Abstract
Background
Inappropriate testing for Clostridioides difficile leads to overdiagnosis of C difficile infection (CDI). We determined the effect of a computerized clinical decision support (CCDS) order set on C difficile polymerase chain reaction (PCR) test utilization and clinical outcomes.
Methods
This study is an interrupted time series analysis comparing C difficile PCR test utilization, hospital-onset CDI (HO-CDI) rates, and clinical outcomes before and after implementation of a CCDS order set at 2 academic medical centers: University of Washington Medical Center (UWMC) and Harborview Medical Center (HMC).
Results
Compared with the 20-month preintervention period, during the 12-month postimplementation of the CCDS order set, there was an immediate and sustained reduction in C difficile PCR test utilization rates at both hospitals (HMC, −28.2% [95% confidence interval {CI}, −43.0% to −9.4%], P = .005; UWMC, −27.4%, [95% CI, −37.5% to −15.6%], P < .001). There was a significant reduction in rates of C difficile tests ordered in the setting of laxatives (HMC, −60.8% [95% CI, −74.3% to −40.1%], P < .001; UWMC, −37.3%, [95% CI, −58.2% to −5.9%], P = .02). The intervention was associated with an increase in the C difficile test positivity rate at HMC (P = .01). There were no significant differences in HO-CDI rates or in the proportion of patients with HO-CDI who developed severe CDI or CDI-associated complications including intensive care unit transfer, extended length of stay, 30-day mortality, and toxic megacolon.
Conclusions
Computerized clinical decision support tools can improve C difficile diagnostic test stewardship without causing harm. Additional studies are needed to identify key elements of CCDS tools to further optimize C difficile testing and assess their effect on adverse clinical outcomes.
Keywords: C difficile infection, diagnostic stewardship, Clostridioides difficile, computerized clinical decision support, interrupted time series analysis
Implementation of a computerized clinical decision support (CCDS) order set significantly reduced C difficile PCR test utilization rates and was not associated with an increase in severe CDI or CDI-related complications including ICU transfer, 30-day mortality, and toxic megacolon.
Clostridioides difficile is the most commonly reported pathogen responsible for healthcare-associated infections [1]. However, an emerging body of literature suggest that a substantial portion of reported C difficile infections (CDI) are related to inappropriate testing of colonized patients [2, 3]. Testing of patients without true disease leads to overdiagnosis of asymptomatically colonized patients as having CDI and unnecessary treatment, which may result in adverse drug effects, further disruption of the gut microbiota, and excess healthcare costs [4–6]. The 2017 Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children and the American Board of Internal Medicine (ABIM) Choosing Wisely initiative recommend testing only those patients who are likely to have CDI [7–9]. Although several studies have assessed the role of electronic decision support tools on process outcomes such as C difficile test orders [10–16], few have evaluated the impact on adverse clinical outcomes [17].
On August 29, 2018, a computerized clinical decision support (CCDS) enteric pathogen order set was implemented at 2 hospitals within UW Medicine to guide providers towards appropriate use of the stand-alone C difficile polymerase chain reaction (PCR) and a multiplex enteric pathogen panel. In this study, we evaluated the effect of the CCDS enteric pathogen order set on C difficile PCR test utilization and clinical outcomes.
METHODS
Setting and Study Population
This was a quasi-experimental study conducted at 2 academic medical centers within the UW Medicine system. The University of Washington Medical Center (UWMC) is a 570-bed tertiary care center that also serves a large population of immunosuppressed patients including solid organ and hematopoietic stem cell transplant recipients. Harborview Medical Center (HMC) is a 413-bed acute care hospital that serves as a public safety-net hospital for King County, and the level 1 trauma and burn center for Washington, Wyoming, Alaska, Montana, and Idaho. Medical housestaff rotate between the 2 different institutions.
Patient Consent Statement
This study was approved by the University of Washington Institutional Review Board Committee with a waiver of informed consent.
Intervention
We developed an electronic order set to guide enteric pathogen test ordering within a Cerner platform (Cerner Corp., Kansas City, MO). Providers were automatically directed to use the order set when testing for C difficile and other enteric pathogens. The order set includes guidelines that preferentially recommend testing patients with new-onset, hospital-associated diarrhea (≥3 loose stools/day) in the absence of laxative use with C difficile PCR (Supplemental Figure 1). The order set prompts providers to order a multiplex enteric pathogen panel only for those presenting with community-onset diarrhea (hospitalization for ≤3 days). Computerized clinical decision support tools were implemented as part of the order set to guide appropriate testing for C difficile. The order set identified patients who received laxatives within the preceding 48 hours and included a “hard stop” alert indicating the laxative name and time administered, along with a message “C. difficile testing is generally NOT indicated for patients receiving laxatives. Contact Lab Medicine Resident on-call to place order, if testing is still indicated.” (Supplemental Figure 2). Repeat testing within 14 days of a positive C difficile test and within 7 days of a negative test was actively discouraged via an alert that fired if an order was placed within this time window advising the provider to contact the lab medicine resident on-call to place the order if testing was believed to be indicated (Supplemental Figure 3). Approximately 2 months before implementation, an educational campaign informed providers and nurses of the planned changes including a memo distributed through the medical director’s office at the 2 hospitals and presentations to key stakeholders. The CCDS enteric pathogen order set went live on August 29, 2018.
Before implementation of the order set, a multidisciplinary C difficile reduction program was implemented at HMC on September 18, 2017, which included provider education, nursing engagement, and restrictions on repeat C difficile PCR testing for nurse-driven orders. Although UWMC had a comprehensive CDI prevention and antimicrobial stewardship program throughout the 2 time periods, there were no programmatic efforts specifically targeting C difficile testing until the implementation of the CCDS order set.
Data Collection
We electronically extracted the following data between January 1, 2017 and August 31, 2019: C difficile PCR test orders and results as well as laxative use 48 hours before C difficile PCR test order. Hospital-onset CDI (HO-CDI) rates and HO-CDI cases during the study period were provided by the infection prevention teams. Among the HO-CDI cases, we electronically extracted the following: white blood cell count (WBC) > 15 000 cells/mL, serum creatinine >1.5 mg/dL, and intensive care unit (ICU) transfer within 7 days after a positive C difficile PCR test result, hospital stay beyond 7 days after a positive C difficile PCR test result, and deaths within 30 days after a positive C difficile PCR test result. Among HO-CDI cases, we electronically captured those in which the keywords “toxic megacolon” and “colectomy” were documented during the same hospitalization as the positive C difficile PCR test result; chart review was conducted (J.Z.) and verified by a physician (C.L.) to determine whether the patient experienced toxic megacolon and/or underwent colectomy due to toxic megacolon as a complication of their CDI.
Laboratory Methods
Stool samples tested for C difficile using the Xpert C. difficile PCR Assay (Cepheid, Sunnyvale, CA) were collected in a sterile media-free container. Formed specimens and those from patients <2 years of age were rejected for testing on the Xpert platform. The enteric pathogen panel consisted of the FilmArray Gastrointestinal Panel (Biofire, Salt Lake City, UT) and limited culture on blood agar, primarily for detection of Aeromonas. Samples for the enteric pathogen panel were primarily received in Cary-Blair medium, and therefore their formed status could not be assessed in the laboratory.
Statistical Analysis
Data were summarized by calendar month of the study period. The preintervention period was defined as January 1, 2017 to August 31, 2018, and the postintervention period was defined as September 1, 2018 to August 31, 2019. At HMC, the preintervention period was further divided into a pre-C difficile reduction program period from January 1, 2017 to September 17, 2017 and a post-C difficile reduction program period from September 18, 2017 to August 31, 2018. The primary outcome of this study was C difficile PCR test utilization rate as measured by number of tests ordered per 10 000 patient days. We conducted an interrupted time series (ITS) analysis using segmented regression with negative binomial distribution to test for changes in the slope and level of C difficile test rate from pre- to postintervention [18]. The dependent variable was the number of tests each month, and the logarithm of the total number of patient-days was included as an offset. Given that UWMC and HMC consist of different patient populations with some differences in preintervention practices, the medical centers were modeled separately (Supplemental Methods). For UWMC, we included a single linear term for the preintervention slope, whereas for HMC, we included an additional term to allow for separate preintervention slopes before and after implementation of the C difficile reduction program. Models for both sites included a term for a level change after the intervention and a single linear term for postintervention slope. We tested for autocorrelation by examining patterns in residuals over time and using the Durbin-Watson test [19]. Model estimates were exponentiated to compute the incidence rate ratio (IRR) and presented as the percentage change in rates by computing IRR − 1. Slope estimates were presented for preintervention and postintervention time periods and represent the percentage change per month.
Secondary outcome measures included C difficile testing within 48 hours after laxative administration, percentage of positive C difficile test results over all orders, and HO-CDI rates. These outcomes were analyzed using segmented regression models similar to those described for the primary outcome. We used quasi-Poisson models to evaluate the total number of C difficile tests ordered within 48 hours after laxative use among all patient-days and logistic regression models to evaluate the proportion of all C difficile tests that were ordered within 48 hours of previous laxative administration. Logistic regression models were also used for the proportion of C difficile tests that were positive; these model estimates were presented as odds ratios (ORs) with 95% confidence intervals (CIs). Negative binomial models were used for HO-CDI rates among all patient-days.
We evaluated clinical outcomes among patients with HO-CDI before and after the intervention including WBC > 15 000 cells/mL, serum creatinine >1.5 mg/dL, and ICU transfer within 7 days after a positive C difficile test, hospital stay beyond 7 days after a positive C difficile test, deaths within 30 days after a positive C difficile test, and diagnosis of toxic megacolon with or without colectomy after a positive C difficile test that occurred during the same hospitalization. Each of these binary outcomes was tested separately, and each measurement of the outcome type was considered independent; aggregate frequencies before and after the intervention were compared using a χ 2 test or Fisher’s exact test. All analyses were performed using the R computing environment (version 3.5.1).
RESULTS
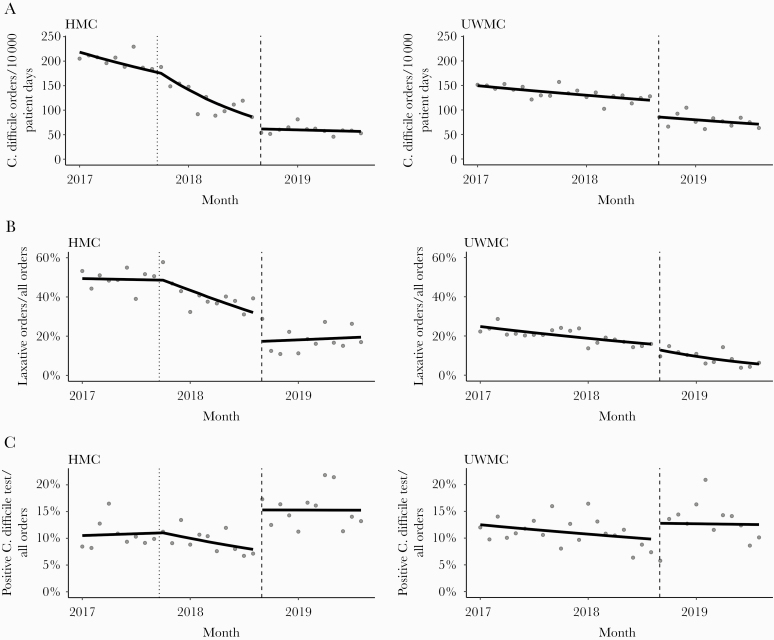
During the preintervention period (January 1, 2017–August 31, 2018), 6053 C difficile PCR tests were ordered over 417 100 patient days (145.1 tests/10 000 patient-days); during the postintervention period (September 1, 2018–August 31, 2019), 1812 C difficile PCR tests were ordered over 259 742 patient days (69.8 tests/10 000 patient-days). Table 1 describes results of the ITS analysis and indicates a significant decline in C difficile PCR order rate in the preintervention period at both hospitals. With implementation of the CCDS order set in August 2018, there was an immediate level change or drop in test utilization at both hospitals (Figure 1A). After accounting for the initial decreasing trend in the preintervention period, the negative binomial segmented regression model estimated a 27.4% and a 28.2% reduction in the rates of C difficile PCR tests ordered at UWMC and HMC, respectively, after the intervention. For UWMC, postintervention trends in test rates (1.7% decline per month) were similar to preintervention trends (1.2% decline per month; P = .58 for change in slopes). In contrast, HMC postintervention test rates stabilized with no evidence of additional decline (−0.8% per month), which was significantly different from the steeper slopes observed in the preintervention period (−2.4% and −6.8%; P < .001 for change in slopes from pre- to postintervention).
Table 1.
Percentage Rate Change for All Clostridioides difficile PCR Test Orders
| Model Parameter | Percentage Change (95% CI) | P Value |
|---|---|---|
| Harborview Medical Center | ||
| Intervention level change | −28.2% (−43.0% to −9.4%) | .005 |
| Preintervention, pre-CDI program slope (per month) | −2.4% (−4.3% to −0.5%) | .016 |
| Preintervention, post-CDI program slope (per month) | −6.8% (−8.6% to −5.0%) | <.001 |
| Postintervention slope (per month) | −0.8% (−3.3% to 1.8%) | .55 |
| University of Washington Medical Center | ||
| Intervention Level Change | −27.4% (−37.5% to −15.6%) | <.001 |
| Preintervention slope (per month) | −1.2% (−1.8% to −0.5%) | .001 |
| Postintervention slope (per month) | −1.7% (−3.4% to 0.1%) | .07 |
Abbreviations: CDI, C difficile infection; CI, confidence interval; PCR, polymerase chain reaction.
Figure 1.
(A) Fitted Trends in Monthly Rate of C. difficile PCR Test Orders. HMC = Harborview Medical Center; UWMC = University of Washington Medical Center. Filled circles represent observed data and solid lines represent fitted trends from a negative binomial segmented regression model. The black dashed vertical line in each figure represents the date of the CCDS order set implementation. The dotted vertical line in HMC represents the implementation date of the C. difficile reduction program; (B) Monthly Proportions of C. difficile PCR Test Orders Obtained within 48 Hours of Laxative Use among all C. difficile PCR Test Orders; (C) Fitted Trends in Monthly Proportions of Positive C. difficile Test Results among all C. difficile PCR Test Orders.
Next, we evaluated the effect of the CCDS order set on the relative contribution of C difficile tests ordered in the setting of laxatives among all tests ordered. After the intervention, the proportion of C difficile tests ordered in the setting of laxative use dropped from 39.3% and 16% at HMC and UWMC, respectively, in August 2018 to 28.9% and 9.6% in September 2018. A significant reduction, or level change, in the proportion of C difficile tests ordered in the setting of laxatives among all C difficile test orders was observed at HMC (OR = 0.45; 95% CI, 0.29–0.69; P < .001) but was not observed at UWMC (OR = 0.78; 95% CI, 0.53–1.15; P = .45) (Table 2; Figure 1B). We also evaluated the effect of the CCDS order set on C difficile testing rates in the setting of laxative use, among all patient days. Although a decline in C difficile testing in the setting of laxatives was observed preintervention, a significant reduction in the rate of C difficile PCR orders within 48 hours of laxative administration of greater magnitude was observed after order set implementation (HMC, −60.8% [95% CI, −74.3% to −40.1%], P < .001; UWMC, −37.3% [95% CI, −58.2% to −5.9%], P = .02) (Supplemental Table 1).
Table 2.
Odds Ratios for Proportion of Clostridioides difficile PCR Test Orders Within 48 Hours of Laxative Use
| Model Parameter | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Harborview Medical Center | ||
| Intervention level change | 0.45 (0.29–0.69) | <.001 |
| Preintervention, pre-CDI program slope (per month) | 1.0 (0.97–1.02) | .8 |
| Preintervention, post-CDI program slope (per month) | 0.93 (0.91–0.96) | <.001 |
| Postintervention slope (per month) | 1.01 (0.96–1.07) | .67 |
| University of Washington Medical Center | ||
| Intervention level change | 0.78 (0.53–1.15) | .45 |
| Preintervention slope (per month) | 0.97 (0.96–0.99) | <.001 |
| Postintervention slope (per month) | 0.92 (0.87–0.98) | .01 |
Abbreviations: CDI, C difficile infection; CI, confidence interval; PCR, polymerase chain reaction.
To assess the effect of the CCDS order set on C difficile test positivity rate, we evaluated monthly trends in proportion of positive C difficile tests among all tests ordered. A significant increase, or level change, in the C difficile test positivity rate was observed at HMC (OR = 2.1; 95% CI, 1.2 to 3.6; P = .01) but was not observed at UWMC (OR = 1.3; 95% CI, 0.9 to 2.0; P = .18) (Supplemental Table 2 and Figure 1C).
Hospital-onset CDI rates were evaluated during the study period. No significant differences were observed in rates of HO-CDI at the 2 hospitals between the 2 study periods (Supplemental Table 3 and Supplemental Figure 4). We did not detect significant residual autocorrelation in any of the segmented regression models we examined, indicating that our model assumption of independent observations was not violated.
We assessed the impact of reduced testing on delayed diagnoses of severe CDI by evaluating potential complications of CDI. Among the 385 HO-CDI cases in the preintervention period and 177 HO-CDI cases in the postintervention period, we observed no significant differences in the proportion of patients with WBC > 15 000 cells/mL, serum creatinine >1.5 mg/dL, or ICU transfer within 7 days after a positive C difficile test, length of hospital stay beyond 7 days after a positive C difficile test, and the proportion of patients who died within 30 days of a positive C difficile test (Table 3). In the preintervention period, 5 (1.3%) of 385 patients had a diagnosis of HO-CDI that was associated with toxic megacolon compared with 0 (0%) of 177 patients in the postintervention period (P = .33).
Table 3.
Clinical Outcomes Associated With Hospital-Onset Clostridioides difficile Cases in the Pre- and Postintervention Periods
| Clinical Outcomes | Preintervention N = 385 | Postintervention N = 177 | P Value |
|---|---|---|---|
| WBC > 15 000 cells/mL within 7 days | 152 (39.5%) | 67 (37.9%) | .78 |
| Serum creatinine >1.5 mg/dL | 97 (25.2%) | 43 (24.3%) | .90 |
| 30-day all-cause mortality | 34 (8.8%) | 8 (4.5%) | .10 |
| ICU admission within 7 days | 12 (29.1%) | 45 (25.4%) | .42 |
| Length of hospital stay beyond 7 days after positive C diff PCR test | 245 (63.6%) | 122 (69.5%) | .21 |
| Toxic megacolon identified as complication of CDI | 5 (1.3%) | 0 (0%) | .33 |
Abbreviations: C diff, C difficile; CDI, C difficile infection; ICU, intensive care unit; PCR, polymerase chain reaction; WBC, white blood cells.
DISCUSSION
We demonstrated that implementation of a CCDS enteric pathogen order set as a diagnostic test stewardship strategy led to a significant and sustained reduction in the rate of C difficile PCR test orders in the 12-month postintervention period. We observed a reduction in testing in the setting of laxative use and an increase in the proportion of positive C difficile tests among all C difficile tests ordered at one hospital and a trend towards increased C difficile test positivity rate at the other hospital. This suggests that the CCDS enteric pathogen order set directed clinicians towards more appropriate testing of those patients with an increased pretest probability of CDI. Finally, despite a reduction in tests ordered, we did not observe any adverse effects on patient outcome among those diagnosed with HO-CDI.
A variety of strategies have been implemented in different settings to improve C difficile diagnostic stewardship (Table 4). With the exception of a few studies that primarily used a laboratory-initiated intervention [20, 21] in conjunction with clinician education to limit inappropriate testing, CCDS tools to direct appropriate testing by clinicians have emerged as an important strategy to improve C difficile diagnostic test stewardship. Our findings are consistent with several studies demonstrating the value of CCDS tools in directing appropriate C difficile testing [11–13, 15, 16, 22]. For example, Mizusawa et al [13] demonstrated that an Epic platform-based CCDS best practice alert (BPA) activated by orders placed in the setting of recent laxative administration and early repeat testing resulted in a significant reduction in rates of C difficile testing.
Table 4.
Clostridioides difficile Diagnostic Stewardship Strategies
| Author, Year | Provider Directed CCDS-Based Intervention | Prospective Audit and Feedback | EHR Platform | Intervention | Outcomes |
|---|---|---|---|---|---|
| Truong, 2017 | No | No | Epic | RN stool documentation dropdown menu created with education on use; real-time Epic data tracking report including stool frequency, consistency, and laxative use within 48 hours; laboratory staff trained to review report and cancel C difficile orders not meeting testing criteria and repeat orders within 7 days | ↓ C difficile test order rate, HO-CDI rates, oral vancomycin days of therapy; No difference in clinical complication rates among those with canceled orders compared with those with diarrhea and negative C difficile results |
| White, 2017 | Yes | No | Sunrise Clinical Manager | C difficile order set created with CCDS tool identifying patients receiving prior laxatives within 36 C difficile and alert displayed asking providers to consider stopping laxative and reassessing in 24 hours before C difficile test order | ↓ Proportion of C difficile test orders in setting of laxative use; nonsignificant increase in proportion of patients with C difficile-related complications |
| Khoury, 2018 | Yes | Yes, IP team | Epic | CCDS tool prompting ordering provider to answer questions related to prior laxative use, whether diarrhea is only symptom present, whether patient is receiving medication active against C difficile; if answer is yes to any of these questions, alert is displayed suggesting potential inappropriate testing and justification is required. IP team daily review, cancels orders deemed to be inappropriate, notifies MD, justification required if testing desired. Laboratory canceled orders for formed stool specimens, and orders placed within 7 days of negative test or 14 days of positive test | ↓ HO-CDI rates |
| Yen, 2018 | No | No | Not specified | Hospital-wide educational campaign; laboratory canceled C difficile orders if stool sample not received within 24 hours or if stool did not meet the “stick test” | ↓ C difficile monthly test orders and HO-CDI rates; no increase in C difficile complications observed postintervention |
| Quan, 2018 | Yes | No | Not specified | Real-time CPOE alert requiring clinician attestation of appropriate testing criteria: ≥3 liquid stools in 24 hours, no alternate cause for diarrhea, and autopopulation of selected criteria: no laxative use within 24 hours, no previous test within 7 days, age >1 year; if testing outside criteria desired, “hard stop” requiring ID or GI approval. | ↓ C difficile test order rate, testing while on laxatives, HO-CDI rates |
| Madden, 2018 | Yes | No | Not specified | Educational campaign targeting GME trainees; CCDS algorithm to guide providers to test only symptomatic patients and avoid duplicate testing; financial incentive provided to GME trainees to reduce testing | ↓ C difficile test order rates, HO-CDI rates, and SIR |
| Mizusawa, 2019 | Yes | No | Epic | CCDS BPA activated if laxatives given within 48 hours, negative C difficile test within prior 7 days, positive test within prior 14 days | ↓ C difficile test order rates; no CDI-associated complications |
| Christensen, 2019 | Yes | Yes, AMS team | Cerner | CPOE C difficile PCR order modified to include criteria for testing; antimicrobial stewardship program directed provider education and prospective clinical review and preauthorization of all C difficile orders placed after calendar day 4 of hospital admission. CCDS built later incorporating information about testing in prior 7 days and required provider attestation of appropriate testing criteria | ↓ C difficile test order rates, HO-CDI rates, and SIR |
| Munson, 2019 | Yes | No | Not specified | C difficile order set developed containing alert recommending restricting testing to (1) patients with colonoscopic or pathologic findings of pseudomembranous colitis and (2) clinically significant diarrhea (change in stool consistency and/or frequency without other identified cause). | ↓ C difficile test order volume, positivity rate, HO-CDI, and HO-CDI NHSN SIR |
| Fleming, 2019 | Yes | Yes | Cerner | A decision support matrix to assist providers with appropriate orders was developed; monthly feedback provided to hospital leadership for dissemination to providers | ↓ C difficile test order volume, positivity rate, HO-CDI, HO-CDI NHSN SIR |
Abbreviations: AMS, antimicrobial stewardship; BPA, best practice alert; CCDS, computerized clinical decision support; C difficile, Clostridioides difficile; CDI, C difficile infection; CPOE, computer physician order entry; EHR, electronic health records; GI, gastrointestinal; GME, Graduate Medical Education; HO, hospital onset; ID, infectious diseases; IP, infection prevention; MD, medical doctor; NHSN, National Healthcare and Safety Network; PCR, polymerase chain reaction; RN, registered nurse; SIR, Standardized Infection Ratio.
An important consideration when implementing diagnostic stewardship interventions is assessing the potential for unintended adverse consequences by more restrictive testing approaches. A recent study found similar rates of serious outcomes among patients who tested positive for C difficile who did and did not receive laxatives and raises concern that exclusion of patients receiving recent laxatives from testing could lead to delayed or missed diagnoses of severe CDI [23]. As many institutions have incorporated CCDS tools that restrict testing in the setting of laxatives, the findings of the above study may have important implications. Although a number of studies have demonstrated that implementation of such tools has led to a decline in C difficile testing and HO-C difficile events [11, 12, 15, 22], very few have systematically evaluated the potential impact of CCDS interventions on clinical and safety outcomes [17]. A CCDS tool directing providers to consider stopping laxatives and reassess in 24 hours before ordering C difficile testing decreased testing in the setting of laxative use [16]; however, a small, but nonsignificant increase in the proportion of patients with C difficile-related complications was observed between the preintervention and postintervention periods [16]. In contrast, another study of 139 patients managed by providers who followed an Epic platform-based CCDS BPA and did not pursue C difficile testing found no adverse events including CDI-associated death, or delayed diagnosis of CDI or associated ileus or megacolon [13]. In our study, we did not observe any differences in the pre- and postintervention periods with respect to severe CDI or complications associated with CDI suggesting that any potential delays in testing resulting from the intervention did not have unintended adverse consequences. Although we were unaware of any changes in our patient population during the study period, we were unable to account for potential ward/service level differences that may have affected these outcomes.
The CCDS enteric pathogen order set did not seem to have an impact on HO-CDI rates. Although the order set provided guidance directing clinicians away from testing in the absence of having 3 or more loose stools in a 24-hour period, due to inconsistent documentation of stool frequency and quality, we were not able to incorporate CCDS alerts targeting inappropriate testing among patients who did not meet these criteria. It is possible that a CCDS tool that directly incorporated such criteria into an alert or that required provider attestation of appropriate testing criteria could have had a greater impact on HO-CDI rates as observed in other studies [12, 15].
Based on data from the first year postintervention, we estimated an annualized cost-savings of $67 935 based solely on the cost per test using the 2020 Medicare fee schedule; because the Medicare reimbursement rate is well below true laboratory costs, the savings has the potential to be much higher for institutions implementing similar CCDS tools. Furthermore, this estimate does not account for savings related to reduction in isolation days and CDI treatment.
CONCLUSIONS
Our analysis was limited as a retrospective study at a single health system. However, the CCDS tool was deployed at 2 different centers within the same health system serving different patient populations and led to a significant reduction in testing rates in both suggesting generalizability to other institutions.
This study demonstrates that CCDS tools in the EHR can be effectively leveraged to improve C difficile diagnostic test stewardship without causing harm. Additional studies are needed to further assess the effect of CCDS tools on adverse clinical outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully acknowledge Deb Wahl, Andrew White, and Patrick Mathias for technical assistance in development of the computerized clinical decision support order set. We also thank Michelle McIntosh, Michelle Swetky, and Wesley Wang for assistance with data obtained through our infection prevention surveillance systems.
Financial support. This work was funded by the University of Washington Department of Medicine Value, Quality, and Safety Accelerator Grant Program.
Potential conflicts of interest. S. A. P. reports grant support from Global Life Technologies, Inc., participates in research trials with Chimerix, Inc., and Merck & Co., and currently participates in a clinical trial sponsored by National Institute of Allergy and Infectious Diseases (U01-AI132004); vaccines for this trial are provided by Sanofi-Aventis; all outside of this submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol 2014; 35:667–73. [DOI] [PubMed] [Google Scholar]
- 3. Kamboj M, Brite J, Aslam A, et al. Artificial differences in Clostridium difficile infection rates associated with disparity in testing. Emerg Infect Dis 2018; 24:584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rock C, Maragakis LL. Diagnostic stewardship for Clostridiodes difficile testing: from laxatives to diarrhea and beyond. Clin Infect Dis 2020; 71:1479–80. [DOI] [PubMed] [Google Scholar]
- 5. Buckel WR, Avdic E, Carroll KC, et al. Gut check: Clostridium difficile testing and treatment in the molecular testing era. Infect Control Hosp Epidemiol 2015; 36:217–21. [DOI] [PubMed] [Google Scholar]
- 6. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan DJ, Croft LD, Deloney V, et al. Choosing wisely in healthcare epidemiology and antimicrobial stewardship. Infect Control Hosp Epidemiol 2016; 37:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship-leveraging the laboratory to improve antimicrobial use. JAMA 2017; 318:607–8. [DOI] [PubMed] [Google Scholar]
- 10. Fleming MS, Hess O, Albert HL, et al. Test stewardship, frequency and fidelity: Impact on reported hospital-onset Clostridioides difficile. Infect Control Hosp Epidemiol 2019; 40:710–2. [DOI] [PubMed] [Google Scholar]
- 11. Khoury JA, Sistrunk WW, Hixson F, et al. Sustained reduction in rates of hospital-onset Clostridium difficile infection using an automated electronic health record protocol. Am J Infect Control 2018; 46:542–8. [DOI] [PubMed] [Google Scholar]
- 12. Madden GR, German Mesner I, Cox HL, et al. Reduced Clostridium difficile tests and laboratory-identified events with a computerized clinical decision support tool and financial incentive. Infect Control Hosp Epidemiol 2018; 39:737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mizusawa M, Small BA, Hsu YJ, et al. Prescriber behavior in Clostridioides difficile testing: a 3-hospital diagnostic stewardship intervention. Clin Infect Dis 2019; 69:2019–21. [DOI] [PubMed] [Google Scholar]
- 14. Munson E, Rodriguez S, Riederer N, et al. Outcome of electronic order alert intervention relative to toxigenic Clostridium difficile PCR analysis and hospital-onset C difficile infection in a multihospital health care system. Am J Clin Pathol 2019; 151:622–7. [DOI] [PubMed] [Google Scholar]
- 15. Quan KA, Yim J, Merrill D, et al. Reductions in Clostridium difficile infection (CDI) rates using real-time automated clinical criteria verification to enforce appropriate testing. Infect Control Hosp Epidemiol 2018; 39:625–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White DR, Hamilton KW, Pegues DA, et al. The impact of a computerized clinical decision support tool on inappropriate Clostridium difficile testing. Infect Control Hosp Epidemiol 2017; 38:1204–8. [DOI] [PubMed] [Google Scholar]
- 17. Dunn AN, Radakovich N, Ancker JS, et al. The impact of clinical decision support alerts on Clostridioides difficile testing: a systematic review [published online ahead of print February 15, 2020]. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa152. [DOI] [PubMed] [Google Scholar]
- 18. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durbin J, Watson GS. Testing for serial correlation in least squares regression I. Biometrika 1950; 37:409–28. [PubMed] [Google Scholar]
- 20. Truong CY, Gombar S, Wilson R, et al. Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 2017; 55:1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yen C, Holtom P, Butler-Wu SM, et al. Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 2018; 39:734–6. [DOI] [PubMed] [Google Scholar]
- 22. Christensen AB, Barr VO, Martin DW, et al. Diagnostic stewardship of C. difficile testing: a quasi-experimental antimicrobial stewardship study. Infect Control Hosp Epidemiol 2019; 40:269–75. [DOI] [PubMed] [Google Scholar]
- 23. White NC, Mendo-Lopez R, Papamichael K, et al. Laxative use does not preclude diagnosis or reduce disease severity in Clostridiodes difficile infection [published online ahead of print October 4, 2019]. Clin Infect Dis 2019; doi: 10.1093/cid/ciz978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.