Abstract
Background
The population age 90 years and older is the fastest growing segment of the U.S. population. Only recently is it possible to study the factors that portend survival to this age.
Methods
Among participants of the Cardiovascular Health Study, we studied the association of repeated measures of cardiovascular risk factors measured over 15–23 years of follow-up and not only survival to 90 years of age, but also healthy aging outcomes among the population who reached age 90. We included participants aged 67–75 years at baseline (n = 3,613/5,888) to control for birth cohort effects, and followed participants until death or age 90 (median follow-up = 14.7 years).
Results
Higher systolic blood pressure was associated with a lower likelihood of survival to age 90, although this association was attenuated at older ages (p-value for interaction <.001) and crossed the null for measurements taken in participants’ 80’s. Higher levels of high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and body mass index (BMI) were associated with greater longevity. Among the survivors to age 90, those with worse cardiovascular profile (high blood pressure, LDL cholesterol, glucose, and BMI; low HDL cholesterol) had lower likelihood of remaining free of cardiovascular disease, cognitive impairment, and disability.
Conclusion
In summary, we observed paradoxical associations between some cardiovascular risk factors and survival to old age; whereas, among those who survive to very old age, these risk factors were associated with higher risk of adverse health outcomes.
Keywords: Cardiovascular, Blood, Longevity, Successful aging, Nonagenarians
The population age 90 years and older is the fastest growing segment of the U.S. population (1). This group with exceptional survival is expected to grow and comprise over 10% of the 65 and older population in 2050 (2). Despite this growth, little is known about risk factor patterns that portend survival to this age. Some research has demonstrated paradoxical relationships between risk factors and survival in old age; factors that are associated with survival in early old age differ from those associated with longevity to age 90. For example, high blood pressure is an established risk factor for mortality in middle-aged and younger elders, but is associated with a lower risk of mortality among the very old and those who are frailer (3–5). Studies have observed that among older adults (>65 years), overweight body mass index (BMI) and higher low-density lipoprotein (LDL) cholesterol are associated with a reduced risk of cardiovascular events and death (4,6,7). Older adults with tightly controlled hemoglobin A1c have may have increased risk of hypoglycemia and mortality, with little cardiovascular benefit (8).
Survival to age 90 is a function of not only maintenance of health into old age, but also the life course exposures. By examining risk factor exposures over multiple years of follow-up, we may better understand the pathways to exceptional survival. Additionally, most prior investigations posit a multiplicative model, such as a logistic regression or Cox proportional hazards model, for risk factors since these models have favorable statistical properties and are popular in the literature. However, a relative measure of risk is affected by the incidence of an outcome in the unexposed group (9). Multiplicative models can be problematic when testing for effect modification by age. The increased risk of mortality with age thus leads to the appearance of an attenuated relative risk of a risk factor with age, even when the risk difference is constant.
Some have suggested that the paradoxical relationships observed in old age are due to selective survival. Studies of old age, by definition, condition on survival to old age, and risk factor exposures as well as health status are causes of survival to old age. Conditioning on the common effect of survival to old age can lead to survival bias, specifically termed collider stratification bias (10–12). Some have argued that concerns about the magnitude of this bias have been overstated, and the typical effect sizes of this bias are minimal (13–16). However, these claims have been made based on simulation studies, and not examined in empirical data. In this study, we knowingly subject our analysis to this bias by studying risk factor relationships with health status only among those surviving to age 90. If collider stratification bias is the explanation for the inverted associations of risk factors and outcomes in old age, we would expect to see this bias exaggerated in a study of those age 90 or older. An alternative explanation is that the paradoxical associations are not an artifact of bias, but that the aging process alters the association of risk factors and outcomes in some subpopulations (17).
In this investigation, we explored the association of repeated measurements of cardiovascular risk factors over 15–23 years of follow-up and survival to 90 years of age. We hypothesized that the association between risk factors measured in late old age (>80 years) and survival to 90 would be attenuated relative to those measured in early old age. Risk factors included systolic and diastolic blood pressure, LDL and high-density lipoprotein (HDL) cholesterol, fasting glucose, and BMI. Because the relationships of these risk factors with longevity are not well studied, we assessed multiple classifications of the risk factors including linear, categorical, and splines. We evaluated additive and multiplicative models of survival to 90. As a second aim, we explored the relationships between the same risk factors and health status among those reaching 90 years, assessed by survival to 90 free of cardiovascular disease, cognitive impairment, and limitation in one or more activities of daily living (ADLs). The goal of this analysis was to study a selected population by conditioning on survival to age 90. We hypothesized that this would not induce paradoxical associations of risk factors and outcomes, since this bias is likely to have a minimal contribution to these associations. This investigation was conducted in the Cardiovascular Health Study, a cohort study of black and white adults aged 65 years and older.
Methods
Study Population
The CHS is a community-based study of 5,888 black and white adults aged 65 and older at baseline. Its primary aim is to evaluate risk factors for the development and progression of cardiovascular disease in older adults (18). The study recruited persons from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania in 1989–1990. Additional Black participants were recruited during a supplemental enrollment in 1992–1993; overall, Black participants constitute 15% of the CHS cohort. Eligible participants had to be aged 65 and older and not institutionalized, expected to remain in the current community for 3 years or longer, not be under active treatment for cancer, and able to provide informed consent without requiring a proxy respondent. Participants completed study visits at enrollment and annually through 1999; these visits included an interview, health questionnaire, physical examination, and collection of blood specimens. An additional follow-up visit occurred in 2005–2006, which included the same components. In addition, follow-up contacts for functional status, cognitive function, depressive symptoms, medication use, hospitalizations, and other health outcomes were conducted by telephone every six months through 2015 for this analysis.
In order to assure at least 15 years of follow-up to age 90, and minimize birth cohort effects, in the analysis of survival to 90, participants were included if they were 67–75 years old at baseline. We excluded 86 participants who were alive, but not yet 90 at the end of follow-up. There were 3,613 eligible participants. In the analysis of outcomes among those reaching age 90 and older, participants were included if they reached 90 years of age during CHS follow-up (n = 2,125).
Outcomes
Survival to 90 was the primary outcome of interest. Deaths were identified by consulting obituaries, medical records, death certificates, household contacts, National Death Index (2), and Centers for Medicare and Medicaid Services data; 100% follow-up for ascertainment of mortality status was achieved.
The prevalent cardiovascular disease status of CHS participants was classified at baseline using a combination of hospital records and physician confirmation, and a CHS outcome-assessment committee adjudicated all incident cardiovascular disease events (19,20). Events included coronary heart disease, cerebrovascular disease, claudication, or heart failure. Coronary heart disease was defined as a history of myocardial infarction, angina pectoris, or revascularization; cerebrovascular disease was defined as a history of transient ischemic attack, or stroke. A participant was defined as free of cardiovascular disease if they had no history of any cardiovascular disease event by age 90.
Cognitive function was assessed at annual visits and phone calls by the Modified Mini Mental State Exam (3MS) which ranges from 0 to 100 with <80 indicating cognitive impairment. Beginning in 1996, participants who did not attend the in-person visit were contacted by phone and asked to complete the Telephone Interview for Cognitive Status; the Informant Questionnaire on Cognitive Decline in the Elderly was administered to a proxy if needed. An internal validation equation was developed to convert Telephone Interview for Cognitive Status and Informant Questionnaire on Cognitive Decline in the Elderly scores to 3MS scores (21). A participant was defined as free of cognitive impairment at 90 if his or her 3MS measure closest to age 90 was ≥80 points.
Participants were asked to report at annual visits and phone calls whether they had difficulty with or were unable to perform any of six ADL: bathing, eating, dressing, using the toilet, getting out of bed or chair, and walking around home. A participant was defined as free of disability at 90 if they had no ADL limitations at the assessment closest to age 90.
Risk Factors
Age, sex, and race were determined by self-report at baseline; race was categorized as Black or White/other; <1% of participants identified as other race. Blood pressure (systolic and diastolic) was measured in seated participants after a 5-minute rest. Trained study personnel obtained three blood pressure readings, and the average of the last two readings was recorded. Blood pressure was assessed at baseline and annually through 1995, years 1996–1999, and at the 2005/2006 visit. HDL cholesterol was measured in fasting blood samples, and LDL cholesterol was calculated according to the Friedewald equation (22). Lipids were measured at baseline and the 1992/1993 visit and 2005/2006 visit. Height and weight were measured and BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Fasting glucose was measured in serum by the enzymatic method. BMI and glucoses measures were measured at baseline, and the 1992/1993, 1996/1997, and 2005/2006 visits.
A priori, we chose to use a data-driven approach for the functional form of the risk factors. We explored all risk factors as continuous, categorical, and using a linear spline with one knot. Categorical cutpoints were selected as those that are clinically relevant or from prior research in the CHS (23): systolic BP >140 mmHg, diastolic BP >60 mmHg, LDL cholesterol >130 mg/dL, HDL cholesterol <40 mg/dL in men, <50 mg/dL in women, BMI <18.5 (underweight), 18.5–25 (normal), 25–30 (overweight) and 30+ (obese), fasting glucose ≥126 mg/dL. The knots used for the linear spline analyses were equivalent to the cut point above, except BMI, for which the knot was placed at 30.
Statistical Analysis
Descriptive statistics were stratified by survival to 90 and presented using means and standard deviation or counts and percentages.
We used generalized estimating equations (GEE) to estimate parameters of a logistic regression model with repeated measures of the risk factors within a person; the primary outcome of interest was survival to age 90. Each risk factor was measured in a separate model to account for the fact that the risk factors may confound, mediate, or interact with one another (24). All models were age-adjusted and we tested whether the association varied by the age of risk factor measurement (age interaction); the age interaction was retained if the p-value was <.10. We explored multiple risk factor classification as linear, linear spline, and cut points and selected the exposure classification based on the model with the lowest quasilikelihood criterion (QIC), which is a model-selection method for GEE (25) (Supplementary Table S1). Subsequent models were adjusted for demographics (sex, race, and education level) and medication (blood pressure, LDL cholesterol, and glucose-lowering). To account for the possibility that the risk factor and age interaction may depend on the scale, we repeated the age and demographic adjusted GEE models using a binomial model with the identify link function to estimate risk differences.
To evaluate whether changes in risk factors were associated with survival to 90, we estimated a person-specific level and slope of each of the risk factors. This was done by estimating a random effects multilevel model of each risk factor on study time. Person-specific level and change in each of the risk factors was then included in an age-adjusted logistic regression model of survival to 90.
Among 2,125 participants surviving to age 90, we used GEE logistic regression models to evaluate the factors associated with staying free of cardiovascular disease, cognitive impairment, and disability to age 90.
We plotted the age-adjusted results from the best-fit models of survival to 90. Age-interactions that were found to be attenuated in adjusted models were not included because they were likely an artifact of confounding. We compared the graphical relationships between the risk factors and survival to 90 and the risk factors and the other aging outcomes (free of cardiovascular disease, cognitive impairment, and disability).
All statistical analyses and graphical displays were performed using STATA/IC 14.0 (StataCorp, College Station, TX) and R-3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 5,802 participants who were followed until age 90 or death, 2,125 (37%) survived to age 90 or older. Of the 3,613 participants aged 67–75 years at baseline, 1,160 (32%) survived to age 90 or older. Women, those reporting white/other race, those with greater education, and those from the Sacramento County study site were more likely to reach age 90 years. Additionally, those with better self-rated health, never smokers, and those with no instrumental activities of daily living and ADL limitations were more likely to survive to 90 or older. Those who reached age 90 years in the restricted birth cohort had higher LDL-, HDL-, and total cholesterol, lower BMI, systolic blood pressure, glucose, and were less likely to have a history of MI, stroke, and heart failure in early old age (Table 1).
Table 1.
Baseline Characteristics by Survival to 90+ Among CHS Participants Aged 67–75 Years at Baseline
| Total N = 3,613* | Did not Survive to Age 90 y N = 2,453 | Survived to Age 90 y N = 1,160 | p-value | |
|---|---|---|---|---|
| Age, years | 71.0 (2.5) | 71.0 (2.5) | 71.1 (2.5) | .48 |
| Female, % | 58.3 | 53.7 | 68.1 | <.01 |
| Black race, % | 14.5 | 15.8 | 11.9 | <.01 |
| Education,% | ||||
| <HS | 27.4 | 30.0 | 21.8 | <.01 |
| HS/Equivalency | 28.1 | 27.2 | 30.1 | |
| >HS | 44.5 | 42.8 | 48.1 | |
| Study site, % | ||||
| Forsyth County, NC | 25.4 | 26.2 | 23.9 | <.01 |
| Sacramento County, CA | 25.8 | 24.5 | 28.5 | |
| Washington County, MD | 22.0 | 23.2 | 19.5 | |
| Pittsburgh, PA | 26.7 | 26.1 | 28.1 | |
| Smoking status, % | ||||
| Current | 13.3 | 16.1 | 7.3 | <.01 |
| Former | 43.5 | 45.7 | 38.8 | |
| Never | 43.3 | 38.2 | 53.9 | |
| IADL limitations, % | 6.7 | 8.0 | 3.9 | <.01 |
| ADL limitations, % | 22.5 | 25.5 | 16.0 | <.01 |
| BMI categories, % | ||||
| <25 | 35.6 | 35.3 | 36.3 | .34 |
| 25–30 | 42.7 | 42.4 | 43.5 | |
| ≥30 | 21.7 | 22.3 | 20.2 | |
| LDL cholesterol, mg/dL | 131 (36) | 129 (36) | 134 (35) | <.01 |
| HDL cholesterol, mg/dL | 54 (16) | 53 (16) | 56.2 (15) | <.01 |
| Total cholesterol, mg/dL | 213 (39) | 210 (39) | 218 (38) | <.01 |
| SBP, mmHg | 135 (21) | 136 (21) | 133 (20) | <.01 |
| DBP, mmHg | 71 (11) | 71 (12) | 71 (11) | .08 |
| Glucose, mg/dL | 111 (35) | 114 (40) | 103 (21) | <.01 |
| History of MI | 9.0 | 11.7 | 3.5 | <.01 |
| Stroke | 3.8 | 5.2 | 0.8 | <.01 |
| Heart failure | 3.7 | 5.1 | 0.8 | <.01 |
| Antihypertensive Medications | 46.0 | 50.1 | 37.8 | <.01 |
| Statins | 2.5 | 2.6 | 2.3 | .62 |
| Diabetes Medications | 6.4 | 8.2 | 2.8 | <.01 |
Note: Values shown mean (SD) or %.
Sample sizes for individual rows may be lower than the total due to missing values.
p-values were calculated using a t-test for continuous variables and chi-squared tests for categorical variables.
ADL = activities of daily living; BMI = body mass index; DBP = diastolic blood pressure; HDL = high-density lipoprotein; HS = high school; IADL = instrumental activities of daily living; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Factors Associated with Survival to Age 90
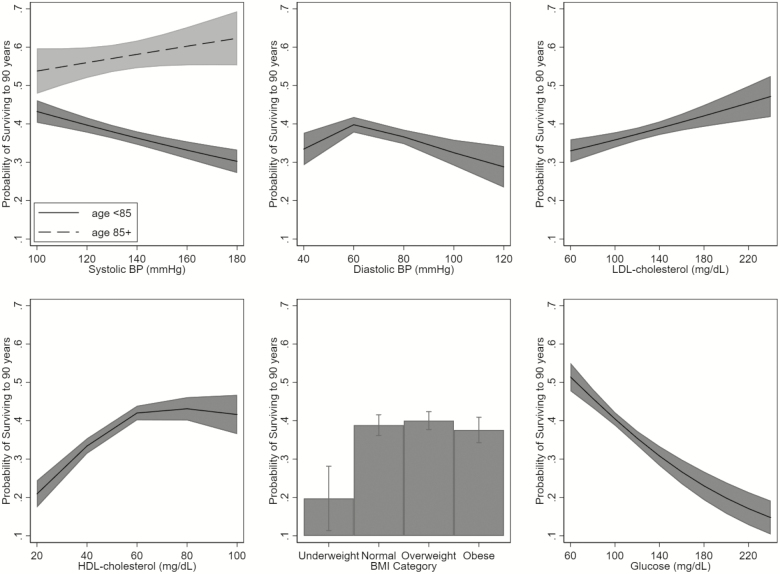
There were a median of eight blood pressure measurements, two lipid measurements, and three BMI and glucose measurements per person prior to age 90 years. All of the risk factors were associated with survival to 90 (Table 2, Figure 1). Higher systolic blood pressure was associated with a lower likelihood of survival to age 90, although this association was attenuated at older ages (p-value for interaction <.001) and crossed the null at 82 years of age. The best-fit model for diastolic blood pressure and survival to 90 was a spline with a cutpoint at 60 mmHg. Among persons with diastolic blood pressure levels lower than 60 mmHg, higher blood pressure was associated with greater likelihood of survival to 90; whereas among persons with diastolic levels ≥ 60 mmHg, higher diastolic blood pressure was associated with lower likelihood of survival to 90. Higher LDL cholesterol was associated with greater likelihood of survival to age 90, as well as higher levels of HDL cholesterol among those with low levels (<40 for men and <50 for women). There was the appearance of an interaction with age for both, although the interaction with LDL cholesterol was diminished after adjustment for demographic factors and medication use. Underweight BMI was associated with lower likelihood of surviving to 90; overweight and obese were not associated with longevity. Higher glucose was associated with lower likelihood of survival to age 90; the association was attenuated with age (p-value for interaction <.001), but did not cross the null when the risk factors were measured prior to 90 years.
Table 2.
The Association of Repeated Measures of Risk Factors and Survival to 90+ in a Multiplicative Model
| Exposure | Age-adjusted | Adjusted for Demographics | Adjusted for All Demographics and Medication Use |
|---|---|---|---|
| OR (95% CI) | |||
| Systolic blood pressure—continuous† | |||
| SBP | 0.79 (0.74, 0.85)*** | 0.79 (0.74, 0.85)*** | 0.79 (0.72, 0.88)*** |
| Age × SBP | 1.05 (1.03, 1.08)*** | 1.04 (1.02, 1.07)*** | 1.05 (1.01, 1.09)* |
| Diastolic blood pressure—spline† | |||
| DBP < 60 | 1.09 (1.06, 1.12)*** | 1.09 (1.07, 1.13)*** | 1.10 (1.03 1.17) |
| DBP ≥ 60 | 0.91 (0.88, 0.94)*** | 0.93 (0.90, 0.96)*** | 0.86 (0.78, 0.94) |
| LDL cholesterol—continuous† | |||
| LDL | 1.17 (1.09, 1.26)*** | 1.13 (1.05, 1.22)*** | 1.14 (1.06, 1.24)** |
| Age × LDL | 0.97 (0.95, 1.00)* | 0.97 (0.95, 1.00)* | 0.99 (0.97, 1.03) |
| HDL cholesterol—spline at 40 (men) or 50 (women)† | |||
| HDL < 40/50 | 1.46 (1.37, 1.55)*** | 1.30 (1.18, 1.42)*** | |
| HDL ≥ 40/50 | 0.97 (0.92, 1.02) | 0.97 (0.92, 1.03) | |
| Age × HDL < 40/50 | 0.96 (0.94, 0.99)** | 0.97 (0.94, 0.99)** | |
| BMI categories (ref = normal weight) | |||
| Underweight | 0.37 (0.22, 0.65)** | 0.32 (0.18, 0.56)*** | |
| Overweight | 1.05 (0.90, 1.21) | 1.15 (0.99, 1.34) | |
| Obese | 0.95 (0.78, 1.14) | 1.01 (0.83, 1.22) | |
| Glucose—continuous† | |||
| Glucose | 0.62 (0.54, 0.70)*** | 0.66 (0.59, 0.75)*** | 0.63 (0.54, 0.72)*** |
| Age × Glucose | 1.06 (1.02, 1.10)** | 1.06 (1.02, 1.10)** | 1.08 (1.03, 1.13)** |
Note: BMI = body mass index; CI = confidence interval; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; OR = odds ratio; SBP = systolic blood pressure.
*p < .05, **p < .01, ***p < .001.
†All continuous predictors are shown per Z score, standardized to baseline visit mean and standard deviation.
Demographic adjusted model was adjusted for sex, black race, education level (<HS, HS/GED, >HS), and study site.
Figure 1.
Age-adjusted association of risk factors and survival to 90. Associations with systolic blood pressure are stratified based on the age of blood pressure measurement < 85 and 85+ years. BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
The patterning of risk factor associations with longevity was similar when we used an additive risk model (Supplementary Table S2), and the attenuated effect of systolic blood pressure measured at older ages persisted. The age interactions with LDL- and HDL cholesterol and glucose were no longer statistically significant.
Change (slope) in the risk factors was not associated with survival to 90 with the exception of BMI (Supplementary Table S3). A 1-unit increase in BMI per year was associated with an over twofold increase in the odds of reaching 90 (odds ratio [OR]: 2.11, 95% confidence interval [CI]: 1.12, 4.00). This is equivalent to an association between a falling BMI and lower odds of reaching 90 years: a 1-unit decrease in BMI per year was associated with an OR of 0.47 (95% CI: 0.25, 0.89). The median change in BMI over follow-up was 0, meaning that about half of participants increased in BMI and half decreased.
Factors Associated with Health Status at Age 90
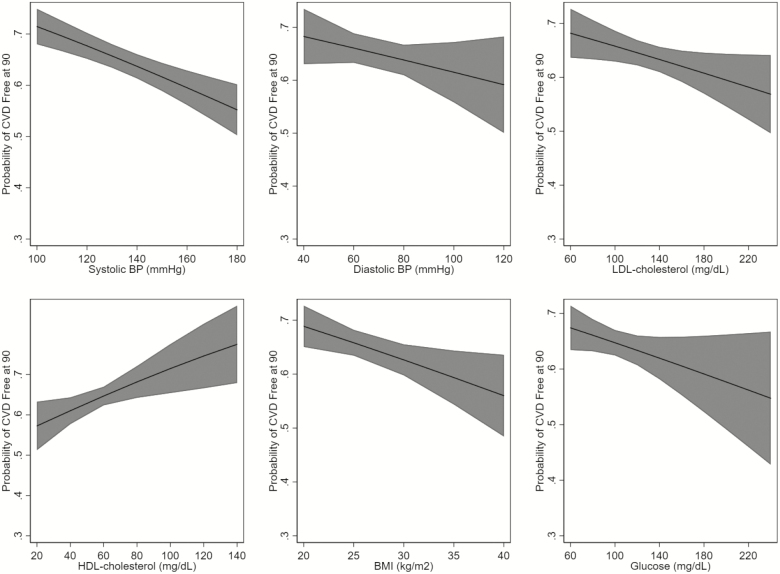
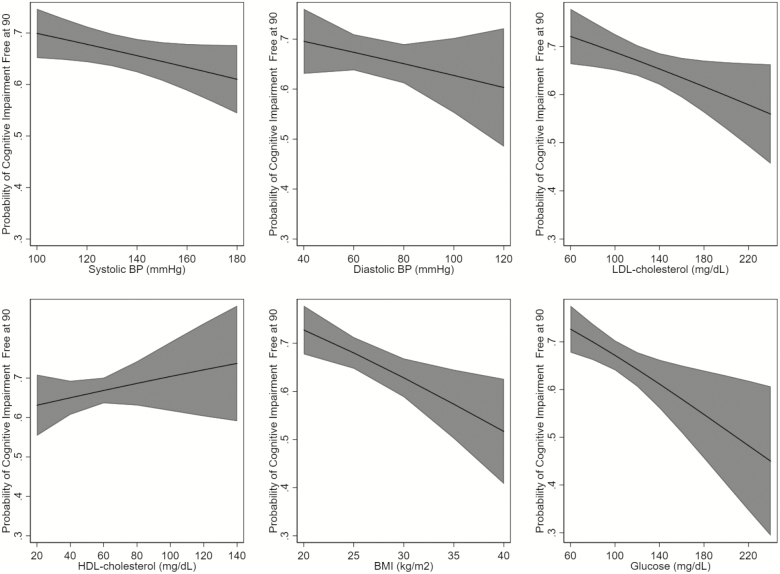
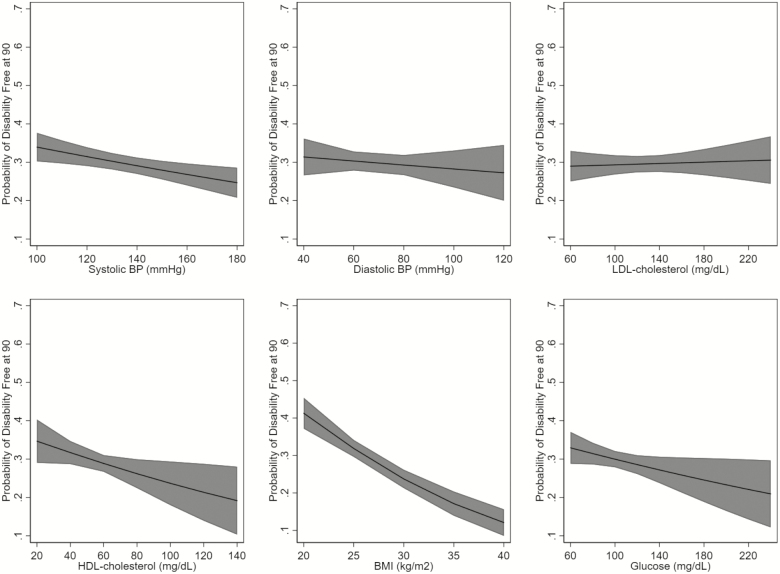
Among 2,125 participants who reached age 90, we observed a different association of the risk factors and health status at age 90. The best-fit models for all risk factors were the models that classified the risk factors as linear. Higher systolic blood pressure and BMI were associated with a lower likelihood of being free of cardiovascular disease at age 90 (Figure 2). Higher LDL cholesterol, glucose, and BMI were associated with a lower likelihood of being free of cognitive impairment at age 90 (Figure 3). Higher systolic blood pressure and BMI, and paradoxically, HDL cholesterol, were associated with a lower likelihood of being free of disability at age 90 (Figure 4). Although many of the effect sizes were not statistically significant, the direction of the association with the outcomes at 90 were in the directions that are traditionally thought to convey risk. That is, higher systolic and diastolic blood pressure, LDL cholesterol, BMI, and glucose, and lower HDL cholesterol were associated with worse outcomes. The exceptions were the associations of LDL and HDL cholesterol and disability.
Figure 2.
Age-adjusted association of risk factors and being cardiovascular disease free at 90, among those surviving to 90. BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Figure 3.
Age-adjusted association of risk factors and being free of cognitive impairment at 90, among those surviving to 90. BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Figure 4.
Age-adjusted association of risk factors and being free of ADL impairment at 90, among those surviving to 90. ADL = activities of daily living; BMI = body mass index; BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Discussion
In this prospective study of cardiovascular risk factors measured over approximately 20 years, we found a different pattern of associations of risk factors with survival to age 90, compared with the pattern of associations with health status at age 90. The association of systolic blood pressure with survival to age 90 crossed the null with aging such that for measures taken in the mid-to-late 80’s, higher systolic blood pressure was associated with greater likelihood of reaching 90. As expected, higher levels of HDL cholesterol were associated with greater longevity among those participants with low levels of HDL cholesterol. In contrast to their cardiovascular risk profiles, low LDL cholesterol and underweight BMI were associated with lower likelihood of survival to 90 when measured after age 67 years. When we restricted analyses to those who reached age 90, the traditional cardiovascular risk factors were largely associated with worse health status at age 90, and there were no apparent interactions with the age at the time of risk factor measurement.
Our findings are consistent with previous reports of an attenuated effect of systolic blood pressure with survival in older age. Multiple studies demonstrated an attenuated association of blood pressure levels with survival among older adults (26–28), and some data from trials suggested a modestly harmful effect of blood pressure lowering on survival among adults aged 80 and older (29). In contrast, the recent SPRINT trial demonstrated a strong protective effect of intensive blood pressure lowering on mortality, even among participants aged 75 and older (30). Given that the present study is observational, our ability to comment on causal relationships is limited. However, our data suggest that those blood pressure measurements taken late in life have an inverse association with longevity. Our finding that low diastolic blood pressure is associated with lower likelihood of survival among those with levels below 60 mmHg is consistent with studies of younger elders that have found elevated risk of events at this level (31). However, in our study, this association was attenuated when adjusting for other risk factors.
In a previous study in the CHS, systolic blood pressure and glucose were associated with cardiovascular events in adults 65–75, and these associations were attenuated at older age (23). Notably, in the present study, the interaction with glucose and age was dependent on the scale, suggesting that the apparent attenuated association in older age was due to the change in likelihood of survival with age (9). The associations between lipids and BMI and survival to 90 were expected. Previous research in CHS demonstrated no association between LDL cholesterol and survival (7), and substantial literature has shown a relationship between underweight and mortality (6). There is controversy regarding the importance of overweight and obesity when observed in very old age, and some studies have not observed an association of overweight and obesity with adverse outcomes (6).
When restricting to a survivor population of those who reached 90, a favorable cardiovascular risk profile was associated better health status. That is, participants with higher systolic and diastolic blood pressure, glucose, and BMI had lower likelihood of remaining free of cardiovascular disease, cognitive impairment, and disability at 90. Exceptions were that adverse lipid factors were unexpectedly associated with lack of disability. These results do not support collider stratification bias as an explanation for the paradoxical association between higher blood pressure measured in old age and greater likelihood of survival to 90. If collider stratification bias were present, we would expect this inverted association to be exaggerated among those surviving to 90. Instead, our observations are consistent with the idea that robust older adults (those likely to reach age 90) are physiologically more similar to younger adults in whom we see these traditional cardiovascular risk factor associations. Older adults are a mix of those aging well and those aging poorly, and survival to 90 may be a marker of robustness. The association of risk factors and outcomes may differ by robustness or other characteristics of the individual. If confirmed, these observations would support risk factor control in robust older adults of any age. However, a future challenge is determining how to prospectively identifying these individuals, as survival to 90 can only be evaluated retrospectively.
There is an alternative explanation for the attenuated association of systolic blood pressure with age, and the null associations of high diastolic blood pressure, LDL cholesterol and obesity with longevity. We suggest that during the life of an individual, some risk factors may undergo a switch over time from being a cause of disease to being a consequence of disease, although the timing of this switch may depend on the health and longevity of the individual. Whether the risk factor functions as a marker of cumulative disease or actually has an inverted causal effect is unknown. However, some have proposed protective mechanisms for higher risk factors in older age. For example, higher blood pressure may allow for sustained perfusion of the essential organs in the presence of vascular disease (17,31). Cholesterol is involved in neurotransmission, thus some have suggested that higher levels may support neuronal functioning in some persons (32,33). Higher glucose may limit episodes of hypoglycemia among those who are very old or frail (34). Further investigations of the physiological alterations that accompany the aging process may lend insight into why these unexpected relationships are observed.
Strengths of this study include a large sample of adults reaching age 90 and the availability of repeated measures of risk factors across the old-age life course. Additionally, we examined the associations of risk factors with survival across both multiplicative and additive scales, thus enabling us to ensure that the apparent age-interactions were robust to the scale of measurement. The primary limitation is for the possibility of unmeasured and residual confounding, which limits the causal interpretation of our findings. There may be time-dependent confounding by medication use or other nonpharmacologic interventions that could bias the risk factor estimates. Implementation of g-methods to account for confounding would be necessary to address this bias. Additionally, we had more repeated measures of the blood pressure measurements compared with lipid, BMI, or glucose, thus power to detect a change in the associations was lower for the non-blood pressure risk factors. Finally, older age is a period of heterogeneity, which often leads to wide standard errors. This resulted in variable estimates for many of our parameters of interest, and we may have been underpowered to detect effect modification by age at the time of risk factor measurement, especially for the analyses restricted to those who reached 90.
In summary, in this large sample of older adults followed from early old age, we identified the cardiovascular risk factor patterns that were associated with survival to 90. Higher levels of systolic blood pressure were associated with lower likelihood of survival to 90, although this association was diminished when blood pressure was measured when participants were in their late 80’s. Low diastolic blood pressure, HDL cholesterol, LDL cholesterol, and BMI, and high glucose were associated with lower likelihood of survival to 90. Interestingly, in the survivor population of participants who reached 90, traditional cardiovascular risk profiles were associated with adverse health status. A better understanding of the factors that are associated with survival and health at 90 may lend insight into the physiologic process of aging.
Supplementary Material
Acknowledgments
M.C.O., A.M.R., B.M.P., and A.B.N. designed the research. M.C.O. and A.M.R. analyzed the data. M.C.O. drafted the manuscript. A.M.A., M.C., and M.L.B. provided subject matter and data expertise. All authors contributed to interpreting the data and edited, reviewed, and approved the manuscript. M.C.O. is responsible for the final content of the manuscript.
Funding
This work was funded by National Institutes of Health grant R21HL135869. The Cardiovascular Health Study is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
M.C.O. serves as a consultant for Cricket Health, Inc. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
References
- 1. He W, Muenchrath MN. ACS-17,90+ in the United States: 2006–2008. Washington, DC: U.S. Government Printing Office; 2011. [Google Scholar]
- 2. U.S. Census Bureau. U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin.www.census.gov/ipc/www/usinterimproj. Published 2008. Accessed August 5, 2009.
- 3. Bejan-Angoulvant T, Saadatian-Elahi M, Wright JM, et al. Treatment of hypertension in patients 80 years and older: the lower the better? A meta-analysis of randomized controlled trials. J Hypertens. 2010;28:1366–1372. doi: 10.1097/HJH.0b013e328339f9c5 [DOI] [PubMed] [Google Scholar]
- 4. Odden MC, Covinsky KE, Neuhaus JM, Mayeda ER, Peralta CA, Haan MN. The association of blood pressure and mortality differs by self-reported walking speed in older Latinos. J Gerontol A Biol Sci Med Sci. 2012;67:977–983. doi: 10.1093/gerona/glr245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014;64:472–480. doi: 10.1161/HYPERTENSIONAHA.114.03831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153:1065–1073. [PubMed] [Google Scholar]
- 8. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61:2020–2026. 10.1111/jgs.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Psaty BM, Koepsell TD, Manolio TA, et al. Risk ratios and risk differences in estimating the effect of risk factors for cardiovascular disease in the elderly. J Clin Epidemiol. 1990;43:961–970. doi: 10.1016/0895-4356(90)90079-5 [DOI] [PubMed] [Google Scholar]
- 10. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 11. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 12. Pearce N, Richiardi L. Commentary: three worlds collide: Berkson’s bias, selection bias and collider bias. Int J Epidemiol. 2014;43:521–524. 10.1093/ije/dyu025 [DOI] [PubMed] [Google Scholar]
- 13. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14:300–306. doi: 10.1097/01.EDE.0000042804.12056.6C [DOI] [PubMed] [Google Scholar]
- 14. Liu W, Brookhart MA, Schneeweiss S, Mi X, Setoguchi S. Implications of M bias in epidemiologic studies: a simulation study. Am J Epidemiol. 2012;176:938–948. doi: 10.1093/aje/kws165 [DOI] [PubMed] [Google Scholar]
- 15. Pizzi C, De Stavola B, Merletti F, et al. Sample selection and validity of exposure-disease association estimates in cohort studies. J Epidemiol Community Health. 2011;65:407–411. doi: 10.1136/jech.2009.107185 [DOI] [PubMed] [Google Scholar]
- 16. Whitcomb BW, McArdle PF. Collider-stratification bias due to censoring in prospective cohort studies. Epidemiology. 2016;27:e4–e5. doi: 10.1097/EDE.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 17. Goodwin JS. Embracing complexity: a consideration of hypertension in the very old. J Gerontol A Biol Sci Med Sci. 2003;58:653–658. doi: 10.1093/gerona/58.7.m653 [DOI] [PubMed] [Google Scholar]
- 18. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 19. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 20. Psaty BM, Delaney JA, Arnold AM, et al. Study of cardiovascular health outcomes in the era of claims data: the Cardiovascular Health Study. Circulation. 2016;133:156–164. doi: 10.1161/CIRCULATIONAHA.115.018610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The Cardiovascular Health Study. Neuroepidemiology. 2009;33:55–65. doi: 10.1159/000215830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23. Odden MC, Shlipak MG, Whitson HE, et al. Risk factors for cardiovascular disease across the spectrum of older age: the Cardiovascular Health Study. Atherosclerosis. 2014;237:336–342. doi: 10.1016/j.atherosclerosis.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x [DOI] [PubMed] [Google Scholar]
- 26. Cupples LA, D’Agostino R. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Heart Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garnson RJ, eds. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 34. Vol NIH publication 87–2703. Washington, DC: National Heart, Lung and Blood Institute, US Dept of Health and Human Services Public Health Services; 1987. [Google Scholar]
- 27. Mattila K, Haavisto M, Rajala S, Heikinheimo R. Blood pressure and five year survival in the very old. Br Med J (Clin Res Ed). 1988;296:887–889. doi: 10.1136/bmj.296.6626.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satish S, Freeman DH Jr, Ray L, Goodwin JS. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc. 2001;49:367–374. doi: 10.1046/j.1532-5415.2001.49078.x [DOI] [PubMed] [Google Scholar]
- 29. Gueyffier F, Bulpitt C, Boissel JP, et al. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group. Lancet. 1999;353:793–796. doi: 10.1016/s0140-6736(98)08127-6 [DOI] [PubMed] [Google Scholar]
- 30. Williamson JD, Supiano MA, Pajewski NM. Intensive vs standard blood pressure control for older adults-reply. JAMA. 2016;316:1923. doi: 10.1001/jama.2016.14936 [DOI] [PubMed] [Google Scholar]
- 31. Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005 [DOI] [PubMed] [Google Scholar]
- 32. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z [DOI] [PubMed] [Google Scholar]
- 33. Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 2001;15:1858–1860. doi: 10.1096/fj.00-0815fje [DOI] [PubMed] [Google Scholar]
- 34. American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.