
- Horton JD, Horton TL, Ritchie P & Varley CA (1992). Skin xenograft rejection in Xenopus--immunohistology and effect of thymectomy. Transplantation 53, 473–476. [DOI] [PubMed] [Google Scholar]
- Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F & Colling A (2007). Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dise. Aquat. Org 73, 175–192. [DOI] [PubMed] [Google Scholar]
- James TY, Toledo LF, Rodder D, da Silva Leite D, Belasen AM, Betancourt-Roman CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV, Ruggeri J, Collins JP, Burrowes PA, Lips KR, Zamudio KR & Longcore JE (2015). Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: Lessons from the first 15 years of amphibian chytridiomycosis research. Ecol. Evol 5, 4079–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungreis AM, Huibregtse WH & Ungar F (1970). Corticosteroid identification and corticosterone concentration in serum of Rana pipiens during dehydration in winter and summer. Comp Biochem Physiol 34, 683–689. [DOI] [PubMed] [Google Scholar]
- Kapcala LP, Chautard T & Eskay RL (1995). The protective role of the hypothalamic-pituitary-adrenal axis against lethality produced by immune, infectious, and inflammatory stress. Ann. N. Y. Acad. Sci 771, 419–437. [DOI] [PubMed] [Google Scholar]
- Kearns P, Fischer S, Fernández-Beaskoetxea S, Gabor CR, Bosch J, Bowen JL, Tlusty MF & Woodhams DC (2017). Fighting fungi with fungi: The mycobiome contribution to emerging disease in amphibians. PeerJ Preprints 5, e3017v1. [Google Scholar]
- Kerney R, Leavitt J, Hill E, Zhang H, Kim E & Burns J (2019). Co-cultures of Oophila amblystomatis between Ambystoma maculatum and Ambystoma gracile hosts show host-symbiont fidelity. Symbiosis 10.1007/s13199-018-00591-2 [DOI] [Google Scholar]
- Kikuyama S, Kawamura K, Tanaka S & Yamamoto K (1993). Aspects of amphibian metamorphosis: Hormonal control In International review of cytology: 105–148: Elsevier. [DOI] [PubMed] [Google Scholar]
- Kirschman LJ, Crespi EJ & Warne RW (2018). Critical disease windows shaped by stress exposure alter allocation trade-offs between development and immunity. J. Anim. Ecol 87, 235–246. [DOI] [PubMed] [Google Scholar]
- Kueneman JG, Woodhams DC, Harris RN, Archer HM, Knight R & McKenzie VJ (2016). Probiotic treatment restores protection against lethal fungal infection lost during amphibian captivity. Proc. Roy. Soc. B 283, 20161553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüddecke T, Schulz S, Steinfartz S & Vences M (2018). A salamander’s toxic arsenal: review of skin poison diversity and function in true salamanders, genus Salamandra. Naturwissenschaften 105, 56. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Bonier F, Romero LM & Moore IT (2019). Glucocorticoids and “stress” are not synonymous. Integ. Organ. Biol 1, obz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfoy C, Danosus D, Sandit R, Jones TH, Garraffo HM, Spande TF & Daly JW (2005). Alkaloids of anuran skin: Antimicrobial function? Z. Naturforsch. C. J. Biosci 60, 932–937. [DOI] [PubMed] [Google Scholar]
- Martel A, Blooi M, Adriaensen C, Van Rooij P, Beukema W, Fisher MC, Farrer RA, Schmidt BR, Tobler U, Goka K, Lips KR, Muletz C, Zamudio KR, Bosch J, Lotters S, Wombwell E, Garner TW, Cunningham AA, Spitzen-van der Sluijs A, Salvidio S, Ducatelle R, Nishikawa K, Nguyen TT, Kolby JE, Van Bocxlaer I, Bossuyt F & Pasmans F (2014). Recent introduction of a chytrid fungus endangers western palearctic salamanders. Science 346, 630–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F & Pasmans F (2013). Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences U S A 110, 15325–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum ML (2007). Amphibian decline or extinction? Current declines dwarf background extinction rate. Journal of Herpetology 41, 483–491. [Google Scholar]
- McEwen BS & Wingfield JC (2003). The concept of allostasis in biology and biomedicine. Horm. Beha 43, 2–15. [DOI] [PubMed] [Google Scholar]
- Mendonça M, Licht P, Ryan M & Barnes R (1985). Changes in hormone levels in relation to breeding behavior in male bullfrogs (Rana catesbeiana) at the individual and population levels. Gen. Comp. Endocrinol 58, 270–279. [DOI] [PubMed] [Google Scholar]
- Muletz-Wolz CR, Almario JG, Barnett SE, DiRenzo GV, Martel A, Pasmans F, Zamudio KR, Toledo LF & Lips KR (2017). Inhibition of fungal pathogens across genotypes and temperatures by amphibian skin bacteria. Front. Microbiol 8, 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon SJ, Rieux A, Farrer RA, Rosa GM, Waldman B, Bataille A, Kosch TA, Murray KA, Brankovics B, Fumagalli M, Martin MD, Wales N, Alvarado-Rybak M, Bates KA, Berger L, Boll S, Brookes L, Clare F, Courtois EA, Cunningham AA, Doherty-Bone TM, Ghosh P, Gower DJ, Hintz WE, Hoglund J, Jenkinson TS, Lin CF, Laurila A, Loyau A, Martel A, Meurling S, Miaud C, Minting P, Pasmans F, Schmeller DS, Schmidt BR, Shelton JMG, Skerratt LF, Smith F, Soto-Azat C, Spagnoletti M, Tessa G, Toledo LF, Valenzuela-Sanchez A, Verster R, Voros J, Webb RJ, Wierzbicki C, Wombwell E, Zamudio KR, Aanensen DM, James TY, Gilbert MTP, Weldon C, Bosch J, Balloux F, Garner TWJ & Fisher MC (2018). Recent asian origin of chytrid fungi causing global amphibian declines. Science 360, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M, Licht P & Crews D (1988). Plasma steroid concentrations change in response to sexual behavior in Bufo marinus. Horm. Behav 22, 338–350. [DOI] [PubMed] [Google Scholar]
- Pasmans F, Van Rooij P, Blooi M, Tessa G, Bogaerts S, Sotgiu G, Garner TW, Fisher MC, Schmidt BR, Woeltjes T, Beukema W, Bovero S, Adriaensen C, Oneto F, Ottonello D, Martel A & Salvidio S (2013). Resistance to chytridiomycosis in European plethodontid salamanders of the genus Speleomantes. PLoS One 8, e63639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Steffen JE, Reinert LK, Cobine PA, Appel A, Rollins-Smith L & Medonca MT (2013). Host stress response is important for the pathogenesis of the deadly amphibian disease, chytridiomycosis, in Litoria caerulea. PLoS One 8, e62146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S & Sarkar D (2014). R core team (2014) nlme: Linear and nonlinear mixed effects models. R package version 3.1–118 R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Prest TL, Kimball AK, Kueneman JG & McKenzie VJ (2018). Host-associated bacterial community succession during amphibian development. Molec. Ecol 27, 1992–2006. [DOI] [PubMed] [Google Scholar]
- Preusser HJ, Habermehl G, Sablofski M & Schmall-Haury D (1975). Antimicrobial activity of alkaloids from amphibian venoms and effects on the ultrastructure of yeast cells. Toxicon : official journal of the International Society on Toxinology 13, 285–289. [DOI] [PubMed] [Google Scholar]
- Raffel TR, Halstead NT, McMahon TA, Davis AK, & Rohr JR (2015). Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proc. Roy. Soc. B Biol. Sci 282, 20142039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JP, Reinert LK, Harper LK, Woodhams DC & Rollins-Smith LA (2010). Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun 78, 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollar EA, Woodhams DC, LaBumbard B, Kielgast J & Harris RN (2017). Prevalence and pathogen load estimates for the fungus Batrachochytrium dendrobatidis are impacted by its DNA copy number variation. Dis. Aquat. Org 123, 213–226. [DOI] [PubMed] [Google Scholar]
- Reeder NM, Pessier AP & Vredenburg VT (2012). A reservoir species for the emerging amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. PLoS One 7, e33567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallick RWR & Miera V (2004). Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis. Aquat. Organ 75, 201–207. [DOI] [PubMed] [Google Scholar]
- Richgels KL, Russell RE, Adams MJ, White CL & Grant EH (2016). Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA. R. Soc. Open Sci 3, 150616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA (1998). Metamorphosis and the amphibian immune system. Immunol Rev 166, 221–230. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA (2001). Neuroendocrine-immune system interactions in amphibians: Implications for understanding global amphibian declines. Immunol. Res 23, 273–280. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA (2009). The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim. Biophys. Acta 1788, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Doersam JK, Longcore JE, Taylor SK, Shamblin JC, Carey C & Zasloff MA (2002). Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev. Comp. Immunol 26, 63–72. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Fites JS, Reinert LK, Shiakolas AR, Umile TP & Minbiole KP (2015). Immunomodulatory metabolites released by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun 83, 4565–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Ramsey JP, Pask JD, Reinert LK & Woodhams DC (2011). Amphibian immune defenses against chytridiomycosis: Impacts of changing environments. Integ. Comp. Biol 51, 552–562. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Justus J, Fuller T, Kelley C, Garson J & Mayfield M (2005). Effectiveness of environmental surrogates for the selection of conservation area networks. Cons. Biol 19, 815–825. [Google Scholar]
- Scheele BC, Hunter DA, Brannelly LA, Skerratt LF & Driscoll DA (2017). Reservoir-host amplification of disease impact in an endangered amphibian. Cons.n Biol 31, 592–600. [DOI] [PubMed] [Google Scholar]
- Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J 3rd, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel MO, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR & Canessa S (2019). Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463. [DOI] [PubMed] [Google Scholar]
- Sheafor B, Davidson EW, Parr L & Rollins-Smith L (2008). Antimicrobial peptide defenses in the salamander, Ambystoma tigrinum, against emerging amphibian pathogens. J. Wildl. Dis 44, 226–236. [DOI] [PubMed] [Google Scholar]
- Smith HK, Pasmans F, Dhaenens M, Deforce D, Bonte D, Verheyen K, et al. 2018. Skin mucosome activity as an indicator of Batrachochytrium salamandrivorans susceptibility in salamanders. PLoS ONE 13, e0199295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzen-van der Sluijs A, Martel A, Asselberghs J, Bales EK, Beukema W, Bletz MC, Dalbeck L, Goverse E, Kerres A, Kinet T, Kirst K, Laudelout A, Marin da Fonte LF, Nollert A, Ohlhoff D, Sabino-Pinto J, Schmidt BR, Speybroeck J, Spikmans F, Steinfartz S, Veith M, Vences M, Wagner N, Pasmans F & Lotters S (2016). Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis 22, 1286–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen G, Pasmans F, Schmidt BR, Rouffaer LO, Van Praet S, Schaub M, Canessa S, Laudelout A, Kinet T & Adriaensen C (2017). Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544, 353–356. [DOI] [PubMed] [Google Scholar]
- Stockwell MP, Clulow J, & Mahoney MJ (2015). Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 177, 901–910. [DOI] [PubMed] [Google Scholar]
- Thekkiniath JC, Zabet-Moghaddam M, San Francisco SK & San Francisco MJ (2013). A novel subtilisin-like serine protease of Batrachochytrium dendrobatidis is induced by thyroid hormone and degrades antimicrobial peptides. Fung. Biol 117, 451–461. [DOI] [PubMed] [Google Scholar]
- Therneau T (2015). A Package for Survival Analysis in S. version 2.38, https://CRAN.R-project.org/package=survival.
- Therneau TM (2019). coxme: Mixed effects cox models. R package version 2.2–14 https://CRAN.R-project.org/package=coxme
- Van Rooij P, Martel A, Haesebrouck F & Pasmans F (2015). Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Vet. Res 46, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences M, Sanchez E, Hauswaldt JS, Eikelmann D, Rodriguez A, Carranza S, Donaire D, Gehara M, Helfer V, Lotters S, Werner P, Schulz S & Steinfartz S (2014). Nuclear and mitochondrial multilocus phylogeny and survey of alkaloid content in true salamanders of the genus Salamandra (salamandridae). Mol. Phylogenet. Evol 73, 208–216. [DOI] [PubMed] [Google Scholar]
- Venesky MD, Parris MJ & Storfer A (2009). Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. Ecohealth 6, 565–575. [DOI] [PubMed] [Google Scholar]
- Wake DB & Vredenburg VT (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci 105, 11466–11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne RW, Crespi EJ & Brunner JL (2011). Escape from the pond: Stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol 25, 139–146. [Google Scholar]
- Woodhams DC, Alford RA, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz MC, Daskin JH, Davis LR, Flechas SV, Lauer A, Gonzalez A, Harris RN, Holden WM, Hughey MC, Ibáñez R, Knight R, Kueneman J, Rabemananjara F, Reinert LK, Rollins-Smith LA, Roman-Rodriguez F, Shaw SD, Walke JB & McKenzie V (2015). Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 96, 595. [Google Scholar]
- Woodhams DC, Alford RA & Marantelli G (2003). Emerging disease of amphibians cured by elevated body temperature. Dis. Aquat. Org 55, 65–67. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Barnhart KL, Bletz MC, Campos AJ, Ganem SJ, Hertz A, LaBumbard BC, Nanjappa P & Tokash-Peters AG (2018). Batrachochytrium: Biology and management of amphibian chytridiomycosis. eLS, 1–18.
- Woodhams DC, Brandt H, Baumgartner S, Kielgast J, Kupfer E, Tobler U, Davis LR, Schmidt BR, Bel C, Hodel S, Knight R & McKenzie V (2014). Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS One 9, e96375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams DC, Geiger CC, Reinert LK, Rollins-Smith LA, Lam B, Harris RN, Briggs CJ, Vredenburg VT & Voyles J (2012). Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Dis. Aquat. Org 98, 11–25. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Hyatt AD, Boyle DG & Rollins-Smith LA (2008). The northern leopard frog Rana pipiens is a widespread reservoir species harboring Batrachochytrium dendrobatidis in north america. Herp. Rev 39, 66–68. [Google Scholar]
- Woodhams DC, Rollins-Smith L, Alford R, Simon M & Harris R (2007). Innate immune defenses of amphibian skin: Antimicrobial peptides and more. Anim. Cons 10, 425–428. [Google Scholar]
- Wyngaard G & Chinnappa C (1982). General biology and cytology of cyclopoids. Developmental biology of freshwater invertebrates, 485–533.
- Yap TA, Koo MS, Ambrose RF, Wake DB & Vredenburg VT (2015). Biodiversity. Averting a north american biodiversity crisis. Science 349, 481–482. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Martel A, Wu J, Praet S, Canessa S & Pasmans F (2018). Widespread occurrence of an emerging fungal pathogen in heavily traded chinese urodelan species. Cons. Lett 11, e12436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.