Abstract
Background
The Costa Rica HPV Vaccine Trial has documented cross-protection of the bivalent HPV vaccine against HPV31/33/45 up to 7 years after vaccination, even with one dose of the vaccine. However, the durability of such protection remains unknown. Here, we evaluate the efficacy of different schedules of the vaccine against HPV31/33/45 out to 11 years postvaccination, expanding to other nontargeted HPV types.
Methods
We compared the rates of HPV infection in vaccinated women with the rates in a comparable cohort of unvaccinated women. We estimated the average vaccine efficacy (VEavg) against incident infections and tested for a change in VE over time.
Results
Among 3-dose women, we observed statistically significant cross-protection against HPV31/33/45 (VEavg = 64.4%, 95% confidence interval [CI] = 57.7% to 70.0%). Additionally, we observed borderline, statistically significant cross-protection against HPV35 (VEavg = 23.2%, 95% CI = 0.3% to 40.8%) and HPV58 (VEavg = 21.2%, 95% CI = 4.2% to 35.3%). There was no decrease in VE over time (two-sided Ptrend > .05 for HPV31, -33, -35, -45, and -58). As a benchmark, VEavg against HPV16/18 was 82.0% (95% CI = 77.3% to 85.7%). Among 1-dose women, we observed comparable efficacy against HPV31/33/45 (VEavg = 54.4%, 95% CI = 21.0% to 73.7%). Acquisition of nonprotected HPV types was similar between vaccinated and unvaccinated women, indicating that the difference in HPV infection rates was not attributable to differential genital HPV exposure.
Conclusions
Substantial cross-protection afforded by the bivalent vaccine against HPV31/33/45, and to a lesser extent, HPV35 and HPV58, was sustained and remained stable after 11 years postvaccination, reinforcing the notion that the bivalent vaccine is an effective option for protection against HPV-associated cancers.
Cervical cancer affects more than half a million women annually worldwide, with the highest mortality burden on low-income countries (1). Persistent infection with carcinogenic human papillomavirus (HPV) is a necessary cause of cervical cancer (2). To date, more than 200 HPV types have been identified, with 13 types confirmed to be potentially oncogenic (3). Approximately 70% of cervical cancers are attributable to HPV16 or 18 (4). An additional five HPV types (HPV31/33/45/52/58) account for another 20% of cervical cancer (4).
The prophylactic HPV vaccine is an effective means to protect against oncogenic HPV infection and risk of HPV-associated cancers (5). The three licensed vaccines (Cervarix, targeting HPV16/18; Gardasil, targeting HPV6/11/16/18; and Gardasil-9, targeting HPV6/11/16/18/31/33/45/52/58) contain virus-like particles that are composed of the HPV L1 capsid proteins, displaying epitopes essential for generating high levels of neutralizing antibodies (6). In addition to protecting against targeted HPV types, the vaccine provides cross-protection against phylogenetically related types with sufficient similarities in their epitopes to allow for partial cross-reactive immune responses (7).
Findings from randomized trials and postimplementation surveillance have demonstrated cross-protection afforded by the bivalent vaccine and, to a lesser extent, the quadrivalent vaccine (8–10). In particular, our previous reports on the Costa Rica HPV Vaccine Trial (CVT) and the associated long-term follow-up (LTFU) have shown that the bivalent vaccine reduces the prevalence of HPV31/33/45 at 7 years postvaccination, even among women who had only one dose of the vaccine (11,12). In addition, recent observational data from national vaccination programs implemented in the Netherlands and Scotland using the bivalent vaccine have shown a decrease in the prevalence of HPV31/33/45 up to 6–7 years postvaccination (13,14). Nonetheless, the extent and durability of cross-protection have been questioned (10,15), and cohorts with large sample sizes and extensive follow-up time are needed to address these questions.
Our earlier publications focus on the a priori composite endpoint for HPV31/33/45. However, the PATRICIA trial (NCT00122681) reported more than 90% efficacy against cervical intraepithelial neoplasia grade 3 or greater (16), suggesting a wider extent of cross-protection against other oncogenic HPVs. A comprehensive analysis on cross-protection in CVT and LTFU is needed to identify other cross-protected HPV types.
With growing evidence supporting efficacy of a one-dose regimen, cross-protection afforded by one dose of the bivalent vaccine is of research importance. Here, we extend our CVT and LTFU post hoc analysis on HPV31/33/45 and evaluate the efficacy of different schedules of the bivalent vaccine out to 11 years postvaccination, expanding to other nontargeted HPV types. This efficacy study on the bivalent HPV vaccine has the longest follow-up time reported to date.
Methods
Study Population
During 2004–2005, CVT (NCT00128661) enrolled 7466 young women 18–25 years of age in Costa Rica in a 4-year-long randomized clinical trial to evaluate the safety and efficacy of the bivalent Cervarix vaccine (GlaxoSmithKline Biologicals, Rixensart, Belgium) to reduce HPV incidence and related neoplasia (17). Women were randomized to receive three doses of either Cervarix or the control hepatitis A virus (HAV) Havrix vaccine (GlaxoSmithKline Biologicals). A subset of women received only one or two doses of the vaccine for reasons that were independent of the trial (ie, pregnancy, colposcopic referral, etc.) (17). At enrollment and annual follow-up visits, serum samples were collected (17). For sexually experienced women, cervical cells were collected by a clinician for cytology and HPV DNA testing. Women with low-grade cytologic abnormalities were followed up every 6 months, and those with high-grade disease were referred to colposcopy for evaluation and treatment. After the initial 4 years, participants were offered cross-over vaccination. Women in the vaccination arm of the study living in selected areas and all women who received fewer than three doses of the HPV vaccine were invited to participate in an unblinded LTFU study that extended to 11 years, with biennial cervical and serum sample collection (11). Again, women with low-grade cytologic abnormalities were followed up every 6 months, and those with high-grade disease were referred to colposcopy. Concurrently at year 4, a new unvaccinated control group (UCG) of 2836 women from the same birth cohort and geographical region were recruited and, after intensive screening to identify and treat prevalent disease, followed for 7 years at a schedule comparable with that of the HPV-vaccinated group (see CONSORT diagrams in Supplemental Figure S1, available online) (18). Protocols were approved by the US National Cancer Institute (NCI) Institutional Review Boards and the corresponding Costa Rican Institutional Review Board; all participants signed informed consent.
Laboratory Methods
We used two methods for HPV genotyping (Supplemental Table S1, available online). Both validated methods have similar sensitivity and specificity (19). The initial method amplified the L1 region of HPV using the SPF10 polymerase chain reaction (PCR) primer system and then detected the amplimers using DNA enzyme immunoassay (DDL Diagnostic Laboratory, the Netherlands) (20). The DNA enzyme immunoassay–positive SPF10 amplimers were then used to identify HPV genotype by reverse hybridization with the HPV line probe assay (LiPA25), allowing detection of carcinogenic (HPV16/18/31/33/35/39/45/51/52/56/58/59) and noncarcinogenic (HPV6/11/34/40/42/43/44/53/54/66/68/70/73/74) types (21).
The new NCI-developed in-house method TypeSeq was performed as described previously (22). Briefly, the stage 1 primer pool contained 127 RNase H2-dependent primers (Integrated DNA Technologies, Coralville, IA), targeting one human gene (B2M) and the L1 gene for 51 HPV types. After amplification, reactions were used as a template for stage 2 universal priming site recoding PCR. This primer pool contained two nested B2M primers and 170 nested HPV unmodified primers (Integrated DNA Technologies). After amplification, reactions were used as a template for stage 3 sequencing adapter and dual-barcode addition PCR. All reactions were pooled, purified, then quantitated using a Qubit2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA). Ion S5 Sequencing (Thermo Fisher Scientific) was performed according to the manufacturer’s instructions. Dual barcode demultiplexing, quality filtering, and HPV genotyping were performed using a custom plug-in.
Outcomes
We consider prevalent infections, incident infections, and incident infections that persisted no less than 6 months. A prevalent infection is defined as a type-specific infection that was detected at the study visit of interest. We report results for the primary study visits at years 1, 2, 3, 4, 7, 9, and 11. Sensitivity analyses considering specific time intervals (eg, 301–660 days postvaccination) instead of specific visits (eg, first scheduled visit) show similar results (data not shown). An incident infection is defined as a prevalent infection that was not detected at the prior study visit. Of note, a recurrent infection could be misclassified as an incident infection if viral levels were below the limit of detection at the prior visit. A 6-month persistent infection is defined as an infection that is also detected at any visit more than 150 days later without an intervening negative test result for that type. This outcome excludes transient HPV deposition that does not result in a true breakthrough infection. The second visit used to define persistence is often a 6-month follow-up visit triggered by low-grade abnormalities.
Statistical Analysis
This article focuses on cross-protection afforded by the bivalent HPV vaccine; single-dose vaccine-induced protection against HPV16/18 is the focus of our complementary article by Kreimer et al. (23).
We report demographic and clinical characteristics of the five study groups: women receiving one dose of the HPV vaccine in CVT (1-dose), women receiving two doses (2-dose), women receiving three doses (3-dose), women receiving the control vaccine in CVT (HAV), and the UCG of LTFU. We then tested for differences in the characteristics using the Freeman-Halton method (24).
We report VE against incident infection at each scheduled visit. We start by reporting the number of women with an incident infection, the total number of women eligible for an incident infection, and the rate of infection (number of women with an infection divided by number of eligible women) for each of the five groups at the scheduled visits. We then estimate the VEs by comparing the rates in the vaccinated and unvaccinated groups (HAV arm for years 1–4 and UCG for years 7–11). We calculate the associated 95% confidence intervals (CI) using a two-step approach (25). First, we calculate the exact 95% confidence interval for the proportion of cases who are vaccinated, π, conditioning on the number of cases and using the mid-P correction. Second, letting (πL, πU) denote this confidence interval and letting NV and NU denote the number of vaccinated and unvaccinated participants, respectively, we define the 95% confidence interval for VE by (1-πUNU /[NV(1- πU)]), 1-πLNU /[NV(1- πL)]). Because the comparison for years 7–11 was not part of a randomized study, we also performed a sensitivity analysis adjusting for potential confounders, including age, number of sexual partners, and smoking.
We then report the average VE (VEavg) against incident infection over 11 years and test whether VE changes over time for the 3-dose group. We start by reporting the number of incident infections over 11 years, the number of person-years observed (ie, total number of annual or biennial visits where the woman had no infection at the previously scheduled visit), and the rate of infection (number of incident infections divided by number of person-years) for each of the four study groups at the scheduled visits. We then model the probability of infection using generalized estimating equations (GEEs), where the dependent variable is incident infection and the independent variable is vaccination status. We include visits from years 2, 3, 4, 7, 9, and 11 where the woman was eligible for an incident infection, use a log-link, and assume an unstructured correlation matrix. Again, for the unvaccinated group, the HAV arm was used for years 2–4 and the UCG was used for years 7–11. The VEavg and 95% confidence intervals are estimated by first exponentiating the coefficient and confidence intervals for vaccination status in the model and then subtracting those values from 1. We test for a trend in VE over time by including a vaccination status x year interaction term in a model that excludes year-1 visits and report the P value, Ptrend, for the corresponding Wald statistic. For main composite endpoints such as HPV31/33/45, HPV16/18, and “other HPV types,” we also performed GEEs with a vaccination x time period (years 2–4 vs years 7–11) interaction term and tested for heterogeneity using a Wald test for this interaction term. VE in year 1 is low because of infections missed at baseline and inclusion of these data would bias the results to show an increasing VE over time. When GEEs failed to converge because of zero or only a small number of events, we calculate confidence intervals using the two-step approach.
We repeat analyses for prevalent and 6-month persistent infections and for the 1-dose and 2-dose groups. Finally, to compare VE in 1-dose and 3-dose women, we repeat the GEEs with both women in the 1-dose and 3-dose groups and consider the P value, P1vs3, for the effect of treatment dose; similar analyses compared VE in 2- and 3-dose women to calculate P2vs3. All P values are two-sided, and a P value of less than .05 was considered statistically significant. The statistical package used for our analyses is SAS9.4M4(TS1M4).
Results
Participant Characteristics
The characteristics of the 2-dose, 3-dose, and HAV groups at enrollment in CVT are similar (Supplemental Table S2, available online). However, the 1-dose group had higher percentages of HPV-positive and HPV-seropositive women. The characteristics of the 1-dose, 2-dose, 3-dose, and UCG groups at baseline visit of LTFU and the 11-year visit are presented in Supplemental Table S2 (available online). We note that the 3-dose group had a higher OC usage and fewer pregnancies, as compared with the UCG, and that the 1-dose group had more pregnancies, as compared with either the 3-dose or UCG groups. As expected for an effective HPV vaccine, vaccinated women had fewer clinically necessitated follow-up visits during LTFU.
Vaccine Efficacy
We observed cross-protection against incident infection by HPV31/33/45 in the 3-dose group (VEavg = 64.4%, 95% CI = 57.7% to 70.0%) (Table 1). We noticed higher vaccine efficacy against HPV31 (VEavg = 64.1%, 95% CI = 54.9% to 71.3%) and HPV45 (VEavg = 79.6%, 95% CI = 71.3% to 85.5%) compared with HPV33 (VEavg = 31.3%, 95% CI = 4.0% to 50.8%). Moreover, we observed statistically significant cross-protection against HPV35 (VEavg = 23.2%, 95% CI = 0.3% to 40.8%) and HPV58 (VEavg = 21.2%, 95% CI = 4.2% to 35.3%) but “negative” protection against HPV56 (VEavg = -26.7%, 95% CI = -50.6% to -6.7%). For comparison, the VEavg against HPV16/18 was 82.0% (95% CI = 77.3% to 85.7%).
Table 1.
Average vaccine efficacy (VEavg) against incident HPV infection for years 2–11
| HPV types | Unvaccinated (HAV or UCG) |
HPV-vaccinated (3-dose) |
HPV-vaccinated (1-dose) |
VEavg expressed in % (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of infection | Person-years | Rate of infection (%) | No. of infection | Person-years | Rate of infection (%) | No. of infection | Person-years | Rate of infection (%) | 3-dose | 1-dose | |||
| A priori cross-protected HPV types | |||||||||||||
| HPV31 | 315 | 12 643 | 2.5 | 96 | 10 657 | 0.9 | 10 | 662 | 1.5 | 64.1 (54.9 to 71.3) | 37.7 (−23.1 to 68.4)§ | ||
| HPV33 | 95 | 12 981 | 0.7 | 54 | 10 773 | 0.5 | 3 | 672 | 0.4 | 31.3 (4.0 to 50.8) | 37.1 (−96.8 to 79.9) | ||
| HPV45 | 224 | 12 828 | 1.7 | 38 | 10 756 | 0.4 | 3 | 674 | 0.4 | 79.6 (71.3 to 85.5) | 74.6 (20.8 to 91.9) | ||
| HPV31/33/45 | 562 | 12 324 | 4.6 | 170 | 10 509 | 1.6 | 14 | 658 | 2.1 | 64.4 (57.7 to 70.0) | 54.4 (21.0 to 73.7) | ||
| Vaccine-targeted HPV types | |||||||||||||
| HPV16 | 386 | 12 407 | 3.1 | 63 | 10 641 | 0.6 | 4 | 671 | 0.6 | 80.9 (74.8 to 85.5) | 79.3 (45.4 to 92.1) | ||
| HPV18 | 238 | 12 795 | 1.9 | 26 | 10 774 | 0.2 | 0 | 676 | 0.0 | 87.1 (80.6 to 91.4) | 100.0 (76.0 to 100.0)‡ | ||
| HPV16/18 | 569 | 12 158 | 4.7 | 88 | 10 573 | 0.8 | 4 | 671 | 0.6 | 82.0 (77.3 to 85.7) | 86.4 (64.1 to 94.8) | ||
| Other HPV types | |||||||||||||
| HPV35 | 147 | 12 910 | 1.1 | 94 | 10 741 | 0.9 | 5 | 672 | 0.7 | 23.2 (0.3 to 40.8) | 31.6 (−64.2 to 71.5) | ||
| HPV39 | 250 | 12 762 | 2.0 | 204 | 10 555 | 1.9 | 9 | 660 | 1.4 | 1.4 (−18.5 to 17.9) | 29.5 (−35.0 to 63.2) | ||
| HPV51 | 389 | 12 619 | 3.1 | 342 | 10 390 | 3.3 | 15 | 651 | 2.3 | −6.4 (−22.8 to 7.8) | 20.5 (−30.9 to 51.7) | ||
| HPV52 | 450 | 12 476 | 3.6 | 367 | 10 338 | 3.6 | 29 | 642 | 4.5 | 2.2 (−12.1 to 14.7) | −33.5 (−89.7 to 6.0) | ||
| HPV56 | 248 | 12 769 | 1.9 | 261 | 10 559 | 2.5 | 11 | 663 | 1.7 | −26.7 (−50.6 to -6.7) | 10.9 (−59.7 to 50.3) | ||
| HPV58 | 252 | 12 785 | 2.0 | 163 | 10 610 | 1.5 | 15 | 658 | 2.3 | 21.2 (4.2 to 35.3) | −17.4 (−93.5 to 28.7)§ | ||
| HPV59 | 206 | 12 897 | 1.6 | 176 | 10 674 | 1.6 | 14 | 660 | 2.1 | −4.7 (−27.8 to 14.3) | −32.3(−123.8 to 21.8) | ||
| Other oncogenic HPVs* | 1291 | 11 079 | 11.7 | 1078 | 9107 | 11.8 | 63 | 571 | 11.0 | −1.7 (−9.9 to 5.9) | 4.4 (−22.4 to 25.4) | ||
| HPV6/11 | 202 | 12 866 | 1.6 | 184 | 10 649 | 1.7 | 9 | 669 | 1.3 | −10.6 (−34.7 to 9.2) | 11.8 (−69.1 to 54.0) | ||
| Other nononcogenic HPVs† | 1446 | 11 019 | 13.1 | 1233 | 9027 | 13.7 | 77 | 575 | 13.4 | −5.1 (−13.0 to 2.2) | −1.1 (−26.0 to 18.8) | ||
Other oncogenic HPVs are HPV35, -39, -51, -52, -56, -58, and -59. HAV = hepatitis A virus-vaccinated control group; UCG = unvaccinated control group.
Other nononcogenic HPVs are HPV34, -40, -42, -43, -44, -53, -54, -66, -68, -70, -73, and -74.
Confidence intervals (CI) were calculated using the two-step approach, which did not account for dependence.
P 1vs3 = .11; all other P1vs3 ≥ .15.
For the 1-dose group, statistically significant cross-protection was observed for the composite HPV31/33/45 (VEavg = 54.4%, 95% CI = 21.0% to 73.7%) (Table 1). Statistical significance was maintained at individual HPV levels only for HPV45 (VEavg = 74.6%, 95% CI = 20.8% to 91.9%). However, vaccine efficacies for 1-dose women were not statistically different from those of 3-dose women (P1vs3 > .05) (see Supplemental Table S3, available online, for year-adjusted infections rates, and Supplemental Table S4, available online, for by-year VE analyses with the 2-dose group).
For both the 3-dose and 1-dose groups, the composite VEavg against the other oncogenic and nononcogenic HPV types was not statistically significantly different from VEavg =0%, confirming that these high VEs observed for cross-protected HPV types cannot be attributed to differences in genital HPV exposure (Table 1). Finally, we noted the estimated VE was also similar for prevalent and 6-month persistent infections (see Supplemental Tables S5–7, available online, for by-year VE analyses, and Supplemental Table S8, available online, for VEavg analyses).
To assess the durability of cross-protection in later years of CVT and LTFU, we compared VEavg of years 2–4 with that of years 7–11. For the 3-dose group, VEavg for years 2–4 was 65.4% (95% CI = 56.4% to 72.6%) and VEavg for years 7–11 was 63.3% (95% CI = 52.9% to 71.4%) (Table 2). For the 1-dose group, VEavg for years 2–4 was 33.4% (95% CI = -28.6% to 65.5%) and VEavg for years 7–11 was 69.3% (95% CI = 26.1% to 87.2%). VEavg estimates for years 2–4 and for years 7–11 were not statistically significantly different from each other (P = .73 for the 3-dose group and P = .68 for the 1-dose group). Estimates of VEavg also did not change in the sensitivity analysis adjusting for potential confounders (Supplemental Table S9, available online).
Table 2.
Average vaccine efficacy (VEavg expressed in %) against incident HPV infection by study period
| HPV types | HPV-vaccinated (3-dose) |
P ‡ (VE2–4 vs VE7–11) | HPV-vaccinated (1-dose) |
P ‡ (VE2–4 vs VE7–11) | ||
|---|---|---|---|---|---|---|
| VEavg (Years 2–4) | VEavg (Years 7–11) | VEavg (Years 2–4) | VEavg (Years 7–11) | |||
| A priori cross-protected HPV types | ||||||
| HPV31/33/45 | 65.4 (56.4 to 72.6) | 63.3 (52.9 to 71.4) | .73 | 33.4 (−28.6 to 65.5) | 69.3 (26.1 to 87.2) | .68 |
| Vaccine-targeted HPV types | ||||||
| HPV16/18 | 78.0 (71.0 to 83.4) | 87.3 (81.3 to 91.4) | .02 | 86.1 (44.4 to 96.5) | 88.0 (52.3 to 97.0) | .94 |
| Other HPV types | ||||||
| Other oncogenic HPVs* | 2.6 (−7.9 to 12.0) | −7.0 (−20.1 to 4.8) | .21 | 8.2 (−31.2 to 35.7) | 1.6 (−36.4 to 28.9) | .76 |
| HPV6/11 | −24.8 (−63.0 to 4.4) | 5.3 (−27.8 to 29.9) | .20 | 13.8 (−129.1 to 67.6) | 15.8 (−103.1 to 65.1) | .98 |
| Other nononcogenic HPVs† | −3.2 (−14.1 to 6.6) | −6.4 (−17.9 to 4.0) | .63 | 9.5 (−24.8 to 34.3) | −7.3 (−40.2 to 17.8) | .93 |
Other oncogenic HPVs are HPV35, −39, −51, −52, −56, −58, and −59.
Other nononcogenic HPVs are HPV34, −40, −42, −43, −44, −53, −54, −66, −68, −70, −73, and −74.
Generalized estimating equations were performed with a vaccination × time period (years 2–4 vs years 7–11) interaction term and tested for heterogeneity using a Wald test for this interaction term. P values were two-sided.
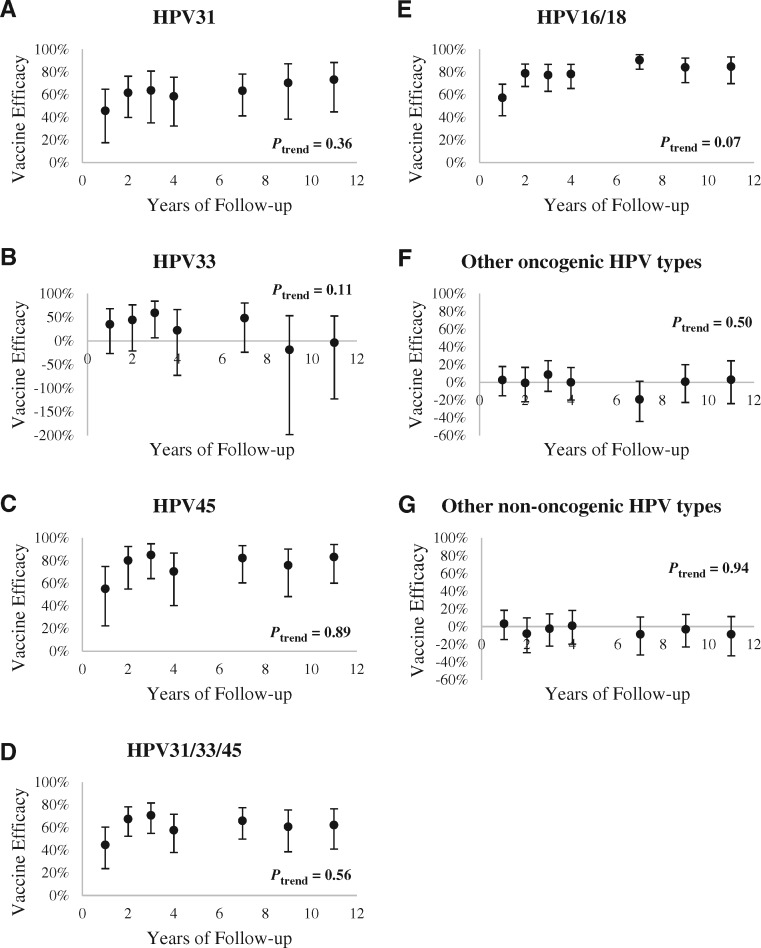
We also assessed VE of the 3-dose group at each study visit between years 1 and 11. Overall, VE against individual HPV31, -33, and -45, and the composite endpoint of HPV31/33/45 indicated that VE has been stable and there was no statistically significant waning in cross-protection up to approximately 11 years postvaccination (Ptrend > .05 for HPV31, -33, and -45 and HPV31/33/45) (Figure 1). Although we observed statistically significant VEavg for HPV33, -35, and -58, VEs by-year for these types were not statistically significant (Supplemental Figure S2 and Supplemental Table S4, available online). Over 11 years, the average infection rate of nononcogenic HPV types were comparable in both HPV-vaccinated and HAV and UCG groups (Ptrend > .05) (Table 1 and Figure 1;Supplemental Table S4, available online), indicating that HPV exposure remained unchanged over time.
Figure 1.
Vaccine efficacy (VE) against incident HPV infection over time. VE against incident infection with (A) HPV31, (B) HPV33, (C) HPV45, (D) HPV31/33/45, (E) HPV16/18, (F) other oncogenic HPVs, and (G) other nononcogenic HPVs over time, in the 3-dose group. Ptrend for VE over 11 years (excluding year 1) is presented to demonstrate stability of protection. All tests were two-sided.
Discussion
We reported here the efficacy of the bivalent vaccine in protecting against new HPV infections at approximately 11 years postvaccination. Our data showed that VE against HPV31/33/45, particularly HPV31 and HPV45, was stable over 11 years, with no evidence of waning. Nearly 64% of incident HPV31 infections and 80% of incident HPV45 infections were prevented in our study. Using HPV16/18 as a benchmark (approximately 82% VE), the bivalent vaccine is approximately 78% and 98% as effective at protecting against HPV31 and HPV45, respectively, as the targeted HPV types, indicating that cross-protection against some HPV types is only marginally lower than protection against the targeted types. Significant cross-protection is of clinical importance because HPV31 and HPV45 account for 3.7% and 5.9% of global cervical cancer cases, respectively (26). Because protection against HPV16/18 remained robust after a decade, the bivalent vaccine could potentially avert 70% of HPV-related cancers through direct protection and an additional 9.6% through cross-protection. Future analyses from CVT and LTFU will continue to assess the efficacy of the vaccine against histologic outcomes.
Although a meta-analysis of vaccination trials has suggested possible waning of cross-protection over time (10), we consistently demonstrate the cross-protective effect of the bivalent vaccine against HPV31/33/45, now more than a decade after vaccination. This is in line with the findings from national vaccination programs in the Netherlands and Scotland, showing steady, statistically significant effectiveness against HPV31/33/45 (13,14). Although evaluation of VE by year, by individual HPV types only showed statistically significant protection against HPV 31 and 45, we believe this could be explained by our sample size and limited power. Combined data from CVT and PATRICIA have shown moderate protection against HPV33 and, to a lesser degree, HPV35 (27). Although the efficacy estimates for these HPV types in CVT-only analyses showed no statistical significance, there was no heterogeneity between results from CVT and PATRICIA, suggesting that more accurate vaccine efficacy estimates could be obtained if the sample size is large enough to detect these less prevalent HPV types.
The World Health Organization recommends vaccination with the bivalent, quadrivalent, or the nonavalent HPV vaccines based on the assessment of their comparable immunogenicity, efficacy, and effectiveness for the prevention of cervical cancer (28). Clinical trials comparing the bivalent and quadrivalent vaccines show greater immunogenicity for the bivalent vaccine (29). One major difference is the adjuvant: the bivalent vaccine uses AS04, and the quadrivalent vaccine uses amorphous aluminum hydroxyphosphate sulfate (28). AS04 includes both aluminum salt and a toll-like receptor 4 agonist and robustly activates both cellular and humoral immune responses (30). We do not yet have a comprehensive understanding of the immune responses to these vaccines. Future studies detailing the avidity of neutralizing antibodies will also help understand these vaccines. Regarding the bivalent and nonavalent vaccines, ongoing trials ESCUDDO (NCT03180034) and PRIMAVERA (NCT03728881) will address the minimum number of doses required to elicit a serologic response effective at preventing HPV infections.
Our data on the 1-dose regimen showed comparable cross-protective estimates against HPV31/33/45 as the recommended 3-dose regimen for the age group in CVT and LTFU. Because of the small sample size for the 1-dose group, the confidence intervals for efficacy estimates were generally wide, making it difficult to draw strong conclusions about individual HPV types. However, our results indicated statistically significant VE for HPV31/33/45 at year 11, suggesting there is no waning in protection. Future results from ESCUDDO will offer definitive proof for cross-protection afforded by one dose of the bivalent vaccine.
We note that participants in CVT attended annual visits, then biennial visits during LTFU, unless abnormal cytology prompted 6-month follow-up visits. Although we believe the efficacy of the vaccine was the reason for the infrequent detection of 6-month persistent HPV infections and not because the vaccinated women had fewer visits, this potential bias could have been eliminated completely with regular 6-month visits.
With the longest follow-up time reported to date, our results show substantial cross-protection against HPV31/33/45 and robust protection against HPV16/18 up to 11 years postvaccination, with no signs of waning. Evidence for durable cross-protection afforded by the bivalent vaccine and emerging evidence showing its efficacy with a 1-dose regimen make this an effective HPV vaccine for protection against HPV-associated cancers.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the 4-year, randomized blinded phase of our study. The long-term follow-up was funded by the NCI with support from the National Institutes of Health Office of Research on Women’s Health.
Notes
The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript.
John T. Schiller and Douglas R. Lowy report that they are named inventors on US government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest. Where authors are identified as personnel of the International Agency for Research on Cancer–WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer–WHO.
Sabrina H. Tsang: conceptualization; formal analysis; investigation; methodology; project administration; writing (original draft, review, and editing). Joshua N. Sampson: conceptualization; formal analysis; investigation; methodology; supervision; writing (original draft, review, and editing). John Schussler: data curation; formal analysis; methodology; project administration; writing (review and editing). Carolina Porras and Sarah Wagner: data curation; investigation; writing (review and editing); methodology. Joseph Boland: methodology. Bernal Cortes: data curation; methodology; writing (review and editing). Douglas R. Lowy: funding acquisition; supervision. John T. Schiller: funding acquisition; investigation; supervision; writing (review and editing). Mark Schiffman: supervision. Troy J. Kemp: writing (review and editing). Ana Cecilia Rodriguez: writing (review and editing). Wim Quint: methodology. Mitchell H. Gail: methodology; writing (review and editing). Ligia A. Pinto: writing (review and editing). Paula Gonzalez: conceptualization; data curation; funding acquisition; investigation; supervision; writing (review and editing). Allan Hildesheim: conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; writing (review and editing). Aimée R. Kreimer: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; writing (review and editing). Rolando Herrero: conceptualization; methodology; supervision; writing (review and editing).
Costa Rica Vaccine Trial Study Group Authors: Bernal Cortés, Paula González, Rolando Herrero, Silvia E. Jiménez, Carolina Porras, Ana Cecilia Rodríguez (Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica); Allan Hildesheim, Aimée R. Kreimer, Douglas R. Lowy, Mark Schiffman, John T. Schiller, Mark Sherman, Sholom Wacholder (NCI, Bethesda, MD, USA); Ligia A. Pinto, Troy J. Kemp (Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD, USA); Mary K. Sidawy, (Georgetown University, Washington, DC, USA); Wim Quint, Leen-Jan van Doorn, Linda Struijk (DDL Diagnostic Laboratory, Netherlands); Joel M. Palefsky, Teresa M. Darragh (University of California, San Francisco, CA, USA); Mark H. Stoler (University of Virginia, Charlottesville, VA, USA).
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Carlos Avila, Loretto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Steve Self, Chair; Adriana Benavides; Luis Diego Calzada; Ruth Karron; Ritu Nayar; and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain and Elizabeth Fontham, Co-Chairs; Diane Davey; Gypsyamber D’Souza; Anne Gershon; Elizabeth Holly; Silvia Lara; Henriette Raventós; Wasima Rida; Richard Roden; Maria del Rocío Sáenz Madrigal; and Margaret Stanley).
Preliminary findings were presented at the 32rd International Papillomavirus Conference in Sydney, Australia, in October 2018.
Supplementary Material
References
- 1. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6(4):204. [DOI] [PubMed] [Google Scholar]
- 3. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 4. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. [DOI] [PubMed] [Google Scholar]
- 5. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6. De Vincenzo R, Ricci C, Conte C, et al. HPV vaccine cross-protection: highlights on additional clinical benefit. Gynecol Oncol. 2013;130(3):642–651. [DOI] [PubMed] [Google Scholar]
- 7. Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol. 2010;118(1):S2–7. [DOI] [PubMed] [Google Scholar]
- 8. Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015;16(7):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skinner SR, Apter D, De Carvalho N, et al. Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and HPV-related diseases. Expert Rev Vaccines. 2016;15(3):367–387. [DOI] [PubMed] [Google Scholar]
- 10. Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–789. [DOI] [PubMed] [Google Scholar]
- 11. Safaeian M, Sampson JN, Pan Y, et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. 2018;110(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1(5):408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donken R, King AJ, Bogaards JA, et al. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis. 2018;217(10):1579–1589. [DOI] [PubMed] [Google Scholar]
- 14. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293–1302. [DOI] [PubMed] [Google Scholar]
- 15. Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines. 2015;14(8):1047–1049. [DOI] [PubMed] [Google Scholar]
- 16. Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. [DOI] [PubMed] [Google Scholar]
- 17. Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine. 2015;33(18):2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagner S, Roberson D, Boland J, et al. Evaluation of TypeSeq, a novel high-throughput, low-cost, next-generation sequencing-based assay for detection of 51 human papillomavirus genotypes. J Infect Dis. 2019;220(10):1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153(6):1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner S, Roberson D, Boland J, et al. Development of the TypeSeq assay for detection of 51 human papillomavirus genotypes by next-generation sequencing. J Clin Microbiol. 2019;57(5):e01794–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreimer AR, Sampson JN, Porras C. et al. Evaluation of durability of a single-dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020;112(10):djaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freeman GH, Halton JH.. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1–2):141–149. [PubMed] [Google Scholar]
- 25. Rothman KJ, Boice JD, Austin H.. Epidemiologic Analysis with a Programmable Calculator. Boston: Epidemiology Resources; 1982. [Google Scholar]
- 26. Serrano B, de Sanjose S, Tous S, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51(13):1732–1741. [DOI] [PubMed] [Google Scholar]
- 27. Tota JE, Struyf F, Sampson JN, et al. Efficacy of the AS04-adjuvanted HPV-16/18 vaccine: pooled analysis of the Costa Rica Vaccine and PATRICIA randomized controlled trials. J Natl Cancer Inst. 2020;112(8):djz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine. 2017;35(43):5753–5755. [DOI] [PubMed] [Google Scholar]
- 29. Einstein MH, Takacs P, Chatterjee A, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a phase III randomized trial. Hum Vaccin Immunother. 2014;10(12):3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giannini SL, Hanon E, Moris P, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33-34):5937–5949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.