Abstract
Background
Impaired recovery of blood pressure (BP) after standing has been shown to be related to cognitive function and mortality in people without dementia, but its role in people with Alzheimer’s disease (AD) is unknown. The aim of this study was to investigate the association of the orthostatic BP response with cognitive decline and mortality in AD.
Methods
In this post hoc analysis of a randomized controlled trial (Nilvad), we measured the beat-to-beat response of BP upon active standing in mild-to-moderate AD. This included the initial drop (nadir within 40 seconds) and recovery after 1 minute, both expressed relative to resting values. We examined the relationship between a small or large initial drop (median split) and unimpaired (≥100%) or impaired recovery (<100%) with 1.5-year change in Alzheimer’s Disease Assessment—cognitive subscale (ADAS-cog) scores and all-cause mortality.
Results
We included 55 participants (age 73.1 ± 6.2 years). Impaired BP recovery was associated with higher increases in ADAS-cog scores (systolic: β [95% confidence interval] = 5.6 [0.4–10.8], p = .035; diastolic: 7.6 [2.3–13.0], p = .006). During a median follow-up time of 49 months, 20 participants died. Impaired BP recovery was associated with increased mortality (systolic: HR [95% confidence interval] = 2.9 [1.1–7.8], p = .039; diastolic: HR [95% confidence interval] = 5.5 [1.9–16.1], p = .002). The initial BP drop was not associated with any outcome. Results were adjusted for age, sex, and intervention group.
Conclusions
Failure to fully recover BP after 1 minute of standing is associated with cognitive decline and mortality in AD. As such, BP recovery can be regarded as an easily obtained marker of progression rate of AD.
Keywords: Alzheimer’s disease, Dementia, Resilience, Blood pressure, Continuous monitoring
With increasing numbers of people to be affected by Alzheimer’s disease (AD) and in the absence of drugs that have a disease-modifying effect, there also is an increasing need to identify potentially modifiable factors that predict the longitudinal course of the disease (1). The progression of AD is very heterogenous, and understanding which factors contribute to this can identify targets for disease modification, help clinical decision making, and inform patients and their families (2). For example, being able to estimate whether someone with dementia will progress quickly or slowly can guide advance care decisions and may result in individualized decisions on the management of this persons’ living conditions and comorbidities (3).
A common comorbidity in AD is hypertension, with an estimated prevalence of 45% (4). Although it is now widely accepted that vascular factors play a crucial role in the development of AD, the optimal treatment targets for blood pressure (BP), but also the prognostic role of BP once AD has been diagnosed, are unclear. Both a high BP and a low BP have been associated with progression of AD (5,6). One of the explanations for this inconsistency could be that most studies focused on static BP measurements. Historically, single BP readings have mostly been used in studies, and variations observed between measurements were often discarded as noise (7). However, recent findings have made us more aware of the importance of the dynamics that we observe in a BP signal, and single BP measurements are no longer the recommended approach (8).
More generally, this fits with the shifting focus in geriatric medicine from using static measurements under basal conditions to quantify a physiological system, toward recognizing that these systems are highly dynamic (9,10). Rather than a static measurement, it may be more meaningful to measure a person’s ability to recover following a stressor to the system (11). This concept can be referred to as physical resilience (12). In the context of the cardiovascular system, physical resilience could be quantified as the ability to recover from an orthostatic challenge. The maintenance of BP during a change in posture is complex, with neurological, cardiovascular, and muscular systems involved (13,14). Impaired function of these systems may result in low BP during standing and reduced organ perfusion, including the brain. Given the frequent occurrence of a posture change during daily life (15), impairments in this response are likely to have clinical implications.
There is indeed evidence that an impaired BP response upon active standing is associated with adverse health outcomes in aging individuals, including mortality (16–18). In addition, an impaired BP response upon standing has been associated with reduced cognitive function (19), higher risk of dementia (20), and conversion from mild cognitive impairment to dementia (21). Whether an orthostatic challenge test also has prognostic value in the progression of established AD is currently unknown. Therefore, we aimed to test the hypothesis that an impaired BP response during an orthostatic challenge test is associated with higher rate of progression of clinical AD. We investigated associations of the BP response with cognitive decline after 1.5 years and with all-cause mortality after a long-term follow-up.
Methods
Study Design and Participants
Data for this study were derived from the cerebral blood flow substudy of the Nilvad trial (22). The Nilvad trial (NCT02017340) was a randomized controlled trial investigating the effects of nilvadipine on cognitive and functional outcomes in 511 people with mild-to-moderate AD. The study found that nilvadipine had no effect on disease progression (23). Between July 2013 and March 2015, the cerebral blood flow substudy included 58 participants from two study sites in the Netherlands (Radboud University Medical Center, Nijmegen and Rijnstate, Arnhem, The Netherlands). Participants were aged 50 and older and had a diagnosis of probable AD according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s disease and Related Disorders Association (24). Using the recent National Institute on Aging—Alzheimer’s Association Research Framework, these participants would now be classified as Alzheimer’s Clinical Syndrome (25). The complete list of inclusion and exclusion criteria for the Nilvad trial can be found in the study protocol (26). Ethical approval was provided by the medical ethics committee (CMO Arnhem-Nijmegen, number 2012–508), and written informed consent was obtained from every patient and a relevant caregiver. The study was carried out according to the Declaration of Helsinki.
Orthostatic Challenge Test
From the evening prior to the study visit, participants refrained from caffeine and alcohol. During testing, beat-to-beat arterial BP was measured in the index or middle finger of the nondominant hand using volume-clamp photoplethysmography (Finapres Medical Systems, Amsterdam, The Netherlands) (27). To prevent hydrostatic errors, an arm sling was used to keep the hand at heart level throughout the procedure, and the Finometer height correction system was used to correct for accidental movements in hand position relative to the heart. The BP signal was calibrated with an upper arm return-to-flow measurement (28). BP was recorded continuously at 200 Hz.
During the orthostatic challenge test, participants sat in a straight-backed chair and were asked to stand up. Participants were aided by research staff if necessary and were instructed to stand calmly. Before the recording started, participants sat quietly for at least 5 minutes. Subsequently, the measurement started with 2 minutes of sitting, followed by 1 minute of standing. This was repeated three times. During the third trial patients remained standing for 5 minutes. Supplementary Figure 1 provides a visual presentation of the protocol.
Semiautomated custom-written Matlab scripts (version 2014b, the MathWorks Inc., Natick, MA) were used to preprocess the data as described previously (29). Briefly, this included automatic detection of systolic and diastolic peaks, visual inspection, and manual correction of this detection, waveform integration for each beat, calculation of the inter-beat-interval and heart rate, and resampling to 10 Hz. Subsequently, systolic BP (SBP), diastolic BP (DBP), and heart rate were filtered using a moving average filter with a 5-second window (30). We extracted the baseline, the initial response, and the recovery values, in line with the normative data published by Finucane and colleagues (see Supplementary Figure 1) (31). Baseline was defined as the average of 30 seconds (−40 to −10) before standing up. The initial orthostatic response was defined as the difference between the baseline and the nadir reached within the first 40 seconds after standing. Orthostatic recovery was defined as the difference between the baseline and the average value after 50–60 seconds of standing. Outcomes were calculated as percentage from baseline, and results of the three trials were averaged to increase reliability. The effect of prolonged standing was investigated by calculating BP recovery after 5 minutes of standing during the third trial (average value after 280–300 seconds of standing).
The normative reference data from the Irish Longitudinal Study on Ageing (TILDA) for the age group of 70–79 years were used to define a cutoff for unimpaired (≥100%) and impaired (<100%) orthostatic recovery after 1 minute (31). A cutoff for the initial drop upon standing could not be derived from the reference data, which was based on the larger initial drop from supine to standing. Therefore, patients were divided into smaller or larger initial drop by a median split.
Study Outcomes
The primary outcome of the Nilvad trial was the 12-item Alzheimer’s Disease Assessment Scale—cognitive subscale (ADAS-cog) (32). The ADAS-cog covers several cognitive domains and ranges from 0 to 80 points, with higher scores indicating worse cognitive performance. Assessments were done at baseline and after 1.5 years and the change from baseline was used as the outcome. To visualize the full BP response, we also compared those with a fast progression on the ADAS-cog to the others. The mean rate of ADAS-cog progression is estimated at 5.5 points/y (33), corresponding to a 1.5-year change of 8.3 points when assuming linearity. Using this information, we defined an ADAS-cog change ≥12 points in 1.5 years as fast progression. The fast progressors were compared with the others, that is, those with ADAS-cog change <12 points.
The trial ended after 1.5 years, after which we passively followed patients for survival status through the database of the Dutch municipal records. Survival data were extracted in March 2019, resulting in a minimal follow-up period of 4 years.
Other Variables
One week prior to their study visit, participants conducted home BP measurements using a validated, memory-equipped, automatic oscillometric device (Microlife WatchBP Home, Microlife, Heerbrugg, Switzerland), following recommendations of the European Society of Hypertension (34,35). During their study visit, the Clinical Dementia Rating (CDR) scale was assessed to describe the stage of dementia (36), and the Disability Assessment for Dementia was used as a measure of daily function (37). Moreover, information on medical history, medication use, and smoking (current, yes/no) was collected by using the patient’s medical record and by consulting the patient’s general practitioner, pharmacy, and informal caregiver.
In addition to the orthostatic challenge test, repeated sit-to-stand challenges were performed at a frequency of 0.05 Hz, that is, alternating 10-second sitting and 10-second standing, for 5 minutes. This was done to induce enhanced BP oscillations at 0.05 Hz for estimation of baroreflex sensitivity (BRS) (29). The majority of the participants also underwent magnetic resonance imaging, from which we extracted total brain volume, gray and white matter volume, white matter lesion volume, microbleeds, infarcts, and global cerebral blood flow. Details on BRS and magnetic resonance imaging assessment and analysis are provided in Supplementary Methods.
Statistical Analyses
Results are presented as mean ± SD or number (percentage), unless stated otherwise. Linear regression was used to assess associations between normal or impaired BP response and change in ADAS-cog. Analyses were adjusted for age, sex, and intervention (8 mg/d nilvadipine or placebo) group. The sample size did not allow adding more covariates.
Next, we divided participants into fast progressors and others, to visualize their full orthostatic BP response. Participants with missing ADAS-cog because of severe disease progression were also classified as fast progressors. Cox proportional hazard models were used to investigate whether an impaired BP response was associated with mortality. The model was adjusted for age, sex, and intervention group. Results are presented as β or hazard ratio (HR) with 95% confidence interval. All analyses were performed for SBP and DBP. Given the explorative nature of the current analyses, p-value’s should be interpreted with caution. All analyses were performed using SPSS software.
Results
Fifty-five participants were included. A flow diagram is provided in Supplementary Figure 2. Nine participants had missing data on the change in ADAS-cog. This was because of severe disease progression at follow-up (n = 5, all CDR 3 after 1.5 years), because of the participant refusing to participate in the cognitive test at baseline and follow-up (n = 2, both CDR 2 at baseline and follow-up), or because the caregiver withdrew consent (n = 2, both CDR 1 at baseline, CDR at follow-up unknown). A magnetic resonance imaging scan was available for 48 participants, and BRS could be calculated in 49 participants. Baseline characteristics of the sample are presented in Table 1.
Table 1.
Baseline Characteristics
| Variable | Total Sample | Fast Progressors | Others |
|---|---|---|---|
| n | 55 | 18 | 33 |
| Age (y) | 73.1 ± 6.2 | 71.7 ± 5.9 | 73.9 ± 6.4 |
| Sex (female) | 56.4 (31) | 55.6 (10) | 63.6 (21) |
| ADAS-cog 12 score | 31.7 ± 10.1 | 35.9 ± 12.9* | 28.7 ± 7.0 |
| DAD score | 33.0 (29.7–38.0) | 32.6 (31.0–37.6) | 33.5 (31.0–37.9) |
| Smoking | 9.1 (5) | 5.6 (1) | 9.1 (3) |
| History of CVD | 16.4 (9) | 22.2 (4) | 15.2 (5) |
| Diabetes mellitus | 5.5 (3) | 0 | 9.1 (3) |
| BMI (kg/m2) | 24.9 ± 3.6 | 25.3 ± 3.1 | 24.8 ± 4.1 |
| Intervention group | 52.7 (29) | 55.6 (10) | 51.5 (17) |
| Antihypertensive drug use | 29.1 (16) | 33.3 (6) | 24.2 (8) |
| Statin use | 18.2 (10) | 16.7 (3) | 21.2 (7) |
| Memantine use | 10.9 (6) | 11.1 (2) | 12.1 (4) |
| Cholinesterase inhibitor use | 81.8 (45) | 77.8 (14) | 84.8 (28) |
| Antidepressants use | 14.5 (8) | 16.7 (3) | 15.2 (5) |
| Home systolic BP (mm Hg) | 135.6 ± 18.3 | 134.6 ± 14.6 | 136.0 ± 19.9 |
| Home diastolic BP (mm Hg) | 77.0 ± 10.0 | 76.9 ± 8.6 | 77.1 ± 10.6 |
| BRS-gain (ms/mm Hg) | 2.60 (2.12–3.57) | 2.44 (1.99–3.26) | 2.87 (2.16–3.68) |
| BRS-down (ms/mm Hg) | 2.55 (1.75–4.64) | 2.91 (2.27–4.20) | 2.37 (1.63–3.50) |
| BRS-up (ms/mm Hg) | 3.87 (2.58–5.53) | 3.96 (2.76–4.77) | 3.59 (2.59–6.10) |
| Brain volume (× 105 mm3) | 13.62 ± 0.67 | 13.57 ± 0.58 | 13.75 ± 0.70 |
| Gray matter volume (× 105 mm3) | 6.58 ± 0.47 | 6.39 ± 0.44* | 6.74 ± 0.43 |
| White matter volume (× 105 mm3) | 7.05 ± 0.42 | 7.18 ± 0.37 | 7.01 ± 0.44 |
| Presence of microbleeds | 20.9 (9) | 15.4 (2) | 25.9 (7) |
| Lacunair infarction | 18.8 (9) | 12.5 (2) | 25.0 (7) |
| WML volume (× 103 mm3) | 9.0 (3.8–23.1) | 11.2 (5.8–26.9) | 8.1 (3.4–14.4) |
| Global CBF (mL/100 g/min) | 84.51 ± 21.25 | 81.00 ± 22.12 | 88.12 ± 20.75 |
Notes: ADAS-cog = Alzheimer’s disease Assessment Scale—cognitive subscale; BMI = body mass index; BP = blood pressure; BRS = baroreflex sensitivity; CBF = cerebral blood flow; CVD = cardiovascular disease; DAD = Disability Assessment for Dementia; WML = white matter lesion volume. Values are mean ± SD, median (interquartile range), or frequency (numbers).
*p < .05 for fast progressors versus others.
Orthostatic Challenge Test
Sitting BP was 137.3/61.8 ± 26.9/14.3 mm Hg. Upon standing, BP decreased with 18.1/8.9 ± 12.2/4.2 mm Hg to 86.7 ± 9.0% of sitting SBP and 85.2 ± 6.7% of sitting DBP. The median drop was 12.0% for SBP and 14.9% for DBP. After 1 minute of standing, in the complete sample BP recovered to 98.2 ± 8.9% for SBP and to 101.9 ± 7.3% for DBP. However, 30 (54.5%) and 20 (36.4%) participants failed to recover to 100% of sitting SBP and DBP, respectively. Baseline characteristics by 1-minute SBP recovery are presented in Supplementary Table 1. Compared with participants with full SBP recovery, those with impaired recovery tended to have lower BRS (BRS-gain, median [interquartile range]: 2.31 [2.00–3.42] vs. 3.17 [2.30–4.46] ms/mm Hg) and higher white matter lesion volume (median [interquartile range]: 11.3 [6.6–27.7] vs. 6.3 [3.4–10.1] × 103 mm3).
Three participants did not complete the 5 minutes of standing and were excluded for analysis of prolonged standing. After 5 minutes of standing, BP recovered to 97.6 ± 11.4% for SBP and to 105.1 ± 10.0% for DBP. For SBP, 28 (52.8%) participants had not recovered to 100% of sitting BP after 5 minutes. For DBP, this number was 14 (26.4%).
Change in ADAS-cog Score
After 1.5 years, ADAS-cog had increased by 8.3 ± 8.6 points. Regression analyses showed that failure to fully recover to sitting SBP (β [95% confidence interval]: 5.6 [0.4–10.8], p = .035) and DBP (7.6 [2.3–13.0], p = .006) within 1 minute was associated with more increase in ADAS-cog scores (Table 2). For SBP, impaired recovery after 5 minutes of standing was also associated with more cognitive decline (6.6 [1.3–11.9], p = .015). There was no association between the initial BP drop and change in ADAS-cog.
Table 2.
The Association Between Orthostatic Blood Pressure Drop and Recovery With Changes in Cognitive Function After 1.5 y of Follow-up
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Initial SBP drop | −0.3 (−5.4 to 4.9) | .922 | −0.5 (−6.2 to 5.3) | .872 |
| Initial DBP drop | 2.3 (−2.8 to 7.4) | .376 | 1.9 (−3.4 to 7.2) | .474 |
| 1-min SBP recovery | 4.7 (−0.3 to 6.8) | .067 | 5.6 (0.4 to 10.8) | .035 |
| 1-min DBP recovery | 5.6 (0.5 to 10.6) | .033 | 7.6 (2.3 to 13.0) | .006 |
| 5-min SBP recovery | 6.3 (1.3 to 7.7) | .015 | 6.6 (1.3 to 11.9) | .015 |
| 5-min DBP recovery | 4.1 (−2.0 to 10.2) | .183 | 4.4 (−2.0 to 10.8) | .176 |
Notes: ADAS-cog = Alzheimer’s disease Assessment Scale—cognitive subscale; CI = confidence interval; BP = blood pressure; DBP = diastolic blood pressure; SBP = systolic blood pressure. Results from regression analyses of BP response and change in ADAS-cog. For the initial drop, groups with a smaller or larger drop were compared (median split). For recovery, impaired recovery (<100% of sitting BP) was compared with unimpaired recovery (≥100% of sitting BP).
aThe adjusted model is corrected for intervention group, age, and sex.
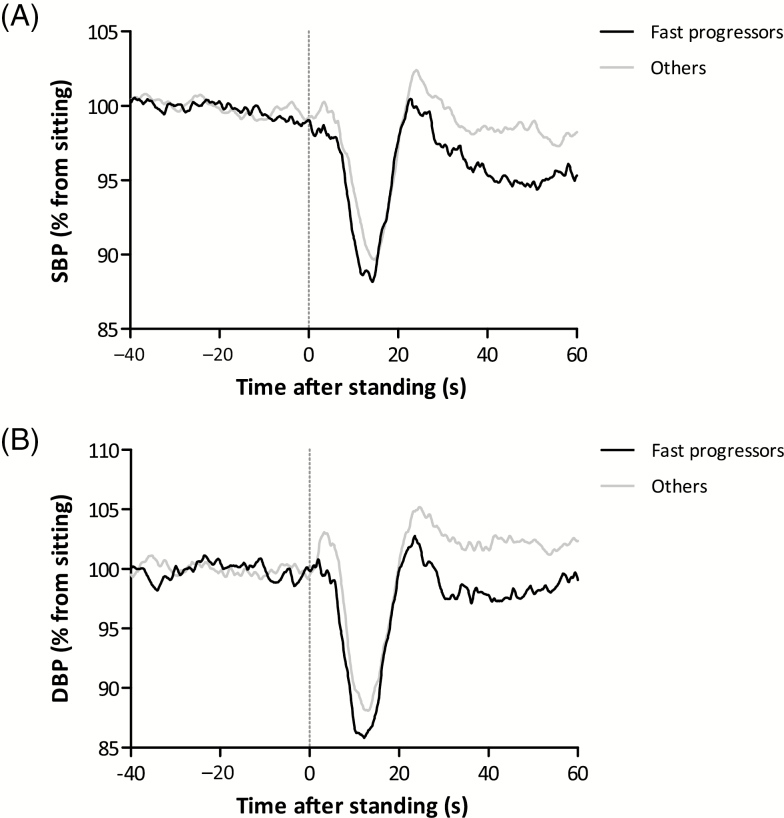
Thirteen participants had an increase in ADAS-cog score of ≥12 and were together with the five participants who could not repeat the ADAS-cog because of severe dementia at follow-up defined as fast progressors. Compared with the others, fast progressors had higher baseline ADAS-cog scores (35.9 ± 12.9 vs. 28.7 ± 7.0 points, p = .039) and lower baseline gray matter volume (6.58 ± 0.47 vs. 6.74 ± 0.43 × 105 mm3, p = .019), see Table 1. Figure 1 shows the full orthostatic BP response for the fast progressors compared with the others.
Figure 1.
Orthostatic challenge response for fast progressors (n = 18, black lines) and others (n = 33, gray lines). (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). Fast progressors were those with an Alzheimer’s Disease Assessment—cognitive subscale (ADAS-cog) increase of ≥12 points (n = 13) or who progressed too severely to perform the ADAS-cog at follow-up (n = 5). Unfiltered results are presented with a sample frequency of 10 Hz. Results of three trials within an individual are averaged.
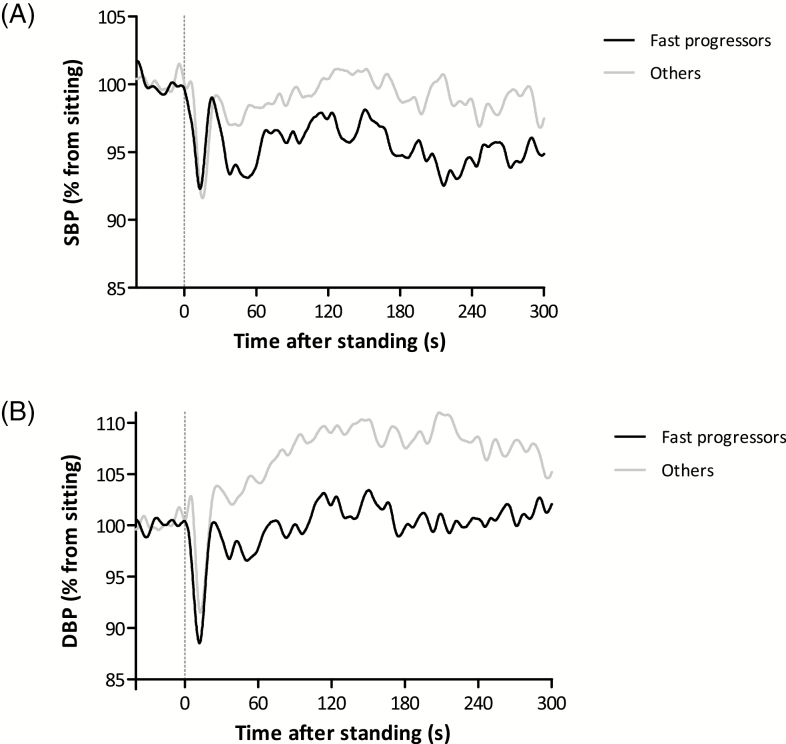
The difference in BP recovery between fast progressors (n = 17) and others (n = 31) remained visible during the complete 5 minutes of standing of the third trial (Figure 2). After 5 minutes, fast progressors had a SBP recovery of 93.6 ± 10.0% compared with 99.4 ± 12.3% for the others (p = .095). DBP recovery was 100.6 ± 8.3% and 107.7 ± 10.4%, for fast progressors and others, respectively (p = .018).
Figure 2.
Prolonged orthostatic challenge response for fast progressors (n = 17, black lines) and others (n = 31, gray lines). (A) Systolic blood pressure (SBP). (B) Diastolic blood pressure (DBP). Fast progressors were those with an Alzheimer’s Disease Assessment—cognitive subscale (ADAS-cog) increase of ≥12 points (n = 13) or who progressed too severely to perform the ADAS-cog at follow-up (n = 4). Filtered results are presented with a sample frequency of 10 Hz.
Similar results were obtained if the five participants with missing ADAS-cog scores were removed from the analysis (Supplementary Figures 3 and 4). Supplementary Figure 5 shows the heart rate response during the orthostatic challenge.
All-Cause Mortality
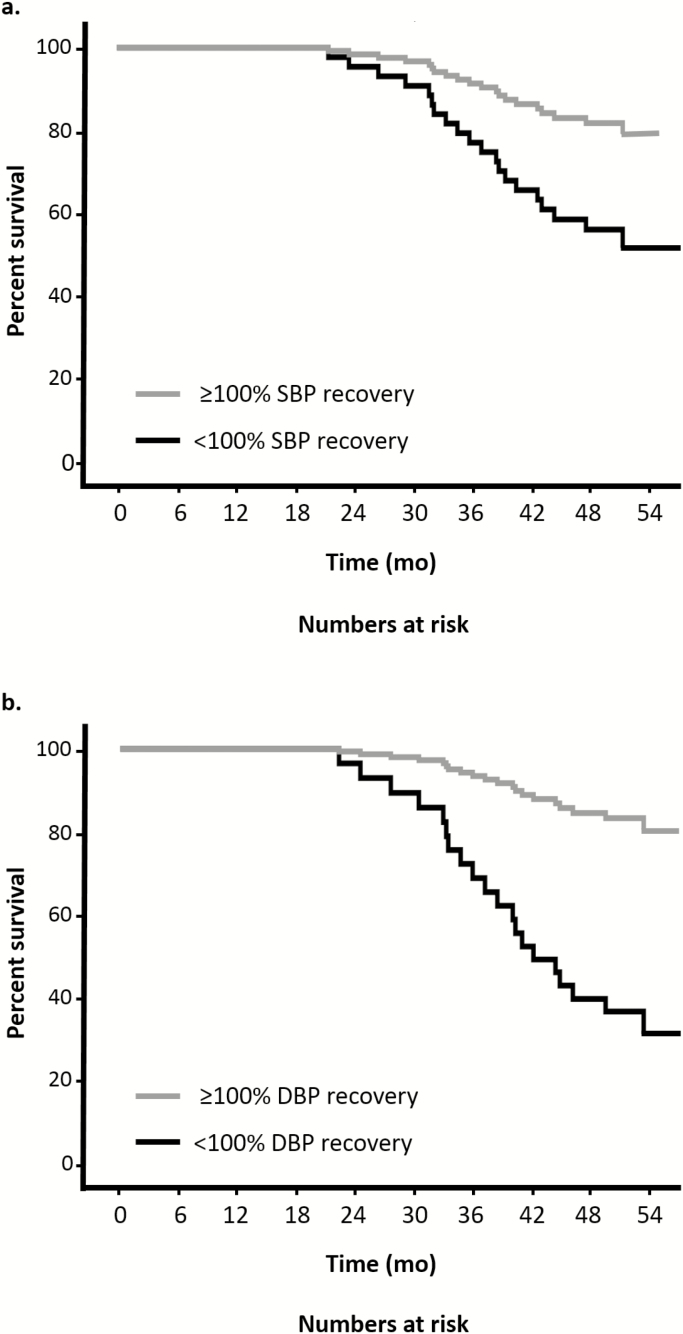
During the follow-up period after the trial ended, 21 participants (37.5%) died, of whom one participant had not performed the orthostatic challenge test at baseline. Participants who died had demonstrated greater ADAS-cog progression in the 1.5 years of active follow-up (mean difference [95% confidence interval]: 7.0 [1.6–12.3] points, p = .012). Table 3 shows the results of the Cox proportional hazard analyses together with the number of events in each group. An impaired BP recovery after 1 minute was associated with a higher mortality risk, for both SBP (adjusted HR [95% confidence interval]: 2.9 [1.1–7.8], p = .039) and DBP (adjusted HR [95% confidence interval]: 5.5 [1.9–16.1], p = .002). Neither a large initial drop in BP nor impaired BP recovery after 5 minutes was associated with a higher mortality risk. Figure 3 shows the adjusted cumulative survival curves for impaired and unimpaired BP recovery after 1 minute.
Table 3.
Results of Cox Proportional Hazard Analyses for All-Cause Mortality
| Events in Impaired Response | Events in Unimpaired Response | Hazard Ratio (95% CI) | p | |
|---|---|---|---|---|
| Initial SBP drop | 11/27 (40.7%) | 9/28 (32.1%) | 1.4 (0.5–3.6) | .506 |
| Initial DBP drop | 12/27 (44.4%) | 8/28 (28.6%) | 1.6 (0.6–3.9) | .328 |
| 1-min SBP recovery | 14/30 (46.7%) | 6/25 (24.0%) | 2.9 (1.1–7.8) | .039 |
| 1-min DBP recovery | 11/20 (55.0%) | 9/35 (25.7%) | 5.5 (1.9–16.1) | .002 |
| 5-min SBP recovery | 11/28 (39.3%) | 9/25 (36.0%) | 1.1 (0.5–2.8) | .794 |
| 5-min DBP recovery | 7/14 (50.0%) | 13/39 (33.3%) | 1.6 (0.6–4.2) | .337 |
Notes: CI = confidence interval; BP = blood pressure; DBP = diastolic blood pressure; SBP = systolic blood pressure. Adjusted for intervention group, age, and sex. For the initial drop, groups with a smaller or larger drop were compared (median split). For recovery, impaired recovery (<100% of sitting BP) was compared to unimpaired recovery (≥100% of sitting BP).
Figure 3.
Cox proportional hazards adjusted cumulative survival curves for unimpaired versus impaired systolic and diastolic blood pressure recovery after 1 min of standing. Adjusted for age, sex, and intervention group.
Discussion
This study presents an examination of the orthostatic challenge test in people with mild-to-moderate AD, who participated in a randomized controlled trial that investigated the effect of nilvadipine on cognitive decline after 1.5 years. In this post hoc analysis, we showed that orthostatic BP recovery was associated with the clinical prognosis in AD. Our main findings are that failure to recover to 100% of baseline BP after 1 minute of standing was associated with greater cognitive decline after 1.5 years and with all-cause mortality. The drop in BP observed immediately after standing was not associated with study outcomes. These results fit in the concept that slowing down of recovery and maladaptive responses to perturbations can be indicators of low physical resilience and loss of complexity in older adults and highlight the potential and relevance of an orthostatic challenge as a test of physical resilience (10,38).
Previous work on the orthostatic BP response in relation to cognitive function did not investigate progression in those with established AD. For example, Hayakawa and colleagues showed that less recovery after 30 seconds was a predictor for conversion from mild cognitive impairment to dementia (21). Also, the average BP recovery of four annual observations was lower in those with dementia when compared with controls (20). On the contrary, in the TILDA study, there was no association between BP recovery and 2-year change in cognition in healthy older adults (39). The latter might be explained by the short follow-up in a relatively healthy sample in combination with global cognitive testing. Our finding that failure to recover to baseline BP predicts all-cause mortality matches with previous work from our group performed in a falls clinic population (18). We now show that this finding also applies to older adults with dementia, thereby extending this observation to a larger part of the geriatric population.
What could explain our observations? The physiology behind the BP response upon active standing is complex. The immediate fall in BP upon standing is mainly the result of reduced venous return due to gravitational forces that cause a sudden shift of central blood volume into the lower trunk and extremities. At the same time, heart rate already starts to increase induced by an exercise reflex (40). Approximately 5 seconds after standing, a further increase in heart rate and an increase in vascular resistance are induced by sympathetic activation, through baroreflex-mediated autonomic regulation, in order to recover the fall in arterial pressure (41). Thus, impaired BP recovery could be related to reduced baroreflex function, specifically reduced sympathetic control of vasoconstriction and heart rate increase. Cardiac BRS was indeed lower in participants with an impaired BP recovery, although not statistically significant. The observations of a lower heart rate response during the recovery phase as well as a continuation of reduced BP recovery during prolonged standing also indicate that autonomic dysfunction may play a role in the pathophysiology. Autonomic dysfunction has been associated with neurodegenerative diseases, including AD (42). The cortical brain structures involved in autonomic regulation are affected by AD pathology in an early phase (43). Conversely, it has been hypothesized that impaired BP recovery contributes to cognitive decline by causing episodes of cerebral hypoperfusion, especially in the presence of impaired cerebral autoregulation. However, in our previous work, neither BRS nor cerebral autoregulation was impaired in AD compared with healthy controls (29).
Impaired BP recovery could also be a marker of vascular disease. Indeed, orthostatic hypotension is associated with an increased risk of cardiovascular and cerebrovascular events, which is independent of other vascular risk factors (44). Underlying vascular disease, identified by incomplete BP recovery, could explain both cognitive progression and increased mortality. Extending this hypothesis, failure to fully recover after standing may be an expression of increased BP variability during daily life, leading to high pulsatility in the brain with microvascular dysfunction as a possible consequence (45).
In our study, AD was diagnosed using clinical criteria, without amyloid biomarkers. This means that some participants may have had other dementia subtypes, including dementia with Lewy bodies (DLB). This might have been clinically diagnosed as AD, as the characteristic motor signs (hypokinetic rigidity) of DLB may be masked in early stages. DLB is associated with neurogenic orthostatic hypotension due to autonomic dysfunction (46). As disease progression and mortality risk may differ between AD and DLB (47), theoretically this could explain part of our observations. However, during the 1.5 years of trial participation, none of our participants developed clinical evidence of DLB.
In summary, a cogent physiological explanation currently remains speculative. Whether our findings are indeed the result of a bidirectional link with more neurodegeneration leading to impaired autonomic dysfunction, which in turns leads to more BP variability and cognitive decline needs to be confirmed in further studies. The same holds for whether it is possible to intervene in this pathway as a way to slow progression. Irrespective of the pathophysiology, our work has clear clinical relevance because it provides the field with an easily performable test to identify a higher risk of disease progression. The clinical course of AD is known to be very heterogenous and being able to identify factors associated with accelerated decline can help care providers and families of people with dementia (1).
Our study, a substudy of the larger Nilvad trial, is limited by its small sample size, hampering the addition of relevant covariates such as hypertension (48). However, our study is enhanced, also compared with the larger Nilvad trial, by the detailed and thorough BP measurements and characterization of participants, including cerebral imaging. Also, we had a complete follow-up for mortality status. ADAS-cog changes were missing for 16% of the participants. This was predominantly because of severe disease progression at follow-up. However, results were similar whether we included these participants as fast progressors or whether we excluded them from the analysis. At baseline, the fast progressors differed from the others in cognitive score and gray matter volume, both factors that could well be associated with faster progression. This underscores why we report impaired BP recovery to be associated with faster progression and not infer a causal relationship. A methodological consideration is the intervention with the calcium-channel blocker nilvadipine that was used in this trial and which may be a confounder in these analyses. However, we previously showed that lowering of BP with nilvadipine in the main Nilvad study did not increase orthostatic hypotension (49). In addition, the intervention had no effect on ADAS-cog scores (26), but in the substudy, we found an effect of nilvadipine on hippocampal cerebral blood flow (50). A particular strength of this study is the use of continuous beat-to-beat finger arterial BP measurements instead of the traditional arm-cuff-based approaches, which allows capturing transient information instead of steady-state measurements (51). The initial drop in BP upon standing in our study (±18/9 mm Hg) was smaller compared with previous studies, for example, in the normative data of people aged 70–79 years from TILDA (±40/25 mm Hg) (31). This is likely the result of studying the BP response using sitting instead of lying as a reference position, which reduces gravitational stress. In addition, we used the average of three sit-to-stand trials, rather than a single measurement as often done in other studies, which also contributes to observing less extreme values. Our sit-to-stand approach has the advantage of being more easily performed by an older population, making it a more feasible test for clinical practice. Furthermore, it is the most frequently occurring posture change during daily activities, making it a relevant test for daily function (15). Despite the smaller initial perturbation in BP, we still observed that the ability to recover BP after 1 minute was associated with cognitive decline and mortality. We did not have data on the presence of cerebral hypoperfusion symptoms upon standing and as such could not study the association with AD progression and initial orthostatic hypotension as defined by Wieling and colleagues (52).
In conclusion, we found evidence for the hypothesis that impaired BP recovery in the first minute after standing is associated with higher rate of cognitive decline and higher all-cause mortality in people with AD. Impaired BP recovery may be regarded as an easily obtained marker of reduced physical and cognitive resilience in dementia. Because of the small sample size and explorative nature of the study, no firm conclusions should be drawn before results have been confirmed in larger studies.
Supplementary Material
Acknowledgments
We are grateful to all study participants and their caregivers for their time and effort. The authors acknowledge the valuable assistance in data collection of William van Aalst, Angelina Santoso, Gerrita van Spijker, and Laura Versteeg. We thank Sanne Gijzel for sharing her vision on the concept of resilience and recovery in older people.
Funding
This work was supported by the Dutch Alzheimer Society (grant no. WE.09-2015-03) and the Alzheimer’s Drug Discovery Foundation (grant no. 20121210). The Nilvad study was funded by the European Commission Framework 7 Programme Health Theme collaborative project (grant no. 279093, awarded to B.L., Principal Investigator).
Conflict of Interest
None reported.
References
- 1. Melis RJF, Haaksma ML, Muniz-Terrera G. Understanding and predicting the longitudinal course of dementia. Curr Opin Psychiatry. 2019;32:123–129. doi: 10.1097/YCO.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haaksma ML, Rizzuto D, Leoutsakos JS, et al. Predicting cognitive and functional trajectories in people with late-onset dementia: 2 population-based studies. J Am Med Dir Assoc. 2019;20:1444–1450. doi: 10.1016/j.jamda.2019.03.025 [DOI] [PubMed] [Google Scholar]
- 3. Doraiswamy PM, Leon J, Cummings JL, Marin D, Neumann PJ. Prevalence and impact of medical comorbidity in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2002;57:M173–M177. doi: 10.1093/gerona/57.3.m173 [DOI] [PubMed] [Google Scholar]
- 4. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19. doi: 10.1186/1471-2318-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein FC, Levey AI, Steenland NK. High blood pressure and cognitive decline in mild cognitive impairment. J Am Geriatr Soc. 2013;61:67–73. doi: 10.1111/jgs.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mossello E, Pieraccioli M, Nesti N, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Internal Med. 2015;175:578–585. doi: 10.1001/jamainternmed.2014.8164 [DOI] [PubMed] [Google Scholar]
- 7. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/s0140-6736(10)60309-1 [DOI] [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/hyp.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 9. Gijzel SMW, Whitson HE, van de Leemput IA, et al. Resilience in clinical care: getting a grip on the recovery potential of older adults. J Am Geriatr Soc. 2019;67:2650–2657. doi: 10.1111/jgs.16149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi: 10.1093/gerona/57.3.b115 [DOI] [PubMed] [Google Scholar]
- 11. Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129:666–670. doi: 10.1016/j.mad.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. doi: 10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol. 2015;11:220–226. doi: 10.3988/jcn.2015.11.3.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frith J, Parry SW. New horizons in orthostatic hypotension. Age Ageing. 2017;46:168–174. doi: 10.1093/ageing/afw211 [DOI] [PubMed] [Google Scholar]
- 15. Dall PM, Kerr A. Frequency of the sit to stand task: an observational study of free-living adults. Appl Ergon. 2010;41:58–61. doi: 10.1016/j.apergo.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 16. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. doi: 10.1093/eurheartj/ehp329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCrory C, Berkman LF, Nolan H, O’Leary N, Foley M, Kenny RA. Speed of heart rate recovery in response to orthostatic challenge. Circ Res. 2016;119:666–675. doi: 10.1161/CIRCRESAHA.116.308577 [DOI] [PubMed] [Google Scholar]
- 18. Lagro J, Schoon Y, Heerts I, et al. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69:471–478. doi: 10.1093/gerona/glt111 [DOI] [PubMed] [Google Scholar]
- 19. Frewen J, Finucane C, Savva GM, Boyle G, Kenny RA. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol A Biol Sci Med Sci. 2014;69:878–885. doi: 10.1093/gerona/glt171 [DOI] [PubMed] [Google Scholar]
- 20. O’Hare C, Kenny RA, Aizenstein H, et al. ; Health ABC Study Cognitive status, gray matter atrophy, and lower orthostatic blood pressure in older adults. J Alzheimers Dis. 2017;57:1239–1250. doi: 10.3233/JAD-161228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayakawa T, McGarrigle CA, Coen RF, et al. Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J Am Geriatr Soc. 2015;63:1868–1873. doi: 10.1111/jgs.13596 [DOI] [PubMed] [Google Scholar]
- 22. Meulenbroek O, O’Dwyer S, de Jong D, et al. European multicentre double-blind placebo-controlled trial of nilvadipine in mild-to-moderate Alzheimer’s disease – the substudy protocols: NILVAD frailty; NILVAD blood and genetic biomarkers; NILVAD cerebrospinal fluid biomarkers; NILVAD cerebral blood flow. BMJ Open. 2016;6:e011584. doi: 10.1136/bmjopen-2016-011584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawlor B, Segurado R, Kennelly S, et al. ; NILVAD Study Group Nilvadipine in mild to moderate Alzheimer disease: a randomised controlled trial. PLoS Med. 2018;15:e1002660. doi: 10.1371/journal.pmed.1002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 25. Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawlor B, Kennelly S, O’Dwyer S, et al. NILVAD protocol: a European multicentre double-blind placebo-controlled trial of nilvadipine in mild-to-moderate Alzheimer’s disease. BMJ Open. 2014;4:e006364. doi: 10.1136/bmjopen-2014-006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res. 1998;38:605–616. doi: 10.1016/s0008-6363(98)00067-4 [DOI] [PubMed] [Google Scholar]
- 28. Guelen I, Westerhof BE, van der Sar GL, et al. Validation of brachial artery pressure reconstruction from finger arterial pressure. J Hypertens. 2008;26:1321–1327. doi: 10.1097/HJH.0b013e3282fe1d28 [DOI] [PubMed] [Google Scholar]
- 29. de Heus RAA, de Jong DLK, Sanders ML, et al. Dynamic regulation of cerebral blood flow in patients with Alzheimer disease. Hypertension. 2018;72:139–150. doi: 10.1161/hypertensionaha.118.10900 [DOI] [PubMed] [Google Scholar]
- 30. van der Velde N, van den Meiracker AH, Stricker BH, van der Cammen TJ. Measuring orthostatic hypotension with the Finometer device: is a blood pressure drop of one heartbeat clinically relevant? Blood Press Monit. 2007;12:167–171. doi: 10.1097/MBP.0b013e3280b083bd [DOI] [PubMed] [Google Scholar]
- 31. Finucane C, O’Connell MD, Fan CW, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831 [DOI] [PubMed] [Google Scholar]
- 32. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 33. Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T; Alzheimer’s Disease Working Group Disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement. 2010;6:39–53. doi: 10.1016/j.jalz.2009.05.665 [DOI] [PubMed] [Google Scholar]
- 34. Stergiou GS, Giovas PP, Gkinos CP, Patouras JD. Validation of the Microlife WatchBP Home device for self home blood pressure measurement according to the International Protocol. Blood Press Monit. 2007;12:185–188. doi: 10.1097/MBP.0b013e3280b083ce [DOI] [PubMed] [Google Scholar]
- 35. Parati G, Stergiou GS, Asmar R, et al. ; ESH Working Group on Blood Pressure Monitoring European society of hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66 [DOI] [PubMed] [Google Scholar]
- 36. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 37. Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471 [DOI] [PubMed] [Google Scholar]
- 38. Olde Rikkert MGM, Melis RJF. Rerouting geriatric medicine by complementing static frailty measures with dynamic resilience indicators of recovery potential. Front Physiol. 2019;10:723. doi: 10.3389/fphys.2019.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feeney J, O’Leary N, Kenny RA. Impaired orthostatic blood pressure recovery and cognitive performance at two-year follow up in older adults: the Irish Longitudinal Study on Ageing. Clin Auton Res. 2016;26:127–133. doi: 10.1007/s10286-016-0340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol. 1982;243:H676–H681. doi: 10.1152/ajpheart.1982.243.5.H676 [DOI] [PubMed] [Google Scholar]
- 41. van Wijnen VK, Finucane C, Harms MPM, et al. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med. 2017;282:468–483. doi: 10.1111/joim.12636 [DOI] [PubMed] [Google Scholar]
- 42. Femminella GD, Rengo G, Komici K, et al. Autonomic dysfunction in Alzheimer’s disease: tools for assessment and review of the literature. J Alzheimers Dis. 2014;42:369–377. doi: 10.3233/JAD-140513 [DOI] [PubMed] [Google Scholar]
- 43. Royall DR, Gao JH, Kellogg DL Jr. Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses. 2006;67:747–758. doi: 10.1016/j.mehy.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 44. Xin W, Mi S, Lin Z, Wang H, Wei W. Orthostatic hypotension and the risk of incidental cardiovascular diseases: a meta-analysis of prospective cohort studies. Prev Med. 2016;85:90–97. doi: 10.1016/j.ypmed.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 45. de Heus R, Olde Rikkert M, Tully PJ, Lawlor B, Claassen J. Blood pressure variability and progression of clinical Alzheimer’s disease. Hypertension. 2019;74(5):1172–1180. doi: 10.1161/HYPERTENSIONAHA.119.13664 [DOI] [PubMed] [Google Scholar]
- 46. Thaisetthawatkul P, Boeve BF, Benarroch EE, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004;62:1804–1809. doi: 10.1212/01.wnl.0000125192.69777.6d [DOI] [PubMed] [Google Scholar]
- 47. Price A, Farooq R, Yuan JM, Menon VB, Cardinal RN, O’Brien JT. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open. 2017;7:e017504. doi: 10.1136/bmjopen-2017-017504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McNicholas T, Tobin K, Carey D, O’Callaghan S, Kenny RA. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4-year follow-up? Data from TILDA (The Irish Longitudinal Study on Ageing). J Am Heart Assoc. 2018;7:e008976. doi: 10.1161/JAHA.118.008976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Heus RAA, Donders R, Santoso AMM, Olde Rikkert MGM, Lawlor BA, Claassen JAHR; Nilvad Study Group Blood pressure lowering with nilvadipine in patients with mild-to-moderate Alzheimer disease does not increase the prevalence of orthostatic hypotension. J Am Heart Assoc. 2019;8:e011938. doi: 10.1161/JAHA.119.011938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Jong DLK, de Heus RAA, Rijpma A, et al. Effects of nilvadipine on cerebral blood flow in patients with Alzheimer disease. Hypertension. 2019;74:413–420. doi: 10.1161/hypertensionaha.119.12892 [DOI] [PubMed] [Google Scholar]
- 51. Finucane C, van Wijnen VK, Fan CW, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res. 2019;29:427–441. doi: 10.1007/s10286-019-00606-y [DOI] [PubMed] [Google Scholar]
- 52. Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond). 2007;112:157–165. doi: 10.1042/CS20060091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.