Abstract
Axial SpA (axSpA), encompassing AS, is a multifactorial disease that localizes to sites of high spinal biomechanical stress. Much has been written on T cells and adaptive immunity in axSpA, which is understandable given the very strong HLA-B27 disease association. Extra-axial disease characteristically involves the anterior uveal tract, aortic root, lung apex and terminal ileum. Under recent classification, axSpA is classified as an intermediate between autoimmunity and autoinflammatory disease, with the latter term being synonymous with innate immune dysregulation. The purpose of this review is to evaluate the ‘danger signals’ from both the exogenous intestinal microbiotal adjuvants or pathogen-associated molecular patterns that access the circulation and endogenously derived damaged self-tissue or damage-associated molecular patterns derived from entheses and other sites of high biomechanical stress or damage that may serve as key drivers of axSpA onset, evolution, disease flares and eventual outcomes.
Keywords: danger signals, pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), axial spondyloarthritis (axSpA), enthesitis
Rheumatology key messages
Subclinical gut barrier dysfunction is an early axial SpA (axSpA) feature, thus incriminating innate immunity.
Entheseal biomechanical stress and resident innate cells also implicate musculoskeletal innate immunity in early axSpA.
Genetics, intestinal permeability/dysbiosis and axSpA tissue tropism collectively incriminate extensive innate immune ‘danger signals’.
Introduction
Axial SpA (axSpA) predominantly affects the spine and sacroiliac joints and encompasses AS and non-radiographic axSpA (nr-axSpA), the chronic prototypic seronegative inflammatory rheumatic disease belonging to the family of SpAs [1]. The enthesis is defined as a region of tough fibrous tissue where tendon, ligament or joint capsule inserts into bone [2, 3]. Enthesitis is understood to be the cardinal lesion in axSpA, with synovitis and osteitis being intimately associated [4].
The innate and adaptive immune system are functionally integrated and usually act together. Much has been written about the role of adaptive immunity in axSpA, which is understandable given the HLA-B27 association and many other genetic pointers towards adaptive immunity [5]. Given the proclivity for simultaneous inflammation in extra-axial biomechanically stressed sites, including the aortic root, lung apex and terminal ileum, and the fact that these tissues are antigenically diverse, it is hard to explain disease in terms of an arthritogenic peptide theory, thus the role of local tissue factors and innate immune responses merits greater consideration. The purpose of this review is to summarize the evidence on the role of innate immunity in disease initiation in axSpA. We will split the role of innate immunity into two major categories, innate immunity in the gut in axSpA and its role at physically stressed sites (Figs 1 and 2).
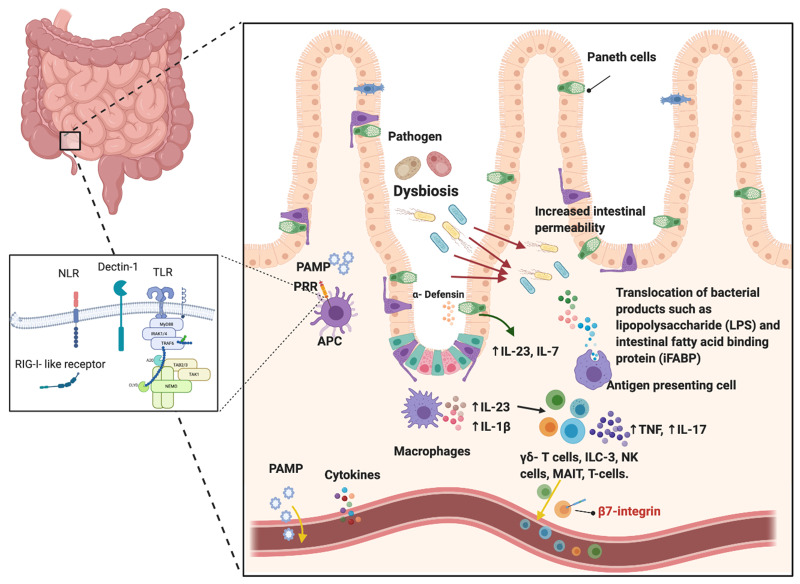
Fig. 1.
Intestinal and gut barrier involvement in early SpA
These bacterial molecules are known as PAMPs and may directly enter the circulation and potentially trigger inflammation at sites of high mechanical stress. Secondly, the impact of DAMPs may activate local innate immune cells to produce pro-inflammatory cytokines and other molecules that may enter the circulation. Thirdly, the possibility remains that the activated innate immune cells may circulate to entheses and bones as part of a gut–enthesis axis. PRR: pattern recognition receptors; PAMPs: pathogen-associated molecular patterns; NLR: nucleotide-binding oligomerization domain (NOD)-like receptors; TLR: Toll like receptors; RIG-I-like: retinoic acid-inducible gene-I-like receptors.
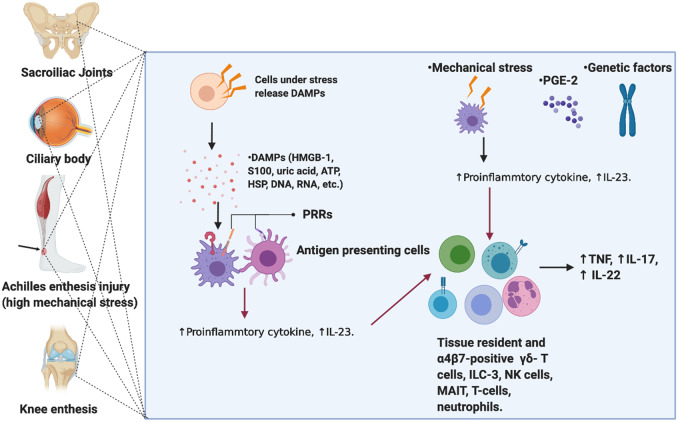
Fig. 2.
Mechanisms involved in early phases of SpA
Injured tissue may activate innate immune responses in many ways. These include fragments of extracellular matrix proteins that collectively activate TLRs, among others. Injured or stressed cells secrete or release proteins that once outside the nucleus can also prime innate immunity. Necrotic cell death also releases nuclear contents that once in the extracellular environment may serve as strong activators of innate immunity and includes RNA and DNA, proteins including HMGB1, and uric acid and HSP, among others. Collectively these likely activate enthesis and bone resident immune cell populations. It is considered that the dysregulated innate immunity in the gut and the spine increase the chances of innate immune dysfunction and clinical development of AS. DAMPs: danger associated molecular patterns; PRR: pattern recognition receptors; HMGB-1: high mobility group protein B1; ILC: Innate lymphoid cells; MAIT: mucosal associated invariant T cell.
Gut, barrier regulation and axSpA
It is increasingly clear that a relationship between intestinal inflammation and axSpA exists. A total of 5–10% of axSpA patients develop clinically diagnosed IBD and up to 60% have evidence of subclinical gut inflammation [6]. A cause-and-effect relationship is best established in reactive arthritis, a member of the SpA family, where enthesitis and synovitis develop after a distant infection, usually genitourinary or gastrointestinal with Campylobacter, Salmonella, Yersinia or Shigella [7]. Severe acute presentations of sacroiliitis have been reported mimicking infection, supporting innate-driven mechanisms [8]. The first line of innate immunity is not the immune cells, but the barrier tissue itself, and there is compelling evidence for barrier dysfunction in animal models and in AS. Increased gut permeability has been demonstrated among patients and first-degree relatives of axSpA [9, 10]. The leakage of epithelial and endothelial membrane forming the gut–epithelial barrier and gut–vascular barrier can result in bacterial translocation with bacterial products such as lipopolysaccharide (LPS), LPS binding protein and fatty acid binding proteins, thus activating resident immune cell populations and inducing inflammation, osteitis and synovitis [11]. In addition to gut-resident myeloid cells and infiltrating neutrophils, Paneth cells, a subset of specialized secretory host-defence epithelial cells located in the small intestines, have been shown to secrete IL-23 and activate key IL-23 responsive cells such as innate lymphoid cells of group 3 (ILC3), γδ T cells and mucosal-associated invariant T (MAIT) cells, which can recirculate from the gut and seek axSpA relevant sites of inflammation [12–16].
While the exact mechanism linking the gut to axSpA pathogenesis is not completely understood, it is believed that the interplay between the microbiome and the intestinal immune system contributes to intestinal innate immune cell activation [17]. It also remains unclear when gut barrier dysfunction with abnormal permeability occurs. However, it is evident that axial disease evolution might be related to changing spinal biomechanics in the later teenage years [18].
It is not understood exactly how intestinal inflammation leads to axial inflammation, but possibilities include innate and adaptive cell circulation and skeletal homing, translocation of adjuvants to the gut or shared dysregulated immune mechanisms [17]. It has been postulated that while IL-23 acts directly on tissue-resident entheseal immune cells, IL-23 might activate circulating immune populations that then migrate and seek entheses, inducing inflammation [4]. Translocation of bacterial products into the gut submucosa and circulating blood provides evidence of the concept of so-called leaky gut, linking bacteria to activation of immune cells in circulating blood and/or enthesis resident immune cells [11]. Similarly, observations highlight the role of the gut as the primary site for the differentiation, expansion and priming of innate cells prior to recirculation to axSpA target sites [19]. Reported de novo severe SpA in ameliorated IBD following α4β7 integrin blockade is further clinical evidence of the intimate connection intertwined at the gut–joint axis and innate-driven mechanisms [20]. Perhaps upregulation of the mucosal vascular cell adhesion molecule 1 (MADCAM-1), the homing integrin (α4β7) ligand, in the gut and inflamed bone marrow of patients with axSpA provides additional evidence of the importance of recirculation of gut-primed innate cells [20]. Finally, microbial products, also known as pathogen-associated molecular patterns, act as adjuvants, defined as substances enhancing antigen-specific immune response [21]. It is possible that bacterial adjuvants act synergistically with mechanical factors in activating innate immune responses and providing necessary co-stimulatory signals [21].
The role of HLA-B27 in shaping the gut microbiome was investigated in an HLA-B27 transgenic rat (murine SpA model) using biome representational in situ karyotyping and 16S rRNA gene sequencing, revealing significant differences in caecal microbiota [22]. A larger proportion of radiographic axSpA patients express HLA-B27 as compared with non-radiographic axSpA [23]. The robust association of HLA-B27 with axSpA provides a strong indication that adaptive immunity may be able to sculpt intestinal innate immunity and hence might provide insights into the molecular influence on phenotype (i.e. radiographic vs non-radiographic axSpA), but adaptive immune mechanisms are not discussed further in this article. Moreover, intestinal dysbiosis is accompanied by alterations of tight junctions between epithelial cells forming the lining of the gut, which is a hot area of research [11].
Pattern recognition receptors in innate immunity in the gut and the enthesis
Pathogen-associated molecular patterns in the gut
The innate immune system possesses a repertoire of germ-line encoded receptors that recognise conserved molecular structures found in pathogens [so-called pathogen-associated molecular patterns (PAMPs)]. Somewhat surprisingly, it was discovered that the same family of receptors become activated on engaging the products released from stressed and damaged cells [termed damage-associated molecular patterns (DAMPs)] [24]. These pattern recognition receptors (PRRs) are subdivided into four major families: Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid–inducible gene 1-like receptor (RLR) and C-type lectin receptor (CLR). Signalling through PRRs triggers a downstream cascade of pro-inflammatory programs within innate immune cells that can initiate antigen-specific adaptive immune responses [24].
TLRs are a group of transmembrane-spanning receptors each having several distinct ligand and cellular localizations. TLRs signal through myeloid differentiation factor 88 (MyD88), except TLR3, which utilizes Toll/IL-1 receptor domain containing adapter-inducing IFN-β (TRIF) for signalling [25]. Subsequent activation of downstream proteins results in upregulation of genes involved in the inflammatory process, antigen presentation, activation of adaptive immunity or induction of cell death [25]. Given the essential role of microbiota and bacterium in axSpA, TLR4, which recognizes LPS from Gram-negative bacteria, was investigated for its association with axSpA, with gene expression profiling showing TLR4 and TLR5 upregulation in axSpA patients [26]. Polymorphisms in TLR4 were associated with axSpA, further adding to the evidence supporting their role in increasing susceptibility to disease [27, 28].
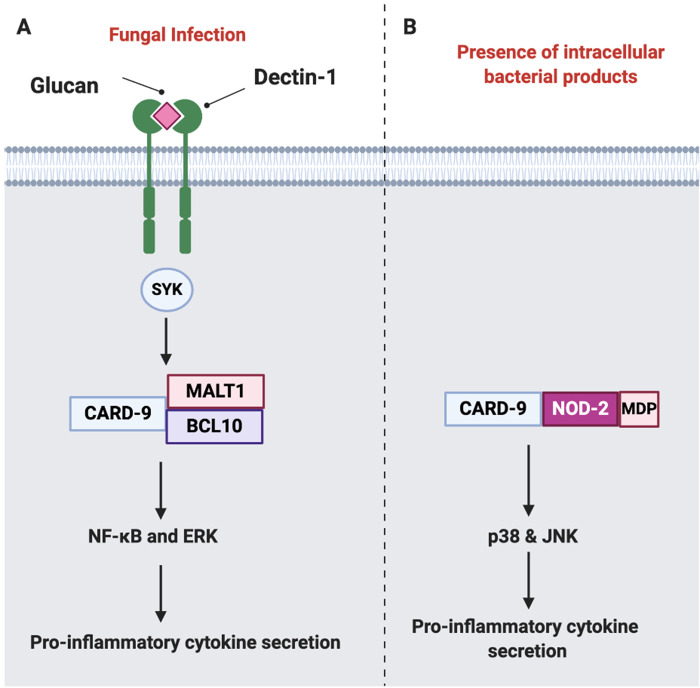
Several reports have identified a nominal association between polymorphisms in caspase recruitment domain-containing protein-9 (CARD-9) and axSpA, placing it as a strong functional candidate [29]. CARD-9 is an adaptor protein expressed exclusively on myeloid cells that mediates downstream signalling of Dectin-1, the NK receptor C-type lectin-like PRR recognizing β-glucan (fungal cell wall polysaccharide) in response to fungal infection, resulting in secretion of pro-inflammatory cytokines including TNF, IL-6 and IL-23, among other cytokines [30]. The strongest evidence for the importance of gut barrier cells in SpA comes from the striking NOD2 association with distal ileal inflammation in Crohn’s disease [31] and it is noted that 50% of AS cases have subclinical ileocolonoscopic or histologic changes reminiscent of Crohn’s disease [32]. NOD2 is an intracellular cytosolic PRR belonging to the NLR family, which mediates the activation of mitogen-activated protein kinase signalling components such as p38 and the c-Jun N-terminal kinase pathway through the amino-terminal CARD domain. NOD2 has two CARD domains and is readily activated by mumaryl dipeptide (MDP), a ubiquitously present peptidoglycan motif found among both Gram-positive and negative bacteria (Fig. 3) [33]. CARD-9 is an essential component of the NOD2 signalling pathway and induces the maturation of dendritic cells (DCs) to antigen presenting cells and priming naïve cells to Th-1 or Th-17 phenotypes [31].
Fig. 3.
Role of CARD-9 in innate signalling
(A) A simplified schematic representation of CARD-9 signalling in response to fungal infection through Dectin-1, which results in recruitment of SYK kinase and the formation of signalosome composed of CARD-9, BCL10 and MALT1, resulting in NF-κB and ERK activation and subsequent production of pro-inflammatory cytokines including IL-6, IL-12, IL-1β among other cytokines. (B) Intracellular NOD2 receptor recognizes MDP (a component of bacterial cell walls) and couples with CARD-9, driving activation of p38 and JNKs and resulting in pro-inflammatory cytokine secretion. MALT1: mucosa-associated lymphoid tissue lymphoma translocation protein 1; BCL10: B cell lymphoma/leukaemia 10.
DAMPs at the enthesis or circulating blood
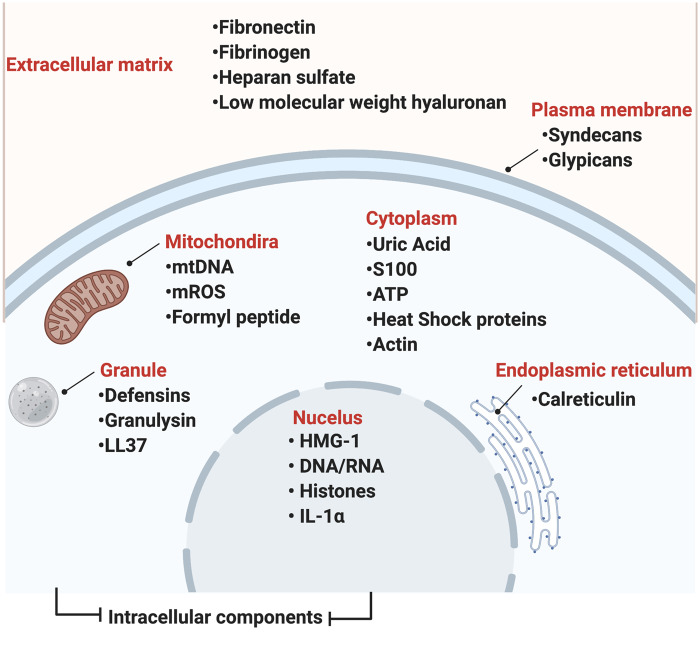
Parallel to its role in identifying non-self and defence against pathogens, the innate immune system has evolved to serve a housekeeping role, including repair and removal of damaged tissue from structures releasing signals, also known as DAMPs [34]. Several molecules that behave as DAMPs have a primary endogenous role, including transport or chaperoning. However, upon release from damaged cells and arriving within the incorrect extracellular compartment they bind to PRRs and activate an innate immune cascade, a response that is identical to that activated by PAMPs. An example of such molecules includes molecules derived from the intracellular compartment, including high-mobility group box 1 (HMGB1) proteins, S100 protein, uric acid, adenosine triphosphate, heat shock proteins (HSPs), DNA and RNA, among others [34]. Following tissue injury, DAMPs can be secreted from extracellular matrix due to degradation; such products include heparan sulphate, low molecular weight hyaluronan, fibrinogen, fibronectin and biglycan, among others, which are capable of directly activating TLRs and even some NLR receptors (Fig. 4) [35].
Fig. 4.
Some DAMPs
Several DAMPs have been identified and are released from both extracellular and intracellular compartments following stress, tissue injury or cell death, all of which might occur at sites of high physical stressing, including the entheses in early SpA. Upon release, DAMPs bind to their respective pattern recognition receptors and induce inflammatory responses that are essential in driving normal repair and homeostasis. Uncontrolled, DAMPs result in hyperactivation of innate immune signals and responses driving and exacerbating inflammatory diseases.
HSP70 plays a central role by acting as a chaperone and folding catalyst, including the folding of synthesized proteins, refolding of aggregated proteins and translocation of secretory proteins and control of activity of regulatory proteins. Extracellular HSPs activate the innate immune system through activation of TLR2, TLR4 and CD91 [36]. The association between HSP70 gene polymorphisms in axSpA was investigated and showed a significant difference in HSP70-1 and HSP70-2 genotypes between axSpA patients and healthy controls [37]. Also, it is possible that impairment of HSP function could result in the accumulation of unfolded protein responses that have been incriminated in SpA pathogenesis [38]. Another important intracellular DAMP, HMGB1, is located in the nucleus and plays an important role in gene expression. When HMGB1 is secreted it binds to TLR2, TLR4, TLR9 and RAGE and results in nuclear factor (NF)-κB activation and inflammation induction [36]. Serum levels of HMGB1 in axSpA patients were reportedly higher than those of controls [39]. Another report demonstrated an association between serum levels, disease activity and inflammatory markers [40]. While these factors may all be linked to axSpA, their role in disease pathogenesis requires further elucidation.
Cytokines as danger signals in both the gut and enthesis
Tissue microdamage, including bone microcracks, and soft tissue microscopic inflammatory and reparative changes have been reported in normal healthy entheses. Injury to tissue is capable of innate immune system activation by the liberation of so-called DAMP receptors, resulting in cytokine release, including IL-23, IL-1β and TNF [41, 42]. Independent of DAMPs, liberation tissue injury, damage or stress may directly liberate key innate immune system cytokines that can also act as danger signals and drive inflammation. The best example of a cytokine danger signal in the experimental setting is the TNF model, where physical stress on the enthesis drives stromal liberation of TNF, resulting in enthesitis, synovitis and also colitis that localizes to the terminal ileum.
Resident immune cells including γδ T cells and ILC3, known targets of IL-23, then respond by producing IL-17 and TNF, among other cytokines [42]. This response drives homeostatic repair at entheseal sites. However, in SpA, repair circuits are dysregulated and failure to resolve inflammation results in disease initiation.
Currently >100 gene polymorphisms have been identified to be associated with axSpA, several of which are associated with innate immune signalling, including interferon regulatory factor 5 (IRF5), TNF, NOS and IL-12B, or are potentially involved in innate immune sensing, such as IL-1R, IL-6R, IL-23R and prostaglandin E2 receptor 4 [43, 44].
IL-1β is a prototypic pro-inflammatory cytokine mainly produced by macrophages in response to PAMPs and exerts pleiotropic effects including immune cell recruitment and activation [45]. Deficiency of IL-1 receptor antagonist (DIRA) is accompanied by joint swelling and inflammatory arthritis, including axial new bone formation, a finding that parallels the development of spontaneous inflammatory arthritis in murine models deficient in IL-1R antagonist [46–48]. Moreover, several single-nucleotide polymorphisms (SNPs) involving the IL1 gene cluster have been associated with axSpA, including IL1α, IL1β, IL-1RN (coding for IL-1RA) and IL-1R [49, 50]. Despite the association of the IL1R SNP with axSpA, IL1R2 gene polymorphism was only demonstrated among patients of European descent, in contrast to the Asian population [51, 52].
Evidence from genetic studies, animal models and therapeutic studies firmly implicates the IL-23–IL-17 axis in the pathogenesis of SpA [53]. Entheseal immune cells, which were only recently discovered, can produce TNF-α transcripts and upregulate IL-17, IL-22 and IL-23 in vitro [54–56]. IL-23, a heterodimeric cytokine of the IL‐12 family, is mainly produced by innate cells including macrophages, DCs and monocytes. IL-23 induces IL-17 production from a wide range of cells, including Th-17, CD8+ T cells and γδ T cells, which results in stromal cells and immune cell activation and promotion of inflammation [2]. It is becoming increasingly evident that IL-17 can be independent of IL-23, probably explaining the ineffectiveness of IL-23 targeting in axSpA [57, 58]. Moreover, IL-23 has been shown to play an important role in the initiation but not the persistence of experimental SpA in the HLA-B27 transgenic murine model [59].
In a recent report, CD14+ cells with the ability to secrete IL-23 were confirmed in the human enthesis [55]. IL-23 overexpression through minicircle DNA injection induced experimental SpA and is essential for enthesitis and entheseal new bone formation by acting on γδ T cells [4]. It is hypothesized that misfolding of HLA-B27 triggers a stress response in the endoplasmic reticulum (ER), known as unfolded protein response, resulting in IL-23 production; however, conflicting reports exist regarding ER stress response [60]. Furthermore, activation of prostaglandin E2 receptor 4 (EP4), the protein product of prostaglandin EP4 receptor (PTGER4) promotes development of Th17 cells by increasing the IL-23 expression level. PTGER4 polymorphisms have been shown to be associated with axSpA [61]. In addition to the increase in IL-23 secretion, it has been shown that prostaglandin E2 (PGE2) levels were significantly elevated in mice patellar and Achilles tendons in response to rigorous treadmill training [62]. Moreover, treatment of tendon stem cells with PGE2 decreased their proliferation ability but induced both adipogenesis and osteogenesis [62]. It is clear that PGE2 plays a central role as a mediator of inflammation, as evidenced by the clinical efficacy of NSAIDs (PGE2 inhibitor) in diminishing the inflammation and pain in patients with axSpA [63].
Innate immune cells involved in axSpA
Macrophages are a heterogeneous population and are broadly subdivided into M1 (classical/pro-inflammatory) and M2 (alternative, anti-inflammatory). Histological assessment of early and active sacroiliitis showed abnormal entheseal architecture with increased vascularity and cellular infiltration with a predominant macrophage cell infiltration in the fibrocartilage [64]. Recently, human enthesis was shown to harbour a population of CD14+ myeloid cells capable of IL-23 and TNF production [55]. Stimulation of CD14+ cells with adjuvants induced chemokine ligand 20 (CCL20) from the enthesis, which functions as a chemoattractant to IL-17-producing cells expressing its ligand chemokine receptor 6 (CCR6) [55]. DCs link innate and adaptive immunity and impaired DC function has been linked to a predisposition to experimental SpA [65], impaired T cell interactions with impaired co-stimulation and altered cytoskeletal dynamics in early disease [66].
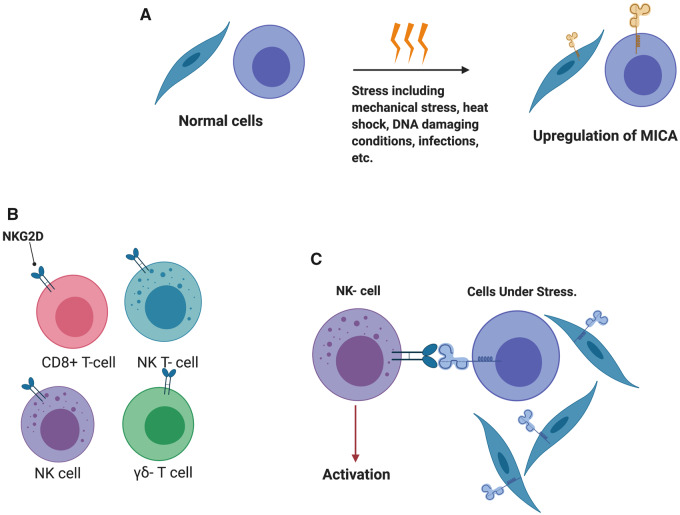
The cellular function of NK cells, a major component of the innate immune system, is defined by the balance of activating and inhibitory signals that recognize the MHC class through the highly polymorphic killer cell immunoglobulin-like receptor (KIR) [67]. The role of HLA-B27 in disease induction is not well understood. One plausible hypothesis implicates the observed ability of HLA-B27 to form heavy chain homodimers and cell surface expression of heterodimers that bind to innate immune receptors on NK cells, including KIR3DL1, KIR3DL2 and LILIRB2 [68, 69]. This interaction results in a downstream pro-inflammatory response in both NK cells and KIR3DL2-expressing T cells, including Th17 and γδ T cells [70, 71]. NKG2D, an activating receptor of CLR, is expressed on NK cells, CD8+ T cells, γδ T cells and NK T cells and has been associated with pro-inflammatory response when activated. MHC class I chain A related (MICA) is a gene located on chromosome 6 close to the HLA-B locus and codes for stress inducible glycoprotein that results in the activation of NKG2D (Fig. 5). Polymorphisms in MICA-129 have been associated with spondylitis, and homozygosity to MICA A5.1 conferred increased risk for axSpA after adjustment for HLA-B27 status [72].
Fig. 5.
Innate cell response during cellular stress
(A) MICA is a gene located on chromosome 6 close to the HLA-B locus and is upregulated during stress response to heat shock and DNA damaging conditions and is influenced by some infectious agents, including Escherichia coli, among others. (B) NKG2D is an activating receptor of the C-type lectin-like family and is expressed on NK cells, CD8+ T cells, γδ T cells and NK T cells. Upon activation, it has been associated with a pro-inflammatory response. (C) MICA serves as a ligand to NKG2D and upon engagement during a stress response, the complex results in the activation of effector cytolytic function of NKG2D-expressing cells against MICA-expressing cells.
Invariant NK T (iNKT) cells represent a small subset of T cells expressing a restricted T cell receptor repertoire, and unlike T cells, iNKT cells recognize glycoproteins through the non-polymorphic MHC class 1 molecule CD1d. iNKT cells secrete IL-17 upon stimulation of either T cell receptor or the constitutively expressed IL-23R, pointing towards an independent and convergent pathway [72]. Interestingly, iNKT cells have been shown to be natural regulators of murine SpA. iNKT cell depletion aggravated gut and joint inflammation in a TNF adenylate–uridylate-rich regulatory element mouse model, which was linked to a greater number of inflammatory DCs [73].
γδ T cells are another distinct subset of the unconventional T cell subpopulation expressing the γδ form of the T cell receptor and capable of recognizing a broad range of antigens through pattern recognition receptors, including TLRs and Dectin-1, supporting their role in the early response to microbes [74]. Several γδ T cell subsets have been identified and have been shown to be potent producers of IL-17, promoting wound healing and osteogenesis in mice [74, 75]. Recently γδ T cells were described in normal human enthesis, with evidence supporting their entheseal residency and expression of transcripts associated with IL-23/IL-17 signalling, including retinoic acid receptor–related orphan receptor C (RORC), IL-23R and CCR6 [58]. Furthermore, IL-17 secretion from γδ T cells was shown to be driven in the absence of IL-23, providing a potential explanation for anti-IL-23 inefficacy in axSpA [58].
ILCs are a family of heterogeneous resident effector cells at the epithelial barrier surfaces of the gut and are involved in tissue remodelling and maintenance of organ homeostasis and inflammation [76], with ILC3 being most relevant in SpA due to IL-17 and IL-22 secretion in response to activation by IL-23 [77]. It has been demonstrated that gut-derived IL-17-producing ILC3 is increased in the peripheral blood, synovial fluid and bone marrow of patients with axSpA and expressed the homing integrin α4β7 [78]. Recently it was shown that peri-entheseal bone and entheseal soft tissue harboured a population of ILC3 that was entheseal resident [79]. The frequency of entheseal ILC3 was lower than that reported in the gut, possibly due to the lack of exposure of enthesis to the external environment [79].
Another innate immune source of IL-17 are MAIT cells, a population of unconventional innate T cells that recognise non-peptide antigens presented by monomorphic MHC class I molecules through a semi-invariant T cell receptor [80]. As the name suggests, MAIT cells are abundant at barrier surfaces, such as mucosal surfaces, and act as part of the first line of defence against bacteria and yeasts by producing inflammatory cytokines, including IFN-γ, TNF, IL-17A and IL-22, among other cytokines [80]. Evidence from a collagen-induced arthritis murine model highlighted the effector role of MAIT cells in augmenting joint inflammation during the effector phase of arthritis, suggesting arthritogenic potency [81]. MAIT cells have also been shown to be enriched in the synovial fluid of patients with axSpA and expression of IL-17 was dependent on priming with IL-7 but not IL-23 or antigen stimulation [16]. IL-22 secretion by MAIT cells has been documented and associated with the induction of genes implicated in the regulation bone formation and osteogenesis [82].
A strong translational perspective in innate immunity in SpA comes from clinical and experimental studies where anti-IL-17A is effective in axSpA but ineffective for gut inflammation. This was shown to be due to γδ T cell intestinal production of IL-17A independent of IL-23 expression, with IL-17A mediating normal barrier function [83, 84].
Conclusions
Innate immune system dysregulation plays a critical role in the pathogenesis of axSpA (Table 1). Evidence from murine models highlight the role of innate immunity in the induction of axSpA. IL-23/IL-17 pathways provide clues to how HLA-B27 contributes to disease pathogenesis through innate immune activation. Several innate immune cells have been identified and their role in the production of disease-relevant cytokines and disease propagation is evident. However, certain areas remain undefined, including the exact role of gut inflammation in axSpA, and further studies are needed to better elucidate the role of innate immunity in the initiation and propagation of inflammation in axSpA.
Table 1.
Summary of the role of innate immune cells in the pathogenesis and progression of axSpA
| Immune cell population | Role in axSpA | Reference |
|---|---|---|
| Macrophages | Active sacroiliitis shows predominant cellular infiltration with macrophages | 64 |
| CD163+ macrophages and CD68+ macrophages from fibrous tissue of axSpA facet joints secrete IL-23 | 85 | |
| Normal enthesis harbours a population of CD14+ cells capable of IL-23 and TNF production | 55 | |
| Dendritic cells | Impaired formation of conjugates between dendritic cells and T cells due to impaired accessory molecule function | 65 |
| Defects in co-stimulation, decreased expression of MHC II and altered cytoskeletal dynamics in axSpA patients | 66, 86, 87 | |
| NK cells | HLA-B27 heavy chain homodimers and heterodimers bind and activate NK cells through killer cell immunoglobulin like receptor | 68, 69 |
| High NK cell cytotoxicity in SpA patients compared with controls | 88 | |
| Lower expression of A20, responsible for NF-κB inhibition on CD56bright cells in patients with axSpA | 89 | |
| MICA serves as a ligand to NKG2D and the complex results in the activation of effector cytolytic function of NKG2D-expressing cells against MICA-expressing cells | 71 | |
| Invariant NK T cells | iNKT depletion worsened joint inflammation in TNF AU-rich regulatory element mouse model | 73 |
| iNKT cells secrete IL-17 upon stimulation of the TCR or IL-23R | 72 | |
| γδ T cells | γδ T cells population has been shown to be the predominant IL-17A producers at the enthesis in the IL-23-dependent mouse model | 4 |
| γδ T cells are resident in the enthesis and express transcripts associated with the IL-23–IL-17 pathway, including RORC, CCR6 and IL-23R | 58 | |
| γδ T cells has been shown to drive IL-17 secretion independent of IL-23 stimulation | 58 | |
| γδ T were enriched within inflamed joints of SpA and acts as a major IL-17 secretors | 90 | |
| Innate lymphoid cells | ILC3 groups are relevant to SpA due to secretion of IL-17 and IL-22 in response to activation by IL-23 | 77 |
| Gut-derived ILC3 were expanded in the synovial fluid and bone marrow of patients with axSpA | 77 | |
| Normal enthesis harbours a population of resident ILC3 | 79 | |
| Psoriatic arthritis is characterized by a skewed ILC homeostasis, with elevated levels of ILC3s, which are potent source of IL-17/IL-22 | 91 | |
| MAIT | Enriched population in the synovial fluid of patients with axSpA | 16 |
| Secretion of IL-17 independent of IL-23. Role in IL-22 secretion and regulation of bone formation | 82 | |
| The number of IL-22+ and IFN-γ+/IL-17A+ MAIT cells was higher in axSpA as compared with healthy controls | 82 |
Acknowledgement
Figures were produced using Biorender.com.
Funding: This research was supported by the Leeds Biomedical Research Council. This paper was published as part of a supplement funded by Novartis.
Disclosure statement: D.M.G. has received speaker fees and honoraria from Roche, Sobi and Novartis and research grants from Novartis. The other authors have declared no conflicts of interest.
References
- 1. Dubash S, McGonagle D, Marzo-Ortega H. New advances in the understanding and treatment of axial spondyloarthritis: from chance to choice. Ther Adv Chronic Dis 2018;9:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D. Interleukin-23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol Rev 2020;294:27–47. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin M, Ralphs JR. Entheses—the bony attachments of tendons and ligaments. Ital J Anat Embryol 2001;106(2 Suppl 1):151–7. [PubMed] [Google Scholar]
- 4. Sherlock JP, Joyce-Shaikh B, Turner SP. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med 2012;18:1069–76. [DOI] [PubMed] [Google Scholar]
- 5. Hanson A, Brown MA. Genetics and the causes of ankylosing spondylitis. Rheum Dis Clin North Am 2017;43:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mielants H, Veys EM, Goemaere S. et al. Gut inflammation in the spondyloarthropathies: clinical, radiologic, biologic and genetic features in relation to the type of histology. A prospective study. J Rheumatol 1991;18:1542–51. [PubMed] [Google Scholar]
- 7. Ajene AN, Fischer Walker CL, Black RE. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr 2013;31:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubash S, Pease C, Aslam A. et al. Acute unilateral sacroiliitis mimicking infection on magnetic resonance imaging with response to nonsteroidal antiinflammatory drugs: a distinct presentation of spondyloarthritis? J Rheumatol 2018;45:1708–10. [DOI] [PubMed] [Google Scholar]
- 9. Martínez-González O, Cantero-Hinojosa J, Paule-Sastre P, Gómez-Magán JC, Salvatierra-Ríos D. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Br J Rheumatol 1994;33:644–7. [DOI] [PubMed] [Google Scholar]
- 10. Vaile JH, Meddings JB, Yacyshyn BR, Russell AS, Maksymowych WP. Bowel permeability and CD45RO expression on circulating CD20+ B cells in patients with ankylosing spondylitis and their relatives. J Rheumatol 1999;26:128–35. [PubMed] [Google Scholar]
- 11. Ciccia F, Guggino G, Rizzo A. et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis 2017;76:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciccia F, Bombardieri M, Principato A. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum 2009;60:955–65. [DOI] [PubMed] [Google Scholar]
- 13. Becker C, Dornhoff H, Neufert C. et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol 2006;177:2760–4. [DOI] [PubMed] [Google Scholar]
- 14. Ciccia F, Accardo-Palumbo A, Alessandro R. et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum 2012;64:1869–78. [DOI] [PubMed] [Google Scholar]
- 15. Kenna TJ, Davidson SI, Duan R. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum 2012;64:1420–9. [DOI] [PubMed] [Google Scholar]
- 16. Gracey E, Qaiyum Z, Almaghlouth I. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis 2016;75:2124–32. [DOI] [PubMed] [Google Scholar]
- 17. Rizzo A, Guggino G, Ferrante A, Ciccia F. Role of subclinical gut inflammation in the pathogenesis of spondyloarthritis. Front Med 2018;5: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacques P, McGonagle D. The role of mechanical stress in the pathogenesis of spondyloarthritis and how to combat it. Best Pract Res Clin Rheumatol 2014;28:703–10. [DOI] [PubMed] [Google Scholar]
- 19. Ciccia F, Guggino G, Rizzo A. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:1739–47. [DOI] [PubMed] [Google Scholar]
- 20. Dubash S, Marianayagam T, Tinazzi I. et al. Emergence of severe spondyloarthropathy-related entheseal pathology following successful vedolizumab therapy for inflammatory bowel disease. Rheumatology (Oxford) 2019;58:963–8. [DOI] [PubMed] [Google Scholar]
- 21. McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy. additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol 2001;28:2155–9. [PubMed] [Google Scholar]
- 22. Lin P, Bach M, Asquith M. et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS One 2014;9:e105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung HY, Machado P, van der Heijde D, D’Agostino MA, Dougados M. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011;70:1930–6. [DOI] [PubMed] [Google Scholar]
- 24. Amarante-Mendes GP, Adjemian S, Branco LM. et al. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol 2018; 9:2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Assassi S, Reveille JD, Arnett FC. et al. Whole-blood gene expression profiling in ankylosing spondylitis shows upregulation of toll-like receptor 4 and 5. J Rheumatol 2011;38:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perica M, Vidović M, Lamot L. et al. Single nucleotide polymorphism of toll-like receptor 4 (TLR4) is associated with juvenile spondyloarthritis in Croatian population. Clin Rheumatol 2015;34:2079–86. [DOI] [PubMed] [Google Scholar]
- 28. Snelgrove T, Lim S, Greenwood C. et al. Association of toll-like receptor 4 variants and ankylosing spondylitis: a case-control study. J Rheumatol 2007;34:368–70. [PubMed] [Google Scholar]
- 29. Ma X, Liu Y, Zhang H. et al. Evidence for genetic association of CARD9 and SNAPC4 with ankylosing spondylitis in a Chinese Han population. J Rheumatol 2014;41:318–24. [DOI] [PubMed] [Google Scholar]
- 30. Drummond RA, Franco LM, Lionakis MS. Human CARD9: a critical molecule of fungal immune surveillance. Front Immunol 2018;9:1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al Nabhani Z, Dietrich G, Hugot J-P, Barreau F. Nod2: the intestinal gate keeper. PLoS Pathog 2017;13:e1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahn SM, Kim Y-G, Bae S-H. et al. Ileocolonoscopic findings in patients with ankylosing spondylitis: a single center retrospective study. Korean J Intern Med 2017;32:916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moreira L, Zamboni D. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol 2012;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw 2018;18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takagi M, Takakubo Y, Pajarinen J. et al. Danger of frustrated sensors: role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements. J Orthop Transl 2017;10:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 2014;289:35237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fraile A, Nieto A, Matarán L, Martín J. HSP70 gene polymorphisms in ankylosing spondylitis. Tissue Antigens 2008;51:382–5. [DOI] [PubMed] [Google Scholar]
- 38. Hjelholt A, Carlsen T, Deleuran B. et al. Increased levels of IgG antibodies against human HSP60 in patients with spondyloarthritis. PLoS One 2013;8:e56210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oktayoglu P, Em S, Tahtasiz M. et al. Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatol Int 2013;33:1327–31. [DOI] [PubMed] [Google Scholar]
- 40. Wang C, Miao Y, Wu X. et al. Serum HMGB1 serves as a novel laboratory indicator reflecting disease activity and treatment response in ankylosing spondylitis patients. J Immunol Res 2016;2016:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sudoł-Szopińska I, Kwiatkowska B, Prochorec-Sobieszek M, Maśliński W. Enthesopathies and enthesitis. Part 1. Etiopathogenesis. J Ultrason 2015;15:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy. Additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol 2001;28:2155–9. [PubMed] [Google Scholar]
- 43. International Genetics of Ankylosing Spondylitis Consortium, Cortes A, Hadler J. et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013;45:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ellinghaus D, Jostins L, Spain SL. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet 2016;48:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 2011;22:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kutukculer N, Puel A, Eren Akarcan S. et al. Deficiency of interleukin-1 receptor antagonist: a case with late onset severe inflammatory arthritis, nail psoriasis with onychomycosis and well responsive to adalimumab therapy. Case Reports Immunol 2019;2019:1902817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arend WP, Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res 2000;2:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akitsu A, Ishigame H, Kakuta S. et al. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2+Vγ6+γδ T cells. Nat Commun 2015;6:6–7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lea W-I, Lee YH. The associations between interleukin-1 polymorphisms and susceptibility to ankylosing spondylitis: a meta-analysis. Joint Bone Spine 2012;79:370–4. [DOI] [PubMed] [Google Scholar]
- 50. Sims AM, Timms AE, Bruges-Armas J. et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirms associations with ankylosing spondylitis. Ann Rheum Dis 2007;67:1305–9. [DOI] [PubMed] [Google Scholar]
- 51. Reveille JD, Sims AM, Danoy P. et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 2010;42:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Momenzadeh P, Mahmoudi M, Beigy M. et al. Determination of IL1R2, ANTXR2, CARD9, and SNAPC4 single nucleotide polymorphisms in Iranian patients with ankylosing spondylitis. Rheumatol Int 2016;36:429–35. [DOI] [PubMed] [Google Scholar]
- 53. Simone D, Al Mossawi MH, Bowness P. Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology (Oxford) 2018;57:vi4–vi9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. El-Zayadi AA, Jones EA, Churchman SM. et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford) 2017;56:488–93. [DOI] [PubMed] [Google Scholar]
- 55. Bridgewood C, Watad A, Russell T. et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann Rheum Dis 2019;78:929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watad A, Rowe H, Russell T. et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis 2020; doi: 10.1136/annrheumdis-2020-217309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 2019;15:747–57. [DOI] [PubMed] [Google Scholar]
- 58. Cuthbert RJ, Watad A, Fragkakis EM. et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis 2019;78:1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Tok MN, Na S, Lao CR. et al. The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin-23 signaling. Front Immunol 2018;9:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith JA. The role of the unfolded protein response in axial spondyloarthritis. Clin Rheumatol 2016;35:1425–31. [DOI] [PubMed] [Google Scholar]
- 61. Klasen C, Meyer A, Wittekind PS. et al. Prostaglandin receptor EP4 expression by Th17 cells is associated with high disease activity in ankylosing spondylitis. Arthritis Res Ther 2019;21:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang J, Wang JH. Production of PGE2 increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res 2009;28:198–203. [DOI] [PubMed] [Google Scholar]
- 63. Kroon FP, van der Burg LR, Ramiro S. et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev 2015;7:CD010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bollow M, Fischer T, Reiβhauer H. et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis—cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 2000;59:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hacquard-Bouder C, Falgarone G, Bosquet A. et al. Defective costimulatory function is a striking feature of antigen-presenting cells in an HLA-B27-transgenic rat model of spondylarthropathy. Arthritis Rheum 2004;50:1624–35. [DOI] [PubMed] [Google Scholar]
- 66. Hacquard-Bouder C, Falgarone G, Bosquet A. et al. Defective costimulatory function is a striking feature of antigen-presenting cells in an HLA-B27-transgenic rat model of spondylarthropathy. Arthritis Rheum 2004;50:1624–35. [DOI] [PubMed] [Google Scholar]
- 67. Pende D, Falco M, Vitale M. et al. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol 2019;10:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bird LA, Peh CA, Kollnberger S. et al. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol 2003;33:748–59. [DOI] [PubMed] [Google Scholar]
- 69. Wong-Baeza I, Ridley A, Shaw J. et al. KIR3DL2 binds to HLA-B27 dimers and free h chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J Immunol 2013;190:3216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kollnberger S, Bird L, Sun M-Y. et al. Cell-surface expression and immune receptor recognition of HLA–B27 homodimers. Arthritis Rheum 2002;46:2972–82. [DOI] [PubMed] [Google Scholar]
- 71. Fechtenbaum M, Desoutter J, Delvallez G. et al. MICA and NKG2D variants as risk factors in spondyloarthritis: a case–control study. Genes Immun 2019;20:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rachitskaya AV, Hansen AM, Horai R. et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 2008;180:5167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jacques P, Venken K, Van Beneden K. et al. Invariant natural killer T cells are natural regulators of murine spondylarthritis. Arthritis Rheum 2010;62:988–99. [DOI] [PubMed] [Google Scholar]
- 74. Lawand M, Déchanet-Merville J, Dieu-Nosjean M-C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol 2017;8:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ono T, Okamoto K, Nakashima T. et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 2016;7:10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Geremia A, Arancibia-Cárcamo CV. Innate lymphoid cells in intestinal inflammation. Front Immunol 2017;8:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Spits H, Artis D, Colonna M. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- 78. Ciccia F, Guggino G, Rizzo A. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:1739–47. [DOI] [PubMed] [Google Scholar]
- 79. Cuthbert RJ, Fragkakis EM, Dunsmuir R. et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol (Hoboken) 2017;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 80. Kjer-Nielsen L, Corbett AJ, Chen Z. et al. An overview on the identification of MAIT cell antigens. Immunol Cell Biol 2018;96:573–87. [DOI] [PubMed] [Google Scholar]
- 81. Chiba A, Tajima R, Tomi C. et al. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum 2012;64:153–61. [DOI] [PubMed] [Google Scholar]
- 82. Toussirot É, Laheurte C, Gaugler B, Gabriel D, Saas P. Increased IL-22- and IL-17A-producing mucosal-associated invariant T cells in the peripheral blood of patients with ankylosing spondylitis. Front Immunol 2018;9:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee JS, Tato CM, Joyce-Shaikh B. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015;43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maxwell JR, Zhang Y, Brown WA. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 2015;43:739–50. [DOI] [PubMed] [Google Scholar]
- 85. Appel H, Maier R, Bleil J. et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum 2013;65:1522–9. [DOI] [PubMed] [Google Scholar]
- 86. Dhaenens M, Fert I, Glatigny S. et al. Dendritic cells from spondylarthritis-prone HLA-B27-transgenic rats display altered cytoskeletal dynamics, class II major histocompatibility complex expression, and viability. Arthritis Rheum 2009;60:2622–32. [DOI] [PubMed] [Google Scholar]
- 87. Slobodin G, Kessel A, Kofman N. et al. Phenotype of resting and activated monocyte-derived dendritic cells grown from peripheral blood of patients with ankylosing spondylitis. Inflammation 2012;35:772–5. [DOI] [PubMed] [Google Scholar]
- 88. Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum 2005;52:3586–95. [DOI] [PubMed] [Google Scholar]
- 89. Yang M, Zhou Y, Liu L. et al. Decreased A20 expression on circulating CD56bright NK cells contributes to a worse disease status in patients with ankylosing spondylitis. Clin Exp Immunol 2019;198:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Venken K, Jacques P, Mortier C. et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in spondyloarthritis patients. Nat Commun 2019;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Soare A, Weber S, Maul L. et al. Cutting edge: homeostasis of innate lymphoid cells is imbalanced in psoriatic arthritis. J Immunol 2018;200:1249–54. [DOI] [PubMed] [Google Scholar]