Abstract
Context
The effects of physiological improvements on cognitive function among persons with type 2 diabetes mellitus (T2DM) are not fully understood.
Objective
To determine whether improvements in physiological markers (body weight, blood sugar control, and physical activity) during intensive lifestyle intervention (ILI) are associated with enhancements in cognitive function in older adults with T2DM.
Design
Multisite randomized controlled trial.
Setting
Academic research centers.
Patients or Other Participants
Participants were aged 45–76 years, with T2DM.
Intervention
The Action for Health in Diabetes (Look AHEAD) study, a randomized, controlled clinical trial of ILI.
Main Outcome Measure
Two to 3 cognitive assessments were collected from 1089 participants, the first and last occurring a mean (standard deviation) of 8.6 (1.0) and 11.5 (0.7) years after enrollment.
Results
Greater improvement in blood sugar control was associated with better cognitive scores (fasting glucose and Rey Auditory Verbal Learning Test [AVLT]: P = 0.0148; fasting glucose and Digit Symbol Coding (DSC): P = 0.0360; HbA1C and DSC: P = 0.0477); but weight loss had mixed associations with cognitive scores (greater body mass index [BMI] reduction and worse AVLT overall: P = 0.0053; and greater BMI reduction and better DSC scores among those overweight but not obese at baseline: P = 0.010). Associations were strongest among those who were overweight (not obese) at baseline, and among those with a history of cardiovascular disease (CVD) at baseline.
Conclusions
Improvements in glycemic control, but not necessarily weight status, during ILI may be associated with better subsequent cognitive performance. These associations may differ by adiposity and CVD history.
Keywords: lifestyle intervention, cognitive function, weight loss, glycemic control, type 2 diabetes, physical activity
Type 2 diabetes mellitus (T2DM) is present in over 25% of US adults aged 65 or older (1, 2). Type 2 diabetes mellitus doubles the risk of cognitive impairment and dementia (including Alzheimer’s disease) and greatly increases health care needs and costs (3). A large body of evidence has established that improvement in glycemic control is attainable in T2DM (4–9), as is significant weight loss (10–12). Such improvements in physiological markers are associated with a reduced risk of several long-term adverse outcomes, including cardiovascular events, retinopathy, nephropathy, and neuropathy (13–15). These data have led many to hope that the effective treatment of T2DM could reduce the elevated risk of cognitive impairment associated with T2DM back down to levels typical of individuals free of T2DM. Such risk reduction could have a major impact on the global case burden of dementia, which is high and expected to rise steadily over the coming decades (16, 17).
But there is limited evidence that effectively treating T2DM reduces the risk of cognitive impairment. Evidence from shorter-term T2DM or pre-T2DM interventions (eg, roughly 9 months or less) targeting diet, physical activity (PA), or glycemic control largely supports the notion that cognitive functioning benefits over the course of such interventions (18–25), with few exceptions (26). But evidence from longer-term interventions (eg, greater than 1 year) and longer postintervention follow-up intervals is mixed. A small number of well-designed, effective, long-term interventions have noted cognitive benefits within the active treatment group over the course of the intervention (27), but several have also shown a lack of such benefit (28–31). Indeed, recent definitive systematic reviews have cited the lack of clarity about long-term cognitive effects of T2DM interventions as a major unmet need (32). This lack of clear cognitive benefit has prevented a clear understanding of the ultimate impact of long-term, effective T2DM treatment on dementia prevalence.
The gap in knowledge about the long-term cognitive impact of T2DM treatment arises from an incomplete understanding of the complex biological processes that connect T2DM to cognitive decline (33–38). Adverse health behaviors (eg, poor diet consumption) induce adverse peripheral changes (eg, fat accumulation, chronic hyperglycemia) as well as adverse changes to the brain (eg, cerebrovascular dysfunction). Peripheral and brain changes exacerbate each other in complex ways that to date are not fully understood. Brain changes may culminate in declines in basic cognitive skills and disruption of affective processes, which in turn may promote the adverse health behaviors. Whether adverse brain changes are driven primarily by mechanisms related to excess fat carriage, glycemic control, PA, or common pathways is not well understood, and it is unclear what specific effects modifying each peripheral factor might have in reducing the risk of adverse brain changes. It is further unclear whether adverse brain changes advance to a “point of no return” after which modification is no longer possible. These complex interactions may be why reports of associations between physiological markers and markers of brain health have been inconsistent (39–46).
This study sought to address this knowledge gap using data from the Action For Health In Diabetes (Look AHEAD) study. The Look AHEAD study tested the relative effectiveness of an intensive lifestyle intervention (ILI) to promote and maintain weight loss and increase PA compared to a diabetes support and education (DSE) control condition (47, 48). Prior analyses have reported that ILI–DSE differences in cognitive measures after 8 to 11 years of follow-up, as well as prevalence of mild cognitive impairment (MCI) and dementia, differed significantly by baseline body mass index (BMI) status and clinical history of cardiovascular disease (CVD) (47–50), with hints that ILI might be beneficial or harmful depending on these baseline variables. This study was designed to determine whether greater improvements in glycemic control, weight, and PA over follow-up were associated with greater cognitive benefits. Our hypothesis was that greater improvements in indicators of blood sugar control, greater increases in PA, and greater weight loss would lead to better subsequent measures of cognitive functioning.
Research Design and Methods
Look AHEAD study design
The design and methods of Look AHEAD have been published previously (51), as have its CONSORT diagram and primary results (12). It was a single-masked randomized, controlled trial that recruited 5145 individuals during 2001 to 2004 with a BMI > 25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin A1c (HbA1c) < 11%, systolic/diastolic blood pressure < 160/100 mmHg, and triglycerides < 600 mg/dl. During the screening process, each prospective participant was required to complete a 2-week run-in, during which they were asked to successfully record information each day about diet and PA. In addition, each participant met with a behavioral psychologist or interventionist to confirm that they understood intervention requirements and to exclude any participant with significant competing life stressors or other issues (depression, alcohol abuse) likely to impair adherence. Participants provided written informed consent. Local Institutional Review Boards approved the protocols.
Look AHEAD intervention
Participants were randomly assigned with equal probability to the ILI or DSE groups. The multidomain ILI included diet modification and PA designed to induce weight loss to an average > 7% at 1 year and maintain this over time (52). Intensive lifestyle intervention participants were assigned a daily calorie goal (1200–1800 based on initial weight), with < 30% of total calories from fat (<10% from saturated fat) and a minimum of 15% of total calories from protein. The PA goal was > 175 minutes per week through activities similar in intensity to brisk walking.
Diabetes support and education participants were invited (but not required) to attend 3 group sessions each year, which focused on diet, PA, and social support (53). These individuals did not receive specific diet, activity, or weight goals—or information on behavioral change strategies.
Interventions were terminated in September 2012. The mean (range) length of intervention for both ILI and DSE participants reported in this manuscript were both 9.9 (8.4–11.0) years.
Subsamples provide cognitive measurements
The Look AHEAD Movement and Memory study invited 1232 Look AHEAD participants at 4 of its 16 centers to participate in an ancillary study to assess cognitive function at follow-up years 8 or 9. Only those who were currently active (ie, had not been lost to follow-up or refused further involvement) and who provided separate informed consent were eligible. A total of 978 individuals enrolled in that ancillary study and provided the cognitive measurements listed below. In addition, the Look AHEAD Brain MRI study invited 875 Look AHEAD participants at 3 of its 16 centers to assess brain structure and function at either follow-up years 10, 11, or 12. A total of 321 individuals enrolled in that study and provided cognitive measurements. Finally, the Look AHEAD Continuation study was offered to all Look AHEAD participants at all 16 centers who were still active at follow-up years 10 through 13. A total of 3751 participants provided cognitive assessments as part of that study. Of the 5145 individuals who were originally randomized by the Look AHEAD study, 3920 participants provided at least 1 cognitive assessment through the 3 ancillary studies. Of those, 2831 provided 1 cognitive test, 774 provided 2 cognitive assessments, and 315 provided 3 cognitive assessments. The current analysis includes only the 1089 individuals who provided 2 or 3 cognitive assessments. Participants at sites that conducted multiple substudies were allowed to be enrolled in more than 1 substudy, but no participant completed cognitive testing more than once per 12-month period.
Assessment of physiological markers
Certified clinic staff, masked to intervention assignment, collected data (51). Digital scales were used throughout follow-up to obtain annual measures of weight. The Paffenbarger Physical Activity Questionnaire was used to estimate weekly minutes of moderate to vigorous PA at enrollment and 4 and 8 years later in a subset of participants (54). The subset came from selected clinical sites that included this questionnaire as part of their assessments. Data collected on the flights of stairs climbed, distance walked, and other fitness, sport, and recreational activities performed during the week prior to the assessment visit were used to compute kcal/week of leisure-time PA. Blood specimens were collected after a > 12-hour fast and analyzed centrally for HbA1c and fasting glucose.
Assessment of cognitive function
Centrally trained, certified, and masked staff conducted standardized assessments of cognitive function across years 8 to 13 of follow-up (55). All cognitive tests were performed after the participant had provided a fasting blood draw and subsequently had a snack; cognitive testing was performed prior to procedures that required physical exertion. Verbal learning and memory were evaluated with the Rey Auditory Verbal Learning Test (AVLT). Speed of processing and working memory were evaluated with the Digit Symbol Coding (DSC) test. Executive function was evaluated with the Modified Stroop Color and Word Test and the Trail Making Test-Part B. Global cognitive functioning was evaluated with the Modified Mini-Mental Status Exam. Test scores were standardized, using z-scores, by subtracting the overall cohort-wide mean of the initial assessments from the individual test score and dividing this by the standard deviation. Scores were ordered so that higher scores reflected better performance (49). The primary cognitive measure for the Look AHEAD trial was an average of these z-scores (composite cognitive function). Among individuals analyzed in this study, the first and last cognitive assessments occurred a mean (standard deviation [SD]) of 8.6 (1.0) and 11.5 (0.7) years after enrollment.
Statistical analysis
Analyses were limited to the 1089 participants who had repeat (ie, 2 or 3) cognitive assessments (which were only done at 6 of the 16 Look AHEAD clinical sites) (55). Demographic characteristics of the sample with repeated cognitive tests are reported by mean (SD) and tested for difference using the Student’s t-test for continuous and frequency (%) and chi-square tests for categorical measures. Mean (SD) and median (interquartile range) are presented for first, second, and third cognitive scores. We examined the association between the change over follow-up in physiological markers (BMI, self-reported leisure-time PA, HbA1c, and fasting plasma glucose) and standardized cognitive scores in mixed effect models adjusted for baseline level of the T2DM marker, randomization arm, age, sex, race/ethnicity, education, clinical site, years from randomization, number of prior cognitive assessments, and the correlation between repeated measurements of the cognitive score. In this analysis, only those measurements of physiological markers collected at clinical evaluations between the baseline evaluation and the clinical evaluation prior to commencement of cognitive testing were utilized. The last analyzed physiological measurements occurred approximately 1.6 to 1.7 years on average prior to the first analyzed cognitive test (see Table 1). Each participant was clinically evaluated approximately once per year over the entire duration of follow-up. Interaction analyses assessed differences in these relationships by randomization arm, obesity category at enrollment (overweight vs obese) and preintervention history of CVD. All associations with a P-value less than 0.05 were considered statistically significant. For each physiological marker and cognitive score, values that were greater than 3 times the interquartile range below or above the first or third quartile were defined as outliers. After fitting our primary models, we removed all such outliers from the data set, refitted all statistical models, and evaluated the effect of outlier removal on findings reported by the models. We also refitted all models that used change in BMI, fasting glucose, and HbA1C as the primary predictor of interest, within the subsample of individuals that provided PA data via the Paffenbarger Physical Activity Questionnaire. We evaluated differences in model coefficients between the full sample and the PA subsample.
Table 1.
Participant characteristics at the time of randomization, broken out by randomization group
| Intensive Lifestyle Intervention (N = 554) | Diabetes Support and Education (N = 536) | P-value | |
|---|---|---|---|
| Age, mean ± SD, years | 58.5 ± 6.8 | 58.2 ± 6.6 | 0.4583 |
| Gender, No. (%) | 0.9796 | ||
| Male | 228 (41.2%) | 221 (41.2%) | |
| Female | 326 (58.8%) | 315 (58.8%) | |
| Race, No. (%) | 0.6505 | ||
| African American | 113 (20.4%) | 111 (20.7%) | |
| American Indian | 5 (0.9%) | 2 (0.4%) | |
| Hispanic | 18 (3.2%) | 23 (4.3%) | |
| Non-Hispanic White | 404 (72.9%) | 383 (71.5%) | |
| Other | 14 (2.5%) | 17 (3.2%) | |
| Education, No. (%) | 0.0482 | ||
| High school | 281 (50.7%) | 280 (52.2%) | |
| College graduate | 133 (24.0%) | 112 (20.9%) | |
| Postcollege | 123 (22.2%) | 110 (20.5%) | |
| Othera | 17 (3.1%) | 34 (6.3%) | |
| BMI, mean ± SD, kg/m2 | 35.6 ± 5.9 | 35.9 ± 5.7 | 0.3515 |
| Obesity group (kg/m2), No. (%) | 0.0283 | ||
| BMI < 30kg/m2 | 99 (17.9%) | 70 (13.1%) | |
| BMI ≥ 30kg/m2 | 455 (82.1%) | 466 (86.9%) | |
| Paffenbarger, mean ± SD, kcal/week | 780.5 ± 958.8 | 853.1 ± 1070 | 0.3325 |
| HbA1c, mean ± SD, % | 7.2 ± 1.1 | 7.2 ± 1.1 | 0.8112 |
| Glucose, mean ± SD, mg/dL | 150.1 ± 45.8 | 149.5 ± 42.1 | 0.8098 |
| Prior CVD, No. (%) | 0.0320 | ||
| No | 471 (85.0%) | 479 (89.4%) | |
| Yes | 83 (15.0%) | 57 (10.6%) | |
| Time from randomization to first cognitive test | 8.6 ± 1.0 | 8.6 ± 1.0 | 0.7968 |
| Time between last analyzed physiological measurement and first cognitive test | 1.6 ± 1.1 | 1.7 ± 1.1 | 0.3363 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; SD, standard deviation.
a “Other” educational attainment referred to a highest level of educational attainment that was less than a high school diploma or general education development (GED), or did not fit into any of the following categories: high school diploma or equivalency (GED), some vocational school, some college, associate degree (junior college), Bachelor’s degree, some graduate school, Master’s degree, doctorate, professional (MD, JD, DDS, etc.).
Results
Characteristics of individuals who received multiple cognitive assessments are shown in Table 1. This sample was fairly well matched across intervention arms (51% ILI, 49% DSE), and there were no statistically significant differences between treatment groups in demographic variables, including age, sex, and race. Within this sample, ILI had slightly higher level of educational attainment (P = 0.048), were less likely to be obese at baseline (ILI: 82%, DSE: 87%; P = 0.028), and were more likely to have a clinical history of CVD (ILI: 15%, DSE: 11%; P = 0.032). Compared to other Look AHEAD participants, those who underwent multiple cognitive assessments (which were only performed in 6 of the 16 Look AHEAD clinical sites among participants with sufficiently long follow-up) were on average 6 months younger, were less likely to be Hispanic and more likely to be African American or Caucasian, were more likely to have completed postcollege education and less likely to have completed other education, and had lower HbA1c and fasting plasma glucose (Table 2).
Table 2.
Comparison of the characteristics at the time of randomization of all randomized Look AHEAD participants and the subset that had more than one cognitive assessment
| Included (N = 1089) | Excluded (N = 4055) | P-value | |
|---|---|---|---|
| Age, mean ± SD, years | 58.3 ± 6.7 | 58.8 ± 6.9 | 0.0269 |
| Gender, No. (%) | 0.5822 | ||
| Male | 449 (41.2%) | 1633 (40.3%) | |
| Female | 641 (58.8%) | 2422 (59.7%) | |
| Race, No. (%) | <0.0001 | ||
| African American | 224 (20.6%) | 580 (14.3%) | |
| American Indian | 7 (0.6%) | 251 (6.2%) | |
| Hispanic | 41 (3.8%) | 639 (15.8%) | |
| Non-Hispanic White | 787 (72.2%) | 2465 (60.8%) | |
| Other | 31 (2.8%) | 120 (3.0%) | |
| Education, No. (%) | <0.0001 | ||
| High school | 561 (51.5%) | 2033 (50.1%) | |
| College graduate | 245 (22.5%) | 873 (21.5%) | |
| Postcollege | 233 (21.4%) | 743 (18.3%) | |
| Other a | 51 (4.7%) | 406 (10.0%) | |
| BMI, mean ± SD, kg/m2 | 35.8 ± 5.8 | 36.0 ± 5.9 | 0.2380 |
| Obesity Group (kg/m2), No. (%) | 0.5063 | ||
| BMI < 30kg/m2 | 169 (15.5%) | 596 (14.7%) | |
| BMI ≥ 30kg/m2 | 921 (84.5%) | 3459 (85.3%) | |
| Paffenbarger, mean ± SD, % | 816.7 ± 1016 | 875.6 ± 1190 | 0.2144 |
| HbA1c, mean ± SD, % | 7.2 ± 1.1 | 7.3 ± 1.2 | 0.0066 |
| Glucose, mean ± SD, mgdL | 149.8 ± 44.0 | 153.9 ± 46.0 | 0.0083 |
| Prior CVD, No. (%) | 0.2841 | ||
| No | 950 (87.2%) | 3483 (85.9%) | |
| Yes | 140 (12.8%) | 572 (14.1%) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; SD, standard deviation.
a “Other” educational attainment referred to a highest level of educational attainment that was less than a high school diploma or general education development (GED), or did not fit into any of the following categories: high school diploma or equivalency (GED), some vocational school, some college, associate degree (junior college), Bachelor’s degree, some graduate school, Master’s degree, doctorate, professional (MD, JD, DDS, etc.).
Raw cognitive test scores at the first cognitive test and cognitive change are shown in Table 3. Mean performance in each test was typical of cognitively normal older adults. Cognitive tests scores were largely stable over time in this cohort. Therefore, we analyzed standardized cognitive test scores adjusting for the order of test across test administrations as our primary outcome of interest.
Table 3.
Cognitive scores at the times of the first, second, and third cognitive assessment among the six tests in the cognitive battery, along with the timepoint at which those scores were collected
| Cognitive Test | First Cognitive Score N = 1089 | Second Cognitive Score N = 1089 | Third Cognitive Score N = 315 | |
|---|---|---|---|---|
| Modified Mini-Mental State Exam | Mean (95% CI) | 92.6 (92.3, 93.0) | 92.7 (92.4, 93.1) | 93.5 (92.9, 94.2) |
| Median (IQR) | 94 (90, 97) | 94 (90, 97) | 95 (91, 98) | |
| Rey Auditory Verbal Learning Test | Mean (95% CI) | 7.4 (7.3, 7.6) | 7.9 (7.6, 8.1) | 8.6 (8.2, 8.9) |
| Median (IQR) | 7 (5, 10) | 8 (5, 10) | 9 (6, 11) | |
| Digit Symbol Coding | Mean (95% CI) | 43.0 (42.3, 43.7) | 41.4 (40.8, 42.1) | 42.5 (41.3, 43.8) |
| Median (IQR) | 43 (36, 50) | 42 (34, 49) | 42 (35, 50) | |
| Trail Making Test – Part B | Mean (95% CI) | 99.5 (96.0, 103.0) | 103.5 (99.8, 107.3) | 100.1 (93.3, 106.8) |
| Median (IQR) | 83 (63, 114) | 84 (63, 116) | 82 (63, 110) | |
| Stroop | Mean (95% CI) | 31.0 (30.1, 32.0) | 32.0 (31.0, 33.0) | 31.8 (29.9, 33.7) |
| Median (IQR) | 28 (21, 37) | 28 (21, 39) | 28 (21, 38) | |
| Cognitive Composite Score | Mean (95% CI) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2) | 0.3 (0.2, 0.4) |
| Median (IQR) | 0.3 (-0.1, 0.7) | 0.3 (-0.2, 0.7) | 0.4 (-0.1, 0.8) |
The larger positive numbers correspond to better performance on all tests, except for the Trail Making Test – Part B. The median (IQR) number of years between randomization and the first, second, and third cognitive tests was 8.1 (8.0, 8.5), 11.1 (10.3, 12.0), and 12.0 (11.1, 12.1).
Abbreviations: CI, confidence interval; IQR, interquartile range.
Baseline BMI, glycemic status, and PA are listed in Table 4, as are summaries of change between randomization and the clinical visit preceding the first cognitive assessment. Mean BMI at baseline was in the range of Class II obesity, mean fasting glucose and HbA1C were above diagnostic thresholds for T2DM, and mean level of PA was below current consensus recommendations for PA attainment. On average, BMI, fasting glucose, and HbA1C decreased over follow-up, while PA increased. However, variability in changes over follow-up were substantial, with both increases and decreases in each of these variables observed. Body mass index change and glycemic change over follow-up were only modestly correlated (Pearson’s rho = 0.18).
Table 4.
Levels of physiological markers at the time of randomization, and change in those markers between randomization and the visit prior to the first cognitive assessment, among participants who received 2 or 3 cognitive assessments
| Diabetes Marker | Baseline | Change Over Follow-up | |||||
|---|---|---|---|---|---|---|---|
| Overall | DSE | ILI | Overall | DSE | ILI | ||
| BMI (kg/m2)a | Mean (95% CI) | 35.75 (35.41, 36.10) | 35.92 (35.44, 36.40) | 35.59 (35.10, 36.09) | -4.69 (-5.14, -4.24) | -2.20 (-2.79, -1.60) | -7.10 (-7.72, -6.49) |
| Median (IQR) | 34.87 (31.44, 39.10) | 35.05 (31.66, 39.14) | 34.75 (31.14, 39.06) | -3.85 (-8.59, 0.17) | -1.48 (-5.78, 2.03) | -6.33 (-11.07, -2.27) | |
| Paffenbarger (kcal/week)b | Mean (95% CI) | 816.68 (743.2, 890.2) | 853.10 (743.3, 962.9) | 780.47 (682.3, 878.6) | 252.51 (190.1, 314.9) | 83.09 (-0.47, 166.7) | 421.00 (331.4, 510.7) |
| Median (IQR) | 448 (84, 1177) | 504 (112, 1183.5) | 420 (56, 1148) | 179.2 (-95.7, 591.4) | 78.6 (-206.4, 378.1) | 296.8 (18.67, 819.2) | |
| Glucose (mg/dL) | Mean (95% CI) | 149.81 (147.2, 152.4) | 149.49 (145.9, 153.1) | 150.13 (146.3, 154.0) | -9.04 (-10.9, -7.14) | -5.44 (-8.06, -2.82) | -12.53 (-15.3, -9.80) |
| Median (IQR) | 140 (120, 170) | 142 (121, 169) | 138.5 (120, 172) | -7.4 (-22.6, 7.6) | -4.1 (-17.2, 10.4) | -11.3 (-25.8, 3.8) | |
| HbA1c, % | Mean (95% CI) | 7.20 (7.13, 7.26) | 7.20 (7.11, 7.30) | 7.19 (7.10, 7.28) | -0.15 (-0.21, -0.10) | -0.07 (-0.14, 0.00) | -0.24 (-0.31, -0.17) |
| Median (IQR) | 7 (6.5, 7.7) | 7 (6.5, 7.7) | 7 (6.4, 7.7) | -0.15 (-0.6, 0.27) | -0.04 (-0.5, 0.37) | -0.21 (-0.69, 0.19) | |
Abbreviations: CI, confidence interval; DSE, Diabetes support and education; HbA1c, hemoglobin A1c; ILI, Intensive lifestyle intervention; IQR, interquartile range.
a BMI change is presented in this table only as percent change in BMI.
b Paffenbarger leisure time physical activity was collected on a subset of individuals.
Associations between cognitive measures and change in the physiological markers over follow-up are presented in Table 5. Greater BMI reduction over follow-up was associated with a poorer mean score over follow-up on the AVLT. There was a trend toward greater weight loss being associated with a poorer mean Modified Mini-Mental Status Exam scores as well. Greater reduction in fasting glucose over follow-up was associated with a better mean AVLT score and a better mean DSC score. There was a trend toward greater reduction in fasting glucose over follow-up being associated with a better mean composite cognitive score as well. Greater reduction in HbA1C over follow-up was associated with a better mean DSC score as well. All other associations between cognitive measures and changes over follow-up in BMI, fasting glucose, and HbA1C were not statistically significant. In addition, associations between changes in PA over follow-up and cognitive measures were not significant. Intervention group assignment was not a statistically significant modifier of the relationship between cognitive function and physiological markers (data not shown). These results were not materially modified by adding smoking status, systolic blood pressure, or diastolic blood pressure at the time of randomization as additional covariates. The results were also not substantially modified by removing outliers from the data set (data not shown). The results for BMI, HbA1C, and fasting glucose were highly similar in the full sample and the subsample that provided Paffenbarger Physical Activity Questionnaire data (data not shown).
Table 5.
Associations between improvement in physiological markers between randomization and the visit prior to cognitive testing and mean standardized cognitive test score
| Cognitive Score | BMIa | Paffenbarger per 1000 kcal/wk Change | ||
|---|---|---|---|---|
| Decrease over follow-up | Change over follow-up | |||
| ß (95% CI) | P-value | ß (95% CI) | P-value | |
| Modified Mini-Mental State Exam | -0.015 (-0.032, 0.002) | 0.0853 | -0.028 (-0.096, 0.039) | 0.4098 |
| Rey Auditory Verbal Learning Test | -0.028 (-0.048, -0.008) | 0.0053 | 0.003 (-0.074, 0.081) | 0.9325 |
| Digit Symbol Coding | 0.015 (-0.005, 0.034) | 0.1358 | 0.051 (-0.025, 0.127) | 0.1909 |
| Trail Making Test – Part B | -0.009 (-0.026, 0.007) | 0.2693 | -0.041 (-0.105, 0.024) | 0.2139 |
| Stroop | 0.008 (-0.011, 0.027) | 0.4086 | 0.054 (-0.022, 0.130) | 0.1612 |
| Cognitive Composite Score | -0.006 (-0.019, 0.007) | 0.3933 | 0.007 (-0.044, 0.059) | 0.7882 |
| Cognitive score | Glucosea per 10 mg/dl change | HbA1c | ||
| Decrease over follow-up | Decrease over follow-up | |||
| ß (95% CI) | P-value | ß (95% CI) | P-value | |
| Modified Mini-Mental State Exam | 0.005 (-0.013, 0.023) | 0.5678 | -0.018 (-0.077, 0.040) | 0.5381 |
| Rey Auditory Verbal Learning Test | 0.026 (0.005, 0.046) | 0.0148 | 0.043 (-0.025, 0.111) | 0.2172 |
| Digit Symbol Coding | 0.022 (0.001, 0.042) | 0.0360 | 0.067 (0.001, 0.134) | 0.0477 |
| Trail Making Test – Part B | 0.004 (-0.013, 0.021) | 0.6287 | -0.012 (-0.068, 0.044) | 0.6748 |
| Stroop | 0.004 (-0.016, 0.024) | 0.6885 | 0.028 (-0.036, 0.093) | 0.3891 |
| Cognitive Composite Score | 0.012 (-0.002, 0.026) | 0.0869 | 0.021 (-0.024, 0.066) | 0.3600 |
All variables are coded such that positive beta values reflect an association between an improvement in the physiological marker and greater performance on the cognitive test. Models adjusted for randomization arm, race, sex, clinical site, education group, years from randomization, order of cognitive test (first, second, or third), level of the physiological marker as randomization, and repeated measures.
Abbreviations: BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; wk, week.
a BMI and glucose were reverse-coded such that increases infer improvement.
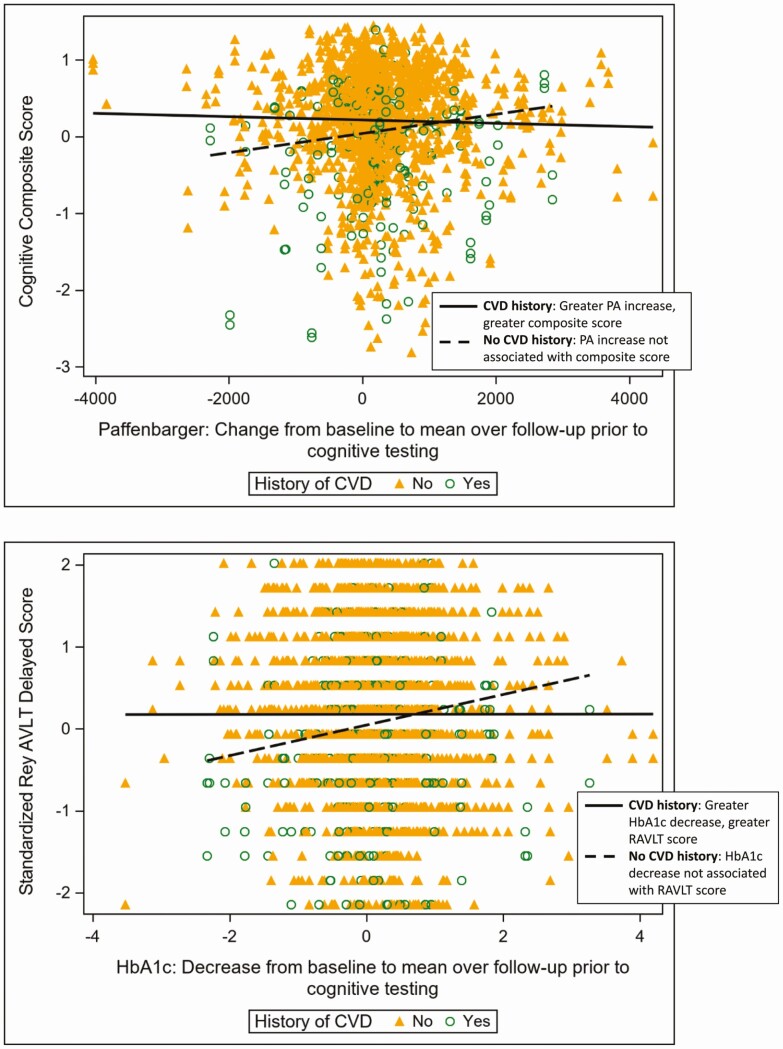
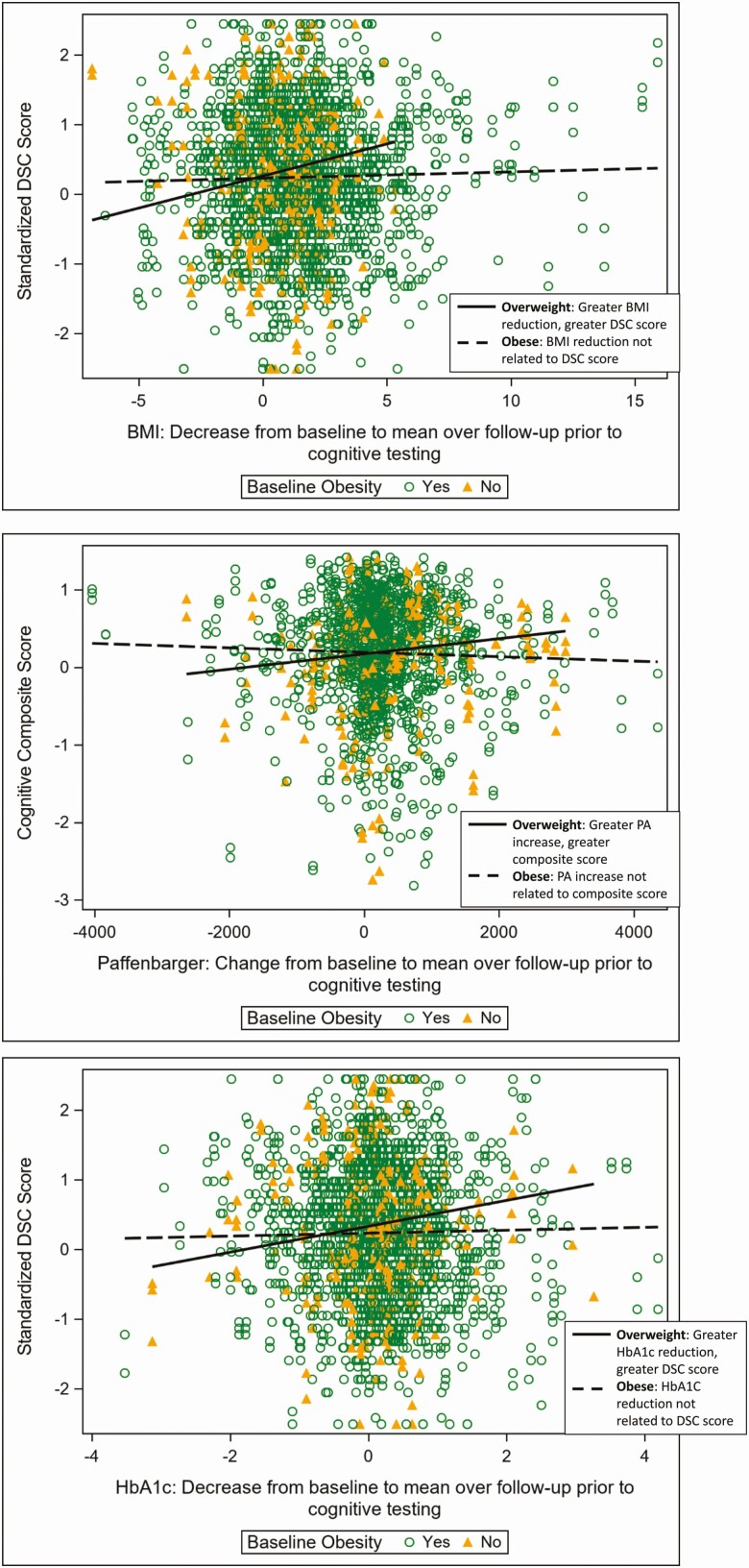
Certain associations between cognitive function and change in physiological markers differed significantly by baseline history of CVD and by baseline BMI category. In particular, greater increase in PA over follow-up was associated with better composite cognitive scores among those who reported a history of CVD at baseline, but among those who did not report a history of CVD, this association was not statistically significant (interaction P-value, 0.0170; Fig. 1). Similarly, greater reduction in HbA1c over follow-up was associated with a better AVLT score among participants who reported a history of CVD at baseline, and the association was not statistically significant among those who did not report a history of CVD at baseline (interaction P-value, 0.0336; Fig. 1). Among those who were overweight at baseline, greater weight loss and HbA1C reduction over follow-up were associated with better DSC scores, while these associations were not statistically significant among individuals who were obese at baseline (interaction P-values, 0.010 and 0.023; Fig. 2). Similarly, among those overweight at baseline, greater increases in PA over follow-up were associated with better cognitive composite scores, but among those obese at baseline this association was not statistically significant (interaction P-value, 0.031; Fig. 2). These interaction effects were not substantially modified by outlier removal (data not shown).
Figure 1.
Top: Greater increase in physical activity (as measured by the Paffenbarger questionnaire) was associated with greater cognitive composite scores among those with a cardiovascular disease (CVD) clinical history prior to randomization but not among those without (interaction P-value = 0.0170). Bottom: Greater reduction in hemoglobin A1c (HbA1c) over follow-up was associated with better Rey Auditory Verbal Learning Test (RAVLT) scores among those with a CVD clinical history but not among those without (interaction P-value = 0.0336). Raw values are presented with unadjusted slopes for each group.
Figure 2.
Among trial participants who were overweight at randomization, greater reductions in body mass index (BMI) and hemoglobin A1c (HbA1C) over follow-up were associated with better digit symbol coding (DSC) score at follow-up, and greater increase in physical activity (PA) over follow-up was associated with better cognitive composite score. However, these associations were not significant among participants who were obese at randomization (interaction P-values: 0.010, 0.023, 0.031). Raw values are presented with unadjusted slopes for each group.
Conclusions
In this study, cognitive function was measured 2 to 3 times in a large subgroup of middle-aged persons with T2DM between 8 and 11 years after their randomization to an ILI or DSE program in the Look AHEAD clinical study. There were 3 key findings. First, greater improvements in glycemic control over follow-up were associated with better cognitive performance in the overall sample. Second, associations between weight loss over follow-up and subsequent cognitive function were mixed, with suggestions that greater weight loss may be associated with either cognitive benefit or harm, depending on the cognitive test. Finally, associations between improvements in glycemic control, PA, and weight over follow-up and cognitive test score differed by baseline adiposity and CVD history, such that benefits were especially evident among those with a history of CVD and those with baseline overweight rather than obesity. Overall, the findings suggest that in overweight or obese individuals with T2DM enrolled in an intervention study, long-term improvements in differing physiological markers may have differing consequences for cognitive functioning, and those consequences may differ based on baseline health factors.
The most consistent finding of this study was that greater improvements in glycemic control over the course of 8 to 11 years of follow-up were associated with better scores on cognitive tests. This finding was observed in the overall sample as well as in subsamples defined by a clinical history of CVD or baseline BMI in the overweight (not obese) range. This finding adds to a mix of literature in which, on one hand, poorer glycemic control in middle age is associated with an increased risk of cognitive decline in old age (3), and short-term (eg, less than 9 months) enhancement of glycemic control is associated with similarly short-term improvement in cognitive function (18–25), but the association between glycemic control and concurrent cognitive function is weak (32), and the ability of long-term glycemic control improvement to improve long-term cognitive function is unclear (27–31). Recent review articles have articulated 1 possible reason for these mixed results: the high complexity and interconnectedness of biological mechanisms that link T2DM to cognitive decline (33–38). Our data adds to this literature by showing that individuals with T2DM who were enrolled in either an ILI or DSE program and showed greater improvements in glycemic control over 8 to 11 years of follow-up also showed greater cognitive performance at follow-up. We speculate that our large sample size, long follow-up duration, and wide distribution of glycemic change values contributed to our ability to detect this association.
Associations between weight loss and cognitive function were inconsistent in this study. In the overall sample, greater weight loss over follow-up was associated with poorer scores on a test of verbal learning and memory, but among those who were overweight (not obese) at baseline, greater weight loss over that period was associated with better scores on a test of processing speed and working memory. These mixed results add to the highly complex literature on weight change late in the lifespan. On one hand, overweight and obesity in middle age are associated with an increased risk of cognitive decline in old age (56, 57). On the other hand, substantial weight loss among cognitively healthy older adults is associated with increased risk of dementia years or even decades in the future (58, 59), and overweight and obesity among elderly individuals may even protect against cognitive decline (56, 60). Complicating this literature is that it is not always clear whether the weight loss is intentional, with intentional weight loss hypothesized to be beneficial and unintentional weight loss hypothesized to represent adverse processes (61, 62). In addition, most prior studies did not report whether weight loss represented primarily a loss of fat mass (which is hypothesized to be beneficial (63)) or lean mass (which represents aging-related sarcopenia, an adverse health condition (64, 65)). Mechanisms relating late-life weight loss to cognitive decline are complex; in particular it is unclear whether clinically-latent neurodegenerative disease modifies the brain circuitry governing energy homeostasis, thus giving rise to weight loss at the same time, as it promotes progressive cognitive decline, or whether aging-related changes to the skeletal muscle, adipose tissue, and gut could cause weight loss directly and thereby exacerbate neurodegenerative changes (66–68). Our data, in a weight loss clinical trial, suggests that weight loss in the context of T2DM can be associated with inconsistent cognitive outcomes. As greater numbers of adults enter their senior years with concurrent overweight and T2DM, future research that clarifies the cognitive impact of weight loss is especially urgent.
Our results regarding baseline health status as a modifier of the effect of improvements in physiological markers on cognitive function were mixed. On one hand, improvements in weight, glycemic control, and PA were primarily associated with better cognition among those who were relatively lighter (overweight but not obese) at baseline. This finding is consistent with a “too little, too late” scenario in which the injury processes associated with obesity may have had more deleterious effects on the brains of obese individuals relative to overweight individuals. In this scenario, neural plasticity and repair mechanisms associated with the improvements in physiological markers may have been exhausted in obese individuals, thus leading to an apparent lack of brain benefit among obese persons. On the other hand, improvements in glycemic control and PA were associated with better cognition among Look AHEAD participants who had a clinical history of CVD, but not among those who did not. One possible explanation for this result is survivor bias (69), that is, the possibility that persons with the combination of T2DM and CVD are also the individuals more likely to have died during follow-up and, therefore, to have been censored from this study. In this scenario, those individuals with concurrent T2DM and CVD who were represented in the study may have been those whose CVD and T2DM cases were relatively mild, thus alleviating the “too little, too late” concern and making cognitive responses to physiological changes possible. Another possible explanation is that treatments for CVD cause their own improvements in cognitive function (70) or potentiate positive cognitive effects of weight loss, PA, and glucose control. In this scenario, it is not the cardiovascular event that makes enhanced cognition possible but rather the exposure to cardiovascular drugs. Overall, our data does not support a simplistic scenario in which the cognitive benefits of improvements in physiological markers are confined to those with better cardiometabolic health at baseline.
Key strengths of this study include its large, diverse, and richly phenotyped cohort, and its long duration of follow-up and repeated standardized assessment of cognitive function. An important limitation was a lack of cognitive assessment at enrollment; however, screening procedures (eg, successful record-keeping, behavioral interview) enhanced the likelihood that enrollees were free of cognitive impairment at baseline. Look AHEAD volunteers came from the subset of community-dwelling individuals for whom an ILI at an academic research center was feasible and safe; thus, the result might not generalize to a more general population of persons with diabetes. While we cannot rule out the potential that differential follow-up may have influenced our findings, covariate adjustment for factors potentially related to this may have limited any such affects. Heterogeneity in the number of cognitive tests per person is an additional limitation to consider when evaluating this work. Finally, cognitive testing occurred after an overnight fast followed by a snack, and the postsnack rise circulating glucose concentration could have varied between individuals. Therefore, we cannot rule out the possibility that interindividual variability in circulating glucose at the time of cognitive testing contributed to cognitive test scores.
In conclusion, in a large sample of older adults with T2DM enrolled in a behavioral clinical trial and greater improvements in glycemic control over 8 to 11 years of follow-up were associated with better cognitive functioning at follow-up, and associations between weight loss and cognitive functioning at follow-up were mixed. The influence of baseline health status on associations between improvements in physiological markers and cognitive function at follow-up were similarly mixed.
Acknowledgments
Financial Support: Funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Additional support was received from the National Institutes of Health through support for the Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153), and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
A full listing of the LookAHEAD Research Group at the end of the Continuation phase of the study is provided below. Adam Spira received an honorarium from Springer Nature Switzerland AG for guest editing a special issue of Current Sleep Medicine Reports.
Look AHEAD Research Group at End of Continuation
Clinical Sites: The Johns Hopkins University: Frederick L. Brancati, MD, MHS1,*; Jeanne M. Clark, MD, MPH1 (Co-Principal Investigators); Lee Swartz2; Jeanne Charleston, RN3; Lawrence Cheskin, MD3; Richard Rubin, PhD3,*; Jean Arceci, RN; David Bolen; Danielle Diggins; Mia Johnson; Joyce Lambert; Sarah Longenecker; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun; Maria Sowers; and Kathy Tyler. Pennington Biomedical Research Center: George A. Bray, MD1; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Timothy Church, MD3; Catherine Champagne, PhD, RD; Valerie Myers, PhD; Jennifer Arceneaux, RN; Kristi Rau; Michelle Begnaud, LDN, RD, CDE; Barbara Cerniauskas, LDN, RD, CDE; Crystal Duncan, LPN; Helen Guay, LDN, LPC, RD; Carolyn Johnson, LPN, Lisa Jones; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Marisa Smith; Lauren Cox; and Monica Lockett, LPN.
The University of Alabama at Birmingham: Cora E. Lewis, MD, MSPH1; Sheikilya Thomas, PhD,MPH2; Monika Safford, MD3; Stephen Glasser, MD3; Vicki DiLillo, PhD3; Gareth Dutton, PhD, Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Sara Hannum; Anne Hubbell, MS; Jane King, MLT; DeLavallade Lee; Andre Morgan; L Christie Oden; Janet Wallace, MS; Cathy Roche, PhD, RN, BSN; Jackie Roche; and Janet Turman.
Harvard Center, Massachusetts General Hospital: David M. Nathan, MD1; Enrico Cagliero, MD3; Heather Turgeon, RN, BS, CDE2; Barbara Steiner, EdM2; Valerie Goldman, MS, RDN2; Linda Delahanty, MS, RDN3; Ellen Anderson, MS, RDN3; Laurie Bissett, MS, RDN; Christine Stevens, RN; Mary Larkin, RN; Kristen Dalton, BS, and Roshni Singh, BS. Harvard Center, Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth McKinney, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Kari Galuski, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; and Kerry Ovalle, MS, RCEP, CDE. Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; and Ann McNamara, RN. University of Colorado Anschutz Medical Campus: James O. Hill, PhD1; Marsha Miller, MS RD2; Holly Wyatt, MD3; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Debbie Bochert; Gina Claxton-Malloy RD Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Loretta Rome, TRS; Terra Thompson, BA, Kirstie Craul, RD, CDE; and Cecilia Wang, MD. Baylor College of Medicine: John P Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Molly Gee, MEd, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Chu-Huang Chen, MD, PhD3; Peter Jones, MD3; Michele Burrington, RD, RN; Allyson Clark Gardner, MS, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Sarah Lee; Sarah Lane Liscum, RN, MPH; Susan Cantu-Lumbreras; Julieta Palencia, RN; Jennifer Schmidt; Jayne Thomas, RD; Carolyn White; Charlyne Wright, RN; and Monica Alvarez, PCT. The University of Tennessee Health Science Center, University of Tennessee East: Karen C. Johnson, MD, MPH□; Karen L. Wilson, BSN□; Mace Coday, PhD3; Beate Griffin, RN, BS; Donna Valenski; Polly Edwards; Brenda Fonda; and Kim Ward. The University of Tennessee Health Science Center, University of Tennessee Downtown: Helmut Steinburg, MD3; Carolyn Gresham, BSN□; Moana Mosby, RN; Debra Clark, LPN; Donna Green RN; and Abbas E. Kitabchi, PhD, MD (retired). University of Minnesota: Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott J. Crow, MD3; Manami Bhattacharya, BS; Cindy Bjerk, MS, RD; Kerrin Brelje, MPH, RD; Carolyne Campbell; Mary Ann Forseth, BA; Melanie Jaeb, MPH, RD; Philip Lacher, BBA; Patti Laqua, BS, RD; Birgitta I. Rice, MS, RPh, CHES; Ann D. Tucker, BA; and Mary Susan Voeller, BA. St. Luke’s Roosevelt Hospital Center: Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Carmen Pal, MD3; Lynn Allen, MD; Janet Crane, MA, RD, CDN; Lolline Chong, BS, RD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; and Raashi Mamtani, MS. University of Pennsylvania: Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2; Robert I. Berkowitz, MD3; Gary Foster, PhD3; Henry Glick, PhD3; Shiriki Kumanyika, PhD RD, MPH3; Yuliis Bell, BA; Raymond Carvajal, PsyD; Helen Chomentowski; Renee Davenport; Lucy Faulconbridge, PhD; Louise Hesson, MSN, CRNP; Sharon Leonard, RD; and Monica Mullen, RD, MPH. University of Pittsburgh: John M. Jakicic, PhD1; David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Daniel Edmundowicz, MD3; Lin Ewing, PhD, RN3; Andrea Hergenroeder, PhD, PT, CCS3; Mary L. Klem, PhD, MLIS3; Mary Korytkowski, MD3; Andrea Kriska, PhD3; Lewis H. Kuller, MD, DrPH3; Amy D. Rickman, PhD, RD, LDN3; Rose Salata, MD3; Monica E. Yamamoto, DrPH, RD, FADA3; Janet Bonk, RN, MPH; Susan Copelli, BS, CTR; Rebecca Danchenko, BS; Tammy DeBruce, BA; Barbara Elnyczky; David O. Garcia, PhD; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Diane Heidingsfelder, MS, RD, CDE, LDN; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Janet Krulia, RN, BSN, CDE; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Lisa Martich, BS, RD, LDN; Meghan McGuire, MS; Tracey Y. Murray, BS; Anna Peluso, MS; Karen Quirin; Jennifer Rush, MPH; Joan R. Ritchea; Linda Semler, MS, RD, LDN; Karen Vujevich, RN-BC, MSN, CRNP; Kathy Williams, RN, MHA; and Donna L. Wolf, PhD. The Miriam Hospital/Brown Medical School: Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; and Jane Tavares, BS. The University of Texas Health Science Center at San Antonio: Helen P. Hazuda, PhD1; Maria G. Montez, RN, MSHP, CDE1; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; and Nora Ramirez, RN, BSN. VA Puget Sound Health Care System/University of Washington: Steven E. Kahn, MB, ChB1; Anne Kure, BS2; Edward J. Boyko, MD, MPH3; Edward Lipkin, MD, PhD3; Dace Trence, MD3; Subbulaxmi Trikudanathan, MD, MRCP, MMSc3; Elaine Tsai, MD3; Brenda Montgomery, RN, MS, CDE; Ivy Morgan-Taggart; Jolanta Socha, BS; Lonnese Taylor, RN, BS; and Alan Wesley, BA. Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico: William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Maria Cassidy-Begay, BSND, RND1; Katie Toledo, MS, LPC2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Sara Michaels, MD3; Paul Bloomquist, MD3; Peter H. Bennett, MB, FRCP3; Bernadita Fallis, RN, RHIT, CCS; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Christina Morris, BA; Julie Nelson, RD; Carol Percy, RN, MS; Patricia Poorthunder; Sandra Sangster; Leigh A. Shovestull, RD, CDE; Miranda Smart; Janelia Smiley; and Teddy Thomas, BS. University of Southern California: Anne Peters, MD1; Siran Ghazarian, MD2; Elizabeth Beale, MD3; Kati Konersman, RD, CDE; Brenda Quintero-Varela; Edgar Ramirez; Gabriela Rios, RD; Gabriela Rodriguez, MA; Valerie Ruelas MSW, LCSW; Sara Serafin-Dokhan; and Martha Walker, RD.
Coordinating Center: Wake Forest University: Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH1; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; David Lefkowitz, MD3; Patrick S. Reynolds, MD3; Denise Houston, PhD3; Mike E. Miller, PhD3; Laura D. Baker, PhD3; Nicholas Pajewski, PhD3; Stephen R. Rapp, PhD3; Stephen Kritchevsky, PhD3; Haiying Chen, PhD, MM3; Valerie Wilson, MD3; Delia S. West, PhD3; Ron Prineas, MD3; Tandaw Samdarshi, MD3; Amelia Hodges, BS, CCRP2; Karen Wall2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Tara D. Beckner; Valery S. Effoe, MD, MS; Melanie Franks, BBA; Katie Garcia, MS; Sarah A. Gaussoin, MS; Candace Goode; Michelle Gordon, MS; Lea Harvin, BS; Mary A. Hontz, BA; Don G. Hire, BS; Patricia Hogan, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Julia T. Rushing, MS; Debbie Steinberg, BS; Jennifer Walker, MS; and Michael P. Walkup, MS.
Central Resources Centers: Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories: Santica M. Marcovina, PhD, ScD1; Jessica Hurting2; John J. Albers, PhD3, and Vinod Gaur, PhD3. ECG Reading Center, EPICARE, Wake Forest University School of Medicine: Elsayed Z. Soliman MD, MSc, MS1; Charles Campbell2; Zhu-Ming Zhang, MD3; Mary Barr; Susan Hensley; Julie Hu; Lisa Keasler; and Yabing Li, MD. Hall-Foushee Communications, Inc.: Richard Foushee, PhD and Nancy J. Hall, MA
Federal Sponsors: National Institute of Diabetes and Digestive and Kidney Diseases: Mary Evans, PhD; Van S. Hubbard, MD, PhD; and Susan Z. Yanovski, MD. National Heart, Lung, and Blood Institute: Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; and Mario Stylianou, PhD. Centers for Disease Control and Prevention: Edward W. Gregg, PhD and Ping Zhang, PhD.
1Principal Investigator; 2Program Coordinator; 3Co-Investigator
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
De-identified versions of the data analyzed in this article are available for public use at the NIDDK Data Repository (https://repository.niddk.nih.gov/home/).
References
- 1. Leung MY, Carlsson NP, Colditz GA, Chang SH. The burden of obesity on diabetes in the United States: medical expenditure panel survey, 2008 to 2012. Value Health. 2017;20(1): 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkman MS, Briscoe VJ, Clark N, et al. ; Consensus Development Conference on Diabetes and Older Adults . Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Association AD. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596–615. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Goodman AM, Group MMS. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995;333(9):541–549. [DOI] [PubMed] [Google Scholar]
- 5. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194–206. [DOI] [PubMed] [Google Scholar]
- 6. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5. [DOI] [PubMed] [Google Scholar]
- 7. Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598–2603. [DOI] [PubMed] [Google Scholar]
- 8. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8): 2261–2267. [DOI] [PubMed] [Google Scholar]
- 9. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. [DOI] [PubMed] [Google Scholar]
- 10. Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22(5):331–339. [DOI] [PubMed] [Google Scholar]
- 11. Wing RR, Lang W, Wadden TA, et al. ; Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Group LAR. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23(10):1499–1504. [DOI] [PubMed] [Google Scholar]
- 14. Control D, Group CTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 15. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. [DOI] [PubMed] [Google Scholar]
- 16. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 18. Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29(2):345–351. [DOI] [PubMed] [Google Scholar]
- 19. Guo M, Mi J, Jiang QM, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41(9):650–656. [DOI] [PubMed] [Google Scholar]
- 20. Novak V, Milberg W, Hao Y, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37(3):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. [DOI] [PubMed] [Google Scholar]
- 22. Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson-Hanley C, Arciero PJ, Westen SC, Nimon J, Zimmerman ENeuropsychological benefits of stationary bike exercise and a cybercycle exergame for older adults with diabetes: an exploratory analysis. J Diabetes Sci Technol. 2012;6(4):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee P, Mazumdar S, Goswami S, et al. Cognitive dysfunction in diabetic patients with special reference to age of onset, duration and control of diabetes. Act Nerv Super. 2012;54(1-2):67–75. [Google Scholar]
- 25. Abbatecola AM, Lattanzio F, Molinari AM, et al. Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care. 2010;33(8):1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiocco AJ, Scarcello S, Marzolini S, et al. The effects of an exercise and lifestyle intervention program on cardiovascular, metabolic factors and cognitive performance in middle-aged adults with type II diabetes: a pilot study. Can J Diabetes. 2013;37(4):214–219. [DOI] [PubMed] [Google Scholar]
- 27. Luchsinger JA, Palmas W, Teresi JA, et al. Improved diabetes control in the elderly delays global cognitive decline. J. Nutr. Health Aging. 2011;15(6):445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koekkoek PS, Ruis C, van den Donk M, et al. Intensive multifactorial treatment and cognitive functioning in screen-detected type 2 diabetes—the ADDITION-Netherlands study: a cluster-randomized trial. J Neurol Sci. 2012;314(1):71–77. [DOI] [PubMed] [Google Scholar]
- 30. Luchsinger JA, Lehtisalo J, Lindström J, et al. Cognition in the Finnish diabetes prevention study. Diabetes Res Clin Pract. 2015;108(3):e63–e66. [DOI] [PubMed] [Google Scholar]
- 31. Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, Martínez G. Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804. doi: 10.1002/14651858.CD003804.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(1):75–89. [DOI] [PubMed] [Google Scholar]
- 33. Stoeckel LE, Arvanitakis Z, Gandy S, et al. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Research. 2016;5:353. doi: 10.12688/f1000research.8300.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Espeland MA, Small DM, Stoeckel LEDiet, obesity, and physical inactivity: Linking diabetes and dementia. In Srikanth V, Arvanitakis Z, eds. Type 2 Diabetes and Dementia. London: Elsevier Academic Press; 2018:117–141. [Google Scholar]
- 35. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. [DOI] [PubMed] [Google Scholar]
- 36. Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7(2):108–114. [DOI] [PubMed] [Google Scholar]
- 37. Luchsinger JA. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimers Dis. 2012;30(2):S185–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11(5):504–510.e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Byun MS, Kim HJ, Yi D, et al. ; KBASE Research Group . Differential effects of blood insulin and HbA1c on cerebral amyloid burden and neurodegeneration in nondiabetic cognitively normal older adults. Neurobiol Aging. 2017;59:15–21. [DOI] [PubMed] [Google Scholar]
- 41. Matsuzaki T, Sasaki K, Tanizaki Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75(9):764–770. [DOI] [PubMed] [Google Scholar]
- 42. Morris JK, Vidoni ED, Wilkins HM, et al. Impaired fasting glucose is associated with increased regional cerebral amyloid. Neurobiol Aging. 2016;44:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55(5):759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thambisetty M, Metter EJ, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70(9):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66(3):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35(1):60–68. [DOI] [PubMed] [Google Scholar]
- 47. Espeland MA, Rapp SR, Bray GA, et al. ; Action for Health In Diabetes (Look AHEAD) Movement and Memory Subgroup; Look AHEAD Research Group . Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69(9):1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Espeland MA, Lipska K, Miller ME, et al. ; LIFE Study Investigators . Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J Gerontol A Biol Sci Med Sci. 2017;72(6):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rapp SR, Luchsinger JA, Baker LD, et al. ; Look AHEAD Research Group . Effect of a long-term intensive lifestyle intervention on cognitive function: action for health in diabetes study. J Am Geriatr Soc. 2017;65(5):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Espeland MA, Luchsinger JA, Baker LD, et al. ; Look AHEAD Study Group . Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88(21):2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Group LAR. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Contemp Clin Trials. 2003;24(5):610–628. [DOI] [PubMed] [Google Scholar]
- 52. Group LAR. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md). 2006;14(5):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8(3):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. [DOI] [PubMed] [Google Scholar]
- 55. Espeland MA, Carmichael O, Hayden K, et al. Long-term impact of weight loss intervention on changes in cognitive function: exploratory analyses from the action for health in diabetes randomized controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2018;73(4):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–746. [DOI] [PubMed] [Google Scholar]
- 59. Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. [DOI] [PubMed] [Google Scholar]
- 60. Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: the Kame Project. Neurology. 2009;72(20):1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–491. [DOI] [PubMed] [Google Scholar]
- 62. Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165(9):1035–1040. [DOI] [PubMed] [Google Scholar]
- 63. O’Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol (1985). 2006;100(5):1584–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4(3):140–142. [PubMed] [Google Scholar]
- 65. Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279(2):E366–E375. [DOI] [PubMed] [Google Scholar]
- 66. Willett WC. Weight loss in the elderly: cause or effect of poor health? Am J Clin Nutr. 1997;66(4):737–738. [DOI] [PubMed] [Google Scholar]
- 67. Sergi G, De Rui M, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer’s disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72(1):160–165. [DOI] [PubMed] [Google Scholar]
- 68. Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. [DOI] [PubMed] [Google Scholar]
- 69. Sy RW, Bannon PG, Bayfield MS, Brown C, Kritharides L. Survivor treatment selection bias and outcomes research: a case study of surgery in infective endocarditis. Circ Cardiovasc Qual Outcomes. 2009;2(5):469–474. [DOI] [PubMed] [Google Scholar]
- 70. Feigin V, Ratnasabapathy Y, Anderson C. Does blood pressure lowering treatment prevents dementia or cognitive decline in patients with cardiovascular and cerebrovascular disease? J Neurol Sci. 2005;229-230:151–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified versions of the data analyzed in this article are available for public use at the NIDDK Data Repository (https://repository.niddk.nih.gov/home/).