Abstract
Background
Over the past several decades, treatment of cancer in adolescents and young adults (AYAs) has evolved substantially, leading to steady improvements in estimated 5-year survival at diagnosis. However, the impact on late mortality in this population is largely unexamined. We investigated temporal trends in mortality among 5-year AYA cancer survivors.
Methods
The Surveillance, Epidemiology, and End Results database was used to identify AYAs (age 15–39 years) diagnosed with cancer during 1975–2011 who survived at least 5 years beyond diagnosis. Survival months were accrued from 5 years postdiagnosis until death or the end of 2016. Cumulative mortality from all causes, the primary cancer, other cancers, and noncancer or nonexternal causes (ie, excluding accidents, suicide, homicide) were estimated according to diagnosis era.
Results
Among 282 969 five-year AYA cancer survivors, 5-year mortality (ie, from 5 through 10 years postdiagnosis) from all-causes decreased from 8.3% (95% confidence interval = 8.0% to 8.6%) among those diagnosed in 1975–1984 to 5.4% (95% confidence interval = 5.3% to 5.6%) among those diagnosed in 2005–2011. This was largely explained by decreases in mortality from the primary cancer (6.8% to 4.2%) between these periods. However, for specific cancer types, including colorectal, bone, sarcomas, cervical/uterine, and bladder, cumulative mortality curves demonstrated little improvement in primary cancer mortality over time. Some reduction in late mortality from noncancer or nonexternal causes was apparent for Hodgkin lymphoma, leukemia, kidney cancer, head and neck cancers, and trachea, lung, and bronchus cancers.
Conclusion
Over the past four decades, all-cause and cancer-specific mortality have decreased among 5-year AYA cancer survivors overall, but several cancer types have not shared in these improvements.
In the United States, the National Cancer Institute defines adolescents and young adults (AYAs) with cancer as patients diagnosed between the ages of 15 and 39 years (1). Malignancies among AYAs make up about 4% of all incident cancer diagnoses in the United States and approximately 70 000 new cases each year (2). Growing recognition of the unique biology of some cancers in AYAs, along with the unique psychosocial considerations associated with late adolescence and early adulthood (3), has led to an increase in research focused on AYAs with cancer as a population distinct from childhood and older adult cancer patients.
Several reports in recent years have investigated trends in 5-year survival among AYAs with cancer in the United States (4–6). Findings from these reports indicate that, although gains have been smaller than those achieved in children and older adults with cancer, early survival among AYAs has improved steadily over the past several decades. Reported 5-year survival gains likely reflect, in large part, the impact of advances in therapy for several of the most common cancers in AYAs. However, although trends in early survival, or that within 5 years of diagnosis, have been well documented, less is known about temporal patterns of late mortality among AYAs who have already survived several years beyond their cancer diagnosis. Given the potential for some cancer therapies to affect health for many years postdiagnosis, through chronic or late adverse effects, it is critical to examine whether and how improvements in treatment for cancer and other noncancer health conditions have altered mortality among long-term AYA cancer survivors.
The objective of this study was to investigate patterns of all-cause mortality among 5-year AYA cancer survivors according to diagnosis era. We also partitioned mortality into that from the primary cancer, other cancers, and noncancer or nonexternal causes in an attempt to assess potential differential impacts of cancer therapy changes on late mortality from cancer vs other health conditions.
Methods
Study Population
We identified AYAs with cancer using data from the Surveillance, Epidemiology and End Results (SEER) program (7), a system of population-based cancer registries in the United States that collects and reports data on cancer incidence and survival. Information collected by SEER includes patient demographics, primary tumor site and morphology, stage, and first course of treatment. SEER also conducts follow-up for patient vital status and reports the number of months survived since cancer diagnosis and the cause of death as determined from death certificates (8).
For these analyses, we identified patients diagnosed with a first malignant primary at ages 15 to 39 years between 1975 and 2011 who had survived at least 5 years after diagnosis (n = 287 050). We excluded cases for whom the state death certificate was not available or was available but lacked cause of death information (n = 1460). We also excluded patients with Kaposi sarcoma to minimize the impact of trends in HIV-related deaths on our mortality estimates (n = 2621). Cancer type was categorized using the AYA-specific recode of International Classification of Diseases for Oncology Third Edition (ICD-O-3) primary site and histology codes (9).
Outcomes
The primary outcome of interest was all-cause mortality, defined based on SEER’s designation of patient vital status. Other outcomes included primary cancer mortality, other cancer mortality, and noncancer or nonexternal mortality; these were defined using two SEER-created variables. Deaths from the primary cancer were identified using the SEER cause-specific death classification, which distinguishes patients whose death was attributable to the cancer of interest (ie, the primary cancer) from those alive, or dead from other causes (10). Using the SEER cause of death recode, which groups ICD 8–10 codes from death certificates into major categories that have been defined consistently over time (11), we defined all other deaths from malignant cancers as “other cancer mortality” (ie, cancer deaths not attributed to the first primary cancer). Deaths from noncancer causes, excluding those from external causes (“accidents and adverse events,” “suicide and self-inflicted injury,” and “homicide and legal intervention”) and “in situ, benign, or unknown behavior neoplasms,” were defined as noncancer/nonexternal mortality.
Statistical Analysis
We first estimated cumulative mortality among 5-year survivors according to diagnosis era (1975–1984, 1985–1994, 1995–2004, 2005–2011), with person-time of follow-up accrued from 5 years after cancer diagnosis until death or end of follow-up in December 2016, whichever occurred first. Estimates for primary cancer, other cancer, and noncancer/nonexternal mortality accounted for deaths from all other causes as competing risks (12). The log-rank test was used to compare all-cause mortality by diagnosis era, and Gray’s test was used in comparisons of cause-specific mortality. In analyses according to cancer type, we also estimated hazard ratios according to diagnosis year, with follow-up restricted to the first 5 years (ie, from 5 to 10 years postdiagnosis), using Cox proportional hazards regression models, adjusting for age at diagnosis, sex, race, and stage. Estimates for hematologic cancers and central nervous system (CNS) tumors were not adjusted for stage because of lack of variation in the summary stage variable. The proportional hazards assumption was checked by visual inspection of log(-log [survival]) plots. Estimates for primary cancer, other cancer, and noncancer/nonexternal-specific mortality were subdistribution hazard ratios (13), with deaths from all other causes modeled as competing risks. Tests for trend were conducted by modeling diagnosis year as a continuous variable.
All tests were two-sided. A P value of less than .05 was considered statistically significant.
Results
A total of 282 969 five-year AYA cancer survivors were included in these analyses. Across diagnosis periods, the majority were white females who were diagnosed between ages 30 and 39 years and had localized stage disease (Table 1). Breast cancer was the most common cancer type (15.6%) within the first three diagnosis periods (1975–1984, 1985–1994, 1995–2004), whereas thyroid cancer was most common (16.7%) in the most recent diagnosis period (2005–2011).
Table 1.
Characteristics of five-year AYA cancer survivors overall and according to diagnosis year, SEER 1975-2011
| Characteristics | Full sample, No. (%) | 1975-1984, No. (%) | 1985-1994, No. (%) | 1995-2004, No (%) | 2005-2011, No. (%) |
|---|---|---|---|---|---|
| Years follow-up, median (IQR) | 8 (4-16) | 29 (20-32) | 20 (17-23) | 10 (8-12) | 3 (2-5) |
| Sex | |||||
| Female | 176994 (62.5) | 20246 (63.4) | 30070 (61.9) | 64410 (62.4) | 62268 (62.8) |
| Male | 105975 (37.5) | 11697 (36.6) | 18520 (38.1) | 38823 (37.6) | 36935 (37.2) |
| Age at diagnosis, y | |||||
| 15-19 | 19168 (6.8) | 2385 (7.5) | 2806 (5.8) | 6671 (6.5) | 7306 (7.4) |
| 20-24 | 31118 (11.0) | 4220 (13.2) | 5170 (10.6) | 10514 (10.2) | 11214 (11.3) |
| 25-29 | 50007 (17.7) | 6660 (20.8) | 8853 (18.2) | 17168 (16.6) | 17326 (17.5) |
| 30-34 | 73896 (26.1) | 8572 (26.8) | 13223 (27.2) | 27141 (26.3) | 24960 (25.2) |
| 35-39 | 108780 (38.4) | 10106 (31.6) | 18538 (38.2) | 41739 (40.4) | 38397 (38.7) |
| Race | |||||
| White | 232517 (82.2) | 27675 (86.6) | 41006 (84.4) | 84399 (81.8) | 79437 (80.1) |
| Black | 24099 (8.5) | 2394 (7.5) | 3646 (7.5) | 9111 (8.8) | 8948 (9.0) |
| Other | 21775 (7.7) | 1587 (5.0) | 3333 (6.9) | 8055 (7.8) | 8800 (8.9) |
| Unknown | 4578 (1.6) | 287 (0.9) | 605 (1.2) | 1668 (1.6) | 2018 (2.0) |
| Summary stage | |||||
| Localized | 148123 (52.3) | 17267 (54.1) | 26043 (53.6) | 54221 (52.5) | 50592 (51.0) |
| Regional | 56929 (20.1) | 5618 (17.6) | 9157 (18.8) | 21228 (20.6) | 20926 (21.1) |
| Distant | 19439 (6.9) | 1742 (5.5) | 2816 (5.8) | 6942 (6.7) | 7939 (8.0) |
| Unstaged | 56602 (20.0) | 6941 (21.7) | 10427 (21.5) | 20681 (20.0) | 18553 (18.7) |
| Unknown | 1876 (0.7) | 375 (1.2) | 147 (0.3) | 161 (0.2) | 1193 (1.2) |
| Cancer type | |||||
| Leukemia | 8634 (3.1) | 586 (1.8) | 1111 (2.3) | 3235 (3.1) | 3702 (3.7) |
| Non-Hodgkin lymphoma | 15439 (5.5) | 1297 (4.1) | 2342 (4.8) | 6061 (5.9) | 5739 (5.8) |
| Hodgkin lymphoma | 20842 (7.4) | 2947 (9.2) | 3960 (8.1) | 7177 (7.0) | 6758 (6.8) |
| Central nervous system | 10212 (3.6) | 1067 (3.3) | 1824 (3.8) | 3733 (3.6) | 3588 (3.6) |
| Osseous and chondromatous | 3504 (1.2) | 375 (1.2) | 600 (1.2) | 1317 (1.3) | 1212 (1.2) |
| Soft tissue sarcomas * | 9498 (3.4) | 1244 (3.9) | 1841 (3.8) | 3519 (3.4) | 2894 (2.9) |
| Germ cell | 30312 (10.7) | 3306 (10.3) | 5599 (11.5) | 11056 (10.7) | 10351 (10.4) |
| Melanoma/skin | 37646 (13.3) | 4401 (13.8) | 7087 (14.6) | 14119 (13.7) | 12039 (12.1) |
| Thyroid | 39014 (13.8) | 3623 (11.3) | 5180 (10.7) | 13600 (13.2) | 16611 (16.7) |
| Head and neck | 7061 (2.5) | 910 (2.8) | 1327 (2.7) | 2523 (2.4) | 2301 (2.3) |
| Trachea, lung, and bronchus | 2175 (0.8) | 315 (1.0) | 413 (0.8) | 743 (0.7) | 704 (0.7) |
| Female breast | 43397 (15.3) | 4983 (15.6) | 7811 (16.1) | 16188 (15.7) | 14415 (14.5) |
| Kidney | 5227 (1.8) | 282 (0.9) | 570 (1.2) | 1776 (1.7) | 2599 (2.6) |
| Bladder | 3736 (1.3) | 702 (2.2) | 860 (1.8) | 1295 (1.3) | 879 (0.9) |
| Cervix/uterus | 21442 (7.6) | 3229 (10.1) | 4155 (8.6) | 7693 (7.5) | 6365 (6.4) |
| Colorectal | 10549 (3.7) | 952 (3.0) | 1438 (3.0) | 4043 (3.9) | 4116 (4.1) |
| Other | 14281 (5.0) | 1724 (5.4) | 2472 (5.1) | 5155 (5.0) | 4930 (5.0) |
Excludes Kaposi sarcoma
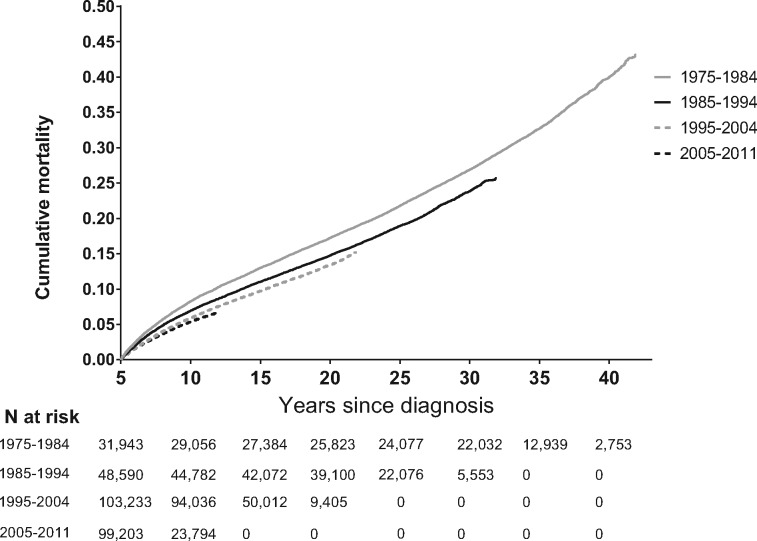
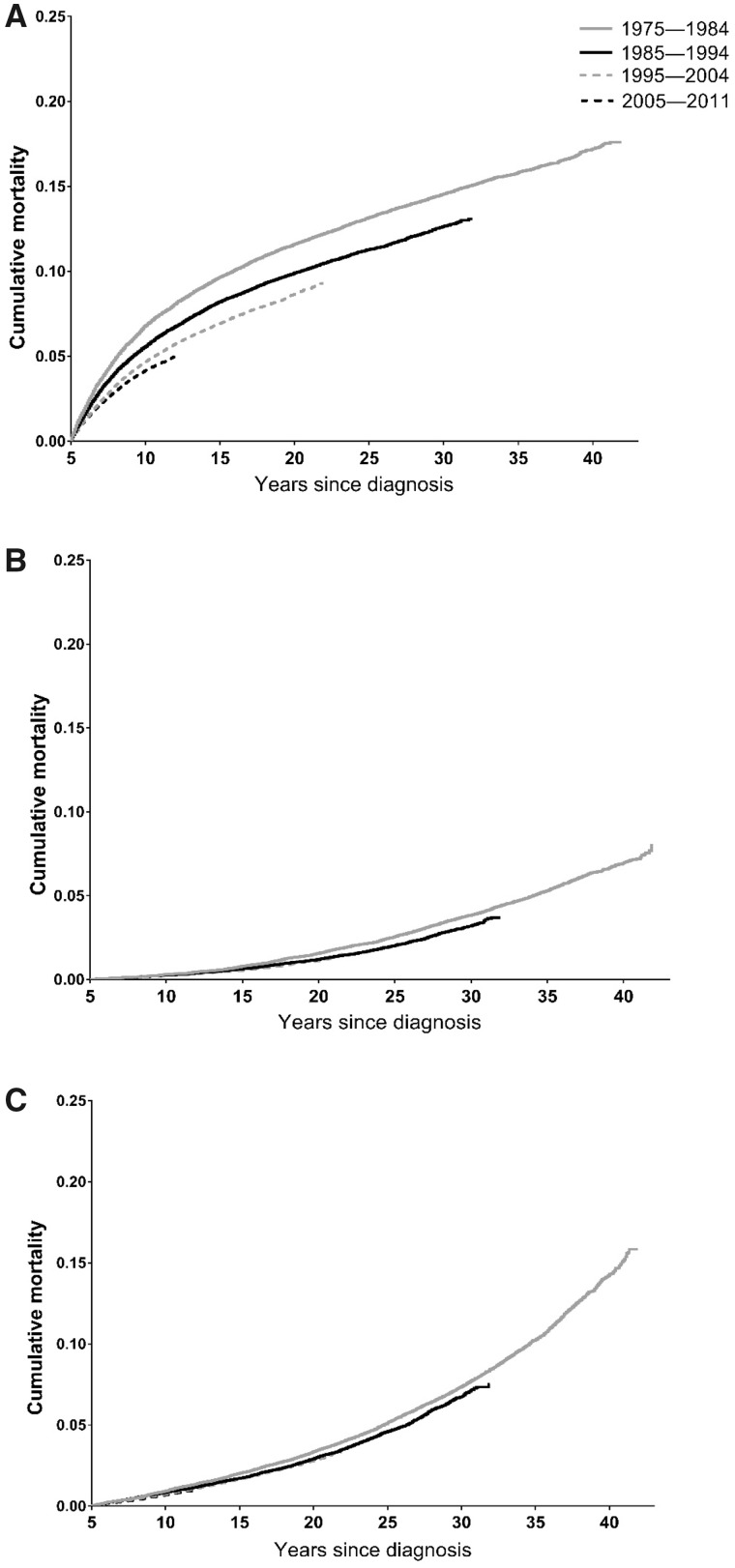
For 5-year survivors of all cancer types combined, 5-year mortality (ie, from 5 to 10 years postdiagnosis) decreased from 8.3% (95% confidence interval = 8.0 to 8.6) to 5.4% (95% confidence interval = 5.3 to 5.6) between those diagnosed in 1975–1984 and those diagnosed in 2005–2011 (Table 2 and Figure 1). This was primarily driven by improvements in 5-year mortality from the primary cancer (6.8% to 4.2%) between these periods, although there was a slight decrease in noncancer/nonexternal mortality as well (0.9% to 0.7%) (Table 2 and Figures 1 and 2). Examination of longer-term trends suggested that these improvements were maintained beyond 5 years of follow-up. Ten-year mortality (ie, from 5 to 15 years postdiagnosis) from all causes decreased from 13.0% for those diagnosed in 1975–1984 to 9.7% for those diagnosed in 1995–2004, whereas primary cancer-specific, other cancer-specific, and noncancer/nonexternal mortality decreased from 9.7% to 6.9%, 0.8% to 0.6%, and 2.0% to 1.7%, respectively, over the same period (Table 2).
Table 2.
Cumulative mortality (95% CI) according to diagnosis year among 5-year AYA cancer survivors
| Cause of death | Cumulative mortality (95% CI) |
|||
|---|---|---|---|---|
| 5-year | 10-year | 20-year | P * | |
| All-cause mortality | <.001 | |||
| 1975–1984 | 8.3 (8.0 to 8.6) | 13.0 (12.7 to 13.4) | 21.8 (21.4 to 22.3) | |
| 1985–1994 | 7.0 (6.7 to 7.2) | 11.1 (10.8 to 11.3) | 19.0 (18.6 to 19.3) | |
| 1995–2004 | 5.9 (5.8 to 6.1) | 9.7 (9.6 to 9.9) | — | |
| 2005–2011 | 5.4 (5.2 to 5.6) | — | — | |
| Primary cancer mortality | <.001 | |||
| 1975–1984 | 6.8 (6.5 to 7.1) | 9.7 (9.4 to 10.0) | 13.2 (12.8 to 13.5) | |
| 1985–1994 | 5.6 (5.4 to 5.8) | 8.2 (8.0 to 8.5) | 11.3 (11.0 to 11.6) | |
| 1995–2004 | 4.7 (4.6 to 4.8) | 6.9 (6.8 to 7.1) | — | |
| 2005–2011 | 4.2 (4.0 to 4.4) | — | — | |
| Other cancer mortality | <.001 | |||
| 1975–1984 | 0.3 (0.2 to 0.4) | 0.8 (0.7 to 0.9) | 2.6 (2.4 to 2.7) | |
| 1985–1994 | 0.3 (0.2 to 0.3) | 0.6 (0.6 to 0.7) | 2.0 (1.9 to 2.2) | |
| 1995–2004 | 0.2 (0.2 to 0.3) | 0.6 (0.5 to 0.6) | — | |
| 2005–2011 | 0.23 (0.2 to 0.3) | — | — | |
| Noncancer/nonexternal mortality | <.001 | |||
| 1975–1984 | 0.9 (0.8 to 1.0) | 2.0 (1.9 to 2.2) | 5.1 (4.9 to 5.4) | |
| 1985–1994 | 0.9 (0.8 to 0.9) | 1.7 (1.6 to 1.8) | 4.6 (4.4 to 4.8) | |
| 1995–2004 | 0.8 (0.7 to 0.8) | 1.7 (1.6 to 1.8) | — | |
| 2005–2011 | 0.7 (0.6 to 0.8) | — | — | |
P value from log-rank test comparing overall survival curves (all-cause mortality) or Gray’s test comparing cumulative mortality curves (primary cancer, other cancer, noncancer/nonexternal mortality). All tests were two-sided. AYA = adolescents and young adults; CI = confidence interval; — = not applicable.
Figure 1.
Cumulative mortality from all causes among 5-year adolescent and young adult (AYA) cancer survivors according to diagnosis year.
Figure 2.
Cumulative mortality according to diagnosis year among 5-year adolescent and young adult (AYA) cancer survivors. A) Primary cancer mortality, (B) other cancer mortality, (C) noncancer/nonexternal mortality.
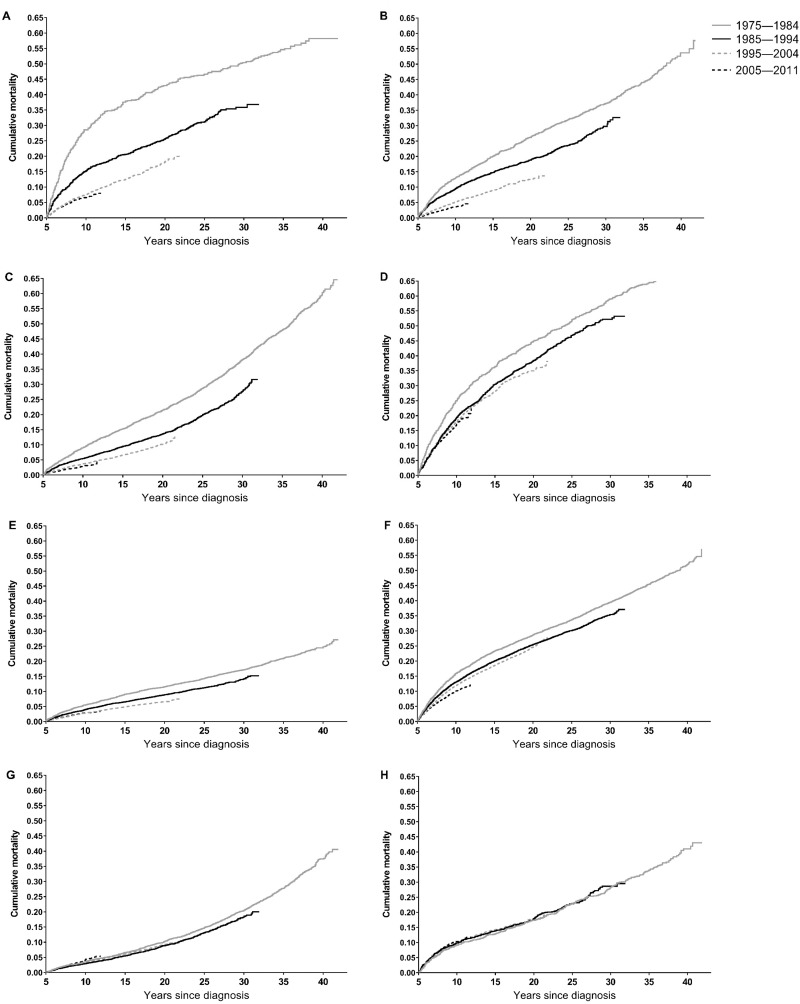
Patterns of late mortality from all causes for selected cancer types are shown in Figure 3 and Supplementary Figure 1 (available online). Substantial decreases in 5-year all-cause mortality between the earliest and most recent diagnosis periods were observed for leukemias (28.6% to 6.6%), non-Hodgkin lymphoma (13.0% to 3.6%), Hodgkin lymphoma (9.1% to 3.0%), CNS tumors (25.0% to 17.2%), melanoma and skin cancers (5.5% to 2.9%), breast cancer (15.9% to 10.1%), and kidney cancer (10.7% to 4.4%) (Table 3 and Figure 3). Multivariable-adjusted hazard ratios also suggested decreases in 5-year all-cause mortality for these cancer types over time (all Ptrend < .05) (Supplementary Table 1, available online). In contrast, for osseous and chondromatous neoplasms, soft tissue sarcomas, bladder cancer, cervical and/or uterine cancers, and colorectal cancer, there was little evidence of improvement in all-cause mortality between the earliest and most recent diagnosis periods. In analyses stratified by age at diagnosis, patterns of all-cause mortality according to diagnosis period were similar between those aged 15–29 years and those aged 30–39 years for most cancer types (Supplementary Table 2, available online).
Figure 3.
Cumulative mortality from all causes among 5-year adolescent and young adult (AYA) survivors of selected cancers. A) Leukemia, (B) non-Hodgkin lymphoma, (C) Hodgkin lymphoma, (D) central nervous system tumors, (E) melanoma/skin cancers, (F) female breast cancer, (G) cervical/uterine cancers, (H) colorectal cancer.
Table 3.
Cumulative mortality (95% CI) from all causes according to diagnosis year among 5-year AYA cancer survivors, stratified by cancer type, age, sex, and race
| Characteristic | All-cause mortality |
|||
|---|---|---|---|---|
| 5-year | 10-year | 20-year | P * | |
| Cancer type | ||||
| Leukemia | <.001 | |||
| 1975–1984 | 28.6 (25.0 to 32.3) | 37.9 (34.0 to 41.8) | 46.6 (42.5 to 50.7) | |
| 1985–1994 | 15.3 (13.2 to 17.5) | 20.6 (18.3 to 23.1) | 31.3 (28.4 to 34.1) | |
| 1995–2004 | 7.6 (6.7 to 8.5) | 12.5 (11.4 to 13.8) | — | |
| 2005–2011 | 6.6 (5.6 to 7.6) | — | — | |
| Non-Hodgkin lymphoma | <.001 | |||
| 1975–1984 | 13.0 (11.2 to 14.9) | 20.1 (18.0 to 22.4) | 32.0 (29.4 to 34.6) | |
| 1985–1994 | 9.6 (8.4 to 10.8) | 14.8 (13.4 to 16.2) | 23.6 (21.8 to 25.4) | |
| 1995–2004 | 5.2 (4.6 to 5.8) | 9.1 (8.3 to 9.9) | — | |
| 2005–2011 | 3.6 (3.0 to 4.3) | — | — | |
| Hodgkin lymphoma | <.001 | |||
| 1975–1984 | 9.1 (8.1 to 10.1) | 15.3 (14.0 to 16.6) | 28.7 (27.1 to 30.4) | |
| 1985–1994 | 5.5 (4.8 to 6.2) | 9.5 (8.6 to 10.4) | 20.0 (18.7 to 21.3) | |
| 1995–2004 | 3.8 (3.4 to 4.2) | 6.7 (6.1 to 7.3) | — | |
| 2005–2011 | 3.0 (2.5 to 3.5) | — | — | |
| Central nervous system | <.001 | |||
| 1975–1984 | 25.0 (22.4 to 27.6) | 36.4 (33.6 to 39.3) | 52.0 (49.0 to 55.0) | |
| 1985–1994 | 19.4 (17.7 to 21.3) | 30.5 (28.4 to 32.6) | 46.8 (44.4 to 49.2) | |
| 1995–2004 | 18.0 (16.8 to 19.3) | 28.1 (26.6 to 29.6) | — | |
| 2005–2011 | 17.2 (15.6 to 18.9) | — | — | |
| Osseous and chondromatous | .43 | |||
| 1975–1984 | 10.4 (7.6 to 13.8) | 14.2 (10.9 to 18.0) | 21.8 (17.8 to 26.2) | |
| 1985–1994 | 8.9 (6.8 to 11.3) | 13.1 (10.6 to 16.0) | 19.3 (16.2 to 22.7) | |
| 1995–2004 | 10.6 (9.0 to 12.3) | 15.1 (13.2 to 17.2) | — | |
| 2005–2011 | 10.1 (7.9 to 12.5) | — | — | |
| Soft tissue sarcomas | .96 | |||
| 1975–1984 | 6.2 (5.0 to 7.7) | 9.8 (8.2 to 11.5) | 17.6 (15.6 to 19.8) | |
| 1985–1994 | 7.3 (6.2 to 8.6) | 10.7 (9.4 to 12.2) | 17.3 (15.6 to 19.1) | |
| 1995–2004 | 6.5 (5.7 to 7.3) | 10.4 (9.3 to 11.4) | — | |
| 2005–2011 | 7.1 (5.9 to 8.4) | — | — | |
| Germ cell and trophoblastic | <.001 | |||
| 1975–1984 | 1.7 (1.3 to 2.2) | 4.4 (3.8 to 5.2) | 11.9 (10.8 to 13.0) | |
| 1985–1994 | 2.2 (1.9 to 2.6) | 4.0 (3.5 to 4.6) | 9.3 (8.5 to 10.1) | |
| 1995–2004 | 1.4 (1.2 to 1.6) | 3.0 (2.7 to 3.4) | — | |
| 2005–2011 | 1.6 (1.3 to 2.0) | — | — | |
| Melanoma/skin | <.001 | |||
| 1975–1984 | 5.5 (4.9 to 6.2) | 9.0 (8.2 to 9.9) | 14.3 (13.3 to 15.4) | |
| 1985–1994 | 4.0 (3.6 to 4.5) | 6.5 (6.0 to 7.1) | 11.2 (10.5 to 12.0) | |
| 1995–2004 | 2.8 (2.6 to 3.1) | 4.8 (4.5 to 5.2) | — | |
| 2005–2011 | 2.9 (2.6 to 3.3) | — | — | |
| Thyroid | .16 | |||
| 1975–1984 | 1.2 (0.9 to 1.6) | 2.5 (2.0 to 3.0) | 6.2 (5.4 to 7.0) | |
| 1985–1994 | 0.8 (0.6 to 1.1) | 1.8 (1.5 to 2.2) | 5.2 (4.6 to 5.9) | |
| 1995–2004 | 0.9 (0.8 to 1.1) | 2.1 (1.9 to 2.4) | — | |
| 2005–2011 | 0.9 (0.7 to 1.1) | — | — | |
| Head and neck | .001 | |||
| 1975–1984 | 7.8 (6.2 to 9.7) | 13.6 (11.4 to 15.9) | 26.9 (24.0 to 29.8) | |
| 1985–1994 | 8.2 (6.7 to 9.7) | 14.3 (12.4 to 16.2) | 27.2 (24.7 to 29.7) | |
| 1995–2004 | 6.4 (5.5 to 7.4) | 10.7 (9.5 to 12.1) | — | |
| 2005–2011 | 6.1 (4.8 to 7.6) | — | — | |
| Trachea, lung, and bronchus | .70 | |||
| 1975–1984 | 14.3 (10.7 to 18.4) | 24.8 (20.2 to 29.7) | 35.3 (30.1 to 40.6) | |
| 1985–1994 | 13.4 (10.3 to 16.9) | 18.4 (14.8 to 22.3) | 32.5 (27.9 to 37.3) | |
| 1995–2004 | 11.8 (9.6 to 14.2) | 18.7 (15.8 to 21.8) | — | |
| 2005–2011 | 10.9 (8.3 to 13.9) | — | — | |
| Female breast | <.001 | |||
| 1975–1984 | 15.9 (14.9 to 17.0) | 23.3 (22.2 to 24.5) | 33.7 (32.4 to 35.0) | |
| 1985–1994 | 13.1 (12.4 to 13.9) | 20.1 (19.2 to 21.0) | 30.1 (29.1 to 31.2) | |
| 1995–2004 | 11.9 (11.4 to 12.4) | 18.6 (18.0 to 19.2) | — | |
| 2005–2011 | 10.1 (9.5 to 10.8) | — | — | |
| Kidney | <.001 | |||
| 1975–1984 | 10.7 (7.4 to 14.6) | 16.8 (12.6 to 21.4) | 31.0 (25.6 to 36.5) | |
| 1985–1994 | 7.2 (5.3 to 9.5) | 14.3 (11.6 to 17.4) | 24.6 (21.1 to 28.4) | |
| 1995–2004 | 5.1 (4.1 to 6.2) | 10.6 (9.1 to 12.2) | — | |
| 2005–2011 | 4.4 (3.4 to 5.6) | — | — | |
| Bladder | .10 | |||
| 1975–1984 | 2.4 (1.5 to 3.8) | 5.0 (3.6 to 6.9) | 11.7 (9.4 to 14.2) | |
| 1985–1994 | 1.9 (1.1 to 2.9) | 4.8 (3.5 to 6.4) | 12.4 (10.2 to 14.8) | |
| 1995–2004 | 3.0 (2.1 to 4.0) | 6.6 (5.2 to 8.1) | — | |
| 2005–2011 | 3.7 (2.3 to 5.6) | — | — | |
| Cervix/uterus | .01 | |||
| 1975–1984 | 3.6 (3.0 to 4.3) | 6.6 (5.8 to 7.5) | 14.8 (13.6 to 16.1) | |
| 1985–1994 | 2.9 (2.5 to 3.5) | 5.5 (4.8 to 6.2) | 13.1 (12.1 to 14.3) | |
| 1995–2004 | 3.4 (3.0 to 3.8) | 6.1 (5.5 to 6.7) | — | |
| 2005–2011 | 4.4 (3.7 to 5.1) | — | — | |
| Colorectal | .84 | |||
| 1975–1984 | 9.2 (7.4 to 11.1) | 12.9 (10.9 to 15.1) | 17.4 (15.0 to 19.9) | |
| 1985–1994 | 9.9 (8.4 to 11.5) | 13.7 (12.0 to 15.5) | 22.9 (20.7 to 25.2) | |
| 1995–2004 | 9.8 (8.9 to 10.7) | 14.3 (13.2 to 15.5) | — | |
| 2005–2011 | 10.3 (9.2 to 11.6) | — | — | |
| Age at diagnosis, y | ||||
| 15–24 | <.001 | |||
| 1975–1984 | 5.8 (5.2 to 6.4) | 8.9 (8.2 to 9.6) | 15.4 (14.5 to 16.3) | |
| 1985–1994 | 4.2 (3.8 to 4.7) | 6.8 (6.2 to 7.3) | 12.0 (11.2 to 12.7) | |
| 1995–2004 | 3.7 (3.4 to 4.0) | 5.9 (5.6 to 6.3) | — | |
| 2005–2011 | 4.0 (3.7 to 4.4) | — | — | |
| 25–29 | <.001 | |||
| 1975–1984 | 6.9 (6.3 to 7.6) | 10.9 (10.2 to 11.7) | 18.2 (17.2 to 19.1) | |
| 1985–1994 | 5.6 (5.1 to 6.1) | 9.0 (8.4 to 9.6) | 15.2 (14.5 to 16.0) | |
| 1995–2004 | 4.6 (4.3 to 4.9) | 7.5 (7.1 to 7.9) | — | |
| 2005–2011 | 4.4 (4.0 to 4.9) | — | — | |
| 30–34 | <.001 | |||
| 1975–1984 | 8.4 (7.8 to 9.0) | 13.3 (12.6 to 14.0) | 21.9 (21.0 to 22.8) | |
| 1985–1994 | 7.2 (6.7 to 7.6) | 11.4 (10.9 to 12.0) | 19.1 (18.4 to 19.8) | |
| 1995–2004 | 5.8 (5.5 to 6.1) | 9.5 (9.2 to 9.9) | — | |
| 2005–2011 | 5.5 (5.1 to 5.8) | — | — | |
| 35–39 | <.001 | |||
| 1975–1984 | 10.8 (10.2 to 11.4) | 17.0 (16.2 to 17.7) | 28.4 (27.5 to 29.2) | |
| 1985–1994 | 8.67 (8.3 to 9.1) | 13.7 (13.2 to 14.2) | 23.7 (23.0 to 24.3) | |
| 1995–2004 | 7.5 (7.2 to 7.7) | 12.4 (12.0 to 12.7) | — | |
| 2005–2011 | 6.5 (6.2 to 6.9) | — | — | |
| Sex | ||||
| Women | <.001 | |||
| 1975–1984 | 8.3 (7.9 to 8.7) | 12.9 (12.4 to 13.4) | 21.0 (20.4 to 21.6) | |
| 1985–1994 | 6.8 (6.6 to 7.1) | 10.9 (10.5 to 11.2) | 18.2 (17.7 to 18.6) | |
| 1995–2004 | 6.0 (5.8 to 6.2) | 9.8 (9.5 to 10.0) | — | |
| 2005–2011 | 5.4 (5.1 to 5.6) | — | — | |
| Men | <.001 | |||
| 1975–1984 | 8.3 (7.8 to 8.8) | 13.3 (12.7 to 13.9) | 23.3 (22.5 to 24.0) | |
| 1985–1994 | 7.2 (6.8 to 7.5) | 11.4 (10.9 to 11.8) | 20.2 (19.6 to 20.8) | |
| 1995–2004 | 5.8 (5.6 to 6.1) | 9.7 (9.4 to 10.0) | — | |
| 2005–2011 | 5.6 (5.2 to 5.9) | — | — | |
| Race | ||||
| White | <.001 | |||
| 1975–1984 | 8.1 (7.8 to 8.4) | 12.7 (12.3 to 13.1) | 21.1 (20.6 to 21.6) | |
| 1985–1994 | 6.6 (6.4 to 6.9) | 10.5 (10.2 to 10.8) | 18.0 (17.6 to 18.4) | |
| 1995–2004 | 5.6 (5.4 to 5.7) | 9.2 (9.0 to 9.4) | — | |
| 2005–2011 | 5.1 (4.9 to 5.3) | — | — | |
| Black | <.001 | |||
| 1975–1984 | 12.0 (10.7 to 13.3) | 18.8 (17.3 to 20.4) | 31.6 (29.7 to 33.5) | |
| 1985–1994 | 11.9 (10.8 to 12.9) | 18.5 (17.3 to 19.8) | 31.1 (29.5 to 32.7) | |
| 1995–2004 | 9.5 (8.9 to 10.1) | 15.2 (14.4 to 16.0) | — | |
| 2005–2011 | 8.9 (8.1 to 9.7) | — | — | |
| Other/unknown | <.001 | |||
| 1975–1984 | 7.0 (5.9 to 8.2) | 11.4 (10.0 to 13.0) | 19.9 (18.1 to 21.8) | |
| 1985–1994 | 6.1 (5.3 to 6.8) | 9.8 (8.8 to 10.8) | 17.5 (16.2 to 18.8) | |
| 1995–2004 | 5.5 (5.1 to 6.0) | 9.3 (8.7 to 9.9) | — | |
| 2005–2011 | 5.1 (4.6 to 5.7) | — | — | |
From log-rank test comparing overall survival. All tests were two-sided. AYA = adolescents and young adults; CI = confidence interval.
For most cancer types, patterns of mortality from the primary cancer across diagnosis periods largely paralleled those of all-cause mortality (Supplementary Table 3, available online). Deaths from other cancers were rare during early follow-up, but, for most cancer types, there was some evidence of decline in 20-year other cancer mortality between survivors diagnosed in 1975–1984 and those diagnosed in 1985–2994. Improvements in 5-year noncancer/nonexternal mortality were most apparent for Hodgkin lymphoma (1.7% to 0.6%) and leukemia (3.3% to 1.4%) (Supplementary Table 4, available online). Cumulative mortality curves also suggested decreases in noncancer/nonexternal mortality for head and neck cancers (1.8% to 1.0%); trachea, lung, and bronchus cancers (2.9% to 0.8%); and kidney cancer (3.2% to 1.4%), although estimates were imprecise. Multivariable-adjusted hazard ratios generally supported these patterns (Supplementary Table 5, available online).
Discussion
In the past several decades, treatment of cancer in AYAs has evolved substantially, leading to steady improvements in estimated 5-year survival at diagnosis (4,5). Yet the potential impact on late mortality in this population has remained largely unexamined. In this study of 5-year AYA cancer survivors, we found substantial improvements in both all-cause and primary cancer-specific mortality for all AYAs combined, and for several individual cancer types. However, for other cancer types, including colorectal, osseous and chondromatous, soft tissue sarcomas, and bladder, cumulative mortality curves suggested little difference in late mortality between patients diagnosed in the 1970s and those diagnosed in more recent decades.
Our analyses indicate that most of the reduction in all-cause mortality among 5-year AYA cancer survivors was attributable to improvements in survival from the primary cancer, particularly between 5 and 10 years postdiagnosis. Likely this reflects the benefit of improved cancer therapies and refined treatment strategies for preventing or delaying late mortality, as well as early mortality, from progression or recurrence of the primary malignancy. Small observed decreases in late mortality from other cancers, defined in our study as deaths from cancer that were not attributed to the primary malignancy, are likely also explained by time-related improvements in cancer treatments. Although we included a different range of diagnosis years and more recent follow-up, our overall analyses largely parallel those from a previous study of 5-year survivors of childhood cancer (diagnosed at ages 0–20 years) identified using SEER data in which reductions in all-cause mortality through 10 years postdiagnosis between patients diagnosed in 1974–1980 and those diagnosed in 1995–2004 (7.1% to 3.9%) were largely driven by reductions in death from recurrence or progression of the primary cancer (5.1% to 2.8%) during this period (14).
Some of the most obvious improvements in primary cancer-specific mortality over time were observed among AYA survivors of hematologic malignancies and CNS tumors—cancer types that are also some of the more predominant among children and have therefore been the focus of considerable childhood cancer research across recent decades. Notably, 5-year cancer-specific mortality among 5-year AYA leukemia survivors dropped from more than 25% to less than 5% between patients treated in the late 1970s and early 1980s and those treated during 2005–2011. Reductions in cancer-specific mortality were also pronounced for survivors of lymphomas and CNS tumors—cancer types with some of the highest mortality from cancer within the period of 5- to 10-years postdiagnosis. These findings demonstrate the impact of more modern treatment regimens on survival beyond the 5-year time point for patients with these cancers—patterns missed by prior AYA studies that have focused only on trends in 5-year survival at diagnosis (4–6).
Time-related trends in mortality were less consistent across cancer types more typical of older adults, which are generally not included in studies of late mortality among childhood cancer survivors (14–18). Improvements over time were evident for 5-year survivors of breast cancer, melanoma and skin cancers, and kidney cancer. However, even in the most recent diagnosis period, cancer-specific mortality remained relatively high between 5 and 10 years postdiagnosis for AYAs with breast cancer, a likely consequence of continued risk of late recurrences, which often present as distant stage disease (19). For AYAs with colorectal cancer, our analyses did not suggest improvement in late cancer-specific mortality over time, a particularly concerning finding given recently reported increases in colorectal cancer incidence among young adults (20). Other cancer types with little evidence of improvement included bladder cancer, cervical and/or uterine cancers, soft tissue sarcomas, and osseous and chondromatous neoplasms; survivors of these cancer types may therefore be priority groups for efforts to improve long-term surveillance to reduce late mortality from cancer.
Recent evidence has suggested that AYAs with cancer have elevated mortality from noncancer causes relative to the general population (21), which is likely due, at least in part, to adverse effects of exposure to toxic cancer therapies. In absolute terms, mortality from noncancer/nonexternal causes among 5-year survivors was generally low through the first 10 to 15 years postdiagnosis, when even the oldest survivors in our analyses were still relatively young adults. However, there was some evidence of a reduction in late mortality across diagnosis eras for particular cancer types. Improvements over the entirety of follow-up in our study were most apparent for Hodgkin lymphoma, a probable consequence of strategies to reduce radiation doses and cumulative exposure to anthracycline-based chemotherapy for patients treated in more recent years in an attempt to reduce cardiac damage (22). Although less clearly and consistently for other cancer types than for Hodgkin lymphoma, cumulative mortality curves for AYA survivors of leukemias, head and neck cancers, kidney cancer, and trachea, lung, and bronchus cancers were also suggestive of some reduction in late mortality from noncancer/nonexternal causes over time, likely reflecting a combination of broader efforts to minimize cancer treatment–related toxicity and improved treatments for other noncancer chronic diseases among long-term survivors.
This population-based study is among the first to examine temporal trends in mortality among long-term survivors of AYA cancers. However, there are some limitations to our analyses. Although SEER registries collect vital status and cause of death information, they do not collect information on disease relapse; therefore, we were unable to account for relapse in our analyses. Detailed cancer treatment information is also not available in SEER, so we were unable to directly evaluate how specific therapies contributed to patterns of late mortality. Additionally, cause of death information in SEER comes from death certificates and is subject to some misclassification. Therefore, some deaths due to cancer may have been misattributed to noncancer causes in our analyses and vice versa. For less common cancer types, the number of 5-year survivors was fairly small, particularly within earlier diagnosis years, and cumulative incidence estimates were imprecise. We also had insufficient sample size for most cancer types to conduct detailed analyses of trends according to characteristics, such as stage and age at diagnosis, or to examine trends in mortality from specific noncancer causes, such as cardiovascular disease.
Over the past four decades, all-cause and cancer-specific mortalities have decreased among 5-year AYA cancer survivors overall, but several cancer types have not shared in these improvements. As cancer therapies continue to advance, it will be critical to continue monitoring late mortality to guide long-term follow-up recommendations.
Funding
HBN was supported by the St. Baldrick’s Foundation.
Notes
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Conflicts of interest: The authors have no disclosures.
Supplementary Material
References
- 1.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance. National Cancer Institute and the LiveStrong Young Adult Alliance. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Report of the Adolescent and Young Adult Oncology Program Review Group. https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf. Published August 2006. Accessed October 1, 2019.
- 2. Sender L, Zabokrtsky KB.. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol. 2015;12(8):465–480. [DOI] [PubMed] [Google Scholar]
- 3. Barr RD, Ferrari A, Ries L, et al. Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr. 2016;170(5):495–501. [DOI] [PubMed] [Google Scholar]
- 4. Keegan TH, Ries LA, Barr RD, et al. ; for the National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 5. Liu L, Moke DJ, Tsai KY, et al. A reappraisal of sex-specific cancer survival trends among adolescents and young adults in the United States. J Natl Cancer Inst. 2019;111(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bleyer A, O’Leary M, Barr R, Ries LA, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000 Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 7.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975–2016 varying). http://www.seer.cancer.gov. Published April 2019. Accessed October 1, 2019.
- 8.Surveillance, Epidemiology, and End Results Program. Overview of the SEER Program. https://seer.cancer.gov/about/overview.html. Accessed October 1, 2019.
- 9.Surveillance, Epidemiology, and End Results Program. AYA Site Recode/WHO 2008 Definition. https://seer.cancer.gov/ayarecode/aya-who2008.html. Accessed October 1, 2019.
- 10.Surveillance, Epidemiology and End Results Program. SEER Cause-specific Death Classification. https://seer.cancer.gov/causespecific/. Accessed December 5, 2019.
- 11.Surveillance, Epidemiology, and End Results Program. SEER Cause of Death Recode 1969+ (04/16/2012). https://seer.cancer.gov/codrecode/1969+_d04162012/index.html. Accessed October 1, 2019.
- 12. Lin GS, Johnston G. Analyzing survival data with competing risks using SAS software. SAS Global Forum 2012 2012. Paper 344-2012; Orlando, FL.
- 13. Fine J, Gray R.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 14. Armstrong GT, Pan Z, Ness KK, et al. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28(7):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. [DOI] [PubMed] [Google Scholar]
- 19. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lui RN, Tsoi KK, Ho JM, et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: a joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol Biomarkers Prev. 2019;28(8):1275–1282. [DOI] [PubMed] [Google Scholar]
- 21. Anderson C, Lund JL, Weaver MA, et al. Noncancer mortality among adolescents and young adults with cancer. Cancer. 2019;125(12):2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shanbhag S, Ambinder RF.. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.