Abstract
Background
The optimal clinical management of oral precancer remains uncertain. We investigated the natural history of oral leukoplakia, the most common oral precancerous lesion, to estimate the relative and absolute risks of progression to cancer, the predictive accuracy of a clinician’s decision to biopsy a leukoplakia vis-à-vis progression, and histopathologic predictors of progression.
Methods
We conducted a retrospective cohort study (1996–2012) of patients with oral leukoplakia (n = 4886), identified using electronic medical records within Kaiser Permanente Northern California. Among patients with leukoplakia who received a biopsy (n = 1888), we conducted a case-cohort study to investigate histopathologic predictors of progression. Analyses included indirect standardization and unweighted or weighted Cox regression.
Results
Compared with the overall Kaiser Permanente Northern California population, oral cancer incidence was substantially elevated in oral leukoplakia patients (standardized incidence ratio = 40.8, 95% confidence interval [CI] = 34.8 to 47.6; n = 161 cancers over 22 582 person-years). Biopsied leukoplakias had a higher oral cancer risk compared with those that were not biopsied (adjusted hazard ratio = 2.38, 95% CI = 1.73 to 3.28). However, to identify a prevalent or incident oral cancer, the biopsy decision had low sensitivity (59.6%), low specificity (62.1%), and moderate positive–predictive value (5.1%). Risk of progression to oral cancer statistically significantly increased with the grade of dysplasia; 5-year competing risk-adjusted absolute risks were: leukoplakia overall = 3.3%, 95% CI = 2.7% to 3.9%; no dysplasia = 2.2%, 95% CI = 1.5% to 3.1%; mild-dysplasia = 11.9%, 95% CI = 7.1% to 18.1%; moderate-dysplasia = 8.7%, 95% CI = 3.2% to 17.9%; and severe dysplasia = 32.2%, 95% CI = 8.1%–60.0%. Yet 39.6% of cancers arose from biopsied leukoplakias without dysplasia.
Conclusions
The modest accuracy of the decision to biopsy a leukoplakia vis-à-vis presence or eventual development of oral cancer highlights the need for routine biopsy of all leukoplakias regardless of visual or clinical impression. Leukoplakia patients, particularly those with dysplasia, need to be closely monitored for signs of early cancer.
Cancers of the oral cavity (lip, oral tongue, gingiva, floor of mouth, palate, and other mouth, including buccal mucosa) account for approximately 250 000 annual incident cases worldwide (1). Most patients with oral cancers are diagnosed at advanced stages, undergo morbid treatments, and have poor survival (1). Oral cancers are believed to be preceded by precancerous lesions, defined clinically based on visual examination (a white patch in the mouth), erythroplakia (a red patch in the mouth), and oral submucous fibrosis (irreversible fibrosis of the submucous tissue) as well as histopathologically based on the presence of dysplasia (2–4). Despite the ease of visual and tactile inspection of the oral mucosa and the availability of precancerous lesions, there are currently no organized programs for screening and secondary prevention of oral cavity cancers in most parts of the world owing to several knowledge gaps regarding the natural history of oral precancer and the clinical management of patients with precancer (5,6).
The natural history of oral leukoplakia, the most common oral precancerous lesion, remains poorly characterized (6,7). The rate of progression of oral leukoplakia to invasive oral cancer has varied widely in the literature, ranging from 0% to 36% (7–10). This variability, in part, arises from differences across studies in the geographic region and risk profile of the populations (smoking vs tobacco chewing), definition of precancer (visual vs histopathologic), study design (clinic based vs population based), duration of follow-up (short vs long term), and completeness of outcome ascertainment (active vs cancer registry based) (7). Only a single study of US elderly patients (aged 65+ years) has addressed the natural history of oral leukoplakia in a large, population-based setting with complete outcome ascertainment (11).
Importantly, the optimal clinical management of patients with oral leukoplakia is unclear (5,7,12). The thresholds for triage and biopsy of leukoplakia are poorly defined (12,13), the predictive value of the histopathologic presence or grade of dysplasia for the identification of lesions most likely to progress to cancer is reportedly equivocal (14), and the appropriate frequency of clinical follow-up of patients with leukoplakia for signs of early cancer is uncertain (7).
We conducted a retrospective cohort study within Kaiser Permanente Northern California (KPNC), a large, integrated health-care system, to investigate the risk of oral cancer in patients with oral leukoplakia. Specifically, we estimated the short- and long-term risks of progression from oral leukoplakia to oral cancer, the association of presence or grade of dysplasia with risk of progression to oral cancer, as well as the predictive value of a clinician’s decision to biopsy the leukoplakia vis-à-vis progression to cancer.
Methods
Study Population
This study was conducted within KPNC, which serves approximately 4 million individuals each calendar year and comprises approximately 28% of the insured northern California population. The KPNC adult population is demographically similar to both the non-Kaiser insured population and the California general population (15). Since 1995, KPNC has maintained electronic medical records for all participants (>10 million participants), which include complete demographic data, detailed medical or clinical histories, and limited behavioral data.
Study Design
We conducted a retrospective cohort study (1996–2012) of patients with clinically diagnosed oral leukoplakia. We investigated the risk of oral cancer in this cohort relative to the entire KPNC population; within patients with oral leukoplakia, we investigated predictors of progression to oral cancer. We also identified a second cohort of patients with oral leukoplakia who received a biopsy; in this second cohort, we conducted a case-cohort study to investigate the association of histopathologic characteristics with risk of progression to oral cancer.
This study was approved by the Kaiser Permanente Division of Research Institutional Review Board (including a waiver of informed consent).
Data Abstraction
Patients with a clinical diagnosis of oral leukoplakia (n = 4886) were identified from electronic medical records through International Classification of Diseases Version-9 (ICD-9) code 528.6. Oral erythroplakia was not included in our evaluation because a separate ICD-9 code did not exist before 2004, and oral submucous fibrosis was not included given its rarity in the United States. We also obtained information regarding whether the leukoplakia was diagnosed by an ear, nose, and throat (ENT) clinician. Oral cancers were identified through the KPNC cancer registry, which participates in the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (16). The Surveillance, Epidemiology, and End Results Program cancer registries are considered the gold standard for cancer registration worldwide, with near-complete case ascertainment and microscopic verification (16). Oral cancers were identified using the International Classification of Diseases for Oncology Version-3 codes: C000-C009 (lip), C020-C028 (oral tongue), C030-C039 (gingiva), C040-C049 (floor of mouth), C050-C059 (palate), and C060-C069 (other mouth, including buccal mucosa). Cancers of the soft palate and uvula, although classified as oropharyngeal cancers, were included because of the ease of visual inspection and the common occurrence of oral leukoplakia at these sites. All histologic subtypes were included, but we note that the majority (approximately 95%) of oral cavity cancers are squamous cell carcinomas. Data on demographics (age, sex, race or ethnicity), socio-economic status (based on residential census tract), ever smoking (based on a report of current smoking at any time), alcohol abuse and dependence (ICD-9 codes: 291, 303, 305.00–305.03), and human immunodeficiency virus (HIV) serostatus were abstracted from patient medical records.
Data on histopathology and the anatomic site of precancer were only available for patients who received a biopsy. Thus, we assembled a second cohort of patients clinically diagnosed with oral leukoplakia who also received a biopsy within ±30 days of the date of first leukoplakia diagnosis (n = 1888; 36% of clinically diagnosed leukoplakias). However, because it was infeasible to manually abstract pathology reports for this entire cohort, we used a case-cohort study design to investigate the association of anatomic and histopathologic characteristics with risk of progression of leukoplakia to cancer.
In the case-cohort study within the 1888 leukoplakia patients who received a biopsy (Supplementary Figure 1 available online), cases included all patients (100%) with leukoplakia who progressed to oral cancer (n = 96) and the subcohort for comparison (controls) included a random sample of the 1888 patients with leukoplakia who received a biopsy (n = 500, 26.5%). Of note, this subcohort of 500 patients also included 25 oral cancer cases, as is the norm in case-cohort studies given the use of the full cohort (cases and noncases) as the sampling frame (17,18). From these patients, we manually abstracted pathology reports for the biopsies conducted ±30 days from leukoplakia diagnosis to collect information on the anatomic site of leukoplakia and the histopathologic diagnosis, classified as no dysplasia (normal, hyperkeratosis, or hyperplasia), mild dysplasia, moderate dysplasia, severe dysplasia, and cancer (carcinoma in situ and invasive cancer). Independent pathology re-review is not routinely performed as part of clinical care in KPNC. Thus, the analyzed histopathologic diagnoses represent review by a single pathologist in most instances. All pathology records were abstracted for patients who received multiple biopsies for one or multiple lesions at one time point. Record abstraction was conducted by one investigator (A. K. Chaturvedi) and independently verified on a 10% random sample by another investigator (M. J. Silverberg); discrepancies were minimal (<2%).
Statistical Analyses
We imposed a minimum health plan membership coverage of 1 year for entry into the cohort. Follow-up for patients with oral leukoplakia started at the date of first leukoplakia diagnosis and ended at the earliest of oral cancer diagnosis, death, end of health plan membership, or end of the study (December 31, 2012).
The risk of oral cancer among patients with oral precancer compared with the entire KPNC population was estimated using standardized incidence ratios (SIRs) and 95% Poisson confidence intervals (CIs) with indirect standardization by age (5-year age groups), sex, and attained calendar year. SIRs were calculated overall and across anatomic site of cancer. Because some prevalent oral cancers could initially be misdiagnosed as oral leukoplakias, we also estimated SIRs stratified by time since leukoplakia diagnosis (<12 months and ≥12 months).
Among patients with oral leukoplakia, we investigated predictors of progression to oral cancer using Cox regression. We estimated the overall hazard ratios (HRs) as well as time-varying hazard ratios by time since leukoplakia diagnosis (<12 months and ≥12 months) to evaluate and account for nonproportionality. Predictors included age (per 10-year increments), sex, race, socio-economic status score, ever smoking, alcohol abuse, HIV serostatus, and biopsy of leukoplakia (yes or no). With oral cancer as the gold standard outcome, we also calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), complement of the NPV (= 1 − NPV), and area under the receiver operating characteristic curve (AUC = [sensitivity + specificity]/2) for the clinician’s decision to conduct a biopsy of the clinically diagnosed leukoplakia. We present the AUC as a familiar transformation of the Youden’s index for binary evaluations (19).
We analyzed the case-cohort component of the study as a weighted two-phase sampling design (20,21). Weighted Cox regression models (overall as well as time varying: <12 months and ≥ 12 months since leukoplakia diagnosis to evaluate and account for nonproportionality) were used to investigate the association of histopathologic characteristics with risk of progression to oral cancer. These models utilized the inverse of the sampling probabilities into the case-cohort subset as weights (weight = 96/96 = 1 for oral cancer cases and weight = 1792/473 = 3.79 for the subcohort minus cases); oral cancer cases in the subcohort (n = 25) were only counted once in these analyses and 2 patients with incomplete pathology reports were excluded (20,21).
We estimated absolute risks of oral cancer in patients with oral leukoplakia after accounting for competing mortality (11). Absolute risks were calculated in the entire leukoplakia cohort (overall and stratified by receipt of biopsy) as well as in the case-cohort component (stratified by histopathologic grade). Jackknife resampling variances were used to calculate 95% confidence intervals around 1-year and 5-year absolute risks (20). All analyses were conducted in SAS (Cary, NC), and χ2, Wald, and jackknife P values less than .05 were considered statistically significant. All tests were two-sided.
Results
The study included 4886 patients diagnosed with oral leukoplakia during 1997–2012 among more than10 million enrollees in KPNC. Patients with leukoplakia had a mean age of 57.8 years and were mostly white (Table 1). Of leukoplakia patients, 42.5% and 5.2% had a history of smoking and alcohol abuse or dependence, respectively. Most leukoplakias (65.5%) were diagnosed by ENT clinicians. Of 4886 leukoplakias, 1888 (38.6%) were biopsied. Leukoplakias in individuals aged 50–59 years, Asians, ever-smokers, and HIV-negative individuals were more likely to be biopsied, as were leukoplakias diagnosed by ENT clinicians (Table 1).
Table 1.
Characteristics of the cohort of individuals with oral leukoplakia in KPNC, 1997–2012
| Patients with oral leukoplakia (n = 4886)n (%)* | Patients with oral leukoplakia not biopsied (n = 2998)n (%)† | Patients with oral leukoplakia who were biopsied (n = 1888)n (%)† | P for not biopsied vs biopsied‡ | |
|---|---|---|---|---|
| Age, y§ | .003 | |||
| <40 | 585 (12.0) | 389 (66.5) | 196 (33.5) | |
| 40–49 | 841 (17.2) | 518 (61.6) | 323 (38.4) | |
| 50–59 | 1250 (25.6) | 716 (57.3) | 534 (42.7) | |
| 60–69 | 1185 (24.3) | 739 (62.4) | 446 (37.6) | |
| 70+ | 1025 (21.0) | 636 (62.0) | 389 (38.0) | |
| Sex | 0.68 | |||
| Male | 2784 (57.0) | 1701 (61.1) | 1083 (38.9) | |
| Female | 2102 (43.0) | 1297 (61.7) | 805 (38.3) | |
| Race or ethnicity | 0.01 | |||
| White, non-Hispanic | 3400 (69.6) | 2102 (61.8) | 1298 (38.2) | |
| Black, non-Hispanic | 207 (4.2) | 123 (59.4) | 84 (40.6) | |
| Hispanic | 446 (9.1) | 279 (62.2) | 167 (37.4) | |
| Asian | 538 (11.0) | 297 (55.2) | 241 (44.8) | |
| Other or unknown | 295 (6.0) | 197 (66.8) | 98 (33.2) | |
| Ever smoking‖ | 0.03 | |||
| No | 2808 (57.5) | 1759 (61.6) | 1049 (37.4) | |
| Yes | 2078 (42.5) | 1239 (59.6) | 839 (40.4) | |
| Alcohol abuse or dependence¶ | 0.17 | |||
| No | 4633 (94.8) | 2853 (61.6) | 1780 (38.4) | |
| Yes | 253 (5.2) | 145 (57.3) | 108 (42.7) | |
| HIV serostatus# | <0.001 | |||
| Negative | 4713 (96.5) | 2843 (60.3) | 1870 (39.7) | |
| Positive | 173 (3.5) | 155 (89.6) | 18 (10.4) | |
| Diagnosed by ENT | <0.001 | |||
| No | 1685 (34.5) | 1196 (71.0) | 489 (29.0) | |
| Yes | 3201 (65.5) | 1802 (56.3) | 1399 (43.7) |
Column percentages. ENT = ear, nose, and throat clinician; HIV = human immunodeficiency virus; KPNC = Kaiser Permanente Northern California.
Row percentages.
χ2P value comparing patients who did not receive a biopsy of leukoplakia vs those who received a biopsy of oral leukoplakia.
Age at precancer diagnosis.
Report of being a current or former smoker in the medical records at any time during follow-up.
Report of alcohol abuse or dependence in the medical records at any time during follow-up.
HIV serostatus at any time during follow-up.
Results in the Entire Leukoplakia Cohort
Among the 4886 leukoplakia patients, during 22 582 person-years of follow-up (mean = 4.62 years of follow-up), 161 oral cavity cancers (149 invasive cancers and 12 carcinoma in situ) occurred at an incidence rate of 713 per 100 000 person-years. Of all oral cancers in the KPNC general population during 1996–2012, only a minority of oral cancers (<5%) were preceded by a leukoplakia diagnosis.
Compared with the KPNC general population, incidence of oral cancer was substantially elevated in patients with oral leukoplakia (SIR = 40.8, 95% CI = 34.8 to 47.6), including enormously high risk in the first year following a leukoplakia diagnosis (SIR = 103.3, 95% CI = 81.0 to 129.9, n = 73 cancers), reflecting prevalent cancer. However, risk was considerably elevated beyond 1 year after leukoplakia diagnosis (SIR = 27.2, 95% CI = 21.8 to 33.5, n = 88 cancers) (Table 2). In both intervals, risks were most strongly elevated for cancers of the tongue, floor of mouth, and other mouth, including buccal mucosa. Tongue cancers accounted for a majority of oral cavity cancers (69.6%). Risk of oral cancer was more strongly elevated when leukoplakia was diagnosed by an ENT clinician vs non-ENT clinician (SIR = 44.8, 95% CI = 37.3 to 53.4 vs SIR = 31.2, 95% CI = 21.9 to 43.2).
Table 2.
Association of oral leukoplakia with risk of oral cavity cancer compared with the KPNC population, 1997–2012
| Anatomic site | Overall |
<12 mo after leukoplakia diagnosis |
≥12 mo after leukoplakia diagnosis |
|||
|---|---|---|---|---|---|---|
| No. observed | SIR (95% CI)* | No. observed | SIR (95% CI)* | No. observed | SIR (95% CI)* | |
| All oral cavity cancers | 161 | 40.8 (34.8 to 47.6) | 73 | 103.3 (81.0 to 129.9) | 88 | 27.2 (21.8 to 33.5) |
| Lip | 9 | 11.3 (5.2 to 21.4) | 3 | 22.1 (4.6 to 64.5) | 6 | 9.3 (3.4 to 20.2) |
| Tongue | 112 | 99.6 (82.0 to 119.9) | 47 | 267.9 (196.8 to 356.2) | 65 | 69.1 (53.3 to 88.1) |
| Gingiva | 5 | 32.3 (10.5 to 75.3) | 2 | 85.8 (10.4 to 309.8) | 3 | 23.2 (4.8 to 67.8) |
| Floor of mouth | 13 | 61.5 (32.7 to 105.1) | 11 | 306.3 (152.9 to 548.0) | 2 | 11.6 (1.4 to 41.7) |
| Palate | 9 | 43.2 (19.8 to 82.0) | 3 | 86.5 (17.8 to 252.8) | 6 | 35.2 (12.9 to 76.5) |
| Other mouth | 13 | 60.9 (32.4 to 104.1) | 7 | 223.8 (90.0 to 461.1) | 6 | 33.4 (12.3 to 72.7) |
SIRs and exact 95% Poisson confidence intervals for the comparison of oral cavity cancer incidence between patients with oral leukoplakia (n = 4886) vs the entire KPNC population. SIRs were adjusted for age, sex, and calendar year. CI = confidence interval; KPNC = Kaiser Permanente Northern California; SIR = standardized incidence ratio.
In multivariable analyses, statistically significant predictors of high risk of progression to oral cancer included older age (Table 3; HR per 10-year increase in age = 1.35, 95% CI = 1.19 to 1.53) and a biopsy of a leukoplakia (adjusted HR = 2.38, 95% CI = 1.73 to 3.28). Associations were similar for less than 12 months vs 12 months and longer following a leukoplakia diagnosis.
Table 3.
Predictors of progression of oral leukoplakia to oral cancer
| Characteristic | Overall |
<12 mo after leukoplakia diagnosis |
≥12 mo after leukoplakia diagnosis |
|||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | P * | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Entire leukoplakia cohort | ||||||
| Age† | 1.35 (1.19 to 1.53) | <.001 | 1.38 (1.15 to 1.65) | <.001 | 1.32 (1.12 to 1.57) | .001 |
| Sex† | .28 | .33 | .49 | |||
| Male | 0.84 (0.60 to 1.16) | 0.80 (0.50 to 1.29) | 0.86 (0.56 to 1.32) | |||
| Female | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Race or ethnicity† | ||||||
| White, non-Hispanic | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Black, non-Hispanic | 0.15 (0.02 to 1.12) | 0.06 | NE | 0.97 | 0.33 (0.05 to 2.37) | 0.27 |
| Hispanic | 1.10 (0.63 to 1.92) | 0.73 | 0.55 (0.20 to 1.53) | 0.25 | 1.70 (0.89 to 3.26) | 0.11 |
| Asian, Pacific Islander | 1.12 (0.69 to 1.80) | 0.65 | 0.84 (0.38 to 1.86) | 0.67 | 1.39 (0.74 to 2.59) | 0.31 |
| Other or unknown | 0.36 (0.09 to 1.49) | 0.16 | 0.31 (0.04 to 2.26) | 0.25 | 0.43 (0.06 to 3.11) | 0.40 |
| Socio-economic status† | 0.29 | 0.96 | 0.18 | |||
| (Quartile score) | 1.04 (0.97 to 1.11) | 1.00 (0.90 to 1.11) | 1.07 (0.97 to 1.71) | |||
| Smoking† | 0.35 | 0.14 | 0.83 | |||
| Never | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Ever | 1.17 (0.85 to 1.61) | 1.43 (0.89 to 2.30) | 0.95 (0.59 to 1.53) | |||
| Alcohol abuse† | 0.73 | 0.55 | 0.92 | |||
| No | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 1.13 (0.57 to 2.22) | 1.33 (0.53 to 3.36) | 0.95 (0.34 to 2.64) | |||
| HIV serostatus† | 0.23 | 0.36 | 0.46 | |||
| Negative | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Positive | 1.79 (0.70 to 4.61) | 1.98 (0.46 to 8.47) | 1.57 (0.47 to 5.23) | |||
| Biopsy of leukoplakia† | <0.001 | <0.001 | 0.003 | |||
| No | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Yes | 2.38 (1.73 to 3.28) | 3.11 (1.91 to 5.07) | 1.92 (1.23 to 2.93) | |||
| Case-cohort component | ||||||
| Histopathology on biopsy‡ | ||||||
| No dysplasia | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Mild dysplasia | 4.9 (2.3 to 10.7) | <0.001 | 9.7 (3.6 to 26.0) | <0.001 | 3.5 (1.7 to 7.4) | 0.001 |
| Moderate dysplasia | 6.0 (2.0 to 17.7) | 0.001 | 9.9 (2.5 to 38.8) | 0.001 | 4.7 (1.4 to 16.2) | 0.013 |
| Severe dysplasia | 15.8 (5.5 to 45.6) | <0.001 | 21.0 (6.2 to 71.3) | <0.001 | 15.0 (4.9 to 46.0) | <0.001 |
Two-sided Wald P values. HIV = human immunodeficiency virus.
Hazard ratios from Cox regression model in the entire precancer cohort (n = 4886). The model included age, sex, race or ethnicity, socio-economic status, smoking, alcohol, HIV status, and biopsy of precancer.
Hazard ratios from weighted Cox regression model in the case-cohort component. Individuals with oral cancer on biopsy (n = 25) were excluded from analyses. The model included age, sex, race or ethnicity, socio-economic status, smoking, alcohol, HIV status, and histopathology on biopsy.
A clinician’s decision to biopsy a leukoplakia had a sensitivity of 59.6%, specificity of 62.1%, AUC of 0.61, PPV of 5.1%, NPV of 97.8%, and a complement of the NPV of 2.2% (Table 4). Results were similar for cancers that occurred during the first year following a leukoplakia or beyond (Table 4).
Table 4.
Predictive accuracy of the decision to biopsy a leukoplakia
| Characteristic | Overall oral cancers (n = 161)*, † | Oral cancers <12 mo after leukoplakia diagnosis (n = 73)* | Oral cancers ≥12 mo after leukoplakia diagnosis (n = 88)* |
|---|---|---|---|
| Sensitivity, % | 59.6 | 65.8 | 54.5 |
| Specificity, % | 62.1 | 62.9 | 62.6 |
| PPV, % | 5.1 | 2.5 | 2.5 |
| NPV, % | 97.8 | 99.2 | 98.7 |
| cNPV (=1-NPV), % | 2.2 | 0.8 | 1.3 |
Versus oral cancer incidence as the gold-standard. Biopsy within +/- 30 days of leukoplakia diagnosis. Analyses were conducted in the entire leukoplakia cohort (n = 4886). cNPV = complement of the negative predictive value; NPV = negative predictive value; PPV = positive predictive value.
Of 161 oral cancers, 96 preceding leukoplakias were biopsied and 65 preceding leukoplakias were not biopsied. See Supplementary Figure 1 (available online) for additional details.
The 5-year competing risk–adjusted absolute risk of oral cancer in patients with oral leukoplakia was 3.3% (95% CI = 2.7% to 3.9%); absolute risks were higher for leukoplakias that were biopsied (5.2%, 95% CI = 4.1% to 6.4%) compared with leukoplakias that were not biopsied (2.2%, 95% CI = 1.7% to 2.9%).
Results in the Case-Cohort Component Restricted to Biopsied Leukoplakias
In the case-cohort study (Supplementary Figure 1 available online), most biopsied leukoplakias included lesions on the tongue, other mouth including buccal mucosa, and gingiva (Table 5). Histopathologically, 15% of clinical leukoplakias had evidence of disease: mild dysplasia = 8.4%, moderate dysplasia = 3.2%, severe dysplasia = 1.8%, and cancer = 1.8%. Anatomic site concordance for leukoplakia and subsequent oral cancer was 84%.
Table 5.
Anatomic site of leukoplakia and histopathology in the case-cohort component of the study
| Characteristic | Subcohort of patients with precancer (n = 500)* | All patients with oral precancer who progressed to cancer (n = 96)* |
|---|---|---|
| No. (%) | No. (%) | |
| Anatomic site of leukoplakia | ||
| Lip | 57 (11.4) | 2 (2.1) |
| Tongue | 193 (38.8) | 66 (68.8) |
| Gingiva | 102 (20.5) | 1 (1.0) |
| Floor of mouth | 17 (3.4) | 11 (11.5) |
| Palate | 26 (5.2) | 8 (8.3) |
| Other mouth | 103 (20.7) | 8 (8.3) |
| Histopathology | ||
| No dysplasia, hyperplasia, or hyperkeratosis | 422 (84.7) | 38 (39.6) |
| Mild dysplasia | 42 (8.4) | 18 (18.8) |
| Moderate dysplasia | 16 (3.2) | 7 (7.3) |
| Severe dysplasia | 9 (1.8) | 8 (8.3) |
| Cancer† | 9 (1.8) | 25 (26.0) |
In the case-cohort study, cases included all patients (100%) with leukoplakia who progressed to oral cancer (n = 96) and controls included a random subcohort of all the 1888 patients with leukoplakia who received a biopsy (n = 500, 26.5%). See Methods for additional details. Information on anatomic site and histopathology was manually abstracted from pathology reports. Numbers do not add to 500 because n = 2 incomplete pathology reports were excluded from analyses.
Denotes a result of cancer on the pathology report of a biopsy initially indicated clinically as oral leukoplakia.
Compared with leukoplakias without histopathologic evidence of dysplasia (Table 3), risk of progression to oral cancer was elevated for all grades of dysplasia and increased statistically significantly with the grade of dysplasia: HR for mild dysplasia = 4.9 (95% CI = 2.3 to 10.7), HR for moderate dysplasia = 6.0 (95% CI = 2.0 to 17.7), and HR for severe dysplasia = 15.8 (95% CI = 5.5 to 45.6). These associations were generally stronger for the occurrence of cancer less than 12 months vs 12 months and longer following leukoplakia, although with overlapping confidence intervals (Table 3).
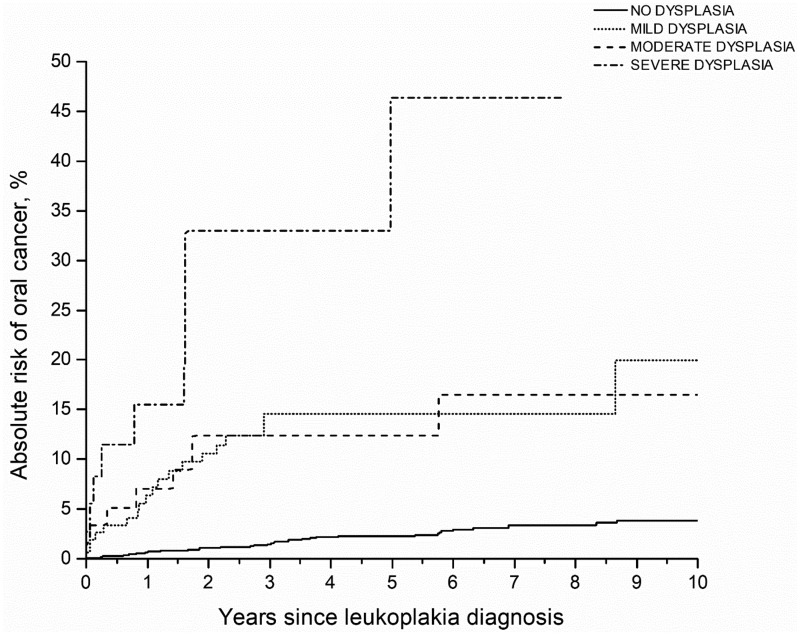
As shown in Figure 1, the 5-year competing risk–adjusted absolute risks of oral cancer were 2.2% (95% CI = 1.5% to 3.1%) for lesions without evidence of dysplasia, 11.9% (95% CI = 7.1% to 18.1%) for mild dysplasia, 8.7% (3.2% to 17.9%) for moderate dysplasia, and 32.2% (8.1% to 60.0%) for severe dysplasia. Despite these strong associations, a substantial proportion of cancers (n = 38, 39.6%; Table 5) arose from precancers histopathologically classified as having no evidence of dysplasia.
Figure 1.
Competing risk–adjusted absolute risks of oral cancer stratified by histopathology in the case-cohort component of the study. Shown are the competing risk–adjusted absolute risks of oral cancer stratified by histopathology (no dysplasia, mild dysplasia, moderate dysplasia, and severe dysplasia). Analyses were conducted in the case-cohort component of the study that was restricted to leukoplakias that were biopsied (n = 1888). Cases included all patients (100%) with leukoplakia who progressed to oral cancer (n = 96) and controls included a random subcohort of all the 1888 patients with leukoplakia who received a biopsy (n = 500, 26.5%).
Discussion
We provide three key observations from a large population-based cohort study of the natural history of patients with oral leukoplakia. First, individuals with oral leukoplakia bear a substantially elevated risk of oral cancer, with enormously high risk within 1 year of a precancer diagnosis (perhaps reflecting prevalent cancers) and notably elevated risk beyond 1 year (incident cancers). Second, a clinician’s decision to biopsy a leukoplakia had modest accuracy with respect to the presence or eventual development of oral cancer. Third, although the presence and grade of dysplasia following a biopsy strongly predicted oral cancer incidence, a large proportion of oral cancers arose from leukoplakias histopathologically diagnosed initially as nondysplastic.
Oral leukoplakia was associated with a 40.8-fold increased risk of oral cancer and a 5-year absolute risk of 3.3% (1 in 30 individuals progressing to cancer over 5 years). These observations are consistent with a recent population-based study conducted by our group within the elderly (ages 65+ years) US Medicare population (11). Our absolute risk estimates (3.3% over 5 years) appear different from high-quality clinical studies (ranging from 13% to 40% over 5 years) such as the Erlotinib Prevention of Oral Cancer (EPOC) Trial, the only clinical prevention trial with oral cancer as a primary endpoint (10). These differences are potentially attributable to three key risk stratification aspects in EPOC: enrichment for patients with dysplastic leukoplakias (73% in the EPOC placebo arm vs 15% in the current study), lesion genomic abnormalities (100% with loss of heterozygosity at a variable combination of chromosomes 3, 4, 8, 9, 11, 13, and 17 in EPOC), and with a prior history of oral cancer (57% in EPOC) (10).
Only a minority of oral cancers (<5%) in the current study were preceded by a clinical diagnosis of leukoplakia. It has been argued that oral cancers can arise de novo and only a variable proportion of oral cancers are preceded by precancer (2,22). This argument is countered somewhat by our observation that the hypothetical histologic intermediates in the step-wise progression of oral cancer follow a pyramidal structure of decreasing prevalence with increasing severity, as would be expected for squamous-epithelial cancers with obligate precancerous states (23). Thus, a more likely explanation of the observed low precedence of cancer by a clinical diagnosis of leukoplakia is the absence of organized screening for oral precancer or cancer in the United States (5). Although the American Dental Association recommends a visual and tactile examination of the oral mucosa on all dental visits (12), adherence to this recommendation is reportedly low (24,25). Likewise, an oral mucosal examination is seldom part of a routine physical examination for internists. Indeed, only 30% of US adults report having ever been screened for oral cancer (26).
Our observations underscore the need for biopsy of all clinically diagnosed leukoplakias. We found that a clinician’s decision to biopsy a leukoplakia (admittedly, interpreted as such vs patient preference or refusal for biopsy) identified a moderate proportion of prevalent or incident cancers (ie, moderate PPV of 5.1%) and missed 40.4% of cancers (low sensitivity of 59.6%). These estimates arise from the fact that visual inspection of oral lesions—the mainstay of opportunistic screening—has low predictive value for the triage of lesions for biopsy (13,27). The literature suggests some discriminating features for the identification of high-risk lesions for biopsy, such as lesion appearance (nonhomogeneous leukoplakia), size (>200 mm2), anatomic location (tongue and floor of mouth), age (older individuals), and sex (females) (2). These general guidelines are supported by some of our observations (associations of older age and high-risk anatomic sites with progression). However, we also show that a clinician’s decision to biopsy, presumably based on these general guidelines, has poor discrimination (AUC = 0.61). Unfortunately, none of the currently marketed visualization adjuncts enable accurate triage of leukoplakias for biopsy (12,28).
Our results also point to potential sampling errors in the biopsy of a leukoplakia. We show that both the presence and the grade of dysplasia were strongly associated with risk of progression to cancer. Yet a substantial proportion of cancers 39.6%, including 34% of cancers that occurred within 1 year of a clinical diagnosis of leukoplakia (ie, prevalent cancers), arose from lesions histopathologically classified as nondysplastic. Some complications in the precise targeting of a biopsy include the large size of precancers (>1 cm in diameter) as well as evidence that visible lesions often underlie a much larger area of somatic molecular changes (29,30). Also, oral pathology grading is known to have poor reproducibility (31), and we utilized histopathologic diagnoses based on a single pathologist’s review. Thus, we note the possibility that misclassification of histopathology diagnoses could have also contributed to the high proportion of cancers arising from nondysplastic leukoplakias.
This study represents the first population-based study, to our knowledge, to incorporate detailed data on demographics, anatomic and histopathologic characteristics, and biopsy procedures to estimate relative and absolute risks of oral cancer in patients with oral leukoplakia. Our results, however, need to be interpreted within the context of some study limitations. We utilized electronic medical records for the identification of patients with oral leukoplakia, and we were dependent on the clinical identification of leukoplakia. This approach could involve confounding by indication in that clinically recorded lesions would be expected to be more severe, leading to an overestimation of the overall relative or absolute risks. Also, our estimates arise from the usual practices for the identification and biopsy of leukoplakia in KPNC, which may not be generalizable to other settings. Notwithstanding, we propose that our absolute risk estimates stratified by histopathologic grade among biopsied leukoplakias are generally transportable to other settings with differential leukoplakia recording or biopsy criteria. We did not have information on precancers diagnosed by dentists; however, a recent review of diagnostic and referral patterns within KPNC showed a high referral rate from dentists to ENTs for precancers and suspected oral cancers (32).
Our results have clinical implications. The observation that many leukoplakias that were not biopsied progressed to cancer underscores the need for routine biopsy of oral leukoplakias regardless of clinical impression. Our results also reinforce the need for adjunctive tools for improved triage and reduced sampling errors in the biopsy of oral leukoplakias. The current lack of organized screening for oral leukoplakia or cancer partly arises from the lack of evidence regarding the secondary prevention value of screening—to date, no randomized trials have evaluated the efficacy of excision of a leukoplakia for reduction in oral cancer risk (5). However, one randomized trial (33) and a few observational studies provide evidence that screening can enable early detection, down-staging, and reductions in oral cancer mortality (11). Thus, the current state of the science, coupled with our observations of high absolute risks of oral cancer as well as the high anatomic site concordance between leukoplakia and cancer, supports at least annual follow-up of patients with oral leukoplakia and perhaps more intensive follow-up of those with dysplasia for signs of early cancer.
Funding
Intramural Research Program, National Cancer Institute.
Notes
Role of the funder: The study sponsor approved submission of the manuscript but did not have a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
Supplementary Material
References
- 1. Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. [DOI] [PubMed] [Google Scholar]
- 2. Napier SS, Speight PM.. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3. Chi AC, Day TA, Neville BW.. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65(5):401–421. [DOI] [PubMed] [Google Scholar]
- 4. Neville BW, Day TA.. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. [DOI] [PubMed] [Google Scholar]
- 5. Moyer VA, Force U.. Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(1):55–60. [DOI] [PubMed] [Google Scholar]
- 6. Speight PM, Epstein J, Kujan O, et al. Screening for oral cancer-a perspective from the Global Oral Cancer Forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):680–687. [DOI] [PubMed] [Google Scholar]
- 7. Mehanna HM, Rattay T, Smith J, McConkey CC.. Treatment and follow-up of oral dysplasia-a systematic review and meta-analysis. Head Neck. 2009;31(12):1600–1609. [DOI] [PubMed] [Google Scholar]
- 8. Warnakulasuriya S, Ariyawardana A.. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med. 2016;45(3):155–166. [DOI] [PubMed] [Google Scholar]
- 9. Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6(5):1702–1710. [PubMed] [Google Scholar]
- 10. William WN Jr, Papadimitrakopoulou V, Lee JJ, et al. Erlotinib and the risk of oral cancer: the Erlotinib Prevention of Oral Cancer (EPOC) randomized clinical trial. JAMA Oncol. 2016;2(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yanik EL, Katki HA, Silverberg MJ, Manos MM, Engels EA, Chaturvedi AK.. Leukoplakia, oral cavity cancer risk, and cancer survival in the U.S. elderly. Cancer Prev Res. 2015;8(9):857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lingen MW, Abt E, Agrawal N, et al. Evidence-based clinical practice guideline for the evaluation of potentially malignant disorders in the oral cavity: a report of the American Dental Association. J Am Dent Assoc. 2017;148(10):712–727.e10. [DOI] [PubMed] [Google Scholar]
- 13. Epstein JB, Guneri P, Boyacioglu H, Abt E.. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc. 2012;143(12):1332–1342. [DOI] [PubMed] [Google Scholar]
- 14. Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E.. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. [DOI] [PubMed] [Google Scholar]
- 15. Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011-12 California Health Interview Survey Oakland, CA: Kaiser Permanente Division of Research; 2015.
- 16.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 18 Regs Research Data, Nov 2017 Sub (1973-2015). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published 2017.
- 17. Langholz B, Thomas DC.. Nested case-control and case-cohort methods of sampling from a cohort: a critical comparison. Am J Epidemiol. 1990;131(1):169–176. [DOI] [PubMed] [Google Scholar]
- 18. Wacholder S. Practical considerations in choosing between the case-cohort and nested case-control designs. Epidemiology. 1991;2(2):155–158. [DOI] [PubMed] [Google Scholar]
- 19. Cantor SB, Kattan MW.. Determining the area under the ROC curve for a binary diagnostic test. Med Decis Making. 2000;20(4):468–470. [DOI] [PubMed] [Google Scholar]
- 20. Korn EL, Graubard BI.. Analysis of Health Surveys: Wiley Series in Probability and Statistics Hoboken, NJ: John Wiley & Sons, Inc.; 1999.
- 21. Mark SD, Katki HA.. Specifying and implementing nonparametric and semiparametric survival estimators in two-stage (sampled) cohort studies with missing case data. J Am Stat Assoc. 2006;101(474):460–471. [Google Scholar]
- 22. Schepman KP, Meij EH, Smeele LE, Waal I, der Waal I. Concomitant leukoplakia in patients with oral squamous cell carcinoma. Oral Dis. 2008;5(3):206–209. [DOI] [PubMed] [Google Scholar]
- 23. Schiffman M, Wentzensen N.. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yellowitz JA, Horowitz AM, Drury TF, Goodman HS.. Survey of U.S. dentists' knowledge and opinions about oral pharyngeal cancer. J Am Dent Assoc. 2000;131(5):653–661. [DOI] [PubMed] [Google Scholar]
- 25. Macek MD, Reid BC, Yellowitz JA.. Oral cancer examinations among adults at high risk: findings from the 1998 National Health Interview Survey. J Public Health Dent. 2007;63(2):119–125. [DOI] [PubMed] [Google Scholar]
- 26. Kravietz A, Angara P, Le M, Sargi Z.. Disparities in screening for head and neck cancer: evidence from the NHANES, 2011-2014. Otolaryngol Head Neck Surg. 2018;159(4):683–691. [DOI] [PubMed] [Google Scholar]
- 27. Walsh T, Liu JL, Brocklehurst P, Glenny AM, Lingen M, Kerr AR.. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev. 2013;(11):CD010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lingen MW, Tampi MP, Urquhart O, et al. Adjuncts for the evaluation of potentially malignant disorders in the oral cavity: diagnostic test accuracy systematic review and meta-analysis-a report of the American Dental Association. J Am Dent Assoc. 2017;148(11):797–813.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomson PJ. Field change and oral cancer: new evidence for widespread carcinogenesis? Int J Oral Maxillofac Surg. 2002;31(3):262–266. [DOI] [PubMed] [Google Scholar]
- 30. Slaughter DP, Southwick HW, Smejkal W.. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. [DOI] [PubMed] [Google Scholar]
- 31. Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P.. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–993. [DOI] [PubMed] [Google Scholar]
- 32. Wang KH, Song BH, Gilde JE, et al. Diagnostic pathway of oral cavity cancer in an integrated health care system. Perm J. 2018;22:17–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927–1933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.