Abstract
Age-related dementia entails impaired blood flow to and throughout the brain due, in part, to reduced endothelial nitric oxide signaling. However, it is unknown whether sex affects cerebrovascular Gq-protein-coupled receptors (GPCRs) and K+ channels underlying endothelium-derived hyperpolarization (EDH) during progressive aging. Thus, we simultaneously evaluated intracellular Ca2+ ([Ca2+]i) and membrane potential (Vm) of intact endothelial tubes freshly isolated from posterior cerebral arteries of young (4–6 mo), middle-aged (12–16 mo), and old (24–28 mo) male and female C57BL/6 mice. Purinergic receptor function (vs. muscarinic) was dominant and enhanced for [Ca2+]i increases in old females versus old males. However, Ca2+-sensitive K+ channel function as defined by NS309-evoked Vm hyperpolarization was mildly impaired in females versus males during old age. This sex-based contrast in declined function of GPCRs and K+ channels to produce EDH may support a greater ability for physiological endothelial GPCR function to maintain optimal cerebral blood flow in females versus males during old age. As reflective of the pattern of cerebral blood flow decline in human subjects, inward-rectifying K+ (KIR) channel function decreased with progressive age regardless of sex. Combined age-related analyses masked male versus female aging and, contrary to expectation, hydrogen peroxide played a minimal role. Altogether, we conclude a sex-based divergence in cerebrovascular endothelial GPCR and K+ channel function while highlighting a previously unidentified form of age-related endothelial dysfunction as reduced KIR channel function.
Keywords: Cerebrovasculature, Endothelial cell, Sex differences, Biology of aging
Approximately 14% of the population in the United States is currently age 65 and older, which is projected to rise to ~21% by 2040 (1). As people age, they become more susceptible to a range of chronic diseases that involve the cardiovascular and nervous systems (eg atherosclerosis, hypertension, coronary artery disease, and Alzheimer’s disease) (2,3). Thus, effective delivery of oxygen and nutrients to and throughout the brain via cerebral blood vessels is crucial for cognitive health during the mammalian lifespan. The endothelium lining the innermost layer of cerebral blood vessels balances coordination of vasodilation and vasoconstriction for optimal blood flow distribution in the brain (4,5). A representative pathway of blood flow regulation is endothelium-derived hyperpolarization (EDH) (6), primarily described by increases in intracellular Ca2+ ([Ca2+]i) upon stimulation of Gq-protein coupled receptors (GPCRs; eg purinergic, P2Y) and/or Ca2+ influx through transient receptor potential (TRP) channels followed by activation of small- and intermediate-Ca2+-activated K+ (SKCa/IKCa) channels and hyperpolarization of membrane potential (Vm) (7,8). Furthermore, independent of rises in [Ca2+]i, cerebrovascular endothelial inward-rectifying K+ (KIR) channels also contribute to EDH (8,9). Although not examined in this study, EDH transmits through myoendothelial gap junctions to smooth muscle cells and ultimately causes relaxation of the blood vessel to ensure sufficient blood flow to active organs and tissues (10). Altogether, EDH ultimately dilates arteries and arterioles to thereby decrease cerebrovascular resistance and promote blood flow throughout the brain (4,7).
In the resistance vasculature, endothelial “dysfunction” has been primarily defined by reduced nitric oxide (NO) signaling that underlies impaired endothelium-dependent vasodilation (2,11). As a parallel vasodilatory pathway, EDH can play a compensatory role for reduced NO bioavailability as shown during conditions of chronic illness that become more prominent with aging such as diabetes [coronary (12) and subcutaneous (13) arteries]. Although recognized to govern cerebral blood flow via regulation of pial arterial (7) and parenchymal arteriolar (14) tone alike, the status of EDH throughout aging adulthood in accord with sex is unknown at the level of endothelial cell function. In contrast to NO, ion channels central to EDH (ie SKCa/IKCa channels) can be “fine-tuned” using selective pharmacology (6,15), and thus, a better understanding of EDH during normal aging is crucial towards advancing therapeutic treatment of the development of cardio- and cerebrovascular diseases (5). Furthermore, hydrogen peroxide (H2O2) is a relatively stable intermediate of the ROS pathway associated with the aging process and development of chronic disease (16) while an endothelial “factor” that can produce EDH (6,17).
The goal of this study was to examine whether and how the function of cerebrovascular endothelial GPCRs and K+ channels alter with progressive aging. Based on previous studies, we also addressed the potential role for oxidative modulation of EDH during aging per actions of H2O2 (18–20). Finally, with a recognized dimorphic effect of sex on endothelial genetics, structure, and systemically integrated function during aging (21,22), we investigated male versus female animals as a function of age. We hypothesized that the function of GPCRs and K+ channels governing endothelium-derived hyperpolarization is altered with progressive age in cerebral arterial endothelium in a sex-specific manner. We examined intact endothelium freshly isolated from posterior cerebral arteries of young (4–6 mo), middle-aged (12–16 mo), and old (24–28 mo) male and female mice (C57BL/6 background strain, continuous laminar flow, 37°C, pH 7.4). This age range of mice encompasses all of adulthood, corresponding to humans living from their early 20s to approximately 80 years old (23). P2Y receptor function was enhanced in females and decreased in males during old age while the sensitivity of SKCa/IKCa channel activation mildly decreased in females versus males. Also, KIR function decreased during progressive aging regardless of sex. In accord with characteristics of EDH in response to exogenous H2O2 or presence of the human mitochondrial catalase transgene (mCAT), functional differences among age and sex did not appear dependent on oxidative signaling per actions of H2O2. Altogether, these observations support several key findings detailing a divergent role for males versus females for cerebrovascular aging while highlighting a previously unknown form of age-related endothelial dysfunction as reduced KIR channel function.
Methods
Animal Care and Use
All animal care use and experimental protocols for this study were approved by the Institutional Animal Care and Use Committee of Loma Linda University and performed in accord with the National Research Council’s “Guide for the Care and Use of Laboratory Animals” (8th Edition, 2011). Experiments were performed using C57BL/6N mice obtained as young (4–6 mo; 34 animals, 17 males and 17 females), middle-aged (12–16 mo; 20 animals, 9 males and 11 females), and old (24–28 mo; 40 animals, 18 males and 22 females) animals from the National Institute on Aging colonies at Charles River Laboratories (Wilmington, MA). In addition, the study utilized old C57BL/6.mCAT mice (24) (24–28 mo; 7 animals, 6 males and 1 female) to examine a potential role of H2O2. Prior to use, all animals were housed on a 12:12 hour light–dark cycle at 22–24°C with fresh water and food available ad libitum. For individual experiments, animals were selected randomly with respect to sex and age.
Solutions
Preparation and composition of all buffers have been described previously (8,25). Consult (25) for a complete list of reagents and chemicals (with vendors and catalog numbers) used for simultaneous measurement of [Ca2+]i and Vm in mouse cerebral endothelium. Briefly, physiological salt solution (control PSS) was used for superfusion of cerebral endothelial tubes [(in mmol/L): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 Glucose]. During tissue dissection to obtain posterior cerebral arteries, PSS contained 0.1% bovine serum albumin while CaCl2 was absent. To isolate arterial endothelial tubes using an enzymatic digestion solution (0.31 mg/mL papain, 0.50 mg/mL dithioerythritol, 0.75 mg/mL collagenase, and 0.13 mg/mL elastase), dissociation buffer contained PSS with reduced Ca2+ (0.1 mmol/L CaCl2) and 0.1% bovine serum albumin. For applications utilizing 15 mmol/L KCl, an equimolar decrease of NaCl was applied accordingly in order to maintain osmolarity.
Dissection of Cerebral Arteries and Isolation of Endothelial Tubes
Preparation of mouse posterior cerebral arteries for isolating endothelial tubes has been previously described and illustrated (8,25). The posterior cerebral artery was used because of the structural integrity of its endothelial tube (width: ~90 µm; length: ≥300 µm) following removal of smooth muscle cells, enabling examination of intra- and intercellular signaling along and among cerebrovascular endothelial cells. In addition, for effective comparison with the literature (26–28), studies of the rodent posterior cerebral artery altogether represent recent studies of cerebrovascular aging and pathology per pial artery endothelial function.
Briefly, each animal was anesthetized via inhalation of isoflurane followed by decapitation. The brain was removed and posterior cerebral arteries arising from the Circle of Willis were carefully dissected from posterior communicating and basilar arteries in chilled (4°C) dissection buffer. Arterial segments were partially digested in dissociation buffer with an enzyme cocktail (described in Solutions) at 34°C for 10 to 12 minutes. Following digestion, adventitia, smooth muscle cells, and internal elastic lamina were removed from each arterial segment. The remaining endothelial tube was then secured in a chamber for continuous superfusion (~7 mL/min, 37°C).
Simultaneous Measurements of Intracellular Ca2+ and Vm
Our protocol for simultaneous measurement of [Ca2+]i and Vm in mouse cerebral endothelium has been previously described and illustrated (25). Briefly, the endothelial tube was loaded with Fura-2 AM (Invitrogen, Carlsbad, CA; 10 µmol/L) in PSS for 40 minutes at room temperature and subsequently washed with PSS for 40 minutes to allow intracellular Fura-2 AM to de-esterify. Experimental temperature was raised and maintained at 37°C using a temperature controller (TC-344C; Warner Instruments, Camden, CT). Ca2+ photometry was performed using an IonOptix system (Milford, MA), whereby an imaging window was focused on ~70 endothelial cells to measure [Ca2+]i. Prior to incubation with Fura-2 AM, autofluorescence was obtained (510 nm during excitation at 340/380 nm) and subtracted from experimental recordings. In order to approximate [Ca2+]i per Fura-2 ratio recordings, minimum and maximum F340/F380 values (Rmin, 0.67 ± 0.02 and Rmax, 3.25 ± 0.12, n = 4) were determined in endothelial tubes as described previously (29). Accordingly, β was calculated as Fmin/Fmax at 380 nm (3.71 ± 0.13). Measured F340/F380 (R) values were converted to [Ca2+]i (in nM) using the equation [Ca2+]i = Kd•β•(R − Rmin)/(Rmax − R) (30) assuming an intracellular Fura-2 Kd of 282 nM (31).
In parallel with [Ca2+]i measurements, Vm of isolated endothelial tubes was recorded using Axoclamp electrometers (2B and/or 900A; Molecular Devices, Sunnyvale, CA) (8,25). An endothelial cell was penetrated with a microelectrode (tip resistance: ~150 MΩ) while viewing the endothelial tube at 400× magnification. Amplifier outputs were connected to a data acquisition system (Digidata 1550A; Molecular Devices), whereby data for simultaneous recordings of [Ca2+]i and Vm were acquired at 10 Hz while temporally synchronized across respective photometry (IonWizard 6.6) and electrometer (Axoscope 10.5; Molecular Devices) software suites.
Pharmacology
To assess maximal cerebrovascular endothelial GPCR function, adenosine 5′‐triphosphate disodium salt hydrate (ATP, 100 μmol/L; Sigma) for purinergic (P2Y) receptors (8,27) and acetylcholine chloride (ACh, 10 μmol/L; Sigma) for muscarinic receptors (8,32) were applied, respectively. Capacity of SKCa/IKCa channels for activation of EDH was measured using NS309 (0.01‐10 μmol/L; Tocris) (8). In turn, the ability for SKCa/IKCa-channel-dependent hyperpolarization to increase [Ca2+]i was simultaneously determined during NS309 treatment (29,33). Elevated extracellular KCl ([K+]o: 15 mmol/L) was used to stimulate endothelial KIR channels (8,34).
Finally, to examine the potential role of oxidative signaling, 200 μmol/L of H2O2 (Sigma) was used (18–20). As a pharmacological note, H2O2 is not a typical ligand for a receptor or ion channel and accordingly, its cellular actions reflect a combination of time and concentration. In such manner, a higher concentration of H2O2 applied corresponds to a quicker cellular response in the form of increasing intracellular Ca2+ towards maximum. Throughout our previous studies (18,20), we have found that an H2O2 concentration of 200 μmol/L was the best compromise for time sensitive (and technically challenging) measurements of [Ca2+]i and/or Vm for ≤40 min. Reduction to lesser concentrations (eg 100 μmol/L) typically entails several hours of continuous recording which was not an option for the current study.
Resting [Ca2+]i and Vm were typically allowed ≥ 2 minutes to stabilize before application of a pharmacological agent, whereby each application was allowed sufficient time (≥ 3 min; except H2O2, ≤ 40 min) to record peak [Ca2+]i and Vm responses. In between individual applications, including the NS309 concentration‐response experiments, the endothelial tube was washed with PSS to baseline conditions.
Catalase Activity Assays
In accord with similar [Ca2+]i responses to H2O2 in young versus old C57BL/6 animals, enzymatic activity decomposing H2O2 into H2O was evaluated using the Catalase Activity Assay Kit (Abcam, Cambridge, UK) in accord with manufacturer’s instructions with modification of H2O2 applied (0.1 mmol/L instead of 1 mmol/L H2O2). In brief, cerebral blood vessels were isolated from the brain using an illustrated protocol (35). Protein was extracted from the blood vessel isolate, whereby the concentration was determined using a Nanodrop OneC (5–7 mg/mL, A260/280~0.45). The fluorimetric assay was then applied in the presence of H2O2.
Data and Statistical Analysis
For Ca2+ photometry, data analyses included fluorescence emission collected at 510 nm and expressed as the ratio during excitation at 340 nm and 380 nm (F340/F380) and change in F340/F380 ratio (ΔF340/F380) = peak response F340/F380 − preceding baseline F340/F380. For electrophysiological recordings, data analyses included resting Vm (mV) and change in Vm (∆Vm) = peak response Vm − preceding baseline Vm. All [Ca2+]i and Vm data represent the number (n) of independent experiments, each representing one endothelial tube from one mouse. All statistical analyses were performed using GraphPad Prism (Version 6.07; GraphPad Software, La Jolla, CA). Effective concentrations (pEC50) for NS309 applications were determined using a sigmoid with variable slope fit (R2 ≥ 0.99).
For simultaneous comparison of young, middle-age, and old data sets per sex, statistical analysis included a one-way Analysis of Variance (Tukey’s post hoc). Unpaired Student’s t-tests were used to analyze comparisons between male and female animal data sets per individual age group. Note that all statistical analyses were applied per response to each concentration of a pharmacological agent or time point during H2O2 treatment. In addition, per the hypothesis and overall experimental design of the study, the data sets were not matched, paired, and/or analyzed using repeated-measures analysis within and among study groups. Differences between groups were accepted as statistically significant with p < .05. All summary data are presented as the mean ± SEM.
Results
The mechanical stability of isolated and secured endothelium allowed for simultaneous measurement of [Ca2+]i and Vm at 37°C for at least 2 hours. Endothelial tubes freshly isolated from mouse (C57BL/6) posterior cerebral arteries were ~90 to 100 μm in width and ≥300 μm in length (8,25). The F340/F380 ratio during resting conditions was similar among groups [young male (0.84 ± 0.04, n = 11) and female (0.86 ± 0.04, n = 11); middle male (0.87 ± 0.03, n = 9) and female (0.91 ± 0.05, n = 11); old male (0.87 ± 0.05, n = 12) and female (0.83 ± 0.02, n = 16); p > .05] as consistent with a resting [Ca2+]i of ~80 to 100 nmol/L. In parallel, slightly increasing resting Vm (in mV) was observed with advancing age [young male (−32 ± 2) and female (−35 ± 1); middle male (−36 ± 2) and female (−37 ± 2); old male (−38 ± 2) and female (−39 ± 2)], whereby significance (p < .05) is noted in old (−39 ± 1) versus young (−34 ± 1) with overall aging (sexes combined). Per our study goal, we simultaneously recorded changes in [Ca2+]i and Vm in response to agonists of endothelial GPCRs and activators of SKCa/IKCa and KIR channels during aging for males and females. In addition, we examined the role of oxidative signaling using H2O2 and old mCAT animals.
Effect of Aging and Sex on Cerebrovascular Endothelial GPCR Function
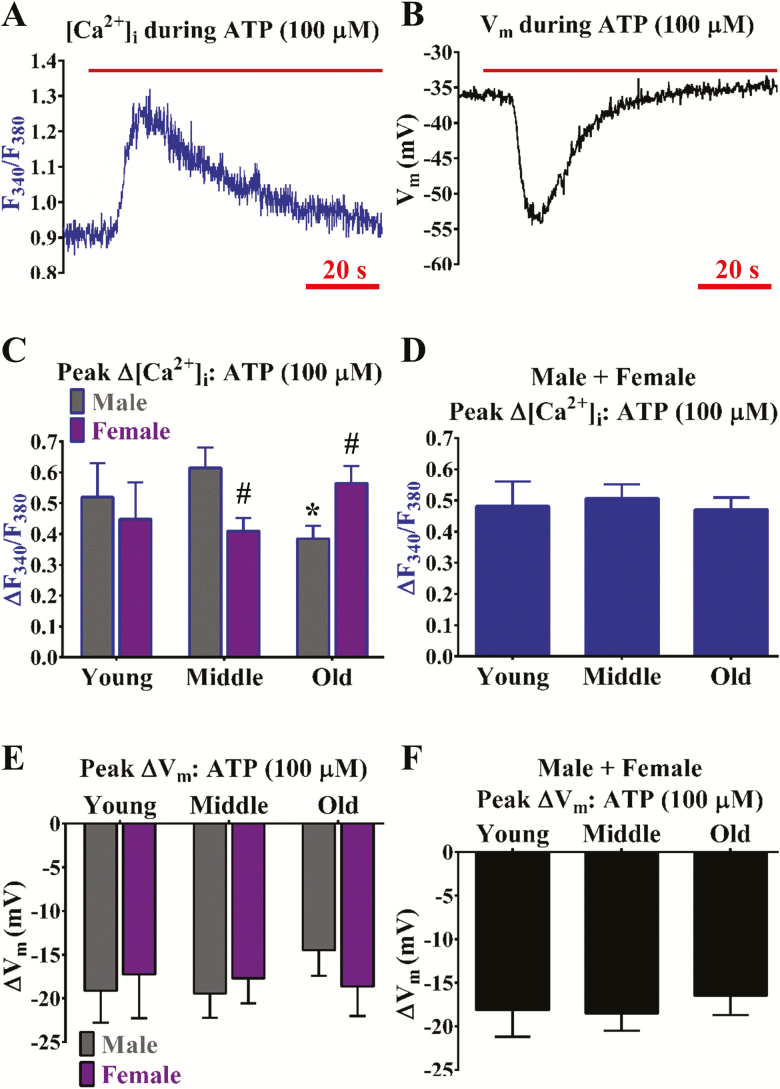
How the span of adult aging impacts cerebrovascular endothelial GPCR function remains unclear. As consistent with a previous study (8), activation of P2Y receptors with ATP (∆F340/F380 ~0.5, ∆[Ca2+]i ~300 nmol/L and ∆Vm ~−15 to −20 mV) is dominant versus muscarinic receptor function during ACh (∆F340/F380 < 0.05 and ∆Vm ≤ −5 mV) in cerebrovascular endothelium (compare Figure 1 with Figure 2). The ∆F340/F380 responses to ATP decreased significantly (p < .05) in old males but not old females (Figure 1C). Accordingly, ∆Vm responses (in mV) to ATP decreased by ~20% in males (middle: −19 ± 3; old: −15 ± 3) but not females (middle: −18 ± 3; old: −19 ± 3) (Figure 1E). Note that old age does not have an apparent effect on P2Y receptor function when data from sexes are combined (Figure 1D and F). In tandem with modest ∆F340/F380 recordings (Figure 2A and C–D), ∆Vm responses to ACh decrease with statistical significance (p < .05) during middle and old age irrespective of sex (Figure 2B and E–F).
Figure 1.
Purinergic receptor function for increasing [Ca2+]i is enhanced in females and declined in males during old age. Representative simultaneous recordings of (A) Fura-2 for [Ca2+]i and (B) a sharp electrode for Vm before and during ATP (100 µmol/L; purinergic receptor agonist and indirect SKCa/IKCa channel activator) in an isolated endothelial tube of a young male. (C) Summary data for ΔF340/F380 during ATP as a function of age (Young, Middle, Old) and sex (Male, Female). (D) As shown in (C) as a function of age only (sexes combined). (E, F) As shown in (C) and (D), respectively, for ∆Vm. n = number of cerebral endothelial tubes (Young, Middle, and Old: 7, 9, and 12 Males and 8, 10, and 11 Females; combined for age only: 15, 19, & 23). *p < .05, Old versus Middle; #p < .05, Female versus Male for same age group. Note that ∆[Ca2+]i and ∆Vm responses to ATP decreased upon old age for males but not females. With data from respective sexes combined, such differences with age are no longer apparent.
Figure 2.
Muscarinic receptor function plays a minor role in EDH and declines with advancing age. Representative simultaneous recordings of (A) Fura-2 for [Ca2+]i and (B) a sharp electrode for Vm before and during ACh (10 µmol/L; muscarinic receptor agonist and indirect SKCa/IKCa channel activator) in an isolated endothelial tube of a young male. (C) Summary data for ΔF340/F380 during ACh as a function of age (Young, Middle, Old) and sex (Male, Female). (D) As shown in (C) as a function of age only (sexes combined). (E, F) As shown in (C) and (D), respectively, for ∆Vm. n = number of cerebral endothelial tubes (Young, Middle, and Old: 4, 9, and 10 Males and 7, 9, and 8 Females; combined for age only: 11, 18, and 18). *p < .05, Middle and/or Old versus Young. Note that ∆[Ca2+]i and ∆Vm responses to ACh are modest (ΔF340/F380 ≤ 0.05, ∆Vm ~5 mV), whereby ∆Vm declined during middle and old age vs. young regardless of sex.
Effect of Aging and Sex on Cerebrovascular Endothelial K+ Channel Function
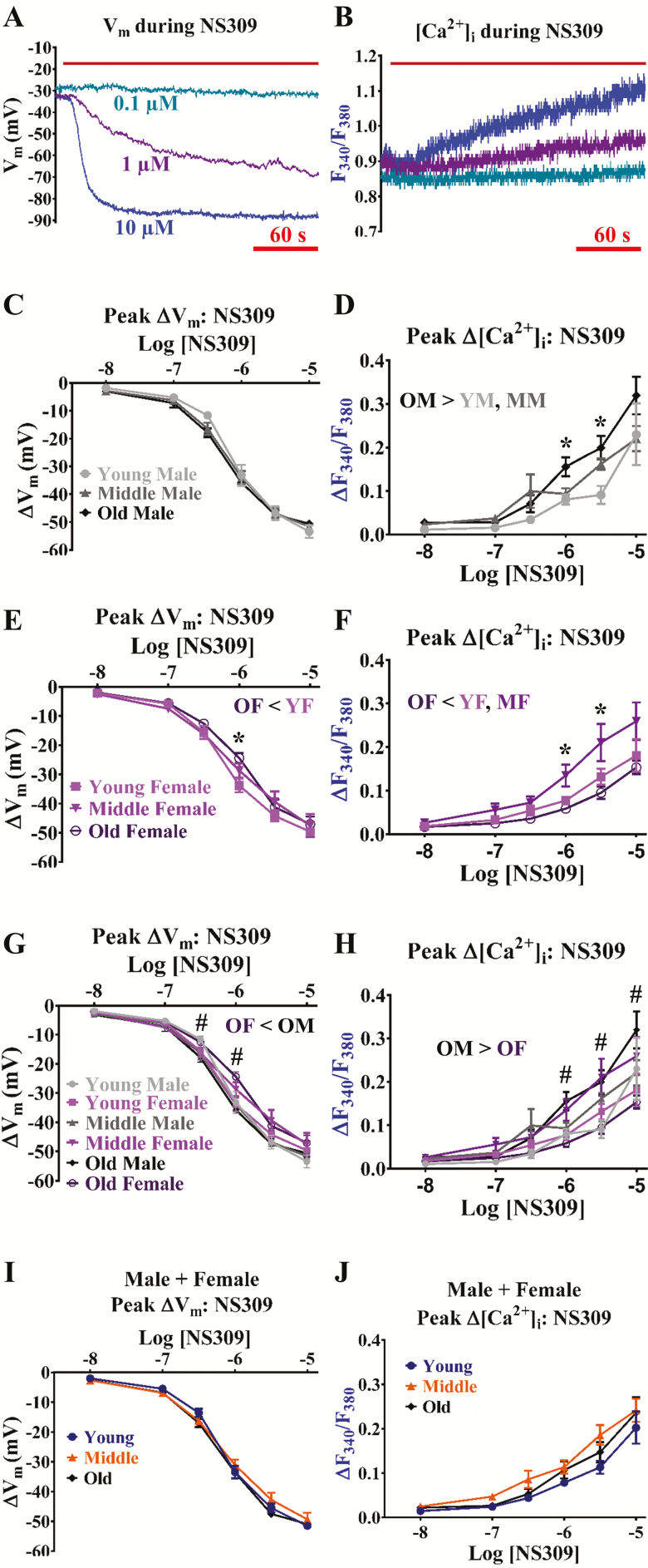
Although previous studies support maintenance of endothelial SKCa/IKCa channel function throughout aging in peripheral skeletal muscle (18,36) and cerebral (37) arteries, combined effects of aging and sex on SKCa/IKCa channel function remain unclear. Activation of SKCa/IKCa channels can finely tune Vm from resting Vm to the equilibrium potential for K+ (EK; ~−90 mV) in cerebrovascular endothelium (8) (Figure 3A). Whereas maximum ∆Vm (~−50 mV) to NS309 was similar throughout groups (Figure 3C, E, and G), responses to effective concentrations of NS309 (0.3 to 1 µmol/L) were less in old females per both aging (< young females; Figure 3E) and sex (< old males; Figure 3G). In addition, the pEC50 corresponding to old females (5.96 ± 0.10) was less than that of young females (6.15 ± 0.08) and old males (6.24 ± 0.06; p < .05; Figure 3G). Note that aging does not have an apparent effect on ∆Vm responses to NS309 when data from sexes are combined (Figure 3I).
Figure 3.
Aging impairs SKCa/IKCa channel function for Vm hyperpolarization in females but not males. Representative simultaneous recordings of (A) a sharp electrode for Vm and (B) Fura-2 for [Ca2+]i before and during NS309 (0.1–10 µmol/L; direct SKCa/IKCa channel activator) in an isolated endothelial tube of a young male. (C) Summary data for ∆Vm during NS309 as a function of age (Young, Middle, Old) according to male sex. (D) Summary data for ΔF340/F380 as a function of age (Young, Middle, Old) according to male sex. (E, F) As shown in (C) and (D), respectively, for female sex. (G) Summary data for ∆Vm as a function of age (Young, Middle, Old) and sex (Male, Female) as (C) and (E) together. (H) As shown in (G) for ΔF340/F380 as (D) and (F) together. (I) Summary data for ∆Vm during NS309 as a function of age only (sexes combined). (J) As shown in I for ΔF340/F380 as a function of age only (sexes combined). N = number of cerebral endothelial tubes (Young, Middle, and Old: 5, 5, and 5 Males and 5, 6, and 5 Females; combined for age only: 10, 11, and 10). *p < .05, Old versus Young and/or Middle for same sex; #p < .05, Female versus Male for Old. YM: Young male; MM; Middle male; OM: Old male; YF: Young female; MF: Middle female; OF: Old female. Note that ∆[Ca2+]i follows ∆Vm responses to NS309. Hyperpolarization responses were decreased to effective concentrations of NS309 (0.3 to 1 µmol/L) in old females as a function of age (vs young) and sex (vs old male). See Results for respective pEC50 values indicating OF < YF or OM. Subsequent ∆[Ca2+]i responses during old age increased for males and decreased for females. With data from respective sexes combined, such differences with age are no longer apparent.
As Vm hyperpolarization may induce Ca2+ entry into endothelial cells via an enhanced electrical driving force (29), we also simultaneously determined whether NS309 indirectly influenced [Ca2+]i signaling following SKCa/IKCa activation. Effective to maximal concentrations of NS309 (1 to 10 µmol/L; ∆Vm: ~−30 to −50 mV) increased F340/F380 (∆F340/F380 ~0.1 to 0.3, ∆[Ca2+]i ~50 to 200 nmol/L; Figure 3B). Remarkably, ∆F340/F380 responses to NS309 were greater (p < .05) in old males vs. younger males (Figure 3D) but less (p < .05) in old females versus the younger female groups (Figure 3F). In such manner, ∆F340/F380 responses to NS309 were greater (p < .05) in old males versus old females (Figure 3H). Note that aging does not have a significant effect on ∆F340/F380 responses to NS309 when data from sexes are combined (Figure 3J).
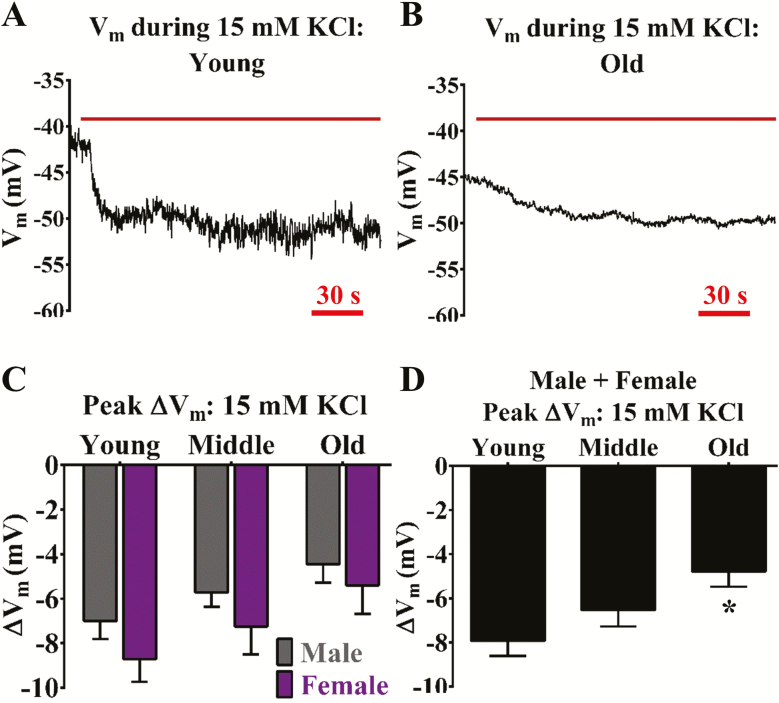
KIR channels may play a crucial role in governing cerebral vasodilation and blood flow (38) but the potential impact of aging on cerebrovascular endothelial KIR channel function remains unknown. Stimulation of cerebrovascular endothelial KIR channels with 15 mM KCl elicits a modest Vm hyperpolarization response [∆Vm ~−5 to −10 mV (8); Figure 4A and B). The ∆Vm responses to 15 mM KCl significantly decreased (p < .05) during old age regardless of sex (Figure 4C and D). Corresponding changes in ∆F340/F380 (≤ 0.03) to 15 mM KCl were inconsistent or altogether absent for detection.
Figure 4.
Aging impairs KIR channel function for Vm hyperpolarization regardless of sex. Representative recordings of Vm before and during 15 mmol/L KCl (KIR channel activator) in an isolated endothelial tube of a (A) young and (B) old male, respectively. (C) Summary data for ∆Vm during elevated KCl as a function of age (Young, Middle, Old) and sex (Male, Female). (D) As shown in (C) as a function of age only (sexes combined). N = number of cerebral endothelial tubes (Young, Middle, and Old: 6, 7, and 9 Males and 7, 8, and 5 Females; combined for age only: 13, 15, and 14). *p < .05, Old versus Young. YM: Young male; MM: Middle male; OM: Old male; YF: Young female; MF: Middle female; OF: Old female. Note lower ∆Vm responses (≥1 mV) in males versus females, whereby ∆Vm decreased progressively with advancing age.
Effects of H2O2 Oxidative Signaling on Cerebrovascular EDH in Male and Female Aging
Enhanced oxidative signaling may occur with progressive aging in peripheral (18) and cerebral (37) arteries. As the most stable intermediate of the reactive oxygen species signaling pathway (16), the role for H2O2 in particular has not been well defined for cerebrovascular EDH. Exogenous H2O2 (200 µmol/L) stimulates robust hyperpolarization of Vm (up to EK) over a 20-minute time period in the endothelium of skeletal muscle arteries in a SKCa/IKCa channel-dependent manner (18) which, in turn, may be dependent on TRP channel-induced Ca2+ influx (20). Calcium-dependent apoptosis of vascular cells is apparent during time periods ≥ 50 minutes (19,20). Thus, we examined the capacity for physiological ∆[Ca2+]i responses to 200 µmol/L H2O2 throughout age and sex within 40 minutes. In addition, we examined identical responses in old mCAT mice as a genetic approach for scavenging endogenous H2O2.
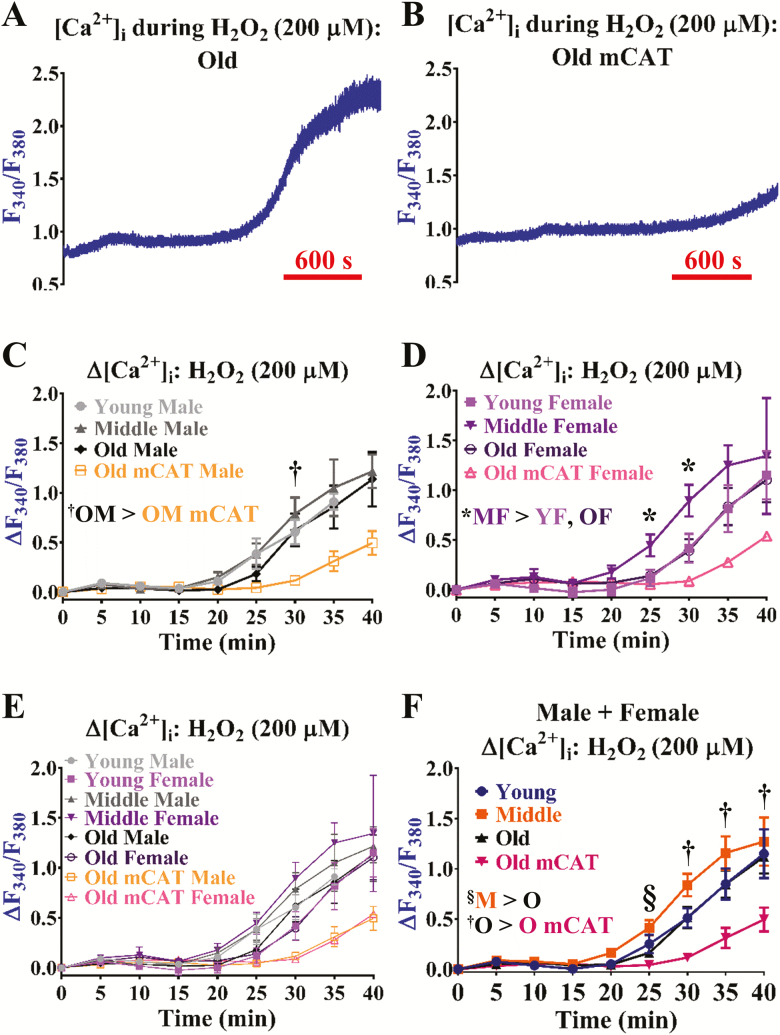
The presence of H2O2 stimulated a large increase in F340/F380 (∆F340/F380 > 0.80), effectively approaching µmol/L concentrations of global [Ca2+]i (Figure 5A). The presence of the mCAT transgene during old age (≥24 mo), decreased this ∆[Ca2+]i response by >60% relative to old C57BL/6 animals (Figure 5B and 5C–F). Accordingly, corresponding ∆Vm responses were virtually absent in old mCAT animals versus aging (eg C57BL/6 groups, −18 ± 3 mV vs. mCAT, 2 ± 2 mV at 30 min). Surprisingly, other than the female middle-age group being the most sensitive in response to H2O2 (Figure 5D), significant differences were not observed for young vs. old C57BL/6 animals (Figure 5E and F). Accordingly, differences were not apparent in young versus old C57BL/6 animals for catalase activity (sexes combined; young, 12.4 ± 2.9 and old, 9.4 ± 1.8 pmol/min/mg, n = 6 experiments from 12 animals per age group, 50% males and 50% females). Per sex, catalase activity was greater in young females versus young males (18.0 ± 2.9 vs. 6.7±0.4 pmol/min/mg; n = 3 experiments from 6 animals per group), whereby catalase activity decreased in old females by ~30% (11.9 ± 2.3) but not old males (7.0 ± 2.3) versus younger groups of like sex.
Figure 5.
Aging does not significantly alter H2O2-mediated oxidative control of Ca2+ signaling underlying EDH. Representative recordings of [Ca2+]i during H2O2 (200 µmol/L; general oxidizing agent, indirect activator of transient receptor potential and K+ channels) in an isolated endothelial tube of an (A) old male C57BL/6 and (B) old male mCAT mouse, respectively. (C) Summary data for ΔF340/F380 as a function of age (Young, Middle, Old) according to male sex. (D) As shown in (C) for females. (E) Summary data for ΔF340/F380 during H2O2 as a function of age (Young, Middle, Old) and sex (Male, Female). (F) As shown in (E) as a function of age only (sexes combined). n = number of cerebral endothelial tubes (Young, Middle, Old, Old mCAT: 6, 7, 8, and 6 Males and 7, 8, 7, and 1 Females; combined for age only: 13, 15, 15, and 7). †p < .05, Old C57BL/6 versus Old mCAT groups. *p < .05, Middle versus Young and/or Old Females; §p < .05, Middle versus Old age; OM mCAT: Old male mCAT. YF: Young female; MF: Middle female; OF: Old female; M: Middle-age mice; O: Old age mice; O mCAT: Old age mCAT mice. Note highest [Ca2+]i responses during middle age with similarities between young and old C57BL/6 in particular. Responses to H2O2 were significantly lower in mCAT versus C57BL/6 animals.
A previous study demonstrated a role for H2O2 in governing endothelial cell resting Vm and activity of K+ channels of skeletal muscle arteries during old age (18). Thus, we also examined whether the mCAT transgene altered resting Vm and hyperpolarization of Vm in response to activation of K+ channels with NS309 (1 µmol/L) and 15 mM KCl in cerebrovascular endothelium of old animals. Resting Vm of old mCAT animals (−29 ± 2 mV) was less than old C57BL/6 animals (−40 ± 2 mV). Hyperpolarization was less in response to the ~EC50 concentration of NS309 (1 µmol/L) in old mCAT animals (∆Vm: −22 ± 3 mV) versus old C57BL/6 (−30 ± 2 mV), whereas ∆Vm responses to 15 mM KCl were similar (−3 ± 1 vs. −5 ± 1 mV, respectively).
Discussion
As young adults (~20–40 yr old), 21% of the cardiac output is received by the brain in female human subjects versus 15% for males (38). This cerebral blood flow-to-cardiac output ratio decreases linearly by ~1.7% in females versus ~0.9% in males per decade of aging throughout middle (mid-40s to early 60s) and old (mid-60s to 80 yr) age and is associated with decreases in cerebral blood flow despite a stable cardiac output (38). Cerebral blood flow regulation is dependent upon the coordination of vasoreactivity of blood vessels throughout the brain (4). In addition to well-characterized barrier function, the endothelium lining the innermost layer of cerebral arteries plays an integral role in cerebral blood flow regulation (5). During aging, “dysfunction” of the endothelial cell layer has been defined in part by a reduction in NO signaling and thereby impaired vasodilation (2,11). As a distinct vascular signaling pathway that is controllable using selective pharmacology (6), the impact of the adult lifespan on cerebrovascular EDH within the context of sex is unknown. Of further significance, EDH can also be used to boost production of NO (6). Thus, in this study, we used male and female C57BL/6 aged throughout the entire healthy adult lifespan to mechanistically dissect indirect (ie via GPCR activated ([Ca2+]i increases) and direct (using K+ ion channel openers) inputs of EDH. Based on previous evidence of the impact of H2O2 oxidative signaling on endothelial function during old age (18–20), we additionally examined old C57BL/6 mice expressing a human mitochondrial catalase transgene (24) as a potentially “positive” control model for vascular aging. As a scientific tool, we employed a developed study model in the form of cerebrovascular endothelial “tubes” (8,25), whereby experimental variables central to EDH function ([Ca2+]i and Vm) were examined simultaneously. In brief, we found that there is a divergence in male versus female aging for sustenance of P2Y receptor and SKCa/IKCa channel function that is minimally dependent on oxidative input per actions of H2O2. When data are combined among sexes for examination of overall aging, disparate effects of male versus female aging are not apparent. With a pattern of slightly higher KIR channel function throughout for females versus males, an overall decrease occurs with age regardless of sex. The scientific basis and clinical relevance of these findings are discussed below.
Purinergic Receptor Activity Is Dominant in Cerebrovascular Endothelium for EDH and Is Enhanced During Female Old Age: Potential Role of Estrogen
Cerebrovascular endothelial P2Y receptors serve as a major GPCR nexus for the neurovascular control of cerebral blood flow (39). The increase in [Ca2+]i that follows P2Y receptor stimulation may favor production of NO, EDH, and/or prostaglandin E2 as vasodilatory signaling mechanisms (40). Our previous work (8) and the current study (Figure 1) illustrate significant ATP activation of EDH (∆Vm: ~−15 to −20 mV) in mouse posterior cerebral arteries. With no significant differences noted for young and middle age, male old age in particular reduces ∆[Ca2+]i by ~40% accompanied by a ~5 mV reduction in the EDH response. Although cerebrovascular P2Y receptor function has been implied to decrease with conditions of aging (39), a precise role for P2Y receptors in regulating EDH during progressive age and sex has been unclear. Estrogen may blunt endothelial P2Y receptor-mediated relaxation of smooth muscle cells in cerebral arteries of ovariectomized females supplemented with exogenous estrogen (41). In our conditions of cerebral endothelium isolated from female mice during normal aging with no prior application of chronic (≥1 mo) surgical or pharmacological interventions, a difference in components of EDH ([Ca2+]i and Vm) does not vary significantly in young female animals versus young males but remains the same or is even enhanced (~30% for ∆[Ca2+]i) during female old age versus female young age. Thus, a potential inverse effect of estrogen on P2Y-receptor generated EDH may explain why female purinergic receptor function is enhanced during old age when long-term estrogen levels may be at their lowest. Regardless, our findings may support a greater ability for physiological endothelial GPCR function to maintain optimal cerebral blood flow in the female sex versus males during old age (42). The similarity in P2Y receptor function throughout aging when data are combined across sexes further highlights the need to examine the role of sex during cerebrovascular aging.
Muscarinic Receptor Activity Plays a Modest Role for Producing EDH in Cerebrovascular Endothelium and Decreases During Middle and Old Age Regardless of Sex: Significance of Peripheral Versus Central Blood Flow Control
As best demonstrated in endothelium of peripheral skeletal muscle arteries (6), activation of muscarinic receptors evokes a robust increase in [Ca2+]i (released from the endoplasmic reticulum) that is then followed by a “plateau” phase described by Ca2+ influx into the cells through TRP channels. Activation of SKCa/IKCa channels, and thereby hyperpolarization of Vm (up to EK with maximal concentrations of ACh, 1 to 10 µmol/L), occurs with the [Ca2+]i response in parallel (29,43). In cerebral endothelium, a reproducible but mild (∆Vm ~−5 mV) and transient (≤ 30 s) activation of EDH occurs in response to 10 µmol/L ACh (8). Regardless of sex, the EDH response declines further to ~−2 to −3 mV during middle and old age (Figure 2). Other than speculation as a potential complement GPCR to P2Y receptor function, the physiological role of muscarinic receptors for controlling cerebral blood flow remains unclear. Indeed, as maximum vasodilation can be achieved with only 10 to 15 mV of EDH (44), perhaps muscarinic receptors govern subtle, necessary changes in blood flow to meet the metabolic demand of the brain parenchyma from moment-to-moment. Also, muscarinic receptors of the brain resistance vasculature may be primarily coupled through production of NO versus EDH (45). The difference in endothelial function of cerebral versus skeletal muscle arteries is not surprising as vascular resistance networks of skeletal muscle are highly dynamic in fluctuations of blood flow (up to 100-fold) requiring rapid engagement and disengagement of endothelial function (6). In contrast, blood flow to and throughout the brain is relatively constant during physical rest and exercise (46).
Demonstration of EDH Via SKCa/IKCa Channel Function During Cerebrovascular Aging: Decreased Sensitivity Upon Female Old Age
A recent study has shown that vasodilation of mouse cerebral arterioles due to NS309 activation of SKCa/IKCa channels remains the same in young (4–5 mo) versus old animal (~22 mo) groups composed of both males and females (45). Our data in the current study are in agreement with this observation for general aging when data from sexes are combined (Figure 3). However, a more focused analysis on the role of biological sex in the current study revealed a decline in EDH responses to effective concentrations of NS309 (0.3 to 1 µmol/L; evoke ~15 to 30 mV of Vm hyperpolarization) and yielded lower pEC50 in old females versus young females and old males. The basis of this sex difference and whether it significantly affects physiological regulation of cerebral blood flow in old female animals is unknown and a subject for future study.
Hyperpolarization of Vm as a result of activated SKCa/IKCa channels may also enhance production of NO (33) via increasing the electrical driving force of Ca2+ entry into endothelial cells through TRP channels (29). Interestingly, this “hyperpolarization-induced Ca2+ entry” mechanism (6,29) was increased and decreased during male and female aging, respectively. Thus, with equal coupling of [Ca2+]i to endothelial NO synthase activation assumed, the ability to restore NO production may be more pharmacologically feasible for males versus females during old age. Although, provided that a high level of Vm hyperpolarization (~30 to 50 mV) is needed to detect significant changes in [Ca2+]i, the physiological relevance or therapeutic utility of this mechanism may be marginal, at least in the absence of submaximal GPCR stimulation (29).
Curious Pattern of a Gradual Decrease in KIR Channel Function With Advancing Age in a Sex Neutral Manner: Central Culprit for Age-Related Decreases in Cerebral Blood Flow?
Cerebrovascular KIR channels have been consistently identified as central regulators of cerebral blood flow (4,47). These channels serve as extracellular K+ sensors to modulate K+ efflux underlying Vm hyperpolarization. In particular, they have been identified for their ability to potentially sustain or “boost” EDH and associated vasodilation (48). Outward K+ current flow through activated SKCa/IKCa channels elevates local extracellular K+ and, in turn, activates KIR channels on either the endothelial or smooth muscle layers of cerebral arteries (36). Prior to the current study, the impact of aging and sex on cerebrovascular endothelial KIR channel function has been unknown. Our findings (Figure 4C) illustrate slightly higher endothelial KIR channel function in females versus males throughout life, whereby a gradual decrease in KIR channel function occurs in both sexes with advancing age. Remarkably, this similar pattern has been observed for the cerebral blood flow-to-cardiac output ratio in aging human subjects in males versus females [(38); see Figure I, Panel b of reference]. Collectively, the data suggested across respective studies support KIR channel function as integral to the control of cerebral blood flow during resting conditions. Either interdependently with the EDH mechanism or not, therapeutic control of KIR channel function in the long term may be most effective for ameliorating age-related decrements in cerebral blood flow regardless of sex. Indeed, hyperpolarization responses to endothelial KIR channel activation are significant (~5 to 10 mV) but not extreme in terms of the range of physiological vasoreactivity needed to govern cerebral blood flow.
Minimal Role of H2O2 During Cerebrovascular Aging
Per previous findings observed for skeletal muscle artery endothelium (18–20), we expected to locate a central role of H2O2 for explaining potential differences in cerebrovascular endothelial GPCR and K+ channel function in old versus young animals. In contrast to skeletal muscle arteries (19,20), we did not observe significant differences in [Ca2+]i responses to H2O2 or overall catalase activity in old versus young animals (Figure 5). The old mCAT animals (vs. old C57BL/6 animals) nearly abolished such increases in [Ca2+]i as consistent with elevated catalase expression and activity to decompose H2O2. Consistent with skeletal muscle endothelium treated with catalase, old mCAT animals indicated significantly decreased resting Vm and ∆Vm responses to NS309 versus old C57BL/6 animals (18). However, findings from mCAT mice were supraphysiological relative to the differences observed with “normal” cerebrovascular aging in C57BL/6 animals, and thus, of limited utility for illuminating subtle age-related differences. The physiological basis of our observation of a minimal role for H2O2 oxidative signaling in cerebrovascular versus skeletal muscle endothelium is unknown. However, it is possible that a central role of oxidative signaling during the aging process is most pertinent for organs that undergo robust and dynamic changes in blood flow distribution such as skeletal muscle.
Study Model Considerations
The current study utilized a study model (cerebrovascular endothelial “tubes”) whereby key physiological components of endothelial cell function are retained (eg Ca2+ signaling, hyperpolarization of Vm up to EK via SKCa/IKCa channel activation). In addition, other confounding factors such as perivascular nerve input, smooth muscle voltage-gated channel function and contractility, and the circulation of blood have been removed (6,8,25). As such, our simultaneous determination of endothelial [Ca2+]i and Vm measurements has been altogether consistent or complementary for vasoreactivity determinations using intact vessels [(36,45) as examples]. However, there are other factors to consider with cerebrovascular aging including potential alterations in the transmission of myoendothelial coupling (6,10) and vascular structure (26). These are topics of ongoing investigation.
Summary and Conclusions
Maintenance of cerebrovascular health is a major determinant of a functional brain during aging. Endothelium lines the inner most layer of cerebral resistance vessels, which regulates the blood flow throughout the brain via coordination of vasodilation and vasoconstriction to meet the metabolic demand of neurons. Although cerebrovascular endothelial “dysfunction” has been identified with advancing age for impaired blood flow to and throughout the brain, the underlying mechanisms have been unclear especially within the context of biological sex. In this study, we addressed whether GPCR and K+ channel function governing endothelium-derived hyperpolarization is maintained with progressive age in cerebral arterial endothelium in males versus females. Using a mouse model that encompasses all of adulthood corresponding to humans ~20 to 80 years old (23), we found that purinergic receptor activity was dominant while enhanced in females but decreased in males during old age. In contrast, changes in membrane potential in response to direct activation of Ca2+-sensitive K+ channels were mildly impaired with female versus male aging. Thus, there is a sex-based contrast in declined function of GPCRs (indirect, physiological) and K+ channels (direct, pharmacological) to produce EDH, which may support a greater ability for physiological endothelial GPCR function to maintain optimal cerebral blood flow in females versus males during old age. There was a pattern of slightly higher KIR channel function in females versus males throughout adult life, whereby a similar rate of decline occurred in both sexes with progressive age. A similar pattern has been observed in human subjects for decreases in cerebral blood flow-to-cardiac output ratio observed in human subjects (38), and thus, KIR channels may be a major indicator of age-related endothelial “dysfunction” in the brain. Contrary to expectation, the presence of H2O2 could not account for key differences among groups. Altogether, we conclude that there is a clear divergence between sexes for regulating endothelial function in the central circulation during aging that cannot be accounted for by oxidative signaling per the actions of H2O2 alone.
Funding
This research has been supported by National Institutes of Health grants R00AG047198 & R56AG062169 (to E.J.B.). The content of this original article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
M.A.H. and E.J.B. designed the study, analyzed and interpreted data, prepared figures, drafted, and revised the manuscript. P.P.C. and M.A.H. performed biochemical and functional experiments, respectively. M.A.H., P.P.C., J.N.B., and E.J.B. edited the manuscript and approved the final version.
Conflict of Interest
None reported.
References
- 1. Payne CF. Aging in the Americas: disability-free life expectancy among adults aged 65 and older in the United States, Costa Rica, Mexico, and Puerto Rico. J Gerontol B Psychol Sci Soc Sci. 2018;73:337–348. doi:10.1093/geronb/gbv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133:159–176. doi:10.1016/j.pharmthera.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Abete P, Della-Morte D, Gargiulo G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res Rev. 2014;18:41–52. doi:10.1016/j.arr.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 4. Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab. 2016;36:492–512. doi:10.1177/0271678X15616138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behringer EJ, Hakim MA. Functional interaction among KCa and TRP channels for cardiovascular physiology: modern perspectives on aging and chronic disease. Int J Mol Sci. 2019;20(6):E1380. doi:10.3390/ijms20061380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behringer EJ. Calcium and electrical signaling in arterial endothelial tubes: new insights into cellular physiology and cardiovascular function. Microcirculation. 2017;24(3). doi:10.1111/micc.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi:10.1152/ajpheart.00376.2003 [DOI] [PubMed] [Google Scholar]
- 8. Hakim MA, Buchholz JN, Behringer EJ. Electrical dynamics of isolated cerebral and skeletal muscle endothelial tubes: differential roles of G-protein-coupled receptors and K+ channels. Pharmacol Res Perspect. 2018;6:e00391. doi:10.1002/prp2.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22:183–196. doi:10.1111/micc.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behringer EJ, Segal SS. Spreading the signal for vasodilatation: implications for skeletal muscle blood flow control and the effects of ageing. J Physiol. 2012;590:6277–6284. doi:10.1113/jphysiol.2012.239673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Félétou M. Endothelium-dependent hyperpolarization and endothelial dysfunction. J Cardiovasc Pharmacol. 2016;67:373–387. doi:10.1097/FJC.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 12. Yada T, Shimokawa H, Tachibana H. Endothelium-dependent hyperpolarization-mediated vasodilatation compensates nitric oxide-mediated endothelial dysfunction during ischemia in diabetes-induced canine coronary collateral microcirculation in vivo. Microcirculation. 2018;25:e12456. doi:10.1111/micc.12456 [DOI] [PubMed] [Google Scholar]
- 13. Mokhtar SS, Vanhoutte PM, Leung SW, et al. Endothelium dependent hyperpolarization-type relaxation compensates for attenuated nitric oxide-mediated responses in subcutaneous arteries of diabetic patients. Nitric Oxide. 2016;53:35–44. doi:10.1016/j.niox.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 14. Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186. doi:10.1038/jcbfm.2010.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels. Circ Res. 2012;110:1311–1321. doi:10.1161/CIRCRESAHA.111.262592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi:10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chidgey J, Fraser PA, Aaronson PI. Reactive oxygen species facilitate the EDH response in arterioles by potentiating intracellular endothelial Ca(2+) release. Free Radic Biol Med. 2016;97:274–284. doi:10.1016/j.freeradbiomed.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol. 2013;33:1892–1901. doi:10.1161/ATVBAHA.113.301514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norton CE, Sinkler SY, Jacobsen NL, Segal SS. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J Physiol. 2019;597:3801–3816. doi:10.1113/JP278255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL, Segal SS. Advanced age protects microvascular endothelium from aberrant Ca(2+) influx and cell death induced by hydrogen peroxide. J Physiol. 2015;593:2155–2169. doi:10.1113/JP270169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huxley VH, Kemp SS. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol. 2018;1065:307–328. doi:10.1007/978-3-319-77932-4_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teixeira SC, Madureira JB, Azevedo EI, Castro PM. Ageing affects the balance between central and peripheral mechanisms of cerebrovascular regulation with increasing influence of systolic blood pressure levels. Eur J Appl Physiol. 2019;119:519–529. doi:10.1007/s00421-018-4036-3 [DOI] [PubMed] [Google Scholar]
- 23. Flurkey K, Currer J, Harrison D. Mouse models in aging research. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, eds. The Mouse in Biomedical Research. 2nd ed Burlington: American College of Laboratory Animal Medicine (Elsevier); 2007:637–672. [Google Scholar]
- 24. Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi:10.1126/science.1106653 [DOI] [PubMed] [Google Scholar]
- 25. Hakim MA, Behringer EJ. Simultaneous measurements of intracellular calcium and membrane potential in freshly isolated and intact mouse cerebral endothelium. J Vis Exp. 2019;( 143). doi:10.3791/58832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol. 2016;310:H365–H375. doi:10.1152/ajpheart.00562.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kochukov MY, Balasubramanian A, Abramowitz J, Birnbaumer L, Marrelli SP. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J Am Heart Assoc. 2014;3:e000913. doi:10.1161/JAHA.114.000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br J Pharmacol. 2013;170:661–670. doi:10.1111/bph.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Behringer EJ, Segal SS. Membrane potential governs calcium influx into microvascular endothelium: integral role for muscarinic receptor activation. J Physiol. 2015;593:4531–4548. doi:10.1113/JP271102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 31. Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 (Pt 1):199–209. doi:10.1111/j.1469-7793.1998.199br.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamada M, Lamping KG, Duttaroy A, et al. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:14096–14101. doi:10.1073/pnas.251542998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerr PM, Wei R, Tam R, et al. Activation of endothelial IKCa channels underlies NO-dependent myoendothelial feedback. Vascul Pharmacol. 2015;74:130–138. doi:10.1016/j.vph.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 34. Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM Jr. Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke. 1998;29:1469–1474. doi:10.1161/01.str.29.7.1469 [DOI] [PubMed] [Google Scholar]
- 35. Boulay AC, Saubamea B, Decleves X, Cohen-Salmon M. Purification of mouse brain vessels. J Vis Exp. 2015;( 105):e53208. doi:10.3791/53208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sancho M, Samson NC, Hald BO, et al. KIR channels tune electrical communication in cerebral arteries. J Cereb Blood Flow Metab. 2017;37:2171–2184. doi:10.1177/0271678X16662041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi:10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xing CY, Tarumi T, Liu J, et al. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab. 2017;37:2848–2856. doi:10.1177/0271678X16676826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toth P, Tarantini S, Davila A, et al. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi:10.1152/ajpheart.00463.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guerra G, Lucariello A, Perna A, Botta L, De Luca A, Moccia F. The role of endothelial Ca2+ signaling in neurovascular coupling: a view from the lumen. Int J Mol Sci. 2018;19(4):E938. doi:10.3390/ijms19040938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi:10.1111/j.1440-1681.2007.04683.x [DOI] [PubMed] [Google Scholar]
- 42. Tarumi T, Ayaz Khan M, Liu J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34:971–978. doi:10.1038/jcbfm.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Behringer EJ, Segal SS. Impact of aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for mitochondria. J Gerontol A Biol Sci Med Sci. 2017;72:1627–1637. doi:10.1093/gerona/glx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi:10.1161/01.res.86.1.94 [DOI] [PubMed] [Google Scholar]
- 45. De Silva TM, Modrick ML, Dabertrand F, Faraci FM. Changes in cerebral arteries and parenchymal arterioles with aging: role of rho kinase 2 and impact of genetic background. Hypertension. 2018;71:921–927. doi:10.1161/HYPERTENSIONAHA.118.10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Musch TI, Friedman DB, Pitetti KH, et al. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol (1985). 1987;63(6):2269–2277. doi:10.1152/jappl.1987.63.6.2269 [DOI] [PubMed] [Google Scholar]
- 47. Longden TA, Dabertrand F, Koide M, et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. doi:10.1038/nn.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith PD, Brett SE, Luykenaar KD, et al. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi:10.1113/jphysiol.2007.145474 [DOI] [PMC free article] [PubMed] [Google Scholar]