Abstract
The repurposing of existing drugs has emerged as an attractive additional strategy to the development of novel compounds in the fight against cancerous diseases. Inhibition of phosphodiesterase 5 (PDE5) has been claimed as a potential approach to target various cancer subtypes in recent years. However, data on the treatment of tumors with PDE5 inhibitors as well as the underlying mechanisms are as yet very scarce. Here, we report that treatment of tumor cells with low concentrations of Sildenafil was associated with decreased cancer cell proliferation and augmented apoptosis in vitro and resulted in impaired tumor growth in vivo. Notably, incubation of cancer cells with Sildenafil was associated with altered expression of HSP90 chaperone followed by degradation of protein kinase D2, a client protein previously reported to be involved in tumor growth. Furthermore, the involvement of low doses of PU-H71, an HSP90 inhibitor currently under clinical evaluation, in combination with low concentrations of Sildenafil, synergistically and negatively impacted on the viability of cancer cells in vivo. Taken together, our study suggests that repurposing of already approved drugs, alone or in combination with oncology-dedicated compounds, may represent a novel cancer therapeutic strategy.
Introduction
Cancer represents one of the leading causes of death worldwide, and the development of novel therapies is still challenging. The number of new drugs reaching market maturity after long development times is small, the costs are rising and the outcome is often unsatisfying. An alternative that has become more attractive in recent years is the repurposing of existing medications already on the market with a high safety record and cost efficacy. An example for this approach is represented by the well-established phosphodiesterase 5 (PDE5)-inhibiting drugs which exhibit potential antioncogenic effects (1,2). Sildenafil is a potent PDE5-inhibiting drug that achieved clinical approval in the US already back in 1998 (3,4). Inhibition of PDE5 causes alterations in a broad range of signaling pathways due to an increased intracellular concentration of cyclic GMP and the resulting augmented activity of cyclic guanosine monophosphate (cCMP)-dependent protein kinase (5–7). Although originally developed to treat heart disease and nowadays predominantly used to counter erectile dysfunction in male patients, PDE5 inhibitors have since been approved for the treatment of lower urinary tract symptoms and pulmonary arterial hypertension and have been implicated in numerous other clinical applications (8). These include cardiovascular, pulmonary, reproductive, neurologic, gastrointestinal disorders and others (9). Interestingly, various recent studies claim a potential beneficial effect of PDE5 inhibitors in the treatment of different cancer types via suppressing tumor growth, especially in combination with other therapeutic approaches, e.g. chemotherapy (10–16). However, the underlying molecular mechanisms of these observations are not fully understood and currently still being under investigation. It has been reported in the literature that upregulation of cGMP-dependent protein kinase following PDE5 inhibition leads to a decreased activity of heat shock protein 90 (HSP90) (17,18).
HSP90 is a ubiquitously expressed ATP-dependent molecular chaperone that aids in the correct folding (19), processing and activity of a large variety of cellular proteins, which are therefore also often referred to as HSP90 ‘clients’ (20,21). These include transcription factors, hormone receptors and protein kinases. The interaction of active HSP90 with its clients is mediated by cochaperones such as CDC37 stabilizing the resulting protein complex (20,21). In aberrantly proliferating cancer cells, HSP90 plays an important role for tumor growth and hypoxia evasion through angiogenesis by maintaining the stability of proteins upregulated in tumor cells, such as protein kinases C and D, SRC (22), AKT (23), PDK-1 and STK33 (24). Several small molecule inhibitors directed against HSP90, including antitumorigenic agent PU-H71, are currently undergoing clinical trials (25). Our laboratory has reported in the past a signaling pathway that interconnects HSP90 expression and activation levels with protein kinase D2 (PKD2) stabilization (26). PKD2 is a serine/threonine kinase from the calcium/calmodulin family of kinases whose activation via phosphorylation can be mediated by various stimuli including receptor tyrosine kinases, reactive oxygen species and hypoxia (26,27). Versatile functions for PKD in tumorigenesis, cancer cell invasion, migration and secretion have been reported in the literature (27,28). In this context, PKD2 contributes to tumor angiogenesis via stabilization of hypoxia inducible factor 1α, activation of nuclear factor-κB and vascular endothelial growth factor A and subsequent tissue vascularization (27,28).
In this study, we investigated the effect of Sildenafil on tumor growth as well as the molecular mechanisms involved in various cancer cell lines. Our data indicate a link between PDE5 inhibition and decreased protein levels of HSP90 leading to impaired tumor proliferation and viability. Altered HSP90 expression was associated with degradation of PKD2. We found that treatment of cells with Sildenafil potentiated tumor growth-impairing effects of HSP90 inhibitor PU-H71, promising a novel potential way to target cancer by administering a combination of PDE5 and chaperone inhibitors.
Materials and methods
Cell lines and reagents
MDA-MB-231, SW480, MIA PaCa-2, Panc1, BxPC3 and A549 cancer cell lines were cultured either in Dulbecco’s modified Eagle's medium or RPMI1640 medium supplemented with 10% fetal bovine serum, 2% glutamine and 1% penicillin/streptomycin (all Sigma, Germany). HCT-116 was cultivated in McCoy media (Sigma, Germany). Cancer cell lines were obtained from DSMZ-German Collection of Microorganisms and culture cells (Braunschweig, Germany) or authenticated at the Multiplexion (Heidelberg, Germany). Early passages (after purchase or authentication) only were used for the current study. Sildenafil citrate was purchased from Sigma (#PZ0003) and used as indicated. For subsequent experiments, cells were preincubated with either 50 nM HSP90 inhibitor, PU-H71 (8-[(6-iodo-1,3-benzodioxol-5-yl)sulfanyl]-9-[3-(propan-2-ylamino) propyl] purin-6-amine) or vehicle control. PU-H71 was synthesized and characterized as previously reported (29).
Plasmids and lentiviral transduction
The pLenti6.2-V5-DEST-PKD2 overexpression vectors were generated using the pDONR-223-PKD2 entry clone from Addgene (PKD2, #23490). High-titer virus-containing supernatants of HEK 293FT cells after cotransfection of lentiviral vectors with pMD2.G and psPAX2 packaging plasmids were used for lentiviral-mediated transduction of cancer cells.
Fluorescence-activated cell sorting analysis
Cancer cells were incubated with Sildenafil for 3 days. After washing with phosphate buffered saline, cells were subjected to Annexin V (Abcam #Ab14082)/Propidium Iodide (Abcam #ab14085) staining according to the manufacturer’s recommendations. Acquisition was conducted with a Beckman Coulter Gallios 3/10 AT01005 machine; analysis of data was performed with Kaluza Analysis 2.1. software.
Western blot analysis
Whole cell extracts were prepared using a lysis buffer containing 10 mM Tris–HCl, 5 mM ethylenediaminetetraacetic acid, 50 mM NaCl, 50 mM NaF and 1% Triton X100 supplemented with Complete Protease inhibitor Cocktail (Roche). Lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and proteins transferred to polyvinylidene difluoride membranes (Millipore, MA). Membranes were blocked with 5% non-fat dry milk in phosphate buffered saline containing 0.2% Tween 20 and incubated overnight at 4°C with specific antibodies. For subsequent washes 0.2% Tween 20 in phosphate buffered saline was used. The following antibodies were used: PKD2 (Bethyl Laboratories, #A300-073A), cleaved poly (ADP ribose) polymerase (PARP; Cell Signaling, #9542S), HSP90β (D-19; Santa Cruz Biotechnology, #sc-1057) and β-actin (Sigma, #A1978). Detection of chemiluminescence signal was conducted with a Fusion (PeqLab/Vilber) unit. Quantification of 16 bit TIF format images was performed with ImageJ software 1.42/Gel Analysis.
Chorioallantoic membrane assay
MIA PaCa-2, SW480 or HCT-116 pancreatic cancer cells (1.5 × 106) were xenografted within silicon ring on the surface of the chorioallantoic membrane (CAM) of chicken eggs 8 days after fertilization. Four days after implantation (day 12 after fertilization), tumors were retrieved, fixed in formalin and further subjected to immunohistochemistry. Macro photographs were taken with a Sony Alpha 77 photo camera. Tumors were delineated as shown in macro photographs. The known diameter of the silicon ring (i.e. 5 mm) was used to set the scale. Using the free drawing tool in ImageJ, tumor size (in pixels converted to mm2) was calculated.
Immunohistochemistry of CAM tumors
Formalin-fixed tumors were embedded in paraffin using standard procedures. The 5 µm sections were processed and stained with an antibody directed against Ki67 (1:100; Dako, clone MIB-1).
Statistical analysis
Statistical analysis as well as graphing was accomplished using Prism 7.03 (GraphPad Software, USA). Mean and statistical significance were calculated and analyzed using t-test. All data are representative of at least three experiments.
Results
Sildenafil treatment impairs proliferation in a time- and dose-dependent manner
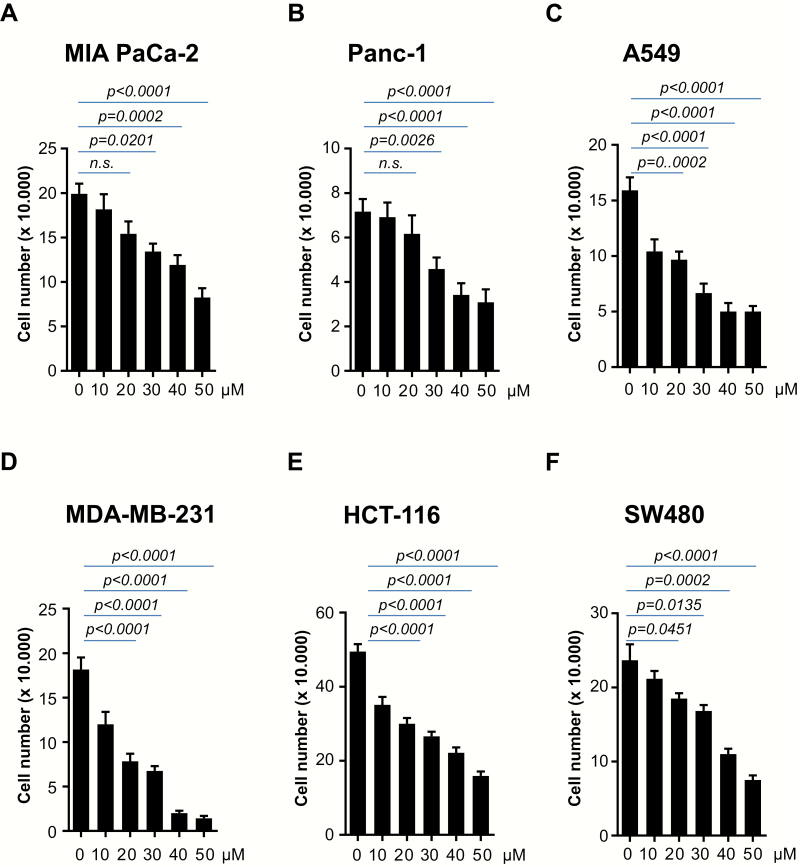
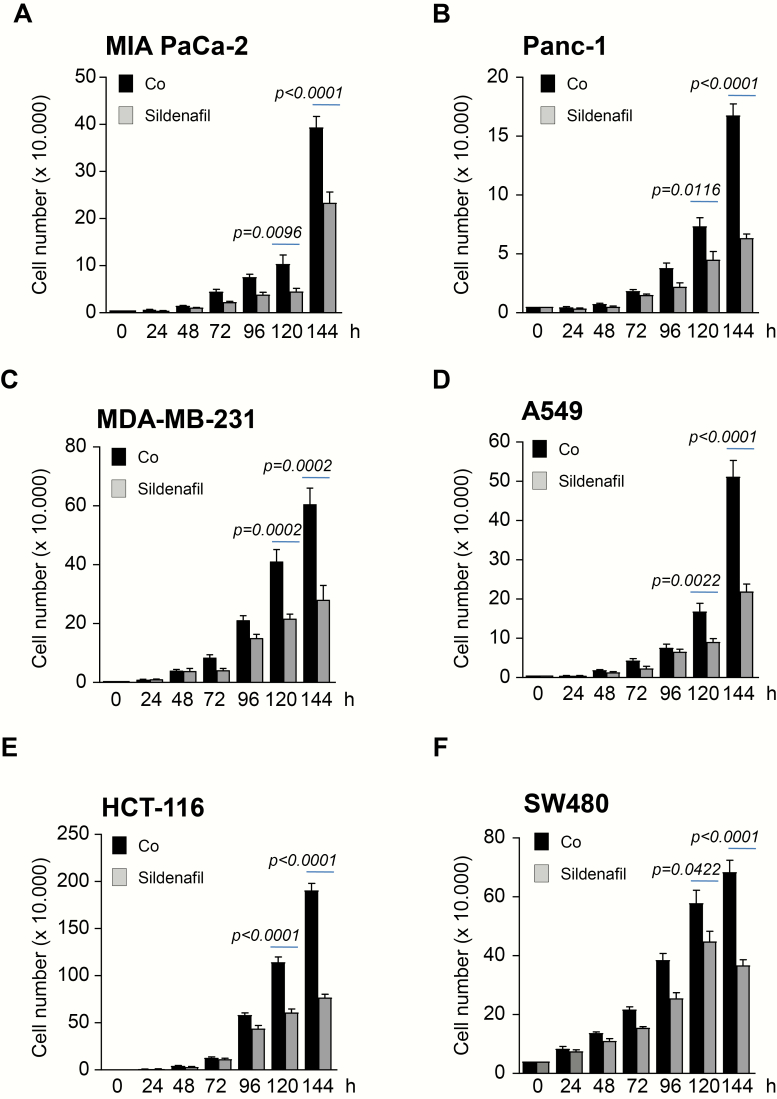
The PDE5 inhibitor Sildenafil has been previously reported to inhibit the proliferation of various human colon cancer cell lines in a concentration-dependent manner with the inhibitory concentration 50 values ranging from 190 to 271 µM (30). We sought to investigate whether the findings of this study could be extended to other tumor entities upon using lower concentrations of Sildenafil. A selection of several cancer cell lines including colon (HCT-116, SW480), pancreatic (MIA PaCa-2, Panc1, BxPC3), breast (MDA-MB-231) and lung (A549) were examined. Treatment with 10–50 µM Sildenafil resulted in impaired proliferation in a dose-dependent manner (Figure 1A–F and Supplementary Figure 1A, available at Carcinogenesis Online). Cancer cell lines taken into the study displayed different sensitivity to Sildenafil. Thus, some cancer cell lines such as A549 or MDA-MB-231 (Figure 1C and D) showed substantially impaired proliferation already at 10 µM. At this concentration, Sildenafil had little effect on proliferation of MIA PaCa-2 and Panc1 (Figure 1A and B). Based on these results, a concentration of 25 µM was used for the subsequent experiments. Cancer cell lines were subjected to a treatment with 25 µM Sildenafil for 6 days. Again, all cancer cell lines taken into the study showed impaired proliferation upon incubation with Sildenafil (Figure 2A–F and Supplementary Figure 1B, available at Carcinogenesis Online).
Figure 1.
Treatment with Sildenafil is associated with impaired proliferation of cancer cells in a dose-dependent manner. (A–F) 2 × 104 MIA PaCa-2 and Panc1 pancreatic, MDA-MB-231 breast, HCT-116 and SW480 colon and A549 lung cancer cells were plated in 12 well dishes and subjected to various concentration of Sildenafil as indicated. Cell number was quantified after 72 h. One of three independent experiments performed in triplicate is shown.
Figure 2.
Sildenafil decreases proliferation of cancer cells in time-dependent manner. (A–F) 2 × 104 MIA PaCa-2 and Panc1 pancreatic, MDA-MB-231 breast, HCT-116 and SW480 colon and A549 lung cancer cells were plated in 12 well dishes and subjected to 25 µM Sildenafil. Cells were allowed to grow for the next 6 days when the number was quantified. One of three independent experiments performed in triplicate is shown.
Cancer cells undergo augmented apoptosis upon incubation with Sildenafil
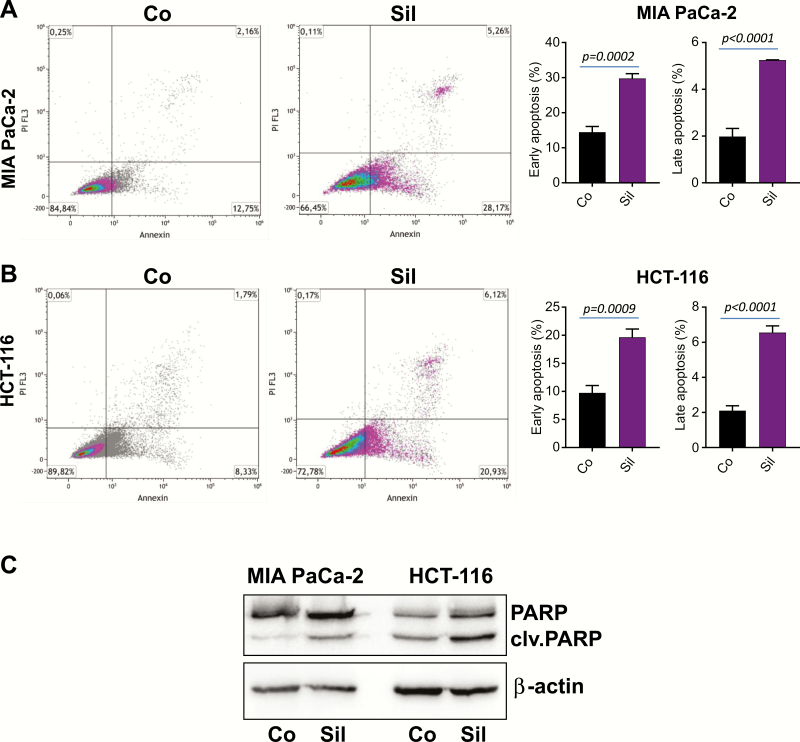
To extend our observations in Figures 1 and 2, the effect of 25 µM Sildenafil on cell viability was analyzed. HCT-116 colon and MIA PaCa-2 pancreatic cancer cells were incubated for 3 days before being stained with Annexin V and subsequently subjected to fluorescence-activated cell sorting analysis. As shown in Figure 3A and B, incubation with Sildenafil significantly increased the proportion of apoptotic cancer cells. In order to substantiate these findings, lysates of MIA PaCa-2 and HCT-116 cancer cells were subjected to western blot analysis. Incubation with Sildenafil for 3 days resulted in a substantial increase in cleaved PARP levels as presented in Figure 3C.
Figure 3.
Sildenafil treatment triggers cancer cell death. (A) MIA PaCa-2 pancreatic and (B) HCT-116 colon cancer cells were treated with 25 µM Sildenafil (Sil) for 3 days. Cells were subjected to Annexin V/Propidium Iodide (PI) staining and subsequent flow cytometry. The fluorescence-activated cell sorting analysis was conducted in triplicate. Data are shown as mean ± SEM. One of the three experiments is presented. (C) Protein lysates of MIA PaCa-2 and HCT-116 cells were subjected to western blot analysis with PARP antibody. β-Actin was used as loading control.
Sildenafil treatment results in impaired tumor growth in ovo
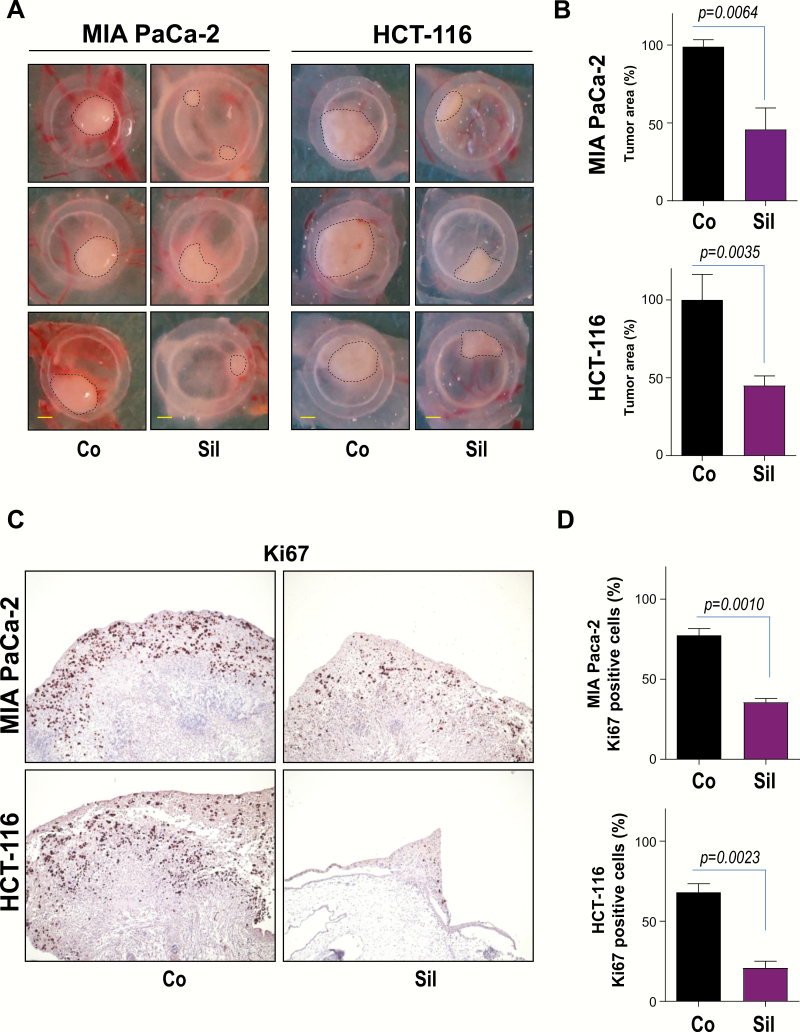
Having determined that Sildenafil treatment results in impaired cancer cell proliferation and augmented cell death in vitro, we further evaluated the effect of this PDE5 inhibitor in tumor formation using the chicken CAM, an established model of tumor growth (31,32). MIA PaCa-2 and HCT-116 cancer cells were seeded on the surface of CAM 8 days after egg fertilization. Xenografted tumors were treated with Sildenafil after 24 and 48 h, respectively. Four days after implantation, tumors were excised, photographed and analyzed by IHC. MIA PaCa-2 and HCT-116 control cancer cells formed substantial tumors on CAM (Figure 4A and B). Treatment with Sildenafil was associated with a significant decrease of tumor size in this experimental model (Figure 4A and B). Immunohistological staining demonstrated that decreased tumor size was associated with a significantly reduced proliferation index as evidenced by impaired Ki67 immunoreactivity (Figure 4C and D).
Figure 4.
Sildenafil treatment suppresses tumor growth in ovo. (A) 1.5 × 106 HCT-116 colon or MIA PaCa-2 pancreatic cancer cells were implanted 8 days after fertilization. Tumors were treated 24 and 48 h with 25 µM Sildenafil (Sil). Tumor photographs 96 h after implantation are presented. Scale bar, 1 mm. (B) Quantification of tumor area is presented. Error bars represent mean ± SEM of four to seven tumors. (C) IHC of HCT-116 and MIA PaCa-2 cells growing on CAM using specific antibodies directed against Ki67 are presented. (D) Quantification of Ki67 positive cells is shown. Error bars represent mean ± SEM of at least six microscopic fields, each containing at least 200 cells.
Impaired proliferation of cancer cells following Sildenafil treatment is associated with altered HSP90 expression and PKD2 degradation
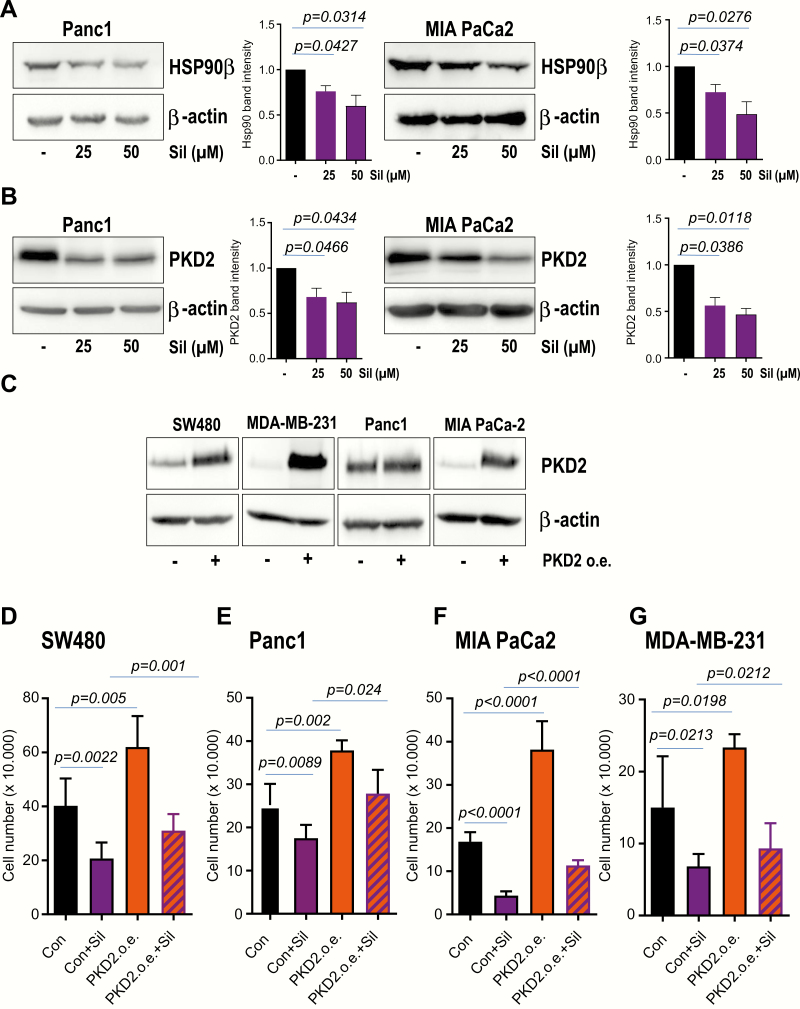
We next sought to investigate the potential mechanism through which Sildenafil might regulate tumor cell proliferation and viability. Recently, Sildenafil was reported to contribute to tumor cell killing by altering the expression of several chaperone proteins (13). In that study, overexpression of HSP90, HSP70 or GRP78 significantly reduced the lethality induced by pemetrexed + Sildenafil (13). To find out whether treatment with Sildenafil affects the expression of HSP90 in our experimental setting, cancer cells were incubated with different concentrations of Sildenafil and cleared lysates were subjected to western blot analysis with HSP90β-specific antibody. Our western blot experiments followed by densitometry analysis demonstrate that treatment with Sildenafil results in decreased HSP90 expression in a dose-dependent fashion (Figure 5A). PKD2 was previously reported to be involved in migration, invasion and growth of various tumor types including glioblastoma and pancreatic cancer (28,32,33). Our lab demonstrated PKD2 to interact with and to be stabilized by HSP90 (26). Interestingly, treatment with Sildenafil did not only alter the expression of HSP90 (Figure 5A) but was also mirrored by a substantial degradation of PKD2 (Figure 5B). In order to substantiate these findings, PKD2 contribution to the proliferation of cancer cells subjected to Sildenafil intervention was assayed in proliferation experiments. PKD2 was stably expressed using lentiviral-mediated transduction (Figure 5C). As expected, Sildenafil treatment negatively impacted on proliferation whilst lone PKD2 overexpression boosted the growth of cancer cells in our experimental model (Figure 5D–G). Most importantly, ectopic PKD2 was able to partially restore the proliferation after treatment with Sildenafil (Figure 5D–G). Altogether, these data suggest that one potential mechanism toward cancer cell death following Sildenafil treatment is represented by the altered HSP90 expression and destabilization of at least one client protein such as PKD2.
Figure 5.
Sildenafil treatment is associated with altered HSP90 expression and PKD2 degradation. (A) Lysates of cancer cell lines incubated with 25 µM Sildenafil for 3 days were subjected to western blot analysis with HSP90β antibody. β-Actin was used as loading control. Densitometry analysis of three to four blots is presented. (B) Cleared lysates of tumor cell lines subjected to 25 µM Sildenafil for 3 days were used for western blot analysis with PKD2 antibody. β-Actin was used as loading control. (C) PKD2 overexpression was achieved via lentiviral-mediated transduction. Western blot analysis with PKD2-specific antibody after ending the selection is presented. (D–G) Cancer cells stably transduced with empty vector (Con) or PKD2 (PKD2 o.e.) were seeded in 12 well dishes. Cell proliferation was monitored for the next 3 days in the presence or absence of Sildenafil (Sil) when cell number was quantified. One representative of three experiments conducted in triplicate is shown.
Potentiated effect of PDE5 and HSP90 inhibitors toward impaired tumor growth in ovo
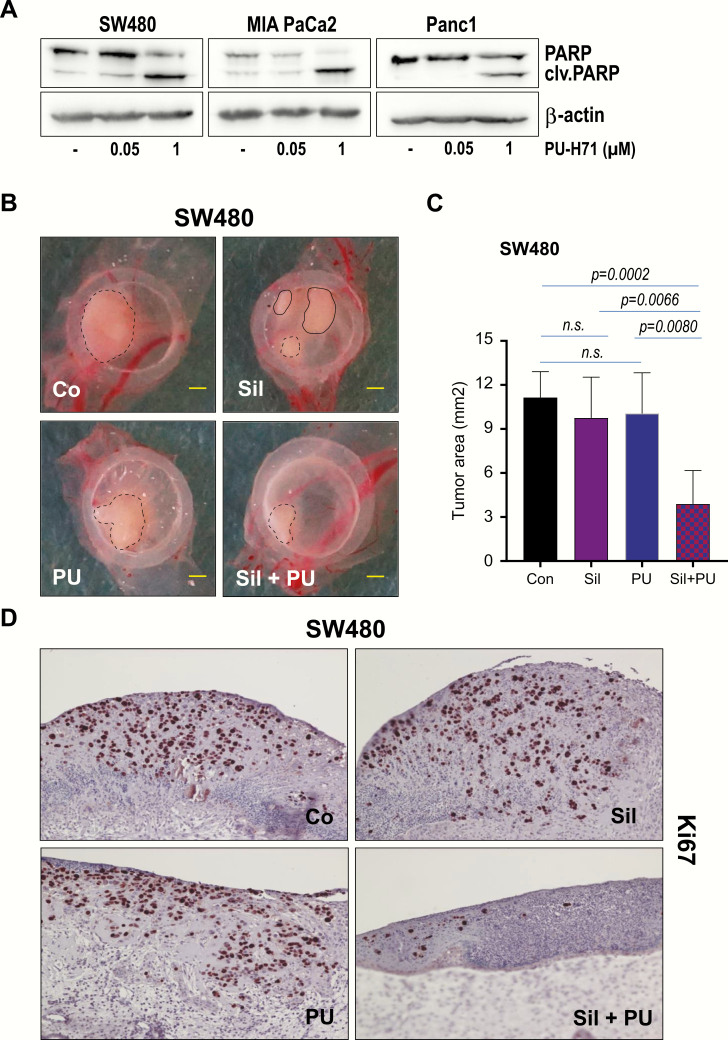
The findings above urged us to involve PU-H71, an ATP-competitive HSP90 inhibitor currently in the clinical trials (34,35). Our previous experiments showed that treatment of tumor cells with 1 µM PU-H71, not only results in PKD2 degradation but is also associated with augmented apoptosis (26,36). Indeed, we could confirm our previous data and detected substantial cell death in cancer cells treated with PU-H71 as demonstrated by increased levels of cleaved PARP (Figure 6A). In our experimental setting we involved however, a PU-H71 concentration 20 times less (i.e. 50 nM) than that used in the previous studies. At this concentration no cleaved PARP was detected (Figure 6A). Next, we sought to combine this concentration with 15 µM Sildenafil, in an attempt to reveal a potential synergistic effect between the two compounds. We employed the CAM experiments and xenografted tumor cells as described in Materials and methods. A cocktail of both compounds was delivered 24 and 48 h after tumor implantation. Tumors were retrieved and measured at 4 days following the implantation (Figure 6B and C). Treatment with either PU-H71 (50 nM) or Sildenafil (15 µM) did not have a significant effect on tumor growth. In combination however, PDE5 inhibition substantially potentiated the effects of PU-H71 on tumor growth (Figure 6C). Tumors subjected to dual therapy showed decreased size and impaired immunoreactivity of Ki67 proliferation marker (Figure 6D).
Figure 6.
Sildenafil potentiates PU-H71 effects toward impaired tumor growth. (A) Lysates of cancer cell lines incubated with different amounts of PU-H71 for 24 h days were subjected to western blot analysis with PARP-specific antibody. β-Actin was used as loading control. (B) 1 × 106 SW480 colon cancer cells were implanted inside a silicon ring on chicken CAM 8 days after fertilization. Tumors were treated either with 50 nM PU-H71, 15 µM Sildenafil or combination of both compounds at 24 and 48 h after implantation. Tumors were allowed to growth for additional 24 h. Scale bar, 1 mm. (C) Quantification of tumor area is presented. Error bars represent mean ± SEM of five to seven tumors. (D) IHC of SW480 cancer cells growing on CAM using specific antibodies directed against Ki67 is presented.
Discussion
Despite continuous advances in medicine and technology, cancer remains a major threat to human society. In order to avoid the harmful and poisonous effect of drugs used in targeting and killing cancer cells, in the last few years, the scientific community started to explore new avenues by repurposing existing drugs with reduced toxicity and diminished side effects. Lately, PDE5 inhibitor Sildenafil has been reported to negatively impact colon cancer cell proliferation and viability. Although the results are promising, the intimate molecular mechanism of how therapy with PDE5 inhibitors impacts on tumor cell viability remains largely unknown.
In this study, we have demonstrated that treatment of cancer cells with Sildenafil resulted in impaired cancer cell proliferation, augmented cell death and decreased tumor growth. Furthermore, treatment with Sildenafil was associated with altered expression of HSP90 chaperone which impacted on the degradation of one of its client proteins, PKD2, a serine/threonine kinase previously reported to be essential for cancer cell proliferation and viability. Finally, our study revealed that the combination of very low doses of PDE5 and HSP90 inhibitors potentiated the decrease in tumor growth.
Initially, PDE5 inhibitors have been used for the treatment of erectile dysfunction by preventing the breakdown of cGMP which subsequently triggers a prolonged activation of protein kinase G and generation of nitric oxide (5). Lately, scientists sought to elucidate the roles of this pathway in the regulation of cell proliferation, tumor development and progression. These efforts were motivated by detecting augmented PDE5 expression in multiple tumor entities (37–39) and multiple cancer cell lines such as breast, prostate, bladder and colorectal (40). Mei et al. showed that PDE5 inhibitor, Sildenafil, inhibited the growth of colorectal cancer cells in vitro and in subcutaneous xenografts, induced G1 cell cycle arrest and apoptosis by generating reactive oxygene species (30). In that study, inhibition of colon cancer cell growth occurred in a concentration-dependent manner with the inhibitory concentration 50 ranging from 190 to 271 µM (30). This prompted us to investigate whether these findings could be extended to other tumor entities upon using lower concentrations of Sildenafil. Several cancer cell lines including colon, pancreatic, breast and lung were treated with increasing concentrations of Sildenafil. Our findings indicate that incubation of cancer cells with 25 µM Sildenafil not only resulted in impaired proliferation but was also corroborated with augmented apoptosis. To note, some of the cancer cell lines taken in our study (i.e. A549 or MDA-MB-231), were sensitive to as less as 10 µM Sildenafil with respect to proliferation. Interestingly, Sildenafil was also reported to enhance the killing effect of other chemotherapeutics agents including cisplatin, gemcitabine and doxorubicin (28). Another study of the same group demonstrated that Sildenafil augments the lethality of pemetrexed through inhibition of multiple chaperone proteins (28). In that study, overexpression of HSP90, HSP70 or GRP78 significantly reduced the lethality induced by pemetrexed + Sildenafil (41). Particularly, HSP90 contributes to the stabilization and activation of around 200 protein clients, many of which are oncoproteins, implicated in various cancer hallmarks. Our findings are in line with a study (13) demonstrating that Sildenafil treatment is associated with the alteration of HSP90 expression.
We previously reported PKD2 as a client of HSP90 (26). Therefore, we sought to investigate whether altered HSP90 expression following Sildenafil treatment is associated with destabilization of PKD2. Indeed, our study shows that PKD2 is degraded following exposure of various cancer cell lines to Sildenafil. Rescue experiments substantiated the requirement of PKD2 (at least in some cancer cell lines) for the viability of cancer cells exposed to Sildenafil and demonstrate an impaired cancer cell growth upon alteration of HSP90 expression and subsequent PKD2 degradation following PDE5 inhibition. Very recently, we could demonstrate that cold physical plasma-induced reactive oxygene species promoted tumor cell death by affecting HSP90/PKD2 signaling (42). These findings are in line with data showing that Sildenafil inhibited the growth of colorectal cancer by generating reactive oxygene species (30). Together, these data reinforce HSP90 as an essential target when developing strategies and designing drugs to induce cancer cell death through the destabilization of client proteins. Since additional potential signaling pathways have been described in the literature, we do not exclude that cell death following Sildenafil treatment was not solely credited to HSP90/PKD2 signaling. For example, Sildenafil was reported to markedly enhance the sensitivity to chemotherapy in p53 mutant breast tumor cells (14), while Sildenafil sensitized prostate cancer cells to doxorubicin-mediated cell apoptosis through CD95 (15). Furthermore, PDE5 inhibitors were reported to suppress cell growth by activating the cGMP/cGMP-dependent protein kinase pathway and subsequent suppression of Wnt/β-catenin signaling (7,16).
Our recent results show that 1 µM PU-H71 is sufficient to promote apoptosis as a result of HSP90 inhibition-triggered client degradation (24,36). In an attempt to mimic subminimal drug doses in clinical setting we used for our experiments as little as 50 nM PU-H71. At this concentration no apoptosis was detected. However, in a therapy cocktail, a more physiological concentration of 15 µM Sildenafil was sufficient to substantially potentiate (the as little as 50 nM) PU-H71 to negatively impact the tumor growth. We also envisage that even lower concentrations of Sildenafil in combination with various drugs already on the market may have a beneficial effect during cancer therapy.
Altogether, our study suggests a new potential therapy avenue targeting essential chaperones and their clients by repurposing existing medications with high safety record. However, further studies using more tumor entities and in vivo animal models are needed to provide information about the generalization of our findings and their relevance in biological systems.
Funding
This work was supported by the German Research Foundation (DFG, grant AZ.96/1-3 to N.A.). L.C is supported by the Chinese Scholarship Council (CSC). G.C. is supported in part by the US National Institutes of Health (NIH) (R01 CA172546, R56 AG061869, R01 CA155226, P01 CA186866, P30 CA08748 and P50 CA192937).
Conflict of Interest Statement: Memorial Sloan-Kettering Cancer Center holds the intellectual rights to PU-H71. Samus Therapeutics, of which G. Chiosis has partial ownership, has licensed PU-H71.
Supplementary Material
Glossary
Abbreviations
- CAM
chorioallantoic membrane
- cCMP
cyclic guanosine monophosphate
- PDE5
phosphodiesterase 5
- PKD2
protein kinase D2
References
- 1. Domvri K. et al. (2017) Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J. Cancer, 8, 3648–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Das A. et al. (2015) PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther., 147, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallis R.M. (1999) The pharmacology of sildenafil, a novel and selective inhibitor of phosphodiesterase (PDE) type 5. Nihon Yakurigaku Zasshi, 114 (suppl. 1), 22P–26P. [DOI] [PubMed] [Google Scholar]
- 4. Bi Y. et al. (2001) The discovery of novel, potent and selective PDE5 inhibitors. Bioorg. Med. Chem. Lett., 11, 2461–2464. [DOI] [PubMed] [Google Scholar]
- 5. Subbotina A. et al. (2017) Inhibition of PDE5A1 guanosine cyclic monophosphate (cGMP) hydrolysing activity by sildenafil analogues that inhibit cellular cGMP efflux. J. Pharm. Pharmacol., 69, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tinsley H.N. et al. (2009) Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol. Cancer Ther., 8, 3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N. et al. (2013) Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol. Cancer Ther., 12, 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersson K.E. (2018) PDE5 inhibitors—pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol., 175, 2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mostafa T. (2016) Useful implications of low-dose long-term use of PDE-5 inhibitors. Sex. Med. Rev., 4, 270–284. [DOI] [PubMed] [Google Scholar]
- 10. Sponziello M. et al. (2015) PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine, 50, 434–441. [DOI] [PubMed] [Google Scholar]
- 11. Tinsley H.N. et al. (2011) Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin-mediated transcription in human breast tumor cells. Cancer Prev. Res. (Phila), 4, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Islam B.N. et al. (2017) Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev. Res. (Phila), 10, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Booth L. et al. (2017) PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget, 8, 1449–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di X. et al. (2010) Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res. Treat., 124, 349–360. [DOI] [PubMed] [Google Scholar]
- 15. Das A. et al. (2016) Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget, 7, 4399–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li N. et al. (2015) Suppression of β-catenin/TCF transcriptional activity and colon tumor cell growth by dual inhibition of PDE5 and 10. Oncotarget, 6, 27403–27415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walton-Diaz A. et al. (2013) Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity. Future Med. Chem., 5, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papapetropoulos A. et al. (2005) Interaction between the 90-kDa heat shock protein and soluble guanylyl cyclase: physiological significance and mapping of the domains mediating binding. Mol. Pharmacol., 68, 1133–1141. [DOI] [PubMed] [Google Scholar]
- 19. Walter S. et al. (2002) Molecular chaperones—cellular machines for protein folding. Angew. Chem. Int. Ed. Engl., 41, 1098–1113. [DOI] [PubMed] [Google Scholar]
- 20. Vaughan C.K. et al. (2008) Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol. Cell, 31, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith J.R. et al. (2009) Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene, 28, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Y. et al. (1999) Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA, 96, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basso A.D. et al. (2002) Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem., 277, 39858–39866. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y. et al. (2017) STK33 participates to HSP90-supported angiogenic program in hypoxic tumors by regulating HIF-1α/VEGF signaling pathway. Oncotarget, 8, 77474–77488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matthew T. et al. (2015) PU-H71: an improvement on nature’s solutions to oncogenic Hsp90 addiction. Pharmacol. Res., 99, 202–216. [DOI] [PubMed] [Google Scholar]
- 26. Azoitei N. et al. (2014) HSP90 supports tumor growth and angiogenesis through PRKD2 protein stabilization. Cancer Res., 74, 7125–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azoitei N. et al. (2010) Protein kinase D2 is a crucial regulator of tumour cell-endothelial cell communication in gastrointestinal tumours. Gut, 59, 1316–1330. [DOI] [PubMed] [Google Scholar]
- 28. Wille C. et al. (2014) Protein kinase D2 induces invasion of pancreatic cancer cells by regulating matrix metalloproteinases. Mol. Biol. Cell, 25, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodina A. et al. (2016) The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature, 538, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mei X.L. et al. (2015) Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am. J. Cancer Res., 5, 3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 31. Vogler M. et al. (2005) Inhibition of clonogenic tumor growth: a novel function of Smac contributing to its antitumor activity. Oncogene, 24, 7190–7202. [DOI] [PubMed] [Google Scholar]
- 32. Azoitei N. et al. (2011) Protein kinase D2 is a novel regulator of glioblastoma growth and tumor formation. Neuro Oncol., 13, 710–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernhart E. et al. (2013) Protein kinase D2 regulates migration and invasion of U87MG glioblastoma cells in vitro. Exp. Cell Res., 319, 2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taldone T. et al. (2008) Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr. Opin. Pharmacol., 8, 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trepel J. et al. (2010) Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer, 10, 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azoitei N. et al. (2012) Targeting of KRAS mutant tumors by HSP90 inhibitors involves degradation of STK33. J. Exp. Med., 209, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bender A.T. et al. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev., 58, 488–520. [DOI] [PubMed] [Google Scholar]
- 38. Karami-Tehrani F. et al. (2012) Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch. Med. Res., 43, 470–475. [DOI] [PubMed] [Google Scholar]
- 39. Eggen T. et al. (2012) Increased gene expression of the ABCC5 transporter without distinct changes in the expression of PDE5 in human cervical cancer cells during growth. Anticancer Res., 32, 3055–3061. [PubMed] [Google Scholar]
- 40. Zhu B. et al. (2007) The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr. Top. Med. Chem., 7, 437–454. [DOI] [PubMed] [Google Scholar]
- 41. Booth L. et al. (2014) Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol. Pharmacol., 85, 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bekeschus S. et al. (2019) Physical plasma-triggered ROS induces tumor cell death upon cleavage of HSP90 chaperone. Sci. Rep., 9, 4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.