Abstract
Background
Diffusion magnetic resonance (MR) characteristics are a predictive imaging biomarker for survival benefit in recurrent glioblastoma treated with anti-vascular endothelial growth factor (VEGF) therapy; however, its use in large volume recurrence has not been evaluated.
Objective
To determine if diffusion MR characteristics can predict survival outcomes in patients with large volume recurrent glioblastoma treated with bevacizumab or repeat resection.
Methods
A total of 32 patients with large volume (>20 cc or > 3.4 cm diameter) recurrent glioblastoma treated with bevacizumab and 35 patients treated with repeat surgery were included. Pretreatment tumor volume and apparent diffusion coefficient (ADC) histogram analysis were used to phenotype patients as having high (>1.24 μm2/ms) or low (<1.24 μm2/ms) ADCL, the mean value of the lower peak in a double Gaussian model of the ADC histogram within the contrast enhancing tumor.
Results
In bevacizumab and surgical cohorts, volume was correlated with overall survival (Bevacizumab: P = .009, HR = 1.02; Surgical: P = .006, HR = 0.96). ADCL was an independent predictor of survival in the bevacizumab cohort (P = .049, HR = 0.44), but not the surgical cohort (P = .273, HR = 0.67). There was a survival advantage of surgery over bevacizumab in patients with low ADCL (P = .036, HR = 0.43) but not in patients with high ADCL (P = .284, HR = 0.69).
Conclusion
Pretreatment diffusion MR imaging is an independent predictive biomarker for overall survival in recurrent glioblastoma with a large tumor burden. Large tumors with low ADCL have a survival benefit when treated with surgical resection, whereas large tumors with high ADCL may be best managed with bevacizumab.
Keywords: Diffusion MRI, ADC histogram analysis, T1 subtraction, Recurrent glioblastoma, Bevacizumab
ABBREVIATIONS
- ADC
apparent diffusion coefficient
- BTIP
brain tumor imaging protocol
- MGMT
06-methylguanine-DNA methyltransferase
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OS
overall survival
- VEGF
vascular endothelial growth factor
- VOI
volume of interest
Glioblastoma continues to be a devastating disease with a uniformly poor prognosis of 14 to 21 mo from diagnosis1-3 and only a small proportion of patients survive more than 5 yr.1-3 Standard of care for patients with newly diagnosed glioblastoma includes concurrent temozolomide plus radiation therapy, followed by temozolomide with or without tumor treating fields.1-3 Upon recurrence few effective treatment options exist, and this is acutely true in patients with large volume recurrence, who are more likely to be transitioned to supportive care.4 Bevacizumab was approved for use in recurrent GBM in 2009 after it was shown to improve progression-free survival5,6; however, randomized phase II trials have not demonstrated an overall survival (OS) benefit when including all patients with recurrent GBM5,7 despite widespread exploration of a number of anti-vascular endothelial growth factor (VEGF) therapies in recurrent GBM.
Although bevacizumab remains an important therapeutic agent for treatment of recurrent GBM, as almost all patients will be exposed to bevacizumab sometime during treatment of their disease, contemporary use in the academic centers in the US tends to be limited to patients who have had multiple relapses or treatment failures, patients who have large tumors or extensive cerebral edema, and/or patients who otherwise have no other treatment or clinical trial options, with additional use among less specialized practitioners.8 Therefore, there is a significant clinical need to identify large volume recurrent GBM patients that may have a significant survival benefit from treatment of anti-VEGF therapy to guide therapy at this clinical junction.
One strategy to identify these patients is to use pretreatment imaging as a proxy for underlying biologic characteristics. One example of this is the use of diffusion magnetic resonance imaging (MRI). Diffusion MRI serves as biomarker for glioma cellularity, with clinical apparent diffusion coefficient (ADC) and tumor cell density being negatively correlated.9 These areas, hypothesized to be areas of active and rapid cell growth are important targets for surgical resection, with residual low ADC/high cellularity areas serving as a poor prognostic marker.4,10,11 The connection between differences in diffusion imaging and underlying tumor biology may allow diffusion MRI to serve as a prognostic marker.
Extensive retrospective data in both single-center and multi-center phase II trials in recurrent GBM have suggested that pretreatment diffusion MRI characteristics are predictive of overall survival when treated with anti-VEGF therapies including bevacizumab, cediranib, cabozantinib, and aflibercept; but are not predictive of overall survival when treated with cytotoxic chemotherapies.12-14 Specifically, patients with elevated ADCL, the mean value of the lower peak in a double Gaussian mixed model applied to ADC measurements in contrast enhancing tumor, were found to have a significant survival benefit in recurrent GBM treated with anti-VEGF therapy compared to patients with a low ADCL. Therefore, we hypothesize contemporary recurrent GBM patients with large tumor burden exhibiting high ADCL will have a significant survival advantage when treated with bevacizumab compared to large tumors with low ADCL. Additionally, since low ADCL may imply a more densely packed tumor, we hypothesize that patients with low ADCL may benefit from an additional surgery compared to treatment with bevacizumab. Therefore, the objective of the current retrospective study was to first confirm that diffusion magnetic resonance (MR) phenotypes can predict survival in large recurrent GBMs treated with bevacizumab and second to test whether these same phenotypes can predict patients who may have value a survival benefit from an additional surgical resection.
METHODS
Patient Population
Institutional review board approval was obtained for this study. Written consent was obtained from all patients prior to treatment. We retrospectively gathered clinical and imaging data for patients treated with recurrent glioblastoma at our institution. Clinical decision making to treat with bevacizumab or repeat surgery was not informed by diffusion MRI phenotypes analysis and was the decision of the care provider team.
Bevacizumab-Treated Recurrent GBM
Written consent was obtained from all patients prior to treatment. A total of 80 recurrent glioblastoma patients from our institution who were treated with bevacizumab over the past 5 yr with high quality anatomic and diffusion MRI data were included. Specifically, inclusion criteria included: 1) pathologically confirmed glioblastoma with recurrence based on MR imaging; 2) no previous exposure to anti-VEGF therapy; 3) treatment with bevacizumab (5-10 mg/kg body weight with or without adjuvant chemotherapy) occurring at least 3 mo after completion of radiation therapy to decrease the chance of treatment-induced pseudoprogression; and 4) pretreatment MR imaging including diffusion MR images available. Of the 80 patients identified, 32 had large contrast enhancing tumors (>20 cc or > 3.4 cm diameter, group average) for use in final analyses. Of these 32 patients, 19% patients were treated with bevacizumab monotherapy while 81% of patients were treated with bevacizumab and concurrent chemotherapy including temozolomide and small molecular inhibitors.
Recurrent GBM Treated with Additional Surgical Resection
A total of 71 patients with recurrent GBM underwent a repeat surgical resection and met the following inclusion criteria: 1) pathologically confirmed glioblastoma with recurrence based on MR imaging; 2) no previous exposure to anti-VEGF therapy; 3) pretreatment MR imaging including diffusion MRI available; and 4) underwent repeat second surgical resection at least 3 mo after completion of radiation therapy. Of these 71 patients, 35 patients had large (>20 cc or 3.4 cm diameter) contrast enhancing tumors for use in the final analysis. Of these 35 patients, 60% received bevacizumab sometime during the course of their disease, after repeated resection surgery, while 40% did not receive bevacizumab.
Anatomic and Diffusion MRI Acquisition
Standard and diffusion MR data were acquired in a manner previously used for ADC analysis.12-14 Specifically, MR data were acquired using either a 1.5T or a 3T MR scanner from an MR scanner manufactured by Siemens Healthcare (Erlangen, Germany) or GE Medical Systems (Waukesha, Wisconsin). All patients received pre- and postcontrast T1-weighted images (gadopentetate dimeglumine [Magnevist; Berlex], at a concentration of 0.1 mmol/kg) along with T2-weighted and T2-weighted FLAIR images according to the standardized brain tumor imaging protocol (BTIP).15 In addition, all patients received either diffusion weighted imaging according to BTIP recommendations15 (3 mm slice thickness with no interslice gap with b-values of 0, 500, and 1000 s/mm2) or diffusion tensor imaging with 64 directions and 2 mm isotropic resolution with b-values of 0 and 1000 s/mm2. ADC maps were calculated offline using b = 0 s/mm2 and b = 1000 s/mm2 images. Anatomical T2-weighted and T2-weighted FLAIR images were not used in the current study. All examinations were acquired within 14 d of starting therapy.
Contrast-Enhanced T1-Weighted Digital Subtraction Maps (T1 Subtraction Maps)
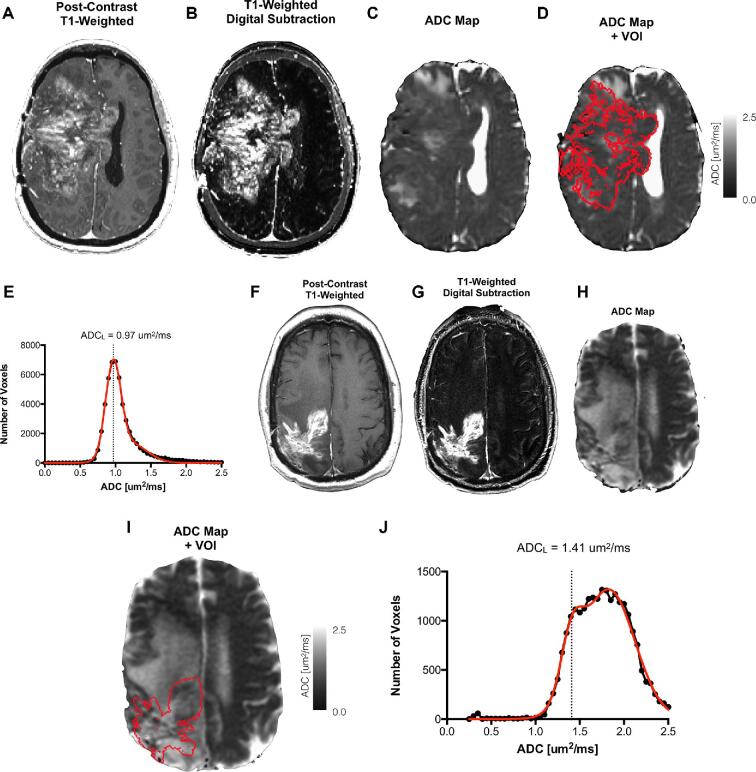
We have previously described our methods for creation of T1 subtraction maps.12,16,17 Briefly, T1-weighted MR images with and without contrast were registered using FSL (FLIRT; FMRIB Software Library, Oxford, England; http://www.fmrib.ox.ac.uk/fsl/). Intensities were normalized using the National Institutes of Mental Health Magnetoencephalography 3Core Facility (3dNormalize; NIMH MEG Core, Bethesda, Md; kurage.nimh.nih.gov/meglab/Med/3dNormalize) and the following equation: [SNor(x, y, z) = S (x, y, z)/σWB] (SNor = normalized image intensity; S = unnormalized image intensity; WB = whole brain). These normalized images underwent voxel by voxel subtraction of intensities (Figure 1A and 1B). The resultant subtraction map was manually reviewed for quality control and contrast enhancing regions were identified to signify a volume of interest for further analysis (Figure 1C and 1D).
FIGURE 1.
ADC Histogram Analysis. A, Postcontrast T1-weighted image; B, T1-weighted digital subtraction map; C, ADC map; D, fused ADC map and volume of interest (VOI) defined from T1 subtraction maps; and E, resulting ADC histogram with double Gaussian fit showing low ADCL phenotype (ADCL = 0.97 μm2/ms). F, Postcontrast T1-weighted image; G, T1-weighted digital subtraction map; H, ADC map; I, fused ADC map and VOI; and J, resulting ADC histogram showing high ADCL phenotype (ADCL = 1.41 μm2/ms).
ADC Histogram Analysis
We have previously described our methods for ADC Histogram Analysis.12,16,17 ADC characteristics within T1 subtraction-defined enhancing tumor volumes were used for ADC histogram analysis (Figure 1E-1H). Nonlinear regression using a double Gaussian mixed model was performed to parameterize ADC histograms using GraphPad Prism, Version 4.0c (GraphPad Software, San Diego, California) or MATLAB (Release 2018b Version 9.5.0). The double Gaussian equation used was:
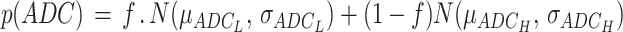 |
In this equation, p(ADC) = ADC probability; f = lower histogram members (voxels); N(μ, σ) = normal Gaussian distribution; ADCL = lower Gaussian distribution; ADCH = upper Gaussian distribution (Figure 1I, 1J). These regressions were manually reviewed for quality control and fit was evaluated with adjusted R2 > 0.7.12,13,18-20
Statistical Analyses and Interpretation
Analysis was carried out between ADCL phenotypes (low or high ADCL) and treatment groups (bevacizumab or surgery). Unpaired t-tests with and without Welch's correction for unequal variance were used to test differences in tumor measurements and demographic characteristics between treatment groups. Continuous values of volume and ADCL phenotypes using a previously described threshold of 1.24 μm2/ms were used to predict OS for each therapy using a combination of log-rank analyses on Kaplan Meier data and Cox proportional hazard models of age, tumor volume and ADCL phenotypes. For all analyses, P < .05 was considered statistically significant. No corrections for multiple comparisons were performed. Statistical analyses were performed with Stata 12 (2011; College Station, Texas) or GraphPad Prism v6.0 h (GraphPad Software, Inc., La Jolla, California) All errors are presented in standard error of the mean.
RESULTS
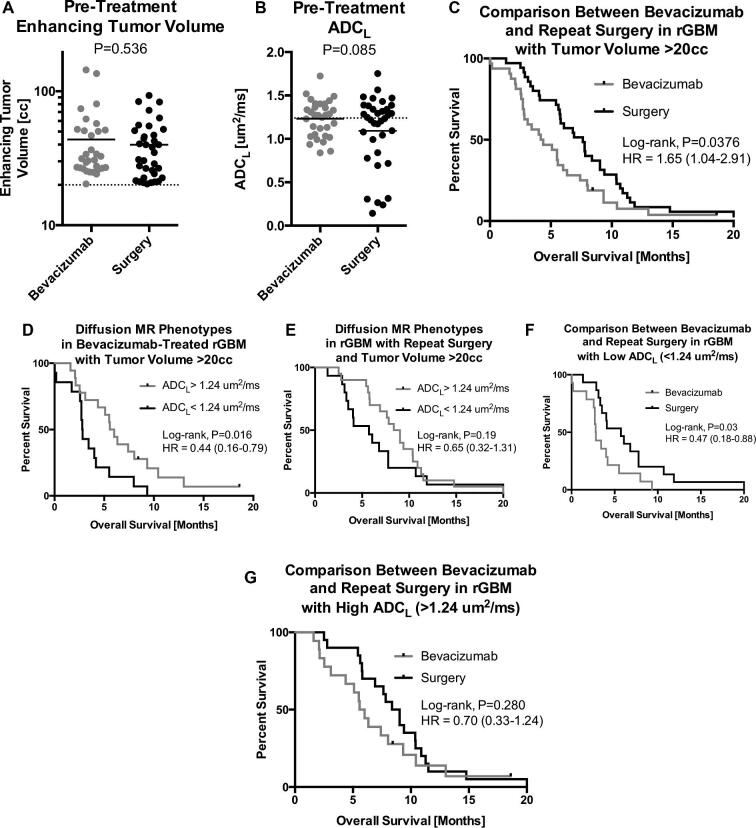
We identified 80 patients receiving bevacizumab and 71 patients receiving repeat surgery within our Neuro Oncology Database in whom the initial inclusion criteria were satisfied; of which 32 bevacizumab patients (40%) and 35 surgical patients (49%). The overall group average was 20 cc or ∼3.4 cm in diameter and this was used as a cut off for large tumor recurrence. No difference in volume (Figure 2A and Table 1; P = .536) was observed between the bevacizumab-treated patients with large tumors (average = 43.7 cc, range 20-144 cc) or large tumors treated with repeat resection (average = 39.9 cc, range 20-93 cc), nor was there any significant difference in ADCL (Figure 2B; P = .085) or average age (Table 1; P = .982). Patients treated with bevacizumab had an average of 2.0 recurrences prior to treatment whereas surgical patients had an average of 1.3 (P < .001). There were no significant differences in prognostic genetic characteristics between two groups including IDH mutation (3.1% vs 5.7%; P = .615) and 06-methylguanine-DNA methyltransferase (MGMT) promoter methylation (31.3% vs 25.7%; P = .622). High ADCL tumors were associated with higher rates of IDH mutation, but this was not statistically significant (5.3% vs 3.5% P = .727). There was no significant difference in prior salvage therapy. This included lomustine (28.1% vs 20%; P = .444), Toca 511/FU (9.4% vs 8.6%; P = .910), checkpoint inhibitors (pembrolizumab, nivolumab, 18.8% vs 20%; P = .899), and EGFR inhibitors (9.8% vs 5.7%; P = .576). There was no difference between time from initial treatment to treatment for large volume recurrence (either bevacizumab or repeat surgery) (16.51 vs 16.50 mo; P = .999). According to our data, patients with large enhancing tumors, in general, had a significant survival advantage if treated with repeat surgical resection compared with bevacizumab (Figure 2C; Log-rank, P = .0376, HR = 1.65, median OS = 7.6 vs 4.3 mo). Given the importance of initial extent of resection on overall survival in glioblastoma, we evaluated whether initial extent of resection affected overall survival after large volume recurrence. Gross total resection was achieved in 41 (62%) patients, subtotal resection in 22 (33%) and biopsy only in 4 (6%). There was no significant difference in overall survival from recurrence in patients who had initial gross total or subtotal resection (median survival 5.8 vs 5.5 mo; Log-Rank P = .732). However, there was a trend towards increased survival advantage was larger in patients with gross total resection relative to subtotal resection on repeat surgery (median survival 8.3 vs 5.8 mo; Log Rank P = .516). There was no difference in ADC phenotypes in patients with gross total resection or subtotal resection (42.8% each; P > .99). There were no new permanent postoperative neurologic deficits after repeat surgery.
FIGURE 2.
A, Comparison of pretreatment tumor volume between bevacizumab-treated recurrent GBM and recurrent GBM treated with repeat surgical resection (P = 6.53). B, Comparison of ADCL between bevacizumab-treated recurrent GBM and recurrent GBM treated with repeat surgical resection (P = .085). C, Kaplan-Meier curves comparing survival between bevacizumab and surgery in recurrent GBM with large tumor burden (>20 cc) (P = .0376). D, Kaplan-Meier curves comparing survival differences between diffusion MR phenotypes in large, recurrent GBM treated with bevacizumab (P = .016). E, Kaplan-Meier curves comparing survival differences between diffusion MR phenotypes in large, recurrent GBM treated with repeat surgical resection (P = .19). F, Kaplan-Meier curves comparing survival differences between bevacizumab and surgery within large, recurrent GBM with low ADCL (P = .03). G, Kaplan-Meier curves comparing survival differences between bevacizumab and surgery within large, recurrent GBM with high ADCL (P = .280).
TABLE 1.
Cohort Characteristics
| Bevacizumab | Surgical | ||
|---|---|---|---|
| Characteristic | (n = 32) | (n = 35) | P value |
| Age (y) | 55.1 | 55.2 | .982 |
| Tumor volume (cc) | 43.7 | 39.9 | .536 |
| ADCL (μm2/ms) | 1.23 | 1.09 | .092 |
| Initial GTR | 47% | 74% | .021 |
| IDH mutation | 3.1% | 5.7% | .615 |
| MGMT methylation | 31.3% | 25.7% | .622 |
| Prior salvage therapy | |||
| Chemotherapy | 28.1% | 20% | .444 |
| Toca 511/FU | 9.4% | 8.6% | .910 |
| Immunotherapy | 18.8% | 20% | .899 |
| EGFR inhibitors | 9.8% | 5.7% | .576 |
| Time from initial treatment to recurrence (mo) | 16.51 | 16.50 | .999 |
| Adjuvant therapy after 2nd resection | |||
| Chemotherapy | 40% | ||
| Immunotherapy | 29% | ||
| EGFR inhibitors | 17% | ||
| Bevacizumab | 60% |
Effects of Pretreatment Tumor Volume on Survival
Cox proportional hazard regression analyses considering the effects of age and pretreatment tumor volume were carried out separately for bevacizumab and surgical patients. Results indicated that tumor volume was a significant predictor of survival in both groups (Table 2); however, bevacizumab-treated patients had worse outcome with increasing tumor burden (Cox, P = .009, HR = 1.02), whereas patients treated with repeated resection had better outcome if they were larger prior to surgery (Cox, P = .006, HR = 0.96).
TABLE 2.
Volume Survival Analysis
| Variable | P value | Hazard ratio | CI |
|---|---|---|---|
| Bevacizumab cohort | |||
| Age | .423 | 0.985 | 0.95-1.02 |
| Tumor volume | .009 | 1.02 | 1.01-1.04 |
| Surgical cohort | |||
| Age | .379 | 1.01 | 0.99-1.04 |
| Tumor volume | .006 | 0.96 | 0.94-0.99 |
CI, Confidence interval.
Bold indicates statistical significance (P < .05).
Diffusion MR Phenotypes Predict Survival in Recurrent GBM Treated With Bevacizumab
A total of 15 of the 32 (47%) bevacizumab treated patients and 15 of the 35 (43%) patients treated with surgery had a low ADCL phenotype (<1.24 μm2/ms). Cox regression including age, tumor volume, and ADCL phenotype confirmed that both pretreatment tumor volume (Table 3; Cox, P = .021, HR = 1.02) and ADCL phenotype (P = .049, HR = 2.25) were predictive of survival in patients treated with bevacizumab (Figure 2D). Log-rank analysis based only on ADCL phenotype also stratified long and short-term survivors (P = .016, HR = 0.44, median OS = 5.8 vs 2.8 mo). Results also indicated that presurgical tumor volume was an independent predictor of survival benefit in patients treated with repeated resection (Table 3; Cox, P = .008, HR = 0.97), with larger tumors benefiting more than smaller tumors, but ADCL phenotype (P = .273) and age (P = .586) were not independently predictive of outcome. Unlike bevacizumab-treated patients, ADCL phenotype alone was not predictive of outcome in patients treated with surgery (Figure 2E), although there was a similar trend toward better outcomes in patients with higher ADCL (Log-rank, P = .19, HR = 0.65, median OS = 8.7 vs 5.7 mo).
TABLE 3.
Diffusion Phenotype Survival Analysis
| Variable | P value | Hazard ratio | CI |
|---|---|---|---|
| Bevacizumab cohort | |||
| Age | .930 | 0.99 | 0.96-1.04 |
| Tumor volume | .021 | 1.02 | 1.01-1.04 |
| ADCL | .049 | 2.25 | 1.03-5.49 |
| Surgical cohort | |||
| Age | .586 | 1.01 | 0.98-1.04 |
| Tumor volume | .008 | 0.97 | 0.94-0.99 |
| ADCL | .273 | 1.50 | 0.73-3.08 |
CI, Confidence interval.
Bold indicates statistical significance (P < .05).
Recurrent GBM With Low ADCL Benefits From Repeat Surgical Resection
Patients with large enhancing tumors and a low ADCL phenotype appear to have a significant survival advantage when treated with surgery compared with bevacizumab treatment (Figure 2F and Table 4; Log-rank, P = .03, HR = 0.47). No survival difference was observed between treatment arms in tumors with high ADCL (Figure 2G; Log-rank, P = .280, HR = 0.70).
TABLE 4.
Survival Analysis Stratified by Diffusion Phenotype
| Variable | P value | Hazard ratio | CI |
|---|---|---|---|
| ADCL < 1.24 Cohort | |||
| Surgery | .036 | 0.433 | 0.20-0.95 |
| ADCL > 1.24 cohort | |||
| Surgery | .284 | 0.693 | 0.36-1.36 |
CI, Confidence interval.
Bold indicates statistical significance (P < .05).
DISCUSSION
This study shows that preoperative MR phenotype using diffusion sequences of large (>20 cc) recurrent GBM can predict overall benefit when treated with bevacizumab. This is consistent with numerous reports demonstrating this to be the case with anti-VEGF therapy for recurrent GBM,12,13,19,20 but also suggests this to be the case even among contemporary patients with very large tumors, which are often treated with bevacizumab when other treatment options are off the table. Additionally, previous studies have suggested this diffusion phenotype is predictive for anti-VEGF therapies but not prognostic for all therapies including systemic chemotherapies12,13 (eg, lomustine or temozolomide). Results from the current study support these previous findings, suggesting that diffusion MRI is predictive of outcome in recurrent GBM treated with bevacizumab, but not repeat surgical resection.
While a significant difference in survival was observed between the two treatment arms regardless of similar distributions in tumor size, age, and diffusion characteristics, patients with unfavorable diffusion phenotypes4,10,11 (“low ADC”, ADCL < 1.24 μm2/ms) may significantly benefit from surgical resection, if safely possible, compared with bevacizumab treatment. It is important to note, however, that there also appeared to be a trend toward better survival in patients treated with surgery in patients with a favorable diffusion phenotype; this benefit to repeat resection has been previously described.21
While both tumor and treatment related factors effect survival, certain tumor related characteristics such as location, infiltration, gliomatosis, and volume are extremely important in prognostication.22 Multiple studies have demonstrated that patients with larger tumors generally do worse when treated with systemic chemotherapies than patients with smaller tumors in both newly diagnosed GBM22 and at first or second relapse.17 Interestingly and perhaps counterintuitively, results from the current study suggest patients with larger tumors actually have a better outcome than smaller tumors when treated with repeat surgical resection. Despite our efforts to match patient demographics (age, previous salvage therapy, initial extent of resection) and tumor imaging characteristics, this difference may speak to some potential bias in our data, as patients with large tumor burden that appear eligible for re-resection are likely to have slower growing tumors or tumors distal from eloquent structures.23
Our study provides data to help clinicians make evidence-based treatment decisions in recurrent GBM. However, management strategy in recurrent large volume GBM is a complex clinical decision that includes several factors. One major consideration is relief of current or expected symptomatic mass effect that could help prevent loss of functional independence and therefore quality of life.21,24,25 As we identified in this study, patients with repeat craniotomy had further adjuvant therapy including repeat chemotherapy, small molecular inhibitors, and immunotherapy and therefore repeat surgery could allow for re-analysis of recurrent tumor for selection and monitoring of adjuvant therapy. Lastly, as seen in this study, several patients with repeat surgery received adjuvant bevacizumab, but the reverse was not seen as in many cases this precludes surgery even if there was increased symptomatic mass effect. Therefore, our results represent a quantitative tool in the complex management paradigm of recurrent GBM that requires combination of survival data and clinical situation.
Limitations
In addition to potential bias in the surgical cohort, recent evidence suggests MGMT promoter methylation status may predict response to bevacizumab.26,27 Although a potential limitation, this was not examined in the current study as there is no evidence to suggest MGMT status has an impact on surgical efficacy. Another potential limitation to the current retrospective study is the potential impact from the sequence of therapies in each patient. As mentioned above, approximately 60% of patients in the surgical arm actually received bevacizumab sometime during their care. Potential changes in diffusion MR phenotypes at the time of bevacizumab initiation were not considered but may have changed as a result of prior therapy or resection. In addition, further treatment after bevacizumab therapy may not affect survival in a significant manner. Last, given small sample size, statistical control for multiple comparisons could not be performed and these data should be evaluated in a larger, prospective manner.
CONCLUSION
Diffusion MR phenotypes predict overall survival in recurrent GBM patients with large tumor burden (>20 cc) that are treated with bevacizumab or surgical resection. Patients with low ADCL have a significant survival advantage when treated with repeat surgery, while patients with high ADCL have favorable survival when treated with bevacizumab.
Disclosures
Funding was received from the American Cancer Society (ACS) Research Scholar Grant (RSG-15-003-01-CCE) (Ellingson); American Brain Tumor Association (ABTA) Research Collaborators Grant (ARC1700002)(Ellingson); UCLA SPORE in Brain Cancer (NIH/NCI 1P50CA211015-01A1) (Ellingson, Liau, Nghiemphu, Lai, Pope, Cloughesy); NIH/NCI 1R21CA223757-01 (Ellingson). Dr Ellingson is on the advisory board for Hoffman La-Roche, Siemens, Nativis, Medicenna, MedQIA, Bristol Meyers Squibb, Imaging Endpoints, and Agios; is a paid consultant for Nativis, MedQIA, Siemens, Hoffman La-Roche, Imaging Endpoints, Medicenna, and Agios; and has grant funding from Hoffman La-Roche, Siemens, Agios, and Janssen. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Cloughesy is on the Advisory Board for Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab and MedQIA.
Contributor Information
Kunal S Patel, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Neurosurgery, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Richard G Everson, Department of Neurosurgery, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Jingwen Yao, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Catalina Raymond, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Jodi Goldman, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Jacob Schlossman, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Joseph Tsung, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Caleb Tan, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Whitney B Pope, Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Matthew S Ji, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Nhung T Nguyen, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Albert Lai, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Phioanh L Nghiemphu, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Linda M Liau, Department of Neurosurgery, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Timothy F Cloughesy, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
Benjamin M Ellingson, UCLA Brain Tumor Imaging Laboratory (BTIL), Center for Computer Vision and Imaging Biomarkers, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Department of Radiological Science, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California.
COMMENTS
The authors report a retrospective series of patients in which they investigate the apparent diffusion coefficient (ADC) on MRI and its relationship to responsiveness of large (>20 cc) recurrent glioblastoma to bevacizumab and surgery. They found that patients with high ADC (>1.24 μm2/ms) had better response to bevacizumab than those with low ADC and that patients with low ADC responded better to surgery than those with high ADC in terms of survival.
This study illustrates how magnetic resonance imaging sequences can be related to tumor biology and how understanding this biology can contribute to understanding patient outcome. As the authors explain in their introduction, diffusion MRI can indicate the tumor cell density. In a simplistic view, high ADC is associated with less tumor cell density and low ADC with high tumor cell density. In keeping with this simplistic explanation, it makes sense that bevacizumab would have more efficacy than surgery when tumor cell density is less (and therefore more normal brain may be interspersed amongst the tumor cells) and that surgery would be more effective than pharmacology when a dense block of tumor cells can be removed. Additional insights could be gained by examining the effects on neurological functioning tumor resections involving eloquent portions of the brain. One might hypothesize that neurological deficits would be increased in surgical resection of high ADC tumors compared to low ADC tumors. Thus, MRI characteristics of tumors, beyond just examining enhancement patterns, can help guide clinical decision-making and lead to better patient outcomes.
N. Scott Litofsky
Columbia, Missouri
This retrospective study looks at the role of diffusion MR phenotypes in predicting survival in recurrent GBMs treated with bevacizumab and repeated surgical resection.
Every clinical scenario of recurrent GBM is unique. Variable factors include the biological profile of the tumor, patient characteristics, tumor location, and extent of first tumor resection. Treatment scenarios similarly vary case by case and it is still unclear how each different treatment ultimately affects the OS/PFS. While definitive answers will not come without prospective and multicentric studies, small retrospective and meta-analytical ones, such as this one, can furnish important new insights.
This study confirms that pre-treatment diffusion MR characteristics within large (>20cc) contrast enhancing tumors are a predictive imaging biomarker for overall survival benefit in recurrent GBM patients treated with bevacizumab. The authors also suggest that unfavorable diffusion phenotypes (low ADCL) may significantly benefit from surgical resection.
While both tumor-related and treatment-related factors play a role in clinical outcomes, certain tumor-related characteristics have a greater effect on tumor progression and survival than reoperation itself. The small sample size of this study is an important limit in this regard, but it is balanced out by the rigorous reporting of all the tumor-related features (including MGMT/IDH status, timing of repeated surgery, extent of first and next resections, treatment received in between surgeries, patient age, etc).
This study highlights how emerging advancements in radiomics can enhance our understanding of tumor and patient variables and can thus improve the management and prognosis of each different case of recurrent GBM.
Antonella Mangraviti
Milan, Italy
Rome, Italy
Federico G. Legnani
Milan, Italy
REFERENCES
- 1. Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Taillibert S, Kanner A et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma. JAMA. 2017;318(23):2306-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang M, Gulotta B, Thomas A et al. Large-volume low apparent diffusion coefficient lesions predict poor survival in bevacizumab-treated glioblastoma patients. Neuro Oncol. 2016;18(5):735-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733-4740. [DOI] [PubMed] [Google Scholar]
- 6. Kreisl TN, Kim L, Moore K et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert MR, Pugh SL, Aldape K et al. NRG oncology RTOG 0625: a randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J Neurooncol. 2017;131(1):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies J, Reyes-Rivera I, Pattipaka T et al. Survival in elderly glioblastoma patients treated with bevacizumab-based regimens in the united states. Neurooncol Pract. 2018;5(4):251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellingson BM, Malkin MG, Rand SD et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qu J, Qin L, Cheng S et al. Residual low ADC and high FA at the resection margin correlate with poor chemoradiation response and overall survival in high-grade glioma patients. Eur J Radiol. 2016;85(3):657-664. [DOI] [PubMed] [Google Scholar]
- 11. Elson A, Bovi J, Siker M, Schultz C, Paulson E. Evaluation of absolute and normalized apparent diffusion coefficient (ADC) values within the post-operative T2/FLAIR volume as adverse prognostic indicators in glioblastoma. J Neurooncol. 2015;122(3):549-558. [DOI] [PubMed] [Google Scholar]
- 12. Ellingson BM, Gerstner ER, Smits M et al. Diffusion MRI phenotypes predict overall survival benefit from anti-VEGF monotherapy in recurrent glioblastoma: converging evidence from phase II trials. Clin Cancer Res. 2017;23(19):5745-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellingson BM, Sahebjam S, Kim HJ et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. Am J Neuroradiol. 2014;35(4):673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellingson BM, Kim HJ, Woodworth DC, Cloughesy TF. Contrast-enhanced T1-weighted digital subtraction maps combined with diffusion MRI to identify recurrent glioblastoma patients that benefit from bevacizumab therapy. J Clin Oncol. 2014;32(suppl; abstr 2008):5s. [Google Scholar]
- 15. Ellingson BM, Bendszus M, Boxerman J et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellingson BM, Kim HJ, Woodworth DC et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellingson BM, Harris RJ, Woodworth DC et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woodworth DC, Pope WB, Liau LM et al. Nonlinear distortion correction of diffusion MR images improves quantitative DTI measurements in glioblastoma. J Neurooncol. 2014;116(3):551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pope WB, Kim HJ, Huo J et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182-189. [DOI] [PubMed] [Google Scholar]
- 20. Pope WB, Qiao XJ, Kim HJ et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108(3):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu VM, Jue TR, McDonald KL, Rovin RA. The survival effect of repeat surgery at glioblastoma recurrence and its trend: a systematic review and meta-analysis. World Neurosurg. 2018;115:453-459.e453. [DOI] [PubMed] [Google Scholar]
- 22. Ellingson BM, Abrey LE, Nelson SJ et al. Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018;20(9):1240-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Awad AW, Karsy M, Sanai N et al. Impact of removed tumor volume and location on patient outcome in glioblastoma. J Neurooncol. 2017;135(1):161-171. [DOI] [PubMed] [Google Scholar]
- 24. Robin AM, Lee I, Kalkanis SN. Reoperation for recurrent glioblastoma multiforme. Neurosurg Clin N Am. 2017;28(3):407-428. [DOI] [PubMed] [Google Scholar]
- 25. Montemurro N, Perrini P, Blanco MO, Vannozzi R. Second surgery for recurrent glioblastoma: a concise overview of the current literature. Clin Neurol Neurosurg. 2016;142:60-64. [DOI] [PubMed] [Google Scholar]
- 26. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943-953. [DOI] [PubMed] [Google Scholar]
- 27. Cloughesy T, Finocchiaro G, Belda-Iniesta C et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O6-methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol. 2017;35(3):343-351. [DOI] [PubMed] [Google Scholar]