Abstract
BACKGROUND
Meningioma growth rates are highly variable, even within benign subgroups, with some remaining stable, whereas others grow rapidly.
OBJECTIVE
To identify molecular-genetic markers for more accurate prediction of meningioma recurrence and better-targeted therapy.
METHODS
Microarrays identified microRNA (miRNA) expression in primary and recurrent meningiomas of all World Health Organization (WHO) grades. Those found to be deregulated were further validated by quantitative real-time polymerase chain reaction in a cohort of 172 patients. Statistical analysis of the resulting dataset revealed predictors of meningioma recurrence.
RESULTS
Adjusted and nonadjusted models of time to relapse identified the most significant prognosticators to be miR-15a-5p, miR-146a-5p, and miR-331-3p. The final validation phase proved the crucial significance of miR-146a-5p and miR-331-3p, and clinical factors such as type of resection (total or partial) and WHO grade in some selected models. Following stepwise selection in a multivariate model on an expanded cohort, the most predictive model was identified to be that which included lower miR-331-3p expression (hazard ratio [HR] 1.44; P < .001) and partial tumor resection (HR 3.90; P < .001). Moreover, in the subgroup of total resections, both miRNAs remained prognosticators in univariate models adjusted to the clinical factors.
CONCLUSION
The proposed models might enable more accurate prediction of time to meningioma recurrence and thus determine optimal postoperative management. Moreover, combining this model with current knowledge of molecular processes underpinning recurrence could permit the identification of distinct meningioma subtypes and enable better-targeted therapies.
Keywords: Meningioma, miRNA, Prognosis, Recurrence
ABBREVIATIONS
- AEG-1
astrocyte-elevated gene-1
- AKT
protein kinase B
- CBTRUS
Central Brain Tumor Registry of the United States
- CI
confidence interval
- FFPE
formalin-fixed paraffin-embedded
- GLA
gamma-linolenic acid
- HR
hazard ratio
- HER2
tyrosine-protein kinase erbB-2
- MRR+
meningiomas with evidence of radiographic recurrence up to 8 years after surgery
- MRR−
meningiomas without evidence of radiographic recurrence up to 8 years after surgery
- miRNA
microRNA
- NRP-2
neuropilin-2
- NF-κB
nuclear factor kappa B
- PI3K/AKT
phosphoinositide-3-kinase/AKT
- PTEN
phosphatase and tensin homolog
- qPCR
quantitative polymerase chain reaction
- STAT3
signal transducer and activator of transcription 3
- TTR
time to relapse
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
Among CBTRUS (Central Brain Tumor Registry of the United States) specific histology groupings, meningiomas are the most frequently reported primary intracranial tumors with the annual incidence rate of 8.33 per 100 000 population,1 accounting for about 23.8% and 46.8% of all intracranial neoplasms in males and females, respectively.1,2 Their recurrence cannot be predicted reliably based on histopathology itself, because even benign lesions can include tumors with distinct biological behavior and oncogenic drivers, as demonstrated in both mesenchymal (fibroblastic) and epithelial (meningothelial) lineages of benign meningiomas.3,4 Recurrence within 5 and 10 yr after gross total resection of World Health Organization (WHO) grade I meningiomas occurs in 12% and 19% of all cases, respectively,5,6 whereas 5- and 10-yr recurrence rates of subtotally resected benign lesions range from 37% to 60%, and 55% to 100%, respectively.6 This suggests that there might be key regulators with a significant effect on meningioma biology irrespective of their histopathological degree, the identification of which would allow a more accurate prediction of their behavior. The expression of miRNAs was recently proposed as just such a predictor. These noncoding small RNAs can act as oncogenes or tumor suppressors in various tumors.4,7 A recent study4 identified sets of miRNAs that were also deregulated in benign and high-grade meningiomas. Although a few miRNA signatures have been suggested to predict meningioma recurrence, no mutual miRNA sequences have been reported so far.7-9 The present study was therefore conducted to shed light on these inconsistencies and extend our knowledge of the relationship between miRNA expression and meningioma recurrence.
METHODS
Patients’ Description
The study was approved by the Institutional Research Ethics Committee. Of 302 patients who underwent meningioma surgery between 1990 and 2012, only 172 from whom sufficient tissue for miRNA analysis and comprehensive clinical data were available were selected. All patients signed their informed consent. Imaging/clinical follow-up were performed at 3 and 12 mo after surgery, and approximately every 24 to 72 mo thereafter if no recurrence/regrowth was detected. When meningioma recurrence/regrowth was found, additional follow-ups were scheduled. Recurrence, after total or gross total (Simpson grades I, II, and III) and incomplete (Simpson grade >III) resection, has been defined as the reappearance of any new lesion in which meningioma tissue had previously been removed, or when any remnants of tumor after primary surgery were noticed on the follow-up imaging to have grown. If significant tumor growth was noted at follow-up or meningioma became symptomatic, patients were reoperated on or underwent radiation therapy when indicated. Because of overall follow-up of the whole cohort, the 8-yr limit was used as an adequate cutoff time for recurrence for simple 2-sample analyses. This cutoff time is for descriptive purposes only and has no influence on the main results of the study.
miRNA Expression Analysis
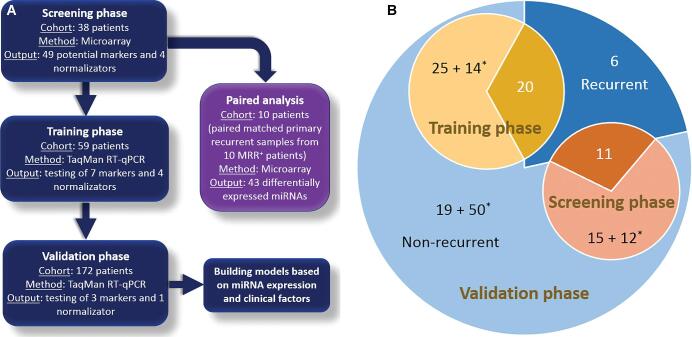
Briefly, in the screening phase, formalin-fixed paraffin-embedded (FFPE) tumor samples were obtained from 38 patients for microarray analysis following RNA purification. Of these, 11 had primary samples of meningiomas with evidence of radiographic recurrence up to 8 yr after surgery (MRR+); 15 had meningiomas without evidence of radiographic recurrence within the same time period (MRR−); and 12 had meningiomas without radiological recurrence within the follow-up of <8 yr. Moreover, paired-matched primary and recurrent samples from 10 MRR+ patients were also analyzed. In the training phase, 59 previously unanalyzed samples were assembled for quantitative polymerase chain reaction (qPCR) analyses (20 MRR+ [primary samples], 25 MRR−, and 14 meningiomas without radiological recurrence within the follow-up of <8 yr). Finally, the validation phase included all 172 patients, including cohorts from the screening and training phases, the total comprising 37 with MRR+ (primary samples), 59 with MRR−, and 76 with meningiomas without radiological recurrence within the follow-up of <8 yr (Table 1 and Figure 1). For detailed information about RNA purification (QIAGEN, Hilden, Germany), miRNA array, and TaqMan assay (Applied Biosystems, Foster City, California), see Text, Supplemental Digital Content 1.
TABLE 1.
The Clinical-Pathological Characteristics of Meningioma Patients Included in the Data Set at All Phases of the Study
| Screening phase | Training phase | Validation phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | n | 38 | 59 | 172 | |||||||||
| MRR− | MRR+ | P value | Totala | MRR− | MRR+ | P value | Totala | MRR− | MRR+ | P value | Totala | ||
| Female/male | n (%) | 13/2 (86.7/13.3) | 7/4 (63.6/36.4) | .348 | 28/10 (73.7/26.3) | 21/4 (84.0/16.0) | 10/10 (50.0/50.0) | .034 | 40/19 (67.8/32.2) | 47/12 (79.7/20.3) | 19/18 (51.4/48.6) | .007 | 115/57 (66.9-33.1) |
| Age (yr) | Median (IQR) | 54 (43-61) | 53 (45-61) | .812 | 54 (43-64) | 51 (43-61) | 48 (41-61) | .912 | 50 (43-60) | 56 (45-62) | 52 (42-64) | .596 | 56 (45-65) |
| WHO grade I/II/III | n (%) | 9/2/3 (64.3/14.3/21.4) | 3/5/3 (27.3/45.5/27.3) | .128 | 14/12/11 (37.8/32.4/29.7) | 24/1/0 (96.0/4.0/0.0) | 16/4/0 (80.0/20.0/0.0) | .155 | 51/8/0 (86.4/13.6/0.0) | 51/3/4 (87.9/5.2/6.9) | 21/11/4 (58.3/30.6/11.1) | .001 | 125/31/14 (73.5/18.2/8.2) |
| Convexity/skull base meningioma | n (%) | 6/9 (40.0/60.0) | 7/4 (63.6/36.4) | .427 | 17/21 (44.7/55.3) | 8/17 (32.0/68.0) | 8/12 (40.0/60.0) | .807 | 23/36 (39.0/61.0) | 20/39 (33.9/66.1) | 18/19 (48.6/51.4) | .221 | 72/100 (41.9/58.1) |
| Simpson grade I-III/>III | n (%) | 12/3 (80.0/20.0) | 8/3 (72.7/27.3) | 1 | 30/7 (81.1/18.9) | 21/2 (91.3/8.7) | 9/11 (45.0/55.0) | .003 | 40/17 (70.2/29.8) | 47/8 (85.5/14.5) | 20/17 (54.1/45.9) | .002 | 127/39 (76.5/23.5) |
| Add-on radiotherapy (yes/no) | n (%) | 4/11 (26.7/73.3) | 6/5 (54.5/45.5) | .228 | 13/25 (34.2/65.8) | 3/22 (12.0/88.0) | 12/8 (60.0-40.0) | .002 | 15/44 (25.4/74.6) | 10/49 (16.9/83.1) | 22/15 (59.5/40.5) | <.001 | 36/136 (20.9/79.1) |
| Follow-up (mo) | Median (IQR) | 149.3 (113.7-198.1) | 99.8 (87.6-123.4) | .005 | 100.9 (66.8-144.7) | 161.2 (124.4-202.2) | 138.3 (102.3-191.8) | .233 | 124.4 (85.5-181.5) | 146.2 (124.5-202.6) | 120.0 (87.3-173.4) | .004 | 95.7 (65.8-145.7) |
| Time to relapseb | % | – | – | – | 64.5 ± 8.8 | – | – | – | 61.3 ± 6.9 | – | – | – | 72.5 ± 4.0 |
IQR, interquartile range (first quartile through third quartile); MRR+, patients with meningiomas with evidence of radiographic recurrence up to 8 yr after surgery; MRR−, patients with meningiomas without evidence of radiographic recurrence up to 8 yr after surgery; WHO, World Health Organization.
aIncluding patients with follow-up <8 yr without recurrence.
bKaplan-Meier estimate of survival at the time of 8 yr after surgery: 8-yr survival ± standard error.
FIGURE 1.
Overview of the present study. A, Design of the study (screening phase, training phase, validation phase, and separately performed paired analyses). B, Description of the patient's cohort in each phase. MRR+, primary samples of meningiomas with evidence of radiographic recurrence up to 8 yr after surgery; *follow-up <8 yr.
Statistical Methods
Statistical analyses were performed with R (R Foundation for Statistical Computing, Vienna, Austria) and Bioconductor. Wilcoxon exact one-sample tests and Cox regression analysis were applied to the microarray data to identify differentially expressed miRNAs and miRNAs with a significant time-to-relapse (TTR) effect. Univariate adjusted and nonadjusted Cox regression models were selected for qPCR data following the building of a full multivariate model and consequent stepwise selection method, which provided factors for the final multivariate model. Each prognostic factor was characterized by HR (hazard ratio) and P value. For more information, see Text, Supplemental Digital Content 1.
RESULTS
Screening Phase
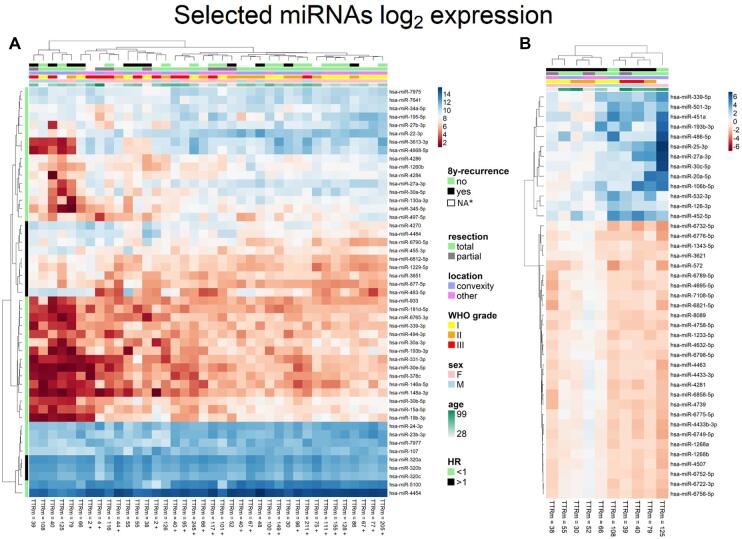
The microarray analysis revealed 49 miRNAs with their expression strongly dependent on TTR in meningiomas at various risks of recurrence (Figure 2A), according to the Cox regression model of TTR. Only mature miRNA with higher abundance (intensity of fluorescence >5) were analyzed in all microarray experiments. Decreasing gene expression as risk of recurrence increases is typical for most miRNAs. Only 12 miRNAs increased expression following recurrence; among them were members of the miR-320 family showing the highest HR values. Seven highly abundant and biologically relevant miRNAs were subsequently selected for further validation by qPCR. That set included hsa-miR-15a-5p, hsa-miR-19b-3p, hsa-miR-30e-5p, hsa-miR-107, hsa-miR-146a-5p, hsa-miR-320c, and hsa-miR-331-3p; their characteristics are shown in Figure, Supplemental Digital Content 2. Additionally, 4 miRNAs that exhibited stable expression and did not correlate with recurrence status or other clinical characteristics were selected for qPCR normalization (let-7b-5p, miR-324-5p, miR-181b-5p, and miR-1281) (Figure, Supplemental Digital Content 3).
FIGURE 2.
Microarray data. A, Unsupervised hierarchical clustering of 49 differentially expressed miRNAs in MRR+ and MRR− based on logarithmic values (log2) of miRNA’s expression compared to recurrence in 8 yr of follow-up, sex, histopathological grade, type of resection, tumor location, and age at diagnosis. Hazard ratio (HR) is also marked for each miRNA. B, Differentially expressed miRNAs between primary and recurrent samples of MRR+ from paired analyses. TTRm, time to relapse in months; + censored if there is no event during follow-up period; * no evidence of radiographic recurrence, but follow-up <8 yr.
Paired Analysis
A total of 41 mature miRNAs exhibited differential expression in paired-matched primary and recurrent MRR+ samples (Figure 2B). Most of these were expressed less strongly in the recurrent samples, with only about 13 of them expressing more strongly in recurrent samples. Interestingly, the sample pairs formed 2 main clusters according to differentially expressed miRNAs, with some miRNAs changing their level of expression in opposite ways. One of these clusters contained only patients with convexity meningiomas and who were generally older and presented with higher histopathological WHO grades at diagnosis. Only 2 miRNAs, miR-193b-3p and miR-27a-3p, exhibited deregulation between paired-matched primary and recurrent samples of MRR+ and also showed dependence on TTR within the screening phase. These were not chosen for further validation of relapse prediction. Interestingly, miR-30c-5p was deregulated in recurrent samples of MRR+, whereas the expression of the closely related miR-30e-5p, miR-30b-5p, and miR-30a-5p was found to be dependent on TTR in the screening phase, which indicates that miR-30 is significantly involved in meningioma pathogenesis; it was therefore also selected for further validation.
Training Phase
The expression of candidate miRNAs was normalized against miR-181, which exhibited the most stable basal expression over all samples within the qPCR data (Figure, Supplemental Digital Content 4). A univariate analysis confirmed the differences in some of the miRNA’s expression in primary samples of MRR+ observed in microarray experiments. The following miRNAs exhibited significant dependence on TTR within the samples using adjusted or nonadjusted models in their expression: hsa-miR-15a-5p, hsa-miR-146a-5p, and hsa-miR-331-3p (Table 2). Nonadjusted models used only certain miRNAs as prognostic factors, whereas the adjusted model also included clinical factors such as age, sex, WHO grade, tumor location, and type of resection. All 3 miRNAs were selected for final validation on the extended cohort.
TABLE 2.
Univariate Adjusted and Nonadjusted Cox Regression Models of Time to Relapse From Training Phase for Each Measured miRNA
| Nonadjusted model | Adjusted model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | HR | 1/HR | 95% Cl range | P value | HR | 1/HR | 95% Cl range | P value | ||
| miR-107 | 1.25 | 0.80 | 0.86 | 1.80 | .240 | 0.94 | 1.06 | 0.63 | 1.41 | .761 |
| miR-331-3p | 1.76 | 0.57 | 1.14 | 2.70 | .010 | 1.57 | 0.64 | 0.93 | 2.65 | .090 |
| miR-15a-5p | 1.15 | 0.87 | 0.97 | 1.35 | .103 | 1.25 | 0.80 | 1.01 | 1.55 | .038 |
| miR-19b-3p | 1.27 | 0.79 | 0.90 | 1.78 | .175 | 1.25 | 0.80 | 0.88 | 1.77 | .214 |
| miR-30e-5p | 1.11 | 0.90 | 0.89 | 1.38 | .357 | 1.21 | 0.83 | 0.90 | 1.64 | .209 |
| miR-320c | 1.23 | 0.81 | 0.79 | 1.92 | .364 | 0.89 | 1.12 | 0.49 | 1.62 | .710 |
| miR-146a-5p | 1.55 | 0.65 | 1.13 | 2.13 | .007 | 1.42 | 0.70 | 1.00 | 2.02 | .053 |
Significant miRNAs in at least one model are marked in bold.
Validation Phase
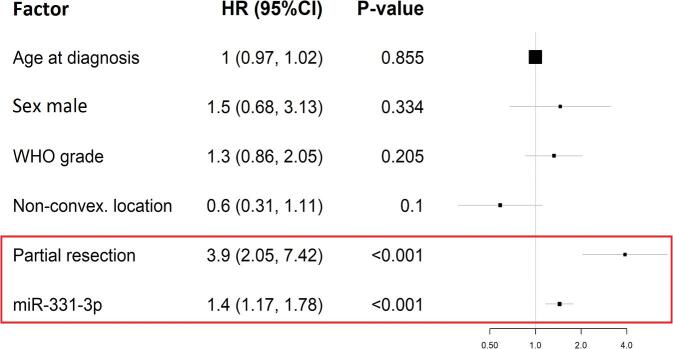
The 3 miRNAs selected in the training phase were tested in 172 patients, including cohorts from the screening and training phases. The final model was developed using the complete set of ΔCt values for the cohort that had a realistically unbalanced ratio of MRR+ and MRR− patients. Adjusting clinical factors were included in the Cox regression model for the final analysis of TTR in patients. Univariate analyses once again confirmed the miR-331-3p as the most promising prognostic factor. In the analysis of each marker separately, miR-331 gave the highest HR and the lowest P value among other miRNA-based univariate models. This analysis was also performed on the total resection subgroup in order to exclude the influence of such a strong prognostic factor that probably has no molecular background. Notably, this analysis produced similar results. Moreover, miR-15a was not a statistically significant candidate in the present models. Investigating the influence of clinical adjusting factors, the type of resection had the strongest prognostic value in all models. However, WHO grade in the subgroup of patients with a total resection was the strongest prognostic factor within the miR-146a- and miR-15a-based models. The results from the univariate Cox regression models are summarized in Table 3. The multivariate model was built in order to test whether miRNA represents real additional value in meningioma recurrence prognostication. The stepwise selection method with fixed clinical adjusting factors was used to select the most important prognostic factors in the multivariate model. The only significant (P < .001) candidate identified by the model was miR-331-3p, the extent of resection being the only other significant clinical factor, other factors having only supportive functions. The final model is shown in Figure 3. Among all tested models, WHO grades and miR-146a-5p also appeared to be predictors. The question remains, whether balanced cohorts in terms of Simpson grades in the validation phase would lead to similar results, or would alternatively confirm the role of the extent of tumor resection as a predictor of recurrence.
TABLE 3.
Univariate Adjusted Cox Regression Models of Time to Relapse From Validation Phase for Each Measured miRNA
| All patients | Patients after total resection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | HR | 1/HR | 95% Cl range | P value | Factor | HR | 1/HR | 95% Cl range | P value | ||
| miR-146a-5p based model | |||||||||||
| Age at diagnosis | 0.99 | 1.01 | 0.97 | 1.01 | .436 | Age at diagnosis | 1.01 | 0.99 | 0.98 | 1.04 | .709 |
| Sex: male | 1.68 | 0.59 | 0.84 | 3.37 | .142 | Sex: male | 1.15 | 0.87 | 0.47 | 2.82 | .759 |
| WHO grade | 1.63 | 0.61 | 1.07 | 2.50 | .024 | WHO grade | 1.86 | 0.54 | 1.06 | 3.24 | .029 |
| Nonconvex. location | 0.63 | 1.60 | 0.33 | 1.18 | .148 | Nonconvex. location | 0.54 | 1.85 | 0.23 | 1.25 | .150 |
| Partial resection | 3.16 | 0.32 | 1.67 | 6.00 | 4.24E-04 | – | – | – | – | – | – |
| miR-146a-5p | 1.34 | 0.74 | 1.10 | 1.63 | .003 | miR-146a-5p | 1.37 | 0.73 | 1.07 | 1.76 | .014 |
| miR-15a-5p based model | |||||||||||
| Age at diagnosis | 0.99 | 1.01 | 0.97 | 1.01 | .254 | Age at diagnosis | 0.99 | 1.01 | 0.97 | 1.02 | .656 |
| Sex: male | 2.77 | 0.36 | 1.45 | 5.27 | .002 | Sex: male | 1.69 | 0.59 | 0.72 | 3.95 | .228 |
| WHO grade | 1.37 | 0.73 | 0.91 | 2.08 | .132 | WHO grade | 1.85 | 0.54 | 1.07 | 3.18 | .027 |
| Nonconvex. location | 0.69 | 1.46 | 0.37 | 1.28 | .236 | Nonconvex. location | 0.65 | 1.55 | 0.28 | 1.50 | .310 |
| Partial resection | 3.67 | 0.27 | 1.92 | 7.02 | 8.52E-05 | – | – | – | – | – | – |
| miR-15a-5p | 0.94 | 1.06 | 0.84 | 1.05 | .283 | miR-15a-5p | 0.96 | 1.04 | 0.82 | 1.11 | .573 |
| miR-331-3p based model | |||||||||||
| Age at diagnosis | 1.00 | 1.00 | 0.97 | 1.02 | .827 | Age at diagnosis | 1.01 | 0.99 | 0.98 | 1.04 | .443 |
| Sex: male | 1.43 | 0.70 | 0.67 | 3.03 | .354 | Sex: male | 1.12 | 0.89 | 0.44 | 2.87 | .811 |
| WHO grade | 1.33 | 0.75 | 0.86 | 2.05 | .200 | WHO grade | 1.53 | 0.65 | 0.86 | 2.73 | .146 |
| Nonconvex. location | 0.58 | 1.72 | 0.31 | 1.10 | .095 | Nonconvex. location | 0.46 | 2.17 | 0.19 | 1.11 | .085 |
| Partial resection | 3.87 | 0.26 | 2.03 | 7.38 | 4.11E-05 | – | – | – | – | – | – |
| miR-331-3p | 1.45 | 0.69 | 1.17 | 1.79 | .001 | miR-331-3p | 1.43 | 0.70 | 1.10 | 1.87 | .007 |
Significant factors are marked in bold.
FIGURE 3.
Final multivariate Cox regression model built with factors selected by stepwise selection with data from validation phase for 161 patients with complete records. Characteristics from the Cox regression model are visualized as a forest plot. HR, hazard ratio.
DISCUSSION
miRNA Profile and Meningioma Recurrence
A link between aberrant miRNA expression and meningioma recurrence has so far been identified in only 3 studies.7-9 However, none of the miRNAs overlaps with the set of 49 deregulated miRNAs identified during the screening phase of the present study. This discrepancy might stem from differences in sample size or cohort homogeneity.8 For example, in contrast to the present study, the other cited studies7-9 included Asian cohorts comprising various numbers of patients ranging from 103 to 230 and a mixture of recurrent and nonrecurrent tumors (see Table, Supplemental Digital Content 5). Other differences between the approaches exist in the methodology, study design, and sample type. For example, only one study performed miRNA-profiling analysis incorporating all 3 phases,7 whereas the screening phase was omitted in the other 2 studies8,9; moreover, one of them selected only one candidate miRNA for analysis without utilizing screening and training dataset.9 These factors combined might have led to the eventual selection of different miRNAs. Regarding tissue samples, one of the cited studies prospectively analyzed circulating miRNAs from patients’ serum,7 whereas the others8,9 used tumor tissue either as snap-frozen or FFPE samples, and collected data retrospectively. Moreover, although quantitative reverse transcription PCR was used in the training and validation phases of all 3 cited studies,7,8 we used microarrays in our screening phase, which is a more advanced technique, as it targets all miRNAs. However, the differences between our results and those of other studies might also stem from the fact that other gene regulation mechanisms play a more robust role in meningioma recurrence.10 The previous studies also reported that the upregulation of miR-190a8 and miR-409-3p7 and downregulation of miR-29c-3p8 and miR-219-5p8 were associated with increased recurrence rates, whereas the effect of miR-224 expression was inconsistent in the different studies. Its downregulation was associated with increased recurrence,7 whereas Wang et al9 found the opposite, which they attributed to the activation of the ERG2-BAK-induced apoptosis pathway. Based on the high biological relevance and differential expression profile resulting from the Cox regression model and hierarchical clustering analysis, 7 miRNAs were selected for qPCR validation on an independent cohort, namely miR-15a-5p, miR-19b-3p, miR-30e-5p, miR-107, miR-146a-5p, miR-320c, and miR-331-3p. Following normalization against stably expressed miR-181, adjusted and nonadjusted models were used to test for effects on the TTR with estimated P values < .1. Among the miRNAs, the most significant positive prognostic factors were selected as being miR-15a-5p (P = .038), miR-146a-5p (P = .053), and miR-331-3p (P = .09). Subsequent testing of the 3 miRNAs on an expanded cohort using stepwise selection in the multivariate model identified the most effective predictive model to be the miR-331-3p-based model. The model which incorporated clinical factors identified those patients with a high miR-331-3p expression (HR 1.44; P < .001) and total/gross total meningioma resection (HR 3.90; P < .001) as cases with a significant influence on TTR. Other clinical factors played only a supportive role.
Having compared various models, the second most effective model was identified to be the one that included miR-146a-5p. In addition to the miRNA, the WHO grades and the extent of tumor resection were also good prognosticators. Factors identified as indicating a low propensity to recur were totally/gross totally removed (HR 3.16; P < .001) benign meningiomas (HR 1.63; P = .024) with upregulated miR-146a-5p expression (HR 1.34; P = .003). In a total/gross total resection subgroup analysis, both miRNAs remained significant predictive factors. Additionally, in the model with miR-146a-5p, the WHO grading system still functioned as a prognosticator.
miR-15a
miR-15 has been reported to suppress tumors in colon11 and prostate cancer.12 Following miR-15 transfection of tumor cells, the apoptosis rate increased significantly, probably because of the inhibition of nuclear factor kappa B (NF-κB) that promotes the transcription of antiapoptotic factors, such as Bcl-2 and Bcl-XL.11 Additionally, miR-15b has been shown to reduce the invasion of glioma cells and angiogenesis by downregulation of neuropilin-2 (NRP-2) that interacts with vascular endothelial growth factor (VEGF), or through deactivation of the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway signaling pathway.13 NRP-VEGF interactions have been shown to promote developmental angiogenesis and metastases.14 Thus, further investigations are warranted in order to verify which of these mechanisms, if any, is applied in meningioma biology. MEK-ERK as a key signaling pathway15 and a highly expressed VEGF/VEGF receptor16 have already been found in meningiomas.
miR-146a-5p
miR-146a is known to suppress gastric cancer cell invasion and metastasis,17 and knocking out its expression in C57BL/6 mice leads to the development of myeloid sarcomas and lymphomas.18 A study on gliomas19 found that a combined treatment with gamma-linolenic acid (GLA) and irradiation led to miR-146a overexpression. Because surgical resection supplemented with irradiation is a standard therapy for recurrent meningiomas,6 it would be desirable to determine whether this combined therapy has a similar beneficial effect on miR-146a-5p expression in meningiomas. The importance of miR-146 in meningioma recurrence is suggested by the existence of a negative feedback loop between signal transducer and activator of transcription 3 (STAT3) and NF-κB involving miR-146b,20 and the fact that STAT3 expression was significantly higher in recurrent WHO grade I and/or grade II meningiomas than in nonrecurrent ones.21STAT3 targets miR-146b, which reduces IL-6 production by downregulating NF-κB. This is the final step in the negative feedback loop because IL-6 activates STAT3.20 Upon miR-146b downregulation, its uninhibited target gene NF-κB induces increased IL-6 production followed by STAT3 activation. Notably, Johnson et al21 reported that STAT3 activation was markedly stronger in WHO grade II meningiomas than those of lower grade. High STAT3 activation was also identified in 2 out of 3 recurrent WHO grade I meningiomas and in none out of 3 nonrecurrent lesions. Additionally, a strong STAT3 phosphorylation/activation signal was observed in 2 out of 4 recurrent WHO grade II meningiomas and one out of 3 nonrecurrent tumors.21 Although the suppression of IL-6 by miR-146a leading to reduced STAT3 expression has not yet been demonstrated in meningiomas,22 these findings highlight the potential therapeutic value of miR-146a in these tumors. Combining GLA with irradiation might be an effective treatment strategy for overexpression of the miRNA, deactivation of the STAT3 pathway, and, thus, reducing the likelihood of meningioma recurrence.
miR-331-3p
The pronounced downregulation of miR-331-3p in glioblastomas suggests its role as tumor suppressor, possibly by upregulating NRP-2 expression23 or influencing targets such as receptor tyrosine-protein kinase erbB-2 (HER2),24 deoxyhypusine hydroxylase,25 phosphatase and tensin homolog/protein kinase B (PTEN/AKT), astrocyte-elevated gene-1 (AEG-1),26 transcription factor E2F1,27HER2/PI3K/AKT,28 or kallikrein-related peptidase 4.29 However, which proteins are targets of miR-331-3p in meningiomas remains unclear. Phosphoinositide-3-kinase (PI3K)/AKT, as a key regulator of cell survival in cancers, has already been associated with meningiomas.30 Moreover, AEG-1-depleted meningioma cells undergo apoptosis via phospho-AKT and Bcl-2 suppression.31 It is therefore rational to suspect that a negative impact of miR-331-3p deregulation on meningioma might be carried out through these signaling pathways.
miRNA Profile in Paired Analyses
Recent findings regarding the variability of gene mutations being dependent on meningioma localization,32 along with the observations that some genetic factors found in these tumors are important embryonic stem cell regulators,33 suggest that meningiomas may derive from early progenitors/cancer stem cells, whereas their histogenetic origin may be site specific. Of note, based on a differential expression of 41 miRNAs in the paired analyses, 2 clusters were created, one of them predominantly comprising convexity meningiomas with 13 miRNAs highly expressed in their recurrent samples. Because miRNAs are involved in the regulation of embryonic stem cell development and signaling,34 these findings further support the view that biological properties of meningiomas may be derived from site-specific progenitor/cancer stem cells regulated by respective miRNAs. Moreover, as WHO grade II and III meningiomas are significantly more frequent in the younger patients,35 it seems that a specific expression profile of the 13 miRNAs can recognize recurrent high-grade meningiomas in older subjects as a biologically distinct tumor subgroup. Basically, this may indicate 2 distinct biological mechanisms required for tumor recurrence, with the mechanism observed in older subjects requiring just the upregulation of the miRNAs. However, because of a small cohort in the paired analyses, all this remains speculative and requires further validation.
Limitations of the Study
The main shortcomings of the present study were its retrospectivity, the limited size of the validation phase cohort, and the lack of an external dataset. As the dataset was unbalanced in terms of Simpson grades, the question remains whether such balancing of the cohort would lead to results similar to those of an unbalanced one. Moreover, miRNA expression profiles in the MRR+ group might be prejudiced by the fact that only some symptomatic patients with meningioma recurrence underwent reoperation, whereas others were irradiated, and those without clinical manifestation and significant tumor growth were left untreated.
CONCLUSION
Our findings indicate that the miRNA-based model can serve as a novel predictor of meningioma recurrence and can thus help in determining an optimal postoperative surveillance regime to identify patients who may benefit from early retreatment. Moreover, combining the model with molecular mechanisms governing meningioma recurrence, such as miRNA targets and associated signaling pathways, might help to identify clinically distinct meningiomas and better target their treatment. Finally, because the literature indicates that no mutual miRNA predictors have yet been identified, a prospective randomized multicenter controlled trial is justified in order to resolve this ongoing discrepancy.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. This work was supported by funding from the Ministry of Health of the Czech Republic (15-29021A); Palacký University Olomouc (LF 2019_003); Ministry of Education, Youth and Sports of the Czech Republic (LM2015091); Czech Technology Agency: Center of Competence for Molecular Diagnostics and Personalized Medicine (TE02000058); and European Regional Development Fund (ENOCH CZ.02.1.01/0.0/0.0/16_019/0000868).
Supplementary Material
Contributor Information
Hanus Slavik, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Vladimir Balik, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic; Department of Neurosurgery, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Jana Vrbkova, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Alona Rehulkova, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Miroslav Vaverka, Department of Neurosurgery, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Lumir Hrabalek, Department of Neurosurgery, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Jiri Ehrmann, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic; Institute of Clinical and Molecular Pathology, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic, Czech Republic.
Monika Vidlarova, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Sona Gurska, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Marian Hajduch, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Josef Srovnal, Laboratory of Experimental Medicine, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, Czech Republic.
Supplemental Digital Content 1. Text. Detailed description of the RNA extraction procedure, miRNA array, TaqMan qPCR assay, and statistical analyses.
Supplemental Digital Content 2. Figure. miRNA markers selected for further experimental phases according to the screening phase. Characteristics from a univariate Cox regression model of time to relapse are visualized as a forest plot. HR, hazard ratio.
Supplemental Digital Content 3. Figure. miRNA references: stable expression of the 4 miRNAs identified in the screening phase presented as a heat map.
Supplemental Digital Content 4. Figure. miRNA references measured within training phase using qPCR. T-test revealed miR-181b-5p as the molecule with the most stable expression among MRR+ and MRR− samples. MRR+, primary samples of meningiomas with evidence of radiographic recurrence up to 8 years after surgery; MRR−, primary samples of meningiomas without evidence of radiographic recurrence up to 8 years after surgery.
Supplemental Digital Content 5. Table. Description of the studies reporting a link between aberrant miRNA expression and meningioma recurrence.7-9
REFERENCES
- 1. Ostrom QT, Gittleman H, Xu J et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl_5):v1-v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29(3):197-205. [DOI] [PubMed] [Google Scholar]
- 3. Durand A, Champier J, Jouvet A et al. Expression of c-Myc, neurofibromatosis type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: relation to grades or histotypes. Clin Neuropathol. 2008;27(5):334-345. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig N, Kim YJ, Mueller SC et al. Posttranscriptional deregulation of signaling pathways in meningioma subtypes by differential expression of miRNAs. Neuro Oncol. 2015;17(9):1250-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Longstreth WT Jr, Dennis LK, McGuire VM, Drangsholt MT, Koepsell TD. Epidemiology of intracranial meningioma. Cancer. 1993;72(3):639-648. [DOI] [PubMed] [Google Scholar]
- 6. Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26(5):461-469. [DOI] [PubMed] [Google Scholar]
- 7. Zhi F, Shao N, Li B et al. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Sci Rep. 2016;6:32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhi F, Zhou G, Wang S et al. A microRNA expression signature predicts meningioma recurrence. Int J Cancer. 2013;132(1):128-136. [DOI] [PubMed] [Google Scholar]
- 9. Wang M, Deng X, Ying Q, Jin T, Li M, Liang C. MicroRNA-224 targets ERG2 and contributes to malignant progressions of meningioma. Biochem Biophys Res Commun. 2015;460(2):354-361. [DOI] [PubMed] [Google Scholar]
- 10. El-Gewely MR, Andreassen M, Walquist M et al. Differentially expressed microRNAs in meningiomas grades I and II suggest shared biomarkers with malignant tumors, Cancers (Basel). 2016;8(3):E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu L, Wang D, Qiu Y, Dong H, Zhan X. Overexpression of microRNA-15 increases the chemosensitivity of colon cancer cells to 5-fluorouracil and oxaliplatin by inhibiting the nuclear factor-κB signalling pathway and inducing apoptosis. Exp Ther Med. 2018;15(3):2655-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zidan HE, Abdul-Maksoud RS, Elsayed WSH, Desoky EAM. Diagnostic and prognostic value of serum miR-15a and miR-16-1 expression among Egyptian patients with prostate cancer. IUBMB Life. 2018;70(5):437-444. [DOI] [PubMed] [Google Scholar]
- 13. Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geretti E, Klagsbrun M. Neuropilins: novel targets for anti-angiogenesis therapies. Cell Adh Migr. 2007;1(2):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson MD, O’Connell M, Vito F, Bakos RS. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neurooncol. 2009;92(2):129-135. [DOI] [PubMed] [Google Scholar]
- 16. Ragel BT, Jensen RL. Aberrant signaling pathways in meningiomas. J Neurooncol. 2010;99(3):315-324. [DOI] [PubMed] [Google Scholar]
- 17. Hou Z, Yin H, Chen C et al. microRNA-146a targets the L1 cell adhesion molecule and suppresses the metastatic potential of gastric cancer. Mol Med Rep. 2012;6(3):501-506. [DOI] [PubMed] [Google Scholar]
- 18. Zhao JL, Rao DS, Boldin MP, Taganov KD, O’Connell RM, Baltimore D. Nf-B dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108(22):9184-9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antal O, Hackler L Jr, Shen J et al. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiang M, Birkbak NJ, Vafaizadeh V et al. Stat3 induction of mir-146b forms a feedback loop to inhibit the Nf-B to il-6 signaling axis and stat3-driven cancer phenotypes. Sci Signal. 2014;7(310):ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson M, O’Connell M, Walter K. STAT3 activation and risk of recurrence in meningiomas. Oncol Lett. 2017;13(4):2432-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ye EA, Steinle JJ. miR-146a suppresses STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in primary human retinal microvascular endothelial cells in high glucose conditions. Vision Res. 2017;139:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Epis MR, Giles KM, Candy PA, Webster RJ, Leedman PJ. miR-331-3p regulates expression of neuropilin-2 in glioblastoma. J Neurooncol. 2014;116(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284(37):24696-24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Epis MR, Giles KM, Kalinowski FC, Barker A, Cohen RJ, Leedman PJ. Regulation of expression of deoxyhypusine hydroxylase (DOHH), the enzyme that catalyzes the activation of eIF5A, by miR-331-3p and miR-642-5p in prostate cancer cells. J Biol Chem. 2012;287(42):35251-35259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen L, Ma G, Cao X, An X, Liu X. MicroRNA-331 inhibits proliferation and invasion of melanoma cells by targeting astrocyte-elevated gene-1. Oncol Res. published online: February 17, 2018 (doi:10.3727/096504018X15186047251584). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo X, Guo L, Ji J et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398(1):1-6. [DOI] [PubMed] [Google Scholar]
- 28. Zhao D, Sui Y, Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35(2):1075-1082. [DOI] [PubMed] [Google Scholar]
- 29. White NM, Youssef YM, Fendler A, Stephan C, Jung K, Yousef GM. The miRNA-kallikrein axis of interaction: a new dimension in the pathogenesis of prostate cancer. Biol Chem. 2012;393(5):379-389. [DOI] [PubMed] [Google Scholar]
- 30. Miller R Jr, DeCandio ML, Dixon-Mah Y et al. Molecular targets and treatment of meningioma. J Neurol Neurosurg. 2014;1(1):1000101. [PMC free article] [PubMed] [Google Scholar]
- 31. Park KJ, Yu MO, Song NH et al. Expression of astrocyte elevated gene-1 (AEG-1) in human meningiomas and its roles in cell proliferation and survival. J Neurooncol. 2015;121(1):31-39. [DOI] [PubMed] [Google Scholar]
- 32. Karsy M, Azab MA, Abou-Al-Shaar H et al. Clinical potential of meningioma genomic insights: a practical review for neurosurgeons. Neurosurg Focus. 2018;44(6):E10. [DOI] [PubMed] [Google Scholar]
- 33. Clark VE, Harmancı AS, Bai H et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, Zhuang L, Lin CP. Roles of MicroRNAs in establishing and modulating stem cell potential. Int J Mol Sci. 2019;20(15):E3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ressel A, Fichte S, Brodhun M, Rosahl SK, Gerlach R. WHO grade of intracranial meningiomas differs with respect to patient's age, location, tumor size and peritumoral edema. J Neurooncol. 2019;145(2):277-286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.