Abstract
Women who carry the BRCA mutation are at high lifetime risk of breast cancer, but there is no consensus regarding an effective and safe chemoprevention strategy. A large body of evidence suggests that 3,3-diindolylmethane (DIM), a dimer of indole-3-carbinol found in cruciferous vegetables, can potentially prevent carcinogenesis and tumor development. The primary aim of this prospective single-arm study was to investigate the effect of DIM supplementation on breast density, a recognized predictive factor of breast cancer risk. Participants were 23 healthy female BRCA carriers (median age 47 years; 78% postmenopausal) who were treated with oral DIM 100 mg × 1/day for 1 year. The amount of fibroglandular tissue (FGT) and background parenchymal enhancement (BPE) on magnetic resonance imaging (MRI) performed before and after the intervention was scored by two independent expert radiologists using the Breast Imaging and Reporting Data System. The results showed a decrease in the average score for FGT amount from 2.8 ± 0.8 at the onset to 2.65 ± 0.84 after 1 year (P = 0.031), with no significant change in BPE (P = 0.429). A group of DIM-untreated age- and menopausal-status-matched women from the BRCA clinic did not show a significant change in FGT amount (P = 0.33) or BPE (P = 0.814) in a parallel year. Mean estradiol level decreased from 159 to 102 pmol/l (P = 0.01), and mean testosterone level decreased from 0.42 to 0.31 pmol/l (P = 0.007). Side effects were grade 1. In conclusion, 1 year’s supplementation with DIM 100 mg × 1/day in BRCA carriers was associated with a significant decline in FGT amount on MRI. Larger randomized studies are warranted to corroborate these findings.
The impact of 1 year’s supplementation with oral 3,3-diindolylmethane 100 mg × 1/day on breast density and estrogen metabolism was evaluated in 23 healthy BRCA carriers. MRI showed a significant decrease in average Breast Imaging and Reporting Data System score for fibroglandular tissue from before to after treatment.
Introduction
Women who carry the BRCA gene mutation are at high risk of the development of breast cancer, reaching 80% over their lifetime (1,2). Currently, there is no consensus regarding an effective and safe chemoprevention strategy. Therefore, in addition to active surveillance, BRCA mutation carriers are referred for bilateral salpingo-oophorectomy and instructed to consider bilateral mastectomy as a risk-reduction strategy (3).
There is a large body of evidence suggesting that 3,3-diindolylmethane (DIM), a dimer of indole-3-carbinol found in cruciferous vegetables, can potentially prevent carcinogenesis, tumor development (4–7) and shift estrogen metabolism in healthy postmenopausal women (8). Estrogen metabolism plays a causative role in breast cancer (9–11). The ovaries produce estrogen in the form of the parent molecules estrone and estradiol, which can be irreversibly hydroxylated via various pathways (12–15). Hydroxylation at the C-2, C-4 or C-16 positions of the steroid ring produces estrogen metabolites that differ in their bioavailability to breast tissues and activation of estrogen receptors. Numerous studies have shown that the ratio between the different metabolites is correlated with the risk of breast cancer (12–15). For example, more extensive 2-hydroxylation of the parent estrogens (2:16 ratio) is associated with a lower risk of breast cancer, and less extensive methylation of potentially genotoxic 4-hydroxylation pathway catechols is associated with a higher risk of breast cancer (10). Moreover, several studies showed that DIM induces apoptosis in cancer cells and prevents the development of cancer in animal models (16–18). These findings, also confirmed by others (19–22), support the rationale for investigating the use of DIM to prevent breast cancer development or recurrence. Some studies suggested possible other benefits in BRCA carriers (23,24).
Breast density is a well-established surrogate for predicting the risk of breast cancer (25–27). The pivotal preventive trial, NSABP–P1, revealed that among the women in the tamoxifen arm, a decrease in mammographic density by at least 10% was associated with a 63% reduction in breast cancer risk, whereas a lesser decrease in density was not beneficial (28). Later studies showed that breast density could also be measured on magnetic resonance imaging (MRI) scans by the amount of fibroglandular tissue (FGT) and background parenchymal enhancement (BPE), the matching definition to mammogram breast density. An increase in these factors is associated with a higher probability of the development of breast cancer (29,30). The aim of the present study was to investigate the impact of 1 year of DIM supplementation on MRI breast density and estrogen metabolism in healthy BRCA carriers. We sought to determine the potential of DIM as an effective and safe strategy to decrease the risk of the development of breast cancer.
Setting and women
A prospective single-arm interventional study was conducted in the Davidoff Center of Rabin Medical Center, a tertiary university-affiliated hospital. Participants were healthy female BRCA carriers attending the Davidoff BRCA clinic. Inclusion criteria were age over 18 years and baseline MRI FGT amount and BPE of >1 (Breast Imaging Reporting and Data System [BI-RADS] score 1–4). Exclusion criteria were previous preventive breast mastectomy, finding of a potentially cancerous lesion on breast imaging, current or planned pregnancy, presence of a severe comorbidity, such as renal or hepatic disease, and known allergy to DIM. Each woman in the cohort was matched by age (within 3 years) and menopausal status to a woman attending the clinic who was not participating in the study and who underwent breast MRI in a parallel year.
Dalessandri et al. reported that DIM supplementation at a dose of 108 mg/day for 30 days increased urinary 2-hydroxyestrone excretions in postmenopausal women with a history of breast cancer (22). Pharmacokinetics data demonstrated a linear dose–exposure relationship for DIM over the range of 50–300 mg. Doses of DIM of up to 200 mg were well tolerated by healthy subjects. The dosage was set to 100 mg × 1 daily to represent the best bioavailability with the least reported side effects. (31)
The DIM dietary supplement (DIM-EvailTM) was donated by Designs for Health (Suffield, CT). Each softgel capsule contained 100 mg diindolymethane and the additional ingredients were medium chain triglycerides, vitamin E, sunflower lecithin; gelatin, purified water, glycerin and annatto (softgel ingredients). The company did not have any access to women or data. The study was approved by the local Institutional Ethics Committee (ClinicalTrials.gov Identifier: NCT02197000).
Study endpoints
Primary endpoint
The primary endpoint of the study was a change in MRI breast FGT amount and BPE measurements from before to after 1 year of daily oral supplementation with 100 mg × 1/day DIM. The primary outcome was assessed using the BI-RADS (see MRI technique). The MRI scans of the matched DIM-untreated women attending the clinic in a parallel year were evaluated for the same primary endpoints.
Secondary endpoints
The secondary endpoints of the study were a change in urinary estrogen metabolism and serum hormone profile from before to after 1 year of DIM supplementation, side effects of DIM and malignancy events during the intervention.
Biomarker measurement
Participants were tested using the 0142 Estronex® Profile kit (Metametrix, Duluth, GA) at study initiation and again after 1 year. The first morning urine was collected. Premenopausal women were instructed to perform urine collection on days 18–25 of the menstrual cycle; follow-up testing was performed on corresponding days of the cycle. Samples were shipped to Genova Diagnostics (Duluth, GA) and analyzed for six urine estrogen metabolites based on the ultra performance liquid chromatography tandem mass spectrometry method as follows: 2-hydroxyestrone, 2-hydroxyestradiol, 4-hydroxyestrone, 16a-hydroxyestrone, 2-methoxyestrone and 4-methoxyestrone. The test report also provided the results for 2-hydroxyestrone+ 2-hydroxyestradiol, 2/16 hydroxyestrogen ratio and 2-hydroxyestrone/2-methoxyestrone.
The serum hormone profile was assessed at the laboratory in Rabin Medical Center at study initiation and after 1 year. The analysis included levels of follicle-stimulating hormone, luteinizing hormone, estradiol, progesterone, prolactin, testosterone, sex hormone-binding globulin and thyroid-stimulating hormone.
MRI technique
We performed breast MRI at baseline and after 1 year of DIM supplementation. Imaging was conducted according to the guidelines of the National Comprehensive Cancer Network/American Society of Clinical Oncology.
Breast MRI was carried out with a 3T or 1.5T machine (3T Ingenia and 1.5T Achieva, Philips Medical Systems, Best, Netherlands). Women were examined in the prone position using a bilateral, 16-channel breast coil (Mammotrak, Philips Medical Systems). Initially, an axial T2w turbo spin-echo (TSE) sequence was used for both field strengths, with echo time (TE) 120 ms, in-plane resolution 1 mm and slice width 3 mm with zero gap. This was followed by a T2w TSE sequence with SPAIR fat suppression. For 3T imaging, a 2D sequence was used with the same resolution and TE 70 ms. At 1.5T, a 3D Vista sequence was used with repetition time/TE 2000/280 ms, in-plane resolution 0.8 mm and slice width 2 mm reconstructed to 1 mm. The dynamic 3D sequence with SPAIR fat suppression was performed using six dynamics with approximately 64 s per dynamic for both field strengths. At 3T, the flip angle was 12° with repetition time/TE 6.3/3.0 ms, in-plane resolution 0.8 mm and slice width 1.8 mm reconstructed to 0.9 mm. At 1.5T, the flip angle was 10° with repetition time/TE 6.6/3.2 ms, in-plane resolution 1.2 mm and slice width 1.8 mm reconstructed to 0.9 mm.
Qualitative FGT amount and BPE were assessed on the MRI images by two fellowship-trained radiologists with a specialty in breast imaging (A.G. and Y.R.) who were blinded to each other’s report, as well as to the timing of the test in relation to the DIM intervention. The amount of FTG was scored in accordance with the BI-RADS categories as follows: (i) almost entirely fat, (ii) scattered areas of FGT, (iii) heterogeneous FGT or (iv) extreme FGT amount. The BPE findings were reviewed on the basis of the amount of FGT enhancement on the first postcontrast images and categorized, in accordance with the BI-RADS, as (i) minimal, (ii) mild, (iii) moderate or (iv) marked. Subtraction and maximum intensity projection images were created by subtracting the unenhanced T1-weighted image from the initial contrast-enhanced image. In cases of substantial patient motion between examinations, BPE was determined by visual comparison of the initial unenhanced and contrast-enhanced images (32). In premenopausal women, MRI was performed on the recommended days 7–15 of the cycle.
Follow-up
Women were asked to report all side effects immediately. During the study period, women were contacted by phone once a month to verify that indeed all side effects were reported and to check adherence. Physical examination was performed at the clinic every 4 months. Breast imaging was repeated annually.
Statistical analysis
Since each woman served as her own control, we performed a per-protocol analysis for those who completed the study. Matched t-test was used to compare mean BI-RADS scores of FGT amount and BPE from before to after 1 year of DIM supplementation. The type I error probability associated with this test of the null hypothesis was set at 0.05.
The data of the matched clinic women were used for the sensitivity analysis. Differences in hormone profile from before to after treatment were tested using Fisher’s exact test. SAS version 9.2 was used for all analyses.
Results
Patient characteristics
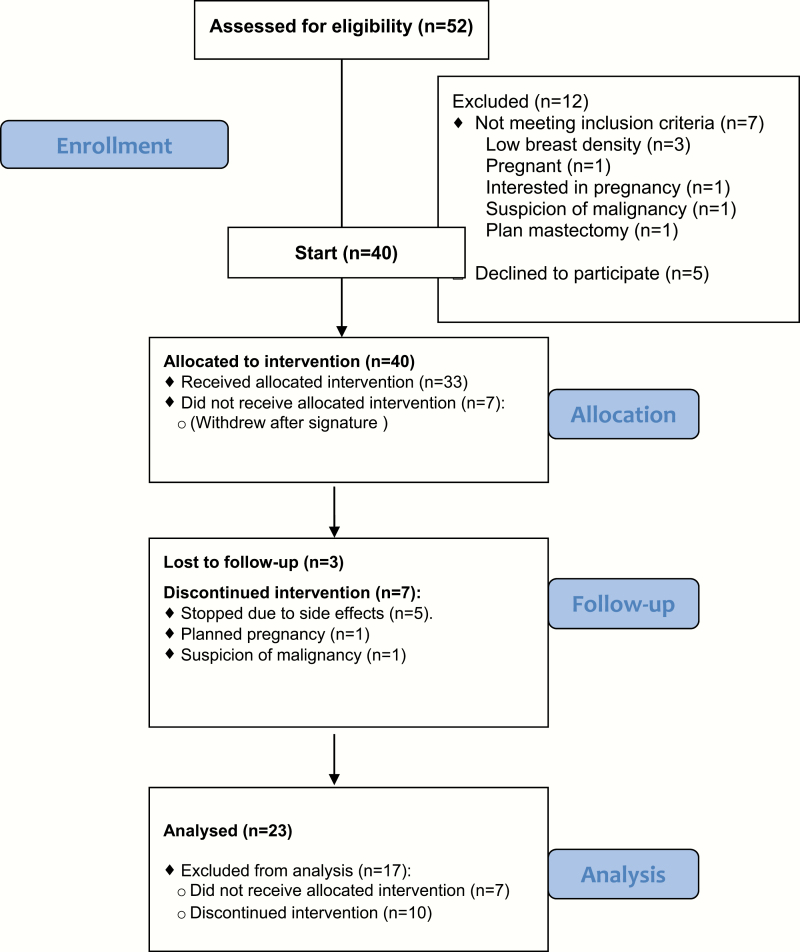
Of the 52 women who were assessed for eligibility for the study, 40 signed the informed consent form. Twenty-three women completed 1 year of intervention. The CONSORT flow diagram in Figure 1 depicts the selection of the final cohort for the study.
Figure 1.
Consort flow diagram showing selection of patients for the study.
Median age of the study group was 47.1 years (range: 35–71.3). Eighteen women carried the BRCA1 mutation, and five women carried the BRCA2 mutation. Eighteen women (78%) were postmenopausal at study onset, of whom three were using hormone replacement therapy (HRT) during the study period. The patient characteristics, including comorbidities and risk factors, are summarized in Table 1.
Table 1:
Characteristics of the BRCA carriers (Intervention+Control)
| Characteristics | Intervention group (n = 23) | Control group (n = 23) |
|---|---|---|
| Age (year), median (range) | 47.1 (35–71.3) | 47.3 (33.4–72.0) |
| Mutation | ||
| BRCA1 | 18 | 10 |
| 185delAG | 15 | 6 |
| 5382insC | 1 | 3 |
| c.3835delG | 1 | 0 |
| Tyr978X | 1 | 1 |
| BRCA2 | 5 | 13 |
| 6174delT | 4 | 13 |
| c.5549C>T;p.P1774L | 1 | 0 |
| Menopausal status | ||
| Postmenopausal | 18 | 19 |
| Premenopausal | 5 | 4 |
| Family historya, n | ||
| Any cancer cases | 23 | 23 |
| Breast cancer cases | 20 | 22 |
| Ovarian cancer cases | 13 | 11 |
| Pancreas cancer cases | 2 | 4 |
| BMI, median (range) | 24.46 (18.7–34.6) | 24.91 (18.9–35) |
| BSO | 17 | 18 |
| Smoking status, n | ||
| Never smoked | 20 | 21 |
| Current smoker | 1 | 1 |
| Past smoker | 2 | 1 |
| No. pregnancies, median (range) | 4 (1–9) | 3 (1–6) |
| No. children, median (range) | 3 (1–6) | 3 (1–5) |
| Physical activity (h/week), median (range) | 2 (0–5) | 2 (0–4) |
| Comorbidities, n | ||
| Hypothyroidism | 3 | 2 |
| Hypertension | 3 | 1 |
| Dyslipidemia | 9 | 4 |
| Ischemic heart disease | 1 | 0 |
| Asthma | 2 | 4 |
| COPD | 1 | 1 |
| Diabetes | 2 | 1 |
| Osteopenia/osteoporosis | 6 | 3 |
BMI, body mass index; BSO, bilateral salpingo-oophorectomy; COPD, chronic obstructive pulmonary disease.
aFirst- or second-degree relative.
Primary outcome
The average BI-RADS score for the amount of FGT decreased during the DIM intervention from 2.80 ± 0.8 to 2.65 ± 0.84, P = 0.031 (Table 2). FGT amount decreased by 0.6 in 30% of women. The three women receiving HRT showed no change in breast tissue density. In no woman was there an increase in density. Figure 2 shows MRI imagings before and after 1 year of intervention.
Table 2.
FGT amount on MRI before and after intervention (BI-RADS score)
| Patient no. | Baseline | After 12 months | Direction of change |
|---|---|---|---|
| 1 | 3.0 | 2.0 | ↓ |
| 2 | 3.0 | 2.5 | ↓ |
| 3 | 4.0 | 4.0 | — |
| 4 | 3.0 | 3.0 | — |
| 5 | 2.5 | 2.0 | ↓ |
| 6 | 2.5 | 2.5 | — |
| 7 | 2.0 | 2.5 | ↑ |
| 8 | 2.0 | 2.0 | — |
| 9 | 2.5 | 2.0 | ↓ |
| 10 | 2.0 | 2.0 | — |
| 11 | 2.0 | 2.0 | — |
| 12 | 2.0 | 2.0 | — |
| 13 | 3.5 | 3.5 | — |
| 14 | 4.0 | 3.5 | ↓ |
| 15 | 4.0 | 4.0 | — |
| 16 | 3.0 | 3.0 | — |
| 17 | 1.5 | 1.0 | ↓ |
| 18 | 3.0 | 3.0 | — |
| 19 | 3.0 | 2.5 | ↓ |
| 20 | 2.0 | 2.0 | — |
| 21 | 2.0 | 2.0 | — |
| 22 | 4.0 | 4.0 | — |
| 23 | 4.0 | 4.0 | — |
BI-RADS : Breast Imaging Reporting and Data System (score range: 1–4)
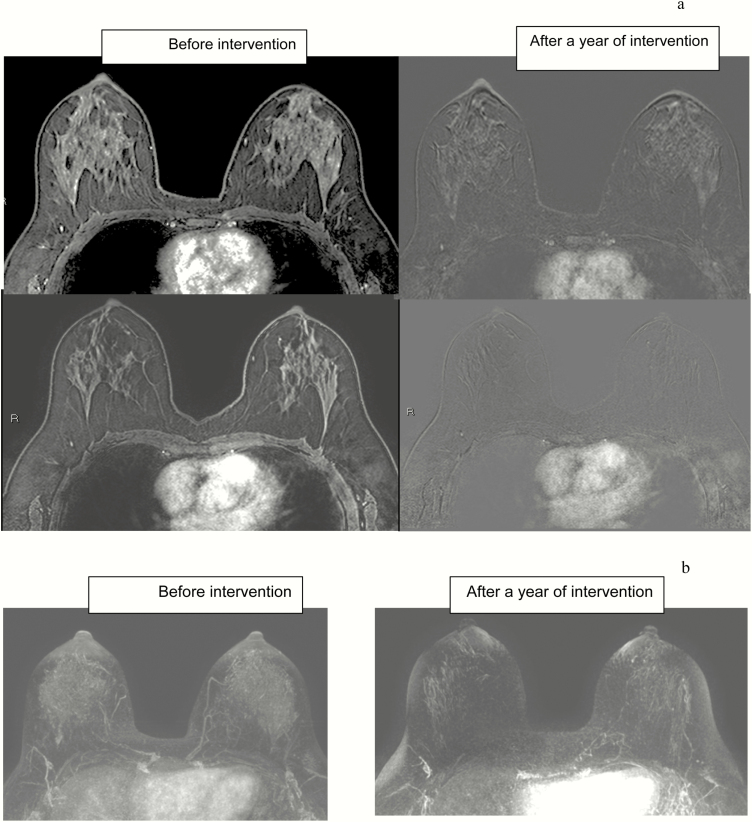
Figure 2.
Screening breast MRI in 43-year-old BRCA carrier at baseline and after 1 year of DIM treatment. (a) Axial T1-weighted fat-suppressed first contrast-enhanced and subtracted images. (b) Minimal intensity projection (MIP), reconstruction images. Note the reduction in BPE, as well as in the amount of FGT.
There was no statistically significant change in BPE scores from before to after completion of the intervention (1.3 ± 5.7 versus 1.3 ± 1.73, P = 0.43; Table 3). Two women showed an increase in BPE scores.
Table 3.
BPE on MRI before and after intervention (BI-RADS score)
| Patient no. | Baseline | After 12 months | Direction of change |
|---|---|---|---|
| 1 | 1.5 | 1.0 | ↓ |
| 2 | 1.0 | 1.0 | — |
| 3 | 1.0 | 1.0 | — |
| 4 | 2.0 | 2.0 | — |
| 5 | 1.0 | 1.0 | — |
| 6 | 2.5 | 2.5 | — |
| 7 | 1.0 | 1.0 | — |
| 8 | 1.0 | 1.0 | — |
| 9 | 1.0 | 1.0 | — |
| 10 | 1.0 | 1.0 | — |
| 11 | 1.0 | 1.0 | — |
| 12 | 2.0 | 1.0 | ↓ |
| 13 | 2.5 | 3.0 | ↑ |
| 14 | 1.0 | 1.0 | — |
| 15 | 1.0 | 1.0 | — |
| 16 | 1.0 | 1.0 | — |
| 17 | 1.0 | 1.0 | — |
| 18 | 2.0 | 2.0 | — |
| 19 | 1.0 | 1.0 | — |
| 20 | 1.0 | 1.0 | — |
| 21 | 1.0 | 1.0 | — |
| 22 | 1.0 | 1.0 | — |
| 23 | 2.5 | 3.5 | ↑ |
BI-RADS : Breast Imaging Reporting and Data System (score range: 1–4).
The matched group showed no significant difference in FGT amount: 2.28 ± 0.9 and 2.3 ± 0.89 after 1 year (P = 0.33) or in BPE score: 1.48 ± 0.66 versus 1.5 ± 0.6 (P = 0.81) after a year of follow-up. Inter-rater agreement (kappa value) between the two radiologists was 0.82 for density and 0.814 for BPE.
Secondary outcome
Mean estradiol level of the study group decreased from 159 ± 116 to 102 ± 29 pmol/l (P = 0.03), and mean testosterone level decreased from 0.42 ± 0.37 to 0.31 ± 0.26 pmol/l (P = 0.03). No significant changes were seen in levels of follicle-stimulating hormone, luteinizing hormone, progesterone, prolactin, sex hormone-binding globulin or thyroid-stimulating hormone. There were no significant changes in any of the urine metabolites evaluated.
Side effects
Three women reported a change in bowel movements. Four women complained of headache and one had nausea. In one patient with known atopic dermatitis, erythema developed which required treatment with systemic steroids. All side effects were grade 1.
Malignancy events
One patient was diagnosed with node-negative invasive breast cancer (7 mm) at age 58 years, 5 months after starting DIM. A review of her previous MRI revealed a nonspecific 3-mm focus, which was interpreted as benign. One patient aged 44 years was referred for prophylactic oophorectomy and was diagnosed with stage I ovarian cancer at 16 months after starting DIM.
Discussion
This is the first study to prospectively examine the impact of DIM (100 mg × 1/day) on the amount of FGT and BPE on breast MRI in BRCA carriers. The results showed a significant reduction in the amount of FGT after 1 year of the intervention, with no change in BPE. In addition, mean estrogen and testosterone levels decreased. No safety issues were encountered, and all side effects were grade 1.
By contrast, a previous randomized, placebo-controlled trial in 98 tamoxifen-treated patients with breast cancer reported no impact of DIM on breast density measured on both mammograms and MRI scans (33). However, there are several differences between this study and ours. Our cohort consisted of women with no history of breast cancer, whereas the earlier study investigated DIM as a secondary preventive measure in patients with breast cancer. The authors did not mention if any of the patients was a BRCA carrier. Moreover, in the present study, DIM was the only intervention, whereas in the randomized study, all patients were also receiving tamoxifen, so the impact of DIM could not be isolated. Lastly, breast density was the primary endpoint in our study but the secondary endpoint in the earlier study.
Little is known about DIM and its effect on BRCA carriers.
Kostopauolos et al. (23) followed healthy BRCA1 carriers taking DIM for 4–6 weeks. An increased level of messenger RNA was noted, which the authors suggested could mitigate the deleterious effect of the BRCA mutation. Fan et al. (24) suggested that low concentrations of DIM protected against oxidative stress in BRCA1 carriers.
Others reported an impact of DIM on urinary estrogen metabolites (20,21). This was not shown in our study, perhaps owing to the low DIM dose (100 mg × 1/day). Our results are in accordance with the study of Nikitina et al. (34), wherein 4–6 weeks of DIM 300 mg/day in 15 BRCA carriers had no effect on urinary 2:16 hydroxyestrogen ratio.
Most of the literature to date on breast density was based on mammography. As some of the BRCA carriers in our clinic were too young for screening mammography, we used FGT amount and BPE on MRI scans as the primary endpoints. In this manner, we could offer participation in the study to all BRCA carriers in the clinic, regardless of age. This approach was supported by studies showing substantial agreement between automated volumetric FGT measured from screening digital mammograms and from MRI scans, suggesting that MRI could be used in clinical practice for risk prediction and prevention (35–37). One study of the effect of lifestyle changes prospectively assigned BI-RADS breast density scores to 301 955 women aged 30 years and older who were not on postmenopausal HRT (38). At least two screening mammography examinations were performed in each case. The results showed an increase in breast density category in 19.6% of the cohort. Although these authors assessed the breast density changes over time using mammography, it is still interesting to note the difference from our study in which none of the women taking DIM showed an increase in the FGT amount. A smaller study used 3D MRI to evaluate breast density in 16 tamoxifen-treated women (39). After 2 years, there was a mean reduction of 5.8% in the percentage of breast density, which positively correlated with the baseline percentage of breast density, supporting the use of MRI to assess breast density during systemic treatment.
There are other factors that may impact breast density, such as day of the menstrual cycle, alcohol consumption and use of HRT. In the present study, there were no changes in the women’s habits during the intervention. Most had undergone bilateral salpingo-oophorectomy and, in those who were still premenopausal, MRI was performed on the recommended days 7–15 of the cycle. Hence, the most dominant known factor that may have impacted the FGT amount was the 1-year increase in age (27). It is noteworthy that none of the three women in our cohort who used HRT showed a decrease in the amount of FGT.
Although the decline observed in blood estradiol and testosterone levels may be part of aging (40), and the difference from before the intervention was statistically significant, the level of decline was probably insufficient to impact FGT amount. In conclusion, our results suggest that DIM supplement may have a potential role in the primary prevention of breast cancer in healthy BRCA carriers.
The main limitations of this study were the nonrandomized design, 1-year duration of the intervention in a lifetime relative high-risk cohort, the relatively small sample size and the higher FGT/BPE baseline values in the matched clinic women. However, each woman served as her own control which made it possible to achieve statistically valid results despite the small number of women. The comparison with the matched women supports the potential of DIM intervention to have a real impact on breast density. In addition, two highly experienced radiologists separately examined the MRI scans, with similar results.
In summary, the need of healthy BRCA carriers for a safe and effective means of preventing breast cancer is currently unmet. The present study showed that the administration of DIM capsules, 100 mg × 1/day, to BRCA carriers led to a significant decline in the FGT amount on MRI scans. These findings, together with the accumulating data on the potential anticancer effect of DIM in the general population and in individuals with different genetic mutations (23,41), justify further investigations in randomized clinical studies of the primary preventive role of DIM supplementation in women with BRCA mutations.
Funding
The study was partially sponsored by the Israel Cancer Association (grant no. 20161385).
Acknowledgements
The DIM supplement used in the study was contributed by Designs for Health, Inc. The company received no information on the study participants.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BI-RADS
Breast Imaging Reporting and Data System
- BPE
background parenchymal enhancement
- DIM
3,3-diindolylmethane
- FGT
fibroglandular tissue
- HRT
hormone replacement therapy
- MRI
magnetic resonance imaging
- TSE
turbo spin-echo
References
- 1. Easton D.F. et al. (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast cancer linkage consortium. Am. J. Hum. Genet., 56, 265–271. [PMC free article] [PubMed] [Google Scholar]
- 2. Antoniou A. et al. (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet., 72, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NCCN. Clinical Practice Guidelines in Oncology, Version 3 http://www.nccn.org/professionals/physiciangls/pdf/genetics_screening.pdf (15 November, 2015, date last accessed).
- 4. Rogan E.G. (2006) The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo, 20, 221–228. [PubMed] [Google Scholar]
- 5. Graham S. et al. (1982) Diet in the epidemiology of breast cancer. Am. J. Epidemiol., 116, 68–75. [DOI] [PubMed] [Google Scholar]
- 6. Higdon J.V. et al. (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol. Res., 55, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verhoeven D.T. et al. (1996) Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomarkers Prev., 5, 733–748. [PubMed] [Google Scholar]
- 8. Fowke J.H. et al. (2000) Brassica vegetable consumption shifts estrogen metabolism in healthy postmenopausal women. Cancer Epidemiol. Biomarkers Prev., 9, 773–779. [PubMed] [Google Scholar]
- 9. Yaeger J.D. et al. (2006) Estrogen carcinogenesis in breast cancer. N. Engl. J. Med., 354, 270–282. [DOI] [PubMed] [Google Scholar]
- 10. Fuhrman B.J. et al. (2012) Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst., 104, 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gruber C.J. et al. (2002) Production and actions of estrogens. N. Engl. J. Med., 346, 340–352. [DOI] [PubMed] [Google Scholar]
- 12. Mueck A.O. et al. (2007) Breast cancer: are oestrogen metabolites carcinogenic? Maturitas, 57, 42–46. [DOI] [PubMed] [Google Scholar]
- 13. Im A. et al. (2009) Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis, 30, 1532–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falk R.T. et al. (2013) Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res., 15, R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y. et al. (1998) The equine estrogen metabolite 4-hydroxyequilenin causes DNA single-strand breaks and oxidation of DNA bases in vitro. Chem. Res. Toxicol., 11, 1105–1111. [DOI] [PubMed] [Google Scholar]
- 16. Ahmad A. et al. (2010) Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets, 11, 652–666. [DOI] [PubMed] [Google Scholar]
- 17. Souli E. et al. (2008) Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem. Toxicol., 46, 863–870. [DOI] [PubMed] [Google Scholar]
- 18. Hedrick E. et al. (2019) Potent inhibition of breast cancer by bis-indole-derived Nuclear receptor 4A1 (NR4A1) antagonists. Breast Can. Res. Treat., 177, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michnovicz J.J. et al. (1991) Altered estrogen metabolism and excretion in humans following consumption of I3C. Nutr. Cancer, 16, 59–66. [DOI] [PubMed] [Google Scholar]
- 20. Michnovicz J.J. et al. (1997) Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J. Natl. Cancer Inst., 89, 718–723. [DOI] [PubMed] [Google Scholar]
- 21. Michnovicz J.J. et al. (1998) Increased estrogen 2- hydroxylation in obese women using oral indole-3-carbinol. Int. J. Obesity Relat. Metab. Disor. 22, 227–229. [DOI] [PubMed] [Google Scholar]
- 22. Dalessandri K.M. et al. (2004) Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr. Cancer, 50, 161–167. [DOI] [PubMed] [Google Scholar]
- 23. Kotsopoulos J. et al. (2014) BRCA1 mRNA levels following a 4-6-week intervention with oral 3,3’-diindolylmethane. Br. J. Cancer, 111, 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan S. et al. (2009) Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res., 69, 6083–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tice J.A. et al. (2015) Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J. Clin. Oncol., 33, 3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramón Y. et al. (2015) Mammographic density and breast cancer in women from high risk families. Breast Cancer Res., 17, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerlikowske K. et al. ; National Institutes of Health Breast Cancer Surveillance Consortium (2007) Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J. Natl. Cancer Inst., 99, 386–395. [DOI] [PubMed] [Google Scholar]
- 28. Cuzick J. et al. (2011) Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J. Natl. Cancer Inst., 103, 744–752. [DOI] [PubMed] [Google Scholar]
- 29. Vignesh A. et al. (2019) Population-based assessment of the association between magnetic resonance imaging background parenchymal enhancement and future primary breast cancer risk. J. Clin. Oncol., 37, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Telegrafo M. et al. (2016) Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn. Reson. Imaging, 34, 173–176. [DOI] [PubMed] [Google Scholar]
- 31. Gregory A.R. et al. (2008) Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3’-diindolylmethane in healthy subjects. Cancer Epidemiol. Biomarkers Prev., 17, 2619–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American College of Radiology (2013) ACR BI-RADS® Atlas: Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA. [Google Scholar]
- 33. Thomson C.A. et al. (2017) A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat., 165, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikitina D. et al. (2015) The effect of oral 3,3’-diindolylmethane supplementation on the 2:16α-OHE ratio in BRCA1 mutation carriers. Fam. Cancer, 14, 281–286. [DOI] [PubMed] [Google Scholar]
- 35. Khazen M. et al. ; Collaborators in the United Kingdom Medical Research Council Magnetic Resonance Imaging in Breast Screening (MARIBS) Study (2008) A pilot study of compositional analysis of the breast and estimation of breast mammographic density using three-dimensional T1-weighted magnetic resonance imaging. Cancer Epidemiol. Biomarkers Prev., 17, 2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J. et al. (2013) Agreement of mammographic measures of volumetric breast density to MRI. PLoS One, 8, e81653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tagliafico A. et al. (2013) Comparative estimation of percentage breast tissue density for digital mammography, digital breast tomosynthesis, and magnetic resonance imaging. Breast Cancer Res. Treat., 138, 311–317. [DOI] [PubMed] [Google Scholar]
- 38. Brand J.S. et al. (2013) Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS One, 8, e81876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J.-H. et al. (2011) Reduction of breast density following tamoxifen treatment by 3-D MRI: preliminary study. Magn. Reson. Imaging, 29, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacNaughton J. et al. (1992) Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin. Endocrinol. (Oxf)., 36, 339–345. [DOI] [PubMed] [Google Scholar]
- 41. Lee Y.-U. et al. (2019) Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science, 17, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]