Abstract
Background
In this study, we sought to estimate the association between oral oncology parity law adoption and anticancer medication use for patients with chronic myeloid leukemia or multiple myeloma.
Methods
This was an observational study of administrative claims from 2008 to 2017. Among individuals initiating tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia or immunomodulatory drugs for multiple myeloma, we compared out-of-pocket spending, adherence, and discontinuation before and after parity among individuals in fully insured plans (subject to parity) vs self-funded plans (exempt from parity) using propensity-score weighted difference-in-differences regression models.
Results
Among patients initiating TKIs (N = 2082) or immunomodulatory drugs (N = 3326) there were no statistically significant differences in adherence or discontinuation associated with parity. The proportion of patients with initial out-of-pocket payments of $0 increased in fully insured plans after parity from 5.7% to 46.1% for TKIs and from 10.9% to 48.8% for immunomodulatory drugs. Relative to changes in self-funded plans, those in fully insured plans were 4.27 (95% CI = 2.20 to 8.27) times as likely to pay nothing for TKIs and 1.96 (95% CI = 1.40 to 2.73) times as likely to pay nothing for immunomodulatory drugs after parity. Similarly, the proportion paying more than $100 decreased from 30.3% to 24.7% for TKIs and 30.6% to 27.5% for immunomodulatory drugs in fully insured plans after parity. Relative to changes in self-funded plans, those in fully insured plans were 0.74 (95% CI = 0.54 to 1.01) times as likely to pay more than $100 for TKIs and 0.85 (95% CI = 0.68 to 1.06) times as likely to pay more than $100 for immunomodulatory drugs after parity.
Conclusions
Among patients initiating TKIs or immunomodulatory drugs, parity was not associated with better adherence or less discontinuation of therapy but yielded decreased patient out-of-pocket payments for some patients.
Orally administered anticancer medications represent an increasing share of new treatment options for cancer. These therapies often require chronic use by patients, making treatment adherence and continuation, as well as out-of-pocket payments, topics of great importance. For patients with chronic myeloid leukemia (CML) and multiple myeloma, orally administered therapies are critical to disease management and, once initiated, patients often remain on therapy indefinitely (1,2).
Unlike infused anticancer medications offered under outpatient medical benefits, coverage for orally administered anticancer medications is typically provided under the pharmacy benefit. Advocates have raised concerns regarding the potential for patients to pay more for products obtained through pharmacy benefits because of cost-sharing arrangements such as deductibles and coinsurance, which require patients to pay a percentage of the drug price (3–5). Prior work has demonstrated that patients facing higher out-of-pocket costs are less likely to start treatment (6–8) and, among those who do start, are more likely to discontinue or have poorer adherence than patients with lower out-of-pocket costs, even for anticancer therapy (9–11).
To address discrepancies in coverage for infused and orally administered anticancer treatments, 43 states and Washington, DC, have passed laws to improve affordability of orally administered anticancer drugs. These laws generally specify that commercially insured patients in fully insured plans should pay no more for oral anticancer therapy than for intravenous therapy offered by the same plan (ie, “oral oncology parity” laws) (4). Notably, the state legislation applies only to fully insured private plans, exempting Medicare, Medicaid, and self-funded plans governed by the Employee Retirement Income Security Act and exempt from state mandates.
In a prior study, we found that oral oncology parity laws had limited impact on the use of orally administered anticancer therapy across a subset of cancers studied in 2008–2012 (12). The laws reduced out-of-pocket spending for some users but not for the highest spenders. Although this prior work suggested that the overall impact of parity on out-of-pocket costs was mixed, we were unable to account for individual-level changes in adherence and discontinuation in that analysis given our inclusion of treatments for multiple cancer types and stages. In the current study, we focus on individuals needing chronic treatment with high-cost anticancer drugs, including tyrosine kinase inhibitors (TKIs) for patients with CML or immunomodulatory drugs for patients with multiple myeloma, which we believe will provide a greater understanding of the association between these state laws and medication access and use. Our objective was to compare anticancer medication adherence, discontinuation, and out-of-pocket payments among patients in fully insured plans (subject to parity) and self-funded plans (exempted from parity via Employee Retirement Income Security Act) before and after parity implementation in states that passed parity laws between 2008 and 2017.
Methods
Data Source and Sample Selection
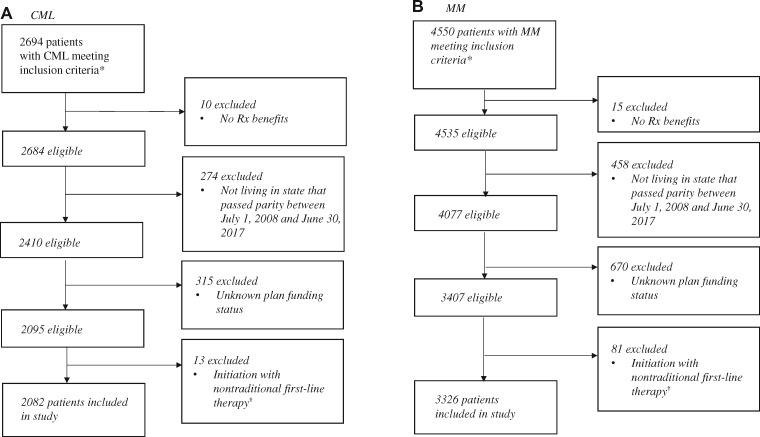
We used 2008–2017 national health plan claims from Aetna, Humana, and UnitedHealthcare aggregated by the Health Care Cost Institute. We included patients filling a TKI for CML (imatinib, dasatinib, nilotinib) or an immunomodulatory drug for multiple myeloma (thalidomide or lenalidomide). We further required the presence of a diagnosis code for CML or multiple myeloma during the study period and continuous enrollment in medical and pharmacy coverage for 3 months before and 6 months after their first observed (index) prescription fill date (with no fills for TKIs or immunomodulatory drugs in the prior 3 months) to ensure adequate follow-up for all outcomes. We also required individuals to be younger than 65 years of age at their index fill (because parity laws do not extend to Medicare). We excluded those without prescription drug benefits, those who did not live in a state that passed parity between July 1, 2008, and June 30, 2017, and those with missing plan funding status. We focused on individuals initiating therapies used as first-line treatment over our study period and excluded the small number of patients whose first observed fill was for nontraditional first-line therapy (13 individuals who initiated either bosutinib or ponatinib for CML and 81 individuals who initiated pomalidomide for multiple myeloma). This study received an institutional review board exemption from the Vanderbilt University School of Medicine.
Primary Outcomes
The primary dependent variables of interest were patient out-of-pocket spending on their first observed orally administered anticancer medication fill, and anticancer medication adherence and discontinuation. We defined adherence using the proportion of days covered (PDC) over the first 180 days following treatment initiation for patients with CML and over the first 120 days following treatment initiation for patients with multiple myeloma. We chose the shorter period of follow-up for multiple myeloma to reflect expected initial treatment and discontinuation for those who go on to receive stem cell transplant. The PDC is a commonly used adherence measure, recommended by the Pharmacy Quality Alliance for administrative claims–based studies (13). We allowed within-class medication switches to avoid overestimating nonadherence. We defined discontinuation as a gap of at least 60 days of therapy after all available drug supply was exhausted (9). Patients were considered adherent if they had at least 80% of days with drug available during the follow-up period (13); otherwise, they were considered nonadherent. Because adherence and discontinuation are related measures, we evaluated medication adherence among all patients and separately among patients who did not discontinue (9).
Finally, we evaluated whether out-of-pocket spending for the index anticancer medication fill changed before and after parity. We calculated out-of-pocket spending summing copayments, coinsurance, and deductibles paid by the patient at the point of sale. For each fill, we standardized out-of-pocket spending to a 30-day fill equivalent price. Next, we inflation adjusted spending to 2017 dollars using the medical component of the Consumer Price Index. In addition to measuring changes in the mean and distribution of out-of-pocket spending, we also evaluated changes in the proportion of patients paying $0 or paying more than $100 per fill. We selected $100 or more as having “high” out-of-pocket spending because prior evidence suggests that rates of treatment abandonment increase substantially at this spending level (7).
Key Independent Variables
We included the following key independent variables: whether the plan was fully insured vs self-funded, time (before vs after parity legislation), and the interaction between plan funding and time (the difference-in-differences estimator).
Statistical Analysis
Using a difference-in-differences design (14,15), we compared outcomes before vs after parity legislation for individuals in fully insured vs self-funded plans. For analyses of adherence and discontinuation, we estimated binomial or modified Poisson regression models with a log link using a generalized estimating equation approach with sandwiched standard errors (16). We controlled for potential confounders using inverse probability of treatment propensity score weights (hereafter described as propensity score–weighted models) for our primary outcome models. Covariates in the propensity score included age group, sex, ZIP-code-level socioeconomic variables from the 2010 American Community Survey (proportion unemployed, proportion insured, proportion living below the poverty level, proportion black race), specific drug initiated, quarter of treatment initiation, and whether the individual was enrolled in a high-deductible or consumer-driven health plan. Separate models were used for CML and multiple myeloma. For these models, we estimated unadjusted and propensity score weighted difference-in-differences risk ratios and 95% confidence intervals. Statistical tests were two-sided with P less than .05 denoting statistical significance.
For spending outcomes, we used propensity score–weighted quantile regression (17) to examine changes in the distribution of out-of-pocket spending at the 25th, 50th, 75th, 90th, and 95th percentiles of spending. We used linear regression (with an identity link and normal distribution) for estimating mean out-of-pocket spending and modified Poisson regression (16) with a log link to estimate the probability of paying $0 or, separately, paying more than $100 per fill, both applied using a generalized estimating equation approach with sandwiched standard errors.
For all outcomes, we tested for parallel trends in outcomes by plan funding status in the 20 months preparity, finding no difference in preparity trends (P > .10 for each outcome for CML and multiple myeloma).
Sensitivity Analyses
We used several robustness checks to confirm our study findings. First, because propensity score weighting can result in extreme weights for some observations, we revised our analyses and used propensity score trimming to exclude weights below the 5th and above the 95th percentiles of the propensity score distribution. We also estimated multivariable regression models instead of propensity score–weighted analysis for all outcomes. Next, because generic imatinib was available only in the later part of our study period, we excluded it in sensitivity analyses to avoid attributing effects of parity to effects of generic entry (instead of only controlling for treatment type initiated). Results of these analyses were similar to the primary analysis and, therefore, are not shown.
Results
Cohort Description
We identified 2082 TKI initiators and 3326 immunomodulatory drug initiators over our study period (Figure 1). Baseline characteristics of individuals in the cohort were generally similar before adjustment (Table 1), although patients in self-funded plans were more likely to be enrolled in high-deductible or consumer-driven health plans before (23.5% vs 15.8%) and after (27.3% vs 16.0%) parity than patients in fully insured plans. Compared with patients in fully insured plans, a slightly lower proportion of patients in self-funded plans used branded imatinib (66.8% vs 70.6%) before parity, and a slightly higher proportion used nilotinib post parity (33.1% vs 29.9%).
Figure 1.
Sample flow diagram. A) Patient selection for individuals with chronic myeloid leukemia and (B) Patient selection for individuals with multiple myeloma. Source: Authors’ analysis of Health Care Cost Institute Claims, 2008–2017. *Inclusion criteria: filled an orally administered anticancer drug of interest; was continuously enrolled in a health plan for 3 months pre- and 6 months postinitiation; aged 64 years or younger at index fill. We used an intent-to-treat approach, ignoring switching plan funding status changes postindex treatment (<1% of individuals meeting inclusion criteria switched plan funding types during the study period). †We excluded individuals with chronic myeloid leukemia (CML) who initiated bosutinib (n = 9) or ponatinib (n = 4) and individuals with multiple myeloma (MM) who initiated pomalidomide (n = 81) because these therapies are not typically used in the first-line setting for the disease of interest.
Table 1.
Unadjusted characteristics of patients initiating TKIs for CML or immunomodulatory drugs for multiple myeloma by plan funding status and time before and after parity*
| Characteristics | CML cohort |
Multiple myeloma cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Preparity |
Postparity |
Preparity |
Postparity |
|||||
| Fully insured | Self-funded | Fully insured | Self-funded | Fully insured | Self-funded | Fully insured | Self-funded | |
| (n = 523) | (n = 638) | (n = 432) | (n = 489) | (n = 879) | (n = 961) | (n = 755) | (n = 731) | |
| Patient characteristics | ||||||||
| Age category, y, % | ||||||||
| <45 | 40.3 | 38.6 | 36.3 | 35.4 | 8.8 | 8.1 | 7.3 | 8.8 |
| 45–54 | 31.4 | 28.8 | 30.6 | 33.7 | 29.9 | 30.6 | 31.0 | 27.6 |
| 55–64 | 28.3 | 32.6 | 33.1 | 30.9 | 61.3 | 61.3 | 61.7 | 63.6 |
| Sex, % female | 43.6 | 45.3 | 39.4 | 42.1 | 40.5 | 42.0 | 40.9 | 41.6 |
| Census-level SES, % | ||||||||
| Unemployed | 5.6 | 5.6 | 5.5 | 5.6 | 5.6 | 5.7 | 5.3 | 5.2 |
| Black population | 12.6 | 14.6 | 13.2 | 11.9 | 14.5 | 16.6 | 12.8 | 11.4 |
| Insured | 86.7 | 86.5 | 85.9 | 86.7 | 86.3 | 86.6 | 87.3 | 87.6 |
| Under FPL | 12.4 | 13.0 | 13.2 | 12.2 | 13.2 | 12.8 | 11.9 | 11.4 |
| Drug initiated | ||||||||
| Brand imatinib | 70.6 | 66.8 | 31.7 | 34.2 | — | — | — | — |
| Generic imatinib | — | — | 13.9 | 12.5 | — | — | — | — |
| Dasatinib | 16.4 | 16.9 | 29.9 | 33.1 | — | — | — | — |
| Nilotinib | 13.0 | 16.3 | 24.5 | 20.3 | — | — | — | — |
| Lenalidomide | — | — | — | — | 85.2 | 83.7 | 96.7 | 96.0 |
| Thalidomide | — | — | — | — | 14.8 | 16.3 | 3.3 | 4.0 |
| Year treatment initiated | ||||||||
| 2008 | 17.4 | 16.9 | — | — | 17.5 | 18.7 | — | — |
| 2009 | 22.4 | 18.0 | — | — | 21.1 | 21.4 | — | 0.3 |
| 2010 | 15.9 | 16.8 | 0.2 | 0.8 | 15.1 | 17.4 | 0.9 | 1.1 |
| 2011 | 14.2 | 15.7 | 1.9 | 3.3 | 15.8 | 16.8 | 3.3 | 4.4 |
| 2012 | 9.8 | 13.8 | 13.0 | 10.0 | 12.2 | 11.4 | 10.9 | 9.0 |
| 2013 | 9.2 | 8.5 | 10.7 | 12.7 | 9.2 | 7.8 | 9.8 | 11.8 |
| 2014 | 9.6 | 7.5 | 13.0 | 10.6 | 7.2 | 5.6 | 10.9 | 10.8 |
| 2015 | 1.7 | 2.7 | 18.3 | 18.2 | 1.9 | 0.8 | 18.3 | 20.1 |
| 2016 | — | 0.2 | 25.0 | 20.9 | — | — | 26.4 | 26.4 |
| 2017 | — | — | 18.1 | 23.5 | — | — | 19.6 | 16.1 |
| High-deductible health plan member | ||||||||
| Yes | 13.4 | 15.8 | 16.9 | 23.5 | 12.3 | 15.9 | 18.0 | 28.2 |
| Quarter diagnosed | ||||||||
| Q1 | 21.4 | 24.5 | 32.9 | 37.4 | 22.1 | 19.6 | 25.8 | 32.8 |
| Q2 | 28.9 | 27.7 | 29.9 | 23.9 | 26.6 | 28.8 | 31.3 | 27.8 |
| Q3 | 26.0 | 24.0 | 19.0 | 19.0 | 28.7 | 26.9 | 20.5 | 17.5 |
| Q4 | 23.7 | 23.8 | 18.3 | 19.6 | 22.6 | 24.7 | 22.4 | 21.9 |
CML = chronic myeloid leukemia; FPL = federal poverty level; SES = socioeconomic status; TKI = tyrosine kinase inhibitor.
Medication Adherence and Discontinuation
When comparing adherence and discontinuation among TKI users, we found that both fully insured and self-funded initiators saw increased adherence and decreased discontinuation in the postparity period. However, there were no statistically significant changes in adherence or discontinuation of anticancer therapy because of parity (Table 2). For patients initiating TKIs, adherence increased from 71.3% before parity to 74.7% after parity for those in fully insured plans. Over the same period, adherence increased from 73.5% to 74.7% in self-funded plans (adjusted difference-in-differences risk ratio [aDD RR] = 1.02, 95% CI = 0.91 to 1.15). We also observed relatively low rates of TKI discontinuation, ranging from 10.2% to 13.8% across all contrasts. Because of this, adherence results were similar when considering patients who did and did not discontinue (Table 2).
Table 2.
Association between parity legislation and orally administered anticancer medication adherence* and discontinuation
| Utilization outcomes | Fully insured, % |
Self-funded, % |
Ratio of risk ratios |
|||
|---|---|---|---|---|---|---|
| Preparity | Postparity | Preparity | Postparity | Adjusted DD risk ratio | 95% CI | |
| Total, n | 523 | 432 | 638 | 489 | — | — |
| TKI discontinuation, unadjusted | 14.3 | 10.2 | 13.9 | 9.4 | 1.05 | 0.65 to 1.71 |
| TKI discontinuation, propensity score weighted† | 13.7 | 10.2 | 13.8 | 10.8 | 0.96 | 0.53 to 1.71 |
| Adherence, unadjusted | 70.9 | 75.7 | 72.6 | 75.1 | 1.03 | 0.93 to 1.14 |
| Adherence, propensity score weighted† | 71.3 | 74.3 | 73.5 | 74.7 | 1.02 | 0.91 to 1.15 |
| Adherence among continuers, unadjusted | 82.8 | 84.3 | 84.3 | 82.8 | 1.04 | 0.95 to 1.12 |
| Adherence among continuers, propensity score weighted† | 82.6 | 82.7 | 85.3 | 83.7 | 1.02 | 0.93 to 1.16 |
| Total, n | 876 | 755 | 960 | 731 | — | — |
| Immunomodulatory drug discontinuation, unadjusted | 16.6 | 13.8 | 16.3 | 13.7 | 0.99 | 0.71 to 1.37 |
| Immunomodulatory drug discontinuation, propensity score weighted† | 16.3 | 15.7 | 15.5 | 15.8 | 0.95 | 0.66 to 1.36 |
| Adherence, unadjusted | 39.0 | 51.3 | 36.9 | 51.7 | 0.94 | 0.80 to 1.09 |
| Adherence, propensity score weighted† | 39.6 | 49.5 | 37.1 | 50.2 | 0.92 | 0.78 to 1.09 |
| Adherence among continuers, unadjusted | 46.8 | 59.5 | 44.0 | 59.9 | 0.93 | 0.81 to 1.08 |
| Adherence among continuers, propensity score weighted† | 47.3 | 58.7 | 44.0 | 59.6 | 0.92 | 0.79 to 1.06 |
Adherence and discontinuation were measured over 180 days postinitiation for the TKI users and from 120 days postinitiation for immunomodulatory drug users to account for differences in clinical use of products. Adherence was defined as 80% of days covered or more during the defined interval. Discontinuation was defined as having a gap of 60 or more days with no drug available during the defined interval. DD = difference-in-differences; TKI = tyrosine kinase inhibitor.
Generalized estimating equations with log links and binominal distributions. Models were estimated using PROC GENMOD in SAS 9.4. Adjusted models were propensity score weighted (PS model included age group, sex, ZIP code demographic variables [poverty, unemployment, race, insurance coverage], enrollment in a high-deductible or consumer-driven health plan, drug initiated, and quarter of the year treatment was initiated).
For patients initiating immunomodulatory drugs, adherence for 120 days postinitiation increased from 39.0% to 51.3% in fully insured plans and from 36.9% to 51.7% in self-funded plans, with no statistically significant differences due to parity (aDD RR = 0.94, 95% CI = 0.80 to 1.09). There were only minor changes in discontinuation over the same period in both groups. When restricting our adherence analysis to patients who continued treatment, we found the proportion of patients who are fully adherent reached 58.7% and 59.6% postparity in fully insured and self-funded plans, respectively.
Out-of-Pocket Spending
Before parity, the mean out-of-pocket spending for a 30-day supply of therapy was $480 (SD = $970) for TKIs and the median was $44 (interquartile range = $36–$273) (Table 3). For immunomodulatory drugs, the mean was $493 (SD = $1199) and the median was $65 (interquartile range = $41–$118). Parity was associated with average unadjusted differences in spending between $34 to $381 per fill for TKIs and from $12 to $424 for immunomodulatory drugs (Table 3). After adjustment, we observed savings consistently among the 25th and 50th percentiles of the out-of-pocket spending distribution but nonstatistically significant differences among higher spenders. Further, for patients with higher spending (90th and 95th percentiles), savings appeared to be driven by larger increases in spending among patients in self-funded plans than by reductions in spending in fully insured plans. Mean spending was not statistically different in either unadjusted or multivariable adjusted models.
Table 3.
Estimated out-of-pocket spending for the index prescription fill by spending quartile*
| Spending outcomes | Fully insured |
Self-funded |
Unadjusted difference-in-differences |
Adjusted difference-in-differences |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Preparity | Postparity | Preparity | Postparity | DD, $ | 95% CI, $ | aDD, $ | 95% CI, $ | P † | |
| TKIs, n | 523 | 432 | 638 | 489 | — | — | — | — | — |
| Mean‡ | $480 | $498 | $312 | $507 | −178 | −369 to 12.92 | −74 | −268 to 120 | .46 |
| 25th percentile | $37 | $0 | $29 | $30 | −38 | −38 to −37 | −35 | −35 to −34 | <.001 |
| 50th percentile | $44 | $20 | $46 | $55 | −34 | −44 to −25 | −17 | −24 to −9 | <.001 |
| 75th percentile | $273 | $88 | $103 | $150 | −232 | −342 to −122 | −181 | −270 to −91 | <.001 |
| 90th percentile | $1736 | $2621 | $920 | $1805 | −381 | −1206 to 444 | −96 | −741 to 549 | .77 |
| 95th percentile | $2488 | $3566 | $1877 | $2956 | −187 | −1198 to 824 | 73 | −343 to 1289 | .26 |
| Immunomodulatory agents, n | 523 | 432 | 638 | 489 | — | — | — | — | — |
| Mean | $493 | $474 | $369 | $433 | −82 | −248 to 83 | −14 | −186 to 158 | .87 |
| 25th percentile | $41 | $0 | $35 | $16 | −23 | −23 to −23 | −24 | −24 to −24 | <.001 |
| 50th percentile | $65 | $4 | $61 | $58 | −59 | −67 to −50 | −33 | −42 to −23 | <.001 |
| 75th percentile | $118 | $110 | $119 | $122 | −12 | −49 to 26 | 3 | −32 to 37 | .88 |
| 90th percentile | $2230 | $2539 | $961 | $1475 | −205 | −1100 to 690 | 833 | 219 to 1448 | .008 |
| 95th percentile | $3060 | $3240 | $2224 | $2841 | −438 | −1100 to 225 | 115 | −492 to 722 | .71 |
Quantile regression was estimated using PROC QUANTREG in SAS 9.4. Both models were adjusted using inverse probability of treatment propensity score weights. DD = diference-in-differences; TKI = tryosine kinase inhibitor.
Two-sided difference-in-difference P value.
Means were estimated via linear regression using a generalized estimating equation with an identity link and normal distribution. Models were estimated using PROC GENMOD in SAS 9.4.
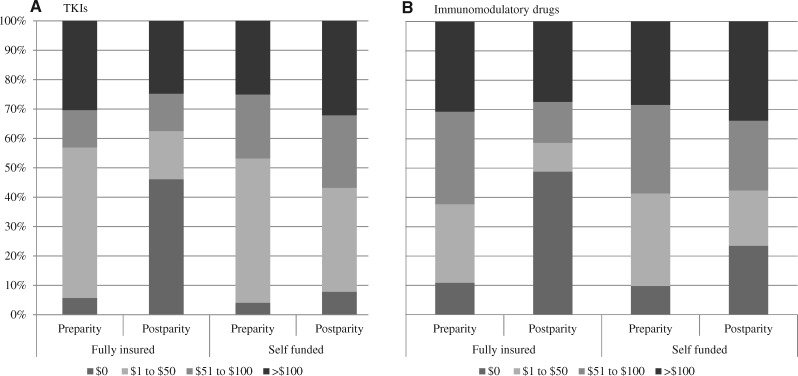
When considering changes in out-of-pocket spending, a key target of parity legislation, the proportion of TKI fills requiring out-of-pocket spending more than $100 was 30.3% before parity and 24.7% after parity in fully insured plans. This proportion increased in self-funded plans during the same period from 25.1% before parity to 32.1% after parity (aDD RR = 0.74, 95% CI = 0.55 to 1.01) (Figure 2A). During the same period, similar smaller changes were observed for immunomodulatory drug fills requiring out-of-pocket spending more than $100 (decreasing from 30.6% to 27.4% in fully insured plans and increasing from 28.4% to 33.8% in self-funded plans, respectively; aDD RR = 0.85, 95% CI = 0.68 to 1.06) (Figure 2B).
Figure 2.
Per-fill out-of-pocket spending on orally administered anticancer medications pre- and postparity, by plan funding status. A) Tyrosine kinase inhibitors and (B) Immunomodulatory drugs. Source: Authors’ analysis of Health Care Cost Institute Claims, 2008–2017. Per-fill out-of-pocket spending standardized to a 30-day supply equivalent. Dollars were inflation adjusted using the medical component of the Consumer Price Index to 2017 US dollars. Models were adjusted using inverse probability of treatment propensity score weights. Adjusted risk ratio (aRR) for paying more than $100 per fill: aRR chronic myeloid leukemia (CML) = 0.74, 95% confidence interval [CI] = 0.54 to 0.1.01, P = .06; aRR multiple myeloma (MM) = 0.85, 95% CI = 0.68 to 1.06, P = .15. aRR for paying $0 per fill: aRR CML = 4.27, 95% CI = 2.20 to 8.27, P ≤ .001; aRR MM = 1.96, 95% CI = 1.40 to 2.73, P ≤ .001. TKI = tyrosine kinase inhibitor.
At the same time, for both TKIs and immunomodulatory drugs there was a substantial increase in the proportion of fills with $0 out-of-pocket spending for patients in fully insured plans after parity (from 5.7% to 46.1% for TKIs and from 10.7% to 49.5% for immunomodulatory drugs). Compared with changes in self-funded plans during the same period, this resulted in an approximate quadrupling of $0 fills for TKIs (aDD RR = 4.27, 95% CI = 2.20 to 8.27) and doubling for immunomodulatory drugs (aDD RR = 1.96, 95% CI = 1.40 to 2.73).
Discussion
Among a cohort of privately insured patients initiating TKIs for CML or immunomodulatory drugs for multiple myeloma, we found modest increases in adherence over time but no association between parity and adherence or discontinuation. In fully insured plans, up to one-half of patients initiating treatment after parity required no out-of-pocket spending; however, approximately 25% required per-fill spending more than $100. Postparity, we observed that many fully insured and self-funded individuals continued to pay high initial prices for anticancer treatment. Prior research has shown that orally administered anticancer medications with out-of-pocket prices of $100 or more have abandonment rates among commercially insured patients ranging from 29% to 67%, including among the agents studied here, making it an important threshold for considering treatment access (7). Perhaps more concerning is our finding that out-of-pocket spending for high spenders (those at the 90th and 95th percentile of spending) increased postparity. Although spending increased less among those in fully insured plans, both groups saw large out-of-pocket spending increases. This could be due to increases in drug prices and general trends in health insurance benefit designs over time such as increased use and size of deductibles and greater use of coinsurance (paying a percentage of the drug’s price) rather than flat copayments. Such changes may apply similarly to medical and pharmacy benefits, which would meet parity’s technical requirements that medical and pharmacy benefits be the same without specifying that the benefits be generous.
Importantly, during the later years of our study period (2014–2017), the Affordable Care Act required commercial health plans to cap total out-of-pocket spending, including out-of-pocket spending on prescription drugs, at $7900 for an individual enrollee and $15 800 for a family in 2019. Patients could still face higher out-of-pocket spending for drugs relative to medical benefits before reaching the out-of-pocket cap, but once the cap is reached patient out-of-pocket spending is no longer required. This could result in patients having more consistent access to drugs in all health plans studied (fully insured and self-funded) in these later years, lessening the estimated impact of parity. Although these limits act to shield patients from very high out-of-pocket spending, it is important to recognize that out-of-pocket maximums for commercial health plans still require substantial out-of-pocket spending. For patients needing chronic use of anticancer medications, where point-of-sale prices can exceed $14 000 per fill (18), affordability may continue to be a problem.
Our finding of improved adherence and reductions in discontinuation in both fully insured and self-funded patients over the study period might have been influenced by out-of-pocket maximums offered under the Affordable Care Act or through increases in other sources of cost-sharing subsidies such as coupons or patient assistance programs. To the extent that coupon use or funding for assistance programs increased over our study period, it might explain similar improvements in these outcomes for individuals in our cohort in both fully insured and self-funded plans. Alternatively, increasing adherence could be related to increased efforts by providers to promote adherence or expanded use of automatic refilling by pharmacies and pharmacy benefit managers.
A strength of this analysis was our use of within-state controls for understanding the impact of parity on patients in plans that were subject to parity. Nevertheless, this evaluation could not account for detailed clinical characteristics of patients. Unlike registry-linked databases, claims data have very limited information regarding disease severity, the date of initial diagnosis, and prior treatments. Also, we were unable to identify decisions to stop treatment that are clinically indicated, such as due to an adverse event, disease progression, or planned discontinuation for stem cell transplantation (for multiple myeloma). We restricted our follow-up period for measuring discontinuation and adherence to 180 days postinitiation for TKI users and 120 days for immunomodulatory drug users to partially account for expected clinical use but recognize that there may be patients for whom stopping therapy is appropriate during that period. Further, we used the PDC to measure adherence to therapy (13), a measure of refill adherence. However, we cannot be sure whether the patient ingested the medication. We were also unable to observe use of manufacturer coupons or patient assistance programs and only observed prescription fills. This means that patients with very high cost-sharing who did not fill their prescriptions are unobserved (6,7). We also could not fully account for deductibles or other spending that might have resulted in patients having low or no initial out-of-pocket costs for their initial treatment (such as having already reached the out-of-pocket cap). We used health plan–derived information on enrollment in a high-deductible or consumer-driven health plan and the quarter of the year when the prescription was filled as a proxy for these measures instead. We also did not evaluate changes in out-of-pocket spending for infused drugs offered under the medical benefit, which is an important area for future research when considering access to anticancer treatments. Finally, despite the millions of covered lives represented by the data source, only a small number of individuals used the drugs of interest, which could limit power and generalizability of our findings. That said, no other data source includes adequate sample size and plan funding data necessary to complete this study. Although each of these limitations is important, they introduce bias into our work only if they are differentially captured over time within the treatment group (fully insured patients) and the control group (self-funded patients), which we believe is unlikely.
States have rapidly expanded their adoption of oral oncology parity laws since 2008, and the Cancer Drug Coverage Parity Act (H.R.2739, S.1566), which would expand parity to all states and individuals in self-funded plans, was introduced in Congress in 2015. Our results suggest that the impact of parity on treatment discontinuation and adherence may be limited, like prior studies and reviews on the topic (12,19,20). Parity is unlikely to be a complete solution for improving access to anticancer drugs, particularly as commercial plans move towards greater use of deductibles and coinsurance, although it may help to limit price increases for high spenders. Additional efforts may be needed to ensure that chronic-use, high-value anticancer drugs are accessible and affordable for patients needing them.
Funding
This project was supported by a Research Scholar Grant, RSGI-14-030-01-CPHPS, from the American Cancer Society (SBD). NLK was also supported by K24CA181510 from the National Cancer Institute. ANW was supported by a National Center for Advancing Translational Sciences, National Institutes, Award Number KL2TR001438. AO was supported by 128608-RSGI-15-211-01-CPHPS from the American Cancer Society.
Notes
Dr Dusetzina had full access to the data and takes responsibility for the integrity of the data and accuracy of the analysis. All authors contributed to the design, analysis, drafting, and critical revision of this manuscript, and all authors have approved the final version.
The study sponsor had no role in the design, analysis, interpretation of the findings; drafting the manuscript; or the decision to submit this manuscript for publication. The authors acknowledge the assistance of the Health Care Cost Institute and its data contributors in providing the claims data analyzed in this study.
Drs Dusetzina, Huskamp, Keating, Winn, and Olszewski and Ms Jazowski have no conflicts to disclose. Dr Wood reports receiving research funding from Genentech and Pfizer. Ms Jazowski is a predoctoral fellow at the Duke Department of Population Health Sciences.
Dr Basch receives research funding from the National Cancer Institute and the Patient Centered Outcomes Research Institute, research consultant funds from Memorial Sloan Kettering Cancer Center and Dana Farber Cancer Institute, editorial funds from the Journal of the American Medical Association, and scientific advisor fees from Sivan, Self Care Catalyst, and CareVive.
References
- 1. Kumar SK. Management of multiple myeloma. J Natl Compr Canc Netw. 2018;16(5S):624–627. [DOI] [PubMed] [Google Scholar]
- 2. Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(9):1108–1135. [DOI] [PubMed] [Google Scholar]
- 3. Wang B, Joffe S, Kesselheim AS.. Chemotherapy parity laws: a remedy for high drug costs? JAMA Intern Med. 2014;174(11):1721–1722. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser Family Foundation. Some states mandate better coverage of oral cancer drugs. http://www.kaiserhealthnews.org/features/insuring-your-health/2012/cancer-drugs-by-pill-instead-of-iv-michelle-andrews-051512.aspx. Published 2012. Accessed September 18, 2013.
- 5. Raborn ML, Pelletier EM, Smith DB, Reyes CM.. Patient out-of-pocket payments for oral oncolytics: results from a 2009 US claims data analysis. J Oncol Pract. 2012;8(3S):9s–15s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Streeter SB, Schwartzberg L, Husain N, Johnsrud M.. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011;7(3S):46s–51s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doshi JA, Li P, Huo H, Pettit AR, Armstrong KA.. Association of patient out-of-pocket costs with prescription abandonment and delay in fills of novel oral anticancer agents. J Clin Oncol. 2018;36(5):476–482. [DOI] [PubMed] [Google Scholar]
- 8. Winn AN, Keating NL, Dusetzina SB.. Factors associated with tyrosine kinase inhibitor initiation and adherence among Medicare beneficiaries with chronic myeloid leukemia. J Clin Oncol. 2016;34(36):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL.. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 10. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim YA, Rascati KL, Prasla K, Godley P, Goel N, Dunlop D.. Retrospective evaluation of the impact of copayment increases for specialty medications on adherence and persistence in an integrated health maintenance organization system. Clin Ther. 2011;33(5):598–607. [DOI] [PubMed] [Google Scholar]
- 12. Dusetzina SB, Huskamp HA, Winn AN, Basch E, Keating NL.. Out-of-pocket and health care spending changes for patients using orally administered anticancer therapy after adoption of state parity laws. JAMA Oncol. 2018;4(6):e173598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hess LM, Raebel MA, Conner DA, Malone DC.. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. [DOI] [PubMed] [Google Scholar]
- 14. Dimick JB, Ryan AM.. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. [DOI] [PubMed] [Google Scholar]
- 15. Wooldridge JM. What's new in econometrics? Difference-in-differences estimation. http://wwwnberorg/WNE/slides 7–3 l-07/slides_10_diffindiffspdf2007. Accessed November 4, 2018.
- 16. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 17. Le Cook B, Manning WG.. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry. 2013;25:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dusetzina SB, Huskamp HA, Keating NL.. Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA. 2019;321(20):2025–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin AL, Bentley JP, Pollom EL.. The impact of state parity laws on copayments for and adherence to oral endocrine therapy for breast cancer. Cancer-Am Cancer Soc. 2019;125(3):374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kircher SM, Meeker CR, Nimeiri H, et al. The parity paradigm: can legislation help reduce the cost burden of oral anticancer medications? Value Health. 2016;19:88–98. [DOI] [PubMed] [Google Scholar]