Abstract
In recent years, significant progress has been made in improving the early diagnosis of spondyloarthritides (SpA), including axial SpA. Nonetheless, there are still issues related to the application of classification criteria for making the primary diagnosis of SpA in the daily practice. There are substantial conceptional and operational differences between the diagnostic vs classification approach. Although it is not possible to develop true diagnostic criteria for natural reasons as discussed in this review, the main principles of the diagnostic approach can be clearly defined: consider the pre-test probability of the disease, evaluate positive and negative results of the diagnostic test, exclude other entities, and estimate the probability of the disease at the end. Classification criteria should only be applied to patients with an established diagnosis and aimed at the identification of a rather homogeneous group of patients for the conduction of clinical research.
Keywords: axial spondyloarthritis, ankylosing spondylitis, classification, diagnosis, magnetic resonance imaging
Rheumatology key messages
Diagnostic and classification approaches have conceptual and operational differences.
The diagnostic approach is aimed at the estimation of the probability of a suspected disease.
The classification approach should be applied to patients with an established diagnosis to define a research group.
Introduction
Spondyloarthritis (SpA) is a term covering a family of diseases with similar clinical and genetic characteristics such as involvement of the axial skeleton, association with HLA-B27 antigen, typical involvement of peripheral joints (asymmetric oligoarthritis, enthesitis, dactylitis), as well as common extra-musculoskeletal manifestations such as acute anterior uveitis, psoriasis and inflammatory bowel disease [1, 2]. Based on the dominant clinical leading symptom, patients with SpA can be divided into two groups: (i) axial SpA – mainly axial symptoms (back pain, morning stiffness in the spine); and (ii) peripheral SpA – predominantly peripheral symptoms such as arthritis or enthesitis or dactylitis.
Axial SpA includes two forms, which can also be considered as two stages of the same disease: the non-radiographic axial SpA (i.e. axial SpA without definite radiographic sacroiliitis fulfilling the radiographic criterion of the modified New York criteria [3]) and radiographic axial SpA (also referred to as ankylosing spondylitis, both terms are being currently used interchangeably [4]).
Peripheral SpA encompasses forms of SpA with predominant peripheral musculoskeletal involvement (arthritis, enthesitis and dactylitis). As already mentioned, both axial and peripheral SpA can be associated with psoriasis that results in a natural overlap with psoriatic arthritis.
The diagnosis of SpA (and especially of axial SpA) is still frequently delayed by many years [5, 6]. The introduction of MRI of the axial skeleton in the diagnostic and classification approaches in axial SpA substantially improved early diagnosis, as the disease can be detected before the development of structural damage visible on X-rays. At the same time, serious concerns have been raised in recent years about the specificity of the method and related false-positive calls. This problem is directly related to an even bigger issue of classification criteria application for making the diagnosis of SpA in clinical practice. In this review, we discuss differences between the diagnostic and classification approaches in SpA with a focus on axial SpA.
Which parameters are relevant for the diagnosis and classification of spondyloarthritis?
Not surprisingly, the diagnostic and the classification approaches operate with the same set of parameters that are usually obtained during the routine diagnostic workout.
Clinical parameters
Back pain
Back pain (in the majority of cases – low back pain) is a leading symptom of axial SpA and can also be present in peripheral SpA, though peripheral manifestations dominate in the latter case. Because the disease usually starts in the 3rd or 4th life decade and has a chronic course, the presence of back pain lasting for three months or longer (chronic back pain) and back pain onset before 45 years of age is an important starting point for the identification of patients with a high probability of axial SpA in the primary care [7], for initiation of the diagnostic procedures [8, 9] and for the classification of patients according to the Assessment of Spondyloarthritis International Society (ASAS) criteria for axial SpA [10]. The estimated prevalence of axial SpA among patients with chronic back pain is ∼5% [11], while in patients with so-called ‘inflammatory back pain’, the probability of axial SpA can go up to ∼30% [12]. Inflammatory back pain is a syndrome describing back pain with specific characteristics that is frequently (but not necessarily) related to an inflammatory process in the axial skeleton. The following symptoms are typical for inflammatory back pain: insidious onset; improvement with exercise; no improvement with rest; pain at night, especially in the 2nd half of the night; morning stiffness in the back of 30 minutes or more; and alternating buttock pain.
Usually, the majority of the symptoms should be present to make a clinical conclusion about the presence of inflammatory back pain. For clinical studies, three sets of classification criteria for inflammatory back pain [13–15] were developed, with broadly comparable performance in terms of sensitivity and specificity.
In a recent systematic evaluation of the diagnostic performance of inflammatory back pain, the sensitivity for the diagnosis of axial SpA ranged 74.4%–81.1% across the different criteria set, whereas the specificity was rather low, with a range 25.1%–43.9% [16]. These data were recently confirmed in an independent cohort [17]. Thus, although the presence of inflammatory back pain alone is not sufficient for the diagnosis of SpA, its presence is an important initial step in the diagnostic approach and can be used for the preselection of patients with a high probability of axial SpA among patients with chronic back pain [7].
Good response to non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are considered as the first-line therapy in patients diagnosed with axial SpA because of a strong effect on main symptoms (back pain and stiffness) that is confirmed in a number of clinical trials [18]. The usual definition of a good response to NSAIDs is incorporated in the classification criteria: 24–48 h after a full dose of an NSAID, back pain is not present anymore or is much better [10]. A good response to NSAIDs is frequently considered in the context of inflammatory back pain, and it can be expected that the presence of inflammatory back pain related to axial SpA increases the probability of the presence of good treatment response to anti-inflammatory therapy.
Peripheral manifestations (arthritis, enthesitis, dactylitis)
In contrast to rheumatoid arthritis, the SpA-like arthritis is a mono- or oligoarthritis involving predominantly lower extremities, although a polyarticular disease is also possible. Arthritis in SpA is normally non-erosive and is considered to be secondary to enthesitis in SpA [19]. The frequency of peripheral arthritis in SpA varies in a substantial range (26–62%) and seems to be more common in the South American population as compared with the European one [8]. The involvement of hip joints (coxitis) is frequently referred to as axial instead of peripheral disease. In contrast to other forms of SpA-associated peripheral arthritis, it might have a severe destructive course and is often associated with younger age of disease onset and more severe axial disease [20].
Inflammation of the entheses, or enthesitis, can potentially affect any entheseal structure, including axial and peripheral regions of the musculoskeletal system; however, in the context of diagnosis/classification of SpA, usually peripheral enthesitis is considered. The clinical diagnosis of enthesitis might be challenging; ultrasound and MRI can be helpful in objective confirmation of entheseal inflammation.
Dactylitis is a typical manifestation of SpA with inflammation of all joints of a finger or a toe accompanied by tendonitis. Similarly to peripheral arthritis, there are substantial geographic differences in the frequency of enthesitis and dactylitis that result in a substantial variation of the sensitivity of these parameters for the diagnosis of SpA. At the same time, the specificity of enthesitis (around 90% for heel enthesitis) and of dactylitis (around 96%) is rather high [8, 21].
Extra-musculoskeletal manifestations (uveitis, psoriasis, inflammatory bowel disease)
The most common (occurring in the course of the disease in about 20% of patients with SpA [8]) extra-musculoskeletal manifestation of SpA is acute anterior uveitis (iritis) that is usually unilateral and responds well to local treatment, though severe, frequently recurrent and treatment-refractory cases are possible. Psoriasis can be found in about 10% of patients with SpA [8, 22], while clinically manifest inflammatory bowel disease (Crohn’s disease or ulcerative colitis) is present in 2% to 7% of SpA patients [8, 22].
Positive family history
Given an association of SpA with a certain genetic background (with HLA-B27 being the strongest contributor followed by interleukin-23 receptor and endoplasmic reticulum aminopeptidase 1 genes [23]), it is not surprising that the presence of SpA and related disorders (anterior uveitis, psoriasis, inflammatory bowel disease) in 1st and 2nd-degree relatives is associated with an increased risk of SpA. For the same reason, family history cannot be considered an independent diagnostic parameter for SpA, and the diagnostic value of this parameter is low if HLA-B27 status is known [24]. This might, however, be different in regions with a weaker association of SpA with HLA-B27-positivity.
Lab parameters
So far, only HLA-B27 and acute phase reactants are used routinely in the diagnosis and classification of SpA. A high prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial SpA was reported a few years ago [25]. In subsequent studies, low specificity of anti-CD74 antibodies was demonstrated that resulted in a conclusion of a low diagnostic value of the test [26]. The diagnostic value of the test might, however, be higher in some regions (e.g. the Middle East and North Africa) with a low HLA-B27 prevalence among SpA patients [27].
HLA-B27
This is one of the most important diagnostic tests in the Caucasian population due to high sensitivity (>80% for axial SpA, lower for peripheral SpA) and specificity (∼90% in the central European population based on the estimated background prevalence of HLA-B27 of about 9% [28]) [8]. However, there are substantial geographic differences in both the background population prevalence of HLA-B27 and the strength of association with the disease that substantially affects the diagnostic value of the test across the globe [29].
Acute phase reactants
CRP in serum and ESR as markers of systemic inflammation are usually included in the routine diagnostic workup in the case of suspicion of an inflammatory disease such as SpA. Although the specificity of these markers (especially of CRP) is rather high [8], the sensitivity is low: CRP is elevated in 50–60% of the patients with radiographic axial SpA and 30–40% of the patients with non-radiographic axial SpA only [22].
Imaging
Imaging is an essential part of the diagnostic workup in axial SpA because clinical signs of affection of the axial skeleton are rather non-specific.
As a first-line imaging assessment in suspected axial SpA, an X-ray examination of the sacroiliac joints (pelvis) is still recommended [30]. This is related to the fact that conventional radiography is widely available and is associated with relatively low costs (compared with CT or MRI). The presence of definite radiographic sacroiliitis (bilateral grade II or unilateral grade III – according to the grading system of the modified New York criteria [3]) has a high specificity (>90%) for the diagnosis of axial SpA, although the sensitivity is low, especially in patients with short symptom duration (around 30% in patients with symptom duration less than one year and about 50% in patients with symptom duration 2–6 years [31]). Another important issue related to this method is low reliability related to the complex anatomy and individual variability of the appearance of sacroiliac joints on conventional radiographs.
Although CT of sacroiliac joints allows a more precise (compared with standard X-rays) assessemnt of structural changes, it is not suitable for early SpA diagnostics because active inflammation cannot be depicted. Furthermore, this method is associated with a relatively high radiation exposure if conventional CT is applied. The diagnostic significance of skeletal scintigraphy [32], ultrasound [33] and positron emission tomography [34] for the early diagnosis of axial SpA is low or unclear and, therefore, these methods are not recommended for use in daily clinical practice.
If there is no evidence of sacroiliitis on conventional radiographs and if axial SpA is still suspected, an MRI examination of the sacroiliac joints (with or without spine, depending on clinical presentation and suspected differential diagnosis) is usually considered [30]. For the diagnosis of axial SpA itself, MRI of the spine does not add much to MRI of sacroiliac joints [35]. The major advantage of MRI is in the detection of active inflammatory changes (osteitis or bone marrow oedema), which occur months to years before structural damage is visible on X-rays. On the other hand, structural changes in the sacroiliac joints are also well captured with MRI.
Osteitis/bone marrow oedema can be captured with the following MRI sequences: Short Tau Inversion Recovery (STIR, other terms are TIRM and SPIR) sequence or a T2-weighted sequence with fat saturation [36]. Contrast-enhanced sequences do not normally increase the sensitivity or specificity of MRI for the detection of active sacroiliitis [37]. Structural changes in sacroiliac joints (such as erosions, fat lesions, backfill, bode buds, ankylosis and sclerosis) can be visualized using the T1-weighted sequence [36]. In daily practice, the combination of STIR and T1-weighted sequences is usually sufficient for diagnostic purposes when axial SpA is suspected. Recently, a volumetric interpolated breath-hold examination (VIBE) sequence was found to be comparable to computer tomography (a gold standard for detection of bony changes) for detection of erosions that might be highly relevant for the differential diagnosis of axial and contextual interpretation of bone marrow oedema and that is not always well captured by conventional STIR and T1-weighted sequences [38].
The Assessment of Spondyloarthritis International Society (ASAS) has developed and recently updated the definition of active SpA-related sacroiliitis on MRI (ASAS definition of positive MRI of sacroiliac joints) [36, 39, 40]. Importantly, this definition is intended for use as a part of the classification and not the diagnostic approach. According to this definition, MRI of sacroiliac joints is positive if there is bone marrow oedema clearly present in a typical anatomical area (subchondral bone), and the appearance of bone marrow oedema is highly suggestive of SpA [36]. Other active inflammatory changes such as enthesitis, capsulitis, joint space enhancement, inflammation at the site of erosion, and joint space fluid can also be manifestations of SpA-related inflammatory involvement of sacroiliac joints.
There is currently no widely accepted definition of structural changes on MRI of sacroiliac joints compatible with SpA. Nevertheless, the presence of structural changes such as erosions, fat lesions, and/or ankylosis supports the diagnosis of axial SpA, even if active inflammatory changes are not present at the time of examination. Furthermore, structural changes provide important contextual information for the interpretation of bone marrow oedema as suggestive of SpA [36, 40].
Although the sensitivity of MRI for the detection of changes (active inflammatory and structural) compatible with SpA is considered to be high, there are concerns related to the specificity of this imaging method in light of the fact that subchondral bone marrow oedema might also be a consequence of mechanical stress in the sacroiliac joints and, therefore, not related to an inflammatory disorder [40]. Indeed, several recent publications have reported that subchondral bone marrow oedema can also occur in healthy volunteers [41, 42] and in athletes [41, 43], giving a current estimation of the specificity of the method on the level of 80–85%.
In summary, although imaging should not receive the all-decisive role in the diagnostic approach for axial SpA (especially in the light of specificity issues described below), one point should be clearly stated – currently, imaging is the only method to detect inflammatory changes in the axial skeleton objectively that is essential if we make a diagnosis of an inflammatory condition affecting this part of the musculoskeletal system.
ASAS has recently developed a new educational project: the ASAS Case Library (https://cases.asas-group.org/), in which imaging findings are discussed in detail in the context of clinical findings and laboratory test results.
X-ray, MRI and CT of the spine play a secondary role in the early diagnosis of a SpA, because active and chronic changes in the spine usually occur later than in the sacroiliac joints. However, the use of imaging of the spine may be necessary in the context of differential diagnosis (e.g. with the degenerative spinal disease). The specificity of imaging findings in the spine seems to be lower compared with the sacroiliac joints [42]. The ASAS definition of a positive spinal MRI (the presence of at least three active inflammatory lesions in the vertebral bodies corresponding to anterior or posterior spondylitis) [44] was developed again for classification and not for diagnostic purposes.
Classification approach in spondyloarthritis
More than ten years ago, ASAS developed classification criteria for axial and peripheral SpA [10, 45, 46] that were intended to replace older sets of SpA criteria such as Amor and the European Spondyloarthropathy Study Group (ESSG) criteria. The ASAS criteria for axial SpA cover both non-radiographic and radiographic axial SpA (ankylosing spondylitis) and have, therefore, potential to replace also the modified New York criteria for ankylosing spondylitis [3].
It is important to emphasize that classification criteria are not intended for use in clinical practice for the primary diagnostic purpose (the diagnosis should be already established) and should not be considered synonymous with diagnostic criteria.
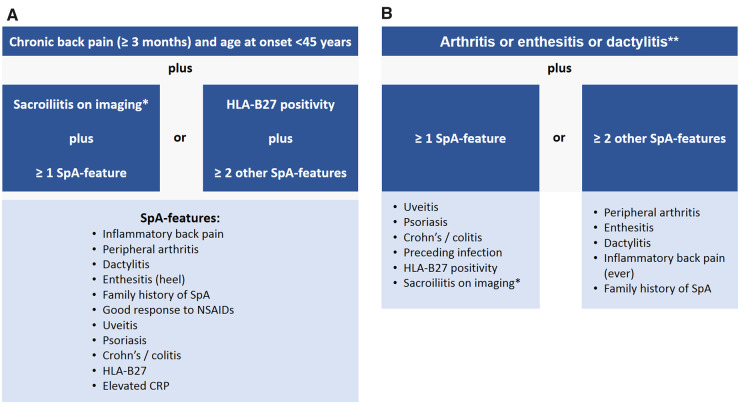
The starting point of the ASAS classification criteria for axial SpA [10, 45] is the presence of chronic back pain (duration >3 months) and onset of back pain before the age of 45 (Fig. 1A). Patients can meet the criteria either via the imaging arm or the clinical arm. The imaging arm requires the presence of sacroiliitis (radiographic—according to the modified New York criteria [3] or MRI—according to the ASAS definition [36, 39, 40]) in conjunction with at least one typical SpA parameter. The clinical arm was originally intended for situations when imaging is not available. The criteria are met if the HLA-B27 is positive and at least two other SpA parameters are present. The overall sensitivity of the criteria is 82.9%, the specificity: 84.4%, with the imaging arm performing slightly better (sensitivity: 66.2%, specificity: 97.3%) than the clinical arm (sensitivity: 56.6%, specificity: 83.3%) [10]. Recently, ASAS and the North American Spondyloarthritis Research and Treatment Network (SPARTAN) started a new study (Classification of Axial Spondyloarthritis Inception Cohort – CLASSIC) aimed at validation and possibly improvement of the specificity of the ASAS classification criteria.
Fig. 1.
ASAS classification criteria for axial and peripheral spondyloarthritis
(A) ASAS classification criteria for axial spondyloarthritis; (B) ASAS classification criteria for peripheral spondyloarthritis. *Sacroiliitis on imaging refers to definite radiographic sacroiliitis according to the modified New York criteria or active sacroiliitis on MRI according to the ASAS definition. **Peripheral arthritis: usually predominantly lower limbs and/or asymmetric arthritis; enthesitis: clinically assessed; dactylitis: clinically assessed. SpA: spondyloarthritis.
The ASAS classification criteria for peripheral SpA (Fig. 1B) [46] are intended for patients with predominant peripheral manifestations (SpA-typical arthritis or enthesitis or dactylitis). Overall, the same parameters can be found in the axial and peripheral criteria sets except for preceding infection in the peripheral SpA set that should cover cases of reactive arthritis, usually manifesting with peripheral involvement. The criteria are met if one or two other SpA parameters are present in addition to arthritis and/or enthesitis and/or dactylitis. The criteria for peripheral SpA have a sensitivity of 77.8% and a specificity of 82.2% [46].
Diagnostic approach in spondyloarthritis
Classification criteria are frequently but wrongly used in the daily clinical practice to confirm or to exclude a diagnosis that is particularly true for axial SpA. There are several important differences between the classification and diagnostic approaches [47, 48] – Table 1.
Table 1.
The main differences between the diagnostic and the classification approaches
| Diagnostic approach | Classification approach | |
|---|---|---|
| Aim | To establish the diagnosis of a disease in clinical practice | To define a homogeneous group of patients for research purposes |
| The starting point | Suspicion of a disease with a certain level of a pre-test probability | Established diagnosis of a disease |
| Differential diagnoses or other conditions that might explain symptoms | Always considered | Not considered |
| Values of the positive diagnostic tests | Different and depend on the test itself, earlier screening or diagnostic tests performed, geographic region and background population | Few levels with the same value of parameters on the same level |
| Values of the negative diagnostic tests | Negative test results are considered; their diagnostic values depend on the same factors as for positive test results | Not considered except the situation that there are not enough positive test results to fulfil the criteria |
| Outcome | Probability of the disease presence | Yes or no answer (classification criteria fulfilled or not fulfilled) with a certain level of sensitivity and specificity |
| External reference (‘gold standard’) | None | Expert opinion derived during classification criteria development |
The first difference is related to the overall aim of the application of the respective approach. The diagnostic approach is applied to identify the disease, causing certain symptoms. In the case of suspicion of axial SpA, this is usually back pain; in peripheral SpA – peripheral musculoskeletal symptoms. The classification approach aims to establish a homogeneous patient population for the sake of clinical research. Therefore, there is a major difference in terms of the starting point in both approaches. With the diagnostic approach, the doctor faces a patient without an established diagnosis but with symptoms, which results in suspicion of a certain disease (or a group of the diseases) that can be called the pre-test-probability. The pre-test probability reflects the probability of a disease in a patient presenting with suspicious symptoms. For instance, in a broad population of patients with chronic back pain, the probability of axial SpA is estimated at the level of 5% [8, 49]. However, it is rather unusual that rheumatologists are dealing with an unselected population of patients with chronic back pain. A much more frequent scenario is a pre-selected patient population based on certain referral rules resulting in an increase of the pre-test probability from 5% to up to 30–40% [7]. In the classification approach, the diagnosis should be established before the application of the classification criteria; i.e. ASAS classification criteria for axial SpA cannot be applied to a patient without an established diagnosis of axial SpA.
The next important point is dealing with differential diagnoses. In the classification approach, other potential explanations of back pain are not considered because of the starting point – an established diagnosis of axial SpA responsible for patient symptoms. In the diagnostic approach, other potential explanations of back pain (such as degenerative disorders, infection, tumour, etc.) should certainly be ruled out to make the diagnosis of axial SpA.
Different diagnostic tests applied in the diagnostic approach have different diagnostic value. The sensitivity and specificity of the test results can be combined in so-called positive and negative likelihood ratios (LR+ and LR) using the following formulae:
The higher the value of LR+, the higher the probability of diagnosis if the parameter is positive; the lower the value of LR, the lower the probability of diagnosis if the parameter is negative [8, 21, 47, 50]. Around 15 years ago Rudwaleit et al. proposed a diagnostic approach based on multiplication of the corresponding LRs; the resulting LR product can be converted in a disease probability [21]. The disease probability estimation can also be done using a simplified addition approach proposed a few years later [50]. There are, however, several issues related to these approaches. Firstly, the sensitivity and the specificity of the test represent averaged values obtained from the literature. It can be expected that there is a substantial variation of the diagnostic performance of the tests depending on the background population (e.g. HLA-B27 has high sensitivity and specificity in the Caucasian population that is different in North African and Arabian populations) and referral practices (e.g. use of inflammatory back pain as a test for the preselection of patients with a high probability of axial SpA on the primary level diminishes the diagnostic value of the test on the rheumatologist level). Secondly, the pre-test probability of 5% is assumed in this approach that is obviously only true for the unselected population of patients with chronic back pain. Finally, the parameters are considered independent from each other, which is frequently not the case as discussed above; for instance, the value of the family history as a SpA parameter is dependent on whether or not the HLA-B27 status is known. The same limitations are related to simplified algorithm approaches – the original one [8] and the ASAS-modified one [9].
In the classification approach, test parameters of the same level have the same value (e.g. inflammatory back pain and presence of peripheral arthritis), while the negative results of the tests are not considered. Negative results of the tests can also be ignored sometimes in the diagnostic approach – in the case of manifestations that are not necessarily present at the beginning of the disease and can evolve over time, such as peripheral and extra-musculoskeletal manifestations of SpA.
Finally, the outcome of the diagnostic approach is the diagnosis with a certain level of probability that can be expressed in percent or in categories such as diagnosis is unlikely, uncertain, possible, likely, etc. Often, the rheumatologist starts in a situation where the diagnosis is possible, but other tests are needed to confirm (or to exclude) it. There is normally no external reference for the diagnosis; the rheumatologist’s opinion is, in fact, the ‘gold standard’ that can change over time with increasing experience and external factors such as new developments or concepts related to the diagnosis of a disease.
In the classification approach, the outcome is the fulfilment of the classification criteria (‘yes’ or ‘no’) with a certain level of sensitivity and specificity. The sensitivity and specificity are calculated on the basis of a ‘gold standard’ that is the diagnosis established by an expert who included patients in the study on the development and/or validation of the classification criteria. Obviously, the ‘gold standard’ might be very heterogeneous and might also change over time for the reasons mentioned above. Thus, classification criteria should only be applied to patients with an established diagnosis.
So far, we discussed the diagnostic and classification approaches in a cross-sectional way: one patient, one moment in time (within several days to weeks needed to collect all the relevant information). The cross-sectional view is correct within the classification approach because recruitment for a particular research project has normally limited duration; however, factor time sometimes plays an important role in the diagnostic process. There are progressive conditions (such as pregnancy, cancer), in which time is the main factor that can solve initial doubts (if any) in the diagnosis. In axSpA, the situation is more complicated because disease progression can occur slowly, the structural damage in the sacroiliac joints and spine is only one—though important—diagnostic parameter, and the disease can have a course with relapses and long remission periods. Nonetheless, new manifestation can occur over time that would increase the probability of axSpA, while stable course, no structural damage, and no objectively detected inflammatory changes measured at different time points would decrease the probability of axSpA.
There are also several additional factors that are often taken into account in the daily practice and which can affect the outcome of the diagnostic approach. These factors are related to therapeutic and prognostic consequences of not making (are there risks associated with a ‘wait and see’ strategy?) or making (are there additional therapeutic options and what is their risk/benefit ratio?) a diagnosis at the time point of the diagnostic evaluation.
Due to variations in the pre-test probability and the diagnostic value, it is virtually impossible to develop a universal diagnostic tool/diagnostic criteria for a certain disease. It is possible, however, to establish a diagnostic framework that can be adapted to local conditions taking different pre-test probabilities and different values of diagnostic tests into account.
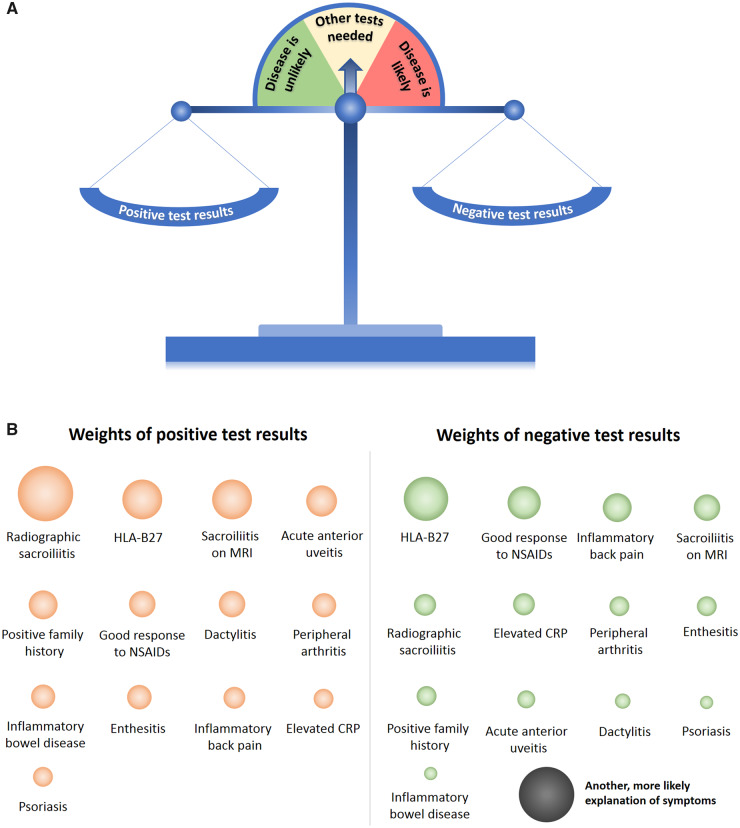
There have been several attempts to visualize the diagnostic process; one of the examples is the well-known diagnostic pyramid [47]. Another possibility to depict the diagnostic process is a ‘diagnostic scale’ – Fig. 2. The scale weighs positive results of the diagnostic tests against negative test results (and the presence of another, more likely explanation of symptoms) with the starting point of an undetermined situation, in which other tests are needed. With the increasing number of positive or negative weights, the situation might come to an unlikely or to a likely diagnosis, but can also remain somewhat undetermined, even if all available tests are applied. The latter case happens in the clinical practice and normally requires a follow-up of a patient – the time factor as discussed above.
Fig. 2.
The diagnostic scale and weights of diagnostic test results for axial spondyloarthritis
The balanced scale (A) indicates an undetermined diagnostic situation, in which other tests are normally needed. Positive and negative test results depending on their weights would change the balance towards more or less likely diagnosis. The weights of the positive and negative diagnostic tests (B) reflected by the size of the balls represent examples based on approximation of the literature data [8]. The real diagnostic weight might be different depending on the region, background population, and referral structures – see article text for further details. An alternative, more likely explanation of symptoms (differential diagnosis), is attributed to the weights of negative test results. SpA: spondyloarthritis.
The differences between the diagnostic and classification approaches described above can be illustrated by the following two clinical cases.
Patient A is a 55-year-old male patient referred by an ophthalmologist because of an episode of acute anterior uveitis and inflammatory back pain of intermittent intensity for about 15–20 years. The last uveitis flare occurred two months ago, the first one – 10 years ago with a flare-free time in between. No other SpA manifestations, no family history. No history of NSAIDs intake in an adequate dose; thus, no judgement on the NSAIDs response was possible. HLA-B27 was positive. The serum level of CRP was within the normal range.
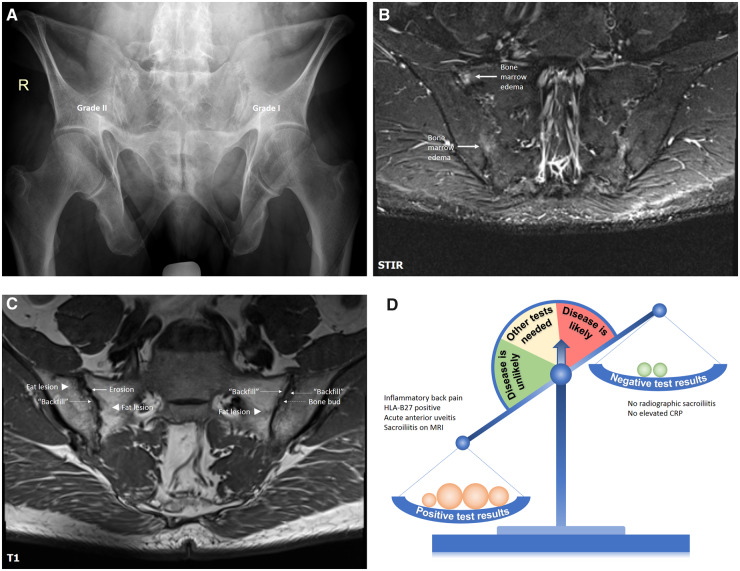
Conventional radiography of sacroiliac joints (Fig. 3A) showed subchondral sclerosis and erosive changes in the right sacroiliac joint corresponding to grade II and suspicious changes corresponding to grade I in the upper portion of the left sacroiliac joint with some overlap with intestinal gas. MRI-STIR of the sacroiliac joints (Fig. 3B) showed bone marrow oedema (osteitis) in the right sacroiliac joint, while MRI-T1 (Fig. 3C) demonstrated prominent structural changes compatible with SpA. Overall, the presence of inflammatory back pain, HLA-B27, uveitis and MRI changes compatible with SpA clearly outweighs the negative test results – the absence of radiographic changes and the negative CRP (Fig. 3C). The absence of peripheral manifestations and other extra-articular manifestations can be ignored as discussed above; the same is also true for the family history if HLA-B27 status is known. The diagnosis of axial SpA can be made in this case, the ASAS classification criteria for axial SpA are also fulfilled.
Fig. 3.
Conventional radiography, MRI-STIR, MRI-T1 of sacroiliac joints, and a diagnostic scale for patient A
A 55-year-old male patient referred by an ophthalmologist because of an episode of acute anterior uveitis and inflammatory back pain of intermittent intensity for about 15 to 20 years. HLA-B27 is positive, CRP is normal. In addition to some suspicious radiographic changes, MRI of sacroiliac joints showed definite active inflammatory and structural changes compatible with SpA. A diagnosis of axial SpA was made. See the article text for further details. SpA: spondyloarthritis; STIR: short tau inversion recovery.
Patient B has a very similar history. This is a 53-year-old male also who had been referred by an ophthalmologist because of acute anterior uveitis and inflammatory back pain of intermittent intensity with onset 20 years ago. There were two episodes of unilateral acute anterior uveitis in recent years and another three episodes over the last 20 years. No other SpA manifestations, no family history. No history of NSAIDs intake. HLA-B27 was positive. The serum level of CRP was within the normal range.
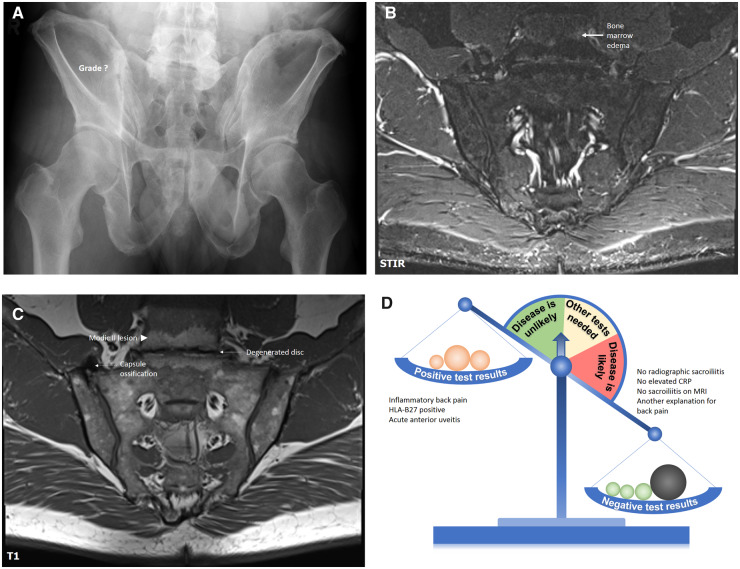
Conventional radiography of sacroiliac joints (Fig. 4A) showed suspicious changes in the right sacroiliac joint – subchondral sclerosis and no clearly visible joint space in the upper portion of the joint that can be potentially related to true bony ankylosis as a manifestation of SpA but can also represent degenerative changes – a bulky osteophyte or a capsule ossification mimicking true ankylosis. MRI-STIR (Fig. 4B) showed no active inflammatory changes in the sacroiliac joints, but some bone marrow oedema in the vertebral bodies L5 and S1. MRI-T1 (Fig. 4C) showed no SpA compatible structural changes, but: (i) ossification of the capsule in the right sacroiliac joint that was responsible for the ankylosis-like appearance of the joint on the conventional radiography, and (ii) degenerative changes of the intervertebral disc L5/S1 with fatty metaplasia of the bone marrow of L5 and S1 vertebrae – degenerative changes compatible with the Modic type II lesion. Bone marrow oedema visible on STIR has, therefore, also degenerative origin. In this case, the presence of uveitis, HLA-B27, and inflammatory back pain is overweighed by the absence of SpA typical changes on imaging and – even more importantly – by the presence of degenerative changes explaining long-lasting back pain (Fig. 4D). Of note, an inappropriate application of the ASAS classification criteria instead of the diagnostic approach in this case would have resulted in a conclusion of the presence of axial SpA due to the presence of chronic back pain that started before 45 years of age, HLA-B27-positivity, presence of inflammatory back pain and acute anterior uveitis.
Fig. 4.
Conventional radiography, MRI-STIR, MRI-T1 of sacroiliac joints, and a diagnostic scale for patient B
A 53-year-old male patient referred by an ophthalmologist because of two recent episodes of acute anterior uveitis and inflammatory back pain of intermittent intensity for about 20 years. HLA-B27 is positive, CRP is normal. Conventional radiography of sacroiliac joints showed suspicious changes, but no SpA-compatible changes could be found on MRI. At the same time, degenerative changes of the intervertebral disc represent the most likely explanation of back pain in this case. See the article text for further details. SpA: spondyloarthritis; STIR: short tau inversion recovery.
Conclusion
Diagnostic and classification approaches are characterized by conceptional and operational differences. Although no universal diagnostic approach can be developed, the main diagnostic principles can be clearly defined: consider the pre-test probability of the disease, evaluate positive and negative results of the diagnostic test, exclude other entities, estimate the probability of the disease at the end. Classification criteria should only be applied to patients with an established clinical diagnosis and aimed at the identification of a more or less homogeneous group of patients for the conduction of clinical research. Thus, the correct diagnosis remains the main challenge in the clinical practice, while correct classification has a high relevance for research.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript. This paper was published as part of a supplement funded by Novartis.
Disclosure statement: The author has declared no conflicts of interest.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Proft F, Poddubnyy D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. The Adv Musculoskelet Dis 2018;10:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 4. Boel A, Molto A, van der Heijde D et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. [DOI] [PubMed] [Google Scholar]
- 5. Redeker I, Callhoff J, Hoffmann F et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology 2019;58:1634–8. [DOI] [PubMed] [Google Scholar]
- 6. Sykes MP, Doll H, Sengupta R, Gaffney K. Delay to diagnosis in axial spondyloarthritis: are we improving in the UK? Rheumatology 2015;54:2283–4. [DOI] [PubMed] [Google Scholar]
- 7. Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D. Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis 2015;74:1483–7. [DOI] [PubMed] [Google Scholar]
- 8. Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis 2004;63:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Berg R, de Hooge M, Rudwaleit M et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann Rheum Dis 2013;72:1646–53. [DOI] [PubMed] [Google Scholar]
- 10. Rudwaleit M, van der Heijde D, Landewe R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 11. Unlu Z, Tarhan S, Gunduz K, Goktan C. Relationship between ossification of the stylohyoid ligament and enthesopathy: a comparative study. Clin Exp Rheumatol 2002;20:661–7. [PubMed] [Google Scholar]
- 12. Hermann J, Giessauf H, Schaffler G, Ofner P, Graninger W. Early spondyloarthritis: usefulness of clinical screening. Rheumatology 2009;48:812–6. [DOI] [PubMed] [Google Scholar]
- 13. Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. JAMA 1977;237:2613–4. [PubMed] [Google Scholar]
- 14. Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. [DOI] [PubMed] [Google Scholar]
- 15. Sieper J, van der Heijde D, Landewe R et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 16. Poddubnyy D, Callhoff J, Spiller I et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD Open 2018;4:e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Hooge M, van Gaalen FA, Renson T et al. Low specificity but high sensitivity of inflammatory back pain criteria in rheumatology settings in Europe: confirmation of findings from a German cohort study. Ann Rheum Dis 2019;78:1605–6. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijde D, Ramiro S, Landewe R et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 19. Schett G, Lories RJ, D'Agostino M-A et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 2017;13:731–41. [DOI] [PubMed] [Google Scholar]
- 20. Calin A, Elswood J. The relationship between pelvic, spinal and hip involvement in ankylosing spondylitis–one disease process or several? Br J Rheumatol 1988;27:393–5. [DOI] [PubMed] [Google Scholar]
- 21. Rudwaleit M, Feldtkeller E, Sieper J. Easy assessment of axial spondyloarthritis (early ankylosing spondylitis) at the bedside. Ann Rheum Dis 2006;65:1251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rudwaleit M, Haibel H, Baraliakos X et al. The early disease stage in axial spondylarthritis: results from the german spondyloarthritis inception cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 23. Cortes A, Hadler J, Pointon JP et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013;45:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Lunteren M, van der Heijde D, Sepriano A et al. Is a positive family history of spondyloarthritis relevant for diagnosing axial spondyloarthritis once HLA-B27 status is known? Rheumatology 2019;58:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baraliakos X, Baerlecken N, Witte T, Heldmann F, Braun J. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann Rheum Dis 2014;73:1079–82. [DOI] [PubMed] [Google Scholar]
- 26. de Winter JJ, van de Sande MG, Baerlecken N et al. Anti-CD74 antibodies have no diagnostic value in early axial spondyloarthritis: data from the spondyloarthritis caught early (SPACE) cohort. Arthritis Res Ther 2018;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziade NR, Mallak I, Merheb G et al. Added value of anti-CD74 autoantibodies in axial spondyloarthritis in a population with low HLA-B27 prevalence. Front Immunol 2019;10:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun J, Bollow M, Remlinger G et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998;41:58–67. [DOI] [PubMed] [Google Scholar]
- 29. Khan MA. HLA-B27 and its subtypes in world populations. Curr Opin Rheumatol 1995;7:263–9. [DOI] [PubMed] [Google Scholar]
- 30. Mandl P, Navarro-Compan V, Terslev L et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 31. Poddubnyy D, Brandt H, Vahldiek J et al. The frequency of non-radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis 2012;71:1998–2001. [DOI] [PubMed] [Google Scholar]
- 32. Song IH, Carrasco-Fernandez J, Rudwaleit M, Sieper J. The diagnostic value of scintigraphy in assessing sacroiliitis in ankylosing spondylitis: a systematic literature research. Ann Rheum Dis 2008;67:1535–40. [DOI] [PubMed] [Google Scholar]
- 33. Klauser A, Halpern EJ, Frauscher F et al. Inflammatory low back pain: high negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum 2005;53:440–4. [DOI] [PubMed] [Google Scholar]
- 34. Strobel K, Fischer DR, Tamborrini G et al. 18F-fluoride PET/CT for detection of sacroiliitis in ankylosing spondylitis. Eur J Nucl Med Mol Imaging 2010;37:1760–5. [DOI] [PubMed] [Google Scholar]
- 35. Ez-Zaitouni Z, Bakker PA, van Lunteren M et al. The yield of a positive MRI of the spine as imaging criterion in the ASAS classification criteria for axial spondyloarthritis: results from the SPACE and DESIR cohorts. Ann Rheum Dis 2017;76:1731–6. [DOI] [PubMed] [Google Scholar]
- 36. Maksymowych WP, Lambert RG, Ostergaard M et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 2019;78:1550–8. [DOI] [PubMed] [Google Scholar]
- 37. van Onna M, van Tubergen A, van der Heijde D, Jurik AG, Landewé R. Gadolinium contrast-enhanced MRI sequence does not have an incremental value in the assessment of sacroiliitis in patients with early inflammatory back pain by using MRI in combination with pelvic radiographs: a 2-year follow-up study. Clin Exp Rheumatol 2014;32:225–30. [PubMed] [Google Scholar]
- 38. Diekhoff T, Greese J, Sieper J et al. Improved detection of erosions in the sacroiliac joints on MRI with volumetric interpolated breath-hold examination (VIBE): results from the SIMACT study. Ann Rheum Dis 2018;77:1585–9. [DOI] [PubMed] [Google Scholar]
- 39. Rudwaleit M, Jurik AG, Hermann K-GA et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 40. Lambert RG, Bakker PA, van der Heijde D et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 41. de Winter J, de Hooge M, van de Sande M et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the assessment of spondyloarthritis international society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol 2018;70:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baraliakos X, Richter A, Feldmann D et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann Rheum Dis 2020;79:186–92. [DOI] [PubMed] [Google Scholar]
- 43. Weber U, Jurik AG, Zejden A et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “Background Noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol 2018;70:736–45. [DOI] [PubMed] [Google Scholar]
- 44. Hermann KG, Baraliakos X, van der Heijde DM et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 2012;71:1278–88. [DOI] [PubMed] [Google Scholar]
- 45. Rudwaleit M, Landewe R, van der Heijde D et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 46. Rudwaleit M, van der Heijde D, Landewe R et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 47. Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. [DOI] [PubMed] [Google Scholar]
- 48. Aggarwal R, Ringold S, Khanna D et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol 1995;34:1074–7. [DOI] [PubMed] [Google Scholar]
- 50. Feldtkeller E, Rudwaleit M, Zeidler H. Easy probability estimation of the diagnosis of early axial spondyloarthritis by summing up scores. Rheumatology 2013;52:1648–50. [DOI] [PubMed] [Google Scholar]