Abstract
Mounting evidence reveals evident sex differences in physiology, disease presentation and response to medication in axial SpA (axSpA). Unfortunately these data are often neglected in clinical practice and research. In this review, myths that still exist on diagnosis, disease manifestation and drug effectiveness were argued against data of the most recent literature. The aim is to increase awareness of sex differences in the clinical aspects of axSpA.
Keywords: spondyloarthritis, biological therapies, epidemiology, inflammation, sex differences, patient reported outcomes
Rheumatology key messages
Women with axSpA have a longer diagnostic delay compared with males.
Women with axSpA show significantly lower TNF inhibitor efficacy and drug survival compared with males.
Men have a higher radiological progression, but the disease burden is similar for both sexes.
Introduction
Many rheumatic diseases show a clear sex difference in prevalence, often with female predominating, as in RA and SLE. In contrast, AS or radiological axial SpA (axSpA) is more frequently diagnosed in men compared with women (3:1), whereas non-radiographic axSpA has an equal sex distribution. This sex distribution might be explained by differences in disease course between the two sexes. Men with axSpA show a higher radiological progression (45 vs 33%) [1], whereas women show higher disease activity scores (mean BASDAI 3.2–5.9 vs 3.9–6.3) [1–6] and extra-articular manifestations (73 vs 82%) [1, 7, 8].
Before we start with the myths, a clarification is needed for the terms sex and gender. In essence, the term sex differences can be described as biological processes that differ between men and women [9]. Gender refers to a person’s self-perception as a man or woman and the behaviour they show during their life or the disease (coping style and disease perception) [7], but in some literature the word gender is also used to refer to physiological differences between sexes. This article aims to create awareness of the impact of sex differences in physiological, pharmacokinetics, disease presentation and treatment efficacy of biologics in axSpA.
Myth 1: Men and women with axSpA are physiologically the same
Sex differences in genes and immune modulation
Sex differences are observed not only in sex chromosomes, X and Y, but also in gene expression, immune modulation and physiological processes between men and women with axSpA. The most important genetic predisposition in axSpA is the association with the HLA-B27 allele. There are indications that women with axSpA are found to be less often positive for the HLA-B27 allele compared with males [1], which might explain the different presentation of axSpA in men and women, such as radiological progression [10–15]. The presence of the HLA-B27 allele is associated with a greater chance for a positive MRI of the SI joints [16]. In addition, HLA-B27 was also found to be a predictor for having a positive treatment response and better drug survival on biologics [17–20].
In addition, there are sex differences in other less familiar gene expressions. An interesting study on genetic expression in AS revealed that 1522 unique genes were expressed in men and 291 genes in women compared with healthy controls [21].
A study considering the ANKH gene, which encodes a protein that is involved in osteogenesis and plays a role in ankylosis in AS, showed that different loci of the ANKH gene were expressed in men and women with AS [22]. Furthermore, in multiplex AS families, a specific tissue-non-specific alkaline phosphatase (TNAP) haplotype, which interplays with the ANKH gene in ossification, was associated with AS in men but not in women [23]. These genetic predispositions in men might explain the higher radiographic progression and higher prevalence of AS in men compared with women.
Immune processes are also influenced by sex hormones. Testosterone decreases TNF-α production but increases the production of anti-inflammatory IL-10 [24]. Oestrogens increase the cell-mediated and humoral immune response and production of IL-1, IL-6 and TNF-α [25], which contributes to increased inflammatory values. Interestingly, syndesmophyte development in men was associated with significantly higher IL-18 levels, whereas in women IL-6 was significantly elevated [26].
In AS, IL-17A and Th17 cells were elevated in male patients but not in female patients [15]. However, the same study did not reveal sex differences in the components of the Th1 axis.
Pain mechanisms
Sex hormones also influence other physiological processes, such as pain transmission. Testosterone increases the pain threshold, whereas conflicting results were found for oestrogen and progesterone [27]. Accumulating data reveal that pain sensation fluctuates with hormonal changes, especially in women during the menstrual cycle, in contrast to men who have more stable hormone levels over time [28, 29]. Besides the influence of hormones, women have a greater number of pain receptors and a different expression of these receptors, for instance, in the opioid receptors [29]. This could explain the overall higher pain sensitivity in women compared with men, which might contribute to higher pain scores reported for patient questionnaires by women with rheumatic diseases.
Body composition
In addition, sex differences in body composition influence the immune modulation indirectly, especially due to fat disposition. Women have greater deposits of subcutaneous fat (SAT), whereas men have more visceral fat (VAT), which is located intra-abdominally [30]. Interestingly, adipose tissue acts as an endocrine organ, secreting not only adipokines, which can act as pro-(leptin) or anti-(adiponectin) inflammatory, but also cytokines, such as the pro-inflammatory cytokine TNF-α [31]. One study reported that female patients with higher disease activity scores [Ankylosing Spondylitis Disease Activity Score (ASDAS) and BASDAI] had a significantly higher percentage of body fat (BF) or fat mass index (FMI) [32]. In contrast, men in this study had significantly higher disease activity scores (ASDAS and BASDAI) when they had low BF or FMI [32]. In addition, several studies have reported an association between a high BMI and a lower TNF inhibitor (TNFi) treatment response [33, 34]. In one study a significant correlation was observed between BMI and the inflammatory marker CRP in female AS patients only [4].
Truth: Besides many sex differences in physiological processes, studies in axSpA have also revealed sex differences in gene expression and body composition. In addition, women with axSpA have different pain mechanisms and hormonal influences that might contribute to higher DASs compared with men.
Myth 2: axSpA is a predominately male disease
Sex differences in axSpA
axSpA encompasses non-radiographic axSpA (nr-axSpA) without radiographic changes and AS with radiological signs of sacroiliitis as classified according to the modified New York criteria [35–37]. For many years AS was considered a predominantly male disease. The initial studies showed a male:female ratio of 10:1 [38], but subsequently this ratio has decreased to ∼3:1 [39]. Recent studies report an even further decline in the male:female ratio among patients with axSpA in Switzerland, from 2.57:1 in 1980 to 1.03:1 by the end of 2016 [40]. In contrast with AS, no sex differences have been encountered in the prevalence of nr-axSpA [41].
Delay in diagnosis
Currently, the average delay to diagnosis in AS is ∼6–8 years [42–44]. Although the age of onset of AS is similar for men and women [2, 22, 23], women have a significantly longer delay in diagnosis compared with men (median 9–14 vs 5–7 years) [45, 46]. These data were confirmed by a recent meta-analysis covering 42 studies and 23 889 patients (32.3% women), revealing a significantly longer diagnostic delay in female patients compared with males (8.8 vs 6.5 years) [47]. So far, only one study has revealed a longer diagnostic delay in men compared with women (9.9 vs 6.3 years) [48]. A longer diagnostic delay was found to be a negative predictor for a positive biologic treatment response [49, 50].
Several explanations were put forward for the longer diagnostic delay among females, such as the differences in presenting symptoms, including more enthesitis-related complaints instead of inflammatory back pain, more prominent widespread pain and a lower prevalence of radiographic changes [43, 45]. Importantly, patients with widespread pain, which occurs in at least 25% of female axSpA patients, are sometimes misdiagnosed as fibromyalgia, as it has some overlapping symptoms with axSpA [33]. In fact, one study reported that widespread pain doubled the delay in diagnosis in women [43]. An additional explanation for the difference in diagnostic delay might be the physician’s bias, because axSpA is considered to be a ‘male disease’ [43]. Consequently, women who show more predisposing factors of axSpA [most importantly a positive family history and acute anterior uveitis (AAU)] might have an increased chance of being diagnosed.
AAU is one of the most important extra-articular manifestations of axSpA [51] and axSpA is the most common associated systemic disease in AAU; they also share the same genetic predisposition, the HLA-B27 antigen. AAU can be the first manifestation of axSpA. Approximately half of axSpA patients experience AAU before the onset of axSpA symptoms and, in addition, in patients presenting with AAU, ∼40% appear to suffer from undiagnosed axSpA [52–54]. Male and female axSpA patients have about the same lifetime risk of developing AAU (∼30%) [34, 51, 55]. However, males are more often diagnosed with SpA many years before AAU occurs, whereas the diagnosis in females is significantly more often made after the first attack of AAU [56]. Therefore screening of AAU patients by a rheumatologist, especially in the case of back pain, could reduce the diagnostic delay, especially in women.
Pitfalls in diagnosis
In AS, radiographic changes of the SI joints, graded according the modified New York criteria, are mandatory for the diagnosis. However, some radiological changes of the pelvis are important for the differential diagnoses, especially in women [35]. For example, iliitis condensans, with bilateral sclerotic lesions around the SI joints, is often accompanied by lower back pain and SI joint tenderness and occurs mainly in women after pregnancy [57, 58].
In addition. imaging of the SI joints by MRI, which can substantiate the diagnosis of non-radiographic axSpA by showing active bone marrow lesions, has some pitfalls as well [59]. Recent studies revealed that other factors, such as intensive sporting activities and pregnancy, can induce SI bone marrow oedema as well. Some studies show that up to 1 year after delivery, bone marrow oedema of the SI joints still can be detected [60].
Truth: AxSpA is not a predominately male disease. Diagnoses of axSpA are often missed or misdiagnosed in female patients, resulting in long diagnostic delays. New referral strategies, such as the occurrence of AAU, might decrease the diagnostic delay in female patients. On the other hand, it is important to be aware of diagnostic pitfalls, especially with MRI of the SI joints, since bone marrow oedema up to 1 year after pregnancy could lead to overdiagnosis.
Myth 3: Men with axSpA have a worse disease outcome compared with women
Radiological progression
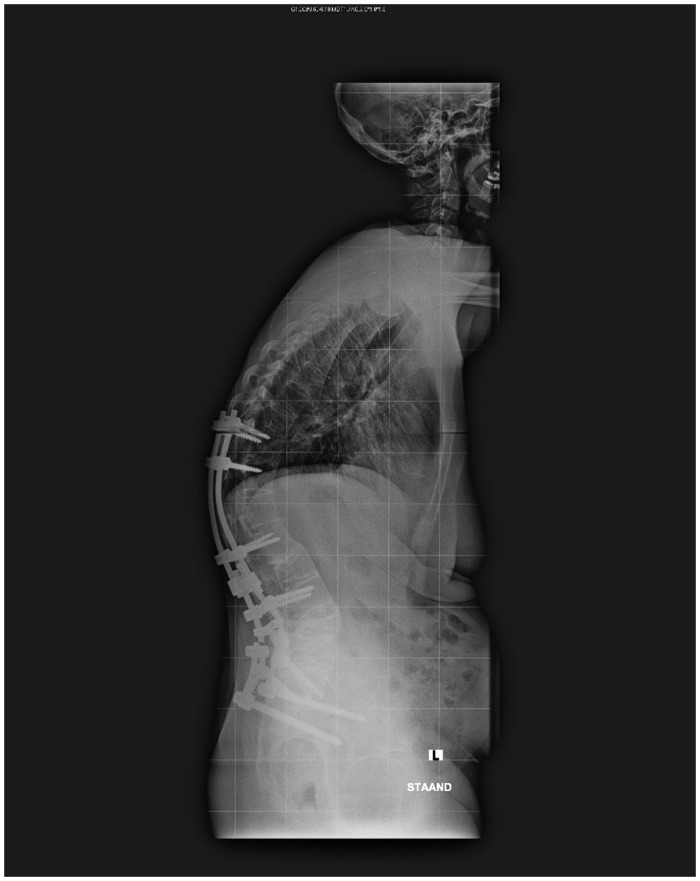
Probably the main reason why men are often considered to have worse disease is the association of male sex with a higher radiological outcome. Most studies have revealed that men are more likely to show worse hip involvement and higher BASRI spine and modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) compared with women [10–15, 61, 62]. However, it is important to note that severe radiographic deformities, including ankylosing, occur in both men and women (Fig. 1). A few studies observed greater radiological progression of the lumbar spine in male AS patients, whereas in female patients this progression was observed mainly in the cervical spine. The fact that most female AS patients seem to have slower radiological progression might be an explanation for the relatively greater number of women diagnosed with nr-axSpA and the longer delay in diagnosis [63]. Comparison studies between nr-axSpA and AS reveal an equal disease burden, independent of sex [64].
Fig. 1.
Woman, 47 years old, with long-standing AS
Extra-articular manifestations and disease manifestations
One of the reasons an equal disease burden is observed and a higher percentage of women are diagnosed with nr-axSpA could be the presence of extra-articular and other disease manifestations, such as enthesitis. Enthesitis was reported to occur more frequently, and be more pronounced, in female patients [1–3, 65–67] (Table 1). In addition, the absence of enthesitis was found to be a predictor for better biologic treatment efficacy [68], which might explain the lower efficacy in female patients.
Table 1.
Extraspinal manifestations and comorbidities in axSpA
| Manifestations and comorbidities | Gender differences |
|---|---|
| Extraspinal manifestations | |
| AAU | No differences |
| Enthesitis | ↑ in women |
| IBD | ↑ in women |
| Psoriasis | ↑ in women |
| Peripheral arthritis | ↑ in women |
| Comorbidities | |
| Cardiovascular diseases | ↑ in men and post-menopausal women |
| Osteoporosis | Equal risk, but underdiagnosis in (young) males |
Extra-articular manifestations seem to have a higher prevalence in women [1, 7, 8] (Table 1), although some studies show conflicting results [43, 61, 66]. Some studies showed a higher prevalence of AAU in men [6, 56, 69], whereas a systemic literature review suggested a somewhat higher prevalence in females (33.3%, vs 28.5% in males) [34]. However, the last study also included other types of uveitis, which could have compromised the results. Three studies, including a meta-analysis, suggested female patients are more likely to develop IBD compared with male patients [1, 8, 51]. In addition, some studies reported a higher risk of psoriasis in female axSpA patients [8, 69].
Comorbidity
Beside extra-articular manifestations, axSpA is also associated with an increased risk of comorbidities, such as cardiovascular diseases and osteoporosis [70]. Unfortunately, cardiovascular diseases in axSpA has not been systematically studied for sex differences (Table 1).
Osteoporosis shows a prevalence range of 19–50%, especially in AS patients with longstanding disease, and can lead to immobilization due to vertebral fractures [71, 72]. Osteoporosis is typically considered a woman’s disease, due to the high prevalence and number of fractures in post-menopausal women compared with men of the same age. For example, at the age of 60 years, the risk in women is 44%, compared with 25% in men [73]. However, in a relatively young male axSpA population with a short disease duration, 51% had a low BMD and 13–16% had osteoporosis [74]. Another study revealed that male patients diagnosed with axSpA had a four times greater risk for low BMD compared with females [75]. A study on osteoporotic fractures in relatively young axSpA patients (mean age 37 years, mean disease duration 7 years) reported at least one osteoporotic fracture in 15% of all patients [76]. Most of these fractures were located at the thoracic spine, which is not included in the regular scoring method of the mSASSS. In relation to peripheral fractures, although women have a higher incidence of fractures, the risk of undertreatment of osteoporosis and mortality after a hip fracture in men is much higher [77, 78].
Inflammatory laboratory values
Studies on sex differences in CRP levels showed significantly higher baseline levels in male patients compared with females [1, 5, 6, 66, 67], but the ESR was inconclusive for sex differences. A possible explanation for finding no clear differences in ESR levels could be the already different cut-off levels for normal ESR levels by sex (15 mm/h for males vs 20 mm/h females).
Disease activity and patient-reported outcomes
In both nr-axSpA and AS, women present themselves in general with higher disease activity, more pain and a worse quality of life (QoL) (Table 2). At baseline, before the start of biologics, BASDAI scores are significantly higher in female patients compared with males, especially the items total back pain, duration of morning stiffness and fatigue [1–3, 5, 6, 65, 66, 79–82]. Interestingly, the ASDAS showed no sex differences [5, 6], which might be due to the fact that men show higher CRP levels, whereas women show higher scores on the other components of the ASDAS-CRP. Sex differences in QoL and overall well-being were inconsistent, depending on the validated questionnaire used. Significantly worse QoL scores were observed in female patients measured with the Ankylosing Spondylitis Quality of Life questionnaire, the Assessment of SpondyloArthritis international Society (ASAS) Health Index and the BAS-G [2, 3, 6, 66, 82]. Other QoL questionnaires, such as the EuroQoL and the 36-item Short Form Health Survey revealed no (large) gender differences [1, 2, 6, 79]. The BASFI showed no large sex differences, except one study that found a higher score in female patients [1] (Table 2).
Table 2.
Sex differences in disease activity, function and physical measures in axSpA
| Disease activity at baseline | Gender differences |
|---|---|
| BASDAI | ↑ in women |
| ASDAS-CRP | No difference |
| CRP-levels | ↑ in men |
| ESR-levels | No difference |
| Function | |
| BASFI | No difference |
| Quality of life | |
| ASQoL | ↓ in women |
| ASASHI | ↓ in women |
| EuroQoL | No difference |
| SF-36 | No difference |
| Physical | |
| BASMI | ↑ in men |
| MASES | ↑ in women |
↑: higher scores; ↓: lower scores; ASQoL: Ankylosing Spondylitis Quality of Life; ASASHI: Assessment of SpondyloArthritis international Society Health Index; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score.
Truth: Overall, men with axSpA show a higher rate of radiological progression compared with women, but severe ankylosis also occurs in female axSpA patients. Women with axSpA have, in general, higher disease activity scores and more peripheral manifestations compared with men. Comorbidities like cardiovascular events have not been studied for sex differences in axSpA, but osteoporosis, even with osteoporotic fractures, a manifestation mainly seen in post-menopausal women, has an unexpectedly high prevalence in young male axSpA patients.
Myth 4: No sex differences are present in efficacy and drug survival of biologics in axSpA
Sex differences in response and efficacy to biologic treatment
Two recent reviews [7, 83] described sex differences in treatment efficacy, but most clinical studies and safety trials are not powered to assess sex differences. For this reason, data from several randomized controlled trials (RCTs) on one biologic, etanercept, were pooled and analysed for sex differences, as the RCT studies separately included too few women to perform the analyses. This study revealed a significantly lower treatment response at 12 weeks according to the BASDAI score in female patients compared with males (−19.2 vs −23.4) [79] (Table 3). In addition, women also had a lower ASDAS-CRP response compared with men (68.4 vs 89.4%) at 12 weeks [5] (Table 3). Currently only two other studies have assessed disease activity for sex differences [83]. A prospective cohort study including the TNFis etanercept, adalimumab, infliximab and golimumab revealed, according to adjusted longitudinal regression analyses for repeated measurements, a significantly higher mean BASCAI score for women (0.9) over a 5 year follow-up period. However, no significant sex differences were observed in the longitudinal analyses for mean ASDAS-CRP. A possible explanation could be because of the high CRP level in men and the higher scores on BASDAI components in women [84]. However, assessment of the ASDAS-CRP clinical response revealed that men achieved the clinical response twice as often as women. The second study included the IL-17 blocker secukinumab and demonstrated no sex differences in treatment response at both 16 (46.9% for men vs 37.5% for women) and 52 weeks (61.7% for men vs 68.4% for women) [49]. Besides differences in response and efficacy, male sex was found to be a predictor for improvement of function (69.9% for men vs 50.0% for women) [50].
Table 3.
Sex differences in efficacy and time on drug in axSpA
| Disease activity (mean) | Differences (range 6–60 months) |
| BASDAI | Remains higher over time in females |
| ASDAS-CRP | No observed differences |
| CRP level | Remains higher over time in males |
| ESR level | No observed differences |
| Treatment response | Differences (range 6–60 months) |
| BASDAI 50% | ↓ in females |
| ASDAS-CRPa | ↓ in females |
| ASAS20/40 | ↓ in females |
| Drug survival | Differences (range 12 weeks–10 years) |
| Time on drug | ↓ in female patients |
| Switch | ↑ in female patients |
Clinically important improvement (ASDAS-CRP ≥ 1.1).
↑: higher scores; ↓: lower scores.
Sex and gender differences in time on drug
In addition to treatment efficacy and response, the reviews also described a clear sex difference in drug survival. Most studies that investigated sex differences in biologic found a significantly lower time on drug in women compared with men, except for the secukinumab study [5, 80, 85–89]. The studies revealed a doubled risk for treatment failure in female patients. A recent study found that 31.1% of males experienced a treatment failure compared with 50.0% of females [50].
Biologics and peripheral manifestations
Although few studies have investigated sex differences separately, a greater number of studies have assessed sex as a possible predictor in relation to treatment efficacy and drug survival [49, 50]. Studies including sex differences in their analyses also described several predictors for treatment efficacy and drug survival, such as presence of HLA-B27 antigen, being TNFi naive, short disease duration and absence of enthesitis [17, 18]. Remarkably, these factors are less prevalent among female patients, as women are less likely to be HLA-B27 positive, more often have enthesitis and a longer disease duration and are less often biologic naive compared with men [1–3, 5, 47, 62, 65–67, 83, 90, 91]. In addition, female patients have a greater fat mass, which is associated with a lower TNFi treatment response [92]. This might be an explanation for the fact that women were found to have a shorter drug survival on biologics compared with men.
Truth: There is substantial evidence found in different studies indicating women have a significantly lower efficacy, response rate and drug survival for TNFis compared with men. Data on sex differences in other biologics, such as IL-17 inhibitors, are limited.
Conclusion
In axSpA, sex differences play a role in biologic processes such as immune responses, pain mechanisms and disease manifestations, such as involvement of the entheses, and disease course, such as radiological progression. Osteoporosis can be overlooked in men and pregnancies can hamper the diagnostic process with pelvis X-rays and MRI of the SI joints. Substantial sex differences were observed in lower TNFi efficacy and drug survival in women compared with men but remain to be determined in other biologics. In conclusion, it is of great importance to be aware of the sex differences in axSpA for diagnosis as well as treatment.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article. This paper was published as part of a supplement funded by Novartis.
Disclosure statement: IEvdH-B has received consulting fees, research or institutional support and educational grants from AbbVie, Eli Lilly, Bristol-Myers Squibb, MSD, Novartis, Pfizer and UCB Pharma. The other authors have declared no conflicts of interest.
References
- 1. Tournadre A, Pereira B, Lhoste A et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res (Hoboken) 2013;65:1482–9. [DOI] [PubMed] [Google Scholar]
- 2. de Carvalho HM, Bortoluzzo AB, Goncalves CR et al. Gender characterization in a large series of Brazilian patients with spondyloarthritis. Clin Rheumatol 2012;31:687–95. [DOI] [PubMed] [Google Scholar]
- 3. Landi M, Maldonado-Ficco H, Perez-Alamino R et al. Gender differences among patients with primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease in an iberoamerican spondyloarthritis cohort. Medicine (Baltimore) 2016;95:e5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubio Vargas R, van den Berg R, van Lunteren M et al. Does body mass index (BMI) influence the Ankylosing Spondylitis Disease Activity Score in axial spondyloarthritis? Data from the SPACE cohort. RMD Open 2016;2:e000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Horst-Bruinsma IE, Zack DJ, Szumski A, Koenig AS. Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann Rheum Dis 2013;72:1221–4. [DOI] [PubMed] [Google Scholar]
- 6. Webers C, Essers I, Ramiro S et al. Gender-attributable differences in outcome of ankylosing spondylitis: long-term results from the Outcome in Ankylosing Spondylitis International Study. Rheumatology (Oxford) 2016;55:419–28. [DOI] [PubMed] [Google Scholar]
- 7. Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zarco P, Gonzalez CM, Rodriguez de la Serna A et al. Extra-articular disease in patients with spondyloarthritis. Baseline characteristics of the spondyloarthritis cohort of the AQUILES study. Reumatol Clin 2015;11:83–9. [DOI] [PubMed] [Google Scholar]
- 9. Tannenbaum C, Day D, Matera A. Age and sex in drug development and testing for adults. Pharmacol Res 2017;121:83–93. [DOI] [PubMed] [Google Scholar]
- 10. Boonen A, vander Cruyssen B, de Vlam K et al. Spinal radiographic changes in ankylosing spondylitis: association with clinical characteristics and functional outcome. J Rheumatol 2009;36:1249–55. [DOI] [PubMed] [Google Scholar]
- 11. Lee W, Reveille JD, Davis JC Jr et al. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann Rheum Dis 2007;66:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramiro S, van der Heijde D, van Tubergen A et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 13. Rudwaleit M, Haibel H, Baraliakos X et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 14. van Tubergen A, Ramiro S, van der Heijde D et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012;71:518–23. [DOI] [PubMed] [Google Scholar]
- 15. Ward MM, Hendrey MR, Malley JD et al. Clinical and immunogenetic prognostic factors for radiographic severity in ankylosing spondylitis. Arthritis Rheum 2009;61:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung HY, Machado P, van der Heijde D, D’Agostino MA, Dougados M. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011;70:1930–6. [DOI] [PubMed] [Google Scholar]
- 17. Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum 2017;47:343–50. [DOI] [PubMed] [Google Scholar]
- 18. Pavelka K, Forejtova S, Stolfa J et al. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol 2009;27:958–63. [PubMed] [Google Scholar]
- 19. Arends S, Brouwer E, van der Veer E et al. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 2011;13:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glintborg B, Sorensen IJ, Ostergaard M et al. Ankylosing spondylitis versus nonradiographic axial spondyloarthritis: comparison of tumor necrosis factor inhibitor effectiveness and effect of HLA-B27 status. An observational cohort study from the nationwide DANBIO registry. J Rheumatol 2017;44:59–69. [DOI] [PubMed] [Google Scholar]
- 21. Gracey E, Yao Y, Green B et al. Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol 2016;68:679–89. [DOI] [PubMed] [Google Scholar]
- 22. Tsui HW, Inman RD, Paterson AD, Reveille JD, Tsui FW. ANKH variants associated with ankylosing spondylitis: gender differences. Arthritis Res Ther 2005;7:R513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsui HW, Inman RD, Reveille JD, Tsui FW. Association of a TNAP haplotype with ankylosing spondylitis. Arthritis Rheum 2007;56:234–43. [DOI] [PubMed] [Google Scholar]
- 24. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol 2019;56:308–21. [DOI] [PubMed] [Google Scholar]
- 25. Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: sex matters. Clin Immunol 2015;159:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang WN, Tso TK, Kuo YC, Tsay GJ. Distinct impacts of syndesmophyte formation on male and female patients with ankylosing spondylitis. Int J Rheum Dis 2012;15:163–8. [DOI] [PubMed] [Google Scholar]
- 27. Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res 2017;95:1271–81. [DOI] [PubMed] [Google Scholar]
- 28. Manson JE. Pain: sex differences and implications for treatment. Metabolism 2010;59:S16–20. [DOI] [PubMed] [Google Scholar]
- 29. Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: modulatory role of gonadal hormones. Life Sci 2019;237:116926. [DOI] [PubMed] [Google Scholar]
- 30. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dias S, Paredes S, Ribeiro L. Drugs involved in dyslipidemia and obesity treatment: focus on adipose tissue. Int J Endocrinol 2018;2018:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ibanez Vodnizza S, Visman IM, van Denderen C et al. Muscle wasting in male TNF-α blocker naive ankylosing spondylitis patients: a comparison of gender differences in body composition. Rheumatology (Oxford) 2017;56:1566–72. [DOI] [PubMed] [Google Scholar]
- 33. Aloush V, Ablin JN, Reitblat T, Caspi D, Elkayam O. Fibromyalgia in women with ankylosing spondylitis. Rheumatol Int 2007;27:865–8. [DOI] [PubMed] [Google Scholar]
- 34. Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis 2008;67:955–9. [DOI] [PubMed] [Google Scholar]
- 35. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 36. Rudwaleit M, van der Heijde D, Landewe R et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 37. Rudwaleit M, van der Heijde D, Landewe R et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 38. West HF. Aetiology of ankylosing spondylitis. Ann Rheum Dis 1949;8:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kennedy LG, Will R, Calin A. Sex ratio in the spondyloarthropathies and its relationship to phenotypic expression, mode of inheritance and age at onset. J Rheumatol 1993;20:1900–4. [PubMed] [Google Scholar]
- 40. Baumberger HK. SAT0417 Gradual progressive change to equal prevalence of ankylosing spondylitis among males and females in Switzerland: data from the Swiss ankylosing spondylitis society (SVMB). Ann Rheum Dis 2017;76:929. [Google Scholar]
- 41. Sieper J, van der Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013;65:543–51. [DOI] [PubMed] [Google Scholar]
- 42. Seo MR, Baek HL, Yoon HH et al. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol 2015;34:1397–405. [DOI] [PubMed] [Google Scholar]
- 43. Slobodin G, Reyhan I, Avshovich N et al. Recently diagnosed axial spondyloarthritis: gender differences and factors related to delay in diagnosis. Clin Rheumatol 2011;30:1075–80. [DOI] [PubMed] [Google Scholar]
- 44. Zwolak R, Suszek D, Graca A, Mazurek M, Majdan M. Reasons for diagnostic delays of axial spondyloarthritis. Wiad Lek 2019;72(9 cz 1):1607–10. [PubMed] [Google Scholar]
- 45. Redeker I, Callhoff J, Hoffmann F et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology (Oxford) 2019;58:1634–8. [DOI] [PubMed] [Google Scholar]
- 46. Calin A, Elswood J, Rigg S, Skevington SM. Ankylosing spondylitis—an analytical review of 1500 patients: the changing pattern of disease. J Rheumatol 1988;15:1234–8. [PubMed] [Google Scholar]
- 47. Jovani V, Blasco-Blasco M, Ruiz-Cantero MT, Pascual E. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and metaanalysis. J Rheumatol 2017;44:174–83. [DOI] [PubMed] [Google Scholar]
- 48. Bandinelli F, Salvadorini G, Delle Sedie A et al. Impact of gender, work, and clinical presentation on diagnostic delay in Italian patients with primary ankylosing spondylitis. Clin Rheumatol 2016;35:473–8. [DOI] [PubMed] [Google Scholar]
- 49. Horst-Bruinsma I, Richard CM, Braun J et al. FRI0418 secukinumab provided similar efficacy in males and females with active ankylosing spondylitis over 52 weeks: post hoc pooled analysis of the measure trials. Ann Rheum Dis 2019;78(Suppl 2):897–8. [Google Scholar]
- 50. Lubrano E, Perrotta FM, Manara M et al. Improvement of function and its determinants in a group of axial spondyloarthritis patients treated with TNF inhibitors: a real-life study. Rheumatol Ther 2020;7:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. [DOI] [PubMed] [Google Scholar]
- 52. Haroon M, O’Rourke M, Ramasamy P, Murphy CC, FitzGerald O. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool). Ann Rheum Dis 2015;74:1990–5. [DOI] [PubMed] [Google Scholar]
- 53. Wach J, Maucort-Boulch D, Kodjikian L et al. Acute anterior uveitis and undiagnosed spondyloarthritis: usefulness of Berlin criteria. Graefes Arch Clin Exp Ophthalmol 2015;253:115–20. [DOI] [PubMed] [Google Scholar]
- 54. Wendling D, Prati C, Demattei C et al. Impact of uveitis on the phenotype of patients with recent inflammatory back pain: data from a prospective multicenter French cohort. Arthritis Care Res (Hoboken) 2012;64:1089–93. [DOI] [PubMed] [Google Scholar]
- 55. Frantz C, Portier A, Etcheto A et al. Acute anterior uveitis in spondyloarthritis: a monocentric study of 301 patients. Clin Exp Rheumatol 2019;37:26–31. [PubMed] [Google Scholar]
- 56. Braakenburg AM, de Valk HW, de Boer J, Rothova A. Human leukocyte antigen-B27-associated uveitis: long-term follow-up and gender differences. Am J Ophthalmol 2008;145:472–9. [DOI] [PubMed] [Google Scholar]
- 57. Jenks K, Meikle G, Gray A, Stebbings S. Osteitis condensans ilii: a significant association with sacroiliac joint tenderness in women. Int J Rheum Dis 2009;12:39–43. [DOI] [PubMed] [Google Scholar]
- 58. Mitra R. Osteitis condensans ilii. Rheumatol Int 2010;30:293–6. [DOI] [PubMed] [Google Scholar]
- 59. Rudwaleit M, Jurik AG, Hermann KG et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 60. de Winter J, de Hooge M, van de Sande M et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of SpondyloArthritis International Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol 2018;70:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cansu DÜ, Çalışır C, Savaş Yavaş U, Kaşifoğlu T, Korkmaz C. Predictors of radiographic severity and functional disability in Turkish patients with ankylosing spondylitis. Clin Rheumatol 2011;30:557–62. [DOI] [PubMed] [Google Scholar]
- 62. Jung Y-O, Kim I, Kim S et al. Clinical and radiographic features of adult-onset ankylosing spondylitis in Korean patients: comparisons between males and females. J Korean Med Sci 2010;25:532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montilla C, Diaz-Alvarez A, Calero-Paniagua I et al. Ankylosing spondylitis without axial progression: analysis of associated factors. J Rheumatol 2014;41:2409–12. [DOI] [PubMed] [Google Scholar]
- 64. Boonen A, Sieper J, van der Heijde D et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556–62. [DOI] [PubMed] [Google Scholar]
- 65. Ibn Yacoub Y, Amine B, Laatiris A, Hajjaj-Hassouni N. Gender and disease features in Moroccan patients with ankylosing spondylitis. Clin Rheumatol 2012;31:293–7. [DOI] [PubMed] [Google Scholar]
- 66. Shahlaee A, Mahmoudi M, Nicknam MH et al. Gender differences in Iranian patients with ankylosing spondylitis. Clin Rheumatol 2015;34:285–93. [DOI] [PubMed] [Google Scholar]
- 67. Lubrano E, Perrotta FM, Manara M et al. The sex influence on response to tumor necrosis factor-alpha inhibitors and remission in axial spondyloarthritis. J Rheumatol 2018;45:195–201. [DOI] [PubMed] [Google Scholar]
- 68. Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum 2017;47:343–50. [DOI] [PubMed] [Google Scholar]
- 69. Mitulescu TC, Popescu C, Naie A et al. Acute anterior uveitis and other extra-articular manifestations of spondyloarthritis. J Med Life 2015;8:319–25. [PMC free article] [PubMed] [Google Scholar]
- 70. Heslinga SC, Van den Oever IA, Van Sijl AM et al. Cardiovascular risk management in patients with active ankylosing spondylitis: a detailed evaluation. BMC Musculoskelet Disord 2015;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. El Maghraoui A, Borderie D, Cherruau B et al. Osteoporosis, body composition, and bone turnover in ankylosing spondylitis. J Rheumatol 1999;26:2205–9. [PubMed] [Google Scholar]
- 72. Ghozlani I, Ghazi M, Nouijai A et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone 2009;44:772–6. [DOI] [PubMed] [Google Scholar]
- 73. Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res 2007;22:781–8. [DOI] [PubMed] [Google Scholar]
- 74. van der Weijden MA, Claushuis TA, Nazari T et al. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol 2012;31:1529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Weijden MA, van Denderen JC, Lems WF et al. Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthropathies. Clin Rheumatol 2011;30:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van der Weijden MA, van der Horst-Bruinsma IE, van Denderen JC et al. High frequency of vertebral fractures in early spondylarthropathies. Osteoporos Int 2012;23:1683–90. [DOI] [PubMed] [Google Scholar]
- 77. Haentjens P, Magaziner J, Colon-Emeric CS et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010;152:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kiebzak GM, Beinart GA, Perser K et al. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002;162:2217–22. [DOI] [PubMed] [Google Scholar]
- 79. Roussou E, Sultana S. Spondyloarthritis in women: differences in disease onset, clinical presentation, and Bath Ankylosing Spondylitis Disease Activity and Functional indices (BASDAI and BASFI) between men and women with spondyloarthritides. Clin Rheumatol 2011;30:121–7. [DOI] [PubMed] [Google Scholar]
- 80. Glintborg B, Ostergaard M, Krogh NS et al. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002–8. [DOI] [PubMed] [Google Scholar]
- 81. Kristensen LE, Karlsson JA, Englund M et al. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 2010;62:1362–9. [DOI] [PubMed] [Google Scholar]
- 82. Ibáñez Vodnizza SE, van Bentum RE, Valenzuela O, van der Horst-Bruinsma IE. Patients with axial spondyloarthritis report significant differences between men and women and high impact of the disease: large websurvey analysis. Joint Bone Spine 2020;87:315–9. [DOI] [PubMed] [Google Scholar]
- 83. Maneiro JR, Souto A, Salgado E, Mera A, Gomez-Reino JJ. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 2015;1:e000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rusman T, Nurmohamed M, Denderen J, Visman I, van der Horst-Bruinsma IE. THU0391 female gender is associated with a poorer response to TNF inhibitors in ankylosing spondylitis. Ann Rheum Dis 2017;76(Suppl 2):354–5. [Google Scholar]
- 85. Flouri ID, Markatseli TE, Boki KA et al. Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts longterm drug persistence. J Rheumatol 2018;45:785–94. [DOI] [PubMed] [Google Scholar]
- 86. Glintborg B, Ostergaard M, Krogh NS et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor alpha inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2013;72:1149–55. [DOI] [PubMed] [Google Scholar]
- 87. Rusman T, ten Wolde S, Euser SM et al. Gender differences in retention rate of tumor necrosis factor alpha inhibitor treatment in ankylosing spondylitis: a retrospective cohort study in daily practice. Int J Rheum Dis 2018;21:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hebeisen M, Neuenschwander R, Scherer A et al. Response to tumor necrosis factor inhibition in male and female patients with ankylosing spondylitis: data from a Swiss cohort. J Rheumatol 2018;45:506–12. [DOI] [PubMed] [Google Scholar]
- 89. Al Arashi W, Iniguez Ubiaga C, Hensor EM et al. Comment on: Tumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis—findings from a United Kingdom cohort. Rheumatol Adv Pract 2018;2:rky036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ortolan A, van Lunteren M, Ramiro S et al. Are gender-specific approaches needed in diagnosing early axial spondyloarthritis? Data from the SPondyloArthritis Caught Early cohort. Arthritis Res Ther 2018;20:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sepriano A, Ramiro S, van der Heijde D et al. What is axial spondyloarthritis? A latent class and transition analysis in the SPACE and DESIR cohorts. Ann Rheum Dis 2020;79:324–31. [DOI] [PubMed] [Google Scholar]
- 92. Ibanez Vodnizza SE, Nurmohamed MT, Visman IM et al. Fat mass lowers the response to tumor necrosis factor-alpha blockers in patients with ankylosing spondylitis. J Rheumatol 2017;44:1355–61. [DOI] [PubMed] [Google Scholar]