Abstract
Skeletal muscle atrophy in the aged causes loss in muscle mass and functions. Naturally occurring antioxidant flavonoid apigenin is able to ameliorate obesity- and denervation-induced muscle atrophies, but its effects on age-related muscle atrophy remain unknown. We hypothesized that apigenin can relieve muscle atrophy in aged mice, probably through special effects on reactive oxygen species and enzymes with antioxidant functions. For the male mice of the study, apigenin showed significant dose-dependent effects in relieving aging-related muscle atrophy according to results of frailty index as indicator of frailty associated with aging, grip strength, and running distance. Apigenin also improved myofiber size and morphological features and increased mitochondria number and volume, as manifested by succinate dehydrogenase staining and transmission electron microscopy. Our tests also suggested that apigenin promoted activities of enzymes such as superoxide dismutase and glutathione peroxidase for antioxidation and those for aerobic respiration such as mitochondrial respiratory enzyme complexes I, II, and IV, increased ATP, and enhanced expression of genes such as peroxisome proliferator-activated receptor-γ coactivator 1α, mitochondrial transcription factor A, nuclear respiratory factor-1, and ATP5B involved in mitochondrial biogenesis. The data also suggested that apigenin inhibited Bcl-2/adenovirus E1B 19kD-interacting protein 3 and DNA fragmentation as indicators of mitophagy and apoptosis in aged mice with skeletal muscle atrophy. Together, the results suggest that apigenin relieves age-related skeletal muscle atrophy through reducing oxidative stress and inhibiting hyperactive autophagy and apoptosis.
Keywords: Apigenin, Apoptosis, Mitophagy, Reactive oxygen species, Skeletal muscle atrophy
Population aging has raised great challenges to health policies and services (1), and aging may pose multiple adverse influences on people, increasing morbidity, disability, mortality, and length of hospital stay (2). Skeletal muscle atrophy can be observed in the aged people due to causes such as neuromuscular disorders, disuse, and aging (3). For preventing and treating skeletal muscle atrophy (sarcopenia) associated with aging, researchers have attempted to explore the underlying mechanisms and develop strategies for prevention and treatment.
Muscle atrophy is often manifested as loss of muscle mass and functions, specifically as smaller myofiber sizes, reduced myofiber mass, transition of myofiber types, and an imbalance between synthesis and degradation of proteins in muscles (4). Muscular protein synthesis and degradation can be regulated by signaling pathways such as oxidative stress, ubiquitin-proteasome system, proinflammatory cytokines, autophagy-lysosome systems, hormones, and so on (5). Specifically, in the old mice with muscle atrophy, oxidative stress markers such as nicotinamide adenine dinucleotide phosphate oxidase are increased, mitochondrial markers such as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) are reduced, ubiquitin-proteasome pathway genes are upregulated, and protein synthesis markers are downregulated when compared with the young mice (6). Treatment with kynurenine increases reactive oxygen species (ROS) and muscle lipid peroxidation, and reduces muscle size and strength in sarcopenia (7). Additionally, studies have suggested that factors such as tumor necrosis factor-α, myostatin, glucocorticoids, and ROS are able to induce protein loss in muscle atrophy (4). Myokine fibroblast growth factor 21 (FGF21) usually having very low content in muscles is inducible by mitochondrial myopathies, FGF21 overexpression in vivo is sufficient to induce autophagy and muscle loss, and Bcl-2/adenovirus E1B 19kD-interacting protein 3 (BNIP3, a mitophagy protein and apoptosis marker) inhibition protects against FGF21-dependent muscle atrophy (8). Mitochondria may play key roles in myocyte loss during aging, because they can induce apoptosis in myocyte (9). Chronic kidney disease is associated with hyperactive autophagy and mitophagy, with elevated mRNA and protein expression of BNIP3, light chain 3 (LC3), and p62 in the skeletal muscle and isolated mitochondria of the chronic kidney disease group with muscle atrophy (10). These studies suggested that oxidative stress, mitochondria dysfunction, autophagy/mitophagy, and apoptosis are closely involved in the age-related skeletal muscle atrophy.
Pharmaceutical and nonpharmaceutical ways have been proposed to prevent and treat skeletal muscle atrophy (5,11). For example, beneficial effect of butyrate on muscle mass during aging is suggested by reduced markers of oxidative stress and apoptosis and improved antioxidant enzyme activity (12). Kynurenine capable of increasing ROS is generated by indoleamine 2,3-dioxygenase (IDO), and myofiber size and muscle strength are increased by treatment with the IDO inhibitor 1-methyl-D-tryptophan in aged mice with sarcopenia (7). Among those with potential for relieving muscle atrophy, apigenin (5,7-dihydroxy-2-(4hydroxyphenyl)-4H-1-benzopyran-4-one) has drawn attention recently (13,14). Apigenin has antioxidant, anti-inflammatory, antimicrobial, antiviral, and antiparasitic functions, and can be used for treating obesity, muscle atrophy, and cancer (13,15–17). Reportedly, apigenin is able to ameliorate obesity-induced and denervation-induced muscle atrophies (13,14), but whether apigenin can be used to prevent and treat aging-related muscle atrophy and its possible mechanisms and effective dose remain unknown.
Considering that aging adversely affects skeletal muscle, we hypothesized that apigenin supplementation relieves such adverse effects by enhancing antioxidant capacity and inhibiting hyperactive mitophagy and apoptosis. Therefore, the aims of this study were to selectively explore mechanisms of aging-associated muscle atrophy in aspects of oxidative stress, mitophagy, and apoptosis and evaluate the preventive effects of apigenin on muscle atrophy in aged mice.
Materials and Methods
Animal Experiments
All animal experiments in this study were approved by the Welfare and Ethical Committee for Experimental Animal Care of Guangzhou University of Traditional Chinese Medicine (Registration No: SYXK (Yue) 2018-0189). Sixty (60) male C57BL/6 mice were purchased from Guangdong Medical Laboratory Animal Center in China. Mice were aged until approximately 16 months for the Old groups (n = 48) and then randomly assigned to 4 groups (12 mice per group): Old (Old control), standard chow diet and distilled water; Old + AP25, apigenin 25 mg·kg−1·day−1 (low dose); Old + AP50, apigenin 50 mg·kg−1·day−1 (middle dose); and Old + AP100, apigenin 100 mg·kg−1·day−1 (high dose). A group of young mice (n = 12) were fed with standard chow diet and distilled water from 6 to 9 months, and served as young adult controls (Young).
All mice were housed in individual ventilated cages under specific pathogen-free conditions at 22 ± 2°C on a 12-h light/dark cycle. Old mice in the treatment groups were intragastrically administered with apigenin at the dose of 25, 50, or 100 mg·kg−1·day−1 (Sigma-Aldrich, St Louis, MI) between 9 and 10 am for 9 months, because there was no appropriate positive control drug to choose. Body weights of all mice were measured twice weekly as bases for calculating the amount of drugs. To assess the changes in food intake, an equal amount of food (5 g/d) was given to each mouse at 10 am. Within each cage, food pellets were stored in mesh hoppers to prevent spillage. After each period of 24 hours, mice were removed from their cages to assess the food intake. By the end of the study, 12 mice in the Old groups (48 mice in total) died of natural causes.
Clinical Frailty Index Assessment, Grip Strength, and Running Distance
A 31-item mouse clinical frailty index (FI) was adopted to assess frailty as described in previous literature in a simplified and noninvasive way (18), because frailty is prevalent in aged population. Clinical assessment included evaluation of the integument, the musculoskeletal system, the vestibulocochlear/auditory systems, the ocular and nasal systems, the digestive system, the urogenital system, the respiratory system, signs of discomfort, the body weight (g), and body surface temperature (°C). Supplementary Table 1 lists clinical signs of deterioration/deficits evaluated in this study. Briefly, mice were taken to a quiet room and assessed at approximately the same time. Each of these frailty assessments was scored as absent (score of 0), mild (score of 0.25), mild to moderate (score of 0.5), moderate (score of 0.75), or severe (score of 1). Values for each deficit were then summed up and divided by the total number of deficits measured to yield an FI score. Old mice were assessed for frailty each month from 16 to 25 months of age according to the needs of administration in a preventive mode, while young mice were appraised with the same method separately at 6 and 9 months. To evaluate muscle functions of mice, grip strength and running distance were measured as described in our previous publication (19).
Myofiber Cross-Sectional Area Measurement and Myofiber-Type Shift Detection by Succinate Dehydrogenase Staining
The frozen sections of the tibialis anterior (TA) muscle were stained with succinate dehydrogenase for measurement of myofiber cross-sectional area and classification of fibers into type I (slow oxidative), IIa (fast oxidative glycolytic), or IIb (fast twitch glycolytic) as we previously reported (20).
Transmission Electron Microscopy for Analysis of Mitochondria
The detailed procedures of transmission electron microscopy for TA muscles were reported previously (19). With a computer imaging analysis system (JEM-1400, JEOL Ltd, Tokyo, Japan), the area, number of cross-section, perimeter, and long axis and short axis of mitochondria were measured. Based on the stereological principles for morphological study, mitochondrial number per unit area, average diameter, and volume density were all further calculated (21).
Mitochondria Isolation and Determination of Mitochondrial Membrane Potential (Δψm)
Skeletal muscle mitochondria were isolated from the quadriceps (QUAD) muscles by differential centrifugation using a mitochondria crude isolation kit (Genemed Scientifics Inc., Shanghai, China) as described (19). The Δψm, an indicator of the inner and outer membrane integrity, was measured using a JC-1 probe (Beyotime, Shanghai, China). In short, the freshly isolated mitochondria were suspended in JC-1 staining solution, and the fluorescence intensity was measured using an Infinite M200 Microplate Reader (Tecan, Mannedorf, Switzerland). Images were acquired by a Zeiss confocal laser scanning microscope, and further analysis of the fluorescence intensity was performed using Zeiss LSM Image Examiner software. The Δψm was represented by the ratio of red to green fluorescence.
Measurement of Oxygen Consumption Rate
The basal oxygen consumption rate (State 4) was measured using the Seahorse XFe24 Extracellular Flux Analyzers (Seahorse Bioscience, Billerica, MA), as we described previously (22). Briefly, 10 μg isolated muscle mitochondria (3–6 μL) were loaded into the XF24 cell culture microplates (Seahorse Bioscience) on ice, and 50 μL of the substrates (5 mM pyruvate plus 5 mM malate) and 440 μL of mitochondrial assay solution (70 mM sucrose, 220 mM mannitol, 5 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% BSA, pH 7.4) were carefully added on the top. All the chemicals loaded in the Seahorse cartridge ports were diluted in mitochondrial assay solution (pH 7.4).
Measurement of Electron Transport Chain Enzyme Activities and ATP Content
The electron transport chain enzyme activities in mitochondria were measured based on specific donor–acceptor oxidoreductase activities. All assays were performed at 30°C with a Shimadzu UV-1601 spectrophotometer. The specific activities of complexes I (#A089-1-1), II (#A089-2-1), and IV (#A089-4-1) were measured using the corresponding kits (Jiancheng Bioengineering Co., Nanjing, China). On the other hand, the ATP levels in gastrocnemius (GAS) muscles were measured with an ATP Assay Kit (Beyotime).
Measurement of Oxidative Stress Indicator Contents and Antioxidant Activities
The content of hydrogen peroxide (H2O2) as an indicator of oxidative stress was measured using a hydrogen peroxide assay kit (Jiancheng Biotech Inc., Nanjing, China) based on reaction with molybdic acid. The malondialdehyde content, as a marker of lipid peroxidation, was determined using an MDA Detection Kit (Beyotime). Moreover, the carbonyl protein level as an indicator of protein oxidative damage was measured using a Protein Carbonyl Content Assay Kit. All of the above 3 measurements were performed with gastrocnemius muscles and as we previously described (23). In addition, the mitochondrial superoxide anion (O2•−) generation was determined by dihydroethidium assay (Beyotime), which is an ethidium-based, redox-sensitive fluorescent probe and will form 2-hydroxyethidium (2-OHE+) if oxidized by O2•−. The fluorescence was measured at an excitation wavelength of 500–530 nm and an emission wavelength of 590–620 nm using a fluorimeter (Tecan).
The total antioxidant capacity (T-AOC) and antioxidant enzymatic activities of superoxide dismutase and glutathione peroxidase in gastrocnemius muscles were determined by use of corresponding assay kits (Beyotime) according to the manufacturer’s instructions.
Determination of Mitochondrial DNA Copy Number
According to the manufacturer’s protocol, mitochondrial DNA (mtDNA) was obtained using a Mito DNA Extraction Kit (Genmed Scientifics, Inc., Delaware). Quantification of mtDNA was performed by use of the ratio of a mitochondrial gene (16S RNA) to a nuclear gene (UCP2) as described (24).
Western Blotting
Snap-frozen quadriceps muscle tissues were homogenized in lysis buffer as reported (19). Cytosolic and mitochondrial proteins were separated. After Western blotting of the samples, protein bands were detected and analyzed using a ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA). Voltage-dependent anion channel protein (VDAC) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, a house keeping gene with relatively stable expression) were used as the loading control for mitochondrial protein and cytosolic protein, respectively. Results were expressed as the integrated optical density (IOD) relative to VDAC or GAPDH. VDAC (1:1000, #4661) and BNIP3 (1:1000, #12396) antibodies were purchased from Cell Signaling Technologies (Danvers, MA). Antibodies for LC3-I/II (1:1000, ab58610), ubiquitin-binding protein p62 (1:1000, ab91526), and cytochrome C (Cyt-C, 1:1000, ab13575) were from Abcam (Cambridge, United Kingdom). ATP5B antibody (1:1000, ARP48185_T100) was from Aviva Systems Biology (San Diego, CA). Mitochondrial transcription factor A (TFAM) (1:100, sc-376672) and nuclear respiratory factor-1 (NRF-1) (1:100, sc-33771) antibodies were from Santa Cruz Biotechnology (CA). PGC-1α antibody (1:2000, NBP1-04676) was from Novus Biological (CO). GAPDH antibody (1:1000, 60004-1-Ig) was from Proteintech (Chicago, IL).
Determination of Apoptosis Markers
The quantitative determination of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) due to cell death was measured using ELISA kit (Roche Diagnostics GmbH, , Mannheim, Germany) (22). Total poly(ADP-ribose) polymerase (PARP) activity was analyzed in gastrocnemius muscle tissue lysates using the Universal PARP Colorimetric Assay (Trevigen Inc., Gaithersburg, MD) (24). Caspase activity was evaluated with the Caspase-3 Assay Kit (Colorimetric) (ab39401). Protein extracts from quadriceps muscles were incubated with YVAD-pNA substrate at 37°C for 2 hours. The pNA emission was quantified with a spectrophotometer at 400 nm (25).
Statistical Analysis
All data were shown as the mean ± standard deviation (SD) or the mean ± standard error of mean (SEM). SPSS (version 25) statistical software package (SPSS Inc., Chicago, IL) was used for statistical analysis. Samples were considered to be normally distributed if p > .05 in the Kolmogorov–Smirnov test. One-way ANOVA was used for comparisons among groups. Heterogeneity of variance was accepted if p < .05, and least significant difference method was used to perform appropriate multiple comparisons among groups. Otherwise, the Tukey’s post hoc test was adopted. p < .05 was considered to be statistically significant.
Results
Apigenin Relieved Frailty and Improved Muscle Function in Aged Mice
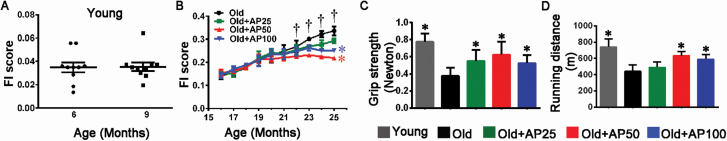
According to the FI assessment, and the measurement of grip strength and running distance, all the old mice performed worse than the Young control, and the old mice treated with apigenin performed better than the Old control, wherein the group treated with apigenin at middle dose performed the best in general (Figure 1A–D). The results suggested that muscle functions deteriorate with aging and apigenin relieves the adverse effects on muscle.
Figure 1.
Apigenin treatment relieved frailty and improved muscle function in aged mice. (A) Mean frailty index (FI) scores were evaluated in young mice and (B) in old mice treated either with apigenin (AP) at dose of 25 mg·kg−1·day−1 (Old + AP25, low dose), 50 mg·kg−1·day−1 (Old + AP50, middle dose), or 100 mg·kg−1·day−1 (Old + AP100, high dose), or without treatment as Old control mice. The muscle function was detected by (C) grip strength and (D) running distance. Data are presented as the mean ± SD (n = 8–10). †p < .05, versus 16 months (in the Old group); *p < .05, versus the Old group (25 months).
Apigenin Attenuated Muscle Atrophy and Myofiber-Type Shift in Aged Mice
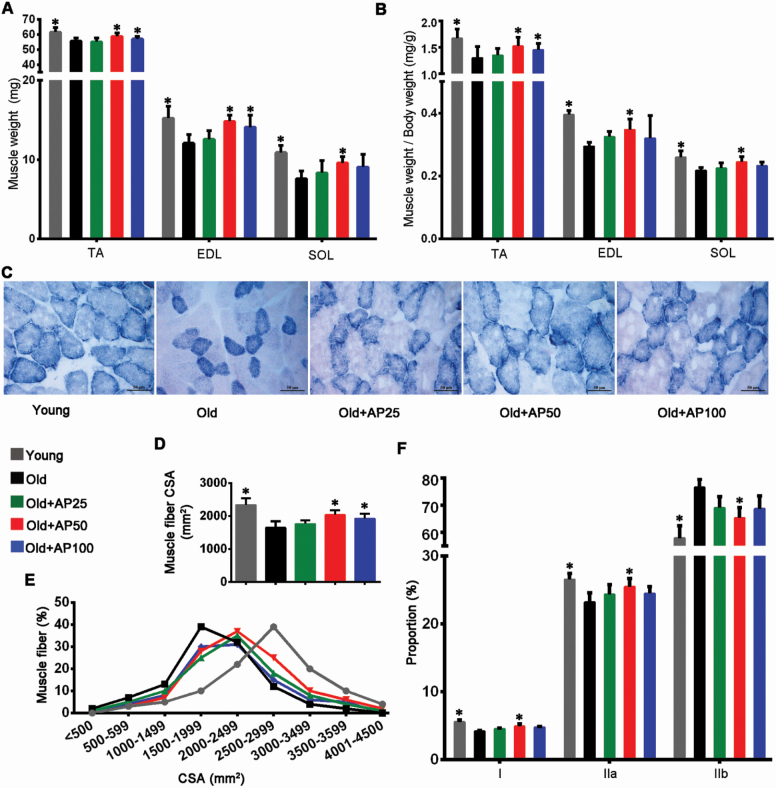
Skeletal muscle atrophy leads to reduced weight and cross-sectional area of muscles (26). Neither the food intake nor the body weight differed significantly among the 5 groups (Supplementary Figure 1A and B). However, the TA, extensor digitorumlongus, and soleus muscle weights and the muscle/body weight ratios of the Old control reduced when compared with the Young control, and the supplementation of apigenin at middle dose effectively relieved the muscle loss (Figure 2A and B). Figure 2C shows images of the TA muscle cross-sections obtained by succinate dehydrogenase staining, where the muscle atrophy of old mice was attenuated in the 3 treatment groups. Similar effects of apigenin on muscle atrophy were demonstrated in the muscle cross-sectional area and muscle fiber frequency distribution results (Figure 2D and E). The succinate dehydrogenase staining showed that type I (slow oxidative) and IIa (fast oxidative glycolytic) muscle fibers rich in mitochondria were significantly decreased, and type IIb (fast twitch glycolytic) fibers with relatively fewer mitochondria were increased in the Old groups. Interestingly, apigenin treatment at middle dose significantly reduced type IIb fibers and increased type I and IIa fibers, suggesting inhibition of fiber-type shift in the treated groups (Figure 2F).
Figure 2.
Apigenin attenuated muscle atrophy in aged mice. (A) Weights of tibialis anterior (TA), extensor digitorum longus (EDL), and soleus (SOL) muscles were normalized to tibia length. (B) Relative weights of TA, EDL, and SOL normalized to body weight. (C) Succinate dehydrogenase (SDH) staining was performed on 10-μm-thick sections from TA muscles frozen in liquid nitrogen-chilled isopentane. Scale bar: 50 μm. (D) Average fiber size (cross-sectional area, CSA) of the SDH-stained TA muscles. (E) Muscle fiber frequency distribution of the SDH-stained TA muscle. (F) Numbers of types I (slow oxidative), IIa (fast oxidative glycolytic), and IIb (fast twitch glycolytic) muscle fibers. Data are presented as the mean ± SD (n = 8–10). *p < .05, versus the Old group (25 months).
Apigenin Improved Mitochondrial Respiratory Function in Skeletal Muscle
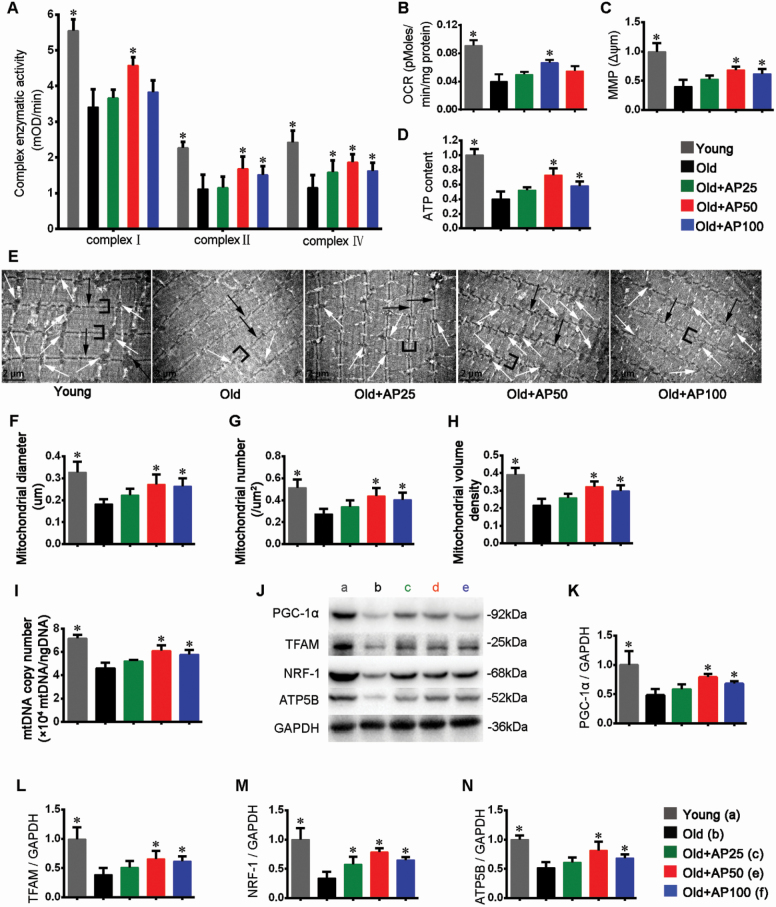
This study found that apigenin at middle dose significantly increased basal oxygen consumption rate in old mice (Figure 3B). Furthermore, activity analysis of mitochondrial respiratory electron transport chain complexes I, II, and IV in gastrocnemius muscle showed that apigenin significantly increased the activities of these complexes (Figure 3A). Stable Δψm is essential for oxidative phosphorylation and generation of ATP (27). In comparison to the Young control, the fold change showed that apigenin at middle and high doses significantly improved Δψm in aged mice (Figure 3C). Concomitantly, Figure 3D indicates that apigenin significantly improved ATP content. Taken together, these results suggested that apigenin improved the muscular mitochondrial functions such as aerobic respiration and ATP synthesis.
Figure 3.
Apigenin improved mitochondrial function and promoted mitochondrial biogenesis in skeletal muscle of aged mice. (A) Activities of mitochondrial complexes I, II, and IV. (B) Oxygen consumption rate (OCR). (C) Mitochondrial membrane potential (MMP) Δψm. (D) ATP content. (E) Transmission electron microscopy micrographs (magnification: 12 000) of tibialis anterior (TA) muscles. White arrows indicate mitochondria, black arrows the Z-line, and brackets the I-bands. Scale bar: 2 μm. Quantitative analysis of mitochondrial (F) size, (G) number, and (H) volume density. (I) The relative mitochondrial DNA (mtDNA) copy number. (J) Representative images and quantification (fold change from Young) of (K) PGC-1α, (L) TFAM, (M) NRF-1, and (N) ATP5B were shown. Data are presented as the mean ± SD (n = 8–10). *p < .05, versus the Old group (25 months).
Apigenin Improved Aberrant Morphological Features and Promoted Mitochondrial Biogenesis in Skeletal Muscle of Aged Mice
To assess the effect of apigenin on morphological features, TA muscles were observed under transmission electron microscopy. The Old control had fewer mitochondria than the Young control, while apigenin improved the overall morphological features (Figure 3E). The quantitative analyses proved that the size (diameter), number, and volume density of mitochondria were significantly improved by apigenin at middle dose (Figure 3F–H).
Additionally, we examined mitochondrial biogenesis in skeletal muscle. Apigenin improved the relative mtDNA copy number (Figure 3I), indicating the mitochondrial expansion in the treated mice. Finally, Western blotting with GAPDH as loading control was used to evaluate protein expression of key genes involved in mitochondrial biogenesis, including PGC-1α, TFAM, NRF-1, and ATP5B, and apigenin at middle and high doses improved their expression levels significantly (Figure 3J–N). In summary, apigenin effectively improved mitochondrial features, increased mitochondrial number, and enhanced the expression of key genes to promote mitochondrial biogenesis.
Apigenin Suppressed Oxidative Stress and Enhanced Antioxidant Capacity
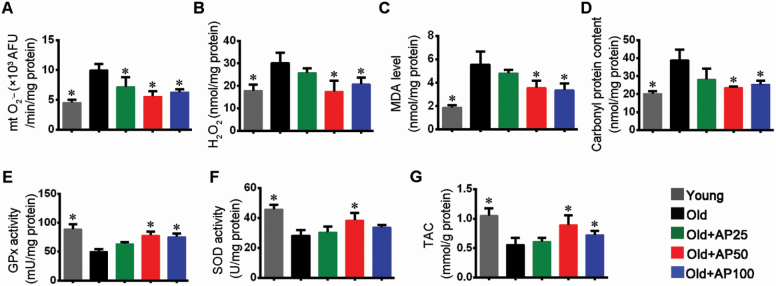
The experiment showed that apigenin reduced mitochondrial superoxide anion (O2•−) as a component contributing to oxidative stress (Figure 4A). Measurement of H2O2 content as an indicator of oxidative stress in proteins in skeletal muscle indicated that apigenin effectively relieved the adverse effect of aging on skeletal muscle (Figure 4B). Additionally, measurement showed that apigenin reduced malondialdehyde content, suggesting inhibition of lipid peroxidation (Figure 4C). On the other hand, carbonyl protein was measured as an indicator of protein oxidative damage, and apigenin was proved to be able to alleviate the damage significantly (Figure 4D). Furthermore, in exploration of mechanism for oxidative stress suppression by apigenin, the T-AOC and activities of superoxide dismutase and glutathione peroxidase in gastrocnemius muscles were determined. The results showed that activities of these 2 enzymes involved in degradation of superoxide were enhanced and the T-AOC was consequently improved in apigenin-treated aged mice (Figure 4E–G).
Figure 4.
Apigenin suppressed oxidative stress and enhanced antioxidant capacity in skeletal muscle of aged mice. (A) Mitochondrial O2•− (mt O2•−) content. (B) H2O2 levels. (C) Malondialdehyde (MDA) levels. (D) Carbonyl protein content. Activities of (E) glutathione peroxidase (GPx) and (F) superoxide dismutase (SOD). (G) Total antioxidative capability (T-AOC). Data are presented as the mean ± SD (n = 8–10). *p < .05, versus the Old group (25 months).
Apigenin Inhibited Hyperactive Mitophagy and Mitochondria-Dependent Apoptosis in Skeletal Muscle of Aged Mice
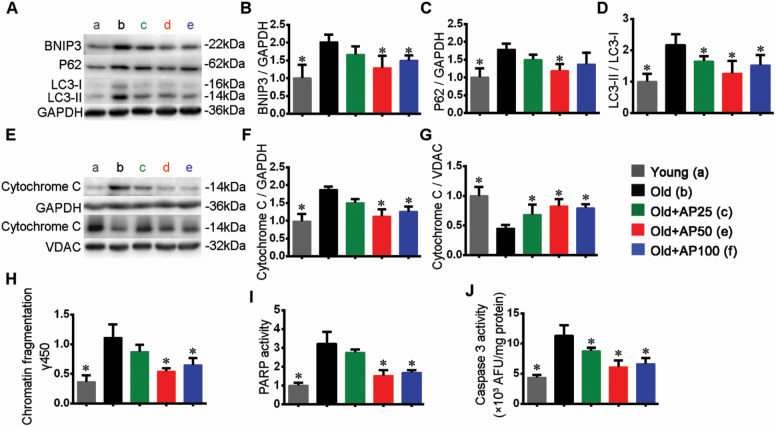
Moderate mitophagy usually serves to remove the damaged or aged mitochondria, and promotes reutilization of constitutional elements (28). Interestingly, expression of BNIP3 as mitophagy marker was enhanced by aging and attenuated by supplementation of apigenin (Figure 5A and B). Ubiquitin-binding protein p62 plays a critical role in selective mitophagy and oxidative stress response (29). Its expression was promoted by aging and inhibited by apigenin (Figure 5A and C). Increase of LC3-II/I ratio in aged mice indicated increase of autophagy, and decrease of the ratio suggested inhibitive effect of apigenin on autophagy (Figure 5A and D). Finally, Figure 5E–G suggest that release of Cyt-C from mitochondria to the cytosol was increased by aging and hindered by apigenin. Such release of Cyt-C is considered as a sign of beginning of apoptosis, and happens before the DNA fragmentation and activation of caspase 3 (30). Damaged mitochondria may generate higher amounts of ROS, and tend to trigger apoptosis (31).
Figure 5.
Apigenin inhibited autophagy and mitochondria-dependent apoptosis in skeletal muscle of aged mice. (A) Representative images and quantification (fold change from Young) were shown for (B) BNIP3, (C) p62, and (D) LC3-II/I. (E) Representative images and quantification (fold change from Young) of cytochrome C in (F) cytosol and (G) mitochondria fractions were shown, respectively. (H) The DNA fragmentation. Activities of (I) PARP (fold change from Young), and (J) caspase 3. Data are presented as the mean ± SD (n = 8–10). *p < .05, versus the Old group (25 months).
DNA fragmentation is an indicator of apoptosis (32), and data showed that DNA fragmentation was increased by aging but reduced by apigenin (Figure 5H). Poly(ADP-ribose) polymerase helps to repair DNA and is a substrate of caspase 3 (33), so we examined their activities to explore the possible mechanism of apigenin. Activities of PARP and caspase 3 were enhanced by DNA fragmentation, while apigenin treatment was associated with inhibition of their activities (Figure 5I and J), which may protect the cells from hyperactive apoptosis. A previous study showed that inhibition of apoptosis partially attenuated denervation-induced muscle atrophy in a Bax knockout mouse model (34). It seems that oxidative stress, hampered mitochondrial functions, and hyperactive mitophagy and apoptosis have complicated roles in the atrophy process.
Discussion
Muscle atrophy leads to reduced muscle mass and impaired muscle functions. Frailty is prevalent in aged population and FI can be used to assess frailty associated with aging. Compared to the young, aged mice in this study showed higher FI, reduced muscle cross-sectional area and weight, weaker grip strength, and shortened running distance, suggesting that aging-related atrophy happened in skeletal muscles of the old mice. Consistent with reports of apigenin playing preventive role in muscle atrophy in obese mice (13), we found that apigenin relieved muscle atrophy in aged mice by inhibiting loss in muscle mass and force. Similarly, previous studies showed the fast-to-slow and slow-to-fast muscle fiber shift in atrophy, and type IIb fibers can shift to type IIa fibers after resistance training (35,36), while our results suggested that apigenin was effective in inhibiting the myofiber-type shift. Sarcopenia is often observed in people with frailty, and loss of muscle strength is very likely to lead to mobility disability and mortality in the aged adults (37,38). As apigenin effectively relieved the relevant symptoms, it seems to be a promising candidate reagent for the prevention of muscle atrophy in the aged.
It is known that mitochondria are crucial for providing energy to muscle contraction, and dramatic reduction in total adenine nucleotides (ATP, ADP, and AMP) is observed in atrophic skeletal muscle (39). Normal mitochondrial membrane potential is prerequisite of the normal functions such as oxidative phosphorylation and ATP generation (27), and our study found that apigenin can help to maintain the membrane potential. Muscle strength is related to muscle contraction involving ATP, and ATP can be replenished by use of the phosphagen system, the glycolytic system, and the mitochondrial oxidative phosphorylation system (40). This study showed apigenin increased the ATP content. Furthermore, the activities of mitochondrial enzymatic complexes in the respiratory electron transport chain for energy supply and the oxygen consumption were improved by apigenin, partly leading to the ATP increase. In this study, apigenin restored oxygen consumption rate to some extent in old mice. In agreement with our findings, another study has shown that the knockdown of optic atrophy protein 3 results in decreased energy metabolism manifested by reduced oxygen consumption rate and ATP, leading to inhibition of cell proliferation (41). In this study, old mice had reduced energy metabolism and impaired mitochondrial functions, while apigenin supplementation improved the ATP content, enzymatic activities, and mitochondrial membrane potential, effectively improving the mitochondrial functions. Furthermore, the mitochondria size, number, volume density, and mtDNA copy number were increased by apigenin as well. Overexpression of PGC-1α, a principal regulator of energy metabolism, ameliorates mitochondrial deficits (29). A recent study has found that testosterone induces the expression of NRF-1 and TFAM, suggesting its role in mitochondrial transcription and biogenesis in muscle (42). Interestingly, the protein expression levels of several key genes involved in mitochondrial biogenesis, including PGC-1α, TFAM, NRF-1, and ATP5B, were improved significantly by apigenin as well.
It is believed that protein synthesis and degradation in muscle are regulated by signaling transduction related to oxidative stress, autophagosome-lysosome systems, hormones, and so on (43). Mitochondria are the aerobic energy production site, and ROS as by-product of mitochondrial metabolism may cause mtDNA mutations and dysfunction, destroy antioxidant defensing system, and cause damage to tissues (28,44). The increased ROS levels can promote autophagy and apoptosis in myoblasts (45). The mechanism may be that dysfunctional and depolarized mitochondria release excessive ROS, the increased ROS results in apoptosis and subsequent mitochondrial depolarization, and the depolarization occurs as defense before mitophagy (46). Therefore, a promising strategy for treating skeletal muscle atrophy is to reduce ROS and relieve oxidative stress. In a study on denervation-induced muscle atrophy, salidroside alleviates the disease by inhibiting oxidative stress, inflammation, expression of atrophic genes, and activation of mitophagy (47). Our study showed that apigenin reduced biomarkers of oxidative stress such as O2•− and H2O2, enhanced activities of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase, and improved T-AOC, thereby protecting muscular proteins and lipids from oxidative damage.
Our previous study has found that Astragalus polysaccharide inhibits the autophagy and apoptosis elicited by peroxide injury (48). Another study on aging-related muscle atrophy reveals elevated expression of genes involved in cellular responses to inflammation and apoptosis (49). In skeletal muscle in the aged, mitophagy protein expression is enhanced possibly due to increased mitochondrial dysfunction, damage, and fission (29). Interestingly, pyrroloquinoline quinone attenuated denervation-induced skeletal muscle atrophy, mitophagy, and fiber-type transition (50). Cytochrome C normally in mitochondria can be released into the cytosol by pro-apoptosis substances and is considered to be sign of beginning of apoptosis (30), and our analysis showed that such release of Cyt-C was inhibited by apigenin. We found that DNA fragmentation as a hallmark of apoptosis, p62 involved in mitophagy and oxidative stress response, LC3-II/I ratio as indicator of autophagy, and BNIP3 as an apoptosis marker were increased in old mice, and apigenin protects the muscles by interfering with the relevant bioprocesses. Concomitantly, inhibition of activities of PARP and caspase 3 implied the protective effect of apigenin on muscle from hyperactive apoptosis.
In summary, this study provided some evidences that antioxidant apigenin relieves aging-related skeletal muscle atrophy, and the mechanism may be tentatively described as below. Aging is associated with T-AOC reduction due to decreased activities of peroxidases, and with oxidative stress increased by higher contents of ROS, causing lipid and protein damages. The electron transport chain enzyme complex activities are inhibited, and ATP content decreases. Meanwhile, the Δψm decreases (mitochondrial depolarization), leading to release of ROS and Cyt-C from mitochondria to cytoplasm. Mitochondrial DNA is fragmented and PARP is induced for DNA reparation. The above processes may trigger hyperactive mitophagy, and the number and size of mitochondria are reduced. The ROS release and mitophagy induce apoptosis, leading to reduced number and size of myofibers. As a result, muscle mass and power are reduced, and muscle atrophy is developed. Antioxidant apigenin helps to prevent the aging-related muscle atrophy at the very beginning. Admittedly, this study only reported the primary results obtained in various selected muscle types in male mice, while female animals are suitable for mimicking postmenopausal women because estrogen deficiency is considered to be a significant factor of muscle atrophy (51). Due to technical, time, and financial limits, we found it not realistic to carry out the above tests on each of the several muscle types used. In the future, new studies can be designed to further evaluate efficacy and side effects of apigenin in prevention and treatment of aging-related muscle atrophy first with other animal models such as female mice and then clinically.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Supplementary Figure 1. (A) The food intake was assessed. (B) Final body weight.
Supplementary Table 1. Clinical Assessment of Deficits in Aging Mice to Create a Frailty Index.
Funding
This study was supported by grants from National Natural Science Foundation of China (81874394, 81673814), Science and Technology Planning Project of Shenzhen Municipality (JCYJ20180306174037820), Science and Technology Planning Project of Guangdong Province (2016A020226032, 2017A020213008), Guangdong Basic and Applied Basic Research Foundation (2020A1515010058), Project of Administration of Traditional Chinese Medicine of Guangdong Province (20180326103756, 20190405225223), Shenzhen Key Medical Discipline Construction Fund (SZXK074), and Innovation and Practice Base for Postdoctoral Researchers of Guangxi International Zhuang Medicine Hospital.
Conflict of Interest
None declared.
Author Contributions
The authors’ responsibilities were as follows: D.W. conceived the experiments; Y.Y. performed the experiments; X.Z., Z.Z, J.Z., and Z.W. analyzed the data; D.W. and Y.Y. wrote the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8 [DOI] [PubMed] [Google Scholar]
- 2. McVeigh TP, Al-Azawi D, O’Donoghue GT, Kerin MJ. Assessing the impact of an ageing population on complication rates and in-patient length of stay. Int J Surg. 2013;11:872–875. doi: 10.1016/j.ijsu.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 3. Sajer S, Guardiero GS, Scicchitano BM. Myokines in home-based functional electrical stimulation-induced recovery of skeletal muscle in elderly and permanent denervation. Eur J Transl Myol. 2018;28:7905. doi: 10.4081/ejtm.2018.7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Pan X, Sun Y, Geng YJ, Yu XY, Li Y. The molecular mechanisms and prevention principles of muscle atrophy in aging. Adv Exp Med Biol. 2018;1088:347–368. doi: 10.1007/978-981-13-1435-3_16 [DOI] [PubMed] [Google Scholar]
- 6. Kadoguchi T, Shimada K, Miyazaki T, et al. Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr Gerontol Int. 2020;20:78–84. doi: 10.1111/ggi.13818 [DOI] [PubMed] [Google Scholar]
- 7. Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:630–642. doi: 10.1002/jcsm.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marzetti E, Hwang JC, Lees HA, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang YY, Gu LJ, Huang J, et al. CKD autophagy activation and skeletal muscle atrophy-a preliminary study of mitophagy and inflammation. Eur J Clin Nutr. 2019;73:950–960. doi: 10.1038/s41430-018-0381-x [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Peng Y, Feng Z, et al. Reloading functionally ameliorates disuse-induced muscle atrophy by reversing mitochondrial dysfunction, and similar benefits are gained by administering a combination of mitochondrial nutrients. Free Radic Biol Med. 2014;69:116–128. doi: 10.1016/j.freeradbiomed.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 11. Walsh ME, Bhattacharya A, Sataranatarajan K, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14:957–970. doi: 10.1111/acel.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser H, Yu K, Pandya C, et al. Kynurenine, a tryptophan metabolite that increases with age, induces muscle atrophy and lipid peroxidation. Oxid Med Cell Longev. 2019;2019:9894238. doi: 10.1155/2019/9894238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi WH, Son HJ, Jang YJ, Ahn J, Jung CH, Ha TY. Apigenin ameliorates the obesity-induced skeletal muscle atrophy by attenuating mitochondrial dysfunction in the muscle of obese mice. Mol Nutr Food Res. 2017;61:1700218. doi: 10.1002/mnfr.201700218 [DOI] [PubMed] [Google Scholar]
- 14. Choi WH, Jang YJ, Son HJ, Ahn J, Jung CH, Ha TY. Apigenin inhibits sciatic nerve denervation-induced muscle atrophy. Muscle Nerve. 2018;58:314–318. doi: 10.1002/mus.26133 [DOI] [PubMed] [Google Scholar]
- 15. Wang M, Firrman J, Liu L, Yam K. A review on flavonoid apigenin: dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed Res Int. 2019;2019:7010467. doi: 10.1155/2019/7010467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su T, Huang C, Yang C, et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol Res. 2020;152:104586. doi: 10.1016/j.phrs.2019.104586 [DOI] [PubMed] [Google Scholar]
- 17. Sharma A, Ghani A, Sak K, et al. Probing into therapeutic anti-cancer potential of apigenin: recent trends and future directions. Recent Pat Inflamm Allergy Drug Discov. 2019;13:124–133. doi: 10.2174/1872213X13666190816160240 [DOI] [PubMed] [Google Scholar]
- 18. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Sun H, Song G, et al. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in STZ-induced diabetic mice. Mol Nutr Food Res. 2018;62:e1700941. doi: 10.1002/mnfr.201700941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Chen J, Liu X, et al. A Chinese herbal formula, Jian-Pi-Yi-Shen decoction, improves muscle atrophy via regulating mitochondrial quality control process in 5/6 nephrectomised rats. Sci Rep. 2017;7:505–521. doi: 10.1038/s41598-017-10027-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Zhang M, Tang J, Li Y, et al. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1α/SIRT3 pathway involved. Chem Biol Interact. 2017;277:168–175. doi: 10.1016/j.cbi.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 22. Rajesh M, Mukhopadhyay P, Bátkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Wei L, Yang Y, Liu H. Dietary supplementation with ketoacids protects against CKD-induced oxidative damage and mitochondrial dysfunction in skeletal muscle of 5/6 nephrectomised rats. Skeletal Muscle. 2018;8:18. doi: 10.1186/s13395-018-0164-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pirinen E, Cantó C, Jo YS, et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dobrowolny G, Aucello M, Molinaro M, Musarò A. Local expression of mIgf-1 modulates ubiquitin, caspase and CDK5 expression in skeletal muscle of an ALS mouse model. Neurol Res. 2008;30:131–136. doi: 10.1179/174313208X281235 [DOI] [PubMed] [Google Scholar]
- 26. Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45:2163–2172. doi: 10.1016/j.biocel.2013.05.036 [DOI] [PubMed] [Google Scholar]
- 27. Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3 [DOI] [PubMed] [Google Scholar]
- 28. Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeo D, Kang C, Gomez-Cabrera MC, Vina J, Ji LL. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1 alpha overexpression in vivo. Free Radical Bio Med. 2018;130:361–368. doi: 10.1016/j.freeradbiomed.2018.10.456 [DOI] [PubMed] [Google Scholar]
- 30. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132 [DOI] [PubMed] [Google Scholar]
- 31. Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tews DS, Goebel HH, Meinck HM. DNA-fragmentation and apoptosis-related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand. 1997;96:380–386. doi: 10.1111/j.1600-0404.1997.tb00302.x [DOI] [PubMed] [Google Scholar]
- 33. Pervaiz S, Hirpara JL, Clément MV. Caspase proteases mediate apoptosis induced by anticancer agent preactivated MC540 in human tumor cell lines. Cancer Lett. 1998;128:11–22. doi: 10.1016/s0304-3835(98)00021-4 [DOI] [PubMed] [Google Scholar]
- 34. Siu PM, Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis. 2006;11:967–981. doi: 10.1007/s10495-006-6315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gannon J, Doran P, Kirwan A, Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur J Cell Biol. 2009;88:685–700. doi: 10.1016/j.ejcb.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 36. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A BiolSci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50A.Special_Issue.11 [DOI] [PubMed] [Google Scholar]
- 37. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 38. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 39. Miller SG, Hafen PS, Brault JJ. Increased adenine nucleotide degradation in skeletal muscle atrophy. Int J Mol Sci. 2019;21:88. doi: 10.3390/ijms21010088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker JS, McCormick MC, Robergs RA. Interaction among skeletal muscle metabolic energy systems during intense exercise. J Nutr Metab. 2010;2010:905612. doi: 10.1155/2010/905612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng N, Glorieux C, Zhang Y, et al. Oncogenic K-ras induces mitochondrial OPA3 expression to promote energy metabolism in pancreatic cancer cells. Cancers. 2019;12:65. doi: 10.3390/cancers12010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pronsato L, Milanesi L, Vasconsuelo A. Testosterone induces up-regulation of mitochondrial gene expression in murine C2C12 skeletal muscle cells accompanied by an increase of nuclear respiratory factor-1 and its downstream effectors. Mol Cell Endocrinol. 2020;500:110631. doi: 10.1016/j.mce.2019.110631 [DOI] [PubMed] [Google Scholar]
- 43. Odeh M, Tamir-Livne Y, Haas T, Bengal E. P38α MAPK coordinates the activities of several metabolic pathways that together induce atrophy of denervated muscles. FEBS J. 2020;287:73–93. doi: 10.1111/febs.15070 [DOI] [PubMed] [Google Scholar]
- 44. Sim MK, Wong YC, Xu XG, Loke WK. Des-aspartate-angiotensin I attenuates ICAM-1 formation in hydrogen peroxide-treated L6 skeletal muscle cells and soleus muscle of mice subjected to eccentric exercise. Regul Pept. 2014;188:40–45. doi: 10.1016/j.regpep.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 45. del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 2006;141:330–335. doi: 10.1104/pp.106.078204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang Z, Fang Q, Ma W, et al. Skeletal muscle atrophy was alleviated by salidroside through suppressing oxidative stress and inflammation during denervation. Front Pharmacol. 2019;10:997. doi: 10.3389/fphar.2019.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yin Y, Lu L, Wang D, et al. Astragalus polysaccharide inhibits autophagy and apoptosis from peroxide-induced injury in C2C12 myoblasts. Cell Biochem Biophys. 2015;73:433–439. doi: 10.1007/s12013-015-0659-8 [DOI] [PubMed] [Google Scholar]
- 49. Giresi PG, Stevenson EJ, Theilhaber J, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21:253–263. doi: 10.1152/physiolgenomics.00249.2004 [DOI] [PubMed] [Google Scholar]
- 50. Ma W, Zhang R, Huang Z, et al. PQQ ameliorates skeletal muscle atrophy, mitophagy and fiber type transition induced by denervation via inhibition of the inflammatory signaling pathways. Ann Transl Med. 2019;7:440. doi: 10.21037/atm.2019.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosa-Caldwell ME, Greene NP. Muscle metabolism and atrophy: let’s talk about sex. Biol Sex Differ. 2019;10:43. doi: 10.1186/s13293-019-0257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.