Abstract
Male infertility secondary to oligozoospermia is surprisingly common. Although a majority of cases are idiopathic, oligozoospermia can be caused by endocrine dysfunction, anatomic abnormalities, medications, or environmental exposures. The work-up includes excluding reversible factors such as hormonal deficiency, medication effects, and retrograde ejaculation and identifying any underlying genetic syndrome and treating reversible medical causes. If no reversible cause is found, appropriate referrals to urology and assisted reproductive technology should be initiated. Lastly, clinicians should be aware of and respond to the psychological and general health ramifications of a diagnosis of oligozoospermia as part of the comprehensive care of men and couples struggling with a diagnosis of infertility.
Keywords: male infertility, gonadotropins, LH, FSH, semen
Male Infertility and Oligozoospermia
Infertility, defined as the inability to achieve a pregnancy after 12 months of regular, unprotected intercourse, affects up to 15% of couples worldwide (1,2). A male factor is thought to contribute to roughly half of cases, as a sole contributor in about 20% of infertile couples, or acting in conjunction with a female infertility factor in 30% of infertile couples (3). In the course of evaluation of the infertile male, following a comprehensive clinical review of the patient, semen analysis is the key initial test. Semen analysis identifies abnormalities in seminal parameters that may be contributing to infertility. The World Health Organization has published data illustrating the distribution of measured semen characteristics in fertile men, noting fifth percentile ranges of semen parameters(Table 1) (4). It is important to recognize that these reference limits do not constitute cutoffs for fertility, but that they merely serve as indices of the distribution of the measured characteristics among the studied population of fertile men. Indeed, it is suspected that there is a significant degree of overlap in semen characteristics between fertile and infertile men. Oligozoospermia refers specifically to the condition in which sperm concentration below the lower reference limit of 15 million sperm/mL of ejaculate. Oligozoospermia can be further classified as mild (between 10 and 15 million sperm/mL), moderate oligozoospermia (between 5 and 10 million sperm/mL), and severe oligozoospermia (less than 5 million sperm/mL) (5). In contrast, azoospermia refers to the condition in which no sperm can be detected in the ejaculate during semen analysis. Of note, the measurement of sperm concentration, being affected by the volume of seminal fluid that dilutes the spermatozoa at ejaculation, does not provide a direct measurement of testicular sperm output; the measurement of total number of sperm in the ejaculate is more suitable for this purpose. The goal of this article is to provide an overview of the nonsurgical management of oligozoospermia, beginning with a discussion of potential etiologies, before delineating the diagnostic approach and available treatment options. To accomplish this, we reviewed the available medical literature in PubMed using combinations of the following search terms: “male infertility,” “gonadotropins,” “oligozoospermia,” “idiopathic,” “spermatogenic failure,” “anti-estrogens,” “diagnosis,” and “treatment.”
Table 1.
Normal (greater than 5th percentile) ranges for semen parameters in men
| Parameter | Value | 95% CI |
|---|---|---|
| Volume (ml) | 1.5 | 1.4-1.7 |
| Sperm concentration (million/mL) | >15 | 12-16 |
| Total sperm number (million/ejaculate) | >39 | 33-46 |
| Morphology (% normal forms)a | >4 | 3-4 |
| Vitality (% alive) | >58 | 55-63 |
| Progressive motility (%) | >32 | 31-34 |
| Total motility (% progressive + nonprogressive) | >40 | 38-42 |
From World Health Organization, ed. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010.
Abbreviation: CI, confidence interval.
aUsing “strict” Tygerberg method (1).
Causes of Oligozoospermia
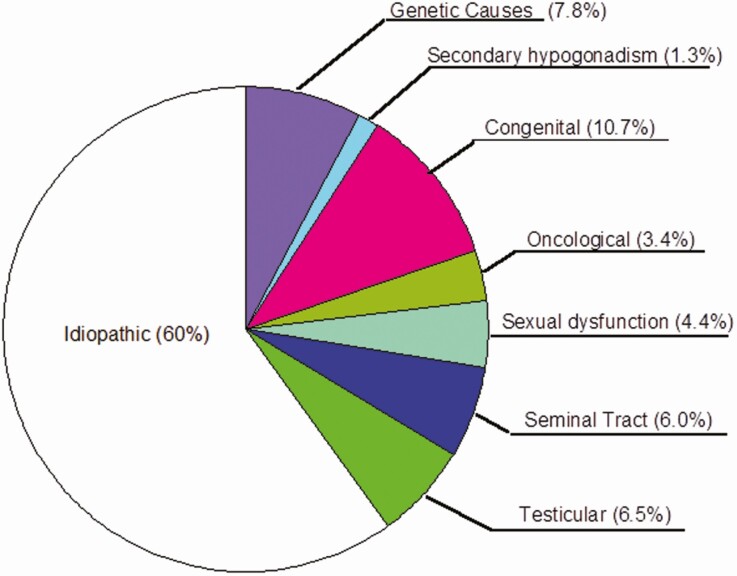
A wide variety of conditions are known to result in oligozoospermia, and the evaluation of patients presenting with oligozoospermia can be challenging. Unfortunately, even with a comprehensive work-up, 60% to 75% of men will not receive a clear diagnosis to explain their oligozoospermia (Fig. 1) (6). Nevertheless, if a diagnosis can be made, it is very helpful in determining appropriate management in many patients as discussed herein. Additionally, obtaining a formal diagnosis can be valuable in providing patients with a reason for their sterility and allaying the feelings of confusion, guilt, and stigma that some may carry. Normally, the hypothalamus produces and secretes gonadotropin-releasing hormone (GnRH) in a pulsatile fashion into the hypothalamo-hypophyseal portal circulation, where it reaches the anterior pituitary and stimulates gonadotroph cells to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the systemic circulation. LH stimulates the Leydig cells of the testes to synthesize and release testosterone, while FSH stimulates the Sertoli cells within the seminiferous tubules to promote spermatogenesis. The testosterone, some of which is aromatized into estradiol, and inhibin B, which is secreted by stimulated Sertoli cells, then provide negative feedback to the hypothalamus and pituitary to decrease secretion of LH and FSH, respectively (7). Disturbance of this carefully regulated hormonal axis gives rise to many of the endocrine-based etiologies of oligozoospermia. In particular, any suppression of gonadotropin secretion will have the downstream effect of disrupting intratesticular testosterone biosynthesis and spermatogenesis.
Figure 1.
The etiology of male infertility in 1737 consecutive men presenting to an infertility clinic. Data from Punab M, Poolamets O, Paju P, et al. Causes of male infertility in 1737 patients. Hum Reprod. 2017;32(1):18-31.
Hypothalamic dysfunction
Deficiency of GnRH secretion is most often a congenital condition. There are variant forms, including an anosmic presentation, Kallmann syndrome, and a normosmic form, which are inherited through different genetic mutations (8). GnRH deficiency is usually diagnosed by the lack of sexual maturation at puberty, although there exist partial forms of the disease that allow for a limited degree of pubertal development or, in rare instances, even complete pubertal development before onset of hypogonadotropic hypogonadism (9).
Pituitary dysfunction
Many forms of pituitary pathology, including mass lesions as well as infiltrative pituitary disease, can interrupt or suppress gonadotropin secretion. Sellar masses, including pituitary adenomas, craniopharyngiomas, and Rathke’s cleft cysts, can exert a mass effect on gonadotroph cells, interfering with hormone secretion. The most common pituitary adenoma subtype, the prolactinoma, or prolactin-secreting pituitary adenoma, additionally causes hyperprolactinemia, which independently suppresses the hypothalamic-pituitary-gonadal axis and causes hypogonadotropic hypogonadism (10). Infiltrative diseases of the pituitary, such as sarcoidosis, hemochromatosis, and Langerhans histiocytosis are known to effect hypopituitarism, as do ischemic insults such as pituitary apoplexy (11-13).
Adrenal dysfunction
While less frequently seen, congenital adrenal hyperplasia is an enzymatic deficiency in the adrenocortical steroidal synthetic pathway, such as 21-hydroxylase deficiency. This condition results in a deficiency in the production of glucocorticoids, and a concomitant excess in production of adrenal androgens driven by high ACTH secretion. The adrenal androgens suppress the hypothalamic-pituitary-gonadal axis. Although somewhat rare, suspicion for this condition is raised when normal to elevated testosterone levels accompany suppressed gonadotropins. The diagnosis is confirmed with elevated serum concentrations of 17-hydroxyprogesterone and androstenedione. Treatment with glucocorticoid replacement can suppress the excessive adrenal steroidogenesis, thereby reducing suppression of gonadotropins and fertility (14,15).
Thyroid disease
Both hyperthyroidism and hypothyroidism can negatively impact spermatogenesis and male fertility. Hyperthyroidism promotes a hyperestrogenic state through increased aromatization of androstenedione and testosterone to circulating estrogens in peripheral tissues (16). The resulting increased estrogen levels suppress the hypothalamic-pituitary-gonadal axis. Additionally, excess thyroid hormone impairs Sertoli cell proliferation, thus decreasing spermatogenic capacity (17,18). Lastly, it is important to note that hyperthyroidism can also result in markedly elevated SHBG levels, which can decrease free and bioavailable serum testosterone levels, despite increasing total serum testosterone measurements (19). Hypothyroidism has been shown to negatively affect testicular development during the prepubertal years (20). Furthermore, in primary hypothyroidism, there is a compensatory increase in thyrotropin-releasing hormone secretion from the hypothalamus. Thyrotropin-releasing hormone stimulates lactotroph cells, resulting in hyperprolactinemia that suppresses the hypothalamic-pituitary-gonadal axis (21). The gonadal axis is typically restored once a euthyroid state is restored.
Genetic syndromes
The diagnosis of severe oligozoospermia should arouse suspicion for a genetic cause of infertility. Klinefelter syndrome (KS) is the most common chromosomal abnormality in men (22). The reported prevalence of KS has been as high as 1 in 500 live births, with a 10% to 15% prevalence within the population of azoospermic men (23,24). While most men with KS are azoospermic, oligozoospermia can be seen in cases of genetic mosaicism. In men with normal karyotypes, deletions on the long arm of the Y chromosome have been recognized as a genetic contributor to male infertility. Such Y chromosome microdeletions have been identified in 5% to 10% of azoospermic men and 2% to 5% of severely oligozoospermic men (25,26). Deletions in specific regions of the affected AZF locus on the Y chromosome impact spermatogenesis differentially, accounting for phenotypic variability. Notably, deletions that are extensive, or that affect the AZFa and AZFb regions, signify that successful surgical sperm retrieval would be highly unlikely and would therefore affect management strategies. Lastly, mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene often lead to male infertility. Most frequently, CFTR mutations lead to congenital bilateral absence of the vas deferens and azoospermia; however, there have been reports of men with CFTR mutations and severe oligozoospermia (27). A low-volume, acidic ejaculate is typically observed in affected patients and reflects seminal vesicular absence.
Anatomic disorders
Varicoceles are pathologic dilations of the venous pampiniform plexus within the spermatic cord. Varicoceles are present in 35% to 40% of infertile men and can result in disordered spermatogenesis with seminiferous tubule germ cell sloughing, testicular atrophy, and decreased testosterone production (28). Although the debate between association versus causation persists, when considering the relationship between varicoceles and infertility, various hypotheses as to how varicoceles may contribute to testicular dysfunction have been made, including testicular hyperthermia, increased testicular oxidative stress, and reflux of renal and adrenal metabolites that exert a toxic effect on spermatogenesis. Ejaculatory duct obstruction is present in about 1% to 5% of infertile men (29). Such men have oligozoospermia or azoospermia and an ejaculate volume of under 1 cc. The ductal obstruction may be partial or complete and unilateral or bilateral, and semen analysis may also reveal a reduced fructose concentration and a low pH, as a result of the loss of the alkalinizing secretions of the seminal vesicles from the ejaculate. Retrograde ejaculation can also occur as a result of spinal cord injury, neurologic disorders, diabetes mellitus, or following surgeries or use of medications that result in bladder neck relaxation such as alpha-antagonists.
Obesity
As a global decline in semen quality over the past half-century (30) has paralleled a burgeoning obesity epidemic, a meta-analysis based on over 13 000 men, assessing the association between male infertility and the metabolic syndrome, demonstrated a J-shaped relationship between body mass index and oligozoospermia, whereby the underweight state was associated with an increased, nonsignificant risk of low sperm counts, while the overweight and obese states were associated with a significantly elevated risk of oligozoospermia, as compared to normal weight men (31). However, not all studies have reported a similar association, which speaks to inconsistency of data and lack of consensus regarding this potential relationship (32-35). Mechanistically, the obese state is associated with an increase in leptin and insulin resistance, which decreases kisspeptin stimulation of GnRH neurons in the hypothalamus. This downregulates GnRH secretion, thereby decreasing gonadotropin secretion. Additionally, increased adiposity increases the expression of aromatase, which converts testosterone into estradiol, thus exacerbating androgen deficiency while furthering hypothalamic-pituitary-gonadal axis suppression (36). Lastly, the epigenetic impact of paternal obesity on the health of the offspring are concerning, with a recent observational study showing significantly higher risks of preterm birth, low birth weight, and needing care in a neonatal intensive care unit in fathers with most of all of the components of the metabolic syndrome (37).
Medications
Many medications are known to impact the functioning of the hypothalamic-pituitary-testicular axis and could lead to infertility from oligozoospermia (Table 2). In particular, the endocrine effects of long-term opioid therapy include suppression of the hypothalamic-pituitary-gonadal axis, via interruption of hypothalamic pulsatile GnRH secretion, which results in hypogonadism, sexual dysfunction, and infertility (38). Opioid-induced hypogonadism is seen in up to 63% of male opioid users (39). Higher doses and longer duration of opioid therapy are associated with increased prevalence of opioid-induced hypogonadism, although the effect tends to be reversible following opioid discontinuation (40). Androgenic steroid abuse is common. Many synthetic androgens dramatically increase muscle mass and strength; however, the resultant supraphysiologic androgen levels inhibit the hypothalamic-pituitary-gonadal axis by negative feedback. Excess testosterone can be aromatized to estradiol, which contributes to side effects such as gynecomastia, while also suppressing pituitary gonadotropin secretion. Testicular atrophy and oligozoospermia commonly result. Although the hypothalamic-pituitary-gonadal axis can recover following discontinuation of anabolic agents, full restoration of sperm production may take months due to the long half-lives of some of these androgens (41). A recent study of the time course of recovery following androgen abuse reported a 7- to 9-month time to recovery for testicular steroidogenesis, with a slower 10- to 14-month recovery period required for spermatogenesis (42). Importantly, when considering hypogonadal issues affecting athletes, it is also essential to recognize the endocrine effects of excessive exercise and overtraining, which tend to manifest in tandem with anorexic tendencies and subsequent nutritional deficits, all of which will contribute to central hypothalamic-pituitary-gonadal axis suppression (43). Glucocorticoids are widely used to treat a variety of autoimmune and inflammatory pathologies. Glucocorticoids have also been implicated as a cause of hypogonadotropic hypogonadism, acting at the hypothalamus to decrease synthesis and release of GnRH. The suppression of the gonadal axis becomes more pronounced with long-term glucocorticoid therapy. Additionally, glucocorticoids may directly inhibit testosterone biosynthesis by Leydig cells (44,45). Lastly, several other medications have the potential to suppress fertility in man, including 5-α reductase inhibitors (46), cannabinoids (47), antipsychotics (48), and possibly antidepressants in the serotonin-selective reuptake inhibitor class (49) (Table 2).
Table 2.
Medications that can suppress fertility in men
| Medication Class | Examples | Mechanism |
|---|---|---|
| GnRH agonists/antagonists | Leuprolide, goserelin | Suppression of gonadotropins |
| Androgens | Testosterone, nandrolone | Suppression of gonadotropins |
| Anti-androgens | Ketoconazole, spironolactone, flutamide | Inhibition of testosterone biosynthesis or binding |
| 5α reductase inhibitors | Finasteride, dutasteride | Inhibition of DHT synthesis from testosterone |
| Opiates | Morphine, methadone | Suppression of gonadotropins |
| Estrogens | Estradiol, lavender bath oils | Suppression of gonadotropins |
| Antipsychotics | Chlorpromazine, risperidone | Elevation of prolactin, leading to suppression of gonadotropins |
| Alpha antagonists | Tamsulosin, silodosin | Retrograde ejaculation |
| Glucocortocoids | Prednisone, dexamethasone | Suppression of hypothalamus and gonadotropin secretion |
| Cannabinoids | tetrahydrocannabinol | Possible anti-motility effect |
| Antidepressants (possible) | Citalopram | Possible direct effect on spermatogenesis |
| Chemotherapeutics and antimetabolites | Cisplatin, cyclophosphamide, vinblastine, methotrexate | Direct effect on spermatogenesis |
Testicular injury
Any process resulting in direct testicular injury can give rise to primary testicular failure and hypergonadotropic hypogonadism. Compromise of testicular architecture can lead to disrupted spermatogenesis. Precipitants in this category would include testicular trauma, torsion, and gonadotoxic exposures. Cancer therapies such as chemotherapy and radiation therapy can cause damage to the seminiferous tubules, resulting in azoospermia or oligozoospermia, with the degree of severity and potential for spermatogenic recovery influenced by agent, dose, and duration of treatment (50). Given the deleterious effects of these treatments on fertility potential, sperm cryopreservation ought to be routinely recommended to patients prior to oncologic therapy, as a paramount component of fertility preservation counseling (51). Additionally, testicular infections such as mumps orchitis can cause parenchymal inflammation and seminiferous tubule destruction, followed by testicular atrophy, with resulting subfertility (52).
Diagnostic Approach to Oligozoospermia
History
The initial assessment of the man in an infertile couple should review a variety of factors that may adversely impact testicular function (see Table 3). Initially, the clinician should ascertain when the patient experienced puberty and his current sexual function, including the capacity for erections and ejaculation, as well as the frequency of sexual intercourse. Additional notable history, such a history of sexually transmitted infections, testicular trauma, prostatitis, or mumps orchitis should be obtained. Furthermore, a thorough medication history should be obtained with particular emphasis on medications known to adversely affect sperm production or sexual function (Table 2). Lastly, exposures to environmental exposures toxins such as pesticides, radiation, lead, or cadmium can contribute to male infertility and should be noted.
Table 3.
Key components of the history and physical examination in men with infertility from oligozoospermia
| History | |
|---|---|
| Pubertal timing | |
| Cryptorchidism/testicular surgery | |
| Sexual function and frequency | |
| Sexually transmitted infections | |
| Infections of prostate or testicle | |
| Current and former medications | |
| Use of alcohol, tobacco, illicit drugs | |
| Systemic disease | |
| Environmental exposures, including radiation, pesticides, and occupational | |
| Cancer diagnosis and treatment | |
| Physical examination | |
| Presence of sexual hair/Tanner stage | |
| Bitemporal hemianopsia (pituitary mass) | |
| Reduced testicular volume (<15 cc) | |
| Testicular mass | |
| Presence of vas deferens bilaterally | |
| Presence of varicocele or other scrotal mass | |
| Presence of cryptorchidism or hypospadias | |
| Synovitis of second and third metacarpal-phalangeal joints, “bronzed” appearance or stigmata of liver disease or heart failure (hemochromatosis) | |
| Presence of marked muscle hypertrophy suggesting anabolic steroid use |
Physical examination
A comprehensive physical examination of the man in an infertile couple should be performed seeking to determine the presence of any systemic disease that could adversely impact fertility (Table 3). For example, the absence of sexual hair and/or presence of gynecomastia suggests hypogonadism. Hyperpigmentation and arthritis of the second and third metacarpal-phalangeal joints are observed in hemochromatosis. A loss of peripheral vision, called bitemporal hemianopsia, suggests a large pituitary mass. Most important, a thorough examination of the penis, testes, and scrotum should be performed using a Prader orchidometer. A testicular volume of less than 15 cc is small for an adult male and suggests either testicular dysfunction, gonadotropin deficiency, or the use of exogenous androgens (53). In addition, the testes should be thoroughly examined to exclude the possibility of testicular cancer. Next, the presence of the vas deferens bilaterally should be noted and any significant abnormality such as hypospadias or the presence of a large varicocele, which is almost always noted in the left hemiscrotum. Lastly, marked muscle hypertrophy coupled with reduced testicular volume is commonly observed in men using androgenic anabolic steroids (54).
Semen analysis
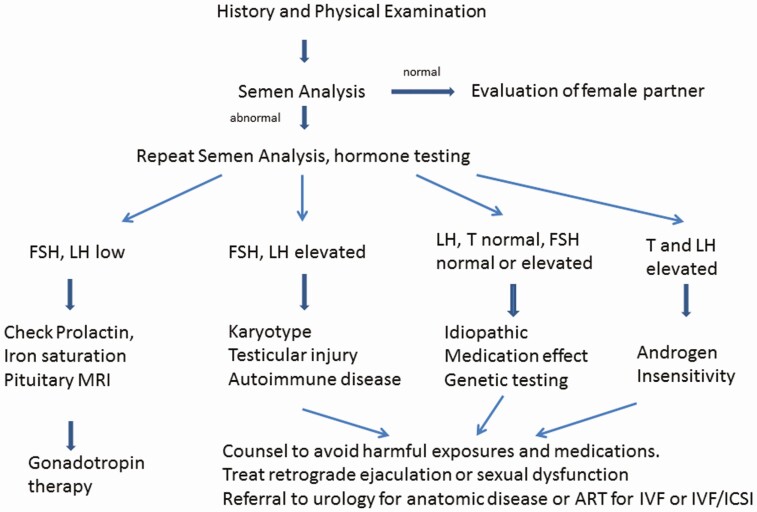
Semen analysis is the first test to obtain in a couple presenting with infertility (Fig. 2). The semen sample should be obtained after 2 to 7 days without an ejaculation. Analysis of the semen sample should be performed using protocols delineated by the World Health Organization; the reference ranges are shown in Table 1 (4). Conventionally, by World Health Organization recommendations, if an initial semen analysis reveals significant abnormalities, the test should be repeated to confirm the initial findings. However, in more recent times, this methodology has been challenged, with high quality of evidence suggesting that analysis of a single ejaculate may be sufficient for determination of further evaluation and management plans (55). In general, men with a single abnormality in sperm concentration, sperm motility or sperm morphology have an approximately 2- to 3-fold risk of infertility, men with 2 abnormalities have a 5- to 7-fold risk of infertility and men with 3 abnormalities have a 16 fold increased risk of infertility (56). However, the fact that men with abnormal sperm parameters may still be fertile, while men with normal sperm parameters may struggle with conception highlights the observational nature of semen analysis, which is not necessarily a test of sperm function. Importantly, for the treatment with in vitro fertilization, only a few million sperm are needed (57). Nonsurgical management options for patients with azoospermia are limited. Patients with obstructive azoospermia should be referred to a urologist who can evaluate them for a testicular biopsy or sperm extraction procedure. Occasionally, abundant round cells are noted in the semen of infertile men. These cells are either white blood cells or immature germ cells (58). White blood cells of greater than 1 million leukocytes/mL of ejaculate may suggest a bacterial infection, although semen cultures or other tests for pathogens are frequently nondiagnostic (59). Lastly, there are several specialized semen and sperm tests such as antisperm antibody assays and assessments of acrosome reactivity and sperm deoxyribonucleic acid (DNA) damage that can be performed (60,61), although given the lack of strong data in support of their implementation and the absence of standardized methodologies, practice guidelines do not endorse their widespread usage.
Figure 2.
An approach to the evaluation and treatment of man with infertility from oligozoospermia. Abbreviations: ART, assisted reproductive technology; FSH, follicle-stimulating hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LH, luteinizing hormone.
Hormonal evaluation
The key hormonal blood assessments in men with oligozoospermia are the gonadotropins, FSH and LH, and testosterone (62). Some providers would also recommend measurement of prolactin and iron saturation to exclude the possibility of hyperprolactinemia or hemochromatosis (63). Ideally, the testosterone should be measured on a sample obtained before 10 am and repeated if low. If the testosterone and gonadotropins are low (hypogonadotropic hypogonadism) or inappropriately normal in the setting of a low testosterone, then additional testing examining for pituitary disease is indicated, including magnetic resonance imaging and testing of the thyroid and adrenal axis (Fig. 2, left side). If the testosterone is low and the FSH and LH are elevated (hypergonadotropic hypogonadism) (Fig. 2, middle), men should have a karyotype and genetic analysis of the Y chromosome (see following discussion). If the genetic tests are normal, other causes of primary hypogonadism such as cryptorchidism or toxic exposures (radiation, chemotherapy or heavy metal exposure, or postpubertal mumps orchitis) should be considered. In many of these men, however, no clear etiology of testicular failure can be identified and autoimmune damage of the testes is presumed (64). The most common presentation for men with infertility from oligozoospermia is either normal gonadotropins and testosterone or elevation in only the FSH (Fig. 2, right side). An FSH level greater than 8 IU/L suggests a loss of inhibin B, the active isoform of inhibin in males (65) and implies significant damage of the seminiferous tubules. This is frequently observed in young men with azoospermia and infertility after childhood or early adult cancers treated with radiation or chemotherapy (66). In the absence of this history, genetic testing is appropriate as for the patient with primary hypogonadism. Very infrequently, a man with oligozoospermia will have an elevated testosterone coupled with an elevated LH that is likely due to an androgen receptor defect, a mild form of androgen insensitivity syndrome (67).
Genetic testing
Genetic testing is indicated in all cases of nonobstructive azoospermia, as well as in cases where sperm densities are found to be below 5 million/mL (68). It is reasonable in men with elevated FSH and LH and small testes to exclude mosaic KS with a karyotype, which affects around 7% to 10% of men with KS (69). This diagnosis is important for both management of the infertility and for the man long-term as KS increases the risk of certain conditions such as diabetes mellitus (70). A karyotype will also diagnose chromosome translations, which can be present in up to 10% of men with severe oligozoospermia (71). Routine karyotyping of both partners prior to utilization of assisted reproductive technologies has been suggested, due to the higher background prevalence of genetic anomalies as compared to the fertile population (72). Men with detected agenesis or atresia of the vasa deferentia have a high likelihood of carrying CFTR mutations and should be referred for genetic counseling, as well as partner genetic screening. Lastly, another 10% of men with sperm concentrations of fewer than 5 million sperm/mL will have detectable microdeletions in their Y chromosome, with attendant implications for infertility management as discussed earlier (73). Additionally, these mutations will be transmitted to any viable male offspring (74), so genetic counseling is mandatory in this situation (75,76).
Imaging
Imaging is useful in men with infertility from oligozoospermia if scrotal mass is discovered on physical examination and the nature of the mass is uncertain (77). Additionally, testicular ultrasound may prove beneficial in patients with a history of cryptorchidism given the associated increased background incidence of testicular cancer in such cases and considering that early tumors may be nonpalpable on scrotal examination. If ejaculatory duct obstruction is suspected on the basis of reduced semen volume and reduce seminal plasma fructose, a transrectal ultrasound may reveal dilation of the seminal vesicles as a result of the obstruction (78).
Treatment of Oligozoospermia
Medication withdrawal
If the medication history reveals the use of one of the medications known or suspected to adversely affect fertility (Table 2), discontinuing the medication on a trial basis, if possible, is reasonable. Given the approximately 64-day maturation time of sperm in men (79), it may take several months for sperm parameters to improve once any offending medications is stopped.
Medical therapy with gonadotropins
For men with infertility and low gonadotropin concentrations in the setting of genuine, organic hypogonadotropic hypogonadism, therapy with gonadotropins can result in significant increases in sperm output over time. Improvements in sperm output are notable after 3 to 6 months of therapy, which can lead to conception in a majority of cases over 1 to 2 years of therapy (80,81). Due to its lower cost and longer half-life, human chorionic gonadotropin (hCG) is universally favored over the shorter-acting recombinant LH for treatment of hypogonadotropic hypogonadism in men. Indeed, hCG treatment alone is sometimes successful in men with adult onset hypogonadotropic hypogonadism and normal testicular volume (82). However, men with congenital idiopathic hypogonadotropic hypogonadism and/or a small baseline testicular volume of less than 4 mL will likely require therapy with both hCG and FSH (80-82). FSH and hCG are proteins, meaning they need to be administered by injection several times a week. Both FSH and LH are available in several formulations (Table 4). Given the duration of therapy required for fertility, the lower cost of the generic preparations is highly favored. In addition, the injections can be given subcutaneously, rather than intramuscularly with good pharmacodynamic effect, which is much less painful for patients (83). The hCG dose is usually titrated to a serum testosterone in the mid-normal range and no higher, as an elevated serum testosterone could suppress any residual endogenous FSH release. A dose that normalizes serum testosterone is likely to significantly improve intratesticular testosterone (84), which is necessary for spermatogenesis. Whether additional serum biomarkers of intratesticular testosterone such as serum 17-hydroxyprogesterone are helpful in hCG dose titration in infertile men is an area of current investigation (85). In men with congenital hypogonadotropic hypogonadism, hCG alone is often administered for 6 months, prior to the addition of FSH to “masculinize” the testes, although this approach has not been tested head to head with concomitant FSH and hCG administration (81). Indeed, some authors have argued that this regimen may be harmful and FSH should be administered first or at least concomitantly with hCG (82). In a man with hypogonadotropic hypogonadism from any cause who is using testosterone, most clinicians would advise discontinuing testosterone therapy when attempting to improve sperm output due to the negative feedback inhibition on FSH secretion. The use of pulsatile GnRH agonist therapy can also be considered in patients with hypogonadotropic hypogonadism (86). This option has improved outcomes compared to gonadotropin therapy (87) but is challenging for patients to use. Moreover, supply of GnRH is limited, so this therapy is not widely utilized outside of research environments. Lastly, some trials have suggested a role for FSH therapy in men with idiopathic infertility and normal circulating FSH concentrations (88). Meta-analysis of several of these trial show benefit, but there is significant heterogeneity across trials (89). As a result, this treatment has not been widely adopted. Of particular note is the notion that FSH therapy may reduce sperm DNA fragmentation in infertile men (90). Hopefully, additional larger-scale, multicenter, randomized trials of FSH will provide more clarity on the potential benefits of this therapy for men with idiopathic infertility.
Table 4.
Medications used to treat oligozoospermia in infertile men
| Medication Class | Indication | Dose | Example Drugs (Trade Names)/Notes |
|---|---|---|---|
| Clear benefit | |||
| β-human chorionic gonadotropin | Hypogonadotropic hypogonadism | 1000–2500 IU SQ 2–3 times weekly (titrated to serum testosterone) | Generica (Novarel®†, Pregnyl®) (Overle®)b |
| Follicle- stimulating hormone | Hypogonadotropic hypogonadism | 75–300 IU SQ 2–3 times weekly | Urofollitropinc (Bravelle®); menotropins with combined FSH/LH activityc (Menopur®); Follitropin alphab (Follistim®, Gonal F®) |
| Human luteinizing hormone | Hypogonadotropic hypogonadism | 75 IU SQ daily | Lutropin alphab (Luveris®)d (not recommended-use hCG) |
| Alpha-agonists | Retrograde ejaculation | 60 mg po q 6 h × six doses prior to ejaculation | Pseudoephedrine (Sudafed®); contraindicated in hypertension |
| Gonadotropin- releasing hormone | Hypogonadotropic hypogonadism | 25-600 ng/kg SQ every 2 h | Generic; requires continuous pulsatile infusion, supply issues |
| Dopamine agonists | Hyperprolactinemia | 0.25-1.5 mg po 1-2 times weekly | Cabergoline (Dostinex) |
| Phosphodiesterase inhibitors | Erectile dysfunction | 25-100 mg po prn 25-100 mg po prn 5-20 mg po prn | Sildenafil (generic); Vardenafil (Levitra®); Tadalafil (Cialis®) |
| Possible benefit | |||
| Selective estrogen receptor modulators | Idiopathic male infertility | 25-50 mg po daily | Clomipheme citratee (generic); potential adverse effects on mood, bone, and sexual function |
| Aromatase inhibitors | Idiopathic male infertility | 1 mg po daily to twice weekly | Anastrazolee (generic); potential mood, sexual function, and bone mineral density concerns |
| Experimental | |||
| Retinoid acid analogs | Idiopathic male infertility | 20 mg po bid × 6 months | Isotretinoine ; adverse events include depression and elevated triglycerides. Requires registration for prescription |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aDerived from urine of pregnant women.
bProduced by recombinant DNA technology.
cDerived from urine of post-menopausal women.
dNot available in the United States.
eNot Food and Drug Administration–approved for the treatment of male infertility.
Treatment of elevated prolactin
If a man with infertility is found to have an elevated prolactin, the infertility may be correctable by normalizing the serum prolactin concentration. Serum prolactin can be elevated either due to certain medications such as dopamine antagonists (antipsychotics, anti-emetics, or certain antihypertensives) or secondary to prolactin-secreting pituitary adenomas. If a suspected medication can be safely discontinued, this should be attempted first. If the elevated prolactin is due to a prolactin-secreting pituitary adenoma, it can be treated with the dopamine agonist cabergoline (Table 4) (91). If a man has both an elevated prolactin and a low serum testosterone concentration, the testosterone should be monitored on treatment to ensure that it normalizes. If not, concomitant treatment with gonadotropins may be required to fully restore fertility (92).
Selective estrogen receptor modulators and aromatase inhibitors
Selective estrogen receptor modulators such as clomiphene citrate (Table 4) have proven to be useful for ovulation induction in infertile women and have been studied in men with oligozoospermia from hypogonadotropic hypogonadism. It should be noted that use of these agents in men is strictly off-label, as they have not been Food and Drug Administration–approved for treatment of male hypogonadism or infertility. While clomiphene has the advantage of being dosed orally, data regarding its use in men with infertility from oligozoospermia from randomized controlled trials are conflicting (93-95). As this approach relies on increasing gonadotropin release from reduced estrogen negative feedback at the pituitary, it should not be used in men with abnormally functioning or absent pituitary glands. Similarly, the use of aromatase inhibitors has shown some promise in men with infertility and hypogonadism (96) but is not recommended in place of gonadotropin therapy for the treatment of men with hypogonadotropic hypogonadism or idiopathic infertility based on insufficient evidence (97). In addition, there exists some concern than antiestrogen therapy may impair male bone health and sexual function (98). In that light, these agents are not intended for long-term use, as studies have demonstrated a significant loss of bone mineral density after as little 12 months of therapy (99). Larger trials are needed before strong conclusions about the efficacy of these approaches can be made.
Antibiotics for leukocytospermia
The semen analysis in some men with infertility demonstrates numerous leukocytes that may suggest a genital infection such as chronic prostatitis. If an infection, such as chlamydia, can be diagnosed, empiric treatment with antibiotics such as doxycycline is reasonable (100). Unfortunately, culture and even DNA tests for known pathogens are usually nondiagnostic, and empiric antibiotics do not demonstrate evidence of benefit.
Sympathomimetics for retrograde ejaculation
The use of sympathomimetics, such as pseudoephedrine (Table 4), can be used to restore antegrade ejaculation in men with retrograde ejaculation by promoting bladder neck contraction (101,102). In cases refractory to medical treatment, urinary sperm retrieval remains an option.
Vitamins and supplements
Semen from men with infertility demonstrates increased evidence of oxidative stress (96). This observation has led to numerous clinical studies examining the administration of various antioxidants as treatments for oligozoospermia with some improvements in sperm parameters (103). Unfortunately, no clear evidence demonstrating benefits to this approach in terms of improved live birth rates have been shown (104). Recently, a large well-done study was performed using folic acid and zinc in over 2000 couples, roughly half of whom had abnormal semen parameters (105). Unfortunately, there was no benefit to treatment in terms of live birth or other outcomes in either the men with abnormal semen or the population as a whole. Nevertheless, this is exactly the type of large, randomized, prospective, double-blinded, placebo-controlled trial that is needed in the areas of male infertility treatment to be able to effectively counsel patients regarding their therapeutic options. Lastly, our group performed a pilot study of isotretinoin, an active form of vitamin A, in a small group of men with oligozoospermia and infertility with promising results (106); however, these results will require validation in a larger, randomized, blinded study before this treatment can be recommended for men with infertility from oligozoospermia.
Urological referral
A large varicocele can often be identified on physical examination of the infertile man. Surgical repair or embolization of the varicocele can result in improvements in seminal parameters, including appreciably increased sperm counts in oligozoospermic men (107). It is important to note that for varicoceles that are only detected radiographically and are not clinically apparent, surgical repair is not routinely recommended, given lack of evidence to suggest a clinical benefit. Transurethral resection of the ejaculatory ducts has also been shown to effect improvements in seminal parameters, with up to 38% of men converting from oligozoospermia or azoospermia to having normal sperm counts (108). As a result, referral of select patients to urology for consideration of a surgical procedure is appropriate in these circumstances, especially for a patient desiring more than 1 child.
Assisted reproductive technologies
For men with oligozoospermia without a treatable cause, referral for consideration of assisted reproductive techniques is appropriate. Indeed, if the female partner’s age is 35 or greater, such a referral should be made expeditiously. Intrauterine insemination is often considered as initial therapy; however, the data supporting this procedure for male infertility from oligozoospermia are weak (109). Therefore, couples may opt to proceed directly to in vitro fertilization with or without intracytoplasmic sperm injection. Fertilization rates in the 50% to 75% range are reported, and the clinical pregnancy rate per cycle is around 20%, with an overall cumulative live birth rate of around 50% after 3 cycles (110,111). Although success rates are generally largely determined by female factors, they can also be impacted by a variety of male-related factors including sperm DNA damage, sperm structural defects, and low sperm vitality. Lastly, men should be counseled regarding the option of using donor sperm, either from an anonymous donor or from a family member. Intrauterine insemination with donor sperm has a high rate of pregnancy and live birth in female partners without an obvious source of reduced fecundity (112).
Psychological and medical ramifications of infertility from oligozoospermia
The diagnosis of infertility can have a significant, detrimental psychological impact on men and their partners (113). Clinicians should expect men with infertility to experience a range of emotions including sexual inadequacy or feelings of “not being a real man” (114). Approaches to the thoughtful delivery of bad news, including delivering the news in person and in clear, easy-to-understand language with adequate time for questions is strongly recommended (115). In addition, referral to mental health colleagues may be appropriate in certain circumstances. More globally, there is emerging evidence suggesting that poor semen parameters and a diagnosis of male infertility may provide a window into a man’s general health, as there appear to be associations between male infertility and cardiovascular, metabolic, oncologic, and autoimmune disease, as well as early mortality (116,117). Such associations may arise from genetic, developmental, and lifestyle factors (37,118). Therefore, an infertility evaluation provides a valuable opportunity for medical assessment and intervention in areas such as cardiovascular risk assessment and cancer screening, which extend far beyond a man’s reproductive health.
Acknowledgments
Financial Support : JKA is supported, in part, by grants K24HD082231 and R01HD098039 from the Eunice Kennedy Shriver National Institute of Child Health and Human Reproduction, a Division of the National Institutes of Health.
Additional Information
Disclosure Summary : JTC has no disclosures. JKA has received research and consultative funding from Clarus Therapeutics.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. ; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520-1524. [DOI] [PubMed] [Google Scholar]
- 2. Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324-1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18-e25. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization, ed. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 5. Shaw W, Padubidri V, Daftary S, Howkins J, Bourne G. Infertility and sterility. In: Shaw’s Textbook of Gynaecology. 16th ed. New Delhi, India: Elsevier; 2015:237-262. [Google Scholar]
- 6. Punab M, Poolamets O, Paju P, et al. Causes of male infertility in 1737 patients. Hum Reprod. 2017;32(1):18-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anawalt BD, Braunstein GD. Testes. In: Gardner DG, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 10th ed. New York, NY: McGraw-Hill Education; 2017. Available at: accessmedicine.mhmedical.com/content.aspx?aid=1144817135. Accessed April 6, 2020. [Google Scholar]
- 8. Cangiano B, Swee DS, Quinton R, Bonomi M. Genetics of congenital hypogonadotropic hypogonadism: peculiarities and phenotype of an oligogenic disease. Hum Genet. 2020. doi: 10.1007/s00439-020-02147-1. [DOI] [PubMed] [Google Scholar]
- 9. Nachtigall LB, Boepple PA, Pralong FP, Crowley WF Jr. Adult-onset idiopathic hypogonadotropic hypogonadism–a treatable form of male infertility. N Engl J Med. 1997;336(6):410-415. [DOI] [PubMed] [Google Scholar]
- 10. Samperi I, Lithgow K, Karavitaki N. Hyperprolactinaemia. J Clin Med. 2019;8(12):E2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langrand C, Bihan H, Raverot G, et al. Hypothalamo-pituitary sarcoidosis: a multicenter study of 24 patients. Qjm. 2012;105(10):981-995. [DOI] [PubMed] [Google Scholar]
- 12. Wahid S, Ball S. The pituitary gland and hereditary haemochromatosis. Lancet. 2001;357(9250):115. [DOI] [PubMed] [Google Scholar]
- 13. Javorsky BR, Aron DC, Findling JW, Tyrrell JB. Hypothalamus and pituitary gland. In: Gardner DG, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 10th ed. New York, NY: McGraw-Hill Education; 2017. Available at: accessmedicine.mhmedical.com/content.aspx?aid=1144814187. Accessed April 6, 2020. [Google Scholar]
- 14. Engels M, Gehrmann K, Falhammar H, et al. ; dsd-LIFE group . Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(3):285-294. [DOI] [PubMed] [Google Scholar]
- 15. Otten BJ, Stikkelbroeck NMML, Hermus ARMM. Hypogonadism in males with congenital adrenal hyperplasia. In: Winters SJ, ed. Male Hypogonadism: Basic, Clinical, and Therapeutic Principles. Contemporary Endocrinology. Totowa, NJ: Humana Press; 2004:125-137. [Google Scholar]
- 16. Southren AL, Olivo J, Gordon GG, Vittek J, Brener J, Rafii F. The conversion of androgens to estrogens in hyperthyroidism. J Clin Endocrinol Metab. 1974;38(2):207-214. [DOI] [PubMed] [Google Scholar]
- 17. Rajender S, Monica MG, Walter L, Agarwal A. Thyroid, spermatogenesis, and male infertility. Front Biosci (Elite Ed). 2011;3:843-855. [DOI] [PubMed] [Google Scholar]
- 18. Wagner MS, Wajner SM, Maia AL. Is there a role for thyroid hormone on spermatogenesis? Microsc Res Tech. 2009;72(11):796-808. [DOI] [PubMed] [Google Scholar]
- 19. La Vignera S, Vita R, Condorelli RA, et al. Impact of thyroid disease on testicular function. Endocrine. 2017;58(3):397-407. [DOI] [PubMed] [Google Scholar]
- 20. Krassas GE, Pontikides N. Male reproductive function in relation with thyroid alterations. Best Pract Res Clin Endocrinol Metab. 2004;18(2):183-195. [DOI] [PubMed] [Google Scholar]
- 21. Ansari MS, Almalki MH. Primary hypothyroidism with markedly high prolactin. Front Endocrinol (Lausanne). 2016;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salzano A, D’Assante R, Heaney LM, et al. Klinefelter syndrome, insulin resistance, metabolic syndrome, and diabetes: review of literature and clinical perspectives. Endocrine. 2018;61(2):194-203. [DOI] [PubMed] [Google Scholar]
- 23. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622-626. [DOI] [PubMed] [Google Scholar]
- 24. Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16(2):163-170. [DOI] [PubMed] [Google Scholar]
- 25. Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660-1665. [DOI] [PubMed] [Google Scholar]
- 26. Krausz C, Casamonti E. Spermatogenic failure and the Y chromosome. Hum Genet. 2017;136(5):637-655. [DOI] [PubMed] [Google Scholar]
- 27. Dohle GR, Halley DJ, Van Hemel JO, et al. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod. 2002;17(1):13-16. [DOI] [PubMed] [Google Scholar]
- 28. Masson P, Brannigan RE. The varicocele. Urol Clin North Am. 2014;41(1):129-144. [DOI] [PubMed] [Google Scholar]
- 29. Modgil V, Rai S, Ralph DJ, Muneer A. An update on the diagnosis and management of ejaculatory duct obstruction. Nat Rev Urol. 2016;13(1):13-20. [DOI] [PubMed] [Google Scholar]
- 30. Levine H, Jørgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3):221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311. [DOI] [PubMed] [Google Scholar]
- 33. Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93(7):2222-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duits FH, van Wely M, van der Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. 2010;94(4):1356-1359. [DOI] [PubMed] [Google Scholar]
- 35. Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90(3):619-626. [DOI] [PubMed] [Google Scholar]
- 36. Fernandez CJ, Chacko EC, Pappachan JM. Male obesity-related secondary hypogonadism–pathophysiology, clinical implications and management. Eur Endocrinol. 2019;15(2):83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kasman AM, Zhang CA, Li S, Stevenson DK, Shaw GM, Eisenberg ML. Association of preconception paternal health on perinatal outcomes: analysis of U.S. claims data. Fertil Steril. 2020;113(5):947-954. [DOI] [PubMed] [Google Scholar]
- 38. Blendon RJ, Benson JM. The Public and the Opioid-Abuse Epidemic. N Engl J Med. 2018;378(5):407-411. [DOI] [PubMed] [Google Scholar]
- 39. de Vries F, Bruin M, Lobatto DJ, et al. Opioids and their endocrine effects: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2019;105(4:1020-1029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antony T, Alzaharani SY, El-Ghaiesh SH. Opioid-induced hypogonadism: pathophysiology, clinical and therapeutics review. Clin Exp Pharmacol Physiol. 2020;47(5):741-750. [DOI] [PubMed] [Google Scholar]
- 41. de Ronde W, Smit DL. Anabolic androgenic steroid abuse in young males. Endocr Connect. 2020;9(4):R102-R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shankara-Narayana N, Yu C, Savkovic S, et al. Rate and extent of recovery from reproductive and cardiac dysfunction due to androgen abuse in men. J Clin Endocrinol Metab 2020;105(6):1827–1839. [DOI] [PubMed] [Google Scholar]
- 43. Hackney AC. Hypogonadism in exercising males: dysfunction or adaptive-regulatory adjustment? Front Endocrinol (Lausanne). 2020;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35(2):109-125. [PMC free article] [PubMed] [Google Scholar]
- 45. Salehian B, Kejriwal K. Glucocorticoid-induced muscle atrophy: mechanisms and therapeutic strategies. Endocr Pract. 1999;5(5):277-281. [DOI] [PubMed] [Google Scholar]
- 46. Amory JK, Wang C, Swerdloff RS, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92(5):1659-1665. [DOI] [PubMed] [Google Scholar]
- 47. du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. 2015;32(11):1575-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 2009;29(1):64-73. [DOI] [PubMed] [Google Scholar]
- 49. Nørr L, Bennedsen B, Fedder J, Larsen ER. Use of selective serotonin reuptake inhibitors reduces fertility in men. Andrology. 2016;4(3):389-394. [DOI] [PubMed] [Google Scholar]
- 50. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shankara-Narayana N, Di Pierro I, Fennell C, et al. Sperm cryopreservation prior to gonadotoxic treatment: experience of a single academic centre over 4 decades. Hum Reprod. 2019;34(5):795-803. [DOI] [PubMed] [Google Scholar]
- 52. Masarani M, Wazait H, Dinneen M. Mumps orchitis. J R Soc Med. 2006;99(11):573-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Overhoff S, Dahm C, Rivero MA, Ferreira U, Schirren C. Andrological findings in hemicastration, lack of a testis, and reduced testicular volume. Andrologia. 1989;21(5):490-497. [PubMed] [Google Scholar]
- 54. Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: a systematic review and meta-analysis. Sports Med. 2017;47(9):1869-1883. [DOI] [PubMed] [Google Scholar]
- 55. Barratt CLR, Björndahl L, De Jonge CJ, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update. 2017;23(6):660-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guzick DS, Overstreet JW, Factor-Litvak P, et al. ; National Cooperative Reproductive Medicine Network . Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345(19):1388-1393. [DOI] [PubMed] [Google Scholar]
- 57. Wolf DP, Byrd W, Dandekar P, Quigley MM. Sperm concentration and the fertilization of human eggs in vitro. Biol Reprod. 1984;31(4):837-848. [DOI] [PubMed] [Google Scholar]
- 58. Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63(6):1143-1157. [DOI] [PubMed] [Google Scholar]
- 59. Lackner J, Schatzl G, Horvath S, Kratzik C, Marberger M. Value of counting white blood cells (WBC) in semen samples to predict the presence of bacteria. Eur Urol. 2006;49(1):148-52; discussion 152. [DOI] [PubMed] [Google Scholar]
- 60. Muller CH. Rationale, interpretation, validation, and uses of sperm function tests. J Androl. 2000;21(1):10-30. [PubMed] [Google Scholar]
- 61. Evenson DP. The sperm chromatin structure assay (SCSA) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Animal Repro Sci. 2016;169:56-75. [DOI] [PubMed] [Google Scholar]
- 62. Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin Reprod Med. 2009;27(2):149-158. [DOI] [PubMed] [Google Scholar]
- 63. Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98(9):3532-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Silva CA, Cocuzza M, Carvalho JF, Bonfá E. Diagnosis and classification of autoimmune orchitis. Autoimmun Rev. 2014;13(4-5):431-434. [DOI] [PubMed] [Google Scholar]
- 65. Anawalt BD, Bebb RA, Matsumoto AM, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81(9):3341-3345. [DOI] [PubMed] [Google Scholar]
- 66. Kenney LB, Antal Z, Ginsberg JP, et al. Improving male reproductive health after childhood, adolescent, and young adult cancer: progress and future directions for survivorship research. J Clin Oncol. 2018;36(21):2160-2168. [DOI] [PubMed] [Google Scholar]
- 67. Batista RL, Costa EMF, Rodrigues AS, et al. Androgen insensitivity syndrome: a review. Arch Endocrinol Metab. 2018;62(2):227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Colpi GM, Francavilla S, Haidl G, et al. European academy of andrology guideline management of oligo-astheno-teratozoospermia. Andrology. 2018;6(4):513-524. [DOI] [PubMed] [Google Scholar]
- 69. Wikström AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25(2):239-250. [DOI] [PubMed] [Google Scholar]
- 70. Calogero AE, Giagulli VA, Mongioì LM, et al. ; Klinefelter ItaliaN Group (KING) . Klinefelter syndrome: cardiovascular abnormalities and metabolic disorders. J Endocrinol Invest. 2017;40(7):705-712. [DOI] [PubMed] [Google Scholar]
- 71. McLachlan RI, O’Bryan MK. Clinical review#: state of the art for genetic testing of infertile men. J Clin Endocrinol Metab. 2010;95(3):1013-1024. [DOI] [PubMed] [Google Scholar]
- 72. Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369-384. [DOI] [PubMed] [Google Scholar]
- 73. Martin RH. Cytogenetic determinants of male fertility. Hum Reprod Update. 2008;14(4):379-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Page DC, Silber S, Brown LG. Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility. Hum Reprod. 1999;14(7):1722-1726. [DOI] [PubMed] [Google Scholar]
- 75. Krausz C, Chianese C. Genetic testing and counselling for male infertility. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):244-250. [DOI] [PubMed] [Google Scholar]
- 76. Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology. 2014;2(3):339-350. [DOI] [PubMed] [Google Scholar]
- 77. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21(1):56-83. [DOI] [PubMed] [Google Scholar]
- 78. Jarow JP. Transrectal ultrasonography in the diagnosis and management of ejaculatory duct obstruction. J Androl. 1996;17(5):467-472. [PubMed] [Google Scholar]
- 79. Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140(3563):184-186. [DOI] [PubMed] [Google Scholar]
- 80. Young J, Xu C, Papadakis GE, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40(2):669-710. [DOI] [PubMed] [Google Scholar]
- 81. Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94(3):801-808. [DOI] [PubMed] [Google Scholar]
- 82. Prior M, Stewart J, McEleny K, Dwyer AA, Quinton R. Fertility induction in hypogonadotropic hypogonadal men. Clin Endocrinol (Oxf). 2018;89(6):712-718. [DOI] [PubMed] [Google Scholar]
- 83. Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod Biomed Online. 2002;4(2): 106-115. [DOI] [PubMed] [Google Scholar]
- 84. Amory JK, Coviello AD, Page ST, Anawalt BD, Matsumoto AM, Bremner WJ. Serum 17-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil Steril. 2008;89(2):380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Patel A, Patel P, Bitran J, Ramasamy R. Can serum 17-hydroxyprogesterone and insulin-like factor 3 be used as a marker for evaluation of intratesticular testosterone? Transl Androl Urol. 2019;8(Suppl 1):S58-S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87(9):4128-4136. [DOI] [PubMed] [Google Scholar]
- 87. Mao JF, Liu ZX, Nie M, et al. Pulsatile gonadotropin-releasing hormone therapy is associated with earlier spermatogenesis compared to combined gonadotropin therapy in patients with congenital hypogonadotropic hypogonadism. Asian J Androl. 2017;19(6):680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Foresta C, Bettella A, Garolla A, Ambrosini G, Ferlin A. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: a prospective, controlled, randomized clinical study. Fertil Steril. 2005;84(3):654-661. [DOI] [PubMed] [Google Scholar]
- 89. Santi D, Granata AR, Simoni M. FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis. Endocr Connect. 2015;4(3):R46-R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Garolla A, Ghezzi M, Cosci I, et al. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine. 2017;56(2):416-425. [DOI] [PubMed] [Google Scholar]
- 91. De Rosa M, Colao A, Di Sarno A, et al. Cabergoline treatment rapidly improves gonadal function in hyperprolactinemic males: a comparison with bromocriptine. Eur J Endocrinol. 1998;138(3):286-293. [DOI] [PubMed] [Google Scholar]
- 92. Salenave S, Trabado S, Maione L, Brailly-Tabard S, Young J. Male acquired hypogonadotropic hypogonadism: diagnosis and treatment. Ann Endocrinol (Paris). 2012;73(2): 141-146. [DOI] [PubMed] [Google Scholar]
- 93. A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility. World Health Organization. Int J Androl. 1992;15(4):299-307. [DOI] [PubMed] [Google Scholar]
- 94. Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1(5):749-757. [DOI] [PubMed] [Google Scholar]
- 95. Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Calogero AE, La Vignera S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert Opin Pharmacother. 2019;20(12):1517-1525. [DOI] [PubMed] [Google Scholar]
- 96. Shoshany O, Abhyankar N, Mufarreh N, Daniel G, Niederberger C. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertil Steril. 2017;107(3):589-594. [DOI] [PubMed] [Google Scholar]
- 97. Del Giudice F, Busetto GM, De Berardinis E, et al. A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J Androl. 2020;22(4):360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94(12):4785-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hamada A, Agarwal A, Sharma R, French DB, Ragheb A, Sabanegh ES Jr. Empirical treatment of low-level leukocytospermia with doxycycline in male infertility patients. Urology. 2011;78(6):1320-1325. [DOI] [PubMed] [Google Scholar]
- 101. Mehta A, Sigman M. Management of the dry ejaculate: a systematic review of aspermia and retrograde ejaculation. Fertil Steril. 2015;104(5):1074-1081. [DOI] [PubMed] [Google Scholar]
- 102. Shoshany O, Abhyankar N, Elyaguov J, Niederberger C. Efficacy of treatment with pseudoephedrine in men with retrograde ejaculation. Andrology. 2017;5(4):744-748. [DOI] [PubMed] [Google Scholar]
- 103. Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50(11):e13126. [DOI] [PubMed] [Google Scholar]
- 104. Duca Y, Calogero AE, Cannarella R, Condorelli RA, La Vignera S. Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin Pharmacother. 2019;20(1):55-67. [DOI] [PubMed] [Google Scholar]
- 105. Schisterman EF, Sjaarda LA, Clemons T, et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA. 2020;323(1):35-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Amory JK, Ostrowski KA, Gannon JR, et al. Isotretinoin administration improves sperm production in men with infertility from oligoasthenozoospermia: a pilot study. Andrology. 2017;5(6):1115-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kroese AC, de Lange NM, Collins J, Evers JL. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. [DOI] [PubMed] [Google Scholar]
- 108. Avellino GJ, Lipshultz LI, Sigman M, Hwang K. Transurethral resection of the ejaculatory ducts: etiology of obstruction and surgical treatment options. Fertil Steril. 2019;111(3):427-443. [DOI] [PubMed] [Google Scholar]
- 109. Bensdorp AJ, Cohlen BJ, Heineman MJ, Vandekerckhove P. Intra-uterine insemination for male subfertility. Cochrane Database Syst Rev. 2007; 4(4):CD000360. [DOI] [PubMed] [Google Scholar]
- 110. Schlegel PN, Girardi SK. Clinical review 87: in vitro fertilization for male factor infertility. J Clin Endocrinol Metab. 1997;82(3):709-716. [DOI] [PubMed] [Google Scholar]
- 111. Gnoth C, Maxrath B, Skonieczny T, Friol K, Godehardt E, Tigges J. Final ART success rates: a 10 years survey. Hum Reprod. 2011;26(8):2239-2246. [DOI] [PubMed] [Google Scholar]
- 112. Hu L, Liao AH, Song S, Xiao N, Xiang WP, Xiong CL. Evaluation of donor semen quality provided by six sperm banks: a retrospective study of 1877 artificial insemination cycles. Andrologia. 2012;44(Suppl 1): 499-504. [DOI] [PubMed] [Google Scholar]
- 113. Luk BH, Loke AY. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: a systematic review. J Sex Marital Ther. 2015;41(6):610-625. [DOI] [PubMed] [Google Scholar]
- 114. Bechoua S, Hamamah S, Scalici E. Male infertility: an obstacle to sexuality? Andrology. 2016;4(3):395-403. [DOI] [PubMed] [Google Scholar]
- 115. Berkey FJ, Wiedemer JP, Vithalani ND. Delivering bad or life-altering news. Am Fam Physician. 2018;98(2):99-104. [PubMed] [Google Scholar]
- 116. Kasman AM, Del Giudice F, Eisenberg ML. New insights to guide patient care: the bidirectional relationship between male infertility and male health. Fertil Steril. 2020;113(3): 469-477. [DOI] [PubMed] [Google Scholar]
- 117. Choy JT, Eisenberg ML. Male infertility as a window to health. Fertil Steril. 2018;110(5):810-814. [DOI] [PubMed] [Google Scholar]
- 118. Murshidi MM, Choy JT, Eisenberg ML. Male infertility and somatic health. Urol Clin North Am. 2020;47(2):211-217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.