Abstract
Objectives
To investigate the relevance of multicopy plasmids in antimicrobial resistance and assess their mobilization mediated by phage particles
Methods
Several databases with complete sequences of plasmids and annotated genes were analysed. The 16S methyltransferase gene armA conferring high-level aminoglycoside resistance was used as a marker in eight different plasmids, from different incompatibility groups, and with differing sizes and plasmid copy numbers. All plasmids were transformed into Escherichia coli bearing one of four different lysogenic phages. Upon induction, encapsidation of armA in phage particles was evaluated using qRT–PCR and Southern blotting.
Results
Multicopy plasmids carry a vast set of emerging clinically important antimicrobial resistance genes. However, 60% of these plasmids do not bear mobility (MOB) genes. When carried on these multicopy plasmids, mobilization of a marker gene armA into phage capsids was up to 10000 times more frequent than when it was encoded by a large plasmid with a low copy number.
Conclusions
Multicopy plasmids and phages, two major mobile genetic elements (MGE) in bacteria, represent a novel high-efficiency transmission route of antimicrobial resistance genes that deserves further investigation.
Introduction
Antimicrobial resistance is recognized today as one of the most serious threats to human health.1 Understanding the mechanisms underlying the emergence and transmission of antimicrobial resistance genes in humans, animals and the environment has become a major goal in microbiological studies worldwide.2–4 Research on horizontal gene transfer in bacteria is of special relevance, as it leads to the rapid spread of known and novel antimicrobial resistance genes. Although a lot of effort has been focused on plasmids and their spread through conjugation or transformation, little is known regarding the relationship between plasmids and phages, two major mobile genetic elements in bacteria. We have recently identified fragments of phages in small multicopy plasmids (MCPs) in enterobacteria.5 This led us to hypothesize that plasmids in general, but probably with more frequency in MCPs, could be transferred between bacteria by phage transduction. Several other facts support this notion: (i) many MCPs lack mobility (MOB) genes, but are widely spread in bacterial populations;5 (ii) each plasmid is present in the cytoplasm in multiple copies, which can rise to 100 copies/cell during antibiotic treatment,6 increasing the theoretical probability of a plasmid or plasmid-borne gene being encapsidated during general transduction; (iii) antimicrobial resistance (AMR) genes normally found on plasmids have been identified in phage particles;7,8 and (iv) like other bacterial DNA, plasmids can be transferred through generalized transduction.9–12
Great attention was focused on MCPs in the 1970s, as they were, and are, essential in biotechnology and gene manipulation.13 However, understanding of the relevance of MCPs in AMR is more recent. We showed that MCPs are responsible for β-lactam resistance in several pathogens, and that MCPs bearing different resistance genes can stably coexist in a single bacterium, giving rise to a novel bacterial strategy for multidrug resistance.14 Further, it is widely known that duplication of genes facilitates gene innovation.15 Notably, a single SNP in the replication origin of an MCP can give rise to a novel variant, with a different plasmid copy number and fitness cost,16 showing how easily gene innovation and spread are facilitated by MCPs. In addition, AMR genes can undergo mutations and further selection in MCPs.17
To test our hypothesis, we have first analysed the relevance of MCPs in bacteria. Second, we have assessed AMR genes present in MCPs. Third, we have tested the efficiency of phage encapsidation of an AMR gene borne on different genetic platforms. To do this, we have used the same AMR gene, armA,18–20 as a marker in eight different plasmids of different sizes (≈5 kb to ≈80 kb), different plasmid copy numbers (1–45), and belonging to different incompatibility groups. To discard generalized transduction events, we have used four temperate phages that lysogenize the donor strains. The amount of armA in phage particles has been quantified after phage induction and the results of packaging of the different plasmids have been compared.
Materials and methods
In silico analysis of plasmids in bacteria
To examine the distribution of plasmids based on their predicted mobility, 16702 plasmid sequences were retrieved from the NCBI nucleotide database, using the PLSDB web server for screening purposes.21 Only plasmid records with circular sequences or complete assemblies were selected. Computational prediction analysis of the mobilization capacity was performed based on the presence of relaxases and mating pair formation systems using the MOB-typer tool included in the MOB-suite software.22 Plasmids lacking a relaxase were classified as ‘non-MOB’, while the presence of a type IV secretion system (T4SS), involved in mating pair formation during conjugation, enabled us to separate potentially ‘conjugative’ and ‘MOB’ plasmids.
Analysis of AMR genes in small plasmids
Small plasmids (ColE1, ColE10 and IncQ) were identified based on plasmid replicon sequences obtained from the PlasmidFinder database,23 using the BLASTn algorithm to look for DNA homologies in the GenBank database. Complete plasmid accessions were retrieved from the NCBI nucleotide database, using an Entrez query with filters to exclude incomplete or non-plasmid sequences. A local, command-line version of AMRfinder tool (https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/AMRFinder/) and ResFinder tool24 with default parameters was used to identify AMR genes in MCPs, allowing enumeration and classification of the genes according to the antimicrobial class they conferred resistance to, along with the plasmid size and GenBank accession number.
Bacteria, strains and culture conditions
Eight different plasmids all carrying the same resistance marker, armA, were used in this study: five conjugative plasmids larger than 47 kb that naturally carry armA, belonging to different incompatibility groups (IncM2, IncFII, IncR, IncX1 and IncN), and three vectors smaller than 12 kb (pACYC184, pCR2.1 and pUC18) into which armA was cloned (Table 1).
Table 1.
Plasmids used in this study
| Plasmid | Group | Size (bp) | Copy number | Reference |
|---|---|---|---|---|
| pACYC184::armA | p15A | 5682 | 8.34 ± 1.07 | This work |
| pCR2.1::armA | ColE1-like | 4929 | 23.11 ± 1.7 | This work |
| pUC18::armA | ColE1-like | 11680 | 41.78 ± 4.23 | 18 |
| pB1362 | IncM2 | 77297 | 2.5 ± 0.13 | ERZ1101256 |
| pB2920 | IncFII | 77960 | 0.78 ± 0.22 | ERZ1079030 |
| pB2948 | IncR | 47627 | 1.11 ± 0.24 | ERZ1079031 |
| pB2954 | IncX1 | 77121 | 1.32 ± 0.14 | ERZ1079029 |
| pMUR050 | IncN | 56634 | 0.99 ± 0.03 | AY522431.4 |
The large plasmids were isolated from WT specimens from different origins that contained the armA gene and were pan-resistant to aminoglycosides. The plasmids were sequenced using Illumina and Nanopore technology (see below) and the complete nucleotide sequence was deposited in the ENA database together with the corresponding metadata on their origin and date of isolation (Table 1). Construction of pUC18::armA is as described by Gonzalez-Zorn et al.18 Cloning using the poly(A) tail was used for pCR2.1::armA only. To clone armA into pACYC184, the gene was amplified with armA400 and armAR, then the product was digested with EcoRI and cloned into the EcoRI site of pACYC184 (Table S1, available as Supplementary data at JAC Online).
Escherichia coli strain WG5 lysogenic for Stx phages 933W, 312 or 557,25 or a Cdt phage,26 and E. coli strain DH5α lysogenic for Stx phages 933W, 312 or 557,25 were used as recipient strains for the plasmids mentioned above to evaluate the packaging of plasmids or plasmid fragments in phage particles. Strain WG5 is a nalidixic acid-resistant mutant of E. coli C (also known as strain CN) and does not contain resistance genes or complete inducible prophages.27 DH5α is a recA-negative E. coli K-12 derivative.28 Stx phages 933W, 312 and 557 are three different phages of the Podoviridae morphological type carrying the Shiga toxin (stx) gene, while Cdt phage is from the Myoviridae morphological type carrying the cytolethal distending toxin (cdt) gene.26 Stx phages and Cdt phages are temperate cos phages that package a fragment of DNA delimited between two cos sites and therefore are not prone to packaging DNA through generalized transduction.29 All lysogens were transformed with MCPs (pACYC184, pCR2.1 or pUC18) and low-copy plasmids (IncM2, IncFII or IncR). Additionally, low-copy plasmids IncX1 and IncN were used to transform some of the lysogens and were also added to the study. Bacterial strains and phages were selected to study whether differences related to the phages or to the host strain could be factors influencing the encapsidation of the different plasmids.
Complete sequence of plasmids with Illumina and MinION technology
Genomic DNA extraction and purification were performed using the Wizard Genomic DNA Purification Kit (Promega Corp., Madison, WI, USA). DNA quality and concentration were measured by NanoDrop (Thermo Fisher Inc., Waltham, MA, USA) and Qubit (Invitrogen Corp., Carlsbad, CA, USA) devices. Short paired-end reads were generated on a HiSeq 2500 platform (Illumina Inc., San Diego, CA, USA). Sequences were processed for subsequent analysis by checking their quality with FastQC version 0.11.3 and trimming end nucleotides of low quality using Trimmomatic version 0.33. Genomes were sequenced using a MinION device (Oxford Nanopore Technologies Ltd., Oxford, UK). Genomic libraries were generated following the 1D Native barcoding genomic DNA protocol, with EXP-NBD103 and SQK-LSK108 kits (Oxford Nanopore Technologies Ltd.), and sequencings were run in a FLO-MIN106 flow cell. Sequencing reads were base-called with MinKNOW software (Oxford Nanopore Technologies Ltd.), demultiplexing was carried out with the Fastq Barcoding workflow of the Epi2Me interface (Metrichor Ltd., Oxford, UK) and trimming of adaptors and barcodes from the reads was assessed by Porechop version 0.2.3. Hybrid assemblies with short and long sequencing reads were achieved using Unicycler version 0.4.0. Closed genomic structures were assessed by Bandage version 0.8.1 and annotated by Prokka version 1.5. Antibiotic resistance genes and plasmid incompatibility groups present in each of the genomic structures were identified using Bandage and applying the ResFinder and PlasmidFinder databases, respectively.
Plasmid copy number quantification and qPCR for armA
The average number of copies of each plasmid was determined by quantitative PCR (qPCR) as described by San Millan et al.6 in triplicate from three independent DNA extractions from DH5α-557. DNA extractions were carried out from 2 mL of LB broth cultures, at an OD600 of approximately 0.6, using a QIAamp DNA Mini Kit (Qiagen, USA). The amount of DNA was quantified using Qubit (Invitrogen Corp., Carlsbad, CA, USA). Since restriction enzyme-digested total DNA is a better template source than non-digested total DNA for plasmid quantification by qPCR,30 100 ng of DNA from each sample was digested using FastDigest PstI (Thermo Fisher Scientific, USA) for 1 h at 37°C. The desired amplification products do not contain the PstI target sequence. We developed a specific qPCR for the armA gene carried in all the plasmids. In order to determine the average plasmid copy number per chromosome, the monocopy gene uidA was amplified to compare the ratio of plasmid to chromosomal DNA (Table 1). A standard curve of each reaction was generated by performing qPCR with five 5-fold dilutions of template DNAs in triplicate working range of DNA concentration, ≈0.2 ng/μL to 320 fg/μL, from which the efficiency of the reaction was calculated. qPCR was performed using a My iQ single-colour real-time PCR detection system (Bio-Rad, USA) and the Bio-Rad iQ SYBR Green Supermix (Bio-Rad, USA) at a final concentration of 20 pg/μL. The amplification conditions were as follows: initial denaturation for 10 min at 94°C, followed by 30 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 49.3°C (armA) or 55°C (uidA), and extension for 1 min at 72°C. To calculate plasmid copy number per chromosome, we used the formula described previously:31 cn = (1 + Ec)Ctc/(1 + Ep)Ctp × Sc/Sp, where cn is the plasmid copy number per chromosome, Sc and Sp are the sizes of the chromosomal and plasmid amplicons in bp, respectively, Ec and Ep are the efficiencies (relative to 1) of the chromosomal and plasmid qPCRs, respectively, and Ctc and Ctp are the threshold cycles of the chromosomal and plasmid reactions, respectively. The armA qPCR assay30 was designed using the sequence of armA in plasmid pMUR050 (AY522431.4) from an E. coli pig isolate.18 pMUR050 was also used to generate standard curves.8 Only qPCR reactions with an efficiency between 95% and 100% were considered as significant. The armA qPCR assay has an average efficiency of 98.4% and a detection limit of 2.74 gene copies. qPCR amplification was conducted with 1 μL of extracted packaged DNA under standard conditions in a Step One Real-Time PCR System (Applied Biosystems, Life Technologies).30 Student’s t-test was used to assess statistical significance.
Phage induction and isolation of phage particles
Cultures (10 mL) of each lysogen were grown on LB broth to the mid-exponential phase (OD600 of 0.3) for optimal phage induction and treated with mitomycin C (final concentration 0.5 μg/mL), which induces the SOS cell response and drives the induction of the lytic cycle of the phage and the production of phage particles. Cultures were incubated overnight in the presence of mitomycin C at 37°C with shaking in the dark because mitomycin C is light sensitive. After incubation, phage particles were extracted as previously described.7 Briefly, the cultures were filtered through low protein-binding 0.22 μm pore-size membrane filters (Millex-GP, Millipore, Bedford, MA, USA) to eliminate cell debris, treated with chloroform (1:10 v/v) to break cell membranes and possible membrane vesicles that might contain nucleic acids, and treated with DNAse I (100 units/mL; Sigma–Aldrich, Spain) for 1 h at 37°C to eliminate non-packaged DNA. At this stage, the phage suspension was used for transmission electron microscopy observations. To confirm the removal of non-packaged DNA, an aliquot of the sample was taken after DNase I treatment and before its de-encapsidation with proteinase K and used to confirm the absence of armA by qPCR.7 Different controls were performed to confirm the stability of the DNase I and its inactivation,32 before extracting packaged DNA by proteinase K digestion, and by phenol chloroform purification as previously described.32,33 Extracted packaged DNA was used for qPCR amplification and Southern blot analysis as described below. The Pearson correlation coefficient (r) was calculated by two-tailed correlation test in order to determine the linear association between the plasmid copy number and phage encapsidation using R version 3.6.1 and P ≤ 0.05 as the critical level of significance.
Southern blotting
The DNA fragment of the armA and the different probes resulting from amplification with the respective primers (Table S1) were labelled with digoxigenin. The probe was labelled by incorporating digoxigenin-11-deoxy-uridine-triphosphate (Roche Diagnostics. Barcelona, Spain) during PCR as described.34
For Southern blot hybridization a higher DNA concentration was required, and for this purpose we extracted packaged DNA from 100 mL cultures of strains WG5-933W, WG5-312 and DH5α-557, selected for being representative of three different phages in two different strains, and containing the different plasmids. We obtained a final volume of 50 μL of DNA that was digested with S1 nuclease (Promega Co., Inc., MA, USA) and analysed by separation on 0.7% agarose gels in 0.5-fold concentrated Tris borate EDTA buffer and stained with ethidium bromide.
Digested DNA was transferred to nylon N+ membranes (Hybond N+, Amersham Pharmacia Biotech, Spain) by capillary blotting.35 The presence of armA and the plasmids was determined in all the digested phage DNA by Southern blot hybridization with the digoxigenin-labelled probes prepared as described above. Stringent hybridization was achieved with the DIG DNA Detection Kit (Roche Diagnostics, Barcelona, Spain) according to the manufacturer’s instructions.
After obtaining armA results, the membranes were incubated with dimethylformamide, heated at 60°C, washed with 0.2 N NaOH and 0.1% SDS solution at 37°C for 10 min to strip the armA probe and hybridized again,35 using the respective digoxigenin-labelled probes targeting regions not close to armA in each plasmid (Table S1). The probes targeting other regions in the small plasmids are located opposite to the insertion site of armA. In large plasmids, the probe is located 5 kbp upstream of armA.
Results and discussion
Plasmid sizes and mobilization genes
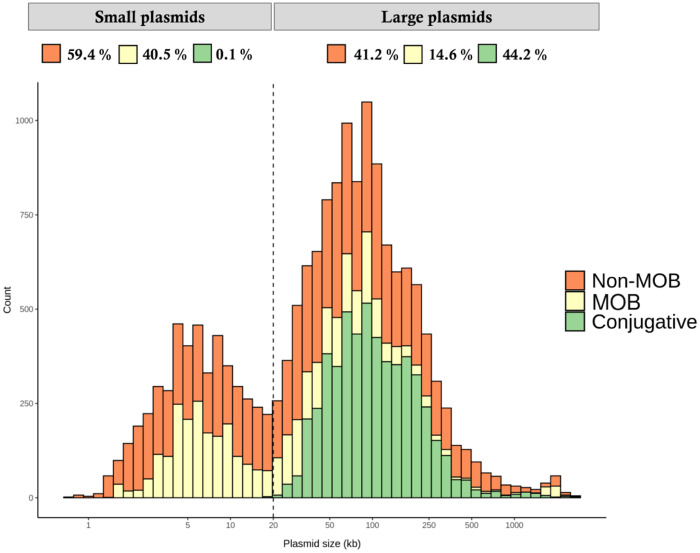
Plasmids are the major vehicle of AMR genes. To evaluate the sizes and potential mobilization capacity of plasmids, we analysed all fully sequenced plasmids available, corresponding to ≈16700 complete circularized plasmid sequences (see Materials and methods). The plot of number of plasmids versus plasmid size is shown in Figure 1. Plasmids follow a bimodal distribution, with a clear differentiation of small plasmids (<20 kb) and large plasmids (>20 kb). It is generally accepted that the main dissemination strategy of plasmids is conjugation. Conjugative plasmids possess the full genetic machinery encoding a T4SS and the relaxases to undergo self-transmission, which is known to require ≈25 kb of genetic information.36,37 We therefore analysed how many plasmids possess a complete conjugative apparatus. In line with their small size, none of the small plasmids are predicted to be conjugative. Interestingly, only 44.2% of the large plasmids possess a full T4SS.
Figure 1.
Histogram of 16702 fully sequenced and circular plasmids. Plasmids follow a bimodal distribution: small plasmids (<20 kb) and large plasmids (>20 kb). Plasmids were predicted to be conjugative (with T4SS), MOB (without T4SS, with a MOB gene) and non-MOB (without T4SS or a MOB gene). Note that most plasmids are not transmissible. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Another set of plasmids carry just a relaxase that enables conjugation using the T4SS of a co-resident conjugative plasmid.38,39 We call them here MOB plasmids and they constitute 40.5% of small plasmids. Among the large plasmids, 14.6% have MOB genes and no T4SS. Consequently, the majority of plasmids are non-MOB plasmids (41.2% of all large plasmids and 59.4% of small plasmids). However, small plasmids do not need to have MOB genes to be efficiently mobilized through conjugation, as it has recently been found that non-MOB plasmids with an origin of transfer (oriT) can be conjugated by relaxases encoded in co-resident plasmids acting in trans.37,40
Small plasmids confer resistance to most antimicrobial classes
The genes borne on small plasmids were listed and classified according to the antimicrobial class they conferred resistance to (Table 2). The table shows different resistance genes found in small plasmids belonging to families of MCPs according to the current literature. These plasmids encode resistance to aminoglycosides, β-lactams, macrolides, lincosamides and streptogramins (MLSs), phenicols, sulphonamides, tetracycline and trimethoprim. The mean plasmid size of all MCPs listed here was 8.3 ± 3.6 kb (SD). Interestingly, emerging genes conferring resistance to clinically relevant or last-resort antibiotics such as fluoroquinolones (qnrS2 and qnrB19), carbapenems, (blaKPC-2, blaKPC-3, blaBKC-1 and blaOXA-656) and colistin (mcr-4 and mcr-5) were also found on small plasmids, highlighting the relevance of these small mobile genetic elements in AMR. Although most of these MCPs (such as pCCK64741 or pMCR_R3445,42) carry a single resistance gene, other resistance genes were aligned in tandem (like in pB1005,43), were found in transposon elements,14 or were contained in a complete integron (like in pQ7,44). Thus, potential transfer of these replicons would lead to resistance not only to a single antimicrobial class, but to several different ones, giving rise to MDR in the recipient bacterium. The bacterial hosts with small plasmids are classical human pathogens (Haemophilus influenzae, Gram-negative opportunistic or pathogenic bacteria, E. coli, Klebsiella pneumoniae or Pseudomonas spp.) and strict animal pathogens such as Haemophilus (Glaesserella) parasuis.
Table 2.
Antimicrobial resistance genes located on small plasmids
| Resistance gene | Plasmid | Plasmid size (bp) | GenBank accession no. |
|---|---|---|---|
| Aminoglycoside | |||
| aac(6')-Ib3 | pCHE-A1 | 8201 | KX244760.1 |
| aac(6')-Ib4 | pJF-789 | 9016 | KX912254.1 |
| aac(6')-Ib-cr | pMdT1 | 5931 | JX457478.1 |
| ant(2'')-Ia | pVAS24-VEB | 9159 | KX575838.1 |
| aph(3')-Ia | pMR0716_ColRNAI | 5310 | CP018108.1 |
| aph(3')-IIIa | pCCK411 | 5265 | FR798946.1 |
| aph(3'')-Ib | pB1005 | 4237 | FJ197818.1 |
| aph(3')-Via | pKPN535a | 14873 | MH595533.1 |
| aph(6)-Id | pLC1477_18-3 | 8398 | CP035011.1 |
| aadA1 | pCCK343 | 5415 | FR687372.1 |
| aadA14 | pCCK647 | 5198 | AJ884726.1 |
| β-Lactam | |||
| blaTEM-1a | pEC404/03-4 | 8599 | AP014807.1 |
| blaTEM-1b | p0.1229_3 | 12894 | CP028323.1 |
| blaTEM-2 | pEC886 | 9261 | HQ659758.1 |
| blaTEM-116 | MK753226 | 5607 | MK753226.1 |
| blaTEM-144 | pST12 | 8275 | HG428760.1 |
| blaBKC-1 | p60136 | 9786 | KP689347.1 |
| blaCTX-M-3 | pPSTRAS1 | 9910 | MH463250.1 |
| blaCTX-M-14 | RCS63_p | 22308 | LT985269.1 |
| blaCTX-M-17 | pIP843 | 7086 | AY033516.1 |
| blaGES-1 | pQ7 | 9042 | FJ696404.1 |
| blaGES-5 | pCHE-A | 7560 | EU266532.1 |
| blaGES-7 | pPCMI3 | 9448 | MH569711.1 |
| blaSHV-12 | RCS35_pII | 14365 | LT985233.1 |
| blaKPC-2 | pKPN535a | 14873 | MH5955331 |
| blaKPC-3 | pKPC_Kp02 | 11930 | KX348145.1 |
| blaKPC-21 | pUR19829-KPC21 | 12748 | MH133192.1 |
| blaCMY-2 | pEC5106 | 14845 | KY612499.1 |
| blaCMY-4 | pQEL231 | 6925 | KP205272.1 |
| blaOXA-256 | pUL3AT | 9005 | HE616889.1 |
| blaOXA-655 | pQGU16 | 14146 | MH718732.1 |
| blaOXA-656 | pQGU13 | 15473 | MH718731.1 |
| blaROB-1 | pB1000 | 4613 | GU080062.1 |
| blaROB-2 | pKKM48 | 4323 | MH316128.1 |
| blaVEB-18 | pVAS24-VEB | 9159 | KX575838.1 |
| Polymyxin | |||
| mcr-4.1 | pMCR_R3445 | 8749 | MF543359.1 |
| mcr-4.2 | pMCR-4.2_AB243 | 9513 | MG800340.1 |
| mcr-4.3 | pEn_MCR4 | 8639 | MH061380.1 |
| mcr-5.1 | pSE11-03671 | 8936 | MK360094.1 |
| mcr-5.2 | pEC2380 | 11708 | MG587004.1 |
| Fluoroquinolone | |||
| qnrS2 | pPH18 | 6388 | KU644672.1 |
| qnrB19 | pPAB19-2 | 3082 | JN979787.1 |
| Macrolide, lincosamide and streptogramin B | |||
| lnu(G) | pIE1115 | 10687 | AJ293027.1 |
| erm(42) | A1_180 | 7406 | CP040385.1 |
| Phenicol | |||
| catA1 | pDT4 | 3716 | HF570110.1 |
| catA3 | pMHSCS1 | 4992 | AJ249249.1 |
| catB2 | pJR1 | 6792 | AY232670.1 |
| floR | p518 | 3937 | KT355773.1 |
| Streptothricin | |||
| sat2 | pCCK343 | 5415 | FR687372.1 |
| Sulphonamide | |||
| sul2 | pB1005 | 4237 | FJ197818.1 |
| Tetracycline | |||
| tet(A) | pLC1477_18-3 | 8398 | CP035011.1 |
| tet(B) | pHS-Tet | 5147 | AY862435.1 |
| tet(C) | pIS2 | 6349 | AY167049.1 |
| tet(G) | pJR1 | 6792 | AY232670.1 |
| tet(H) | pB1018 | 6074 | JQ319774.1 |
| tet(L) | pCCK3259 | 5317 | AJ966516.1 |
| tet(O) | pB1006 | 6033 | FJ234438.1 |
| Trimethoprim | |||
| dfrA1 | pCCK343 | 5415 | FR687372.1 |
| dfrA14 | pABC-3 | 6779 | KT988306.1 |
| dfrB3 | pPCMI3 | 9448 | MH569711.1 |
bla OXA-256/655/656 belong to the OXA-10 class D family.
High-efficiency packaging of DNA from MCPs in phages
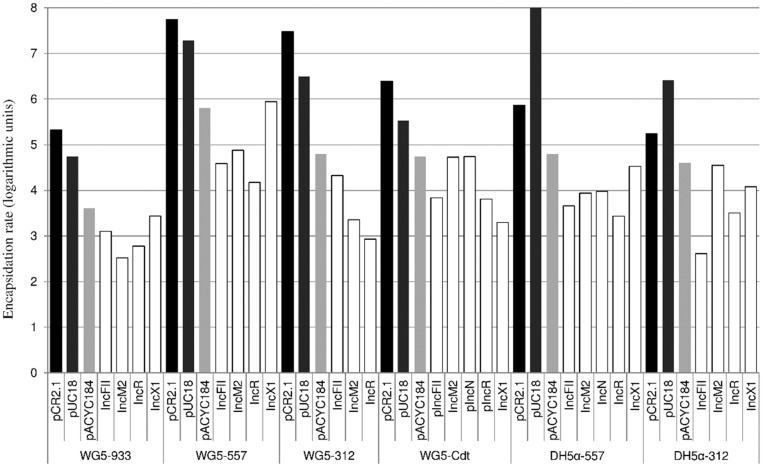
Due to their relevance in AMR, high frequency and lack of mobilization genes, the interaction of MCPs with phages was further investigated. The different E. coli genetic backgrounds (E. coli WG5 or DH5α) were lysogenic for different phages (Podoviridae Stx phages 933W or 312 or Myoviridae Cdt phage). All lysogens were transformed with the same set of plasmids, all bearing the 16S rRNA methyltransferase gene armA, conferring resistance to all clinically relevant aminoglycosides (Table 1). The large plasmids belonged to the IncFII, IncM2, IncR, IncN and IncX1 incompatibility groups, whereas the MCPs belonged to the ColE1 and p15A families (Table 1). The copy number of all plasmids was determined using qPCR and a chromosomal monocopy gene as a marker (see Materials and methods). The plasmid copy number showed that plasmid pUC18 had the highest copy number, with 42 copies/cell, followed by pCR2.1 (23 copies/cell) and pACYC184 (8 copies/cell). All large plasmids, however, had a copy number that ranged between 1 and 3 (Table 1).
Upon induction with mitomycin C and isolation of capsids, all purified phage lysates were shown to have the armA gene (Figure 2). Non-lysogenic DH5α strains transformed with the same set of plasmids did not show the detection of armA in an extract prepared in the same way as for phage (data not shown). Interestingly, the encapsidation rate of armA was significantly higher when the genetic platform of armA was an MCP than when it was carried on a large low-copy plasmid (P < 0.0001). Encapsidation, when armA was borne on MCPs such as pUC18, was up to 4 logarithmic units more efficient than when armA was borne on a large plasmid like IncR (Figure 2).
Figure 2.
Representative experiment showing the detection of armA in the phage DNA fraction of E. coli strains WG5 and DH5α lysogenized with Stx phages 933W, 557 or 312 or Cdt phage and DH5α lysogenized with Stx phages 933W or 312 transformed with the different plasmids. Shaded bars correspond to MCPs and white bars to large, low-copy-number plasmids.
Southern blot analyses were performed with the DNA isolated from the phage fraction purified from some of the lysogens. We selected those showing enough encapsidated DNA to perform Southern blotting. As probes, an internal fragment of armA and probes targeting another region in each plasmid were used (Table S1). After S1 nuclease digestion to remove chromosomal DNA, a positive signal was obtained with both probes in fragments corresponding to 1.6 to >3 kb, confirming that in the phage fraction, not only armA but also other fragments of plasmid DNA were encapsidated (Figure S1). No complete plasmids, only shorter fragments, were detected in the Southern blots (Figure S1). Fragmentation of the DNA could be caused during the extraction process or due to S1 nuclease, which might more frequently cut packaged coiled plasmidic DNA than non-packaged plasmids. It could also be a mechanism for encapsidation, which must be required to package some of the large plasmids of >70 kb, since the phage capsid allows 62 kb for the three Stx phages or 34 kb for the Cdt phage.
Our selection of S1 nuclease may not be optimal, but it allowed the exclusion of chromosomal DNA, confirming the plasmidic nature of the packaged DNA. Moreover, digestion of packaged DNA using other endonucleases resulted in long DNA smears (data not shown).
Phages are able to encapsidate and transfer AMR genes.45–47 This takes place not only in the environment, but also in the human gut, where antimicrobial treatment induces encapsidation of AMR genes.48 Antibiotic resistance genes can be located chromosomally or in plasmids.47 Generalized transduction of low- and high-copy plasmids has previously been shown with Salmonella phage P22, Bacillus phage SPP2 and E. coli phage Mu.9–12 In addition, some strains harbour certain P1 phage derivatives that remain as plasmids during their life cycle without being integrated as prophages.49 Our results confirm that AMR genes, when borne on plasmids, can use phages for potential mobilization. This is especially relevant in the case of MCPs, because they cannot transfer horizontally using conjugation. The difference from previous reports is that phages in our study use a cos-packaging mechanism, which eliminates the possibility of generalized transduction. The recently described chromosomal lateral transduction shows how phage packaging can mediate efficient transduction of complete genomes.50 By lateral transduction, prophages packaging DNA through the cos mechanism can mobilize large fragments of chromosome, but no mobilization of plasmids has been reported by lateral transduction so far.
Southern blotting showed that fragmented plasmids were encapsidated. This could be attributable to fragmentation of DNA during the extraction process or due to S1 nuclease digestion, which may more frequently cut packaged plasmid DNA than non-packaged, coiled plasmids. Nevertheless, all MCPs (or their DNA fragments) were encapsidated with higher frequency than the low-copy plasmids. It should be considered that MCPs do not possess recognizable pac or cos sequences, classically used for transduction,51 and therefore the plasmid packaging mechanism used by the cos phages in this study is not yet known.
The plasmid DNA would therefore mainly use phage capsids to disseminate, as most of them do not possess MOB genes. The potential encapsidation of the plasmids would appear to be independent of the phage induction rates mediated by mitomycin C. This is supported by the fact that, when using a DH5α host that is recA negative,52 encapsidation rates were similar to those obtained with the recA-positive strain WG5. Also, the use of the Cdt phage, which has a higher level of spontaneous induction than the Stx phages,26 did not show marked differences in terms of plasmid packaging. Therefore, the most significant difference between the plasmids is the size and copy number. In fact, there is correlation between the encapsidation rate and the copy number of the MCPs (P = 0.0015, Figure S2); armA in plasmid pACYC184, with the lowest copy number of 8 copies/cell, is potentially encapsidated with lower frequency than when borne on pCR2.1 or pUC18, with 23 and 44 copies/cell. Although at a lower rate, large plasmids could be transferred between bacteria through phages; up to 41% of all large plasmids analysed here also lack mobilization genes (Figure 1).
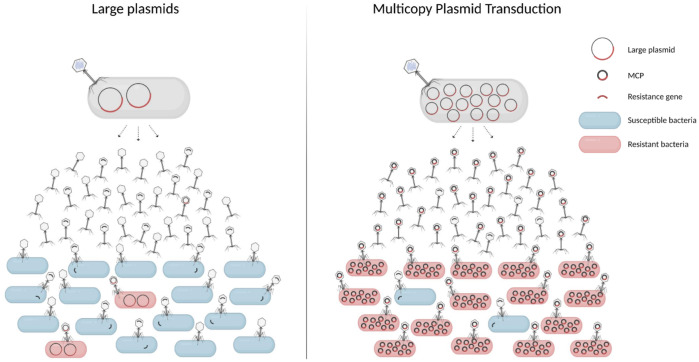
The use of phage therapy may need to take into account these results, to learn about the different mechanisms of AMR transfer, but also to focus efforts on the development of phage-based strategies that do not contribute to the spread of AMR genes.53 Thus, our findings here suggest that MCP transduction (Figure 3) could be an extremely efficient means of mobilization of AMR genes, and although further investigations are needed to determine the mechanism of this phenomenon, our findings have key implications for plasmid evolution and the emergence and spread of AMR.
Figure 3.
High efficiency spread of AMR through multicopy plasmid transduction. AMR genes borne on small MCPs (right) are encapsidated up to 10000 times more efficiently than when borne on large low-copy plasmids (left). As a consequence, the phages can disseminate over distance to transduce susceptible bacteria and transfer resistance genes. We propose a model where multicopy plasmid transduction is a major powerful route for AMR gene dissemination in nature, in which AMR spreads with high efficiency and over distance between bacteria from humans, animals and the environment. As the cargo of plasmids can also be other genes apart from AMR genes, this phenomenon represents a striking coordination between MGEs driving the evolution of bacterial populations. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Funding
This work was supported by ARDIG, from the European Joint Programme One Health EJP, Grant Agreement No 773830, The CARTNET, MSCA-ITN-2017, Grant agreement ID: 765147 and EFFORT, FP7-KBBE-2013-7, Grant agreement 613754 all from European Union's Horizon 2020 research and innovation programme. This work was supported further by the Spanish Ministerio de Innovación y Ciencia (AGL2016-75536-P), the Agencia Estatal de Investigación (AEI) and the European regional fund (ERF) the Generalitat de Catalunya (2017SGR170) and the Centre de Referència en Biotecnologia (XeRBa). L. R.-R is supported by the Beatriu de Pinos postdoctoral programme of the Government of Catalonia’s Secretariat for Universities and Research of the Ministry of Economy and Knowledge. C.S. was funded with an FPU Ph.D. grant from the Spanish Ministry of Education (FPU18/04196).
Transparency declarations
None to declare.
Supplementary data
Table S1, Figures S1 and S2 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1.WHO. WHO Report on Surveillance of Antibiotic Consumption 2016–2018.https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf.
- 2. Hernando-Amado S, Coque TM, Baquero F. et al. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat Microbiol 2019; 4: 1432–42. [DOI] [PubMed] [Google Scholar]
- 3. Johnson AP, Hughes G.. The prospect of untreatable gonorrhoea. BMJ 2017; 358: j3973. [DOI] [PubMed] [Google Scholar]
- 4. Granier SA, Hidalgo L, San Millan A. et al. ArmA methyltransferase in a monophasic Salmonella enterica isolate from food. Antimicrob Agents Chemother 2011; 55: 5262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ares-Arroyo M, Bernabe-Balas C, Santos-Lopez A. et al. PCR-based analysis of ColE1 plasmids in clinical isolates and metagenomic samples reveals their importance as gene capture platforms. Front Microbiol 2018; 9: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. San Millan A, Santos-Lopez A, Ortega-Huedo R. et al. Small-plasmid-mediated antibiotic resistance is enhanced by increases in plasmid copy number and bacterial fitness. Antimicrob Agents Chemother 2015; 59: 3335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown-Jaque M, Rodriguez Oyarzun L, Cornejo-Sánchez T. et al. Detection of bacteriophage particles containing antibiotic resistance genes in the sputum of cystic fibrosis patients. Front Microbiol 2018; 9: 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colomer-Lluch M, Jofre J, Muniesa M.. Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS One 2011; 6: e17549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canosi U, Lüder G, Trautner TA.. SPP1-mediated plasmid transduction. J Virol 1982; 44: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi H, Saito H.. Mechanism of pBR322 transduction mediated by cytosine-substituting T4 bacteriophage. Mol Gen Genet 1982; 186: 497–500. [DOI] [PubMed] [Google Scholar]
- 11. Teifel-Greding J. Transduction of multi-copy plasmid pBR322 by bacteriophage Mu. Mol Gen Genet 1984; 197: 169–74. [DOI] [PubMed] [Google Scholar]
- 12. Mann BA, Slauch JM.. Transduction of low-copy number plasmids by bacteriophage P22. Genetics 1997; 146: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang AC, Cohen SN.. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 1978; 134: 1141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cerdeira LT, Lam MMC, Wyres KL. et al. Small IncQ1 and Col-like plasmids harboring blaKPC-2 and non-Tn4401 elements (NTEKPC-IId) in high-risk lineages of Klebsiella pneumoniae CG258. Antimicrob Agents Chemother 2019; 63: e02140–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins RE, Merz H, Higgs PG.. Origin and evolution of gene families in bacteria and archaea. BMC Bioinformatics 2011; 12: S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos-Lopez A, Bernabe-Balas C, Ares-Arroyo M. et al. A naturally occurring single nucleotide polymorphism in a multicopy plasmid produces a reversible increase in antibiotic resistance. Antimicrob Agents Chemother 2017; 61: e01735–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. San Millan A, Escudero JA, Gifford DR. et al. Multicopy plasmids potentiate the evolution of antibiotic resistance in bacteria. Nat Ecol Evol 2016; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Zorn B, Teshager T, Casas M. et al. armA and aminoglycoside resistance in Escherichia coli. Emerg Infect Dis 2005; 11: 954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Zorn B, Catalán A, Escudero JA. et al. Genetic basis for dissemination of armA. J Antimicrob Chemother 2005; 56: 583–5. [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez B, Escudero JA, San Millan A. et al. Fitness cost and interference of Arm/Rmt aminoglycoside resistance with the RsmF housekeeping methyltransferases. Antimicrob Agents Chemother 2012; 56: 2335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galata V, Fehlmann T, Backes C. et al. PLSDB: a resource of complete bacterial plasmids. Nucleic Acids Res 2019; 47: D195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson J, Nash J.. MOB-suite: software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb Genom 2018; 4: e000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carattoli A, Zankari E, García-Fernández A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serra-Moreno R, Jofre J, Muniesa M.. Insertion site occupancy by stx2 bacteriophages depends on the locus availability of the host strain chromosome. J Bacteriol 2007; 189: 6645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allué-Guardia A, Imamovic L, Muniesa M.. Evolution of a self-inducible cytolethal distending toxin type V-encoding bacteriophage from Escherichia coli O157:H 7 to Shigella sonnei. J Virol 2013; 87: 13665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imamovic L, Misiakou M-A, van der Helm E. et al. Complete genome sequence of Escherichia coli strain WG5. Genome Announc 2018; 6: e01403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 1983; 166: 557–80. [DOI] [PubMed] [Google Scholar]
- 29. Feiss M, Rao VB.. The bacteriophage DNA packaging machine. Adv Exp Med Biol 2012; 726: 489–509. [DOI] [PubMed] [Google Scholar]
- 30. Providenti MA, O’Brien JM, Ewing R. et al. The copy-number of plasmids and other genetic elements can be determined by SYBR-Green-based quantitative real-time PCR. J Microbiol Methods 2006; 65: 476–87. [DOI] [PubMed] [Google Scholar]
- 31. San Millan A, Heilbron K, MacLean RC.. Positive epistasis between co-infecting plasmids promotes plasmid survival in bacterial populations. ISME J 2014; 8: 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colomer-Lluch M, Calero-Cáceres W, Jebri S. et al. Antibiotic resistance genes in bacterial and bacteriophage fractions of Tunisian and Spanish wastewaters as markers to compare the antibiotic resistance patterns in each population. Environ Int 2014; 73: 167–75. [DOI] [PubMed] [Google Scholar]
- 33. Quirós P, Colomer-Lluch M, Martínez-Castillo A. et al. Antibiotic resistance genes in the bacteriophage DNA fraction of human fecal samples. Antimicrob Agents Chemother 2014; 58: 606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muniesa M, Recktenwald J, Bielaszewska M. et al. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect Immun 2000; 68: 4850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown T. Southern blotting. Curr Protoc Mol Biol 1993; 21: 2.9.1–2.9.20. [DOI] [PubMed] [Google Scholar]
- 36. Smillie C, Garcillán-Barcia MP, Francia MV. et al. Mobility of plasmids. Microbiol Mol Biol Rev 2010; 74: 434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramsay JP, Firth N.. Diverse mobilization strategies facilitate transfer of non-conjugative mobile genetic elements. Curr Opin Microbiol 2017; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- 38. Garcillán-Barcia MP, Francia MV, de la Cruz F.. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 2009; 33: 657–87. [DOI] [PubMed] [Google Scholar]
- 39. Garcillán-Barcia MP, Cuartas-Lanza R, Cuevas A. et al. Cis-acting relaxases guarantee independent mobilization of MOBQ4 plasmids. Front Microbiol 2019; 10: 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Traore DAK, Wisniewski JA, Flanigan SF. et al. Crystal structure of TcpK in complex with oriT DNA of the antibiotic resistance plasmid pCW3. Nat Commun 2018; 9: 3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kehrenberg C, Catry B, Haesebrouck F. et al. Novel spectinomycin/streptomycin resistance gene, aadA14, from Pasteurella multocida. Antimicrob Agents Chemother 2005; 49: 3046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carattoli A, Villa L, Feudi C. et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 2017; 22: pii=30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. San Millan A, Escudero JA, Gutierrez B. et al. Multiresistance in Pasteurella multocida is mediated by coexistence of small plasmids. Antimicrob Agents Chemother 2009; 53: 3399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poirel L, Carattoli A, Bernabeu S. et al. A novel IncQ plasmid type harbouring a class 3 integron from Escherichia coli. J Antimicrob Chemother 2010; 65: 1594–8. [DOI] [PubMed] [Google Scholar]
- 45. Muniesa M, Blanco JE, De Simón M. et al. Diversity of stx2 converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 2004; 150: 2959–71. [DOI] [PubMed] [Google Scholar]
- 46. Brown-Jaque M, Calero-Cáceres W, Espinal P. et al. Antibiotic resistance genes in phage particles isolated from human faeces and induced from clinical bacterial isolates. Int J Antimicrob Agents 2018; 51: 434–42. [DOI] [PubMed] [Google Scholar]
- 47. Fillol-Salom A, Alsaadi A, Sousa J. d. et al. Bacteriophages benefit from generalized transduction. PLoS Pathog 2019; 15: e1007888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernández-Orth D, Miró E, Brown-Jaque M. et al. Faecal phageome of healthy individuals: presence of antibiotic resistance genes and variations caused by ciprofloxacin treatment. J Antimicrob Chemother 2019; 74: 854–64. [DOI] [PubMed] [Google Scholar]
- 49. Venturini C, Zingali T, Wyrsch ER. et al. Diversity of P1 phage-like elements in multidrug resistant Escherichia coli. Sci Rep 2019; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen J, Quiles-Puchalt N, Chiang YN. et al. Genome hypermobility by lateral transduction. Science 2018; 362: 207–12. [DOI] [PubMed] [Google Scholar]
- 51. Rao VB, Feiss M.. Mechanisms of DNA packaging by large double-stranded DNA viruses. Annu Rev Virol 2015; 2: 351–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Imamovic L, Muniesa M.. Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PLoS One 2012; 7: e32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yehl K, Lemire S, Yang AC. et al. Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 2019; 179: 459–69.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.