Abstract
Nut intake has been associated with reduced total cancer-related mortality, but evidence for colorectal cancer (CRC) risk is inconclusive. We investigated the associations between nut and peanut butter intake and anatomical CRC subtypes. To account for molecular heterogeneity, associations between nut and peanut butter intake and colorectal tumors harboring APC, KRAS or BRAF mutations, p53 overexpression or microsatellite instability were examined in secondary analyses. In the Netherlands Cohort Study (n = 120 852), lifestyle habits were measured with a questionnaire in 1986. After 20.3 years follow-up, 3567 CRC cases were included in case–cohort analyses. For the analyses of molecular CRC subtypes, 574 cases were included after 7.3 years follow-up. In categorical analyses, total nut intake was not significantly associated with CRC [HR (95% CI) 10+ g/day versus non-consumers = 0.94(0.78–1.15) in men; 0.96(0.75–1.22) in women]. In restricted cubic spline analyses, significant non-linear inverse associations with rectal cancer were observed for total nut, peanut and peanut butter intake in women, and borderline significant non-linear inverse associations for total nut and peanut intake in men. Regarding the molecular CRC subtypes, peanut butter intake was significantly associated with an increased risk of colorectal tumors that did not develop through the serrated neoplasia pathway in men [HR (95% CI) per 5 g/day increment = 1.22(1.07–1.38)]. Nut and peanut butter intake are non-linearly inversely associated with rectal cancer risk in women. In men, nut intake is borderline significantly non-linearly associated with a reduced rectal cancer risk. Peanut butter is associated with an increased risk of colorectal tumors that do not develop through the serrated neoplasia pathway in men.
Nut and peanut butter intake are inversely associated with rectal cancer risk in women, and nut intake potentially in men. Peanut butter intake might increase the risk of CRC-tumors that do not develop through the serrated neoplasia pathway in men.
Introduction
In 2018, colorectal cancer (CRC) was estimated to be the third most common cancer worldwide, accounting for 10.2% of all new cancer cases, and the second most frequent cause of cancer-related mortality (1). The global incidence of CRC has been estimated to rise to more than 2.2 million new cases and 1.1 million deaths by 2030, which is partly caused by changes in the prevalence of lifestyle factors with more people adopting a more Western diet and lifestyle (2).
Nuts represent a food group that has been studied for its potential cancer-chemopreventive activities, because recent studies have shown that nut consumption is inversely associated with total cancer-related mortality (3). Nuts contain numerous bioactive compounds like vitamins B6 and E, folate, selenium, fiber, mono- and polyunsaturated fatty acids and polyphenols. The nutritional composition differs per nut subtype (4,5). Possible mechanisms by which nuts might reduce CRC risk relate to their antioxidant activities and anti-inflammatory effects (4,6,7). Bioactive compounds in nuts might contribute to normal cell differentiation and DNA repair mechanisms, reduced tumor initiation, promotion and angiogenesis, and induced apoptosis (6,7). Fiber in nuts increase fecal volume, dilute fecal carcinogens, and decrease the intestinal transit time, thereby reducing the contact between carcinogens and the intestinal lining (7,8). However, the exact working mechanisms and the differential effects of nut subtypes have yet to be elucidated.
To our knowledge, eight prospective cohort studies (9–16) and eight case–control studies (17–24) have investigated the relation between nut and peanut butter intake and CRC risk. Two cohort studies found no significant associations between nut intake and CRC risk in both sexes (10,11). In men, five cohort studies observed no significant associations (9,11,13–15). In women, one cohort study found significant inverse associations of nut intake with colon and distal colon cancer, but not with colorectal, proximal colon and rectal cancer (11). Another cohort study in women observed significant inverse relations between peanut intake and CRC risk (13), and a third observed significant inverse relations between total nut intake and colon cancer risk (16). Conversely, two cohort studies found no relations in women (12,14,15). No cohort studies were performed on peanut butter. Results from the case–control studies were contradictive as well (17–24).
The contradicting results might partly be explained by the fact that CRC is a heterogeneous disease with several molecular subtypes, each characterized by certain (epi)genetic abnormalities (25). There are minimally two hypothesized pathways to CRC development. The traditional adenoma-carcinoma pathway accounts for 60–90% of all sporadic CRC tumors, which are more often observed in men and the distal colon, and is characterized by chromosomal instability (25). Mutations in the Adenomatous Polyposis Coli (APC) tumor suppressor gene often occur early in this pathway, whereas mutations in the Kirsten ras (KRAS) proto-oncogene and in TP53 are common later events (25). The serrated neoplasia pathway accounts for 10–30% of all sporadic CRC tumors, which are more frequently observed in women and the proximal colon, and is characterized by microsatellite instability (MSI) (25). Mutations in the B-RAF proto-oncogene serine/threonine kinase (BRAF) are early events in this pathway (25).
Diet and lifestyle may play important roles in causing mutations and epigenetic alterations, and can influence tumor growth in tissues that already underwent (epi)genetic changes (25). Therefore, they might be associated with molecular characteristics in CRC. If such associations are observed, this may point to distinct underlying molecular pathways linking diet and lifestyle to cancer and to distinct etiologies of molecular CRC subtypes. This will strengthen the evidence-based needed for prevention.
In previous studies, several nut components have been found to be associated with the molecular CRC subtypes (25). High intake of polyunsaturated fat, especially linoleic acid, has been positively associated with mutated KRAS colorectal tumors (26). In contrast, (marine) omega-3 polyunsaturated fatty acid intake was found to be associated with a lower risk of MSI-high tumors, but not MS-stable (MSS) tumors (27). Regarding folate intake, a positive associations has been found with mutated BRAF colorectal tumors (28), but high folate consumption has also been associated with a reduced risk of mutated KRAS rectal tumors in men (29) and wild-type APC colon tumors, while being positively associated with mutated APC colon tumors in men (30). However, there is no prospective evidence for associations between nut or peanut butter intake and molecular CRC subtypes.
As primary aim, we investigated the associations between total nut, tree nut, peanut and peanut butter intake and the risk of anatomical CRC subtypes in men and women, using data from the Netherlands Cohort Study (NLCS) with 20.3 years of follow-up. As secondary aim, we performed exploratory and hypothesis-generating analyses to examine the associations between nut intake and molecular CRC subtypes, characterized by mutational status of APC, KRAS, BRAF and MSI and p53-status, using an existing database of molecular tumor characteristics of CRC cases who were diagnosed during the first 7.3 years of follow-up in the NLCS.
Materials and methods
The NLCS is a population-based prospective cohort study in the Netherlands, which started on 17 September 1986 (31). In total, 120 852 men and women aged 55–69 years at baseline were included. A case–cohort approach was applied to improve the efficiency of the follow-up and data processing. A subcohort of 5000 participants was randomly sampled from the entire cohort directly after baseline. Cases were obtained from the entire cohort, whereas person-time was estimated in the subcohort as an estimate of the follow-up time in the total cohort.
In September 1986, participants filled in a mailed, self-administered baseline questionnaire on diet, lifestyle habits and other cancer risk factors. This questionnaire included a validated semi-quantitative 150-item food frequency questionnaire (FFQ) that covered information on dietary habits in the year preceding baseline (32). By filling in and returning the baseline questionnaire, participants agreed to participate in the NLCS. The NLCS was conducted in agreement with the Declaration of Helsinki. The institutional review boards of the Netherlands Organization for Applied Scientific Research TNO (Zeist) and Maastricht University (Maastricht) approved the NLCS.
The entire cohort was followed-up for cancer incidence until 31 December 2006 by record linkage to the Dutch Pathology Registry (PALGA) and Dutch Cancer Registry (33), providing a coverage of approximately 96% (34). The subcohort was followed-up biennially for data on vital status, which was estimated to be 100% complete.
Study population
In our primary analyses, the study population consisted of subcohort members and incident microscopically confirmed CRC cases diagnosed during 20.3 years (baseline until December 2006) of follow-up. Participants were excluded if they reported prevalent cancer (excluding skin cancer) at baseline, if they had inconsistent or incomplete dietary data, or if they had missing data on potential confounding variables.
For the anatomical analyses, 3567 CRC cases (ICD-O-3 codes: C18–20) remained eligible after applying the inclusion and exclusion criteria, including 2483 colon cancer cases (C18) and 752 rectal cancer cases (C20) (Supplementary Figure S1 is available at Carcinogenesis Online). Of the colon cancer cases, 1292 were categorized as proximal (C18.0–18.4) and 1120 as distal colon cancer cases (C18.5–18.7). Malignant tumors of the rectosigmoid junction (C19) were only included in the overall CRC analyses, because of the higher risk of misclassification.
For the molecular analyses, paraffin-embedded tumor tissue samples of 732 CRC cases detected during 7.3 years of follow-up (September 1986–December 1993) were collected and analyzed as described in (35). Because of incomplete nationwide coverage by PALGA in the earlier years, the first 2.3 years of follow-up were excluded. The number of CRC cases per molecular subtype are presented in Supplementary Figure S2, available at Carcinogenesis Online.
APC, KRAS and BRAF mutations
The methods of DNA isolation, PCR and sequencing have been described elsewhere (35–37). For the APC gene, tumor material was analyzed for mutations in the mutation cluster region spanning codons 1286–1520 of exon 15. For the KRAS oncogene, codons 12–13 of exon 1 were analyzed, and for the BRAF gene the V600E BRAF mutation in exon 15. For the APC and KRAS genes, a nested PCR method was performed, followed by direct sequencing of purified segments (35,36). For the BRAF gene, a semi-nested PCR and restriction fragment length polymorphism analyses were performed (37).
Microsatellite instability
MSI was determined by a pentaplex PCR using the MSI markers BAT-26, BAT-25, NR-21, NR-22 and NR-24 (38). Tumors were classified as MS-unstable (MSI) if ≥2 markers showed instability and as MSS if ≤1 of the markers showed instability.
TP53 expression
Immunohistochemical staining of p53 was performed using the avidin–biotin–peroxidase complex method, with the DO-7 mouse monoclonal antibody (DAKO A/S, Denmark) (39). Immunostained slides and negative controls were evaluated semi-quantitatively and independently by two observers without knowledge of clinical parameters. Cases were positive for TP53 overexpression if ≥20% of the tumor cell nuclei showed positive staining with the antibody (39).
Exposure measurement
The baseline questionnaire measured lifestyle habits, dietary intakes and other cancer risk factors. In the 150-item FFQ, three items covered intake of ‘peanuts’, ‘other, mixed nuts’ (tree nuts) and ‘peanut butter’ in the preceding year. Participants filled in the intake frequencies and number of portion sizes they consumed per intake. Intake frequencies could range from ‘never or <1×/month’ to ‘6–7×/week’. Assumed standard portion sizes were 28 g for peanuts and tree nuts and 15 g per slice of bread for peanut butter (32). Mean daily intakes were calculated by multiplying intake frequencies and portion sizes. Total nut intake was the sum of tree nut and peanut intake. Peanut butter intake was not included in total nut intake, because its nutrient composition differs from that of nuts (5). NLCS personnel was blinded to the case/subcohort status of participants during the entry, coding and interpretation of the questionnaire data.
Statistical analysis
Age- and multivariable-adjusted Cox regression analyses were performed for men and women separately. In our primary analyses, we investigated the relation between total nut intake and CRC risk. The analyses were also performed for tree nut, peanut and peanut butter intake and for colon, proximal colon, distal colon and rectal cancer. Person-years in the subcohort were calculated from baseline (September 1986) until CRC diagnosis, loss to follow-up, death, migration or end of follow up (December 2006), whichever came first.
The proportional hazards assumption was tested using scaled Schoenfeld residuals (40) and by visually inspecting log-minus-log survival plots. Because a potential violation was observed for age, a time-varying covariate was included for age. Additional variance introduced by sampling the subcohort from the full cohort was taken into account by using the robust Huber–White sandwich estimator (41).
Total nut, tree nut, peanut and peanut butter intakes were analyzed on a categorical and continuous scale. Total nut and peanut intake were categorized into 0, 0.1–<5, 5–<10 and 10+ g/day, and tree nut and peanut butter intake into 0, 0.1–<5 and 5+ g/day because of the smaller number of participants in the higher intake categories. Non-consumers formed the reference category. Linear trends were evaluated by assigning median intakes in the subcohort to the intake categories, fitting these as continuous variables in regression models, and performing Wald tests. In continuous analyses, increments of 5 g/day were analyzed.
Predefined confounders included age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (<18.5/18.5–<25/25–<30/30+ kg/m2), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level [primary or lower vocational education (low)/secondary or medium vocational education (medium)/higher vocational education or university (high)], family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (0/0.1–<5/5–<15/15–<30/30+ g/day). Cigarette smoking frequency and duration were centered to reduce multicollinearity between the smoking variables (42). Potential confounders included height, consumption of fruits, vegetables, whole grains, red meat, processed meat, fish, dairy products, cheese and coffee, nutritional supplement use, post-menopausal hormone replacement therapy (in women), long-term use (>6 months) of non-steroidal anti-inflammatory drugs (including aspirin), history of chronic bowel disease and history of diabetes. Predefined confounders were included in the multivariable-adjusted models irrespective of their effect on the HRs. None of the potential confounders changed the HRs with ≥10% when using a backward selection procedure, so only the predefined confounders were included in the final model.
In nutritional epidemiologic research, linear relations are uncommon because the capacity for absorbing, transporting and metabolizing dietary factors is often limited (43). Therefore, we tested for non-linearity using restricted cubic spline analyses with three fixed knots at 0, 5 and 10 g/day. In sensitivity analyses, this model was compared with models with different knot positions or additional knots using the Akaike Information Criterion score (44).
Heterogeneity in the associations between nut intake and the risk of anatomical CRC subtypes was tested using a competing risk procedure (28), which estimates standard errors using a bootstrapping method (1000 replications) especially designed for the case–cohort design (45).
Stratified analyses were performed to investigate associations between total nut intake and colon and rectal cancer risk across levels of BMI (18.5–<25,/25+ kg/m2), non-occupational physical activity (≤30/30–≤60/60–≤90/>90 min/day), cigarette smoking status (never/former/current), alcohol consumption (0/0.1–<5/≥5 g/day), educational level (low/medium/high) and family history of CRC (no/yes) to exploratively investigate potential effect modification. Participants with a BMI <18.5 kg/m2 were excluded from the interaction analyses, because of the low number of participants in this category. The two highest intake categories were merged to increase statistical power. Interactions were tested by including cross-product terms in the Cox regression models and performing Wald tests.
In sensitivity analyses, we mutually adjusted tree nut, peanut and peanut butter intakes and we additionally adjusted for the alternate Mediterranean diet (aMED) score excluding alcohol and nuts (46). Potential reversed causation was examined by comparing the median total nut intake of cases diagnosed during the first 2 years of follow-up to the median intakes of cases diagnosed later in time, and by excluding the first 2 years of follow-up. Moreover, we restricted the peanut butter analyses to participants with a self-reported constant peanut butter intake in the 5 years before baseline. This information was obtained from the FFQ, in which participants were asked whether they used more, less, or just as much peanut butter 5 years before baseline as they did at baseline. This information was collected for peanut butter intake, but unfortunately not for tree nuts and peanuts.
Two-sided P-values < 0.05 were considered statistically significant. All analyses were performed in Stata 14 software (StataCorp.2015. College Station, TX, USA).
Molecular subtypes
In the molecular analyses, we investigated the relation between nut and peanut butter intake and the following molecular CRC endpoints: the presence of truncating APC mutations (no/yes); activating KRAS mutations (no/yes); p53 overexpression (no/yes); BRAF mutations (no/yes) and MSI (no/yes). The relation between nut intake and CRC developed through the traditional adenoma–carcinoma pathway was investigated by examining truncating APC mutations and/or activating KRAS mutations and/or p53 overexpression as combined endpoint. BRAF mutations and/or MSI were combined as marker of the serrated neoplasia pathway. Unfortunately, the molecular analyses could not be performed for colon or rectal cancer separately, because of the small number of cases and the skewed distribution of nut intake. We did not combine men and women, because the confounder patterns and the relation between nut intake and cancer risk differed between the sexes in previous studies (11,19).
In the molecular analyses, the same statistical approach was used as in the primary analyses. However, the follow-up period ran until December 1993 and excluded the first 2.3 years. Also, nut intake categories were merged and alcohol intake and BMI were included as continuous covariates because of the lower case numbers. There were no violations of the PH assumption, so no time-varying covariates were included in the models. Moreover, the P-values of the trend tests and continuous analyses were adjusted for multiple testing using the method of Benjamini–Hochberg (47), correcting for 44 tests per sex in the analyses of the individual molecular subtypes (Tables 3 and 4) and for 16 tests per sex in the analyses of the traditional adeno-carcinoma and serrated neoplasia pathways (Table 5). This correction was only done in the analyses of the molecular subtypes because of their explorative nature, the limited number of cases and the resulting less stable estimates.
Table 3.
Multivariable-adjusted HRs and 95% CI for the relation between nut and peanut butter intake and the risk of molecular subtypes of CRC in men; NLCS, 1986–1993, excluding the first 2.3 years of follow-up
| Total nuts (g/day) | Tree nuts (g/day) | Peanuts (g/day) | Peanut butter (g/day) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.1–<5/0.1+ | 5–<10/5+ | 10+ | P-trend/FDR-adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1–<5/0.1+ | 5+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1–<5/0.1+ | 5+ | P-trend/FDR-adjusted P-trendb | Per 5 g/day increment | |
| Person-years in subcohort | 2592 | 2767/5897 | 1115/3129 | 2014 | 6175 | 2314 | 2913 | 3001/5576 | 2575 | 6082 | 1458/2407 | 949 | ||||||||
| Total CRC | ||||||||||||||||||||
| N cases | 115 | 103 | 39 | 55 | 240 | 72 | 124 | 114 | 74 | 226 | 41 | 45 | ||||||||
| HRa | 1 | 0.93 | 0.90 | 0.65 | 0.060/ | 0.97 | 1 | 0.84 | 0.236/ | 0.91 | 1 | 1.02 | 0.76 | 0.072/ | 0.98 | 1 | 0.83 | 1.44 | 0.057/ | 1.14 |
| 95% CI | ref | 0.69–1.27 | 0.59–1.37 | 0.45–0.95 | 0.352 | 0.92–1.03 | ref | 0.62–1.12 | 0.528 | 0.71–1.17 | ref | 0.76–1.37 | 0.54–1.06 | 0.352 | 0.92–1.04 | ref | 0.57–1.19 | 0.99–2.09 | 0.352 | 1.02–1.29 |
| APC wild-type | ||||||||||||||||||||
| N cases | 73 | 68 | 61 | 154 | 48 | 77 | 76 | 49 | 142 | 31 | 29 | |||||||||
| HRa | 1 | 1.01 | 0.79 | 0.169/ | 0.99 | 1 | 0.88 | 0.481/ | 0.92 | 1 | 1.15 | 0.84 | 0.264/ | 1.00 | 1 | 0.99 | 1.48 | 0.090/ | 1.12 | |
| 95% CI | ref | 0.70–1.46 | 0.53–1.16 | 0.487 | 0.93–1.06 | ref | 0.62–1.25 | 0.705 | 0.71–1.20 | ref | 0.81–1.63 | 0.56–1.27 | 0.528 | 0.93–1.07 | ref | 0.65–1.52 | 0.94–2.33 | 0.396 | 0.97–1.30 | |
| Truncating APC mutation | ||||||||||||||||||||
| N cases | 42 | 35 | 33 | 86 | 24 | 47 | 38 | 25 | 84 | 10 | 16 | |||||||||
| HRa | 1 | 0.81 | 0.67 | 0.169/ | 0.93 | 1 | 0.76 | 0.269/ | 0.89 | 1 | 0.83 | 0.62 | 0.113/ | 0.93 | 1 | 0.55 | 1.36 | 0.290/ | 1.18 | |
| 95% CI | ref | 0.50–1.31 | 0.40–1.13 | 0.487 | 0.83–1.04 | ref | 0.47–1.23 | 0.528 | 0.54–1.47 | ref | 0.52–1.32 | 0.36–1.09 | 0.440 | 0.82–1.05 | ref | 0.28–1.07 | 0.76–2.42 | 0.532 | 0.99–1.40 | |
| P-hetero-geneity | 0.922 | 0.681 | 0.724 | 0.319 | ||||||||||||||||
| KRAS wild-type | ||||||||||||||||||||
| N cases | 78 | 72 | 28 | 32 | 158 | 52 | 83 | 80 | 47 | 147 | 31 | 32 | ||||||||
| HRa | 1 | 0.94 | 0.95 | 0.54 | 0.043/ | 0.94 | 1 | 0.90 | 0.549/ | 0.92 | 1 | 1.05 | 0.70 | 0.054/ | 0.94 | 1 | 0.95 | 1.55 | 0.047/ | 1.15 |
| 95% CI | ref | 0.66–1.34 | 0.58–1.54 | 0.34–0.87 | 0.352 | 0.87–1.02 | ref | 0.64–1.27 | 0.724 | 0.69–1.22 | ref | 0.75–1.48 | 0.47–1.06 | 0.352 | 0.87–1.02 | ref | 0.62–1.45 | 1.00–2.40 | 0.352 | 1.01–1.32 |
| Activating KRAS mutation | ||||||||||||||||||||
| N cases | 37 | 31 | 11 | 23 | 82 | 20 | 41 | 34 | 27 | 79 | 10 | 13 | ||||||||
| HRa | 1 | 0.92 | 0.81 | 0.91 | 0.630/ | 1.02 | 1 | 0.71 | 0.179/ | 0.89 | 1 | 0.95 | 0.87 | 0.642/ | 1.03 | 1 | 0.59 | 1.21 | 0.557/ | 1.12 |
| 95% CI | ref | 0.55–1.55 | 0.39–1.66 | 0.51–1.62 | 0.724 | 0.94–1.11 | ref | 0.43–1.17 | 0.487 | 0.57–1.39 | ref | 0.58–1.56 | 0.51–1.51 | 0.724 | 0.95–1.12 | ref | 0.30–1.17 | 0.65–2.25 | 0.724 | 0.90–1.40 |
| P-hetero-geneity | 0.466 | 0.323 | 0.613 | 0.386 | ||||||||||||||||
| No p53 over-expression | ||||||||||||||||||||
| N cases | 45 | 44 | 19 | 20 | 98 | 30 | 50 | 49 | 29 | 96 | 16 | 16 | ||||||||
| HRa | 1 | 1.05 | 1.12 | 0.60 | 0.276/ | 0.94 | 1 | 0.89 | 0.605/ | 0.89 | 1 | 1.11 | 0.72 | 0.149/ | 0.94 | 1 | 0.78 | 1.18 | 0.570/ | 1.01 |
| 95% CI | ref | 0.67–1.63 | 0.62–2.04 | 0.33–1.10 | 0.528 | 0.85–1.03 | ref | 0.58–1.38 | 0.724 | 0.60–1.32 | ref | 0.73–1.68 | 0.43–1.22 | 0.487 | 0.84–1.04 | ref | 0.44–1.36 | 0.66–2.09 | 0.724 | 0.80–1.27 |
| p53 over-expression | ||||||||||||||||||||
| N cases | 68 | 58 | 20 | 34 | 138 | 42 | 72 | 64 | 44 | 129 | 23 | 28 | ||||||||
| HRa | 1 | 0.87 | 0.78 | 0.69 | 0.120/ | 1.00 | 1 | 0.83 | 0.323/ | 0.93 | 1 | 0.97 | 0.79 | 0.239/ | 1.00 | 1 | 0.80 | 1.59 | 0.045/ | 1.21 |
| 95% CI | ref | 0.59–1.28 | 0.46–1.35 | 0.44–1.10 | 0.440 | 0.93–1.07 | ref | 0.57–1.20 | 0.547 | 0.70–1.25 | ref | 0.67–1.42 | 0.51–1.20 | 0.528 | 0.93–1.08 | ref | 0.50–1.29 | 1.01–2.52 | 0.352 | 1.06–1.38 |
| P-hetero-geneity | 0.669 | 0.985 | 0.871 | 0.746 | ||||||||||||||||
| BRAF wild-type | ||||||||||||||||||||
| N cases | 94 | 82 | 75 | 195 | 56 | 102 | 90 | 59 | 182 | 69 | ||||||||||
| HRa | 1 | 0.90 | 0.71 | 0.056/ | 0.97 | 1 | 0.80 | 0.188/ | 0.93 | 1 | 0.97 | 0.72 | 0.070/ | 0.98 | 1 | 1.07 | 0.668/ | 1.20 | ||
| 95% CI | ref | 0.65–1.26 | 0.50–1.01 | 0.352 | 0.91–1.04 | ref | 0.57–1.12 | 0.487 | 0.71–1.21 | ref | 0.71–1.33 | 0.50–1.05 | 0.352 | 0.91–1.05 | ref | 0.79–1.46 | 0.724 | 1.06–1.35 | ||
| BRAF mutation | ||||||||||||||||||||
| N cases | 13 | 17 | 14 | 30 | 14 | 14 | 20 | 10 | 34 | 10 | ||||||||||
| HRa | 1 | 1.42 | 0.98 | 0.677/ | 0.93 | 1 | 1.24 | 0.501/ | 0.97 | 1 | 1.62 | 0.89 | 0.446/ | 0.92 | 1 | 0.76 | 0.468/ | 0.81 | ||
| 95% CI | ref | 0.65–3.09 | 0.43–2.26 | 0.724 | 0.83–1.04 | ref | 0.67–2.30 | 0.711 | 0.63–1.51 | ref | 0.76–3.47 | 0.37–2.13 | 0.701 | 0.81–1.05 | ref | 0.37–1.58 | 0.705 | 0.48–1.38 | ||
| P-hetero-geneity | 0.573 | 0.156 | 0.455 | 0.513 | ||||||||||||||||
| MSS | ||||||||||||||||||||
| N cases | 88 | 155 | 187 | 56 | 95 | 148 | 182 | 61 | ||||||||||||
| HRa | 1 | 0.86 | 0.312/ | 0.97 | 1 | 0.82 | 0.242/ | 0.86 | 1 | 0.93 | 0.611/ | 0.98 | 1 | 0.95 | 0.735/ | 1.17 | ||||
| 95% CI | ref | 0.64–1.16 | 0.547 | 0.91–1.04 | ref | 0.59–1.14 | 0.528 | 0.64–1.17 | ref | 0.69–1.25 | 0.724 | 0.92–1.05 | ref | 0.69–1.30 | 0.752 | 1.03–1.34 | ||||
| MSI | ||||||||||||||||||||
| N cases | 12 | 18 | 22 | 8 | 13 | 17 | 20 | 10 | ||||||||||||
| HRa | 1 | 0.79 | 0.594/ | 0.96 | 1 | 1.07 | 0.879/ | 1.23 | 1 | 0.84 | 0.691/ | 0.90 | 1 | 1.37 | 0.405/ | 1.02 | ||||
| 95% CI | ref | 0.34–1.87 | 0.724 | 0.77–1.19 | ref | 0.45–2.51 | 0.879 | 0.93–1.63 | ref | 0.36–1.96 | 0.723 | 0.67–1.21 | ref | 0.65–2.89 | 0.660 | 0.74–1.40 | ||||
| P-hetero-geneity | 0.691 | 0.689 | 0.671 | 0.377 |
aAdjusted for age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (kg/m2; continuous), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level (low/medium/high), family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (g/day; continuous).
bFDR-adjusted P-trends are calculated with adjustment for 44 tests.
Table 4.
Multivariable-adjusted HRs and 95% CI for the relation between nut and peanut butter intake and the risk of molecular subtypes of CRC in women; NLCS, 1986–1993, excluding the first 2.3 years of follow-up
| Total nuts (g/day) | Tree nuts (g/day) | Peanuts (g/day) | Peanut butter (g/day) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.1–<5/0.1+ | 5+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1–<5/0.1+ | 5+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | 0.0 | 0.1–<5/0.1+ | 5+ | P-trend/FDR- adjusted P-trendb | Per 5 g/day increment | |
| Person-years in subcohort | 3641 | 3295/5443 | 2149 | 6378 | 2706 | 4287 | 3360/4797 | 1437 | 6643 | 1599/2441 | 842 | ||||||||
| Total CRC | |||||||||||||||||||
| N cases | 110 | 104 | 48 | 194 | 68 | 125 | 104 | 33 | 194 | 48 | 20 | ||||||||
| HRa | 1 | 1.15 | 0.87 | 0.339/ | 0.97 | 1 | 0.89 | 0.487/ | 0.95 | 1 | 1.19 | 0.92 | 0.658/ | 0.98 | 1 | 1.13 | 0.95 | 0.882/ | 0.92 |
| 95% CI | ref | 0.85–1.56 | 0.59–1.28 | 0.875 | 0.86–1.10 | ref | 0.65–1.22 | 0.875 | 0.73–1.23 | ref | 0.89–1.61 | 0.60–1.42 | 0.922 | 0.86–1.11 | ref | 0.80–1.60 | 0.57–1.59 | 0.970 | 0.73–1.16 |
| APC wild-type | |||||||||||||||||||
| N cases | 77 | 68 | 35 | 135 | 45 | 86 | 94 | 129 | 51 | ||||||||||
| HRa | 1 | 1.10 | 0.94 | 0.716/ | 1.00 | 1 | 0.88 | 0.483/ | 0.94 | 1 | 1.15 | 0.397/ | 1.02 | 1 | 1.28 | 0.165/ | 1.07 | ||
| 95% CI | ref | 0.77–1.57 | 0.60–1.49 | 0.922 | 0.87–1.16 | ref | 0.61–1.27 | 0.875 | 0.65–1.35 | ref | 0.83–1.60 | 0.875 | 0.89–1.17 | ref | 0.90–1.83 | 0.875 | 0.85–1.34 | ||
| Truncating APC mutation | |||||||||||||||||||
| N cases | 33 | 36 | 13 | 59 | 23 | 39 | 43 | 65 | 17 | ||||||||||
| HRa | 1 | 1.27 | 0.73 | 0.238/ | 0.91 | 1 | 0.94 | 0.827/ | 0.97 | 1 | 1.08 | 0.766/ | 0.88 | 1 | 0.68 | 0.187/ | 0.44 | ||
| 95% CI | ref | 0.76–2.12 | 0.36–1.46 | 0.875 | 0.75–1.12 | ref | 0.54–1.64 | 0.958 | 0.68–1.37 | ref | 0.66–1.75 | 0.922 | 0.68–1.14 | ref | 0.38–1.21 | 0.875 | 0.22–0.90 | ||
| P-hetero-geneity | 0.630 | 0.612 | 0.967 | 0.193 | |||||||||||||||
| KRAS wild-type | |||||||||||||||||||
| N cases | 73 | 70 | 33 | 131 | 45 | 83 | 70 | 23 | 131 | 45 | |||||||||
| HRa | 1 | 1.19 | 0.95 | 0.672/ | 0.94 | 1 | 0.91 | 0.633/ | 0.72 | 1 | 1.22 | 1.02 | 0.987/ | 0.97 | 1 | 1.07 | 0.733/ | 0.93 | |
| 95% CI | ref | 0.84–1.71 | 0.60–1.52 | 0.922 | 0.82–1.07 | ref | 0.62–1.34 | 0.922 | 0.44–1.17 | ref | 0.86–1.74 | 0.61–1.71 | 0.987 | 0.85–1.11 | ref | 0.74–1.55 | 0.922 | 0.70–1.24 | |
| Activating KRAS mutation | |||||||||||||||||||
| N cases | 37 | 34 | 15 | 63 | 23 | 42 | 34 | 10 | 63 | 23 | |||||||||
| HRa | 1 | 1.07 | 0.72 | 0.265/ | 1.02 | 1 | 0.88 | 0.588/ | 1.08 | 1 | 1.15 | 0.76 | 0.409/ | 0.98 | 1 | 1.08 | 0.775/ | 0.88 | |
| 95% CI | ref | 0.65–1.77 | 0.38–1.39 | 0.875 | 0.83–1.24 | ref | 0.54–1.41 | 0.922 | 0.94–1.25 | ref | 0.69–1.89 | 0.37–1.56 | 0.875 | 0.76–1.27 | ref | 0.65–1.77 | 0.922 | 0.60–1.30 | |
| P-hetero-geneity | 0.956 | 0.837 | 0.947 | 0.847 | |||||||||||||||
| No p53 over-expression | |||||||||||||||||||
| N cases | 40 | 49 | 27 | 78 | 38 | 48 | 49 | 19 | 78 | 28 | 10 | ||||||||
| HRa | 1 | 1.54 | 1.33 | 0.517/ | 1.06 | 1 | 1.26 | 0.278/ | 1.09 | 1 | 1.52 | 1.35 | 0.391/ | 1.06 | 1 | 1.66 | 1.21 | 0.499/ | 1.04 |
| 95% CI | ref | 0.98–2.42 | 0.78–2.27 | 0.875 | 0.95–1.20 | ref | 0.83–1.90 | 0.875 | 0.96–1.22 | ref | 0.98–2.37 | 0.76–2.41 | 0.875 | 0.93–1.21 | Ref | 1.05–2.64 | 0.58–2.53 | 0.875 | 0.79–1.37 |
| p53 over-expression | |||||||||||||||||||
| N cases | 68 | 55 | 21 | 114 | 30 | 75 | 55 | 14 | 115 | 19 | 10 | ||||||||
| HRa | 1 | 0.97 | 0.62 | 0.084/ | 0.82 | 1 | 0.67 | 0.083/ | 0.54 | 1 | 1.04 | 0.68 | 0.209/ | 0.86 | 1 | 0.75 | 0.78 | 0.444/ | 0.78 |
| 95% CI | ref | 0.66–1.42 | 0.36–1.09 | 0.875 | 0.68–1.00 | ref | 0.43–1.05 | 0.875 | 0.25–1.18 | ref | 0.71–1.51 | 0.36–1.26 | 0.875 | 0.69–1.06 | ref | 0.45–1.24 | 0.39–1.56 | 0.875 | 0.53–1.14 |
| P-hetero-geneity | 0.069 | 0.028 | 0.166 | 0.063 | |||||||||||||||
| BRAF wild-type | |||||||||||||||||||
| N cases | 86 | 77 | 38 | 151 | 50 | 97 | 104 | 150 | 51 | ||||||||||
| HRa | 1 | 1.09 | 0.88 | 0.491/ | 0.99 | 1 | 0.84 | 0.339/ | 0.99 | 1 | 1.10 | 0.562/ | 0.99 | 1 | 1.02 | 0.933/ | 0.86 | ||
| 95% CI | ref | 0.78–1.53 | 0.57–1.37 | 0.875 | 0.87–1.13 | ref | 0.59–1.20 | 0.875 | 0.78–1.25 | ref | 0.80–1.50 | 0.916 | 0.86–1.14 | ref | 0.72–1.44 | 0.977 | 0.66–1.12 | ||
| BRAF mutation | |||||||||||||||||||
| N cases | 17 | 23 | 10 | 33 | 17 | 21 | 29 | 37 | 13 | ||||||||||
| HRa | 1 | 1.70 | 1.20 | 0.931/ | 0.98 | 1 | 1.36 | 0.350/ | 0.87 | 1 | 1.47 | 0.222/ | 1.00 | 1 | 1.20 | 0.609/ | 1.08 | ||
| 95% CI | ref | 0.88–3.28 | 0.53–2.74 | 0.977 | 0.79–1.20 | ref | 0.71–2.60 | 0.875 | 0.42–1.83 | ref | 0.79–2.71 | 0.875 | 0.82–1.20 | ref | 0.60–2.38 | 0.922 | 0.63–1.85 | ||
| P-hetero-geneity | 0.510 | 0.216 | 0.436 | 0.932 | |||||||||||||||
| MSS | |||||||||||||||||||
| N cases | 88 | 116 | 154 | 50 | 100 | 104 | 154 | 50 | |||||||||||
| HRa | 1 | 0.99 | 0.974/ | 0.99 | 1 | 0.81 | 0.239/ | 1.01 | 1 | 1.05 | 0.754/ | 0.98 | 1 | 0.97 | 0.853/ | 0.91 | |||
| 95% CI | ref | 0.73–1.36 | 0.987 | 0.86–1.13 | ref | 0.57–1.15 | 0.875 | 0.84–1.22 | ref | 0.77–1.43 | 0.922 | 0.84–1.14 | ref | 0.68–1.37 | 0.962 | 0.70–1.20 | |||
| MSI | |||||||||||||||||||
| N cases | 13 | 25 | 23 | 15 | 16 | 22 | 27 | 11 | |||||||||||
| HRa | 1 | 1.74 | 0.136/ | 0.87 | 1 | 1.96 | 0.064/ | 0.52 | 1 | 1.72 | 0.134/ | 0.94 | 1 | 1.46 | 0.326/ | 0.85 | |||
| 95% CI | ref | 0.84–3.61 | 0.875 | 0.67–1.14 | ref | 0.96–4.01 | 0.875 | 0.28–0.95 | ref | 0.85–3.50 | 0.875 | 0.74–1.20 | ref | 0.69–3.11 | 0.875 | 0.52–1.38 | |||
| P-hetero-geneity | 0.298 | 0.069 | 0.431 | 0.575 |
aAdjusted for age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (kg/m2; continuous), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level (low/medium/high), family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (g/day; continuous).
bFDR-adjusted P-trends are calculated with adjustment for 44 tests.
Table 5.
Multivariable-adjusteda HRs and 95% CI for the relation between nut and peanut butter intake and the risk of CRC developed through the traditional adenoma-carcinoma pathway (truncating APC mutation and/or activating KRAS mutation and/or p53 overexpression) and the serrated neoplasia pathway (BRAF mutation and/or MSI); NLCS, 1986–1993, excluding the first 2.3 years of follow-up
| Subcohort | Traditional pathway | No traditional pathway | Serrated pathway | No serrated pathway | |||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years | N cases | HR (95% CI) | N cases | HR (95% CI) | N cases | HR (95% CI) | N cases | HR (95% CI) | |
| Men | |||||||||
| Total nuts (g/day) | |||||||||
| 0.0 | 2592 | 95 | 1 (reference) | 19 | 1 (reference) | 19 | 1 (reference) | 81 | 1 (reference) |
| 0.1–<5 | 2767 | 81 | 0.87 (0.63–1.22) | 21 | 1.24 (0.66–2.32) | 22 | 1.25 (0.64–2.45) | 67 | 0.87 (0.61–1.25) |
| 5+ | 3129 | 77 | 0.73 (0.51–1.03) | 16 | 0.81 (0.39–1.67) | 18 | 0.88 (0.43–1.80) | 65 | 0.72 (0.49–1.05) |
| P-trend | 0.086 | 0.387 | 0.502 | 0.104 | |||||
| FDR-adjusted P-trendb | 0.458 | 0.688 | 0.730 | 0.458 | |||||
| Per 5 g/day increment | 0.97 (0.91–1.04) | 0.98 (0.87–1.11) | 0.96 (0.86–1.08) | 0.96 (0.89–1.04) | |||||
| P-heterogeneity | 0.729 | 0.652 | |||||||
| Tree nuts (g/day) | |||||||||
| 0.0 | 6175 | 195 | 1 (reference) | 42 | 1 (reference) | 40 | 1 (reference) | 167 | 1 (reference) |
| 0.1+ | 2314 | 58 | 0.82 (0.60–1.13) | 14 | 0.98 (0.51–1.89) | 19 | 1.31 (0.76–2.25) | 46 | 0.76 (0.53–1.09) |
| P-trend | 0.230 | 0.960 | 0.336 | 0.136 | |||||
| FDR-adjusted P-trendb | 0.613 | 0.983 | 0.688 | 0.458 | |||||
| Per 5 g/day increment | 0.93 (0.72–1.20) | 0.85 (0.47–1.53) | 1.09 (0.82–1.46) | 0.89 (0.66–1.21) | |||||
| P-heterogeneity | 0.745 | 0.093 | |||||||
| Peanuts (g/day) | |||||||||
| 0.0 | 2913 | 103 | 1 (reference) | 20 | 1 (reference) | 20 | 1 (reference) | 88 | 1 (reference) |
| 0.1–<5/0.1+ | 3001/5576 | 90 | 0.95 (0.69–1.31) | 23 | 1.39 (0.76–2.57) | 26 | 1.49 (0.78–2.86) | 73 | 0.92 (0.65–1.30) |
| 5+ | 2575 | 60 | 0.73 (0.50–1.06) | 13 | 0.87 (0.41–1.85) | 13 | 0.83 (0.39–1.79) | 52 | 0.74 (0.50–1.11) |
| P-trend | 0.082 | 0.484 | 0.348 | 0.143 | |||||
| FDR-adjusted P-trendb | 0.458 | 0.730 | 0.688 | 0.458 | |||||
| Per 5 g/day increment | 0.97 (0.91–1.05) | 0.99 (0.88–1.12) | 0.95 (0.83–1.08) | 0.97 (0.89–1.05) | |||||
| P-heterogeneity | 0.732 | 0.383 | |||||||
| Peanut butter (g/day) | |||||||||
| 0.0 | 6082 | 185 | 1 (reference) | 40 | 1 (reference) | 42 | 1 (reference) | 158 | 1 (reference) |
| 0.1+ | 2407 | 68 | 1.02 (0.75–1.38) | 16 | 1.15 (0.61–2.18) | 17 | 1.07 (0.60–1.90) | 55 | 1.00 (0.71–1.40) |
| P-trend | 0.913 | 0.661 | 0.820 | 0.983 | |||||
| FDR-adjusted P-trendb | 0.983 | 0.881 | 0.983 | 0.983 | |||||
| Per 5 g/day increment | 1.18 (1.04–1.33) | 0.85 (0.57–1.26) | 0.93 (0.67–1.27) | 1.22 (1.07–1.38)* | |||||
| P-heterogeneity | 0.805 | 0.654 | |||||||
| Women | |||||||||
| Total nuts (g/day) | |||||||||
| 0.0 | 3641 | 86 | 1 (reference) | 24 | 1 (reference) | 23 | 1 (reference) | 78 | 1 (reference) |
| 0.1–<5 | 3295 | 76 | 1.05 (0.75–1.47) | 28 | 1.55 (0.87–2.78) | 28 | 1.55 (0.87–2.78) | 70 | 1.09 (0.77–1.56) |
| 5+ | 2149 | 34 | 0.76 (0.48–1.18) | 14 | 1.31 (0.63–2.73) | 12 | 1.03 (0.49–2.16) | 31 | 0.82 (0.51–1.31) |
| P-trend | 0.173 | 0.647 | 0.790 | 0.326 | |||||
| FDR-adjusted P-trendb | 0.752 | 0.846 | 0.903 | 0.846 | |||||
| Per 5 g/day increment | 0.98 (0.85–1.13) | 0.93 (0.77–1.12) | 0.95 (0.79–1.15) | 0.98 (0.84–1.15) | |||||
| P-heterogeneity | 0.558 | 0.600 | |||||||
| Tree nuts (g/day) | |||||||||
| 0.0 | 6378 | 149 | 1 (reference) | 45 | 1 (reference) | 44 | 1 (reference) | 132 | 1 (reference) |
| 0.1+ | 2706 | 47 | 0.78 (0.54–1.13) | 21 | 1.30 (0.73–2.29) | 19 | 1.13 (0.62–2.05) | 47 | 0.92 (0.63–1.33) |
| P-trend | 0.188 | 0.374 | 0.687 | 0.651 | |||||
| FDR-adjusted P-trendb | 0.752 | 0.846 | 0.846 | 0.846 | |||||
| Per 5 g/day increment | 0.98 (0.76–1.27) | 0.79 (0.45–1.40) | 0.70 (0.30–1.68) | 1.04 (0.87–1.24) | |||||
| P-heterogeneity | 0.222 | 0.563 | |||||||
| Peanuts (g/day) | |||||||||
| 0.0 | 4287 | 97 | 1 (reference) | 28 | 1 (reference) | 27 | 1 (reference) | 89 | 1 (reference) |
| 0.1–<5/0.1+ | 3360/4797 | 99 | 1.02 (0.74–1.40) | 38 | 1.53 (0.89–2.63) | 26 | 1.49 (0.82–2.69) | 71 | 1.14 (0.81–1.61) |
| 5+ | 1437 | 10 | 1.34 (0.63–2.87) | 19 | 0.77 (0.45–1.32) | ||||
| P-trend | 0.914 | 0.126 | 0.506 | 0.310 | |||||
| FDR-adjusted P-trendb | 0.975 | 0.752 | 0.846 | 0.846 | |||||
| Per 5 g/day increment | 0.98 (0.85–1.14) | 0.95 (0.78–1.16) | 0.99 (0.84–1.16) | 0.97 (0.80–1.16) | |||||
| P-heterogeneity | 0.300 | 0.494 | |||||||
| Peanut butter (g/day) | |||||||||
| 0.0 | 6643 | 149 | 1 (reference) | 45 | 1 (reference) | 47 | 1 (reference) | 134 | 1 (reference) |
| 0.1+ | 2441 | 47 | 0.92 (0.64–1.32) | 21 | 1.62 (0.93–2.81) | 16 | 1.16 (0.63–2.14) | 45 | 1.00 (0.69–1.45) |
| P-trend | 0.655 | 0.087 | 0.623 | 0.990 | |||||
| FDR-adjusted P-trendb | 0.846 | 0.752 | 0.846 | 0.990 | |||||
| Per 5 g/day increment | 0.82 (0.61–1.10) | 1.17 (0.83–1.64) | 1.03 (0.62–1.70) | 0.84 (0.63–1.13) | |||||
| P-heterogeneity | 0.222 | 0.969 |
aAdjusted for age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (kg/m2; continuous), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level (low/medium/high), family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (g/day; continuous).
bFDR-adjusted P-trends are calculated with adjustment for 16 tests per sex.
*P < 0.05 after FDR correction.
Results
In Table 1, baseline characteristics stratified by sex are presented. In men, the mean (SD) total nut intake was 7.9 (13.7) g/day in the subcohort and 7.9 (13.2) g/day in CRC cases. In women, these intakes were 4.4 (8.5) and 4.3 (8.5) g/day, respectively. Tree nut, peanut and peanut butter intakes were comparable in CRC cases and subcohort members. Female rectal cancer cases had lower mean intakes of total nuts, tree nuts and peanuts compared with subcohort members.
Table 1.
Baseline characteristics [mean (SD) or %] of subcohort members and CRC cases; NLCS, 1986–2006
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Subcohort | Colorectal cancer cases | Colon cancer cases | Rectal cancer cases | Subcohort | Colorectal cancer cases | Colon cancer cases | Rectal cancer cases | |
| N | 1834 | 1993 | 1296 | 475 | 1886 | 1574 | 1187 | 277 |
| Age (years) | 61.2 (4.2) | 61.5 (4.1) | 61.7 (4.2) | 61.1 (4.0) | 61.4 (4.2) | 61.9 (4.1) | 61.9 (4.1) | 61.8 (4.0) |
| Body mass index (kg/m2) | 24.9 (2.6) | 25.2 (2.6) | 25.2 (2.7) | 25.1 (2.5) | 25.0 (3.5) | 25.0 (3.5) | 25.0 (3.5) | 25.0 (3.5) |
| Ever cigarette smoker (%) | 86.4 | 87.2 | 86.7 | 88.2 | 41.1 | 41.9 | 40.5 | 45.1 |
| University or higher vocational education (%) | 20.3 | 21.3 | 24.2 | 16.4 | 9.5 | 9.8 | 9.8 | 7.2 |
| Non-occupational physical activity (min/day) | 81.0 (67.4) | 82.5 (67.6) | 82.7 (66.5) | 85.5 (73.0) | 65.5 (50.6) | 62.9 (51.9) | 62.7 (51.4) | 63.7 (57.5) |
| Family history of CRC (%) | 5.6 | 9.1 | 9.5 | 7.0 | 6.0 | 10.3 | 10.5 | 9.8 |
| Daily energy intake (kcal) | 2167 (499) | 2150 (484) | 2132 (488) | 2200 (471) | 1684 (389) | 1681 (375) | 1677 (382) | 1690 (335) |
| Total nut intake (g/day) | 7.9 (13.7) | 7.9 (13.2) | 7.8 (12.2) | 8.0 (14.7) | 4.4 (8.5) | 4.3 (8.5) | 4.5 (8.7) | 3.6 (7.6) |
| Tree nut intake (g/day) | 1.0 (3.4) | 1.0 (3.4) | 1.1 (3.5) | 1.0 (3.4) | 1.1 (4.0) | 1.0 (3.1) | 1.0 (3.0) | 0.9 (3.4) |
| Peanut intake (g/day) | 6.9 (13.0) | 6.9 (12.4) | 6.6 (11.3) | 7.0 (13.9) | 3.3 (7.0) | 3.3 (7.2) | 3.5 (7.4) | 2.7 (6.2) |
| Peanut butter intake (g/day) | 1.4 (4.2) | 1.6 (4.2) | 1.5 (4.1) | 1.4 (3.7) | 1.2 (3.6) | 1.0 (3.5) | 1.1 (3.8) | 0.8 (2.4) |
| Alcohol intake (g/day) | 15.1 (17.1) | 16.3 (17.2) | 15.6 (16.5) | 17.6 (18.6) | 6.0 (9.5) | 6.3 (10.5) | 6.2 (10.2) | 6.5 (10.9) |
On average, CRC cases were somewhat older than subcohort members, more often ever smokers, and higher educated, more often reported a positive CRC family history, and reported higher alcohol intakes (Table 1). Male cases were on average heavier than subcohort members and more non-occupationally physically active. Female cases were less non-occupationally physically active than subcohort members.
Multivariable-adjusted HRs for the associations between nut and peanut butter intake and the risk of CRC and its anatomical subtypes in men and women are presented in Table 2. Age-adjusted results can be found in Supplementary Tables S1 and S2, available at Carcinogenesis Online, for men and women, respectively. Total nut intake was not associated with CRC risk in men or women in categorical and continuous multivariable-adjusted analyses. Compared with non-consumers, the HR (95% CI) for 10+ g total nuts/day was 0.94 (0.78–1.15; P-trend of linear test over all intake categories = 0.494) in men and 0.96 (0.75–1.22; P-trend = 0.458) in women. No associations between tree nut, peanut and peanut butter intake and CRC risk were observed in both sexes. Nut and peanut butter intake were also not associated with colon, proximal colon and distal colon cancer in both sexes, except for a significant positive association between 0.1–<5 g total nut intake/day and distal colon cancer risk in women [HR (95% CI) = 1.31 (1.02–1.67)]. In men, no significant associations were observed between nut and peanut butter intake and rectal cancer, except for a significant inverse association for 5–<10 g peanut intake/day versus non-consumers [HR (95% CI) = 0.61 (0.40–0.92)]. In women, also no significant associations were seen between nut and peanut butter intake and rectal cancer risk, except for a significant inverse association for 5–<10 g total nut intake/day versus non-consumers [HR (95% CI) = 0.42 (0.23–0.76)].
Table 2.
Multivariable-adjusted HRs and 95% CI for CRC and its anatomical subtypes in men and women, according to nut and peanut butter consumption; NLCS, 1986–2006
| Colorectal cancer | Colon cancer | Proximal colon cancer | Distal colon cancer | Rectal cancer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median intakea | Person-years | Cases | Multivariable-adjusted HR (95% CI)b | Cases | Multivariable-adjusted HR (95% CI)b | Cases | Multivariable-adjusted HR (95% CI)b | Cases | Multivariable-adjusted HR (95% CI)b | Cases | Multivariable-adjusted HR (95% CI)b | |
| Men | ||||||||||||
| Total nut intake (g/day) | ||||||||||||
| 0 | 0.0 | 8605 | 614 | 1.00 (reference) | 397 | 1.00 (reference) | 173 | 1.00 (reference) | 218 | 1.00 (reference) | 155 | 1.00 (reference) |
| 0.1–<5 | 2.5 | 9509 | 668 | 1.00 (0.84–1.19) | 426 | 0.99 (0.81–1.20) | 196 | 1.06 (0.83–1.37) | 213 | 0.87 (0.68–1.10) | 168 | 1.02 (0.78–1.32) |
| 5–<10 | 8.5 | 3896 | 252 | 0.93 (0.74–1.17) | 170 | 0.96 (0.74–1.24) | 78 | 1.04 (0.74–1.45) | 82 | 0.80 (0.59–1.11) | 48 | 0.72 (0.49–1.04) |
| 10+ | 21.4 | 6936 | 459 | 0.94 (0.78–1.15) | 303 | 0.98 (0.78–1.22) | 142 | 1.11 (0.84–1.47) | 155 | 0.87 (0.66–1.14) | 104 | 0.82 (0.60–1.11) |
| P-trend | 0.494 | 0.849 | 0.541 | 0.481 | 0.123 | |||||||
| Continuous, per 5 g/day increment | 0.99 (0.97–1.02) | 0.99 (0.96–1.02) | 1.01 (0.97–1.04) | 0.98 (0.95–1.02) | 0.99 (0.95–1.03) | |||||||
| Tree nuts (g/day) | ||||||||||||
| 0 | 0.0 | 20 837 | 1453 | 1.00 (reference) | 935 | 1.00 (reference) | 421 | 1.00 (reference) | 488 | 1.00 (reference) | 346 | 1.00 (reference) |
| 0.1–<5 | 1.6 | 6615 | 439 | 0.97 (0.82–1.14) | 289 | 0.97 (0.80–1.17) | 130 | 0.98 (0.77–1.25) | 147 | 0.92 (0.73–1.16) | 109 | 1.06 (0.82–1.36) |
| 5+ | 8.9 | 1494 | 101 | 0.97 (0.71–1.32) | 72 | 1.06 (0.76–1.49) | 38 | 1.29 (0.86–1.95) | 33 | 0.91 (0.59–1.41) | 20 | 0.81 (0.48–1.35) |
| P-trend | 0.774 | 0.791 | 0.253 | 0.593 | 0.485 | |||||||
| Continuous, per 5 g/day increment | 0.98 (0.89–1.07) | 0.99 (0.90–1.09) | 1.04 (0.93–1.15) | 0.95 (0.83–1.09) | 0.97 (0.82–1.14) | |||||||
| Peanuts (g/day) | ||||||||||||
| 0 | 0.0 | 9711 | 696 | 1.00 (reference) | 448 | 1.00 (reference) | 198 | 1.00 (reference) | 241 | 1.00 (reference) | 177 | 1.00 (reference) |
| 0.1–<5 | 2.5 | 10 376 | 718 | 0.99 (0.84–1.17) | 462 | 1.00 (0.83–1.20) | 216 | 1.07 (0.85–1.37) | 226 | 0.88 (0.70–1.10) | 178 | 0.97 (0.75–1.24) |
| 5–<10 | 8.5 | 3216 | 197 | 0.91 (0.71–1.17) | 133 | 0.98 (0.74–1.29) | 58 | 0.99 (0.69–1.43) | 70 | 0.93 (0.66–1.31) | 35 | 0.61 (0.40–0.92) |
| 10+ | 21.4 | 5643 | 382 | 0.97 (0.79–1.19) | 253 | 1.02 (0.81–1.28) | 117 | 1.13 (0.84–1.51) | 131 | 0.94 (0.71–1.25) | 85 | 0.80 (0.58–1.10) |
| P-trend | 0.704 | 0.858 | 0.527 | 0.944 | 0.112 | |||||||
| Continuous, per 5 g/day increment | 1.00 (0.97–1.02) | 0.99 (0.96–1.02) | 1.00 (0.97–1.04) | 0.99 (0.95–1.03) | 0.99 (0.95–1.04) | |||||||
| Peanut butter (g/day) | ||||||||||||
| 0 | 0.0 | 20 730 | 1427 | 1.00 (reference) | 929 | 1.00 (reference) | 425 | 1.00 (reference) | 476 | 1.00 (reference) | 347 | 1.00 (reference) |
| 0.1–<5 | 1.2 | 4995 | 318 | 0.99 (0.82–1.19) | 208 | 0.98 (0.79–1.20) | 94 | 0.98 (0.75–1.28) | 106 | 0.96 (0.74–1.24) | 70 | 0.91 (0.68–1.22) |
| 5+ | 9.6 | 3220 | 248 | 1.22 (0.98–1.51) | 159 | 1.21 (0.95–1.54) | 70 | 1.17 (0.86–1.60) | 86 | 1.27 (0.95–1.70) | 58 | 1.14 (0.82–1.59) |
| P-trend | 0.076 | 0.125 | 0.318 | 0.109 | 0.429 | |||||||
| Continuous, per 5 g/day increment | 1.07 (0.99–1.15) | 1.06 (0.98–1.15) | 1.07 (0.97–1.19) | 1.05 (0.95–1.16) | 1.01 (0.90–1.13) | |||||||
| Women | ||||||||||||
| Total nut intake (g/day) | ||||||||||||
| 0 | 0.0 | 13 183 | 629 | 1.00 (reference) | 452 | 1.00 (reference) | 276 | 1.00 (reference) | 163 | 1.00 (reference) | 126 | 1.00 (reference) |
| 0.1–<5 | 2.1 | 12 150 | 606 | 1.10 (0.94–1.30) | 462 | 1.18 (0.99–1.41) | 261 | 1.11 (0.90–1.37) | 189 | 1.31 (1.02–1.67) | 106 | 0.95 (0.71–1.27) |
| 5–<10 | 7.8 | 3762 | 149 | 0.94 (0.73–1.22) | 125 | 1.13 (0.86–1.49) | 71 | 1.09 (0.78–1.51) | 49 | 1.18 (0.81–1.73) | 14 | 0.42 (0.23–0.76) |
| 10+ | 15.7 | 4223 | 190 | 0.96 (0.75–1.22) | 148 | 1.07 (0.82–1.39) | 95 | 1.13 (0.83–1.54) | 51 | 1.02 (0.70–1.47) | 31 | 0.74 (0.47–1.16) |
| P-trend | 0.458 | 0.816 | 0.517 | 0.782 | 0.060 | |||||||
| Continuous, per 5 g/day increment | 1.00 (0.96–1.04) | 1.02 (0.97–1.06) | 1.02 (0.97–1.07) | 1.02 (0.95–1.09) | 0.93 (0.83–1.03) | |||||||
| Tree nuts (g/day) | ||||||||||||
| 0 | 0.0 | 23 269 | 1124 | 1.00 (reference) | 835 | 1.00 (reference) | 507 | 1.00 (reference) | 306 | 1.00 (reference) | 207 | 1.00 (reference) |
| 0.1–<5 | 1.6 | 8228 | 368 | 0.96 (0.81–1.14) | 289 | 1.02 (0.85–1.23) | 159 | 0.92 (0.74–1.15) | 122 | 1.19 (0.92–1.53) | 55 | 0.80 (0.57–1.11) |
| 5+ | 8.9 | 1821 | 82 | 0.98 (0.71–1.36) | 63 | 1.03 (0.73–1.46) | 37 | 1.00 (0.66–1.52) | 24 | 1.09 (0.67–1.77) | 15 | 0.99 (0.55–1.79) |
| P-trend | 0.858 | 0.834 | 0.895 | 0.571 | 0.809 | |||||||
| Continuous, per 5 g/day increment | 0.98 (0.89–1.07) | 0.99 (0.91–1.08) | 1.01 (0.91–1.11) | 0.97 (0.85–1.10) | 0.94 (0.72–1.21) | |||||||
| Peanuts (g/day) | ||||||||||||
| 0 | 0.0 | 15 536 | 733 | 1.00 (reference) | 531 | 1.00 (reference) | 316 | 1.00 (reference) | 201 | 1.00 (reference) | 143 | 1.00 (reference) |
| 0.1–<5 | 2.5 | 12 435 | 608 | 1.09 (0.93–1.28) | 473 | 1.18 (0.99–1.40) | 278 | 1.20 (0.97–1.47) | 182 | 1.16 (0.91–1.47) | 101 | 0.93 (0.69–1.25) |
| 5–<10 | 8.5 | 2442 | 96 | 0.89 (0.66–1.20) | 76 | 1.00 (0.73–1.38) | 39 | 0.88 (0.59–1.31) | 33 | 1.12 (0.72–1.74) | 12 | 0.54 (0.29–1.02) |
| 10+ | 17.1 | 2905 | 137 | 1.03 (0.79–1.35) | 107 | 1.13 (0.85–1.52) | 70 | 1.27 (0.90–1.78) | 36 | 0.98 (0.65–1.49) | 21 | 0.78 (0.46–1.30) |
| P-trend | 0.849 | 0.628 | 0.398 | 0.908 | 0.169 | |||||||
| Continuous, per 5 g/day increment | 1.00 (0.96–1.06) | 1.03 (0.97–1.08) | 1.03 (0.96–1.09) | 1.03 (0.95–1.12) | 0.91 (0.80–1.04) | |||||||
| Peanut butter (g/day) | ||||||||||||
| 0 | 0.0 | 24 266 | 1170 | 1.00 (reference) | 866 | 1.00 (reference) | 520 | 1.00 (reference) | 323 | 1.00 (reference) | 214 | 1.00 (reference) |
| 0.1–<5 | 1.2 | 5866 | 269 | 1.00 (0.83–1.20) | 208 | 1.05 (0.86–1.29) | 119 | 0.99 (0.77–1.26) | 86 | 1.19 (0.90–1.57) | 46 | 0.91 (0.65–1.30) |
| 5+ | 6.9 | 3186 | 135 | 0.95 (0.74–1.22) | 113 | 1.08 (0.82–1.41) | 64 | 1.03 (0.74–1.42) | 43 | 1.09 (0.75–1.59) | 17 | 0.63 (0.37–1.09) |
| P-trend | 0.682 | 0.559 | 0.879 | 0.596 | 0.092 | |||||||
| Continuous, per 5 g/day increment | 0.96 (0.85–1.08) | 1.01 (0.89–1.14) | 0.99 (0.84–1.16) | 1.05 (0.89–1.22) | 0.78 (0.60–1.02) |
aMedian intake in the subcohort.
bAdjusted for age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (<18.5/18.5–<25/25–<30/30+ kg/m2), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level (low/medium/high), family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (0/0.1–<5/5–<15/15–<30/30+ g/day).
The tests for heterogeneity across the anatomical CRC subtypes showed significant heterogeneity between overall colon and rectal cancer in women (P-heterogeneity = 0.029), but not in men (P-heterogeneity = 0.263). No significant heterogeneity was found between proximal and distal colon cancer in both sexes.
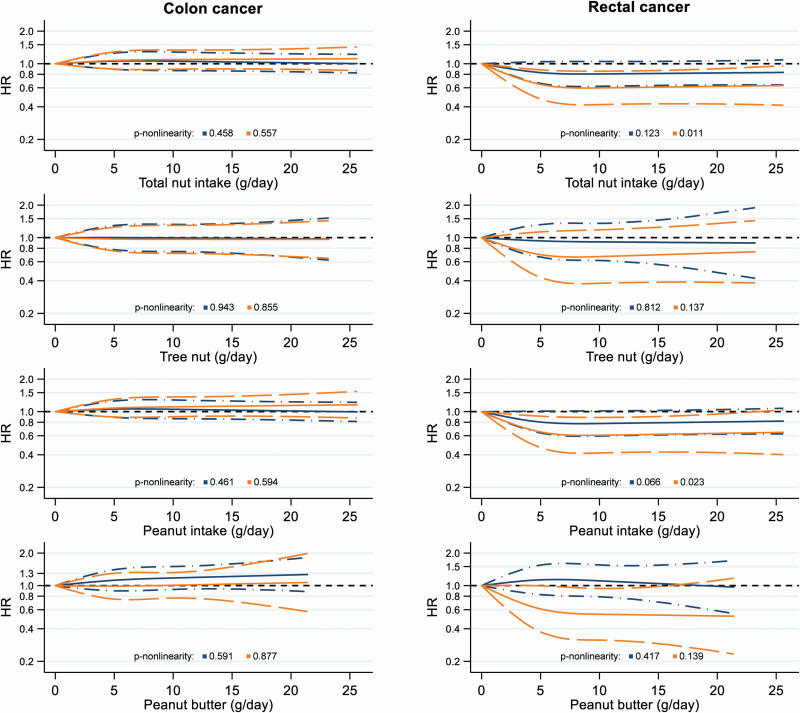
Restricted cubic spline curves for colon and rectal cancer according to nut and peanut butter intake are presented in Figure 1. For colon cancer, no associations were seen with nut or peanut butter intake in both sexes, and no statistical evidence for non-linearity. For rectal cancer, the exposure response curves showed significant inverse associations with total nut, peanut and peanut butter intake in women, with a clear leveling-off of the exposure-response curves at intake of >7.5 g/day. The non-linearity tests were significant for total nut and peanut intake in relation to rectal cancer in women (P-non-linearity = 0.011 and 0.023, respectively), but not for tree nut or peanut butter intake. In men, the inverse associations between total nut and peanut intake and rectal cancer risk were borderline significant and no evidence for non-linearity was found. Based on the AIC score, the model fit of the restricted cubic spline model with three fixed knots at 0, 5 and 10 g nut intake/day did not improve when using additional knots or different knot positions (data not shown).
Figure 1.
Restricted cubic spline curves, with three fixed knots at 0, 5 and 10 g nut or peanut butter intake/day; NLCS, 1986–2006. Blue lines represent males and orange lines females. Solid lines represent HRs and dashed lines 95% CIs. HRs were multivariable-adjusted for age (years; continuous), cigarette smoking [status (never/former/current), frequency (n/day; continuous, centered) and duration (years; continuous, centered)], BMI (<18.5/18.5–<25/25–<30/30+ kg/m2), non-occupational physical activity (≤30/>30–≤60/>60–≤90/>90 min/day), educational level (low/medium/high), family history of CRC (no/yes), total energy intake (kcal/day; continuous) and alcohol consumption (0/0.1–<5/5–<15/15–<30/30+ g/day).
In the analyses stratified by potential effect modifiers, we observed a significant interaction between total nut intake and cigarette smoking status in relation to colon cancer in women (P-interaction = 0.015; Supplementary Table S3 is available at Carcinogenesis Online): we observed no association between total nut intake and colon cancer risk in never and former smokers, and a significant positive association in current smokers. For rectal cancer (Supplementary Table S4 is available at Carcinogenesis Online), significant inverse trends with total nut intake were seen in men and women with this categorization [HR (95% CI) for 5+ g/day versus non-consumers = 0.78 (0.60–1.03), P-trend of linear test over all intake categories = 0.043 and 0.58 (0.39–0.87), P-trend = 0.005, respectively]. A significant interaction by BMI was found in men (P-interaction = 0.002), with a significant inverse association in normal weight men and a significant positive association in overweight men for the second nut intake category.
In sensitivity analyses, mutually adjusting tree nut, peanut and peanut butter intake did not importantly change the results for the anatomical CRC subtypes, and neither did additionally adjusting for the aMED score excluding alcohol and nuts. When comparing the median total nut intake at baseline of colon and rectal cancer cases diagnosed over the follow-up period, we observed no increasing or decreasing trends (Supplementary Table S5 is available at Carcinogenesis Online). However, the Kruskal–Wallis tests was significant for total nut intake in male rectal cancer cases (P = 0.013). Excluding the first two years of follow-up or restricting the analyses of peanut butter to participants with a constant peanut butter intake in the 5 years before baseline did not change the results essentially.
Molecular subtypes
Baseline characteristics of subcohort members and cases per molecular CRC subtype are presented in Supplementary Table S6, available at Carcinogenesis Online. Multivariable-adjusted HRs (95% CI) for the relation between nut and peanut butter intake and the risk of molecular CRC subtypes can be found in Tables 3 and 4. For total nut intake, no associations were observed in both sexes, except for significant inverse associations with total CRC and wild-type KRAS tumors in men [HR (95% CI) for 10+ g/day versus non-consumers = 0.65 (0.45–0.95), P-trend of linear test over all intake categories = 0.060 and 0.54 (0.34–0.87), P-trend = 0.042, respectively]. After FDR correction, the P-trend for wild-type KRAS tumors became non-significant. For tree nut intake, no associations with the molecular subtypes were observed in both sexes in the categorical analyses. In continuous analyses, the HR (95% CI) per 5 g tree nuts/day increment was 0.52 (0.28–0.95) for MSI tumors in women. This inconsistent finding between the categorical and continuous analyses is probably due to chance. Peanut intake was not associated with the molecular CRC subtypes in both sexes. For peanut butter intake, significant positive associations were found in wild-type KRAS tumors and tumors with p53 overexpression in men [HR (95% CI) for 5+ g/day versus non-consumers = 1.55 (1.00–2.40), P-trend = 0.047 and 1.59 (1.01–2.52), P-trend = 0.045, respectively]. After FDR correction, these P-trends became non-significant. In continuous analyses, significant positive associations with peanut butter were also seen in male cases with total CRC, with wild-type KRAS tumors, tumors with p53 overexpression, wild-type BRAF tumors and MSS tumors [HR (95% CI) per 5 g/day increment = 1.14 (1.02–1.29), 1.15 (1.01–1.32), 1.21 (1.06–1.38), 1.20 (1.06–1.35) and 1.17 (1.03–1.34), respectively]. However, the FDR-adjusted P-values were not significant for these estimates. In women, no clear associations were observed with peanut butter intake, except for a significant inverse association in mutated APC tumors in continuous analyses [HR (95% CI) per 5 g/day increment = 0.44 (0.22–0.90)], which became non-significant after FDR correction.
The heterogeneity test was significant for the associations between tree nut intake and the risk of tumors with and without p53 overexpression in women (P-heterogeneity = 0.028), although no significant associations were seen for these molecular subtypes.
When combining a truncating APC mutation and/or activating KRAS mutation and/or p53 overexpression into one endpoint (traditional adenoma–carcinoma pathway), no associations were observed in both sexes, except for a significant positive association for peanut butter intake in men in continuous analyses [HR (95% CI) per 5 g/day increment = 1.18 (1.04–1.33); Table 5]. However, the FDR-adjusted P-value for this continuous estimate was not significant. No relation was observed in colorectal tumors that did not develop through the traditional adenoma–carcinoma pathway. In colorectal tumors with BRAF mutation and/or MSI (serrated neoplasia pathway), also no relation with nut intake was observed. In tumors that did not develop through the serrated neoplasia pathway, a significant positive association with peanut butter intake in men in continuous analyses was found [HR (95% CI) per 5 g/day increment = 1.22 (1.07–1.38)], which remained significant after FDR correction. About 74% of the tumors arising from the traditional adenoma–carcinoma pathway were also defined as tumors that did not develop through the serrated neoplasia pathway.
In both sexes, no heterogeneity was observed in associations between CRC tumors that did or did not develop through the traditional adenoma–carcinoma pathway, or between tumors that did or did not develop through the serrated neoplasia pathway.
Discussion
After 20.3 years of follow-up, we observed significant inverse associations between total nut, peanut and peanut butter intake and rectal cancer risk in women in restricted cubic spline analyses, and the non-linearity tests were significant for total nut and peanut intake. In men, the inverse non-linear relations between total nut and peanut intake and rectal cancer risk were borderline significant. However, in categorical and continuous analyses, no significant associations between nut (subtypes) and peanut butter intake and the risk of CRC or its anatomical subtypes were found in men and women. In the analyses of molecular CRC subtypes after 7.3 years of follow-up, peanut butter intake was associated with an increased risk of CRC that did not develop through the serrated neoplasia pathway in men in continuous analyses, but this was not significantly different from the associations with tumors characterized by the serrated neoplasia pathway. Nut and peanut butter intake were not significantly associated with other molecular CRC subtypes in both sexes.
Our results for the anatomical CRC subtypes are partly in line with the results of eight previous cohort studies (9–16). In five cohort studies, also no significant associations between nut intake and colorectal or colon cancer risk were found in men and/or women (9,10,12,14,15). In another cohort study, an inverse association between peanut product intake and CRC risk was observed in women, but not in men (13). Two other cohorts found inverse associations between nut intake and (distal) colon cancer risk in women (11,16), but not in men (11) or for other anatomical CRC subtypes (11,16). No prospective studies have been performed on peanut butter intake. The results of eight case–control studies were also inconclusive (17–24). Differences in study results might be explained by the low case numbers in some studies because of short follow-up periods (9,10,12,13). Furthermore, few studies investigated rectal cancer (9,11,16–18,20,23) or nut subtypes (16,17) separately, and some combined intake of nuts with other foods, like legumes or pulses (9,18,19,21,22).
In the analyses of the anatomical CRC subtypes, we observed no significant associations in continuous analyses, which is probably caused by the non-linear character of the relations. In restricted cubic spline analyses, which do not assume linearity, we observed significant inverse associations between total nut, peanut and peanut butter intake and rectal cancer risk in women, and borderline significant inverse associations between total nut and peanut intake and rectal cancer risk in men. The non-linear association between tree nut intake and rectal cancer risk in women was not significant, probably because of the low tree nut intake.
The significant inverse associations with nut and peanut butter intake in women were seen for rectal cancer, but not for colon cancer, and the test for heterogeneity by anatomical subtype was significant. The colon and rectum have different physiologic and biochemical characteristics, and several studies have observed that associations with lifestyle factors differ between these anatomical regions, indicating that these have distinct etiologies (48,49).
The observed sex differences were also seen in previous analyses on nut consumption and cancer risk in the NLCS (50). One possible explanation might be differences in nut intake. Total nut and peanut butter intake were on average higher in men than in women in our study. Furthermore, the observed sex differences might have a hormonal basis. Phytoestrogens in nuts, which are structurally similar to estrogens, have a relatively high affinity for estrogen receptor-beta (51). Binding of phytoestrogens activates estrogen receptor-beta, thereby promoting apoptosis of colorectal cells (51). However, in previous analyses in the NLCS, we found no significant associations between nut intake and ovarian, endometrial or estrogen receptor-positive breast cancer (52,53). Lastly, the bowel transit time and frequency of constipation have been reported to be higher in women than in men (54). Consequently, fiber in nuts may have a stronger effect in women by increasing fecal volume, diluting fecal carcinogens, and decreasing the intestinal transit time (7,8).
In the molecular analyses, we observed a positive association in continuous analyses in men between peanut butter intake and colorectal tumors that did not develop through the serrated neoplasia pathway. For tumors that developed through the traditional adenoma–carcinoma pathway, also a positive association with peanut butter intake was observed in continuous analyses in men, although not significant after FDR correction. This can be explained by the fact that 74% of the cases with tumors arising from the traditional adenoma–carcinoma pathway were also defined as tumors that did not develop through the serrated neoplasia pathway. Most molecular and anatomical CRC subtypes were also non-significantly positively associated with peanut butter intake, except for tumors with BRAF mutation, MSI or without p53 overexpression. The mechanism underlying these positive associations is not understood, and requires further studies. However, it is known that peanut butter that was sold in the Netherlands in 1986 contained more sodium, trans fatty acids and vitamin B6, and less niacin than peanuts (5). Trans fatty acids have been hypothesized to increase cancer risk, although evidence for an association between trans-fatty acid intake and CRC risk is limited and inconsistent (55,56).
To our knowledge, no previous studies investigated the relation between nut and peanut butter intake and the risk of molecular CRC subtypes. We performed these analyses to account for molecular heterogeneity, which might dilute the relations between nut and peanut butter intake and overall CRC. However, our results should be interpreted cautiously, because it concerns exploratory analyses in which many tests were performed within small groups.
Our analyses were performed using baseline FFQ data only, although food intake might have changed over time. Nevertheless, dietary habits as measured with the FFQ were stable for minimally 5 years in a reproducibility study (57), and nut intake appeared to be quite constant in a study with repeated measurements (58). Moreover, the NLCS consisted of an older population at baseline, in which dietary habits were relatively stable. Non-differential misclassification of the exposure may have occurred, which potentially attenuated the estimates. Moreover, we cannot exclude residual confounding by (un)measured confounders.
Multiple testing may have resulted in chance findings, especially in the molecular analyses because of their explorative nature, the smaller case numbers and more unstable results. Therefore, we applied the FDR-correction in the molecular analyses. Because of the short follow-up period of 7.3 years in the molecular analyses, the number of CRC cases was low, which made it impossible to investigate molecular subtypes of colon and rectal cancer separately or different mutation types. Moreover, we only looked at functional mutations, which probably contributed to CRC development. The potential impact of non-functional mutations is not yet understood. Consequently, our findings need to be interpreted carefully.
The prospective design and the long and complete follow-up of the NLCS make selection and information bias unlikely. The detailed information on potential confounders allowed us to extensively control for most known risk factors, although these were only measured at baseline and may have changed over time. Moreover, we were able to investigate nut subtypes separately.
In conclusion, we observed significant non-linear inverse relations with rectal cancer risk for nut and peanut butter intake in women. In men, nut intake might be non-linearly associated with a reduced rectal cancer risk, although these associations were borderline significant. Peanut butter intake was associated with an increased risk of CRC that did not develop through the serrated neoplasia pathway in men. However, the results of the molecular analyses should be interpreted cautiously, because this is the first study investigating the relation between nut intake and molecular CRC subtypes, and because many subgroup analyses were performed in small case groups. Therefore, our results need to be replicated in larger studies.
Supplementary Material
Acknowledgements
The authors would like to thank the participants of the Netherlands Cohort Study (NLCS), the Netherlands Cancer Registry and the Dutch Pathology Registry. Furthermore, NLCS staff members are acknowledged for their valuable assistance and advice.
Glossary
Abbreviations
- CRC
colorectal cancer
- ER
estrogen receptor
- FFQ
food frequency questionnaire
- MCR
mutation cluster region
- MSI
microsatellite instability
- NLCS
the Netherlands Cohort Study
- RFLP
restriction fragment length polymorphism
Funding
This work was funded by the Dutch Cancer Society (grant number UM 2015–7860 to P.v.d.B.).
Conflict of Interest Statement: The authors declare that they have no conflict of interest.
References
- 1. Bray F et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut, 66, 683–691. [DOI] [PubMed] [Google Scholar]
- 3. Aune D et al. (2016) Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med., 14, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ros E. (2010) Health benefits of nut consumption. Nutrients, 2, 652–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stichting Nederlands Voedingsstoffenbestand. (1986) NEVO-Table. Dutch Food Composition Table 1986–1987, Nederlands voedingsstoffenbestand. Voorlichtingsbureau voor de Voeding, The Hague, The Netherlands. [Google Scholar]
- 6. Falasca M, et al. (2014) Cancer chemoprevention with nuts. J. Natl. Cancer Inst., 106. doi: 10.1093/jnci/dju238. [DOI] [PubMed] [Google Scholar]
- 7. González CA et al. (2006) The potential of nuts in the prevention of cancer. Br. J. Nutr., 96(Suppl 2), S87–S94. [DOI] [PubMed] [Google Scholar]
- 8. Bingham SA et al. (2003) Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet, 361, 1496–1501. [DOI] [PubMed] [Google Scholar]
- 9. Heilbrun LK et al. (1989) Diet and colorectal cancer with special reference to fiber intake. Int. J. Cancer, 44, 1–6. [DOI] [PubMed] [Google Scholar]
- 10. Singh PN et al. (1998) Dietary risk factors for colon cancer in a low-risk population. Am. J. Epidemiol., 148, 761–774. [DOI] [PubMed] [Google Scholar]
- 11. Jenab M et al. (2004) Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomarkers Prev., 13, 1595–1603. [PubMed] [Google Scholar]
- 12. Lin J et al. (2004) Dietary fat and fatty acids and risk of colorectal cancer in women. Am. J. Epidemiol., 160, 1011–1022. [DOI] [PubMed] [Google Scholar]
- 13. Yeh CC et al. (2006) Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J. Gastroenterol., 12, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reedy J et al. (2008) Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am. J. Epidemiol., 168, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung TT et al. (2010) The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am. J. Clin. Nutr., 92, 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang M et al. (2016) Nut consumption and risk of colorectal cancer in women. Eur. J. Clin. Nutr., 70, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham S et al. (1978) Diet in the epidemiology of cancer of the colon and rectum. J. Natl. Cancer Inst., 61, 709–714. [PubMed] [Google Scholar]
- 18. Pickle LW et al. (1984) Colorectal cancer in rural Nebraska. Cancer Res., 44, 363–369. [PubMed] [Google Scholar]
- 19. Kune S et al. (1987) Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr. Cancer, 9, 21–42. [DOI] [PubMed] [Google Scholar]
- 20. Young TB et al. (1988) Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int. J. Cancer, 42, 167–175. [DOI] [PubMed] [Google Scholar]
- 21. Peters RK et al. (1992) Diet and colon cancer in Los Angeles County, California. Cancer Causes Control, 3, 457–473. [DOI] [PubMed] [Google Scholar]
- 22. Chun YJ et al. (2015) Associations of colorectal cancer incidence with nutrient and food group intakes in Korean adults: a case-control study. Clin. Nutr. Res., 4, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J et al. (2018) The relationship between nut intake and risk of colorectal cancer: a case control study. Nutr. J., 17, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castello A, et al. (2018) Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr., 58, 1495–1505. [DOI] [PubMed] [Google Scholar]
- 25. Hughes LAE et al. (2017) Lifestyle, diet, and colorectal cancer risk according to (Epi)genetic instability: current evidence and future directions of molecular pathological epidemiology. Curr. Colorectal Cancer Rep., 13, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weijenberg MP et al. (2007) Dietary fat and risk of colon and rectal cancer with aberrant MLH1 expression, APC or KRAS genes. Cancer Causes Control, 18, 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song M, et al. (2015) Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J. Natl. Cancer Inst., 107. doi: 10.1093/jnci/djv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Vogel S et al. (2008) Associations of dietary methyl donor intake with MLH1 promoter hypermethylation and related molecular phenotypes in sporadic colorectal cancer. Carcinogenesis, 29, 1765–1773. [DOI] [PubMed] [Google Scholar]
- 29. Brink M et al. (2005) Dietary folate intake and k-ras mutations in sporadic colon and rectal cancer in The Netherlands Cohort Study. Int. J. Cancer, 114, 824–830. [DOI] [PubMed] [Google Scholar]
- 30. de Vogel S et al. (2006) Dietary folate and APC mutations in sporadic colorectal cancer. J. Nutr., 136, 3015–3021. [DOI] [PubMed] [Google Scholar]
- 31. van den Brandt PA et al. (1990) A large-scale prospective cohort study on diet and cancer in The Netherlands. J. Clin. Epidemiol., 43, 285–295. [DOI] [PubMed] [Google Scholar]
- 32. Goldbohm RA et al. (1994) Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr., 48, 253–265. [PubMed] [Google Scholar]
- 33. Van den Brandt PA et al. (1990) Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int. J. Epidemiol., 19, 553–558. [DOI] [PubMed] [Google Scholar]
- 34. Goldbohm RA, et al. (1994) Estimation of the coverage of Dutch municipalities by cancer registries and PALGA based on hospital discharge data. Tijdschr. Soc. Gezondheidsz, 72, 80–84. [Google Scholar]
- 35. Brink M et al. (2003) K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis, 24, 703–710. [DOI] [PubMed] [Google Scholar]
- 36. Lüchtenborg M et al. (2004) APC mutations in sporadic colorectal carcinomas from The Netherlands Cohort Study. Carcinogenesis, 25, 1219–1226. [DOI] [PubMed] [Google Scholar]
- 37. Sieben NL et al. (2006) Clonal analysis favours a monoclonal origin for serous borderline tumours with peritoneal implants. J. Pathol., 210, 405–411. [DOI] [PubMed] [Google Scholar]
- 38. Suraweera N et al. (2002) Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology, 123, 1804–1811. [DOI] [PubMed] [Google Scholar]
- 39. Bongaerts BW et al. (2007) Alcohol consumption and distinct molecular pathways to colorectal cancer. Br. J. Nutr., 97, 430–434. [DOI] [PubMed] [Google Scholar]
- 40. Schoenfeld D. (1982) Partial residuals for the proportional hazards regression model. Biometrika, 69, 239–241. [Google Scholar]
- 41. Lin DY, et al. (1989) The Robust Inference for the Cox Proportional Hazards Model. J. Am. Stat. Assoc., 84, 1074–1078. [Google Scholar]
- 42. Leffondré K et al. (2002) Modeling smoking history: a comparison of different approaches. Am. J. Epidemiol., 156, 813–823. [DOI] [PubMed] [Google Scholar]
- 43. Willett WC.(1998) Nutritional Epidemiology. Oxford University Press, New York. [Google Scholar]
- 44. Akaike H. (1974) A new look at the statistical model identification. IEEE Trans. Automat. Control, AC-19, 716–723. [Google Scholar]
- 45. Wacholder S, et al. (1989) Alternative variance and efficiency calculations for the case-cohort design. Biometrika, 76, 117–123. [Google Scholar]
- 46. Fung TT et al. (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr., 82, 163–173. [DOI] [PubMed] [Google Scholar]
- 47. Benjamini Y, et al. (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol., 57, 289–300. [Google Scholar]
- 48. Wei EK et al. (2004) Comparison of risk factors for colon and rectal cancer. Int. J. Cancer, 108, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murphy N et al. (2019) Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European Countries: a multinational cohort study. Clin. Gastroenterol. Hepatol., 17, 1323–1331.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nieuwenhuis L et al. (2019) Nut and peanut butter consumption and the risk of lung cancer and its subtypes: a prospective cohort study. Lung Cancer, 128, 57–66. [DOI] [PubMed] [Google Scholar]
- 51. Williams C et al. (2016) Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett., 372, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van den Brandt PA et al. (2018) Tree nut, peanut, and peanut butter intake and risk of postmenopausal breast cancer: the Netherlands Cohort Study. Cancer Causes Control, 29, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nieuwenhuis L et al. (2020) Nut and peanut butter intake are not directly associated with the risk of endometrial or ovarian cancer: results from a Dutch prospective cohort study. Clin. Nutr., 39, 2202–2210. [DOI] [PubMed] [Google Scholar]
- 54. Bohlin J et al. (2018) Longer colonic transit time is associated with laxative and drug use, lifestyle factors, and symptoms of constipation. Acta Radiol. Open, 7, 2058460118807232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Islam MA et al. (2019) Trans fatty acids and lipid profile: a serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr., 13, 1643–1647. [DOI] [PubMed] [Google Scholar]
- 56. Thompson AK et al. (2008) Trans-fatty acids and cancer: the evidence reviewed. Nutr. Res. Rev., 21, 174–188. [DOI] [PubMed] [Google Scholar]
- 57. Goldbohm RA et al. (1995) Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur. J. Clin. Nutr., 49, 420–429. [PubMed] [Google Scholar]
- 58. Bao Y et al. (2013) Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med., 369, 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.