Abstract
Despite various challenges that hinder the implementation of high-tech molecular methods in resource-limited settings, we have been able to implement and achieve International Organization for Standardization 15189:2012 accreditation for genotypic HIV drug resistance testing in our facility. At the Center for Human Virology and Genomics, Nigerian Institute of Medical Research, Nigeria has recorded a high sequencing success rate and good quality sequence data. This was achieved by optimizing laboratory processes from 2008 to the current date. We have optimized sample preparation, RT-PCR, several post-PCR processes, and the cycle sequencing to improve the sensitivity of amplification even with limited plasma samples and low viral copy numbers. The optimized workflow maximizes output, minimizes reagent wastage, and achieves substantial cost savings without compromising the quality of the sequence data. Our performance at our last external quality assurance program is a testimonial to the efficiency of the workflow. For the 5-sample panel, each with 67–68 mutation points evaluated, we scored 100% for all 5 specimens. Our optimized laboratory workflow is thus documented to support laboratories and to help researchers achieve excellent results the first time and eliminate contamination while minimizing the wastage of costly sequencing reagents.
Keywords: biomedical research · HIV drug resistance · laboratory workflow · reverse transcriptase polymerase chain reaction
INTRODUCTION
The fundamental goal of antiretroviral therapy (ART) is to effectively suppress the HIV viral load (VL) in patients, restore immunologic functions, improve quality of life, and reduce HIV-associated morbidity and mortality.1, 2 With increased support from diagnosis, treatment, and monitoring programs, there has been a significant decline in HIV/AIDS-associated morbidity and mortality.3 This success is mainly achieved through improved ART coverage and the continuous clinical, virologic, and immunologic monitoring of patients on ART.4 One such monitoring strategy is HIV drug resistance testing (HIVDRT), which helps assess the potential impact of HIV drug resistance (HIVDR) on clinical outcomes.
In sub-Saharan Africa, where standardized ART regimens are used in a public health approach and where VL monitoring and resistance testing are typically not done routinely, there is a crucial need for an efficient HIVDRT protocol, robust enough to eliminate contamination and improve sequence quality while minimizing wastage. Phenotypic HIVDRT is sometimes used to assess the impact of different ART regimens on a specific clone of HIV strain. Here, the sensitivity (phenotype) of a patient’s HIV in response to specific antiretrovirals is measured directly.5 However, genotypic resistance tests (GRTs) can investigate the viral genome mutation that is associated with drug resistance through nucleotide sequencing of the target genes.6
DNA sequencing is the most common method for HIVDR assessment because of its ease of use, quick detection of mutation, and comparatively low cost for developed regions.5, 7, 8 In high-income countries, Sanger GRT is considered cost effective at the time of diagnosis or treatment initiation and virologic failure. However, in low- and middle-income countries, survey prototypes have produced contradictory results regarding the cost effectiveness of Sanger GRT.5 To improve its affordability and accessibility in for resource-limited countries, the World Health Organization (WHO) launched a global action plan against HIVDR. The U.S. Centers for Disease Control and Prevention (CDC) was one of the pioneer inventors to participate in this and developed a robust test, currently recommended and distributed by Thermo Fisher Scientific (Waltham, MA, USA).3, 9 This test is used for genotyping the genetically diverse HIV-1 virus from plasma and dried blood spot samples to detect resistance mutations in the protease and reverse transcriptase genes with a good subtype inclusivity rate compared with other commercial DRT assays in the market.
Phenotypic HIVDRT is sometimes used to assess the impact of different ART regimen on a specific clone of HIV strain. However, genotypic testing, especially DNA sequencing, is the most common method for HIVDR assessment because of its ease of use, quick detection of mutation, and comparatively low cost.5, 7, 8 Laboratory steps involved in the genotypic HIVDRT workflow include specimen preparation, RNA extraction, RT-PCR and nested PCR, gel electrophoresis (to verify correct band size), cycle sequencing, and running on the genetic analyzer. Depending on the laboratory workflow and throughput, Sanger sequencing may take between 2 and 7 d to go from specimen to results. There are various challenges along this long laboratory workflow. Here, we present optimization tweaks observed for HIVDRT that have contributed to the high-quality sequences generated from our facility. These workflow tweaks have helped improve consistency, sensitivity, and efficiency since 2008. They can be used irrespective of the assay (in-house or commercial kits) being used for Sanger sequencing, especially in a resource-limited setting. We thought to share these optimization tweaks that have helped improve consistency, sensitivity, and efficiency since 2008.
MATERIALS, METHODS, AND RESULTS
Facility and personal protective equipment optimization
The Center for Human Virology and Genomics Laboratory is an International Organization for Standardization 15189:2012 accredited facility in the Microbiology Department of the Nigerian Institute of Medical Research. As a national HIV referral laboratory, the Center performs various diagnosis and monitoring tests, including CD4 count, VL, liver function test, and HIVDR, for HIV patient management and research purposes. HIVDRT is a major assay the Center performs for several national HIV projects and surveys. The Center is also preparing for the WHO audit and recognition to perform HIVDRT for WHO-approved surveillance studies.
Consequently, an approved quality management system is in place at the Center, with HIVDRT among the International Organization for Standardization 15189 accredited assays. There is a sequencing suite using 4 separate laboratory workspaces in a unidirectional workflow for 1) reagent preparation, 2) specimen extraction, 3) RT-PCR, and 4) sequencing. Each room within the sequencing suite has dedicated materials necessary to complete the processes in the work area.
Laboratory coats are dedicated to working areas, and gloves are changed frequently to preclude contamination. Each room has an appropriate biosafety cabinet, and work surfaces and equipment are decontaminated before and after work using appropriate solvents such as RNase Away, DNA Away, DNAzap (all from Thermo Fisher Scientific), 70% alcohol, and UV radiation in the biosafety cabinets. We use standard laboratory cold boxes for 1.7–2.0 ml tubes, 0.2 ml tubes, or 96-microwell plates, with dedicated sets in relevant rooms of the sequencing suite. These cold boxes are changed intermittently to maintain reagents at appropriate temperatures.
Laboratory run sheets are used for documentation of events and staff traceability during all steps of the workflow.
Specimen-related challenges and optimization tweaks
Plasma is the most recommended sample type for HIVDR genotyping wherever possible, and dried blood spots can also be used.3 The specimen, the volume available, and the specimen processing and shipping conditions play a significant role in the efficiency of downstream HIVDRT. The routine VL specimen transportation network in the country can be used after filling a HIVDRT request form. This collects, among other data, the last known VL to ascertain eligibility.
Plasma processing involves the use of anticoagulant-containing tubes to prevent clot formation, preferably EDTA anticoagulant and never heparin. Clot formation absorbs and masks a proportion of virus, reducing the amount of virus available for amplification and genotyping.3 This is particularly important for specimens collected from patients on ART who have low-level viremia. Similarly, hemolysis should be avoided when processing the primary specimen into plasma. Specimens for HIVDRT can be processed using the VL specimen processing protocol. The time between the collection of primary specimens to the separation of plasma should be within 4–6 h. After that, plasma can be kept at 2–8°C before shipment the same day. If it is not being shipped immediately, plasma can be stored at −20°C or preferably at −80°C (for >6 mo) before shipment and analysis. Cold chain storage should be maintained during sample shipment using dry ice and special ice packs designed to maintain the temperature for the distance and transit time. Freeze-thawing should be avoided because each cycle is detrimental to downstream processes.3 Improper storage allows RNases to degrade the specimen, especially at elevated temperatures.
Viral RNA extraction challenges and optimization tweaks
A high-quality purified nucleic acid is essential for successful genotyping, which we achieve by implementing proper specimen extraction. Many commercial methods exist; however, it is advisable to use a method validated in the facility for the specimen type and the nucleic acid being collected. RNA is unstable at high temperatures and prone to degradation by RNases, making work with RNA more challenging.10
The fundamental issue with RNA-based studies is the stability and ubiquity of the RNase A enzyme, harbored by personnel and on contact surfaces. Thus, good laboratory practice and use of personnel protective equipment, preferably in a unidirectional workflow, will achieve optimal and reproducible results. Also, segregation of tools and equipment and the use of RNase-free reagents and consumables will improve workflow efficiency.
Given the high number of hands-on manipulations involved, this procedure is prone to errors. Hence, it should be carried out in manageable run sizes to reduce the risk of contamination. Appropriate job aids should be made available at each point of use, and excessive movements and distractions avoided at all points.
VL of the specimen for HIVDRT determines the success of the extraction process. Low HIV VL in plasma can be further complicated by suboptimal preservation and increased freeze-thaw cycles leading to the degradation of the available RNA.11 This is especially important for specimens stored for 6 mo or more. To improve the chances of extraction of quality RNA, we use a higher specimen volume, 2–3 times higher than the input volume, and concentrate via centrifugation. Plasma specimens are centrifuged at 16,400 rpm for 1 h in a refrigerated centrifuge set at 4°C before proceeding with RNA extraction. The extraneous supernatant is removed, leaving only the sample input volume, before starting extraction. The supernatant is removed while avoiding the marked area where concentrated RNA should have pelleted depending on tube placement in an angular centrifuge. This process increases the concentration of HIV viral RNA available for isolation during the extraction process. Recently collected specimens with high VL do not need this concentration step unless they are processed in the same run with stored specimens.
Specimens with VL >1000 copies per milliliter (cp/ml) are ideal for HIVDRT. Identification of resistance mutations is often not guaranteed when VLs are below 1000 cp/ml. Samples with low VL can be concentrated by centrifuging as described above before RNA extraction. The success of the PCR amplification is highly dose-dependent on the initial HIV copy number.
RT-PCR–related challenges and optimization tweaks
After RNA extraction has been completed, the RT-PCR should preferably be initiated the same day. Extracted RNA should be kept on ice or in a 2–8°C cooler box. The RT-PCR amplification converts the RNA to cDNA. After that, another round of nested PCR is performed to amplify the cDNA fragment sufficiently. The nested PCR further amplifies the target’s smaller inner fragment, reducing potential contamination from nonspecific amplification due to inadvertent primer binding during RT-PCR.
PCR primers tend to intertwine and form hairpin loops or dimers with each other due to long-term cold storage. When such primers are used directly, they do not open and anneal to the template strand. Depending on the protocol and reagents, this can give a variable performance from reagents of the same lot or kit. If a PCR mix containing the deoxynucleoside triphosphates (dNTPs) and primers does not come mixed with the enzyme, the variable performance can be minimized and standardized by heating the PCR mix at 95°C for 3–5 min and immediately cooling on ice before adding the enzyme. If a PCR protocol has an initial 95°C step for some minutes, such as when using hot start enzymes, this will take care of such secondary structures, and you may not need this tweak.
It is preferable to use different tubes for the RT-PCR and the nested PCR such that you can repeat the nested PCR without starting afresh from the RNA specimen. Besides, the input RNA volume for the RT-PCR and nested PCR vary for different assays. This input specimen volume for RT-PCR or nested PCR can be increased up to twice the volume to improve the chances of amplification, especially for specimens with low VL. In extreme cases, you may need to increase specimen input volume for both the RT-PCR and the nested PCR, for example, with a stored specimen that has low VL and has been stored for >6 mo, mostly in suboptimal storage in resource-constrained settings. The U.S. CDC and Thermo Fisher Scientific HIVDR kit has a rescue RT-PCR mix containing alternate primers used to amplify specimens failing amplification with the standard RT-PCR mix.
In Nigeria, we have a lot of recombinant HIV strains, and thus, before we conclude a specimen was not sequenced, we must use this rescue RT-PCR mix for repeat testing. After using both the standard and rescue RT-PCR mixes without bands seen on gel electrophoresis, we conclude that a specimen could not be sequenced.
Postamplification challenges and optimization tweaks
After nested PCR, we carry out agarose gel electrophoresis to determine which specimens were amplified and mainly check that it has a fragment with little or no smearing. This is done post-PCR for both the standard and rescue mix testing. Having the right-sized fragment on the gel is very important and guarantees end results no matter how faint the gel band. However, if the right-sized band is not seen on the gel, do not go ahead, irrespective of quantification values, because lower fragment and dimers can contribute to this. In fact, without an appropriate gel band, it is not recommended to go ahead or quantify. Use an appropriate DNA ladder and preferably one of the new, safer DNA stains. If any specimen was not amplified, we repeat testing with the U.S. CDC–Thermo Fisher Scientific rescue RT-PCR mix.
PCR purification
Specimens with the appropriate fragment size and little or no smearing can be purified for downstream analysis. There are several commercial methods to remove unused dNTPs and primers before moving further to the sequencing stage. Suitable purification will reduce background noise in the sequences to a minimum.12 We purify nested PCR products using enzymatic methods [specifically with ExoSAP-IT (Thermo Fisher Scientific)]. Column-based methods are also popular, and we have used these before. However, you cannot wholly elute cDNA from the columns. This is important for specimens with low cDNA copy numbers. Using the enzymatic method keeps all cDNA copies available for downstream processes as the reagent is added to the cDNA product in the specified proportion and heated at 2 different temperature for variable periods using a thermal cycler. The first temperature degrades the excess nucleotides and unused primers, whereas the second temperature inactivates the enzymes. It is essential to keep the purified cDNA cold, even after taking a specimen for immediate processing. Our experience has shown that keeping purified specimens at room temperature may lead to degradation, possibly from residual uracil N-glycosylase activity.
For some assays requiring specimen–cycle sequencing reagent mixing according to cDNA concentration, the use of the enzymatic cleanup method is advantageous because it inadvertently increases the volume of the purified cDNA. Thus, we achieve pure cDNA template while minimizing loss of copy numbers and sequence background noise downstream. Fig. 1 below shows that the use of ExoSAP-IT contributed to achieving clean cDNA for downstream applications. The difference between it and the neat amplicons was not much, showing that we can recover more cDNA copies. Fig. 2 further demonstrates the utility of ExoSAP-IT purification. Two specimens with double bands and specimen NM082741 with low cDNA copies were purified with ExoSAP-IT and successfully sequenced with no background noise. Note that Fig. 2 is a prepurification gel picture, but the use of ExoSAP-IT conserves all available cDNA copies.
FIGURE 1.
Gel electrophoresis results of neat and EXOSAP-IT purified amplicons.
FIGURE 2.
Gel electrophoresis of specimens with low cDNA (NM082741) and double fragments (NM101776; NM101482) successfully sequenced (prepurification gel photo).
Quantification of cDNA in the nested PCR products
Depending on the protocol and assay, you may not need to know the exact quantity of the cDNA. For some assays, if you have a good band on the agarose gel, you can be assured that sequencing will be successful. However, too high cDNA copy number in the cycle sequencing mix can be problematic for cycle sequencing and the software you use for assembly and base-calling. If the cDNA concentration is right, you will have a clear sequence needing few or no base-calling edits. In RECall software (British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada) for HIV sequence assembly and editing, such sequences are automatically approved.
Nested PCR products are quantified using the double-stranded DNA high-sensitivity reagent on the Qubit-4 fluorometer (Thermo Fisher Scientific) following the manufacturer’s protocol. Earlier we quantified before PCR purification, but now we quantify cDNA after PCR purification using the enzymatic method. With the enzymatic purification method, we have successfully sequenced cDNA with a quantification value of 2.2–5.7 ng/μl before purification and another sample with 1.21 ng/μl after purification. The quantification value obtained here guides the optimization of the DNA volume in the cycle sequencing reaction for optimal results.
Optimizing cDNA concentration for cycle sequencing
During sequencing reactions, dNTPs and dideoxynucleoside triphosphates are incorporated into the sequencing product. These products are further purified before being analyzed by the sequencing machine to generate the data report. The concentration of DNA template in each of the sequencing wells determines the amount of sequence reaction occurring at a given time as other factors are constant. The same volume of cDNA from a patient with low VL and another patient with high VL would contain different cDNA concentrations. For reaction efficiency, the HIV-1 cDNA concentration should not be too low nor too high. This promotes an effective sequence reaction allowing the generation of a good quality sequence with minimal background noise.
Early in our sequencing experience, we noticed that sequences with high backgrounds resolve if the same sequencing reaction plate is run a second time in the genetic analyzer, even without removing from the genetic analyzer. We are not sure why this occurs. Consequently, for specimens quantified before purification, if cDNA is ≤30 ng/μl, we use 2 µl of HIV cDNA for the cycle sequencing reaction, whereas 1 µl is used for specimens containing cDNA >30 ng/μl. If cDNAs are quantified after purification, 20 ng/μl is used as the cutoff point. Our experience has shown that this allows for efficient sequence reaction and the generation of good quality sequence data with minimized background noise.
Evidence of the efficiency of the workflow
In a study carried out between 2013 and 2016, we used specimens stored for between 3 and 9 yr, often suboptimally due to power fluctuations.13 This optimized workflow proved so useful that we had a 75% success rate with specimens stored for 9 yr (Fig. 3). HIVDRT kits routinely recommend the use of specimens stored for no more than 6 mo.
FIGURE 3.
Sequencing success rate in a study that used stored specimens.
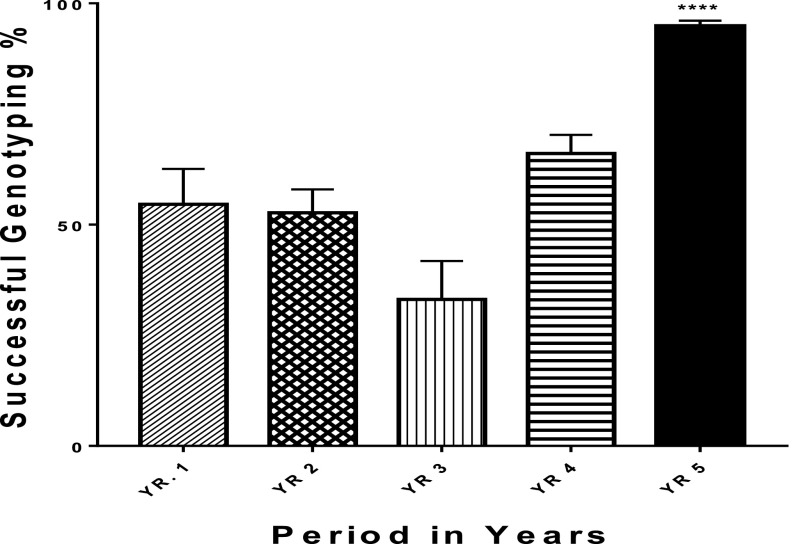
Except for the concentration step, these optimizations were implemented as continual improvements in our sequencing journey since 2008. The data show that our sequencing success rate improved significantly in the last 5 yr (Fig. 4).
FIGURE 4.
Sequencing success rates over the last 5-yr period. Error bars represent se (P = 0.05); ****P < 0.0001.
A t test was used to compare each period with the yr 5 data. The result showed that there was a significant difference (<0.0001) in HIVDRT performance over the years. With training and retraining, various optimization processes were implemented, leading to a significant improvement in the outcome of HIVDRT.
Fig. 5 shows the trace files for 3 specimens successfully sequenced despite challenges during the workflow. Specimen NM-08-2741 had low DNA yield, as seen in the gel electrophoresis picture (Fig. 2). However, this specimen was successfully sequenced with minimal background noise and with up to 4 forward and reverse reads overlapping. Finally, specimen DRT1075 was successfully sequenced even though the purified nested PCR amplicon had a value of 1.21 ng/μl after purification.
FIGURE 5.
Trace files for specimens. A) NM-08-2741 with low DNA yield. B) DRT1075 with 1.21 ng/μl value after purification.
In addition, the Thermo Fisher Scientific HIVDRT assay is recommended for specimens with a VL above 1000 cp/ml. However, in another survey in 2019, we were requested to attempt to sequence 106 samples with a VL below 1000 cp/ml. Using our optimized workflow, we were able to successfully sequence 58 specimens, giving a 54.7% success rate with samples below 1000 cp/ml.
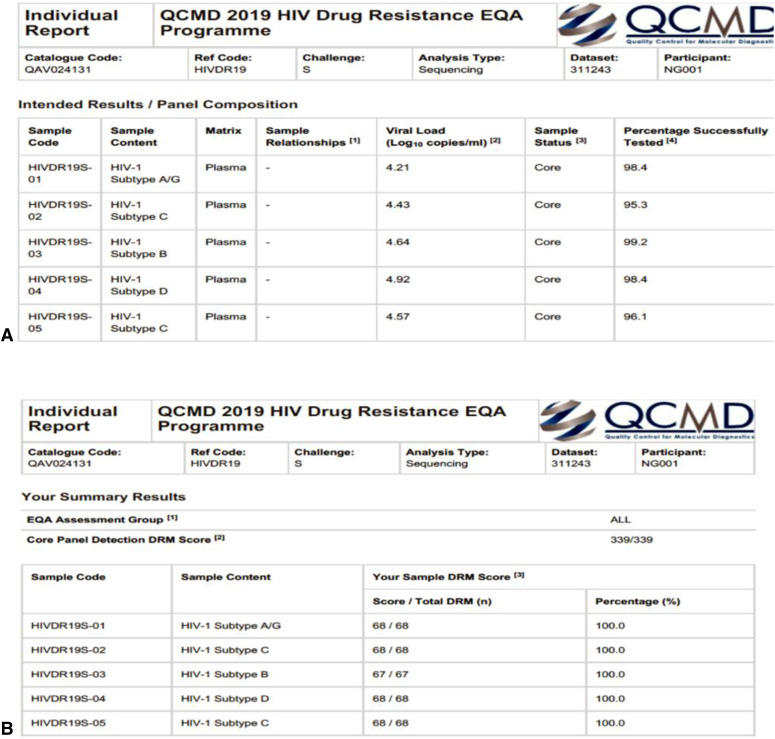
Finally, as an accredited assay in our facility, we participate in an external quality assurance program for HIVDRT run by the Quality Control for Molecular Diagnostics. The assessment panel included 5 specimens with 67–68 points of mutation being monitored per specimen. Our 2019 report (Fig. 6) indicate we scored 100% for all 5 samples, including making the right call for all 67–68 point mutations.
FIGURE 6.
External quality assurance report. A) Panel specimen details. B) Facility results per specimen.
CONCLUSIONS
The laboratory workflow for HIV drug resistance genotypic testing is long and combines many processes and thus requires stepwise quality control and assurance to ensure excellent results. Given the unique challenges of resource-limited settings, we thought to share our laboratory workflow and practices that helped us progressively achieve improved performance in sequencing since 2008. Our optimized laboratory workflow is thus documented to support laboratories and researchers in achieving excellent results the first time, eliminating contamination, and minimizing wastage of costly sequencing reagents.
REFERENCES
- 1.Montaner JSG, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. [DOI] [PubMed] [Google Scholar]
- 2.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO/HIVResNet global HIV drug resistance strategy. 2010. Available at: http://www.who.int/hiv/pub/drugresistance/hiv_reslab_strategy.pdf. Accessed: June 14, 2020.
- 4.de Waal R, Lessells R, Hauser A, et al. HIV drug resistance in sub-Saharan Africa: public health questions and the potential role of real-world data and mathematical modelling. J Virus Erad. 2018;4(Suppl 2):55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzaule SC, Ondoa P, Peter T, et al. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect Dis. 2016;16:e267–e275. [DOI] [PubMed] [Google Scholar]
- 7.Lapointe HR, Dong W, Lee GQ, et al. HIV drug resistance testing by high-multiplex “wide” sequencing on the MiSeq instrument. Antimicrob Agents Chemother. 2015;59:6824–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee SY, Jordan MR, Raizes E, et al. HIV-1 drug resistance mutations: potential applications for point-of-care genotypic resistance testing. PLoS One. 2015;10:e0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Wagar N, DeVos JR, et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One. 2011;6:e28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan, T, Huggett, J, Sanchez, E. Good practice guide for the application of quantitative PCR (qPCR). Teddington, UK: LGC, 2013.
- 11.Pattery T, Verlinden Y, De Wolf H, et al. Development and performance of conventional HIV-1 phenotyping (Antivirogram®) and genotype-based calculated phenotyping assay (virco®TYPE HIV-1) on protease and reverse transcriptase genes to evaluate drug resistance. Intervirology. 2012;55:138–146. [DOI] [PubMed] [Google Scholar]
- 12.Shafer RW, Dupnik K, Winters MA, Eshleman SH. A guide to HIV-1 reverse transcriptase and protease sequencing for drug resistance studies. HIV Seq Compend. 2001;2001:1–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosemary A, Chika O, Jonathan O, et al. Genotyping performance evaluation of commercially available HIV-1 drug resistance test. PLoS One. 2018;13:e0198246. [DOI] [PMC free article] [PubMed] [Google Scholar]