Abstract
Background
Activation of the mammalian target of rapamycin (mTOR) pathway is observed in neurofibromatosis type 1 (NF1) associated low-grade gliomas (LGGs), but agents that inhibit this pathway, including mTOR inhibitors, have not been studied in this population. We evaluate the efficacy of the orally administered mTOR inhibitor everolimus for radiographically progressive NF1-associated pediatric LGGs.
Methods
Children with radiologic-progressive, NF1-associated LGG and prior treatment with a carboplatin-containing chemotherapy were prospectively enrolled on this phase II clinical trial to receive daily everolimus. Whole blood was analyzed for everolimus and markers of phosphatidylinositol-3 kinase (PI3K)/mTOR pathway inhibition. Serial MRIs were obtained during treatment. The primary endpoint was progression-free survival at 48 weeks.
Results
Twenty-three participants (median age, 9.4 y; range, 3.2–21.6 y) were enrolled. All participants were initially evaluable for response; 1 patient was removed from study after development of a malignant peripheral nerve sheath tumor. Fifteen of 22 participants (68%) demonstrated a response, defined as either shrinkage (1 complete response, 2 partial response) or arrest of tumor growth (12 stable disease). Of these, 10/15 remained free of progression (median follow-up, 33 mo). All remaining 22 participants were alive at completion of therapy. Treatment was well tolerated; no patient discontinued therapy due to toxicity. Pharmacokinetic parameters and pre-dose concentrations showed substantial between-subject variability. PI3K/mTOR pathway inhibition markers demonstrating blood mononuclear cell mTOR pathway inactivation was achieved in most participants.
Conclusion
Individuals with recurrent/progressive NF1-associated LGG demonstrate significant disease stability/shrinkage during treatment with oral everolimus with a well-tolerated toxicity profile. Everolimus is well suited for future consideration as upfront or combination therapy in this patient population.
Keywords: everolimus, low-grade glioma, neurofibromatosis, NF1, RAD001, PIK3K/mTOR pathway
Key Points.
1. Neurofibromatosis type 1–associated low-grade gliomas demonstrate mTOR pathway activation.
2. Everolimus results in disease stability/shrinkage in recurrent NF1 low-grade gliomas.
3. Oral everolimus is well tolerated in children with NF1 and recurrent low-grade gliomas.
Importance of the Study.
This study investigates the safety and efficacy of everolimus, an mTOR inhibitor, in children with NF1-associated LGGs. The importance of the study lies in several points—first, there is a lack of effective therapeutic options in children with relapsed/recurrent disease; second, the mechanism of action makes everolimus an ideal compound for use in NF1 LGGs, since mTOR is a known downstream effector of the pathway that is hyperactivated in NF1-deficient tumors; third, prior studies of LGG in children have reported on mixed populations including individuals with and without NF1 using traditional chemotherapy combinations. Oral everolimus demonstrated activity as a single agent for radiographic progressive NF1 LGGs after failure of standard upfront chemotherapy with a favorable toxicity profile.
Low-grade gliomas (LGGs) are the most common brain tumor in children with neurofibromatosis type 1 (NF1). Individuals with NF1-associated optic pathway gliomas are at risk for significant visual loss from tumor progression,1 and LGGs in other locations, including the brainstem, may be associated with significant motor deficits and often require repeated treatment with chemotherapy.2,3 The current chemotherapy regimens for children with NF1-associated LGG, including vincristine/carboplatin or vinblastine,4 often result in arrest of tumor progression but rarely result in recovery of vision or motor function. While prior studies in this population have focused on cytotoxic chemotherapy, targeted molecular approaches upstream of mammalian target of rapamycin (mTOR) are now under way. This is particularly relevant for individuals with progressive and/or symptomatic disease, for whom there is no standard approach to treatment.5
Mammalian TOR is a downstream target of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway, which when activated leads to abnormal cell growth, cell proliferation, and angiogenesis. Everolimus is an oral derivative of rapamycin that acts directly on tumor cells by inhibiting tumor cell growth and proliferation and indirectly by inhibiting angiogenesis, leading to reduced tumor vascularity via inhibition of activity of the tumor cell hypoxia-inducible factor 1, vascular endothelial growth factor (VEGF) production, and VEGF-induced proliferation of endothelial cells.6 The NF1 tumor suppressor protein neurofibromin regulates the mTOR pathway such that mTOR hyperactivation is observed in both NF1-deficent cells and LGGs from NF1 patients and Nf1 genetically engineered mice.7,8 Based on these preclinical observations, we now present the results of a prospective, single arm, phase II clinical trial investigating the efficacy of everolimus in children with NF1 and radiologic progressive LGG previously treated with chemotherapy.
Materials and Methods
Study Population
Study participants were enrolled at one of 10 Department of Defense–funded NF Clinical Trials Consortium sites. Inclusion criteria included age ≥1 year and ≤21 years with a radiologically progressive intracranial LGG. All participants must have met at least 2 of the diagnostic criteria for NF1 and/or were known to have a pathogenic NF1 mutation in peripheral blood-derived DNA. Participants were deemed eligible after failure of a carboplatin-containing regimen and if the treating site had a report of progression on 2 prior imaging studies. Allergy to carboplatin was not considered treatment failure and change in enhancement did not constitute progression. The time period of progression was determined by the individual institution. Before enrollment, the responsible investigator contacted the Study Chair to review patient eligibility and imaging. A biopsy was not mandated and/or required to confirm the diagnosis of LGG. MRI scans were required within 21 days of enrollment, revealing at least one site of measurable disease. Additional eligibility criteria included: Karnofsky performance status ≥50% (if >10 y of age) or Lansky performance status ≥50% (if ≤10 y of age); adequate hematologic, liver, and renal function, fasting serum cholesterol of ≤300 mg/dL OR ≤7.75 mmol/L and fasting triglyceride level of ≤2.5× upper limit of normal. Participants must have recovered from the acute toxic effects of all prior therapies. At least 6 months must have elapsed from involved field radiation therapy, craniospinal radiation, or Gamma Knife radiotherapy if target lesions were included. Participants had to be at least 2 weeks from a major surgical procedure. Exclusion criteria included: uncontrolled significant medical illness (defined by the treating physician); another active tumor requiring concurrent treatment with chemotherapy or radiation therapy; a history of malignancy in the last 3 years; known positive status of human immunodeficiency virus or related illness from acquired immune deficiency syndrome; impaired lung function, with O2 saturation ≤88% on room air; symptomatic cardiac disease; uncontrolled diabetes; uncontrolled hypertension; impairment of gastrointestinal function altering the absorption of everolimus; prior mTOR inhibitor treatment; women who were pregnant, breastfeeding, or of childbearing potential not using contraception.
The study was approved by the Department of Defense Human Research Protection Office, the NF Clinical Trials Consortium organizing institution (University of Birmingham, Alabama) and by the institutional review board (IRB) of each participating institution. Informed assent and consent were obtained from the patient, parent, or guardian according to IRB guidelines.
Treatment
Everolimus was provided by Novartis in pill form as a blister pack. Pills were administered whole by mouth or individually compounded in liquid at the time of use for administration via a gastrostomy or nasogastric tube, once daily, in a fasting state, at 5 mg/m2/dose (max 10 mg), from study day 1 in 28-day cycles continuously for up to 12 cycles. Treatment continued until disease progression, unacceptable toxicity, or 48 weeks of protocol therapy. Toxicity was monitored using Common Terminology Criteria for Adverse Events (CTCAE) v3. Dose delay and modifications were required for persistent thrombocytopenia (<50 000/µL), neutropenia (absolute neutrophil count <750 m3), or any persistent non-hematologic CTCAE grade 2 or 3 toxicity. Everolimus was held until recovery (CTCAE grade ≤1) for any grade ≥3 or greater non-hematologic toxicity possibly, probably, or definitely related to everolimus. Discontinuation of everolimus was required for any grade 4 toxicity or any toxicity requiring treatment interruption for ≥28 days. All interruptions or modification to study drug administration were recorded.
Patient Evaluations
Within 14 days prior to initiating therapy, all participants underwent a baseline evaluation, including medical history, neurologic examination, performance score, laboratory testing, and pulse oximetry. Brain MRI was required within 21 days of enrollment. Participants were followed with physical examination, laboratory testing, and MRIs after cycles 3, 6, 9, at the end of therapy, and then at least yearly following the completion of protocol therapy. MRI measurements pre- and posttherapy were used for assessment of radiographic response and scored in central review by one pediatric neuro-radiologist (S.P.P.) blinded to outcome. Assessment required that the target lesion(s) be measured in at least 2 dimensions for response evaluation. Assessment was performed in 3 dimensions whenever possible, although lesions described in 2 dimensions were adequate for response evaluation. Lesions were assessed by both changes in enhancement (if present) and T2-weighted or fluid-attenuated inversion recovery (FLAIR) changes. Changes in cyst volume were excluded from the measurement of tumor response. Complete response (CR) was defined as disappearance of all measureable disease, partial response (PR) as a 50% reduction in the sum of products of perpendicular diameters of the lesion, and progressive disease (PD) as a 25% increase in the sum of products of all measurable lesions. All others were considered stable disease (SD).
Pharmacokinetic Analysis
Whole blood samples were obtained for pharmacokinetic assessments pre-dose and at 2 and 5 hours post-dose pre-course 2. Everolimus concentrations in blood were determined using a validated liquid chromatography–tandem mass spectrometry assay. Pharmacokinetic analysis was performed using MW/Pharm clinical software (v3.82, Mediware). Pharmacokinetic parameters were Bayesian estimated with a previously published pediatric population pharmacokinetic model as the Bayesian prior.7 Pharmacokinetic parameter estimates such as clearance and volume of distribution were allometrically scaled to body weight to account for body size differences among patients as described previously.9 The trough concentration at 24 hours post-dose (Ctrough) and the 24 h area under the concentration-time curve (AUC0–24h) were estimated for each individual patient.
Pharmacodynamic Analysis
Levels of p70s6 kinase (S6K1), pS6, eukaryotic initiation factor 4E binding protein 1 (4EBP1), and myelocytomatosis oncogene (MYC) activation were analyzed in peripheral blood mononuclear cells using a previously published method.10 The optional pharmacodynamic (PD) samples were obtained pre-drug, day 7, day 14, and pre-odd courses 3, 5, 7, 9, 11, and at completion of therapy. Genetic polymorphism studies for everolimus-associated hypercholesterolemia were obtained and pooled with data from other pediatric studies and will be reported separately.
Statistical Considerations
This study used a one-stage design and assessed best response of progressive LGG in previously treated individuals with NF1. This design was selected because the response for either decrease or increase in tumor volume was predicted to occur slowly, given the biologic nature of these tumors. The treatment regimen was considered promising for further study if after 48 weeks of treatment there was at least a 25% response rate compared with an expected response rate of 5% or less, which was considered evidence of an unpromising regimen. Assuming the true response rate is 5%, the binomial distribution was used to calculate type I errors and power. Based on this approach, enrollment of 20 evaluable participants was estimated for accrual. Evidence of a promising response rate was considered if at least 3 responders were seen among 20 individuals. If the true response rate is 5%, the probability of having 2 or fewer successes is 0.075. Thus, observing 3 or more successes provided sufficient evidence to conclude the response rate is higher than 5%. If the actual rate were 25%, the probability of concluding that the treatment is active (3 or more) was 0.91 (power). Response was based on the presence of CR, PR, or SD after completion of 48 weeks of therapy, including participants who discontinued therapy early for toxicity. SD was included because study entry required only the presence of radiographic progressive tumors, and not clinical progression. Any patient who experienced PD within the 48 weeks of protocol therapy was censored at that point and considered PD for statistical analysis. Progression-free survival (PFS) analysis was performed according to the method of Kaplan–Meier.
Results
Subject Characteristics
Twenty-three individuals were enrolled across 9 institutions between June 2011 and March 2014. Accrual was temporarily halted twice due to shortage of drug supply, resulting in dose recalculations to the closest available formulation for participants on study at that time. Subject characteristics are summarized in Table 1. The median age at time of study enrollment was 9 years (range, 3–21), with 9 males. Tumor location, as determined by central review, included optic pathway (n = 13), temporal lobe/amygdala (n = 4), brainstem (n = 2), and one each in the third ventricle, cerebral peduncle, intraventricular septum, and basal ganglia. All participants had received at least one prior chemotherapy regimen containing carboplatin.
Table 1.
Demographic and clinical characteristics
| Variable | |
|---|---|
| Age, y, mean (range) | 8.8 (3–21) |
| Weight, kg, median (range) | 32.8 (13.8,84.9) |
| Sex, n (%) | |
| Male | 9 (39) |
| Female | 14 (61) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 2 (9) |
| Non-Hispanic | 21 (91) |
| Race, n (%) | |
| White (European, Middle Eastern, or North American) | 21 (91) |
| Black (African American, Haitian) | 0 (0) |
| Native Hawaiian, Other Pacific Islander (Samoan, Guamanian) | 0 (0.0) |
| Asian (Cambodian, Indian, Chinese, Japanese, Korean, Malaysian, Pakistani, Filipino, Vietnamese, and Thai) | 0 (0) |
| American Indian or Alaska Native | 0 (0) |
| Unknown | 1 (4) |
| Other | 1 (4) |
| Tumor location, n (%) | |
| Optic pathway | 13 (57) |
| Temporal lobe | 4 (17) |
| Brainstem | 2 (9) |
| Third ventricle | 1 (4) |
| Cerebral peduncle | 1 (4) |
| Intraventricular septum | 1 (4) |
| Basal ganglia | 1 (4) |
Treatment Response
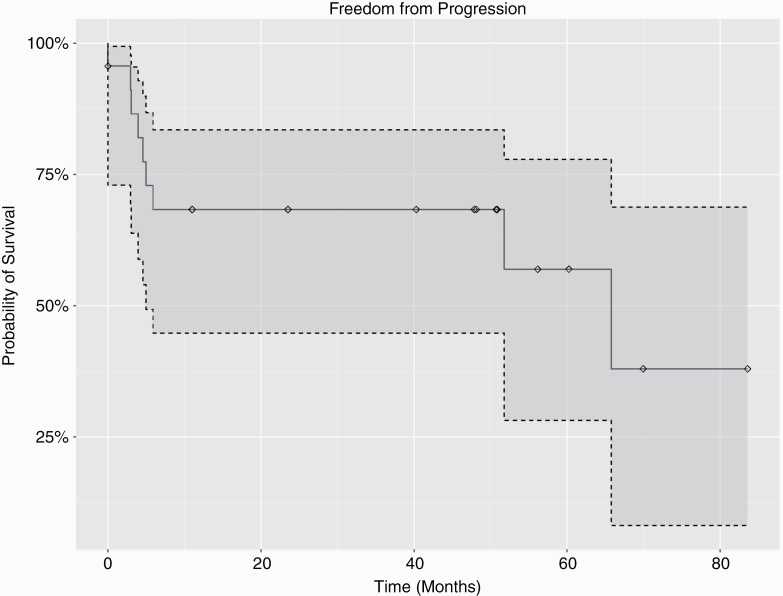
Institutional and blinded central reviews of MR imaging for response to therapy were strongly correlated. Two participants deemed PD at the local site and therefore removed from study participation were not interpreted as PD by independent blinded review. Fig. 1 shows 1- and 2-year PFS. In an exploratory analysis, volumetric response was also calculated by central review using volume of FLAIR hyperintensity and quantitative metrics of tumor size. On 3D/volumetric analysis there was 1 CR, 3 PR, 11 SD, and 8 PD by 48 weeks, and by 2D analysis 1 CR, 2 PR, 12 SD, and 8 PD (Table 2, Supplementary Table 1). Overall 15/22 participants (68%) demonstrated treatment response. With a median follow-up of 33 months, 10/15 (66%) of participants remained free of radiographic progression. All participants, except for the subject with malignant peripheral nerve sheath tumor (MPNST), remained alive at completion of therapy.
Fig. 1.
Progression-free survival. One- and 2-year PFS rates are demonstrated. With a median follow-up of 33 months, 10/15 (66%) of participants with a response remained free of radiographic progression.
Table 2.
Radiographic response by 2D and 3D analysis
| 3D Analysis, n (%) | |||||
|---|---|---|---|---|---|
| 2D Analysis | Complete Response | Partial Response | Stable Disease | Progressive Disease | |
| 2D Analysis | Complete response | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Partial response | 0 (0) | 2 (9) | 0 (0) | 0 (0) | |
| Stable disease | 0 (0) | 1 (4) | 10 (43) | 1 (4) | |
| Progressive disease | 0 (0) | 0 (0) | 1 (4) | 7 (30) | |
| Total | 1 (4) | 3 (13) | 11 (48) | 8 (35) |
Treatment Compliance and Toxicity
Twelve of 23 participants completed all 48 weeks of planned protocol therapy. One participant was removed from therapy after the development of an MPNST. Of the 9 participants who were deemed by local assessment to have PD, treatment durations were 2, 2, 3, 4, 5, 5, 5, 8, and 8 cycles (median, 4.7; range, 2‒8 cycles). No participants discontinued treatment due to toxicity. Overall, treatment was well tolerated (Table 3). The most frequent toxicity reports at least possibly related to everolimus included hematologic, mucositis, and hyperlipidemia/ hypertriglyceridemia. Five participants experienced grade 3 toxicities thought at least possibly related to everolimus therapy, and 1 subject had grade 4 toxicities. The majority of grade 3 and 4 toxicities were experienced by a single subject.
Table 3.
Most common reported toxic effects by CTCAE grade possibly or probably related to everolimus
| Toxicity Type | Grade 2, n | Grade 3, n | Grade 4, n |
|---|---|---|---|
| Hematologic Thrombocytopenia Leukopenia Neutropenia Anemia | 1 4 40 | 1 | |
| Hyperlipidemia | 2 | ||
| Hypertriglyceridemia | 2 | 1 | |
| Transaminitis | 1 | 1 | |
| Hypophosphatemia | 1 | 1 | |
| Pneumonitis | |||
| Mucositis | 9 | 1 | |
| Other non-hematologic toxicity* | 36 | 12 | 4 |
*Twenty-seven other/non-hematologic toxicity reports were from one single subject. These included grade 4 toxicities of electrolyte disturbance (n = 3) and ocular/visual: other (n = 1)
Pharmacokinetic Analysis
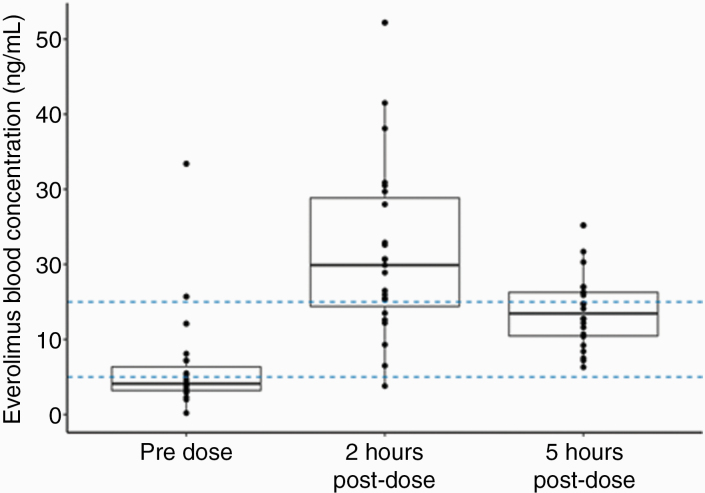
Steady-state pharmacokinetic data were available for all 23 participants. Everolimus pharmacokinetic profiles showed substantial interpatient variability (Fig. 2). Pre-dose trough concentrations (Ctrough) in 73.9% of patients were outside the suggested target range (5–15 ng/mL). This range was based on previous data in patients with tuberous sclerosis complex.11 In 69.6% of patients, trough concentrations were below 5 ng/mL, while in a small number of patients (4.3%) trough concentration was above 15 ng/mL. Pharmacokinetic parameter estimates are summarized in Supplementary Table 1. The estimates of oral clearance and volume of distribution were comparable to pharmacokinetic parameter estimates observed in a phase I study of everolimus in pediatric patients with refractory solid tumors.7
Fig. 2.
Everolimus blood concentrations in patients with NF1 pre-cycle 2. The dashed lines indicate the suggested target range (5–15 ng/mL) of pre-dose trough concentration previously identified in patients with tuberous sclerosis complex.11
PI3K/mTOR Pathway Inhibition
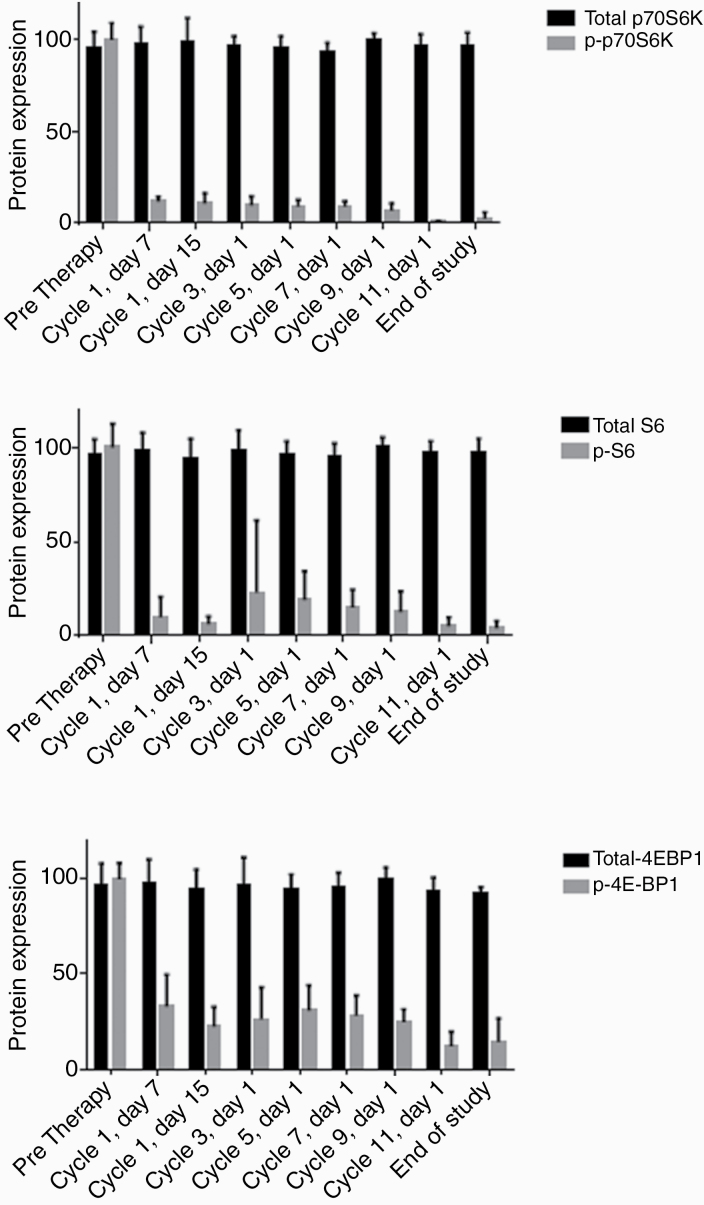
Inhibition of the PI3K/mTOR pathway was assessed. As identified in other pediatric and adult studies, NF1-affected individuals on everolimus demonstrated a rapid reduction in phosphorylated (activated) S6K (pS6K) in peripheral blood mononuclear cells within 2 weeks of initiating therapy, and the suppression was maintained throughout duration of treatment. Similar reductions in 4EBP1 and MYC signaling were also identified (Fig. 3). There was no relationship between downstream mTOR inhibition and treatment response.
Fig. 3.
Everolimus resulted in target inhibition in peripheral blood mononuclear cells within 2 weeks of initiating therapy, and suppression was maintained through the duration of treatment. Expression of downstream markers is attenuated: (A) p-70 S6 kinase (Thr389; p70S6K), (B) S6 Ser235/236 phosphorylation (pS6), and (C) 4EBP14 (EBP1-Thr70; p-4EBP).
Discussion
Everolimus demonstrated activity as a single agent for radiographic-progressive, NF1-associated LGG after progression following standard upfront carboplatin-containing therapy. Fifteen of 22 participants (68%) demonstrated either arrest of tumor growth or a radiologic response. Given the relapsing and remitting course of LGGs in this population, in particular in the region of the optic tracts and chiasm, which are not surgically resectable, identification of well-tolerated therapies is of paramount importance.
The treatment of pediatric LGG has previously relied on traditional chemotherapy combinations.12 A randomized Children’s Oncology Group trial of the 2 standard chemotherapy regimens for pediatric LGG using vincristin/carboplatin versus TPCV (hioguanine, procarbazine, lomustine [CCNU], and vincristine) demonstrated near equal activity in sporadic pediatric LGG,4 with 5-year event-free survival (EFS) in treatment-naïve participants of 45–55%. Treatment-naïve individuals with NF1, who were nonrandomly assigned to the vincristine and carboplatin arm to avoid second tumor risk associated with alkylating agents in the TPCV regimen, had improved outcome (5-y EFS of 65%) compared with those of patients with sporadic disease. A significant proportion with sporadic and NF1-associated LGG progressed after upfront therapy. A number of other chemotherapies have been evaluated in progressive pediatric LGG, including carboplatin alone,13 vincristine and actinomycin-D,14,15 vinblastine,16,17 temozolomide,18 bevacizumab,19 and metronomic chemotherapy.20,21 While overall lower response rates are typically observed in the recurrent setting, these regimens have been formally tested neither against each other nor specifically in the NF1 population. At the time of this study development, mitogen-activated protein kinase kinase (MEK) inhibitors were not yet available and the impetus of the trial was to evaluate for potential therapies based on the known pathways of NF1. More recently, in a phase II study of the MEK1 and MEK2 inhibitor selumetinib for recurrent/refractory or progressive LGG, 10/25 patients with NF1 achieved a partial response;22 however, because this study required radiographic progression for study entry, the 2 patient populations are not the same. Clearly the presence of inhibitors closer to the molecular defect in Ras signaling provides a reduced opportunity for escape.
Whereas PFS is an important endpoint in the assessment of activity of different treatment regimens, it is well known that the majority of participants with NF1 LGGs are long-term survivors.23 As such, cumulative and long-term toxicity becomes increasingly important when selecting optimal treatment strategies. Allergic reactions to carboplatin, hepatotoxicity to actinomycin D, myelosuppression with vinblastine, and significant secondary tumor risk with regimens containing alkylating agents, etoposide and radiation therapy have become important factors in choosing therapy for individuals with NF1. Everolimus has been used extensively in both adults and children, including prolonged use in organ transplant recipients and children with subependymal giant cell astrocytoma (SEGAs).24 The drug can be orally administered as either a tablet or a liquid. Dosing parameters for children (5 mg/m2) are well tolerated in the vast majority; however, the observed interpatient variability in trough concentration suggests that individual dose adjustments based on blood concentration measurements could be helpful to ensure target exposure attainment. Everolimus appears to have sufficient CNS penetration as demonstrated by its FDA-approved activity in individuals with SEGA. The mechanism of action makes it an ideal compound for use in NF1 LGGs, since mTOR is a known downstream effector of the pathway that is hyperactivated in NF1-deficient tumors. Whether some of its activity results from its anti-angiogenic effects is unknown, as both direct tumor cell and anti-angiogenic effects have been reported. Vinblastine has recently undergone phase II testing in pediatric LGG, including a subset with NF1. Importantly, individuals with initial progressive disease were permitted to continue treatment with vinblastine, and several ultimately demonstrated arrest in tumor growth, possibly as a result of a more delayed anti-angiogenic mechanism of action.25 Individuals with early radiographic progression in this study were taken off everolimus, raising the question whether responses may have been realized had they been allowed to continue on therapy. It is very likely that prolonged administration of targeted inhibitors may be necessary among patients with progressive tumors. As yet, however, approaches using lower dose or intermittent dosing as a way to minimize toxic effects while retaining efficacy have not been assessed.
It is difficult to compare response rates between studies of pediatric LGG, because the patient populations, tumors, and tumor locations are heterogeneous. In addition, many studies include participants both with and without NF1. In the current study, all participants had NF1 and were required to have MRI evidence of radiographic progression. Clinical progression alone, such as change in visual function for optic pathway tumors, was not sufficient for study entry. These strict criteria allowed us to estimate the activity of the regimen using either arrest of tumor growth or shrinkage as a measure of true tumor response.
Lack of clinical correlation/functional outcome parameters is a potential limitation of this study, as functional outcome is perhaps more important than any radiologic improvement for translation of clinical benefit. Functional endpoints were not collected as part of the trial due to the heterogeneity of the clinical symptoms, age of patients, tumor location, and pilot nature of the trial. We do agree that functional endpoints are as important as, if not more important than, radiologic endpoints.
As tissue was not mandated or collected as part of this trial, the target inhibition was used as a surrogate of response. The identification of pharmacokinetic profiles and PI3K/mTOR pathway inhibition markers that replicate other studies is encouraging and suggests that this compound can be used safely and at levels that confirm mTOR pathway suppression in this patient population. The lack of direct correlation between mTOR pathway inhibition and tumor outcome may suggest that mTOR inhibition alone does not singularly reflect pathway inhibition within the tumor. It may be helpful for future trials to perform dose adjustment based on everolimus levels. The favorable toxicity profile observed in NF1 populations did not significantly differ from other populations.7 These results are encouraging in the recurrent/progressive population. The combination of dabrafenib plus trametinib has been used in adults with melanoma, where it was associated with improved overall survival.26 This combinatorial approach is now in clinical trials for pediatric low-grade glioma (NCT02684058).
We noted variation in institutional versus central radiographic review. While treatment decisions were based on the institutional report, the fact that 2 of the 22 participants came off study for PD that was not evident on central review, and the reclassification of participants recoded as SD who in central review met the criteria for PR, reinforces the importance of real-time, central radiographic review for these kinds of studies. This may be particularly cogent for those with NF1, where the presence of focal areas of signal intensity complicates radiologic assessments.
In conclusion, everolimus demonstrated significant tumor stabilization and/or shrinkage in radiographically progressive NF1 LGG in this prospective, phase II, single arm, open-label multi-institutional trial. The medication is oral, available as both a liquid and a tablet, does not require intravenous access or a central venous line, and has a very favorable toxicity profile, consistent with the use of this drug in other chronic pediatric conditions. Future studies which compare or combine this agent with other emerging molecularly targeted therapies, such as MEK inhibitors, could be considered if the 2 drugs are tolerable in combination.
Supplementary Material
Acknowledgments
The authors thank the children and parents who participated in this study and acknowledge the hard work by and support from Karen Cole-Plourde and Elizabeth Davis (University of Alabama). We also thank the clinicians and study coordinators at participating sites.
Funding
This work was supported by the US Army Medical Research and Materiel Command, Office of the Congressionally Directed Medical Research Programs, Department of Defense Neurofibromatosis Research Program (W81XWH-05-1-0615); by Novartis (Basel, Switzerland); and by the National Cancer Institute of the National Institutes of Health (P50CA165962 to M.W.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Unpublished papers cited: None.
Conflict of interest statement.
None.
Authorship statement.
Conception and design of the work: All authors. Material preparation, data collection and analysis: N.J.U., S.P.P., A.C., G.C., T.M., A.A.V., M.W.K. First draft: N.J.U. and M.W.K. Revising work critically for important intellectual content: All authors. Final approval of manuscript: All authors.
References
- 1. Farrell CJ, Plotkin SR. Genetic causes of brain tumors: neurofibromatosis, tuberous sclerosis, von Hippel-Lindau, and other syndromes. Neurol Clin. 2007;25(4):925–46, viii. [DOI] [PubMed] [Google Scholar]
- 2. Ullrich NJ, Raja AI, Irons MB, Kieran MW, Goumnerova L. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007; 61(4):762–766; discussion 766–767. [DOI] [PubMed] [Google Scholar]
- 3. Mahdi J, Shah AC, Sato A, et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology. 2017;88(16):1584–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernáiz Driever P, von Hornstein S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. J Neurooncol. 2010;100(2):199–207. [DOI] [PubMed] [Google Scholar]
- 6. Kawachi Y, Maruyama H, Ishitsuka Y, et al. NF1 gene silencing induces upregulation of vascular endothelial growth factor expression in both Schwann and non-Schwann cells. Exp Dermatol. 2013;22(4):262–265. [DOI] [PubMed] [Google Scholar]
- 7. Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–4812. [DOI] [PubMed] [Google Scholar]
- 8. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. [DOI] [PubMed] [Google Scholar]
- 9. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. [DOI] [PubMed] [Google Scholar]
- 10. Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26(10):1603–1610. [DOI] [PubMed] [Google Scholar]
- 11. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. [DOI] [PubMed] [Google Scholar]
- 12. Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15(4):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dodgshun AJ, Maixner WJ, Heath JA, Sullivan MJ, Hansford JR. Single agent carboplatin for pediatric low-grade glioma: a retrospective analysis shows equivalent efficacy to multiagent chemotherapy. Int J Cancer. 2016;138(2):481–488. [DOI] [PubMed] [Google Scholar]
- 14. Reddy AT, Packer RJ. Chemotherapy for low-grade gliomas. Childs Nerv Syst. 1999;15(10):506–513. [DOI] [PubMed] [Google Scholar]
- 15. Cokgor I, Friedman AH, Friedman HS. Gliomas. Eur J Cancer. 1998;34(12):1910–1915; discussion 1916–1918. [DOI] [PubMed] [Google Scholar]
- 16. Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. [DOI] [PubMed] [Google Scholar]
- 17. Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 18. Packer RJ, Pfister S, Bouffet E, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robison NJ, Campigotto F, Chi SN, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61(4):636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helfferich J, Nijmeijer R, Brouwer OF, et al. Neurofibromatosis type 1 associated low grade gliomas: a comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol. 2016;104:30–41. [DOI] [PubMed] [Google Scholar]
- 22. Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7): 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franz DN, Belousova E, Sparagana S, et al. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLoS One. 2016;11(6):e0158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103(12):2636–2642. [DOI] [PubMed] [Google Scholar]
- 26. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.