Abstract
Background
Glioblastoma is associated with poor prognosis and high mortality. Although the use of first-line temozolomide can reduce tumor growth, therapy-induced stress drives stem cells out of quiescence, leading to chemoresistance and glioblastoma recurrence. The specificity protein 1 (Sp1) transcription factor is known to protect glioblastoma cells against temozolomide; however, how tumor cells hijack this factor to gain resistance to therapy is not known.
Methods
Sp1 acetylation in temozolomide-resistant cells and stemlike tumorspheres was analyzed by immunoprecipitation and immunoblotting experiments. Effects of the histone deacetylase (HDAC)/Sp1 axis on malignant growth were examined using cell proliferation–related assays and in vivo experiments. Furthermore, integrative analysis of gene expression with chromatin immunoprecipitation sequencing and the recurrent glioblastoma omics data were also used to further determine the target genes of the HDAC/Sp1 axis.
Results
We identified Sp1 as a novel substrate of HDAC6, and observed that the HDAC1/2/6/Sp1 pathway promotes self-renewal of malignancy by upregulating B cell-specific Mo-MLV integration site 1 (BMI1) and human telomerase reverse transcriptase (hTERT), as well as by regulating G2/M progression and DNA repair via alteration of the transcription of various genes. Importantly, HDAC1/2/6/Sp1 activation is associated with poor clinical outcome in both glioblastoma and low-grade gliomas. However, treatment with azaindolyl sulfonamide, a potent HDAC6 inhibitor with partial efficacy against HDAC1/2, induced G2/M arrest and senescence in both temozolomide-resistant cells and stemlike tumorspheres.
Conclusion
Our study uncovers a previously unknown regulatory mechanism in which the HDAC6/Sp1 axis induces cell division and maintains the stem cell population to fuel tumor growth and therapeutic resistance.
Keywords: glioblastoma, HDAC6, HDAC inhibitor, Sp1, temozolomide
Key Points.
Sp1 is a novel substrate of HDAC6.
Sp1 deacetylation mediated by HDAC1/2/6 affects the malignant behavior of GBM cells.
Targeting of HDAC1/2/6 is a promising treatment approach for chemoresistant GBM.
Importance of the Study.
Temozolomide is the first-line chemotherapeutic drug used to treat glioblastoma, although drug resistance occurs in most patients irrespective of their prior response to initial treatment. However, the molecular mechanisms underlying the stress-induced Sp1 activation leading to drug resistance remains unclear. In this study, we observed that HDAC1/2/6 were interacted with Sp1 in temozolomide-resistant glioblastoma cells and induced Sp1 deacetylation, thereby elevating levels of BMI1, hTERT, and cell cycle genes to trigger therapy resistance. In addition, although Sp1 has been shown to be involved in multiple cellular functions by regulating the expression of downstream genes, most previous studies have mainly focused on one or a few Sp1 target genes. The lack of clinical relevance is also a big issue leading to poor connection between basic research and clinical application. Herein, we performed a systematic approach to provide a “macro perspective” of the HDAC/Sp1 regulatory pathway in glioblastoma recurrence.
Glioblastoma (GBM) generally shows poor prognosis and is not considered curable even after treatment with the first-line chemotherapeutic drug temozolomide (TMZ). In clinical studies, approximately 90% of patients showed disease recurrence within 2 years of treatment, irrespective of their prior response to initial treatment.1 Although the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) is considered a critical player in TMZ resistance,2 recent studies have shown that MGMT is silenced in approximately 50% of GBM patients and the development of resistance is typically inevitable.3 Therefore, development of therapeutic resistance in GBM is complex, especially for patients with MGMT deficiency. A small subset of cells within a malignant tumor, known as cancer stem cells (CSCs), can initiate tumor growth.4 They are also more resistant to anticancer therapeutics than the bulk of tumor cells,5,6 suggesting that most CSCs are capable of surviving the therapy and eventually become the underlying cause of tumor recurrence.
Stress conditions such as inflammation, hypoxia, and therapy induce genotoxic stress/oxidative damage affect CSCs, which occur in niches that maintain the stemlike properties of these cells.7,8 Furthermore, studies have shown that stressful conditions such as serum deprivation and anchorage independence sphere culture preferentially promote the survival of the highly malignant CSC-like cells, enabling selection of subclones within a tumor.9 Previously, we demonstrated that specificity protein 1 (Sp1) is a stress-activated transcription factor, which upregulates genes that protect from stress-induced cell damage.10,11 Recently, we showed that Sp1 expression increases drastically in both TMZ-resistant GBM cells and serum-free/suspension-adapted tumorspheres, which upregulated the expression of antioxidant and CSC-related genes, protecting malignant cells against therapeutic drugs and stress conditions.12,13 Although microenvironmental stress alters the expression of Sp1 target genes, which may lead to the rewiring of TMZ-sensitive GBM cells into a resistant CSC-like state, the molecular mechanisms underlying stress-induced Sp1 activation leading to drug resistance remains unclear. In addition, the number of genes that are targeted by Sp1 to regulate and facilitate the stemlike properties of GBM requires detailed investigation.
Mounting evidence indicates an association between misregulated histone deacetylase (HDAC) activity and many oncological diseases. For example, several HDAC isoforms are upregulated in various tumors, and blockage of their functions by HDAC inhibitors reduces the proliferation and metastatic potential of tumor cells, and even resensitize tumor cells to primary agents, thereby overcoming therapy resistance.14 Several studies have also revealed that HDACs are important for maintaining CSC-related properties in malignant tumors.15,16 Our recent study further demonstrated that the HDAC inhibitor (HDACI) suberoylanilide hydroxamic acid (SAHA) elicits checkpoint activation and apoptosis in GBM cell-derived tumorspheres.17 Interestingly, Sp1 is known to interact with HDAC1, and Sp1 functions, including transcriptional activity, DNA binding, and cofactor recruitment, are tightly controlled by the only known acetylated residue, K703, of Sp1.18,19 Although both Sp1 and HDACs are known as critical risk factors for cancer recurrence after treatment; whether chemotherapy agent-induced stress affects Sp1 acetylation and the identity of the HDAC(s) that specifically alters Sp1 function to mediate GBM cell resistance to TMZ remains unclear.
To determine the regulatory mechanisms of TMZ resistance in malignant gliomas, we hypothesized that exposure of GBM to TMZ may activate the HDAC/Sp1 pathway in malignant progenitor/CSC-like cell populations that are prone to survive TMZ-induced cytotoxicity. By investigating tumorspheres and TMZ-resistant cells, we identified a novel interaction between HDAC6 and Sp1, and observed that HDAC1/2/6 induced Sp1 deacetylation, upregulating expression of B cell-specific Mo-MLV integration site 1 (BMI1), human telomerase reverse transcriptase (hTERT), and several mitotic genes, which promoted CSC-like cell proliferation and suppressed chemotherapy agent–elicited tumor inhibition. However, inhibition of HDAC1/2/6 by an azaindolyl sulfonamide (MPT0B291) caused Sp1 acetylation and GBM cell sensitization to TMZ, and resulted in senescence of both TMZ-resistant cells and tumorspheres.
Materials and Methods
Cell Culture
Human GBM cell lines U87MG and A172 (both American Type Culture Collection), 2 patient-derived GBM lines, P3 and P11, as well as their TMZ-resistant cells and tumorspheres, were cultivated in different culture media as described in previous studies.12,13,17
Experimental Animals
All experiments and animal care were conducted in accordance with the guidelines and under the supervision of Institutional Animal Care and Use Committee (IACUC-106010), the National Health Research Institutes (NHRI, Taiwan). Male NOD.CB17-Prkdcscid/NcrCrlBltw (NOD/SCID) mice (5–6 weeks old, BioLASCO) were maintained at the animal facility of NHRI.
Statistical Analysis
All experiments were performed more than three independent times with duplicate samples in each test. Statistical analyses of the data from the immunoblotting (IB), immunoprecipitation (IP), chromatin IP (ChIP), telomeric repeat amplification protocol (TRAP), and viability assays were performed using the unpaired two-tailed Student’s t-test. The level for statistical significance was set at less than 0.05.
The details of materials and other methods are described in the Supplementary Material.
Results
Sp1 Is Deacetylated by HDAC1/2/6 in Both TMZ-Resistant Cells and GBM Spheroids
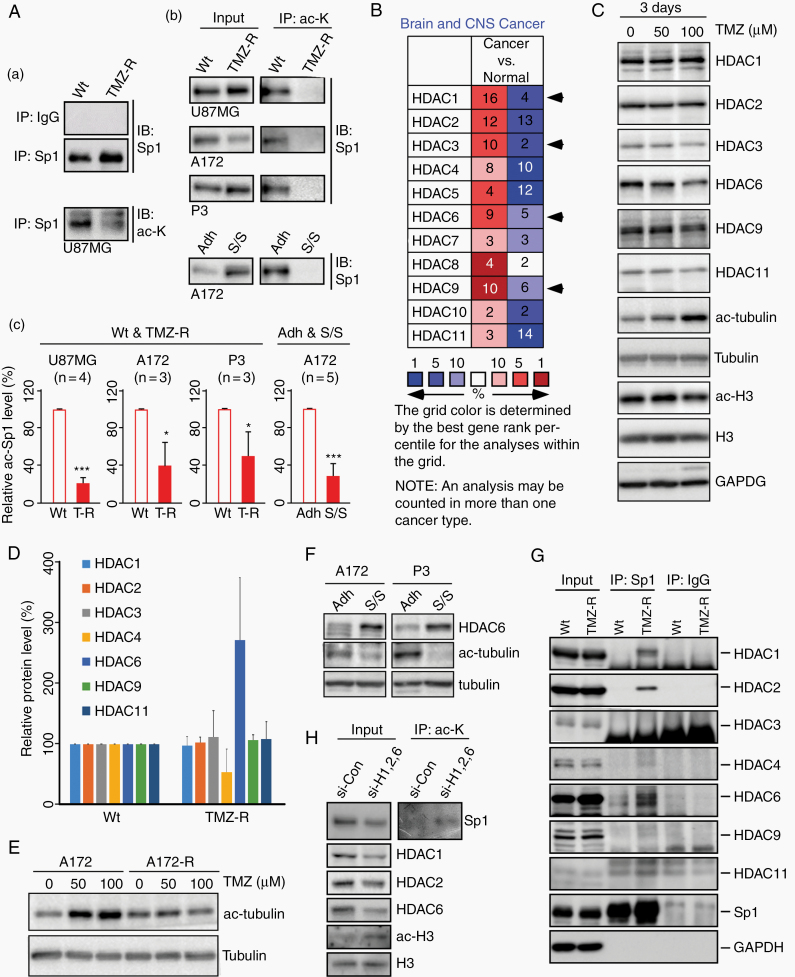
Lysine acetylation within the Sp1 DNA-binding motif is known to alter Sp1 activity. Here, we identified a significant decrease in Sp1 acetylation in several TMZ-resistant GBM cell lines and serum-free/suspension-adapted tumorspheres compared with their normal controls (Fig. 1A). We also investigated the transcript levels of HDAC family members using the Oncomine database (https://www.oncomine.org/) and observed that HDAC1/3/6/9 were overexpressed in brain malignancies in the various microarray datasets (the “n” of each grid is the number of microarray datasets with statistically significant expression changes; Fig. 1B), implying a critical role for certain HDACs in active glioma tumorigenesis. Interestingly, while GBM cell proliferation was inhibited after 3 days of TMZ treatment (data not shown), the expression of certain HDACs, especially that of the tubulin deacetylase HDAC6, was also attenuated, which caused tubulin acetylation (Fig. 1C and Supplementary Figure 1A). However, when cell viability recovered after weeks to one month of TMZ treatment, HDAC6 was upregulated in these resistant cells (Fig. 1D and Supplementary Figure 2A) and TMZ had no effect on tubulin acetylation (Fig. 1E and Supplementary Figure 1B). Furthermore, we observed elevated HDAC6 expression and reduced tubulin acetylation in GBM spheres (Fig. 1F and Supplementary Figure 2B). Co-IP studies were performed to investigate whether HDACs regulate Sp1 acetylation in resistant cells, and Sp1 interaction with HDAC1/2/6 was observed in TMZ-resistant GBM cells (Fig. 1G). In addition, knockdown of HDAC1/2/6 elevated Sp1 acetylation in the resistant cells (Fig. 1H). These data reveal that HDAC6 and/or HDAC1/2 is responsible for the deacetylation of Sp1, which may affect the malignant behavior of GBM cells.
Fig. 1.
Sp1 is deacetylated by HDAC1/2/6 in TMZ-resistant GBM cells. (A) The wild-type (Wt) and TMZ-resistant (TMZ-R) GBM cells,12 as well as GBM spheroids formed in serum-free medium/suspension (S/S) culture and the control attached (Adh) cells13 were used for the immunoprecipitation (IP) assay with rabbit IgG, anti-Sp1 (Sp1, panel a), and anti–acetyl-lysine (ac-K, panels b and c) antibodies, and analyzed using immunoblotting (IB) as indicated. In panel c, the protein level of acetylated Sp1 was normalized to its total protein and quantified. (B) Gene expression profiles of HDACs in brain tumors were analyzed using the Oncomine database. HDAC1/3/6/9, shown by the arrows, were upregulated more in cancer tissues than in normal samples. Red indicates upregulation; blue indicates downregulation. The number in the cell represents the number of datasets that pass the filter criteria (threshold: P < 0.05). (C to F) Cells were harvested and analyzed using IB. The Wt (C and E) and TMZ-R (E) A172 cells were treated with the indicated concentrations of TMZ for 3 days. (D) The protein expression of HDACs in Wt and TMZ-R P11 GBM cells was normalized to the loading control and quantified. (F) The levels of HDAC6 and tubulin acetylation in attached GBM cells and tumorspheres. (G) Wt and TMZ-R P11 cells were used for IP assay with anti-Sp1 antibodies and rabbit IgG, and analyzed using IB as indicated. (H) TMZ-R U87MG cells were transfected with a nontargeting control siRNA or HDAC1/2/6-specific siRNAs as indicated. After knockdown, the cells were used for IP assay. (t-test: *P < 0.05, ***P < 0.001)
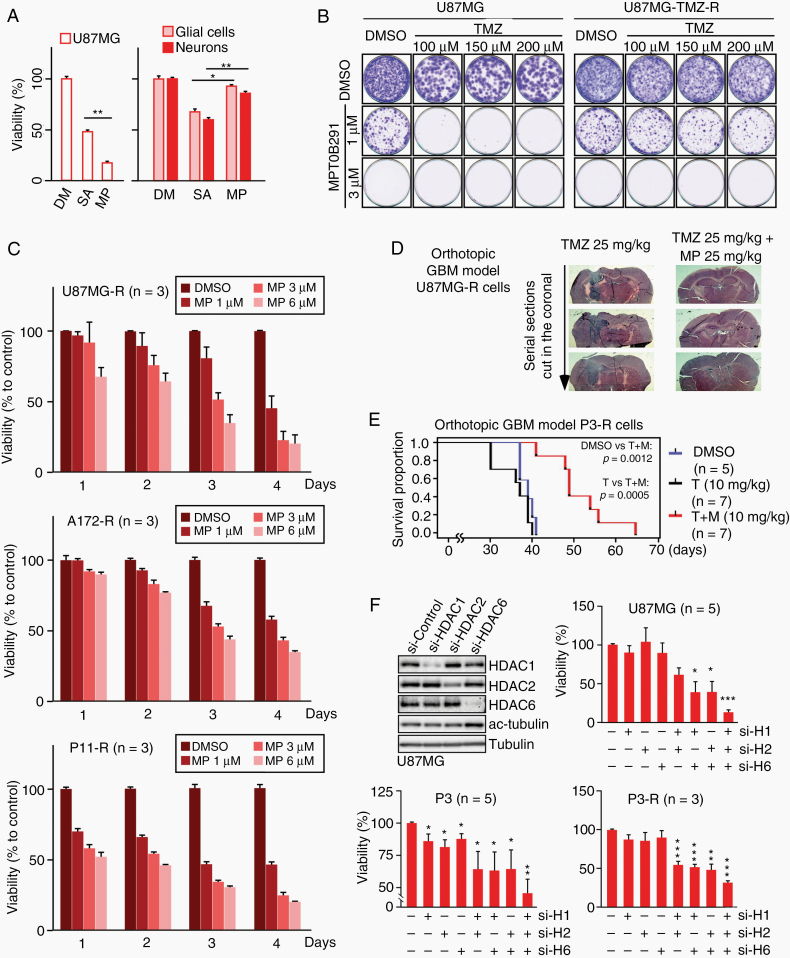
Inhibition of HDAC1/2/6 restricts the growth of both TMZ-resistant GBM cells and their parental TMZ-sensitive cells. We further confirmed the roles of HDAC1/2/6 in TMZ resistance. HDACIs were used, including a pan-HDACI, trichostatin A, a class I selective HDACI SAHA, and 4 potent HDAC6 inhibitors (nexturastat A, tubacin, tubastatin A, and MPT0B291) (Supplementary Table 1 and Supplementary Figure 3). After evaluating the cytotoxic effects of these inhibitors using primary glial cell culture (Supplementary Figure 4A), 2 cytotoxic agents, trichostatin A and tubacin, were excluded. After comparing SAHA with the 3 remaining HDAC6 inhibitors, we identified that MPT0B291 was more potent than nexturastat A and tubastatin A in inhibiting HDAC1/2 (Supplementary Table 1), and exhibited better tumoricidal activity but lower neuronal/glial toxicity than SAHA (Fig. 2A). The effect of MPT0B291 on TMZ-sensitive and TMZ-resistant GBM cells was subsequently investigated, and results showed that treatment with low concentrations (1 μM) of MPT0B291 enhanced the sensitivity of wild-type U87MG cells to TMZ (Fig. 2B). In addition, MPT0B291 also induced a dose- and time-dependent decrease in the number of TMZ-resistant cells (Fig. 2B, C), but only slightly reduced the survival of primary glial cells (Supplementary Figure 4B). Furthermore, orthotopic transplantation models of GBM cells, including TMZ-sensitive and TMZ-resistant cells (Supplementary Figure 5 and Fig. 2D, E), were developed. Consistently, MPT0B291 attenuated tumor growth and prolonged mouse survival in these models. Using small interfering (si)RNAs for reducing HDAC expression, we verified that combined inhibition of HDAC1/2/6, but not of each HDAC, significantly suppressed GBM cell viability (Fig. 2F), suggesting that HDAC1/2/6 are promising targets for brain malignancy.
Fig. 2.
HDAC1/2/6 inhibition significantly reduces the growth rates of TMZ-resistant GBM cells. (A) U87MG cells, as well as primary cultures of neurons and glial cells, were treated with 1 μM SAHA (SA), 1 μM azaindolyl sulfonamide compound 12 (MPT0B291, MP), or dimethyl sulfoxide (DMSO) (DM) for 4 days. After treatment, cell viability was assessed using colorimetric MTT assay. (B) In the focus formation assay, parental and TMZ-resistant (TMZ-R) U87MG cells were seeded at low density onto 60-mm plates, and treated with TMZ or MP alone or in combination at different doses every 3 days. Following a 2-week incubation period, the forming foci were stained using crystal violet. Representative images are shown. (C) TMZ-R GBM cell lines, including U87MG-R, A172-R, and P11-R cells, were treated with DMSO or different doses of MP (1, 3, 6 μM) for various time intervals (1 to 4 days). Cell viability was assessed using the MTT assay. (D) TMZ-R U87MG inoculated orthotopic mice were treated with 25 mg/kg TMZ to maintain a TMZ-resistant phenotype, and co-treated with or without 25 mg/kg MP every 2 days for 3 weeks. The brain tumors were observed using serial histology sections along the tumor using hematoxylin and eosin staining. (E) TMZ-R P3 inoculated orthotopic mice were randomly grouped and treated with DMSO, 10 mg/kg TMZ (T), or TMZ plus 10 mg/kg MP (T+M) every 2 days. Survival was plotted using a Kaplan–Meier curve. (F) Cells were transfected with HDAC1-, HDAC2-, and/or HDAC6-specific siRNAs or a nontargeting control siRNA as indicated for 2 days. After knockdown, cell viability was assessed using the MTT assay. (t-test: *P < 0.05, **P < 0.01, ***P < 0.001)
HDAC1/2/6 Regulate Sp1 Levels
As HDAC1/2/6 interacted with Sp1 in TMZ-resistant cells, the effect of MPT0B291-induced HDAC inhibition on Sp1 was investigated further. In green fluorescent protein (GFP)-Sp1 overexpressing A172 cells and GBM stemlike tumorspheres, an obvious increase in Sp1 acetylation was observed after treatment with MPT0B291 (Supplementary Figure 6A, B). Interestingly, endogenous Sp1 level was attenuated by MPT0B291 in a dose-dependent manner (Supplementary Figure 6B‒D), as well as by HDAC1/2/6 knockdown (Supplementary Figure 6E), implying that Sp1 deacetylation induced by HDAC1/2/6 plays important roles in supporting high expression of Sp1 in TMZ-resistant and tumorsphere cells. Protein posttranslational modifications (PTMs) are well known to be able to impact protein folding, stability, and activation. Thus, we subsequently investigated the effect of HDAC inhibition mediated by MPT0B291 or gene silencing on Sp1 protein stability. Blocking protein synthesis with cycloheximide showed that HDAC inhibition did not affect Sp1 stability (Supplementary Figure 7A). As lysine acetylation affects Sp1 transcriptional activities20 and Sp1 positively regulates its own transcription,21 HDAC inhibition-induced protein acetylation may have an influence on Sp1 to transactivate its own promoter. Hence, we measured Sp1 mRNA level in tumorspheres and resistant cells and observed a significant reduction in Sp1 expression after MPT0B291 treatment or HDAC1/2/6 knockdown (Supplementary Figure 7B). Furthermore, by performing a DNA-affinity precipitation assay, we verified that MPT0B291 treatment reduced binding of Sp1 to DNA (Supplementary Figure 7C). In summary, these results indicate that HDAC1/2/6 are essential for the regulation of Sp1 activity and its expression in GBM cells.
The HDAC1/2/6/Sp1 Axis Mediates Abnormal Expression of Cell Cycle–Related Genes in GBM
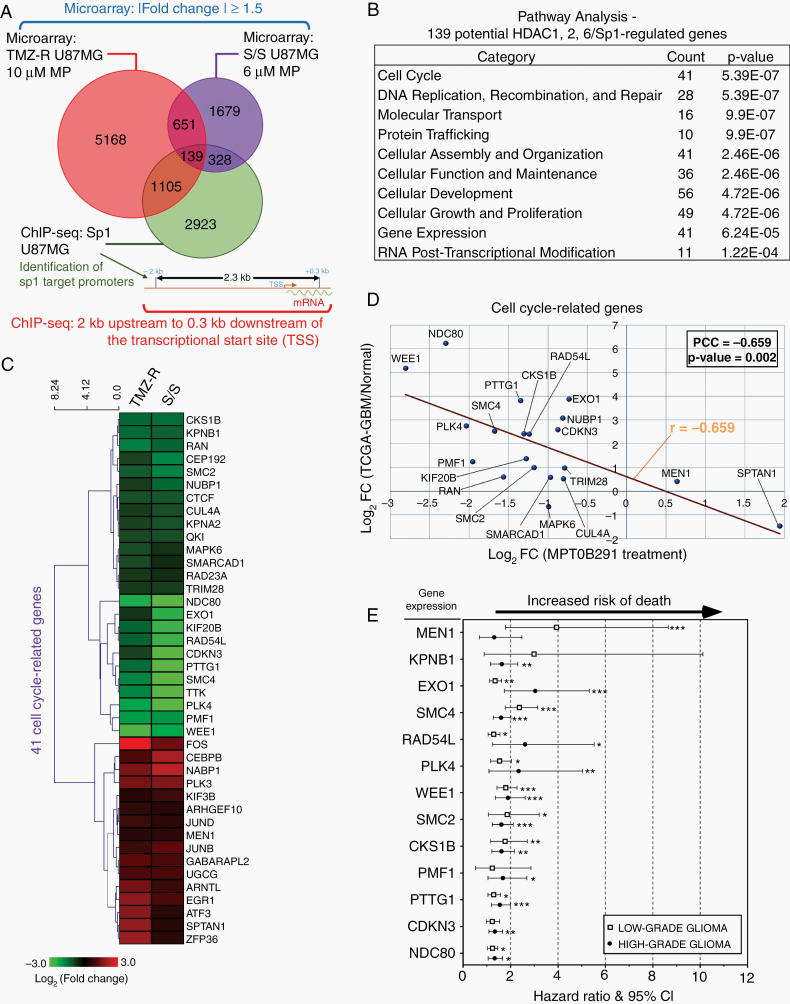
HDAC1/2/6 may not target only the Sp1 promoter. To elucidate the gene expression profiles along the HDAC1/2/6/Sp1 axis, we performed microarray analysis in TMZ-resistant cells and tumorspheres. Comparison of the tumor transcriptomes revealed that the levels of 5168 distinct mRNAs in resistant cells and 1679 distinct mRNAs in tumorspheres were statistically significantly altered by MPT0B291, in which 651 overlapping genes were identified from the 2 categories (Fig. 3A). To also determine the Sp1 target genes, we performed ChIP-seq analysis and generated genome-wide binding profiles of Sp1. Sequencing of Sp1-ChIP–derived DNA fragments identified 2923 potential Sp1 target loci that were within −2000 bp to +300 bp regions flanking the transcription start sites of genes. After the integration of the ChIP-seq data with microarray data, 139 intersection genes were found to exhibit consistent changes in the extent of Sp1 binding and gene expression levels (Fig. 3A). Functional classification of the 139 genes using immunoprecipitation assay (IPA) identified that the highest scored canonical pathway was “cell cycle,” containing 41 genes (Fig. 3B), among which 25 were downregulated and 16 were upregulated after MPT0B291 treatment (Fig. 3C). These results suggested that the HDAC/Sp1 axis may primarily affect cell cycle progression, protecting GBM cells against therapeutic drugs and stress conditions.
Fig. 3.
The HDAC/Sp1 pathway plays an important role in regulating cell cycle progression and proliferation. (A) Venn diagram illustrating overlaps between number of genes that were altered by more than 1.5–fold in TMZ-resistant (TMZ-R) cells and in spheroids (serum-free medium/suspension culture, S/S) following MP treatment and were also targeted by Sp1 in U87MG cells. (B) The IPA software program was applied on 139 potential HDACs/Sp1-regulated genes (the intersection genes of Sp1 ChIP-seq data and gene expression microarray data from MP-treated TMZ-R and S/S in [A]) to identify top 10 scoring canonical pathways. (C) Heat map representing the expression levels of 41 cell cycle–related genes, obtained from IPA analysis in (B), following MP treatment. (D) Relationships between MP-treated microarray data (in horizontal) and TCGA-GBM NGS data (in vertical) using Pearson’s correlation coefficient (PCC, r). Each dot represents the expression value of a cell cycle-related gene. (E) Forest plots showing hazard ratios for risk of death in low-grade and high-grade glioma patients with higher expression of the indicated gene(s). The lines on both sides denote 95% confidence intervals. All the original data (Kaplan–Meier curve) were obtained from PROGgeneV2 (Supplementary Figure 15A). Hazard ratios above 1 indicate a worse outcome. (t-test: *P < 0.05, **P < 0.01, ***P < 0.001)
A recent study showed that reversal of gene expression abnormalities correlates with drug efficacy in several diseases, including breast, liver, and colon cancers.22 Thus, we analyzed The Cancer Genome Atlas (TCGA)-GBM next-generation sequencing (NGS) dataset of 124 patients to investigate the dysregulation and clinical relevance of the 41 cell cycle–related genes. Twenty of these genes showed significant differences (P < 0.05) between solid GBM tumors and normal tissue specimens. Comparison of the expression of 20 genes between MPT0B291-treated samples and GBM patients revealed a strong negative correlation (Pearson’s r = −0.659) (Fig. 3D), indicating that inhibition of HDAC1/2/6 by MPT0B291 may reverse the abnormalities of the cell cycle–related genes in GBM. Moreover, analysis of the clinical outcome data showed that higher expression of several cell cycle–related genes (MEN1, KPNB1, EXO1, SMC4, RAD54L, PLK4, WEE1, SMC2, CKS1B, PMF1, PTTG1, CDKN3, NDC80) in high-grade and/or low-grade gliomas was associated with decreased survival (Fig. 3E). Therefore, these data provided a foundation for clinical practice in the future.
MPT0B291 Induces Premature Senescence in Both Tumorspheres and TMZ-Resistant Cells
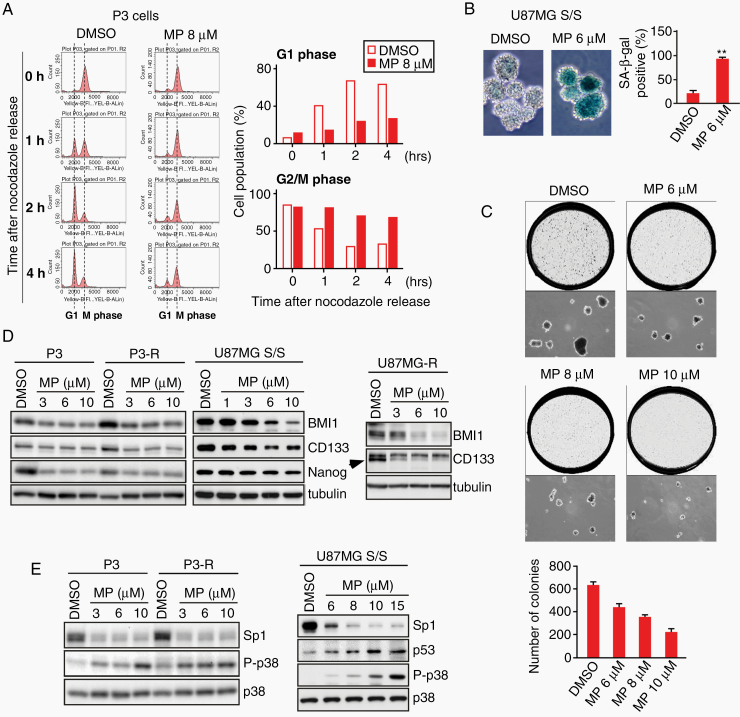
Flow cytometry was performed to assess the cell cycle progression of tumor cells. Results showed that MPT0B291 blocked entry into the G1 phase from mitosis of cells following release from the nocodazole-induced cell cycle synchronization (Fig. 4A). As the HDAC/Sp1 axis also affects cellular development–related genes (Fig. 3B), and a developmentally regulated cell cycle arrest is the fundamental feature of neuro/gliogenesis, we investigated whether MPT0B291 promoted tumorsphere differentiation into glial-like cells. However, changes in the expression of the glial marker glial fibrillary acidic protein were not observed after MPT0B291 treatment (Supplementary Figure 8). Studies have shown that the senescence program may be initiated after cell cycle arrest in G1 and G2 phases.23,24 Therefore, cellular senescence was further investigated by measuring the activity of senescence-associated beta-galactosidase (SA-β-gal), a direct marker of cellular senescence; results showed that about 95% of spheroid bodies became senescent after MPT0B291 treatment (Fig. 4B). In addition, we performed a soft agar assay to investigate whether MPT0B291 affected the self-renewing capacity of single cells dissociated from tumorspheres. As expected, MPT0B291 suppressed the clonogenicity of CSC-like cells in a dose-dependent manner (Fig. 4C). Furthermore, the expression of the stem cell markers CD133, BMI1, and Nanog, known to correlate with the malignant phenotype of GBM, was significantly downregulated by MPT0B291 in both TMZ-resistant cells and tumorspheres (Fig. 4D). A corresponding increase in the levels of the G2/M checkpoint regulators, including p53 and phospho-p38,25 was observed after MPT0B291 treatment (Fig. 4E). These results show that HDAC inhibition by MPT0B291 interrupted cell cycle progression, leading to premature senescence in CSC-like cells.
Fig. 4.
MPT0B291 induces senescence and diminished the expression stemness-related markers in both GBM spheroids and TMZ-resistant (TMZ-R) cells. (A) Mitotic P3 cells were released into the cell cycle by removing nocodazole from the culture, and then harvested at different time points as indicated. Cells were then fixed for cell cycle progression assay using flow cytometry. The percentages of cells in G1 phase and G2/M phase are shown in right panel. (B) U87MG spheroids (S/S) were treated with DMSO or MP. At 4 days posttreatment, senescence was examined using SA β-gal staining. Photomicrographs of spheroids were randomly selected in microscopic fields, and SA-β-gal positive cells were counted. (C) Cells dissociated from U87MG spheroids via trypsinization were grown in soft agar, followed by treatment with different doses of MP every 4 days. After 3 weeks of incubation at 37°C, the colonies that arose from these single cells were photographed randomly, and a histogram of average colony numbers was plotted after performing the experiment in triplicate. (D and E) Cells, as indicated, after 2 days of MP treatment were harvested, and the cell lysates were analyzed using IB with the indicated antibodies. (t-test: **P < 0.01)
MPT0B291 Reduces the Expression of Stemness-Related Genes and Telomerase Activity in an Sp1-Dependent Manner
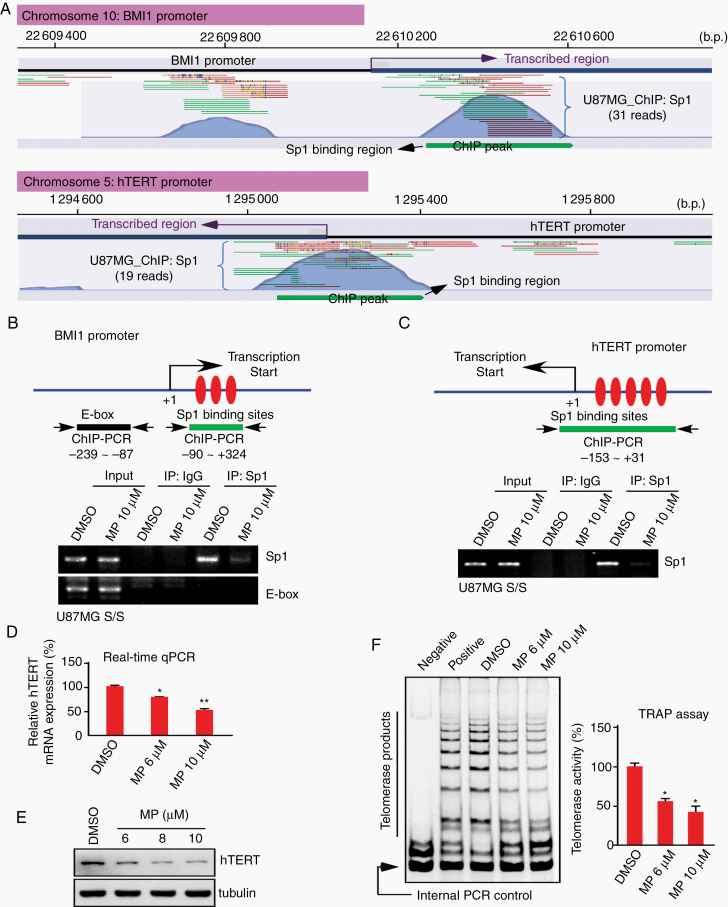
The BMI1-telomerase cascade plays an essential role in cell cycle progression and self-renewal of CSCs.26 Sp1/ChIP-seq data analysis showed that both BMI1 and hTERT are targets of Sp1 (Fig. 5A). Furthermore, the results of ChIP verified that Sp1 binds in vivo to the +90 to +324 region of the BMI promoter and to the −153 to +31 region of the hTERT promoter, although these bindings were significantly reduced when tumorspheres were treated with MPT0B291 (Fig. 5B, C). We observed that MPT0B291 can attenuate BMI1 protein levels (Fig. 4D). The effects of MPT0B291 on hTERT expression and telomerase activity were also investigated; results confirmed that hTERT mRNA, protein, and catalytic activity levels were suppressed by MPT0B291 in a dose-dependent manner (Figures 5D‒F).
Fig. 5.
MPT0B291 decreases Sp1 binding to BMI1 and hTERT promoters. (A) The Sp1 ChIP-seq reads mapped to the promoter region of BMI1 and hTERT. Forward reads are shown in green and reverse reads are shown in red. The significance of ChIP peaks, generated using the CLC Genomics Workbench 10.1.1 software, indicated Sp1 binding loci. (B and C) U87MG spheroids were treated with DMSO or MP for 6 h, and the level of Sp1 binding to the promoter regions of BMI1 and hTERT was assessed using a ChIP assay with rabbit IgG or anti-Sp1 antibodies. DNA was then extracted from the sample for PCR with the primers as indicated. Rabbit IgG acted as a negative control for nonspecific precipitation, and E-box was used as a negative control for nonspecific binding. (D to F) U87MG spheroids were treated with different doses of MP for 2 days. After treatment, the mRNA (D) and protein (E) levels of hTERT in cells were analyzed using real-time PCR and IB, respectively. Furthermore, relative telomerase activity (F) was also detected using the TRAP assay and normalized to the value of the internal PCR control in each reaction. The cell lysis buffer was used as a negative control. The arrow points to the 36-bp internal control. (t-test: *P < 0.05, **P < 0.01)
Subsequently, gene silencing and overexpression assays were performed to assess whether Sp1 plays a direct role in regulating cell proliferation and self-renewal activities of malignant GBM cells. In GBM tumorsphere cells, siRNA-mediated knockdown of Sp1 significantly attenuated the expression of hTERT and stemness-related genes (BMI1, CD133, SOX2) and increased the levels of p53 and phospho-p38 (Supplementary Figure 9A), which suppressed colony formation on soft agar (Supplementary Figure 9B), and spheroid growth/proliferation (Supplementary Figure 9C), resulting in tumor sphere senescence (Supplementary Figure 9D). Furthermore, Sp1 knockdown also inhibited BMI1 and hTERT, upregulated p53, and induced cell senescence in TMZ-resistant cells (Supplementary Figure 9E, F). On the other hand, in a GFP/Sp1-expressing stable clone, Sp1 overexpression not only elevated hTERT basal levels and activity, but also partially restored the inhibitory effect of MPT0B291 on stemness (CD133) and telomere elongation in spheroid cells (Supplementary Figure 10A, B), thereby preventing cellular senescence (Supplementary Figure 10C).
Activation of HDAC1/2/6/Sp1 Is Associated with Poor Clinical Outcome and Tumor Recurrence for GBM
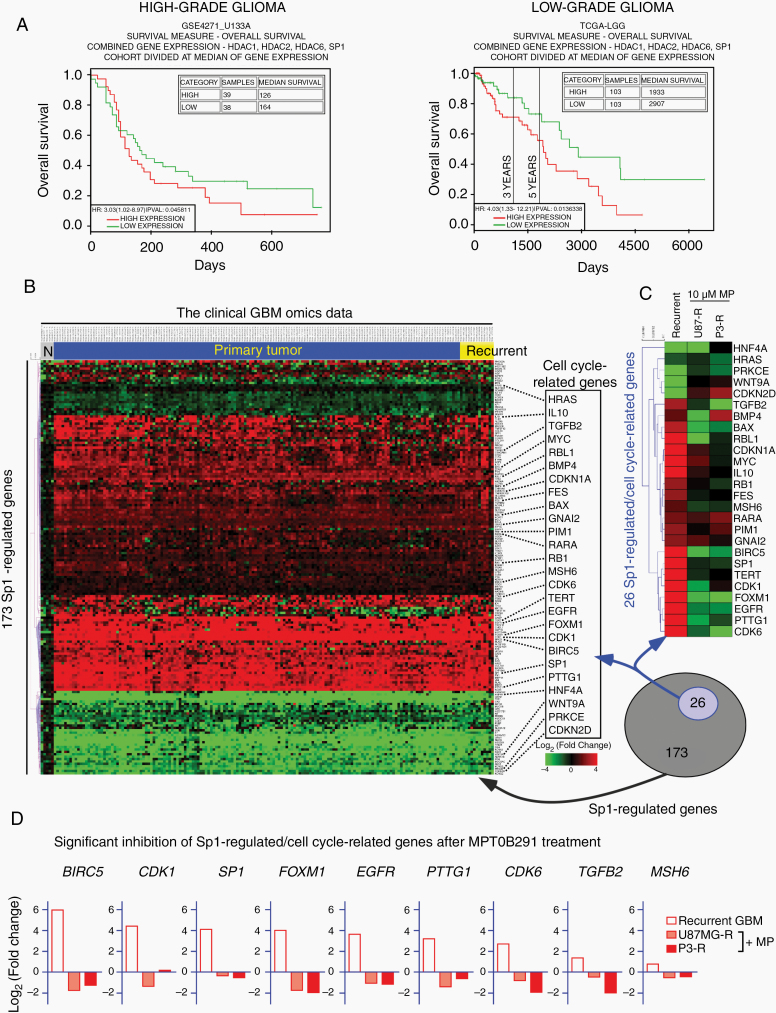
Analysis of the clinical outcome data showed that higher expression of HDAC1/2/6, and Sp1 in both high-grade and low-grade gliomas was associated with decreased survival (Fig. 6A and Supplementary Figure 11). To further confirm the clinical relevance of the HDAC/Sp1 axis and treatment resistance, we used the RNA-seq data of 12 paired primary/recurrent GBM samples obtained from TCGA-GBM and GEO-GSE62153 datasets. Comparison with primary tumors revealed 144 dysregulated Sp1-targeted genes in these recurrent GBM tissues (Supplementary Figure 12A), following integration with the information obtained from the Ingenuity knowledge base annotation, in which 15 cell cycle–related genes were differentially expressed in recurrent tumors. Consistently, 13 of the 15 cycle-related genes (more than 85%) were identified as the targets of the HDAC/Sp1 axis (Fig. 3C). However, comparison with the microarray results of MPT0B291-treated TMZ-resistant cells showed that the expression of these genes was significantly reversed by MPT0B291 (Supplementary Figure 12B). In addition to the RNA-seq data of 12 paired primary/recurrent GBM samples, we further examined clinical GBM omics data, which contain a total of 174 patients, and identified 26 Sp1-regulated/cell cycle–related genes differentially expressed in these GBM tissues, including primary and recurrent tumors (Fig. 6B). Moreover, the expression of certain pro-proliferative genes, such as BIRC5, CDK1, SP1, FOXM1, EGFR, PTTG1, CDK6, TGFB2, and MSH6 was significantly decreased by MPT0B291-induced HDAC inhibition (Fig. 6C, D), suggesting that the HDAC/Sp1 axis is a critical pathway promoting tumor cell growth against current therapies for GBM.
Fig. 6.
The HDAC1/2/6/Sp1 pathway supports GBM pathogenesis and tumor malignancy. (A) Kaplan–Meier survival plots from Phillips et al’s database (GSE4271_U133A, 77 high-grade astrocytomas)35 and from TCGA Brain Lower Grade Glioma database (TCGA-LGG, grades II–III tumors). The gene expression levels of HDAC1/2/6, as well as that of Sp1, were grouped into higher or lower than average (*P < 0.05) groups. (B) Heat map representing the expression levels of 173 Sp1-regulated genes (annotated in Ingenuity knowledge base) in 5 normal brain samples (N), 156 primary tumor, and 13 recurrent tumor samples from TCGA-GBM database (https://portal.gdc.cancer.gov). Compared with the normal brain samples, 26 cell cycle-related genes (based on Gene Ontology annotations) were significantly differentially expressed in recurrent tumors (highlighted in right side). (C) Heat map representing the expression level of 26 Sp1-regulated/cell cycle–related genes in the recurrent GBM tumors and MPT0B291-treated TMZ-R cells. (D) The bar chart further shows the expression profiling (log2 fold change) of 9 significantly downregulated Sp1-regulated/cell cycle–related genes following MPT0B291 treatment.
Discussion
Previous studies have shown Sp1 to be a stress-responsive factor that induces the expression of stemness- and anti-oxidation–related genes.11–13,27 In this study, we observed that HDACs were overexpressed in GBM and that GBM cells can hijack the transcriptional activity of Sp1 via protein deacetylation by HDAC1/2/6, thereby elevating levels of BMI1, hTERT, and cell cycle genes to trigger therapy resistance. BMI1 acts as a repressor of the p16Ink4a/p19Arf senescence pathways and hTERT is critical for maintaining telomere length, both of which are known to promote self-renewal of malignancy (Supplementary Figure 13). Furthermore, the epidermal growth factor receptor/cyclin-dependent kinase (CDK) pathway can trigger DNA replication, RAD54/MSH/EXO1 is involved in the regulation of DNA repair, structural maintenance of chromosomes proteins are essential for chromosomal condensation, pituitary tumor transforming gene 1 is the major effector for chromosome segregation, polo-like kinase 4 induces centrosome amplification, the NDC80 complex is a key complex at the kinetochore-microtubule interface, and forkhead box M1, CDK1, and survivin are important for controlling G2-M transition, all of which are targeted by the HDAC/Sp1 axis (Figures 3D and 6D) and promote cell cycle progression (Supplementary Figure 13). However, inhibition of this axis via pharmacological approaches significantly reduced the proliferation and stemness in TMZ-resistant GBM cells. Therefore, we concluded that the HDAC/Sp1 axis plays an important role in stemlike/drug-resistant GBM cells against TMZ-induced genotoxic stress.
Sp1 has been shown to be involved in multiple cellular functions by regulating the expression of downstream genes. However, most previous studies have mainly focused on one or a few Sp1 target genes. The lack of clinical relevance is also a big issue leading to poor connection between basic research and clinical application. Herein, we performed a systematic approach to provide a “macro perspective” of the HDAC/Sp1 regulatory pathway in GBM recurrence. Analysis of ChIP-seq data showed that 2923 Sp1 target genes were possibly involved in eliciting tumor resistance to TMZ (Fig. 3). Interestingly, we previously showed that the involvement of Sp1 in transcriptional activity, cofactor recruitment, and protein stability is rigorously controlled by distinct PTMs, including phosphorylation, acetylation, methylation, and ubiquitination.20 However, integrated analysis of cDNA microarray and ChIP-seq data revealed that MPT0B291-mediated HDAC1/2/6 inhibition affected the expression of many Sp1 target genes, including 1105 overlapping genes in resistant cells and 328 overlapping genes in tumorspheres (about 45% of total genes identified using ChIP-seq analysis; Fig. 3A), suggesting that acetylation may play a more important role than other PTMs in Sp1 regulation, promoting the malignant behavior of GBM cells. Moreover, MPT0B291-mediated Sp1 acetylation (Supplementary Figure 6A) reduced binding of Sp1 to DNA (Supplementary Figure 7C) and Sp1 acetylation-dead mutant, Sp1-K703A, protected GBM cells against TMZ-induced cell death better than Sp1 wild-type (Supplementary Figure 14), suggesting that Sp1 acetylation indeed affects its activation and TMZ response in GBM cells.
Further investigations regarding the clinical relevance of HDAC1/2/6/Sp1-regulated genes revealed that the expression of these genes, especially those related to cell division, exhibited negative correlation between MPT0B291-treated samples and primary (Fig. 3D) or recurrent tumor samples (Fig. 6 and Supplementary Figure 12) from TCGA-GBM database. These results indicated that HDAC-mediated lysine acetylation/deacetylation plays a critical role in Sp1-induced regulation of cell cycle transitions, which may promote tumor cell division irrespective of treatment-induced “stop” signals, but the HDAC1/2/6 inhibitor MPT0B291 can reverse the cell cycle abnormalities in GBM. In addition, measurements of the parallel artificial membrane permeability assay for blood‒brain barrier (PAMPA-BBB)28 showed that MPT0B291 has better BBB permeability values (PAMPA effective permeability coefficient, Pe: 11.25 ± 0.18 × 10–6 cm/s) than caffeine (Pe: 11.25 ± 0.18 × 10–6 cm/s, data not shown). Thus, MPT0B291 may be a better treatment option for patients with GBM.
Dysregulated HDAC activity and expression are possibly associated with cancer initiation, progression, and recurrence. For instance, a recent study reported that compared with in nonneoplastic controls, the expression of HDAC1/3/6 is significantly increased in GBM samples.29 Furthermore, treatment of GBM cells with trichostatin A, a pan-HDACI, enhanced cellular sensitivity to the alkylating agent lomustine, although the underlying mechanisms remain unclear. In this study, we identified a novel regulatory mechanism via which GBM cells develop resistance against the alkylating agent TMZ; this involves HDAC1/2/6/Sp1-mediated expression of BMI1, hTERT, and cell cycle–related genes in maintaining tumor stemness, telomere length, and cell proliferation. However, how stress conditions can induce activation of the HDAC1/2/6/Sp1 axis remains unclear. Protein kinase C (PKC) is known as a stress sensor important for inducing stress responses in cells.30 Furthermore, previous reports have revealed that PKC delta (δ) is involved in the regulation of histone H3 and H4 deacetylation mediated by HDAC1/2, 31and that PKC alpha (α) and zeta (ζ) phosphorylate HDAC6 to enhance HDAC6 deacetylase activity.32,33 Therefore, we assume that PKCs may act as the upstream regulators of the HDAC1/2/6/Sp1 axis, although whether tumor cells can hijack this axis via PKC to gain therapeutic resistance and uncontrolled proliferation warrants further investigations.
Several cytoplasmic proteins, including α-tubulin, heat shock protein 90, and cortactin, are known to be substrates of HDAC6. Our current results further indicated that nuclear Sp1 is a previously unidentified target of HDAC6, and that HDAC-mediated deacetylation endows Sp1 with the ability to autoregulate its own expression and elevate stemness-/cell cycle–related gene expression, thereby promoting the malignant behaviors of GBM cells (Supplementary Figure 13). Interestingly, stress conditions, such as chemotherapy-induced genotoxicity and oxidative damage, are linked to CSCs,7,8 which upregulate stress signaling pathways to maintain cancer cell survival and stemness.34 The results of Fig. 1 showed that HDAC6 induction, HDAC1/2/6/Sp1 interaction, and Sp1 deacetylation were observed after long-term TMZ treatment or stressful sphere culture, implying that the HDAC1/2/6/Sp1 axis is a stress-response pathway. Notably, we further observed that HDAC1/2/6/Sp1 activation was associated with poor clinical outcome in patients with gliomas. However, inhibition of this axis by MPT0B291 treatment or knockdown approaches caused an obvious induction of G2/M arrest and senescence in both TMZ-resistant cells and stemlike tumorspheres. In conclusion, our study uncovers the HDAC1/2/6/Sp1 axis as an important pathway for cancer chemoprevention/stress protection via maintenance of tumor cell division and stemness. Hence, targeting of this axis can be a promising therapeutic approach for GBM.
Supplementary Material
Funding
This work was financially supported by research grants from the Ministry of Science and Technology in Taiwan (grant numbers 108–2321-B-038–008, and 108–2314-B-182A-052, 108–2320-B-038–013, and 109–2636-B-038–002) and the TMU Research Center of Cancer Translational Medicine from The Featured Areas Research Center Program within the framework of the Higher Education funded by the Ministry of Education in Taiwan.
Conflict of interest statement. The authors declare no competing interests.
Authorship statement. Investigation and validation: W.B.Y., C.C.H., T.I.H., J.P.L., K.Y.C., P.Y.C., J.J.L., and S.T.Y. Data curation: W.B.Y., C.C.H., T.I.H., J.P.L. W.C.C, and J.Y.C. Writing‒original draft preparation: W.B.Y., C.C.H., T.I.H., and J.P.L. Writing‒review and editing: W.C.C. and J.Y.C. Supervision: J.Y.W., S.H.Y., R.M.C., and J.Y.C. Funding acquisition: W.C.C. and J.Y.C.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Wick W, Weller M, van den Bent M, et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 3. Wen PY. Therapy for recurrent high-grade gliomas: does continuous dose-intense temozolomide have a role? J Clin Oncol. 2010;28(12):1977–1979. [DOI] [PubMed] [Google Scholar]
- 4. Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. [DOI] [PubMed] [Google Scholar]
- 5. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 6. Liau BB, Sievers C, Donohue LK, et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell. 2017;20(2):233–246 e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh CH, Lin YJ, Wu CP, Lee HT, Shyu WC, Wang CC. Livin contributes to tumor hypoxia-induced resistance to cytotoxic therapies in glioblastoma multiforme. Clin Cancer Res. 2015;21(2):460–470. [DOI] [PubMed] [Google Scholar]
- 8. Pisco AO, Huang S. Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me’. Br J Cancer. 2015;112(11):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chuang JY, Kao TJ, Lin SH, et al. Specificity protein 1-zinc finger protein 179 pathway is involved in the attenuation of oxidative stress following brain injury. Redox Biol. 2017;11:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh SH, Yang WB, Gean PW, et al. Translational and transcriptional control of Sp1 against ischaemia through a hydrogen peroxide-activated internal ribosomal entry site pathway. Nucleic Acids Res. 2011;39(13):5412–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang KY, Hsu TI, Hsu CC, et al. Specificity protein 1-modulated superoxide dismutase 2 enhances temozolomide resistance in glioblastoma, which is independent of O6-methylguanine-DNA methyltransferase. Redox Biol. 2017;13:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang KY, Huang CT, Hsu TI, et al. Stress stimuli induce cancer-stemness gene expression via Sp1 activation leading to therapeutic resistance in glioblastoma. Biochem Biophys Res Commun. 2017;493(1):14–19. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med. 2016; 6(10):a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witt AE, Lee CW, Lee TI, et al. Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian cancer. Oncogene. 2017;36(12):1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pathania R, Ramachandran S, Mariappan G, et al. Combined inhibition of DNMT and HDAC blocks the tumorigenicity of cancer stem-like cells and attenuates mammary tumor growth. Cancer Res. 2016;76(11):3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu CC, Chang WC, Hsu TI, et al. Suberoylanilide hydroxamic acid represses glioma stem-like cells. J Biomed Sci. 2016;23(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung JJ, Wang YT, Chang WC. Sp1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol Cell Biol. 2006;26(5):1770–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waby JS, Chirakkal H, Yu C, et al. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol Cancer. 2010;9:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang WC, Hung JJ. Functional role of post-translational modifications of Sp1 in tumorigenesis. J Biomed Sci. 2012;19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanai M, Wei D, Li Q, et al. Loss of Krüppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12(21):6395–6402. [DOI] [PubMed] [Google Scholar]
- 22. Chen B, Ma L, Paik H, et al. Reversal of cancer gene expression correlates with drug efficacy and reveals therapeutic targets. Nat Commun. 2017;8:16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mao Z, Ke Z, Gorbunova V, Seluanov A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging (Albany NY). 2012;4(6):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dikovskaya D, Cole JJ, Mason SM, et al. Mitotic stress is an integral part of the oncogene-induced senescence program that promotes multinucleation and cell cycle arrest. Cell Rep. 2015;12(9):1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113(2):175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu CC, Lee PT, Kao TJ, et al. Upregulation of Znf179 acetylation by SAHA protects cells against oxidative stress. Redox Biol. 2018;19:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsinman O, Tsinman K, Sun N, Avdeef A. Physicochemical selectivity of the BBB microenvironment governing passive diffusion-matching with a porcine brain lipid extract artificial membrane permeability model. Pharm Res. 2011;28(2):337–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Staberg M, Michaelsen SR, Rasmussen RD, Villingshøj M, Poulsen HS, Hamerlik P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol (Dordr). 2017;40(1):21–32. [DOI] [PubMed] [Google Scholar]
- 30. Barnett ME, Madgwick DK, Takemoto DJ. Protein kinase C as a stress sensor. Cell Signal. 2007;19(9):1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebenezer DL, Berdyshev EV, Bronova IA, et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax. 2019;74(6):579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Du Y, Seibenhener ML, Yan J, Jiang J, Wooten MC. aPKC phosphorylation of HDAC6 results in increased deacetylation activity. PLoS One. 2015;10(4):e0123191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu J, Coyne CB, Sarkar SN. PKC alpha regulates Sendai virus-mediated interferon induction through HDAC6 and β-catenin. EMBO J. 2011;30(23):4838–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.