Abstract
Background
Hepatitis C virus (HCV) incidence has increased in the worsening opioid epidemic. We examined the HCV preventive efficacy of medication-assisted treatment (MAT), and geographic variation in HCV community viral load (CVL) and its association with HCV incidence.
Methods
HCV incidence was directly measured in an open cohort of patients in a MAT program in New York City between 1 January 2013 and 31 December 2016. Area-level HCV CVL was calculated. Associations of individual-level factors, and of HCV CVL, with HCV incidence were examined in separate analyses.
Results
Among 8352 patients, HCV prevalence was 48.7%. Among 2535 patients seronegative at first antibody test, HCV incidence was 2.25/100 person-years of observation (PYO). Incidence was 6.70/100 PYO among those reporting main drug use by injection. Female gender, drug injection, and lower MAT retention were significantly associated with higher incidence rate ratios. Female gender, drug injection, and methadone doses <60 mg were independently associated with shorter time to HCV seroconversion. HCV CVLs varied significantly by geographic area.
Conclusions
HCV incidence was higher among those with lower MAT retention and was lower among those receiving higher methadone doses, suggesting the need to ensure high MAT retention, adequate doses, and increased HCV prevention and treatment engagement. HCV CVLs vary geographically and merit further study as predictors of HCV incidence.
Keywords: opioid use disorder, people who use drugs, injection drug use, hepatitis C virus, HCV incidence, geographic analysis, epidemiology, epidemics
The current opioid epidemic has led to a rise in hepatitis C virus (HCV) incidence by leading to an increase in drug injection [1–3]. In the United States, HCV incident cases rose more than 300% from 2010 to 2015 [1, 3]. The current HCV epidemic is occurring despite the availability of effective HCV primary prevention and direct-acting antivirals agents (DAAs), which can cure HCV infection in >95% of cases [4]. All-cause mortality among those with HCV infection and HCV-related mortality both remain high [5, 6].
High degrees of coverage of combined prevention with medication-assisted treatment (MAT) (eg, methadone) for opioid use disorders (OUDs), access to sterile syringes at needle/syringe programs and pharmacies, and HCV treatment, what could be referred to as HCV “cure as prevention” (CasP), will be essential for reducing HCV burden among people who use drugs (PWUD) [7–10].
MAT reduces HCV risk through a reduction in the frequency of drug injection [11]; current use of MAT reduces HCV acquisition by 40%–60% [10, 12, 13]. MAT may be most effective in preventing HCV when MAT engagement is continuous and when doses are adequate [13–15]. However, MAT is a complex intervention with significant geographic variation in coverage, and the evidence base addressing potentially modifiable factors likely to affect MAT’s HCV preventive efficacy (eg, dose, degrees of engagement) is incomplete [7, 13, 16–19].
Gaps in the implementation of combined evidence-based programs for HCV prevention persist [9, 20–22]. More than one-third of HCV infected people in New York City (NYC) in 2015–2017 were undiagnosed, and fewer than one-third of those infected received treatment [23, 24]. Furthermore, critical gaps in MAT availability in relation to need persist; in 2015 in the United States <9% of people received needed MAT [25–27].
Community viral load (CVL) is a construct that has been applied to human immunodeficiency virus (HIV) epidemiology [28–31]. HIV CVL is an aggregate measure of HIV viral loads (VLs) of individuals in a program or area and has been shown to be a valuable public health metric; studies have identified geographic disparities in HIV CVL with higher mean HIV CVLs in areas with higher poverty rates, and have found reductions in HIV CVL to be associated with decreased HIV incidence [28–31]. The magnitude of individual HIV VL increases transmission risk associated with an exposure event [32]. Studies of HIV CVL have explored a number of CVL measures of greater or lesser inclusiveness; the former rely on directly measured data, estimation, and imputation, while the latter rely entirely on directly measured data among those engaged in care and have been referred to as engaged-in-care CVLs [33, 34]. Analogously, HCV transmission risk via needlestick is directly correlated with HCV VL magnitude of the source patient [35]. This suggests that HCV VL magnitude in a PWUD engaged in distributive sharing will increase transmission risk per event and that the HCV CVL in an area may be an important driver of HCV incidence and a useful metric of the population-level impact of combined prevention and CasP.
The availability of DAAs has led to discussions of HCV elimination [4]. The World Health Organization established a goal of a 90% reduction in new HCV infections by 2030 [36]. Yet, the anticipated beneficial impact of DAAs on HCV incidence derives primarily from modeling, and direct evidence for their impact on incidence is scarce [37]. In the absence of effective population-level HCV prevention and CasP, the high prevalence of active, untreated HCV results in a high CVL, which serves as a large HCV reservoir, increasing the probability of a nonsterile injection episode with HCV contaminated injection equipment [8]. Thus, accurate estimates of HCV incidence, optimization of the HCV preventive efficacy of MAT, and examination of the potential value of public health metrics, such as HCV CVL, are critical to HCV control.
The objectives of this work were to calculate HCV prevalence, incidence, and engaged-in-care HCV CVL. We examined the impact of methadone dose and of measures of MAT retention on HCV incidence. Furthermore, we examined geographic variation in HCV CVL and the impact of area-level HCV CVL on area-level HCV incidence.
METHODS
This study examined an observational retrospective open cohort of patients receiving MAT for OUDs in an opioid treatment program (OTP) in NYC enrolled at any time between 1 January 2013 and 31 December 2016 [16, 38]. Patient data were collected from electronic medical records and included HCV antibody, HCV VL tests, and demographic information. Patients were routinely offered opt-out HCV antibody testing at program entry and annual evaluations, and were tested unless they declined testing or were known to be antibody positive. This research was approved by the institutional review boards at Mount Sinai Health System and City University of New York.
Definitions
Outcomes of Interest
HCV infection was defined as HCV antibody positivity. Patients were included in HCV incidence calculations if they had ≥1 negative HCV antibody test during the study period followed by ≥1 HCV antibody test; incident HCV was defined as a new positive, after a previous negative, HCV antibody test.
We calculated HCV infection incidence rates (IRs), using person-time of observation, presented as rates per 100 person-years of observation (PYO), with 95% confidence intervals (CIs). In incidence analyses, time zero was defined as the date of first negative HCV antibody test. For those who did not seroconvert, person-time was the time between the first and last documented negative HCV antibody test. For seroconverters, the date of incident HCV infection was assigned at the midpoint between the dates of the last observed negative and the first observed positive HCV antibody test. We also calculated incidence rate ratios (IRRs) using the Wald unconditional maximum likelihood estimation method (with 95% CIs).
Exposures of Interest
Demographic variables were measured at OTP admission if that occurred during the study period, or at the first annual evaluation during the study period if they had been admitted prior to the study period. Methadone dose was calculated as the mean of each patient’s daily dose during the study period, then dichotomized at 60 mg; this threshold was chosen based on data that doses ≥60 mg are most effective in OUD relapse prevention [39]. The degree of MAT engagement was measured both as (1) a dichotomous variable, MAT engagement, reflecting whether a patient was continuously enrolled in MAT or was enrolled, discharged, and readmitted ≥1 time in the study period, and (2) as a continuous variable, MAT retention, measuring the total number of days enrolled in MAT during the study period. Patients were considered as having a main route of drug use by injection if any of their reported primary, secondary, or tertiary drugs of choice were reported to be by injection.
CVL was calculated using data from patients who had ≥1 HCV VL test during the study period 2013–2016 and reflects the mean of the sum of each patient’s most recent HCV VL test in copies/mL with their standard deviations (SDs). CVLs were calculated overall and for geographic area of residence using the 34 United Hospital Fund (UHF) ZIP code aggregations; we then compared the HCV CVLs between different UHF areas [40].
Statistical Analysis
We assessed differences in first HCV antibody serostatus using the χ 2 test and examined factors associated with HCV antibody positivity in multivariate logistic regression. We calculated HCV prevalence, number of HCV seroconversions, IR and IRRs overall and by individual-level factors, and HCV CVL overall, by individual-level factors, and by area. The presence of multicollinearity between variables was examined by the variance inflation factor and in bivariate correlations; multicollinearity was not identified.
Kaplan–Meier plots were drawn to depict the probability of remaining HCV antibody negative during the study period, overall and by main drug use by injection, gender, MAT engagement, and methadone dose; we used the Mantel–Cox log-rank test to examine differences in time to seroconversion. We used univariate and multivariate Cox proportional hazards regression to assess the relationship between time to HCV seroconversion and individual-level variables; associations are presented as unadjusted hazard ratios (HRs) and adjusted HRs (aHRs) with 95% CIs, respectively.
For all analyses, HCV CVLs were transformed to the log10 scale; in the tables HCV CVLs are presented untransformed. Relationships between individual-level factors and HCV CVL were examined using the Kruskal–Wallis test; differences in HCV CVL by UHF area were examined using Spearman correlation coefficient. We then examined the relationship between HCV CVL in UHF areas and HCV incidence among UHF areas using a negative binomial regression where the count of seroconversions was the dependent variable and the natural logarithm of PYO functioned as an offset term in the model to account for differences in time at risk across areas.
Analyses were conducted in R, and P values were reported as statistically significant at a level of P < .05 [41].
RESULTS
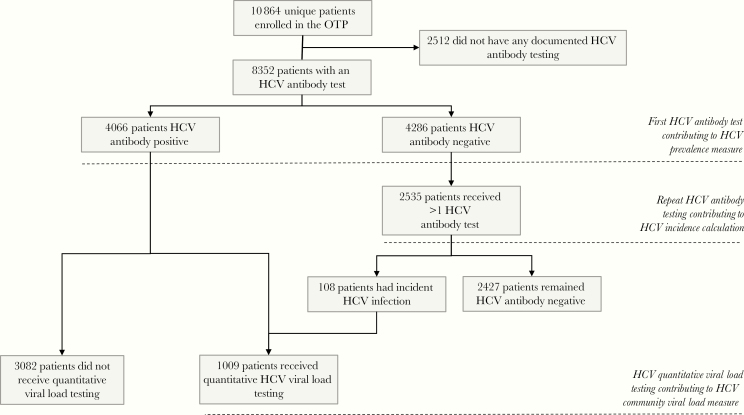
There were a total of 8352 unique patients enrolled in the OTP during the study period who received ≥1 HCV antibody test. There were 4286 patients whose first HCV antibody test was negative; 2535 of these patients received ≥1 subsequent HCV antibody test; this subset of patients was assessed for incident HCV (Figure 1). Patients who did and did not receive antibody testing did not differ significantly; neither did those receiving precisely 2 or >2 HCV antibody tests (data not shown). Overall HCV prevalence was 48.7% (4066/8352; 95% CI, 47.6%–49.7%). HCV prevalence among those reporting, and not reporting, main drug use by injection was 70.5% and 35%, respectively (Table 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of study population and hepatitis C virus measures of interest. Abbreviations: HCV, hepatitis C virus; OTP, opioid treatment program.
Table 1.
Characteristics of Patients in an Opioid Treatment Program in New York City, 2013–2016, and Associations With Hepatitis C Virus Seroprevalence
| Overall Study Population HCV Antibody Tested | HCV Antibody Negative | HCV Antibody Positive | Multivariate Logistic Regression Model of HCV Serostatus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual -Level Characteristica,b |
No. | (%) | No. | No. | HCV Prevalence | Univariate P Valuec | Adjusted OR | 95% CI LL | 95% CI UL | P Value |
| Overall | 8352 | (100.0) | 4286 | 4066 | 48.7% | NA | NA | NA | NA | NA |
| Age, y | < .001 | |||||||||
| 18–24 | 350 | (4.2) | 257 | 93 | 26.6% | ref | … | … | ||
| 25–34 | 1293 | (15.5) | 808 | 485 | 37.5% | 2.11 | 1.59 | 2.81 | < .001 | |
| 35–44 | 1708 | (20.5) | 959 | 749 | 43.9% | 3.62 | 2.72 | 4.81 | < .001 | |
| 45–54 | 2852 | (34.1) | 1593 | 1259 | 44.1% | 5.10 | 3.84 | 6.78 | < .001 | |
| 55–64 | 1851 | (22.2) | 587 | 1264 | 68.3% | 14.39 | 10.69 | 19.36 | < .001 | |
| ≥65 | 291 | (3.5) | 77 | 214 | 73.5% | 16.90 | 11.35 | 25.17 | < .001 | |
| Race/ethnicity | < .001 | |||||||||
| Non-Hispanic white | 2290 | (27.4) | 1191 | 1099 | 48.0% | ref | … | … | ||
| Hispanic | 3739 | (44.8) | 1836 | 1903 | 50.9% | 1.23 | 1.08 | 1.41 | .002 | |
| Non-Hispanic black | 1825 | (21.9) | 1009 | 816 | 44.7% | 0.83 | .71 | .98 | .025 | |
| Other | 498 | (6.0) | 250 | 248 | 49.8% | 0.93 | .73 | 1.17 | .521 | |
| Gender | .28 | |||||||||
| Male | 6073 | (72.7) | 3083 | 2985 | 49.2% | ref | … | … | ||
| Female | 2277 | (27.3) | 1201 | 1076 | 47.3% | 1.05 | .93 | 1.17 | .45 | |
| Employment | < .001 | |||||||||
| Employed | 1532 | (18.3) | 932 | 600 | 39.2% | ref | … | … | ||
| Unemployed | 6820 | (81.7) | 3354 | 3466 | 50.8% | 1.52 | 1.31 | 1.76 | < .001 | |
| Veteran status | < .001 | |||||||||
| Not a veteran | 7897 | (94.6) | 4095 | 3802 | 48.1% | ref | … | … | ||
| Veteran | 455 | (5.4) | 191 | 264 | 58.0% | 1.23 | .98 | 1.54 | .07 | |
| Insurance payor | < .001 | |||||||||
| Medicaid | 4938 | (59.1) | 2429 | 2509 | 50.8% | ref | … | … | ||
| Other | 1012 | (12.1) | 541 | 471 | 46.5% | 1.16 | 1.03 | 1.30 | .02 | |
| Self-pay | 2402 | (28.8) | 1316 | 1086 | 45.2% | 0.80 | .67 | .96 | .02 | |
| Education | < .001 | |||||||||
| Higher education beyond HS | 2018 | (24.2) | 1166 | 852 | 42.2% | ref | … | … | ||
| Less than HS | 3097 | (37.1) | 1536 | 1561 | 50.4% | 1.39 | 1.22 | 1.58 | < .001 | |
| HS diploma or GED | 3129 | (37.5) | 1576 | 1553 | 49.6% | 1.37 | 1.20 | 1.58 | < .001 | |
| Reported main drug use by injection | < .001 | |||||||||
| No | 5131 | (61.4) | 3337 | 1794 | 35.0% | ref | … | … | ||
| Yes | 3221 | (38.6) | 949 | 2272 | 70.5% | 6.68 | 5.97 | 7.48 | < .001 | |
| Methadone dose, mg | .464 | |||||||||
| ≥60 | 5065 | (60.6) | 2625 | 2440 | 48.2% | NA | NA | NA | NA | |
| <60 | 3236 | (38.7) | 1632 | 1604 | 49.6% | NA | NA | NA | NA | |
| MAT engagementd | < .001 | |||||||||
| Continuous engagement in MAT | 4531 | (54.3) | 2208 | 2323 | 51.3% | NA | NA | NA | NA | |
| Interrupted engagement in MAT | 3821 | (45.7) | 2078 | 1743 | 45.6% | NA | NA | NA | NA | |
| MAT retentiond | ||||||||||
| Days active in the OTP, mean (SD) | 1189 | (909.33) | … | … | … | NA | NA | NA | NA | |
| Days not active in the OTP, mean (SD) | 30 | (129.78) | … | … | … | NA | NA | NA | NA | |
| Repeat HCV Ab test after first HCV Ab negatived | ||||||||||
| Only 1 HCV Ab test (row percentages) | 4289 | (51.4) | 1751 | 2560 | 59.7% | NA | NA | NA | NA | |
| >1 HCV Ab test | 4063 | (48.6) | … | … | … | NA | NA | NA | NA | |
Abbreviations: Ab, antibody; CI, confidence interval; GED, graduate equivalent degree; HCV, hepatitis C virus; HS, high school; LL, lower limit; MAT, medication-assisted treatment; NA, not applicable; OR, odds ratio; OTP, opioid treatment program; SD, standard deviation; UL, upper limit.
aNumbers in the columns labeled “No.” reflect those with data available. Missing data were <3%; no multicollinearity identified.
bNo. and % are presented for each individual-level characteristic unless otherwise specified.
cχ 2 test for categorical variables and Wilcoxon test for continuous variables; comparison was between those HCV antibody positive and HCV antibody negative; values in bold were statistically significant based on a priori P value determination of < .05.
dThese individual-level characteristics were not included in the multivariate logistic regression model of HCV serostatus as they include data from the time period after the baseline assessment of serostatus.
In multivariate analyses, HCV antibody prevalence significantly increased with age and was significantly higher among those whose main drug use was by injection (aOR, 6.68 [95% CI, 5.97–7.48]). Further, HCV prevalence was significantly lower among non-Hispanic blacks than among non-Hispanic whites (aOR, 0.83 [95% CI, .71–.98]) and was significantly higher among Hispanics than among non-Hispanic whites (aOR, 1.23 [95% CI, 1.08–1.41]) (Table 2).
Table 2.
Hepatitis C Virus (HCV) Incidence and HCV Incidence Rate Ratios Among an Open Cohort of Patients Receiving Medication-Assisted Treatment for Opioid Use Disorder in New York City
| Incidence Rate | Incidence Rate Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual-Level Characteristicsa | Total No. | Incident HCV No. | PYO | Per 100 PYO | 95% CI LL | 95% CI UL | IRR | 95% CI LL | 95% CI UL | P Valueb |
| Overall | 2535 | 108 | 4859 | 2.25 | 1.82 | 2.68 | … | … | … | |
| Age, y | ||||||||||
| 18–24 | 85 | 5 | 121 | 4.12 | 1.33 | 9.62 | 1.00 | … | … | |
| 25–34 | 387 | 19 | 668 | 2.80 | 1.71 | 4.44 | 0.68 | .26 | 1.84 | .45 |
| 35–44 | 537 | 18 | 991 | 1.80 | 1.08 | 2.87 | 0.44 | .16 | 1.18 | .13 |
| 45–54 | 1056 | 36 | 2149 | 1.67 | 1.17 | 2.31 | 0.41 | .16 | 1.03 | .08 |
| 55–64 | 417 | 27 | 837 | 3.22 | 2.13 | 4.69 | 0.78 | .30 | 2.03 | .59 |
| ≥65 | 48 | 3 | 84 | 3.55 | .73 | 10.40 | 0.86 | .20 | 3.61 | .87 |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 622 | 30 | 1100 | 2.72 | 1.84 | 3.89 | 1.00 | … | … | |
| Hispanic | 1148 | 52 | 2244 | 2.31 | 1.73 | 3.04 | 0.85 | .54 | 1.33 | .48 |
| Non-Hispanic black | 587 | 18 | 1160 | 1.55 | .92 | 2.45 | 0.57 | .32 | 1.02 | .60 |
| Other | 178 | 8 | 354 | 2.25 | .97 | 4.45 | 0.83 | .38 | 1.81 | .66 |
| Gender | ||||||||||
| Male | 1782 | 66 | 3405 | 1.94 | 1.50 | 2.47 | 1.00 | … | … | |
| Female | 751 | 42 | 1450 | 2.89 | 2.08 | 3.91 | 1.49 | 1.02 | 2.20 | .04 |
| Employment | ||||||||||
| Employed | 606 | 23 | 1202 | 1.91 | 1.21 | 2.87 | 1.00 | … | … | |
| Unemployed | 1924 | 85 | 3657 | 2.32 | 1.86 | 2.87 | 1.21 | .77 | 1.92 | .41 |
| Veteran status | ||||||||||
| Not a veteran | 2412 | 102 | 4622 | 2.20 | 1.79 | 2.67 | 1.00 | … | … | |
| Veteran | 123 | 6 | 237 | 2.53 | .93 | 5.50 | 1.15 | .50 | 2.60 | .71 |
| Insurance payor | ||||||||||
| Medicaid | 1869 | 79 | 3676 | 2.14 | 1.70 | 2.67 | 1.00 | … | … | |
| Other | 427 | 19 | 399 | 4.75 | 2.86 | 7.42 | 2.22 | 1.34 | 3.65 | < .001 |
| Self-pay | 240 | 11 | 784 | 1.40 | .70 | 2.50 | 0.65 | .34 | 1.22 | .18 |
| Education | ||||||||||
| Less than HS | 949 | 46 | 1869 | 2.46 | 1.80 | 3.28 | 1.00 | … | … | |
| HS diploma or GED | 939 | 37 | 1770 | 2.10 | 1.47 | 2.88 | 0.84 | .55 | 1.31 | .46 |
| Higher education beyond HS | 639 | 23 | 1209 | 1.90 | 1.20 | 2.85 | 0.78 | .47 | 1.28 | .33 |
| Reported main drug use by injection | ||||||||||
| No | 52 | 52 | 4025 | 1.30 | .96 | 1.70 | 1.00 | … | … | |
| Yes | 492 | 56 | 834 | 6.70 | 5.10 | 8.70 | 5.20 | 3.56 | 7.58 | < .001 |
| Methadone dose, mg | ||||||||||
| ≥60 | 1738 | 68 | 3429 | 1.98 | 1.54 | 2.50 | 1.00 | … | … | |
| <60 | 780 | 40 | 1400 | 2.86 | 2.00 | 3.90 | 1.44 | .97 | 2.13 | .07 |
| MAT engagement | ||||||||||
| Continuous engagement in MAT | 1725 | 78 | 3654 | 2.13 | 1.68 | 2.66 | 1.00 | … | … | |
| Interrupted engagement in MAT | 810 | 30 | 1205 | 2.50 | 1.68 | 3.55 | 1.17 | .77 | 1.78 | .46 |
| MAT retention, d, mean (SD) | 40.8 (139.8) | 70.0 (198.0) | NA | NA | NA | NA | 1.01 | 1.00 | 1.02 | .03 |
Abbreviations: CI, confidence interval; GED, graduate equivalent degree; HCV, hepatitis C virus; HS, high school; IRR, incidence rate ratio; LL, lower limit; MAT, medication-assisted treatment; NA, not applicable; PYO, person-years of observation; SD, standard deviation; UL, upper limit.
aThe numbers in the columns labeled No. reflect those with data available. Missing data were <3%; no multicollinearity identified.
bStatistical significance of rate difference; values in bold were statistically significant based on a P value of < .05.
One thousand nine patients received quantitative HCV VL testing and contributed to the HCV CVL measures (Figure 1).
There were 108 seroconversions during 4859 PYO; 2.5% (95% CI, 2.1%–3.0%) had seroconverted by the end of the study period. Median person-years contributed were 1.98 years (25th, 75th percentiles: 1.05, 2.37 years). HCV incidence in the overall cohort was 2.25/100 PYO (95% CI, 1.82–2.68); incidence was 6.70/100 PYO (95% CI, 5.10–8.78) and 2.89/100 PYO (95% CI, 2.08–3.91) among those reporting main drug use by injection and women, respectively. Lower MAT retention was significantly associated with higher HCV incidence rate ratios (IRR, 1.01 [95% CI, 1.00–1.02], P = .03). HCV incidence was significantly higher among those whose main drug use was by injection (IRR, 5.2 [95% CI, 3.56–7.58]) and for females (IRR, 1.49 [95% CI, 1.02–2.20]). Incidence was slightly higher among those whose methadone doses were <60 mg compared to those with doses ≥60 mg (IRR, 1.44 [95% CI, .97–2.13]). IRs and IRRs did not differ significantly by race/ethnicity (Table 2).
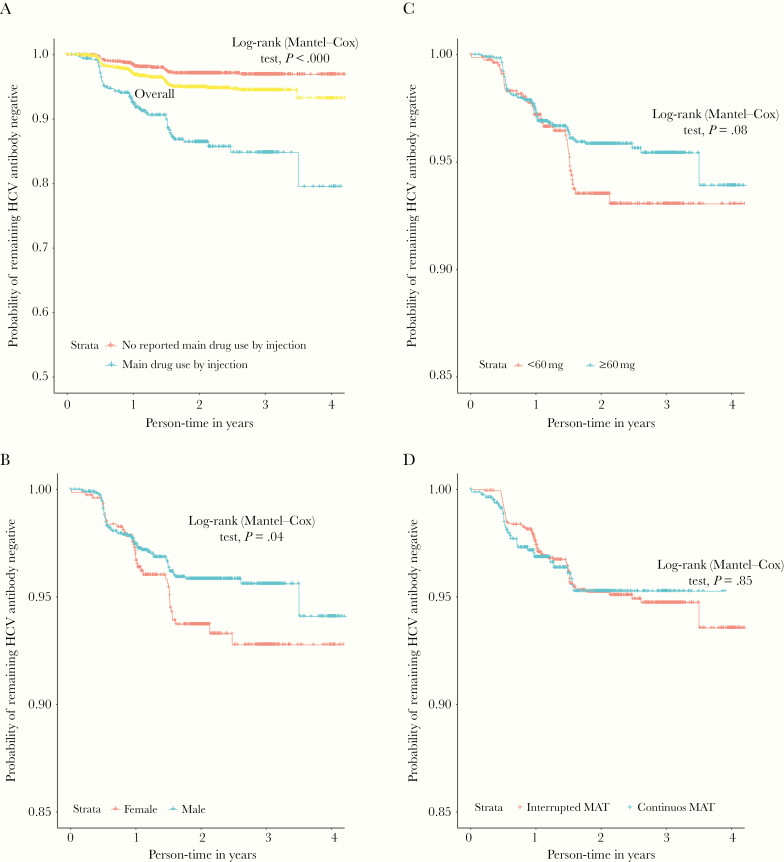
Figure 2 presents the Kaplan–Meier curves and Mantel–Cox log-rank tests for time to HCV seroconversion by gender, methadone dose, and MAT engagement, and main drug use by drug injection. After 4 years of follow-up, >20% of patients reporting main drug use by injection had acquired HCV infection compared to <2% of those not reporting main drug use by injection (HR, 5.11 [95% CI, 3.50–7.46]) (Figure 2A, Table 3).
Figure 2.
Kaplan–Meier time to hepatitis C virus seroconversion, for injection drug use (A), gender (B), methadone dose (C), and dichotomous medication-assisted treatment engagement (D). Abbreviations: HCV, hepatitis C virus; MAT, medication-assisted treatment.
Table 3.
Time to Hepatitis C Virus (HCV) Seroconversion by Characteristics of 2535 HCV Antibody-Seronegative Patients Enrolled in an Opioid Treatment Program in New York City, 2013–2016
| Individual-Level Characteristicsa | No.b | (%)b | Hazard Ratio | 95% CI LL | 95% CI UL | P Value | Adjusted HRc | 95% CI LL | 95% CI UL | P Valued |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 45.33 | (10.70) | 1.03 | 0.98 | 1.02 | .73 | 1.03 | 1.01 | 1.05 | < .001 |
| Age, y | 2535 | |||||||||
| 18–24 | 85 | (3) | ref | … | … | … | … | |||
| 25–34 | 387 | (15) | 0.54 | 0.28 | 1.97 | .74 | … | … | … | |
| 35–44 | 537 | (21) | 0.14 | 0.18 | 1.28 | .48 | … | … | … | |
| 45–54 | 1056 | (42) | 0.09 | 0.18 | 1.15 | .45 | … | … | … | |
| 55–64 | 417 | (16) | 0.76 | 0.33 | 2.25 | .86 | … | … | … | |
| ≥65 | 53 | (2) | 0.76 | 0.19 | 3.35 | .80 | … | … | … | |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 1148 | (45) | ref | … | … | … | ref | … | … | |
| Hispanic | 587 | (23) | 0.66 | 0.39 | 1.13 | .13 | 0.73 | 0.42 | 1.26 | .26 |
| Non-Hispanic black | 622 | (25) | 1.15 | 0.73 | 1.80 | .54 | 0.82 | 0.50 | 1.33 | .41 |
| Other | 178 | (7) | 0.96 | 0.46 | 2.02 | .92 | 0.83 | 0.39 | 1.75 | .62 |
| Gender | ||||||||||
| Male | 1784 | (70) | ref | … | … | ref. | … | … | ||
| Female | 751 | (30) | 1.49 | 1.01 | 2.19 | .04 | 1.52 | 1.03 | 2.23 | .03 |
| Employment | ||||||||||
| Unemployed | 606 | (24) | ref | … | … | … | … | … | ||
| Employed | 1929 | (76) | 0.83 | 0.52 | 1.31 | .43 | … | … | … | |
| Veteran status | ||||||||||
| Not a veteran | 2412 | (95) | ref | … | … | … | … | … | ||
| Veteran | 123 | (5) | 1.15 | 0.50 | 2.61 | .75 | … | … | … | |
| Insurance payor | ||||||||||
| Medicaid | 1868 | (74) | ref | … | … | … | … | … | ||
| Other | 427 | (17) | 1.03 | 0.63 | 1.71 | .90 | … | … | … | |
| Self-pay | 240 | (9) | 1.54 | 0.82 | 2.90 | .18 | … | … | … | |
| Education | ||||||||||
| Higher education beyond HS | 639 | (25) | ref | … | … | … | … | … | ||
| HS diploma or GED | 939 | (37) | 1.10 | 0.66 | 1.86 | .71 | … | … | … | |
| Less than HS | 957 | (38) | 1.36 | 0.83 | 2.24 | .22 | … | … | … | |
| Reported main drug use by injection | ||||||||||
| No | 2043 | (81) | ref | … | … | ref | … | … | ||
| Yes | 492 | (19) | 5.11 | 3.50 | 7.46 | .00 | 5.98 | 3.98 | 8.98 | < .001 |
| Methadone dose, mg, mean (SD) | 81.71 | (41) | 1.03 | 0.99 | 1.00 | .21 | … | … | … | |
| Methadone dose, mg | ||||||||||
| ≥60 | 1755 | (69) | ref | … | … | ref | … | … | ||
| <60 | 780 | (31) | 1.41 | 0.95 | 2.08 | .08 | 1.52 | 1.03 | 2.24 | .03 |
| MAT engagement | ||||||||||
| Continuous engagement in MAT | 1725 | (68) | ref | … | … | … | … | … | ||
| Interrupted engagement in MAT | 810 | (32) | 1.04 | 0.68 | 1.59 | .85 | … | … | … | |
| MAT retention, days inactive or not retained, mean (SD) | 40.87 | (140) | 1.10 | 1.11 | 1.12 | .02 | 1.01 | 1.00 | 1.02 | .07 |
Abbreviations: CI, confidence interval; GED, graduate equivalent degree; HR, hazard ratio; HS, high school; LL, lower limit; MAT, medication-assisted treatment; SD, standard deviation; UL, upper limit.
aThe numbers in the column labeled No. reflect those with data available. Missing data were <3%; no multicollinearity identified.
bNo. and % are presented for each individual-level characteristic except as otherwise specified for the variables age, methadone dose, and MAT retention.
cMultivariate model included age (as a continuous variable), gender, race/ethnicity, reported main drug use by injection, methadone dose (dichotomous), MAT engagement (continuous).
dThe values in bold were statistically significant based on P < .05.
In our multivariate Cox proportional hazards model controlling for individual-level characteristics, female sex (aHR, 1.52 [95% CI, 1.03–2.23]), main drug use by injection (aHR, 5.98 [95% CI, 3.98–8.98]), and doses <60 mg (aHR, 1.52 [95% CI, 1.03–2.24]) all were independently associated with shorter time to HCV seroconversion. Lower MAT retention was slightly associated with a longer time to HCV seroconversion (aHR, 1.01 [95% CI, 1.00–1.02]) (Table 3).
The overall mean HCV CVL was 3 583 742.9 copies/mL (SD, 6 133 314 copies/mL). Mean HCV CVL increased with patient age (P = .005). Non-Hispanic blacks had a higher mean CVL than non-Hispanic whites and than all others, but not significantly so and with a very large SD. UHF level HCV incidence rates ranged from 0.00/100 PYO (95% CI, .00–.03) to 9.89/100 PYO (95% CI, 1.20–35.73) (data not shown). HCV CVLs varied significantly by UHF area (Spearman R = –0.44; P = .008) (Table 4).
Table 4.
Hepatitis C Virus Community Viral Load and Univariate Associations for Patients in an Opioid Treatment Program in New York City, 2013–2016
| Individual-Level Characteristicsa | No. | (%) | Mean CVL | CVL SD | P Valueb |
|---|---|---|---|---|---|
| Overall | 1009 | (100) | 3 583 742.9 | 6 133 314 | NA |
| Age, y | .005 | ||||
| 18–24 | 22 | (2.2) | 2 057 538 | 3 651 791 | |
| 25–34 | 98 | (9.7) | 2 822 991 | 4 057 202 | |
| 35–44 | 175 | (17.3) | 3 162 512 | 4 550 681 | |
| 45–54 | 341 | (33.8) | 3 809 692 | 6 341 650 | |
| 55–64 | 333 | (33.0) | 3 969 522 | 5 450 024 | |
| ≥65 | 38 | (3.8) | 2 932 791 | 3 530 230 | |
| Race/ethnicity | .09 | ||||
| Non-Hispanic white | 252 | (25.0) | 3 549 630 | 5 253 802 | |
| Hispanic | 435 | (43.1) | 3 174 546 | 4 351 025 | |
| Non-Hispanic black | 254 | (25.2) | 4 085 511 | 7 082 772 | |
| Other | 66 | (6.5) | 4 463 736 | 5 679 993 | |
| Gender | .50 | ||||
| Male | 765 | (75.8) | 3 551 500 | 5 397 555 | |
| Female | 242 | (24.0) | 3 681 253 | 5 462 181 | |
| Employment | .17 | ||||
| Employed | 133 | (13.2) | 4 714 177 | 6 391 286 | |
| Unemployed | 874 | (86.6) | 2 717 093 | 2 320 797 | |
| Veteran status | .48 | ||||
| Veteran | 64 | (6.3) | 3 477 293 | 7 831 622 | |
| Not a veteran | 943 | (93.5) | 5 135 516 | 5 205 816 | |
| Insurance payor | .13 | ||||
| Medicaid | 666 | (66.0) | 3 687 084 | 5 630 153 | |
| Other | 102 | (10.1) | 10 035 246 | 7 386 687 | |
| Self-pay | 239 | (23.7) | 2 764 247 | 3 617 036 | |
| Education | .79 | ||||
| Less than HS | 395 | (39.1) | 3 517 255 | 4 710 260 | |
| HS diploma or GED | 395 | (39.1) | 3 492 736 | 5 614 784 | |
| Higher education beyond HS | 204 | (20.2) | 3 815 818 | 5 990 518 | |
| Unknown | 13 | (1.3) | 4 645 185 | 8 423 787 | |
| Reported main drug use by injection | .16 | ||||
| Yes | 544 | (53.9) | 3 441 366 | 5 055 684 | |
| No | 463 | (45.9) | 3 748 720 | 5 820 709 | |
| Methadone dose, mg | .57 | ||||
| ≥60 | 603 | (59.8) | 3 666 703 | 5 433 123 | |
| <60 | 398 | (39.4) | 3 468 724 | 5 407 571 | |
| MAT engagement | .16 | ||||
| Continuous engagement in MAT | 649 | (64.3) | 3 866 577 | 5 963 782 | |
| Interrupted engagement in MAT | 358 | (35.5) | 3 068 023 | 4 301 752 |
Abbreviations: CVL, community viral load; GED, graduate equivalent degree; HS, high school; MAT, medication-assisted treatment; NA, not applicable; SD, standard deviation.
aThe numbers in the column labeled No. reflect those with data available. Missing data were <3%; no multicollinearity identified.
b P value by Kruskal–Wallis test and hepatitis C virus CVL to the log base 10 was used in analyses, values in bold were statistically significant based on P < .05.
In negative binomial regression analysis examining the relationship between area-level HCV CVL and area-level HCV incidence rates, the HCV IRR associated with a 1 log10 increase in HCV CVL was 2.72 (95% CI, .77–11.13; P = .147).
DISCUSSION
We identified an HCV incidence rate of 6.7/100 PYOs among those in MAT whose main drug use was by injection. This rate is lower than incidence rates often observed in out-of-treatment or recently treated PWUD [10, 13, 14, 22]. In our study, female sex was associated both with higher HCV incidence rates and shorter time to HCV seroconversion, corroborating other emerging data [42, 43]; in a recent study that controlled for individual risk behaviors, females were found to have a higher HCV incidence, higher IRR, and a shorter time to HCV seroconversion, suggesting that some combination of biologic, social, and structural factors may contribute to an increased risk among females [42, 44]. We found that non-Hispanic blacks had a lower HCV prevalence and Hispanics had a higher HCV prevalence than non-Hispanic whites but that incidence and IRRs did not differ significantly by race/ethnicity. These observations merit further study and likely merit focused interventions to address race/ethnicity disparities in HCV prevalence and gender disparities in HCV incidence.
Our data demonstrate that PWUD who do not report that injection is among their main routes of drug use have HCV incidence rates higher than the general population. The HCV incidence observed was many fold higher than the 1 per 100 000 population HCV incidence observed in the United States in 2016 [45]. Furthermore, HCV prevalence among those not reporting main drug use by injection was 35%, >10-fold higher than the 3.25% prevalence observed in the baby boomer birth cohort [46]. The 1.3/100 PYO incidence of HCV we observed among those whose main drug use was not by injection may reflect either that these patients did inject nonsterilely (without themselves considering injection one of their main routes of drug use), or may reflect other modes of transmission such as use of contaminated noninjection drug use equipment or sex with blood present [38]. This highlights the importance of MAT to reduce HCV incidence among those with OUDs, both among those who do inject (to reduce injection frequency) and who do not inject (to prevent transitions to injection) and the importance of HCV testing among PWUD regardless of reported route of use.
Our work contributes to the limited literature examining the impact of continuous MAT engagement during follow-up for HCV incidence [10]. While measures reflecting duration of lifetime injection may be most relevant for HCV prevalence and measures reflecting recent injection (eg, ever in the past 6 months) may best reflect current HCV injection risk, individuals transition between periods of injection and noninjection use. In fact, “reverse transitions” from injection to noninjection drug use have been adopted by some PWUD as a means of harm reduction, and such reverse transitions have been associated with decreases in HCV incidence [47].
The HCV incidence rates identified in our study reflect incidence among patients enrolled in MAT and receiving repeated HCV testing; HCV incidence may have been higher in the absence of MAT. Nonetheless, our approach of examining incidence based on the first negative HCV test while enrolled in MAT allows an examination of the potential impact of specific MAT factors: methadone dose, and MAT engagement and retention. Our finding that methadone doses of <60 mg were significantly associated with a shorter time to HCV seroconversion adds to the existing but modest literature identifying this association [15, 39, 48]. This highlights the importance not only of expanding MAT access but also in ensuring high-quality MAT through provision of adequate doses.
Studies examining HCV incidence as a function of degree of MAT retention as a continuous variable are scarce. A recent Cochrane review identified that most studies of the impact of MAT on HCV incidence examined a variable of “current” MAT use; some used a definition of “ever” having been in MAT [10]. We found that the more days a patient was not retained in MAT, the higher the risk for HCV seroconversion, and that more days not retained in MAT was slightly associated with shorter time to HCV seroconversion; these data add support to the growing evidence base suggesting that efforts to improve not only access to MAT but also greater MAT retention (ie, increasing the days that an individual is retained in MAT) will be important to HCV control [7, 12, 14, 48].
While these data are derived from patients receiving MAT for OUDs, they were mostly not in HCV care. One of the major factors contributing to the lesser population-level impact of combined prevention on HCV control (compared to HIV control) is that while that HIV treatment was made available at public health scale, the same has not yet been fully accomplished for HCV treatment [37, 49].
Our study contributes to the literature by applying the CVL construct to HCV epidemiology and by examining area-level variations in HCV CVL and its association with area-level HCV incidence [28, 31]. We found that HCV CVL varied significantly among UHF areas. Geographic variation in HCV CVL has also been recently reported in Houston, Texas [19]. These data suggest that measures of HCV CVL may be valuable in geographically focusing both harm reduction and CasP efforts to achieve HCV control. Our study also found that for each 1 log10 increase in HCV CVL, the HCV incidence rate was expected to be multiplied by about 2.7, although this association was not statistically significant. Our data are consistent with the general inference that the CVL in an area may contribute to incidence by increasing the risk associated with any single nonsterile injection event. As acute HCV infection and HCV incidence are difficult to identify and measure directly, if HCV CVL were found to be a valid predictor of incidence it would be a valuable metric for HCV control efforts. Furthermore, since individuals remain HCV antibody positive after treatment and cure, metrics reflecting active HCV infection, such as CVL, may assume increasing importance as CasP is increasingly implemented. Our findings, and these considerations, suggest the importance of further research using datasets examining a greater number of geographic areas and more person-time, as well as possible refinements in how HCV CVL is measured, including more inclusive HCV CVL measures, to assess the potential value of the HCV CVL concept more precisely [34].
This study has limitations that must be noted. Analyses were of PWUD in MAT in OTP who may differ from those not in OTP, thereby limiting generalizability. Analyses were conducted among those who received HCV antibody testing; while it is plausible that those tested may have differed from those untested, we did not identify differences between these groups.
Our incidence analyses examining the impact of MAT did not adjust for access to sterile syringes through needle/syringe programs or pharmacies as individual-level data on patients’ sterile syringe use were unavailable; however, the OTP studied was in NYC, an area of high sterile syringe access.
Our CVL analyses were limited by the modest number of geographic areas, of incident infections, by modest person-time, and by use of a CVL measure relying exclusively on directly measured data and which therefore may not fully reflect OTP patients who were not fully tested or other PWUD who may be linked to OTP patients through risk networks; more inclusive CVL measures that utilize both directly measured data and estimation and imputation merit further study [34]. A more refined measure of CVL could also reflect the aggregate of both individual VLs and individual degree of distributive risk behavior.
Strengths of this work included the use of longitudinal data to directly calculate incidence, assessment of the impact of methadone dose and MAT retention on the HCV preventive efficacy of MAT, examination of sex disparities in HCV incidence, assessment of incidence among those whose main drug use was not by injection, and the examination of geographic variation in HCV CVL and the impact of area-level HCV CVL on area-level HCV incidence.
CONCLUSIONS
The worsening HCV epidemic causes significant morbidity and mortality despite the availability of known primary prevention and highly efficacious HCV treatment that can result in cure. The incidence rates observed in our study support recommendations for annual HCV testing of all those in MAT, not only of those who report injecting drugs [50]. Interventions (at individual-, provider-, program-, and policy levels) to ensure adequate methadone dosing and enhanced MAT engagement and retention and, when relevant, to link MAT patients to sources of sterile syringes for primary HCV prevention, may be valuable strategies to enhance HCV control [7, 8, 10, 12, 17, 18]. The finding that HCV IRRs and time to seroconversion were shorter among females suggests that further studies of, and efforts to address, these disparities are needed. HCV CVLs vary geographically, suggesting a need to geographically focus prevention and treatment efforts. Further study of potential racial/ethnic and gender disparities in HCV prevalence and HCV CVL are warranted. HCV CVLs merit further study as predictors of HCV incidence and as metrics of HCV prevention and treatment efforts.
The large reservoir of chronically infected persons and current HCV incidence rates reinforce the need for vigorous efforts to expand MAT access and ensure appropriate doses, continuous engagement, and improved retention and the need to expand and geographically focus HCV prevention and treatment to achieve HCV CasP and HCV control.
Notes
Disclaimer. The views expressed are the authors’ own and do not necessarily represent the views of the National Institutes of Health, the United States Government, or the authors’ institutions.
Financial support. This work was supported in part by the National Institute on Drug Abuse/National Institutes of Health (T32 training grant number 5T32DA007233-35 and P30 center grants P30DA011041 and P30DA040500).
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Perlman DC, Jordan AE. The syndemic of opioid misuse, overdose, HCV, and HIV: structural-level causes and interventions. Curr HIV/AIDS Rep 2018; 15:96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis 2014; 59:1411–9. [DOI] [PubMed] [Google Scholar]
- 3. Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW. State HCV incidence and policies related to HCV preventive and treatment services for persons who inject drugs—United States, 2015-2016. MMWR Morb Mortal Wkly Rep 2017; 66:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 2019; 393:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moorman AC, Rupp LB, Gordon SC, et al. CHeCS Investigators Long-term liver disease, treatment, and mortality outcomes among 17,000 persons diagnosed with chronic hepatitis C virus infection: current Chronic Hepatitis Cohort Study status and review of findings. Infect Dis Clin North Am 2018; 32:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emmanuel B, Shardell MD, Tracy L, Kottilil S, El-Kamary SS. Racial disparity in all-cause mortality among hepatitis C virus-infected individuals in a general US population, NHANES III. J Viral Hepat 2017; 24:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis 2011; 204:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57(Suppl 2):S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linas BP, Barter DM, Leff JA, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Platt L, Reed J, Minozzi S, et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016; 2016. pii:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011; 8:CD004145. [DOI] [PubMed] [Google Scholar]
- 12. Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106:1978–88. [DOI] [PubMed] [Google Scholar]
- 13. Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat 2005; 28:321–9. [DOI] [PubMed] [Google Scholar]
- 16. Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS 2005; 19(Suppl 3):S20–25. [DOI] [PubMed] [Google Scholar]
- 17. de Vos AS, van der Helm JJ, Matser A, Prins M, Kretzschmar ME. Decline in incidence of HIV and hepatitis C virus infection among injecting drug users in Amsterdam; evidence for harm reduction? Addiction 2013; 108:1070–81. [DOI] [PubMed] [Google Scholar]
- 18. MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy 2014; 25:34–52. [DOI] [PubMed] [Google Scholar]
- 19. Arnold RM, Yang B, Yu Q, Arafat RR. Refocusing hepatitis C prevention through geographic viral load analysis. Online J Public Health 2018. http://ojphi.org/ojs/index.php/ojphi/article/view/6501. Accessed 30 August 2019.
- 20. Jordan AE, Perlman DC, Reed J, Smith DJ, Hagan H. Patterns and gaps identified in a systematic review of the hepatitis C virus care continuum in studies among people who use drugs. Front Public Health 2017; 5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perlman DC, Des Jarlais DC, Feelemyer J. Can HIV and hepatitis C virus infection be eliminated among persons who inject drugs? J Addict Dis 2015; 34:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis C virus infection among persons who inject drugs in New York City: 2006-2013. Drug Alcohol Depend 2015; 152:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bocour A, Greene SK, Laraque F, Winters A. Estimating the prevalence of chronic hepatitis C virus infection in New York City, 2015. Epidemiol Infect 2018; 146:1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. New York City Department of Health and Mental Hygiene. Working toward a hep free NYC: HAV, HBV, and HCV in NYC—2017 annual report. New York: New York City Department of Health and Mental Hygiene, 2018. [Google Scholar]
- 25. Abraham AJ, Andrews CM, Grogan CM, et al. The Affordable Care Act transformation of substance use disorder treatment. Am J Public Health 2017; 107:31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Foundation for AIDS Research. amfAR report highlights huge gaps in access to opioid addiction treatment 2018. https://www.amfar.org/amfar-report-highlights-huge-gaps-in-access-to-opioid-addiction-treatment/. Accessed 13 August 2019.
- 27. Lipari RN, Park-Lee E, Van Horn S. America’s need for and receipt of substance use treatment in 2015. The CBHSQ report. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013:1–7. [PubMed] [Google Scholar]
- 28. Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das M, Raymond HF, Chu P, et al. Measuring the unknown: calculating community viral load among HIV-infected MSM unaware of their HIV status in San Francisco from National HIV Behavioral Surveillance, 2004-2011. J Acquir Immune Defic Syndr 2013; 63:e84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castel AD, Befus M, Willis S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS 2012; 26:345–53. [DOI] [PubMed] [Google Scholar]
- 31. Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014; 28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laraque F, Mavronicolas HA, Robertson MM, Gortakowski HW, Terzian AS. Disparities in community viral load among HIV-infected persons in New York City. AIDS 2013; 27:2129–39. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Guidance on community viral load: a family of measures, definitions, and method for calculation 2011. http://www.ct.gov/dph/lib/dph/aids_and_chronic/surveillance/statewide/community_viralload_guidance.pdf. Accessed 30 August 2019.
- 35. Yazdanpanah Y, De Carli G, Migueres B, et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 2005; 41:1423–30. [DOI] [PubMed] [Google Scholar]
- 36. Popping S, El-Sayed M, Feld J, et al. Report from the International Viral Hepatitis Elimination Meeting (IVHEM), 17-18 November 2017, Amsterdam, the Netherlands: gaps and challenges in the WHO 2030 hepatitis C elimination framework. J Virus Erad 2018; 4:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Makarenko I, Artenie A, Hoj S, et al. Transitioning from interferon-based to direct antiviral treatment options: a potential shift in barriers and facilitators of treatment initiation among people who use drugs? Int J Drug Policy 2019; 72:69–76. [DOI] [PubMed] [Google Scholar]
- 38. Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trafton JA, Minkel J, Humphreys K. Determining effective methadone doses for individual opioid-dependent patients. PLoS Med 2006; 3:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. New York City Department of Health and Mental Hygiene. New York City United Hospital Fund (UHF) neighborhoods and NYC ZIP code areas 2006. http://www.nyc.gov/html/doh/ downloads/pdf/survey/uhf_map_100604.pdf. Accessed 1 September 2019.
- 41. R Foundation for Statistical Computing. R: a language and environment for statistical computing. 2013. http://www.R-project.org/. Accessed 30 August 2019. [Google Scholar]
- 42. Esmaeili A, Mirzazadeh A, Morris MD, et al. InC3 Collaborative The effect of female sex on hepatitis C incidence among people who inject drugs: results from the international multicohort InC3 collaborative. Clin Infect Dis 2018; 66:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iversen J, Wand H, Topp L, Kaldor J, Maher L. Reduction in HCV incidence among injection drug users attending needle and syringe programs in Australia: a linkage study. Am J Public Health 2013; 103:1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009; 200:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm. Accessed 15 September 2019. [Google Scholar]
- 46. Smith BD, Morgan RL, Beckett GA, et al. Centers for Disease Control and Prevention Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012; 61:1–32. [PubMed] [Google Scholar]
- 47. Des Jarlais DC, McKnight C, Arasteh K, et al. Transitions from injecting to non-injecting drug use: potential protection against HCV infection. J Subst Abuse Treat 2014; 46:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kapadia SN, Johnston CD, Marks KM, Schackman BR, Martin EG. Strategies for improving hepatitis C treatment access in the United States: state officials address high drug prices, stigma, and building treatment capacity. J Public Health Manag Pract 2019; 25:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. American Association for the Study of Liver Diseases/Infectious Diseases Society of America. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018; 67:1477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]