Abstract
Despite the publication of various recommendations, quality standards and referral strategies to promote early diagnosis in axial SpA (axSpA) over the last decade, there remains a significant delay to diagnosis, leading to a lost tribe of undiagnosed, untreated patients with persistent back pain and axSpA symptoms. This review discusses the various factors contributing to diagnostic delay in axSpA, while providing recommendations to improve the diagnostic pathway, for example use of the online Spondyloarthritis Diagnosis Evaluation (SPADE) tool (http://www.spadetool.co.uk/). Significant shortcomings exist at both the primary and secondary care level, with healthcare professionals often lacking knowledge and awareness of axSpA. Myths regarding the classical signs and symptoms still prevail, including the perception of axSpA as a male disease, only occurring in individuals who are HLA-B27 positive with raised inflammatory markers. Individuals within this lost tribe of undiagnosed patients are likely lacking adequate treatment and are thereby at risk of worse clinical outcomes. It is therefore vital that public health initiatives are implemented to improve education of healthcare professionals and to ensure early specialist referral, to ultimately improve the lives of patients with axSpA.
Keywords: axial spondyloarthritis, diagnosis, healthcare professional awareness, early referral, extra-articular manifestations
- There remains a concerning delay to diagnosis and treatment in axial SpA.
- Significant shortcomings exist at the primary and secondary care level.
- Improved education of healthcare professionals and implementation of early referral strategies is required.
Introduction
Axial SpA (axSpA) is a chronic, inflammatory, rheumatic disease, characterized by fluctuating periods of flare and remission [1], often resulting in spine fusion and significant disability. The term axSpA encompasses both AS, whereby clear structural changes to the spine and/or SI joints can be observed via X-ray, and non-radiographic axSpA (nr-axSpA), whereby axSpA is instead diagnosed from other clinical features and MRI [2]. Whilst the natural history of axSpA remains unclear, it is evident from follow-up studies that the majority of people with nr-axSpA will not go on to develop structural changes detectable by X-ray [3–5]. Patients more likely to progress radiographically may be smokers, male, HLA-B27 positive, have higher baseline levels of structural changes (e.g. presence of syndesmophytes) or raised CRP and/or ESR[5–8].
AxSpA is estimated to effect ∼1 in 200 adults in the UK—twice the prevalence of multiple sclerosis or Parkinson’s disease [9, 10]. The primary symptom of axSpA is chronic lower back pain (CLBP), however other symptoms such as fatigue, morning stiffness, sleep disturbance and reduced function and/or mobility are often present [11, 12]; this leads patients with axSpA to experience considerable physical, emotional and economic burden [13–17], with the mean retirement age of people with AS estimated at 36 years [18]. Although primarily affecting the axial skeleton and SI joints, axSpA is frequently associated with a number of peripheral extra-articular manifestations (EAMs), including uveitis, enthesitis, psoriasis, dactylitis and IBDs [19–21].
Increased delay to diagnosis has been associated with worse outcomes in axSpA; a recent systematic review found that individuals with a delayed diagnosis had higher disease activity, worse physical function, increased structural damage, greater likelihood of work disability, and higher direct and indirect healthcare costs than those who received earlier diagnosis [13]. Delayed diagnosis is associated with an increased likelihood for worse quality of life and negative psychological consequences [13], in addition to worse treatment outcomes [22, 23], fatigue, difficulty sleeping and prevalence of psychosomatic disorders [24]. Several factors are independently associated with a long diagnostic delay: including female sex, HLA-B27 negativity, presence of psoriasis and young age of symptom onset [25]. Previous misdiagnosis of FM and psychosomatic disorders is suggested to be higher in women compared with men (20.7 vs 6.6% and 40.8 vs 23.0%, respectively) [24]. Presence of peripheral arthritis and IBD have been associated with earlier diagnosis [26–28]—earlier diagnosis and treatment leading to better outcomes and treatment responses [22, 23].
The mean diagnostic delay in AS has often been reported as between 8–10 years [23, 29–33]. However, some recent reports suggest this delay may now be <6 years [25, 28, 34–36], although the methodology of one paper has been queried [35]. This reduction is likely multifactorial, but key factors include the recent implementation of MRI in the diagnosis of axSpA and the 2009 publication of updated classification criteria by the Assessment of SpondyloArthritis International Society (ASAS). It is important to note that such criteria are not intended for use in diagnosis—the primary objective of classification criteria is to identify a homogeneous population for clinical trials and research, whereby patients are similar in terms of clinical characteristics. However, the ASAS classification criteria were seminal in that they formally recognized the concept of nr-axSpA, placing emphasis on early disease and use of MRI to identify early inflammatory changes, to allow for earlier detection of patients with the condition and inclusion of patients with nr-axSpA in clinical trials [2, 19]. In addition, in recent years there has been greater education of healthcare professionals (HCPs) through training initiatives introduced by groups such as the National Axial Spondyloarthritis Society and British Society for Spondyloarthritis (BRITSpA) [37].
Despite advances in our understanding of axSpA and improved education initiatives for HCPs, a recent survey of 2846 patients across 13 countries reported that the mean diagnostic delay has remained in the region of ∼8 years [38]. While an additional UK study published in 2015 found a stable mean diagnostic delay of 8–9 years and a median delay of 5 years prior to and after the 2009 updated classification criteria [26]. Furthermore, in England, a recent 2019 inquiry led by the All-Party Parliamentary Group for AxSpA found that significant shortcomings remain in axSpA care [39]. For example, just 21% of the 191 clinical commissioning groups and 99 provider Trusts investigated had a specific inflammatory back pain pathway in place. Without such a pathway, rapid referral to specialist care and potential early diagnosis for someone with symptoms of axSpA is unlikely.
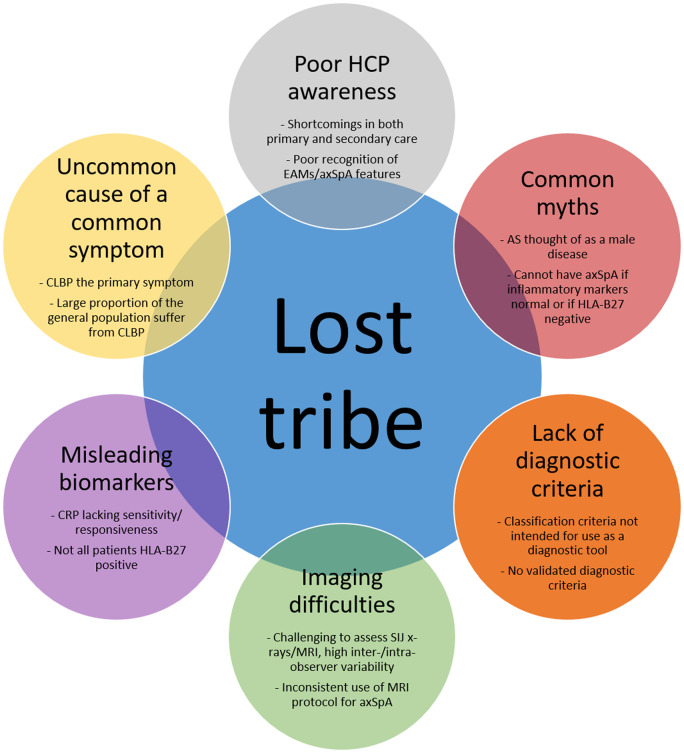
Ten years on from the publishing of updated ASAS classification criteria for axSpA, this maintained diagnostic delay has resulted in a ‘lost tribe’ of undiagnosed, untreated individuals with persistent back pain. The present review will describe some of the key factors contributing to this delay (Fig. 1) and challenges that must be addressed, in addition to outlining some of the policies and recommendations that should be implemented in order to reduce the concerning delay to diagnosis in axSpA.
Fig. 1.
Summary figure: source of the axial SpA lost tribe
AxSpA is a relatively uncommon cause of a common symptom
The primary symptom of axSpA is CLBP. Worldwide, 19.6% of individuals in the general population aged 20–59 years are reported to suffer from CLBP [40] and up to 80% of the population experience back pain at some point within their lifetime [41–43]. In contrast, prevalence of AS and axSpA as a whole have been estimated at between 0.01–0.54% and 0.13–1.40%, respectively [44–46]. In primary care, prevalence of axSpA has been estimated at between 5–24% of patients with CLBP [47–49], or 32–71% of CLBP patients in secondary care [50–53], therefore representing a relatively uncommon cause of a common symptom. As such, upon presentation to a primary care setting, the CLBP experienced in axSpA may instead be associated with other, more common or well-known pain disorders, particularly in the absence of ‘classical’ AS symptoms and obvious radiographic sacroiliitis [13, 54].
Worldwide, individuals often first present with CLBP to their general practitioners (GPs) or other non-rheumatology healthcare providers [53, 55–57], so it is vital that GPs are aware of and able to recognize the hallmark symptoms of axSpA. However, awareness of axSpA among GPs, including knowledge of long-term features, axSpA as a disease spectrum and importance of early diagnosis, is lacking [58, 59]. Due to the high prevalence of CLBP and low awareness among GPs and other non-rheumatology healthcare providers, particularly regarding the differences between mechanical back pain and inflammatory back pain [55, 57–62] (IBP, key for identifying axSpA), it is unsurprising that the delay to diagnosis remains high for axSpA.
It is also important to note that IBP should not be considered as a mandatory criterion for diagnosis, but as an axSpA feature. In fact, it has been estimated that only 70–80% of patients with axSpA have typical IBP symptoms [52, 63–67]. Although IBP should remain an important characteristic for screening patients in primary care, primary HCPs should bear in mind that absence of IBP does not exclude a diagnosis of axSpA [52]. Indeed, axSpA is a complex disease with heterogeneous presentation, therefore knowledge of all hallmark symptoms is crucial for early referral. While individual symptoms in isolation are insufficient to diagnose or rule out axSpA, identification of a combination of symptoms in an individual with chronic back pain (CBP) allows for a more confident diagnosis [see Recommendations for improved referrals, with reference to the recently developed Spondyloarthritis Diagnosis Evaluation (SPADE) tool [68] and 2015 ASAS-endorsed recommendations for referral [69]].
Lack of diagnostic criteria
Age at onset (<45 years) and type of back pain (chronic—present for >3 months) are key to screening patients with suspected axSpA. However, after this screening, diagnosis becomes challenging due to lack of validated diagnostic criteria [57].
The aforementioned 2009 ASAS classification criteria for axSpA were developed to facilitate the conduct of clinical trials and observational studies in early axSpA through the identification of uniform patient populations and to help guide a flexible approach to earlier diagnoses [2, 70–72]. While not intended for use as a diagnostic tool, many practitioners may inappropriately use classification criteria as a surrogate for diagnostic criteria [73], potentially leading to over- or under-diagnosing of axSpA. Indeed, discrepancies have been observed between diagnosis by a rheumatologist and satisfaction of the ASAS classification criteria [52, 74].
Imaging difficulties
Assessment of conventional SI joint X-rays is challenging, with high inter- and intra-observer variability [75–80]. Of particular concern, the reproducibility and performance of identification of radiographic sacroiliitis does not significantly improve with training [75].
While MRI has transformed axSpA diagnosis and allowed for much earlier detection of inflammatory and structural changes, there remains some debate around what constitutes a ‘positive’ MRI suggestive of axSpA [81, 82], potentially leading to over-diagnosis or misclassification if used for diagnostic purposes without context [81, 83–90]. Interpretation of MRI is challenging and will depend on the expertise of the radiologist; inconsistencies have also recently been found regarding its use in clinical practice [91]. Utilizing the survey responses of 269 UK radiologists, Bennett and colleagues found that just 75% of radiologists were aware of the term axSpA; a concerning 31% and 25% were aware of the ASAS definitions of positive MRI for the SI joints and spine, respectively [91]. Furthermore, it has been reported that just one-third of musculoskeletal radiologists perform the recommended MRI protocol for axSpA [92, 93]. Recent efforts by groups such as BRITSpA to provide consensus recommendations for the acquisition and interpretation of MRI in the diagnosis of axSpA should help standardize practice and in future allow for a more consistent, reliable approach to diagnosis [94]. Such efforts should reduce inevitable false-positive and false-negative inference of axSpA from MRI, in part, contributing to the lost tribe of undiagnosed patients with axSpA.
Misleading biomarkers
No accurate biomarkers or immune-phenotyping tools currently exist for the identification of axSpA. HLA-B27 and CRP serum biomarkers are commonly used, whereby there is a strong genetic association with HLA-B27 [2]. However, not all axSpA patients are HLA-B27 positive and this often leads to delayed diagnosis in HLA-B27-negative patients [95]. Furthermore, CRP, despite being a widely used laboratory marker for axSpA and used as a criterion for determining treatment, is thought to be lacking in sensitivity and responsiveness, while the natural degree of fluctuation is not well understood. A recent study found frequent fluctuation in CRP levels, whereby 50% of patients with normal CRP at baseline had at least one elevated CRP result within the following 16 weeks [96]. Its use as a one-off diagnostic tool is therefore challenging, and may lead to under-diagnosis of axSpA, particularly in those who have no signs of inflammation on MRI yet experience high disease activity. These factors may have led to a lost tribe of HLA-B27-negative patients with normal inflammatory markers and undiagnosed axSpA.
Myths that need to be dispelled
Historically, X-rays were an important part of diagnosing AS, whereby diagnosis required evidence of significant radiographic changes. This therefore led to long delays from symptom onset to diagnosis. Furthermore, many people with nr-axSpA may never develop the level of radiographic change that would have been previously required for a diagnosis of AS, so these diagnoses would have previously been missed, despite symptoms and disability consistent with AS. Interestingly, radiographic progression is more evident in males, therefore AS was traditionally thought of as a male disease. However, a significant proportion of female patients also suffer from AS or axSpA (Fig. 2) [2, 97–105]. Unfortunately, despite the evidence, common myths prevail; a recent study reported that all interviewed GPs believed AS was almost exclusively diagnosed in men, expressing that practical referral measures would be useful [58].
Fig. 2.
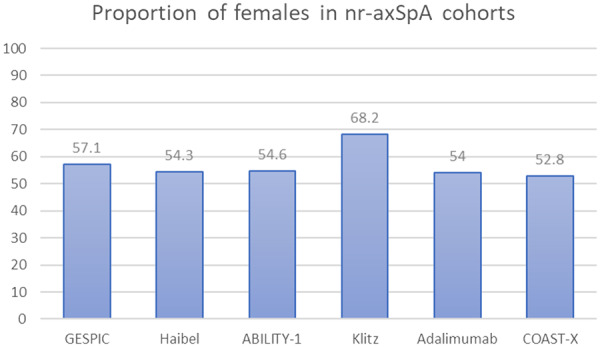
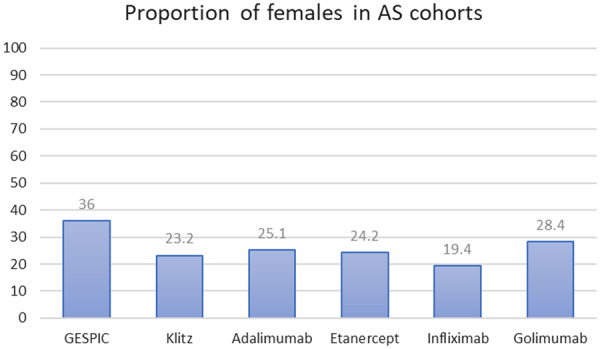
Females prevalent with axial SpA [2, 97–105]
GESPIC: German Spondyloarthritis Inception Cohort [97]; Haibel [98]; ABILITY-1 (Study of Adalimumab in Patients With Axial Spondyloarthritis) [99]; Klitz [100]; Adalimumab [101]; COAST-X [A Study of Ixekizumab (LY2439821) in Participants with Nonradiographic Axial Spondyloarthritis] [105]; Etanercept [102]; Infliximab [103]; Golimumab [104]. GESPIC focusses on patients with primarily axial symptoms but includes patients with peripheral SpA.
Another common misconception is that a patient cannot have axSpA if presenting with normal inflammatory markers or if tested as HLA-B27 negative, potentially leading to missed diagnoses of axSpA (see Misleading biomarkers and Secondary care awareness).
Delayed referral to specialist rheumatology care
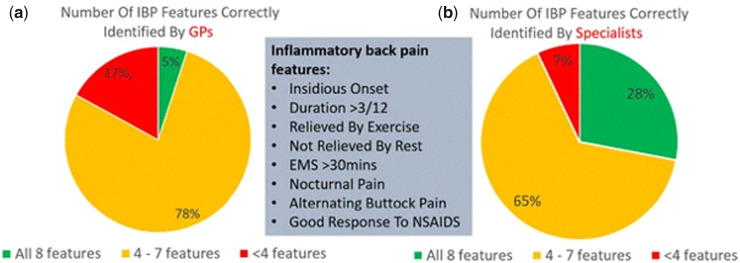
Sixty-two percent of patients report contacting a HCP within 12 months of developing axSpA symptoms [30]. Therefore, one of the significant delays to diagnosis appears to occur after initial presentation to a healthcare provider, with failures both at the primary [39, 59, 61, 106–108] and secondary care [52, 75, 91, 93] level. Evidence suggests that despite various guidelines and recommendations in place relating to referral and investigations of patients with CLBP, there is often a disparity between the guidelines and what is implemented in clinical practice, particularly regarding referral for appropriate imaging when AS or axSpA may be indicated [39, 93, 109–113]. Knowledge, awareness, confidence and clinical assessment for the signs, symptoms and risk factors of suspected axSpA among medical professionals in both primary and secondary care is often poor (Fig. 3) [52, 57, 59, 106, 114], including among musculoskeletal radiologists who often are involved in the interpretation of imaging results during the diagnostic pathway [91–93]. This lack of awareness has a major impact on patients; some report not feeling ‘listened to’ or ‘believed’ about their symptoms, with others feeling helpless and withdrawing themselves from care completely, leading to further diagnostic delay (Fig. 4) [115]. A recent 2018 study found that although the interval between initial presentation and diagnosis varied greatly, one-third of patients diagnosed in the past 5 years waited over 10 years from initial presentation to formal diagnosis [93].
Fig. 3.
GPs [59] vs specialistsa—number of correctly identified features of IBP [114]
aSecondary care consultants working in the following specialties: orthopaedics (n = 64), ophthalmology (n = 40), A&E (n = 35), gastroenterology (n = 27), genitourinary medicine (n = 16), spinal surgery (n = 13) and dermatology (n = 10). IBP: inflammatory back pain; GPs: general practitioners.
Fig. 4.
Qualitative exploration of patient experience: delay to diagnosis [115]
Primary care awareness
The majority of patients with CLBP will initially seek care from primary care physicians such as GPs or physiotherapists, orthopaedics, pain clinics, chiropractors, or complementary or alternative medicine practitioners (e.g. osteopaths, massage therapists, acupuncturists [116]), before being referred to rheumatology if displaying musculoskeletal symptoms [55, 60, 117–123]. Although musculoskeletal complaints can account for up to 20% of all consultations in primary care [124], as discussed previously, distinguishing axSpA from other forms of CLBP can be difficult [55, 57–62] (Fig. 3), with a concerning lack of awareness of other axSpA features and EAMs [58, 59, 106]. Collaboration between practitioners is also lacking—in a recent survey of chiropractors and osteopaths, the principal perceived barrier to onward referral was reluctance by the GP to accept their professional opinion [106].
A recent 2019 study found that the majority of HCPs failed to recognize IBP in patients with CLBP, with many of those who did (24.2%) opting to treat the symptoms before referral to a specialist [55, 60]. Forty-one percent of HCPs stated that patients had seen another specialist before consulting them. Upon referral, the top barriers preventing patients from seeing a specialist were the long wait (77%), insurance restrictions (47.1%—will not be applicable in some countries), lack of adequate specialist nearby (35.1%), patient reluctance or resistance (25.2%) and travel distance to specialist (21.5%). Just 7.4% reported no issues with the referral process. Patient reluctance or resistance may be due to denial or perhaps simply other commitments—axSpA frequently presents in the second or third decade of a patient’s life, often a critical time for attempting to establish careers and relationships [106]. Patients initially referred for treatment by a physiotherapist to address symptoms may also suffer a delay to therapy access; one study reporting problems with access to physiotherapy for 32% of GPs [59].
Following access to physiotherapy, a further diagnostic delay may occur. In 2019, McCrum and colleagues found that the average time from initial physiotherapy visit to diagnosis with SpA was 6.4 years [108]. Forty-four percent of these patients received three or more physiotherapy episodes prior to diagnosis; the number of contacts within each episode ranged from 3 (47 people) to 58 (1 person), a median of 11 contacts per episode (10 people). As in the recently published National Institute for Health and Care Excellence (NICE) guidelines for referral [107, 125], this highlights the importance of physiotherapists for recognition and referral of axSpA, while emphasizing that identification of axSpA is missed at multiple timepoints across a patients journey to diagnosis. Some people have described having to truly fight for their diagnosis, resulting in distress and often feelings of sadness, frustration or anger [126].
Secondary care awareness
Another source of this lost tribe of undiagnosed individuals are those presenting to secondary care with EAMs [52, 127–136]. A 2018 study by Sykes et al. [135] explored the prevalence of axSpA among 366 individuals with acute anterior uveitis (AAU). Minimum prevalence was identified as 20.2% of patients with AAU; nearly one-quarter of these patients was previously undiagnosed despite years of back pain, representing a substantial hidden burden of disease. This supports the work of Haroon and colleagues, where 40% of patients presenting with AAU had undiagnosed SpA [134]. HLA-B27 diagnosis is often the trigger for referral of patients with AAU to rheumatology. The Dublin Uveitis Evaluation Tool (DUET) algorithm developed by Haroon and colleagues indeed prompts referral if a patient is HLA-B27 positive, or has co-existing psoriasis or peripheral arthritis—with high sensitivity and specificity (96% and 97% respectively)—potentially implicating HLA-B27 as the ‘anchor criterion’ for the ASAS classification criteria clinical arm [134, 135]. However, importantly, in the study by Sykes et al. nearly half of patients identified as missed diagnoses were HLA-B27 negative. These patients would therefore have remained lost if utilizing the DUET algorithm. Similarly, nearly two-thirds of new diagnoses would have been missed if using IBP rather than CBP as a referral strategy, supporting the presence of IBP as an axSpA feature rather than mandatory criterion for referral [52, 137, 138], in line with ASAS-endorsed recommendations for early referral [69]. Due to the high prevalence of axSpA among individuals presenting with AAU, the authors thereby recommend that all individuals with AAU and CBP with onset before the age of 45 years should be referred to rheumatology regardless of HLA-B27 status [135].
Similar concepts have been recommended for individuals presenting with other EAMs, including IBD and psoriasis [127–130, 136]. SpA may occur in up to 13% of individuals with IBD [128]; a recent review reported that prevalence of sacroiliitis ranged from 2.2 to 68% among IBD patient populations [127]. The latter work has informed a prospective observational study of magnetic resonance enterography as a screening tool for axSpA, initiated in March 2019 (ClinicalTrials.gov NCT03817983) [139]. Similarly, the recent ADIPSA [Axial Disease In PSoriatic Arthritis (PsA)] study found that 49/201 (23.9%) PsA patients fulfilled Modified New York criteria for AS [140]. Although due to lack of MRI, fulfilment of the full ASAS criteria could not be assessed, 85/118 (72%) psoriatic SpA cases and 9/127 (7%) peripheral PsA cases fulfilled ASAS clinical or radiographic imaging criteria. In the multicentre SASPIC (Screening for AxSpA in Psoriasis, Iritis, and Colitis) cohort, 47.6% of patients with psoriasis, AAU or colitis ≤45 years of age with ≥3 months of undiagnosed back pain were diagnosed with axSpA when utilizing a proposed three-stage evaluation approach [clinical evaluation; labs (HLA-B27, CRP) and radiography; MRI] [141], while as many as 68.7% were diagnosed after the clinical evaluation alone [142]. These studies suggest a significant lost tribe of undiagnosed axSpA patients among people with AAU, psoriasis and IBD.
It has even been suggested that a lost tribe of axSpA patients could exist within a population of young people undergoing hip arthroplasty; a publication by Waters and colleagues in 2015 identified structural abnormality in 24/92 patients (26.1%) and known inflammatory arthropathy in 3/92 (3.3%) [143]. Inflammatory markers were investigated for 41 patients, 10 (24%) of which had elevated CRP and/or ESR. Eighty-three individuals had radiographs available for investigation; SI joint abnormality observed in nine individuals (10.8%), five (6%) having bilateral grade 2 sacroiliitis. None of these patients was previously diagnosed with axSpA.
Recommendations for improved referrals
Following recommendations from earlier studies, amid growing concerns regarding delay to diagnosis and consequences for the patient, over the past decade we have seen the development, publication and evaluation of multiple early referral and screening strategies, recommendations, quality standards and algorithms for identifying and referring suspected axSpA and/or CLBP [67, 69, 123, 125, 136, 139, 142, 144–149], in addition to the development, introduction and testing of various educational tools for HCPs.
Indeed, education of HCPs in primary and secondary care and the wider use of diagnostic algorithms may improve early recognition and referral of axSpA, thereby improving patient outcomes. Education has been found to substantially improve the recognition and referral of patients with suspected axSpA by GPs [150, 151]—one recent prospective, multicentre study demonstrated over 40% improvement in referral after receiving SpA education or training [150]. In physiotherapists, good awareness of the NICE 2017 guidance on SpA and continuing professional development was associated with better awareness and knowledge of axSpA features [61]. Furthermore, the delay to diagnosis is shortened in individuals with peripheral disease [26–28], likely due to the fact that GPs have been consistently prompted via the early arthritis initiative about the importance of early referral for patients with swollen joints [26]. A recent study of two large UK centres found a 51% increase in new axSpA diagnoses between 2009 and 2013, following the introduction of the ASAS 2009 classification criteria [2], vs the prior 5 years [26].
Closer collaboration is recommended between rheumatology and specialists presented with EAMs (e.g. ophthalmology [134, 135], gastroenterology [127–130, 136], dermatology [131]). As outlined by Sykes and colleagues regarding patients presenting with AAU and CBP [135], there does not appear to be a straightforward mechanism for screening patients. Therefore, specialists should be made aware of axSpA referral guidelines, which should be implemented appropriately based on their existing healthcare infrastructure, perhaps utilizing a referral strategy of all patients with EAMs + CBP with onset at age <45 years old. Knowledge of axSpA and EAMs is advancing all the time, therefore specialists should remain aware of such updates—for example, the recent suggestion of hidradenitis suppurativa (acne inversa) as a possible new EAM [132, 133]. Other secondary healthcare providers such as rheumatologists and radiologists should also work more closely to improve and standardize interpretation of MRI on the pathway to diagnosis, as outlined in recommendations by the BRITSpA collaborators [91, 94]. This also calls for better training of rheumatologists and radiologists in the interpretation of MRI in the context of suspected axSpA [152]. Closer collaboration is also recommended between primary healthcare providers, including GPs, physiotherapists, osteopaths and chiropractors [106].
To summarize Poddubnyy and Sieper (2019) [152]: any screening or referral strategy for SpA is useful, with no existing strategy illustrating outstanding performance (applied referral strategy increases probability of axSpA from 5 to 40–50%) and complex/simple strategies performing equally as well. Importantly, the starting point for referral should be CBP starting at a young age, usually suggested at <45 years. The formal ASAS-endorsed recommendation for early referral of patients with suspected axSpA suggests that patients with CBP (duration ≥3 months) with onset before the age of 45 years should be referred to a rheumatologist if at least one of the following parameters is present: IBP; HLA-B27 positive; sacroiliitis on imaging if available (X-rays or MRI); peripheral manifestations (arthritis, enthesitis, dactylitis); EAMs (psoriasis, IBD, uveitis); positive family history for SpA; good response to NSAIDs; elevated acute-phase reactant (e.g. CRP) [69]. This recommendation may thereby be used in clinical practice by GPs and other primary care HCPs or non-rheumatology specialists as a flexible, universal strategy for referral of patients with suspected axSpA. However, the ideal referral strategy will likely vary depending on the clinical setting and country, due to potential differences in healthcare structure and prevalence of referral parameters (e.g. availability/use of HLA-B27 testing) [144].
Importantly, individual symptoms in isolation are insufficient to either diagnose or rule out axSpA. As outlined in Rudwaleit’s early work on likelihood ratios for diagnosis, it is the identification of a combination of axSpA symptoms that should lead to diagnosis [153]. The online SPADE tool (http://www.spadetool.co.uk/) was recently developed to aid HCPs in their diagnosis of axSpA, whereby probability of axSpA is displayed on a chart based on symptoms entered into the tool, with clear instructions on how to then proceed (e.g. further tests needed, assessment by a rheumatologist is recommended) [68]. Future implementation of such strategies, education tools and quality standards will be paramount for reducing the delay to diagnosis in axSpA, in order to uncover the lost tribe of undiagnosed, untreated individuals with persistent back pain (Fig. 5).
Fig. 5.
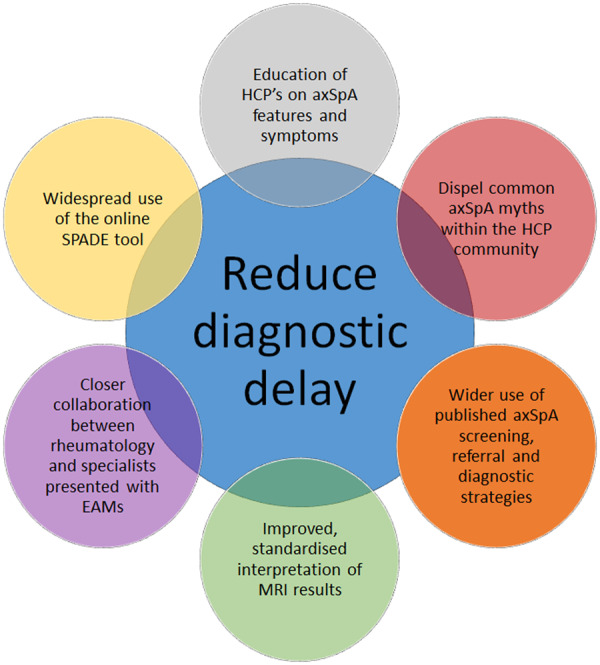
Summary figure: implementable steps to reduce diagnostic delay in axial SpA
Conclusions—the future of axSpA care
Lack of knowledge, awareness and confidence diagnosing axSpA in both primary and secondary care has led to a lost tribe of undiagnosed patients experiencing persistent back pain and ongoing axSpA symptoms (Fig. 1). These individuals may reside within existing patient populations, including those with IBD, psoriasis/PsA, AAU and early hip arthroplasty, or may not exhibit the outdated, preconceived ‘classic’ symptoms of AS if HLA-B27 negative, female or not displaying raised inflammatory markers. Importantly, these individuals are likely lacking optimal treatment and are thereby at risk of worse outcomes and potential complications. It is therefore of the utmost importance that this lost tribe is uncovered, through better education of HCPs and implementation of existing referral strategies, recommendations and quality standards.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article. This paper was published as part of a supplement funded by Novartis.
Disclosure statement: R.S. has received grants and honoraria from Abbvie, Biogen, Lilly, Novartis, Celgene and Union Chimique Belge. The other authors have declared no conflicts of interest.
References
- 1. Wendling D, Prati C. Flare in axial spondyloarthritis. The dark side of the outcome. Ann Rheum Dis 2016;75:950–1. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewé R. et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 3. Poddubnyy D, Sieper J. Radiographic progression in ankylosing spondylitis/axial spondyloarthritis: how fast and how clinically meaningful? Curr Opin Rheumatol 2012;24:363–9. [DOI] [PubMed] [Google Scholar]
- 4. van der Heijde D, Baraliakos X, Hermann K-G. et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sari I, Haroon N. Radiographic progression in ankylosing spondylitis: from prognostication to disease modification. Curr Rheumatol Rep 2018;20:82. [DOI] [PubMed] [Google Scholar]
- 6. Sari I, Lee S, Tomlinson G. et al. Factors predictive of radiographic progression in ankylosing spondylitis. Arthritis Care Res (Hoboken) 2020; doi:10.1002/acr.24104. [DOI] [PubMed] [Google Scholar]
- 7. López-Medina C, Molto A, Claudepierre P, Dougados M. Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. Ann Rheum Dis 2020;79:209–16. [DOI] [PubMed] [Google Scholar]
- 8. Ramiro S, Heijde D, Sepriano A. et al. Spinal radiographic progression in early axial spondyloarthritis: five-year results From the DESIR cohort. Arthritis Care Res (Hoboken) 2019;71:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton L, Macgregor A, Toms A. et al. The prevalence of axial spondyloarthritis in the UK: a cross-sectional cohort study. BMC Musculoskelet Disord 2015;16:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Facts and Figures | National Axial Spondyloarthritis Society. 2019. https://nass.co.uk/about-axial-spondyloarthritis/as-facts-and-figures/ (20 February 2020, date last accessed).
- 11. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 12. Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am 2012;38:387–403. [DOI] [PubMed] [Google Scholar]
- 13. Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther 2020;7:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nikiphorou E, Ramiro S, van der Heijde D. et al. Association of comorbidities in spondyloarthritis with poor function, work disability, and quality of life: results from the Assessment of SpondyloArthritis International Society Comorbidities in Spondyloarthritis Study. Arthritis Care Res (Hoboken) 2018;70:1257–62. [DOI] [PubMed] [Google Scholar]
- 15. Claudepierre P, Fagnani F, Cukierman G. et al. Burden of severe spondyloarthritis in France: a nationwide assessment of prevalence, associated comorbidities and cost. Joint Bone Spine 2019;86:69–75. [DOI] [PubMed] [Google Scholar]
- 16. Macfarlane GJ, Rotariu O, Jones GT, Pathan E, Dean LE. Determining factors related to poor quality of life in patients with axial spondyloarthritis: results from the British Society for Rheumatology Biologics Register (BSRBR-AS). Ann Rheum Dis 2020;79:202–8. [DOI] [PubMed] [Google Scholar]
- 17. Strand V, Singh JA. Patient burden of axial spondyloarthritis. J Clin Rheumatol 2017;23:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cakar E, Taskaynatan MA, Dincer U. et al. Work disability in ankylosing spondylitis: differences among working and work-disabled patients. Clin Rheumatol 2009;28:1309–14. [DOI] [PubMed] [Google Scholar]
- 19. Rudwaleit M, van der Heijde D, Landewe R. et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 20. van der Horst-Bruinsma IE, Nurmohamed MT. Management and evaluation of extra-articular manifestations in spondyloarthritis. Ther Adv Musculoskelet Dis 2012;4:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erol K, Gok K, Cengiz G. et al. Extra-articular manifestations and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis. Acta Reumatol Port 2018;43:32–9. [PubMed] [Google Scholar]
- 22. Sieper J, van der Heijde D, Dougados M. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seo MR, Baek HL, Yoon HH. et al. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol 2015;34:1397–405. [DOI] [PubMed] [Google Scholar]
- 24. Ogdie A, Benjamin Nowell W, Reynolds R. et al. Real-world patient experience on the path to diagnosis of ankylosing spondylitis. Rheumatol Ther 2019;6:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Redeker I, Callhoff J, Hoffmann F. et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology (Oxford) 2019;58:1634–8. [DOI] [PubMed] [Google Scholar]
- 26. Sykes MP, Doll H, Sengupta R, Gaffney K. Delay to diagnosis in axial spondyloarthritis: are we improving in the UK? Rheumatology (Oxford) 2015;54:2283–4. [DOI] [PubMed] [Google Scholar]
- 27. Aggarwal R, Malaviya AN. Diagnosis delay in patients with ankylosing spondylitis: factors and outcomes–an Indian perspective. Clin Rheumatol 2009;28:327–31. [DOI] [PubMed] [Google Scholar]
- 28. Masson Behar V, Dougados M, Etcheto A. et al. Diagnostic delay in axial spondyloarthritis: a cross-sectional study of 432 patients. Joint Bone Spine 2017;84:467–71. [DOI] [PubMed] [Google Scholar]
- 29. Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton L, Gilbert A, Skerrett J, Dickinson S, Gaffney K. Services for people with ankylosing spondylitis in the UK–a survey of rheumatologists and patients. Rheumatology (Oxford) 2011;50:1991–8. [DOI] [PubMed] [Google Scholar]
- 31. Grigg SE, Martin BJ, Buchanan RR, Schachna L. Burden of delay to diagnosis of ankylosing spondylitis ACR/ARHP Annual Scientific Meeting 2011. [Google Scholar]
- 32. Fitzgerald G, Gallagher P, O’Sullivan C. et al. 112. Delayed diagnosis of axial spondyloarthropathy is associated with a higher prevalence of depression. Rheumatology (Oxford) 2017;56(Suppl 2):kex062.112. [Google Scholar]
- 33. Rosenbaum JT, Pisenti L, Park Y, Howard RA. Insight into the quality of life of patients with ankylosing spondylitis: real-world data from a US-based life impact survey. Rheumatol Ther 2019;6:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sørensen J, Hetland ML. Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2015;74:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sykes M, Doll H, Gaffney K. Comment on: ‘Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry’ by Sørensen et al. Ann Rheum Dis 2014;73:e44. [DOI] [PubMed] [Google Scholar]
- 36. Abdelrahman FI, Mortada M. AB0858 Impact of application of ASAS criteria for axial spondyloarthritis on the diagnostic delay in Egyptian patients. Ann Rheum Dis 2018;77(Suppl 2):1556–7. [Google Scholar]
- 37. Healthcare Professional Awareness: We are Working to Raise Healthcare Professional Awareness of Axial SpA. Including Primary Care, Rheumatology and AHPs: NASS. https://nass.co.uk/get-involved/campaign-with-us/healthcare-professional-awareness/(20 February 2020, date last accessed).
- 38. Garrido-Cumbrera M, Poddubnyy D, Gossec L. et al. The European map of axial spondyloarthritis: capturing the patient perspective—an analysis of 2846 patients across 13 countries. Curr Rheumatol Rep 2019;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Axial Spondyloarthritis Services in England: A National Inquiry. All-Party Parliamentary Group for Axial Spondyloarthritis: NASS. https://nass.co.uk/wp-content/uploads/2020/01/Axial-Spondyloarthritis-Services-in-England-FINAL.pdf (20 February 2020, date last accessed).
- 40. Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: systematic review. Rev Saude Publica 2015;49:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med 2001;51:124–35. [DOI] [PubMed] [Google Scholar]
- 42. Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12:E35–70. [PubMed] [Google Scholar]
- 43. Hoy D, Bain C, Williams G. et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012;64:2028–37. [DOI] [PubMed] [Google Scholar]
- 44. Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol 2018;30:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bohn R, Cooney M, Deodhar A, Curtis JR, Golembesky A. Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol 2018;36:263–74. [PubMed] [Google Scholar]
- 46. Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta‐regression analysis. Arthritis Care Res (Hoboken) 2016;68:1320–31. [DOI] [PubMed] [Google Scholar]
- 47. van Hoeven L, Vergouwe Y, de Buck P. et al. External validation of a referral rule for axial spondyloarthritis in primary care patients with chronic low back pain. PLoS one 2015;10:e0131963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Hoeven L, Luime J, Han H, Vergouwe Y, Weel A. Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care Res (Hoboken) 2014;66:446–53. [DOI] [PubMed] [Google Scholar]
- 49. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol 1995;34:1074–7. [DOI] [PubMed] [Google Scholar]
- 50. Sepriano A, Landewé R, van der Heijde D. et al. Predictive validity of the ASAS classification criteria for axial and peripheral spondyloarthritis after follow-up in the ASAS cohort: a final analysis. Ann Rheum Dis 2016;75:1034–42. [DOI] [PubMed] [Google Scholar]
- 51. van den Berg R, de Hooge M, van Gaalen F. et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492–9. [DOI] [PubMed] [Google Scholar]
- 52. Tant L, Delmotte N, Van den Enden M, Gangji V, Mielants H. High prevalence of undiagnosed axial spondyloarthritis in patients with chronic low back pain consulting non-rheumatologist specialists in Belgium: SUSPECT study. Rheumatol Ther 2017;4:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deodhar A, Mease PJ, Reveille JD, Curtis JR. et al. Frequency of axial spondyloarthritis diagnosis among patients seen by US rheumatologists for evaluation of chronic back pain. Arthritis Rheumatol 2016;68:1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kiltz U, Baraliakos X, Regel A, Buhring B, Braun J. Causes of pain in patients with axial spondyloarthritis. Clin Exp Rheumatol 2017;35(Suppl 107):S102–7. [PubMed] [Google Scholar]
- 55. Magrey M, Yi E, Wolin D. et al. Recognition of inflammatory back pain by US healthcare providers and barriers to specialist referral. Arthritis Rheumatol 2019; 71 (suppl 10). https://acrabstracts.org/abstract/recognition-of-inflammatory-back-pain-by-us-healthcare-providers-and-barriers-to-specialist-referral/ (2 August 2020, date last accessed). [Google Scholar]
- 56. Van Der Heijde D, Sieper J, Elewaut D. et al. Referral patterns, diagnosis, and disease management of patients with axial spondyloarthritis: results of an international survey. J Clin Rheumatol 2014;20:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 2019;38:625–34. [DOI] [PubMed] [Google Scholar]
- 58. van Onna M, Gorter S, van Meerendonk A, van Tubergen A. General practitioners’ perceptions of their ability to identify and refer patients with suspected axial spondyloarthritis: a qualitative study. J Rheumatol 2014;41:897–901. [DOI] [PubMed] [Google Scholar]
- 59. Jois RN, Macgregor AJ, Gaffney K. Recognition of inflammatory back pain and ankylosing spondylitis in primary care. Rheumatology (Oxford) 2008;47:1364–6. [DOI] [PubMed] [Google Scholar]
- 60. Magrey M, Yi E, Wolin D. et al. SAT0338 delayed diagnosis of ankylosing spondylitis: results from a survey of 1690 US physicians from 10 specialties. Ann Rheum Dis 2019;78:1247–1249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steen M, Cairns MC, McCrum C. Physiotherapists’ Awareness, Knowledge and Confidence in the Recognition and Referral of Possible Axial Spondyloarthritis: Are We Contributing to Diagnostic Delays? 2019. https://wwwabstractstosubmitcom/wcpt2019/archive/#/viewer/abstract/2061 (20 February 2020, date last accessed).
- 62. Adizie T, Elamanchi S, Prabu A. et al. Knowledge of features of inflammatory back pain in primary care in the West Midlands: a cross-sectional survey in the United Kingdom. Rheumatol Int 2018;38:1859–63. [DOI] [PubMed] [Google Scholar]
- 63. Sieper J, van der Heijde D, Landewe R. et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis International Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 64. Rudwaleit M, Landewe¥ R, van der Heijde D. et al. The development of Assessment of SpondyloArthritis International Society (ASAS) classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 65. Rojas-Vargas M, Munoz-Gomariz E, Escudero A. et al. First signs and symptoms of spondyloarthritis—data from an inception cohort with a disease course of two years or less (REGISPONSER-Early). Rheumatology (Oxford) 2009;48:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rudwaleit M, Van der Heijde D, Khan M, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis 2004;63:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van den Berg R, de Hooge M, Rudwaleit M. et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis International Society (ASAS)-cohort. Ann Rheum Dis 2013;72:1646–53. [DOI] [PubMed] [Google Scholar]
- 68. Habibi S, Doshi S, Sengupta R. THU0413 utility of the spade tool to identify axial spondyloarthritis in patients with chronic backpain. Ann Rheum Dis 2016;75:338.2. [Google Scholar]
- 69. Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D. Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015;74:1483–7. [DOI] [PubMed] [Google Scholar]
- 70. Rudwaleit M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol 2010;22:375–80. [DOI] [PubMed] [Google Scholar]
- 71. Braun J, Baraliakos X, Kiltz U, Heldmann F, Sieper J. Classification and diagnosis of axial spondyloarthritis–what is the clinically relevant difference? J Rheumatol 2015;42:31–8. [DOI] [PubMed] [Google Scholar]
- 72. Navarro-Compán V. An update on diagnosis and classification of axial spondyloarthritis. Curr Rheumatol Rep 2019;21:39. [DOI] [PubMed] [Google Scholar]
- 73. Rosenbaum JT. Evolving diagnostic criteria for axial spondyloarthritis. Ocul Immunol Inflamm 2016;24:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Strand V, Rao SA, Shillington AC. et al. Prevalence of axial spondyloarthritis in United States rheumatology practices: Assessment of SpondyloArthritis International Society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res (Hoboken) 2013;65:1299–306. [DOI] [PubMed] [Google Scholar]
- 75. Van Tubergen A, Heuft-Dorenbosch L, Schulpen G. et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis 2003;62:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hollingsworth P, Cheah P, Dawkins R. et al. Observer variation in grading sacroiliac radiographs in HLA-B27 positive individuals. J Rheumatol 1983;10:247–54. [PubMed] [Google Scholar]
- 77. Christiansen AA, Hendricks O, Kuettel D. et al. Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol 2017;44:70–7. [DOI] [PubMed] [Google Scholar]
- 78. Braun J, Sieper J, Bollow M. Imaging of sacroiliitis. Clin Rheumatol 2000;19:51–7. [DOI] [PubMed] [Google Scholar]
- 79. Yazici H, Turunc M, Ozdogan H. et al. Observer variation in grading sacroiliac radiographs might be a cause of ‘sacroiliitis’ reported in certain disease states. Ann Rheum Dis 1987;46:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sepriano A, Rudwaleit M, Sieper J. et al. Five-year follow-up of radiographic sacroiliitis: progression as well as improvement? Ann Rheum Dis 2016;75:1262–3. [DOI] [PubMed] [Google Scholar]
- 81. Maksymowych WP. The role of imaging in the diagnosis and management of axial spondyloarthritis. Nat Rev Rheumatol 2019;15:657–72. [DOI] [PubMed] [Google Scholar]
- 82. Robinson PC, Sengupta R, Siebert S. Non-radiographic axial spondyloarthritis (nr-axSpA): advances in classification, imaging and therapy. Rheumatol Therapy 2019;6:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weber U, Lambert RG, Østergaard M. et al. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty‐seven subjects. Arthritis Rheum 2010;62:3048–58. [DOI] [PubMed] [Google Scholar]
- 84. Deodhar A. Sacroiliac joint magnetic resonance imaging in the diagnosis of axial spondyloarthritis: “a tiny bit of white on two consecutive slices” may be objective, but not specific. Arthritis Rheumatol 2016;68:775–8. [DOI] [PubMed] [Google Scholar]
- 85. Weber U, Lambert RG, Østergaard M. et al. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 2010;62:3048–58. [DOI] [PubMed] [Google Scholar]
- 86. Weber U, Hodler J, Kubik RA. et al. Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 2009;61:900–8. [DOI] [PubMed] [Google Scholar]
- 87. Marzo-Ortega H, McGonagle D, O’Connor P. et al. Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann Rheum Dis 2009;68:1721–7. [DOI] [PubMed] [Google Scholar]
- 88. Weber U, Jurik AG, Zejden A. et al. OP0117 bone marrow oedema in sacroiliac joints of young athletes shows most frequently in the posterior inferior ilium. Ann Rheum Dis 2017;76:101.
- 89. Agten CA, Zubler V, Zanetti M. et al. Postpartum bone marrow edema at the sacroiliac joints may mimic sacroiliitis of axial spondyloarthritis on MRI. AJR Am J Roentgenol 2018;211:1306–12. [DOI] [PubMed] [Google Scholar]
- 90. Lambert RG, Bakker PA, van der Heijde D. et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 91. Bennett AN, Marzo-Ortega H, Kaur-Papadakis D, Rehman A. The use of magnetic resonance imaging in axial spondyloarthritis: time to bridge the gap between radiologists and rheumatologists. J Rheumatol 2017;44:780–5. [DOI] [PubMed] [Google Scholar]
- 92. Mandl P, Navarro-Compán V, Terslev L. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 93. Derakhshan MH, Pathak H, Cook D. et al. Services for spondyloarthritis: a survey of patients and rheumatologists. Rheumatology (Oxford) 2018;57:987–96. [DOI] [PubMed] [Google Scholar]
- 94. Bray TJP, Jones A, Bennett AN. et al. Recommendations for acquisition and interpretation of MRI of the spine and sacroiliac joints in the diagnosis of axial spondyloarthritis in the UK. Rheumatology (Oxford) 2019;58:1831–8. [DOI] [PubMed] [Google Scholar]
- 95. Fallahi S, Jamshidi AR. Diagnostic delay in ankylosing spondylitis: related factors and prognostic outcomes. Arch Rheumatol 2016;31:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Landewé R, Nurminen T, Davies O, Baeten D. A single determination of C-reactive protein does not suffice to declare a patient with a diagnosis of axial spondyloarthritis ‘CRP-negative’. Arthritis Res Ther 2018;20:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rudwaleit M, Haibel H, Baraliakos X. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 98. Haibel H, Rudwaleit M, Listing J. et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91. [DOI] [PubMed] [Google Scholar]
- 99. Sieper J, van der Heijde D, Dougados M. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kiltz U, Baraliakos X, Karakostas P. et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:1415–22. [DOI] [PubMed] [Google Scholar]
- 101. van der Heijde D, Kivitz A, Schiff MH. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46. [DOI] [PubMed] [Google Scholar]
- 102. Davis JC, Jr, Van Der Heijde D, Braun J, Dougados M. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230–6. [DOI] [PubMed] [Google Scholar]
- 103. van der Heijde D, Dijkmans B, Geusens P. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91. [DOI] [PubMed] [Google Scholar]
- 104. Inman RD, Davis JC, J., Heijde D. et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402–12. [DOI] [PubMed] [Google Scholar]
- 105. Deodhar A, van der Heijde D, Gensler LS. et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. [DOI] [PubMed] [Google Scholar]
- 106. Yong CY, Hamilton J, Benepal J. et al. Awareness of axial spondyloarthritis among chiropractors and osteopaths: findings from a UK Web-based survey. Rheumatol Adv Pract 2019;3(2):rkz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McCrum C. When to suspect spondyloarthritis: a core skill in musculoskeletal clinical practice. Musculoskelet Sci Pract 2019;44:102079. [DOI] [PubMed] [Google Scholar]
- 108. McCrum C, Kenyon K, Cleaton J, Dudley T. An unrecognised masquerader: a retrospective review of people presenting to musculoskeletal physiotherapy with undiagnosed spondyloarthritis. Physiotherapy 2019;105:e102–3. [Google Scholar]
- 109. Hall AM, Scurrey SR, Pike AE. et al. Physician-reported barriers to using evidence-based recommendations for low back pain in clinical practice: a systematic review and synthesis of qualitative studies using the Theoretical Domains Framework. Implement Sci 2019;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Piccoliori G, Engl A, Gatterer D. et al. Management of low back pain in general practice–is it of acceptable quality: an observational study among 25 general practices in South Tyrol (Italy). BMC Fam Pract 2013;14:148.24090155 [Google Scholar]
- 111. González-Urzelai V, Palacio-Elua L, López-de-Munain J. Routine primary care management of acute low back pain: adherence to clinical guidelines. Eur Spine J 2003;12:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Williams CM, Maher CG, Hancock MJ. et al. Low back pain and best practice care: a survey of general practice physicians. Arch Int Med 2010;170:271–7. [DOI] [PubMed] [Google Scholar]
- 113. Baker R, Lecouturier J, Bond S. Explaining variation in GP referral rates for x-rays for back pain. Implement Sci 2006;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mathieson HR, Merashli M, Gaffney K, Marzo-Ortega H; on behalf of BRITSpA (British Society for Spondyloarthritis). Poor awareness of inflammatory back pain and axial spondyloarthritis among secondary care specialists. Clin Rheumatol 2016;35:2627–8. [DOI] [PubMed] [Google Scholar]
- 115. Jordan A, Family H, Blaxall K, Begen FM, Sengupta R. Use of complementary and alternative medicine in axial spondyloarthritis: a qualitative exploration of self-management. J Clin Med 2019;8(5):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sengupta R, Cook D, Gaffney K. The importance of targeting education strategies for complementary therapists dealing with potential axial spondyloarthritis patients. Clin Exp Rheumatol 2014;32:803. [Google Scholar]
- 117.Gallup-Palmer College of Chiropractic Annual Report: Americans’ Perceptions of Chiropractic. 2015. https://www.palmer.edu/getmedia/8a750f7d-ccc4-4c32-accb-92aef43117ca/gallup-report-palmer-college.pdf (20 February 2020, date last accessed).
- 118. Lind BK, Diehr PK, Grembowski DE, Lafferty WE. Chiropractic use by urban and rural residents with insurance coverage. J Rural Health 2009;25:253–8. [DOI] [PubMed] [Google Scholar]
- 119. Weeks WB, Goertz CM, Meeker WC, Marchiori DM. Public perceptions of doctors of chiropractic: results of a national survey and examination of variation according to respondents’ likelihood to use chiropractic, experience with chiropractic, and chiropractic supply in local health care markets. J Manipulative Physiol Ther 2015;38:533–44. [DOI] [PubMed] [Google Scholar]
- 120. Deyo RA, Tsui-Wu Y-J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987;12:264–8. [DOI] [PubMed] [Google Scholar]
- 121. Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS. Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine 2003;28:292–7. [DOI] [PubMed] [Google Scholar]
- 122. Jordan A, Family H, Blaxall K, Begen FM, Sengupta R. Use of complementary and alternative medicine in axial spondyloarthritis: a qualitative exploration of self-management. J Clin Med 2019;8:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Braun J, Mosch T, Fischer I, Kiltz U. Identifikation von Patienten mit axialer Spondyloarthritis in der Primärversorgung (AWARE-Studie). Zeitschrift für Rheumatologie 2019;78:568–76. [DOI] [PubMed] [Google Scholar]
- 124. Jordan K, Clarke AM, Symmons DPM. et al. Measuring disease prevalence: a comparison of musculoskeletal disease using four general practice consultation databases. Br J Gen Pract 2007;57:7–14. [PMC free article] [PubMed] [Google Scholar]
- 125. Spondyloarthritis in over 16s: diagnosis and management: NICE guideline. National Institute for Health and Care Excellence, 2017. https://www.nice.org.uk/guidance/ng65 (20 February 2020, date last accessed). [PubMed]
- 126. Martindale J, Goodacre L. The journey to diagnosis in AS/axial SpA: the impact of delay. Musculoskeletal Care 2014;12:221–31. [DOI] [PubMed] [Google Scholar]
- 127. Evans J, Sapsford M, Raine T. et al. AB0719 prevalence of undiagnosed axial spondyloarthritis in patients with inflammatory bowel disease: a systematic literature review and primary research study. Ann Rheum Dis 2019;78(Suppl 2):1822–3. [Google Scholar]
- 128. Karreman MC, Luime JJ, Hazes JMW, Weel A. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2016;11:631–42. [DOI] [PubMed] [Google Scholar]
- 129. Stolwijk C, Pierik M, Landewé R, Masclee A, van Tubergen A. Prevalence of self-reported spondyloarthritis features in a cohort of patients with inflammatory bowel disease. Can J Gastroenterol 2013;27:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fragoulis GE, Liava C, Daoussis D. et al. Inflammatory bowel diseases and spondyloarthropathies: from pathogenesis to treatment. World J Gastroenterol 2019;25:2162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Avalos-Díaz E, Cherit JD, Herrera-Esparza R. Cutaneous manifestations of spondyloarthritis. Int J Clin Rheumatol 2012;7:55–61. [Google Scholar]
- 132. Schneider-Burrus S, Witte-Haendel E, Christou D. et al. High prevalence of back pain and axial spondyloarthropathy in patients with hidradenitis suppurativa. Dermatology 2016;232:606–12. [DOI] [PubMed] [Google Scholar]
- 133. Rondags A, Arends S, Wink FR, Horváth B, Spoorenberg A. High prevalence of hidradenitis suppurativa symptoms in axial spondyloarthritis patients: A possible new extra-articular manifestation. Semin Arthritis Rheum 2019;48:611–7. [DOI] [PubMed] [Google Scholar]
- 134. Haroon M, O’Rourke M, Ramasamy P, Murphy CC, FitzGerald O. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool). Ann Rheum Dis 2015;74:1990–5. [DOI] [PubMed] [Google Scholar]
- 135. Sykes MP, Hamilton L, Jones C, Gaffney K. Prevalence of axial spondyloarthritis in patients with acute anterior uveitis: a cross-sectional study utilising MRI. RMD Open 2018;4:e000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chan J, Sari I, Salonen D. et al. Prevalence of sacroiliitis in inflammatory bowel disease using a standardized computed tomography scoring system. Arthritis Care Res (Hoboken) 2018;70:807–10. [DOI] [PubMed] [Google Scholar]
- 137. Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol 2006;20:401–17. [DOI] [PubMed] [Google Scholar]
- 138. Braun A, Saracbasi E, Grifka J, Schnitker J, Braun J. Identifying patients with axial spondyloarthritis in primary care: how useful are items indicative of inflammatory back pain? Ann Rheum Dis 2011;70:1782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. MRE as a Screening Tool for axSpA in IBD. Identifier: NCT03817983 ClinicalTrials.gov: ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT03817983 (20 February 2020, date last accessed).
- 140. Jadon DR, Sengupta R, Nightingale A. et al. Axial disease in psoriatic arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 2017;76:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Maksymowych WP, Carmona R, Yeung J. et al. THU0393 performance of the ASAS classification criteria presenting with undiagnosed back pain? Data from the screening in axial spondyloarthritis in psoriasis, iritis, and colitis (SASPIC) cohort. Ann Rheum Dis 2019;78(Suppl 2):482. [Google Scholar]
- 142. Maksymowych WP, Carmona R, Yeung J. et al. SAT0339 what is the impact of imaging on diagnostic ascertainment of patients presenting with undiagnosed back pain and what is the impact of central evaluation? Data from the screening in axial spondyloarthritis in psoriasis, iritis, and colitis (SASPIC) cohort. Ann Rheum Dis 2019;78(Suppl 2):1248–9. [Google Scholar]
- 143. Waters L, Blanckley S, Fountain J, Goodson NJ. THU0480 screening for the presence of sacroiliitis is recommended for young patients undergoing HIP arthroplasty. Ann Rheum Dis 2015;74:373. [Google Scholar]
- 144. Abawi O, van den Berg R, van der Heijde D, van Gaalen FA. Evaluation of multiple referral strategies for axial spondyloarthritis in the SPondyloArthritis Caught Early (SPACE) cohort. RMD Open 2017;3:e000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Poddubnyy D, Vahldiek J, Spiller I. et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol 2011;38:2452–60. [DOI] [PubMed] [Google Scholar]
- 146. Jamal M, Korver AM, Kuijper M. et al. The IMPACT study: A clustered randomized controlled trial to assess the effect of a referral algorithm for axial spondyloarthritis. PLoS one 2020;15:e0227025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Brandt HC, Spiller I, Song I-H. et al. Performance of referral recommendations in patients with chronic back pain and suspected axial spondyloarthritis. Ann Rheum Dis 2007;66:1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kiltz U, Landewé RBM, van der Heijde D. et al. Development of ASAS quality standards to improve the quality of health and care services for patients with axial spondyloarthritis. Ann Rheum Dis 2020;79:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Carvalho PD, Machado PM. How to investigate: early axial spondyloarthritis. Best Pract Res Clin Rheumatol 2019;33:101427. [DOI] [PubMed] [Google Scholar]
- 150. van Onna M, Gorter S, Maiburg B, Waagenaar G, van Tubergen A. Education improves referral of patients suspected of having spondyloarthritis by general practitioners: a study with unannounced standardised patients in daily practice. RMD Open 2015;1:e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. van Onna M, Gorter S, Maiburg B, Waagenaar G, van Tubergen A. GPs’ patterns of clinical assessment when faced with a patient suspected for spondyloarthritis: a prospective educational intervention study. BJGP Open 2017;1:bjgpopen17X100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Poddubnyy D, Sieper J. Current unmet needs in spondyloarthritis. Curr Rheumatol Rep 2019;21:43. [DOI] [PubMed] [Google Scholar]
- 153. Rudwaleit M, Feldtkeller E, Sieper J. Easy assessment of axial spondyloarthritis (early ankylosing spondylitis) at the bedside. Ann Rheum Dis 2006;65:1251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]