Abstract
Background
Patients with opioid use disorder (OUD) are frequently admitted for invasive infections. Medications for OUD (MOUD) may improve outcomes in hospitalized patients.
Methods
In this retrospective cohort of 220 admissions to a tertiary care center for invasive infections due to OUD, we compared 4 MOUD treatment strategies: methadone, buprenorphine, methadone taper for detoxification, and no medication to determine whether there were differences in parenteral antibiotic completion and readmission rates.
Results
The MOUDs were associated with increased completion of parenteral antimicrobial therapy (64.08% vs 46.15%; odds ratio [OR] = 2.08; 95% CI, 1.23–3.61). On multivariate analysis, use of MOUD maintenance with either buprenorphine (OR = 0.38; 95% CI, .17–.85) or methadone maintenance (OR = 0.43; 95% CI, .20–.94) and continuation of MOUD on discharge (OR = 0.35; 95% CI, .18–.67) was associated with lower 90-day readmissions. In contrast, use of methadone for detoxification followed by tapering of the medication without continuation on discharge was not associated with decreased readmissions (OR = 1.87; 95% CI, .62–5.10).
Conclusions
Long-term MOUDs, regardless of selection, are an integral component of care in patients hospitalized with OUD-related infections. Patients with OUD should have arrangements made for MOUDs to be continued after discharge, and MOUDs should not be discontinued before discharge.
Keywords: injection drug use, medications for opioid use disorder, opioids, opioid use disorder, people who inject drugs (PWID)
The rise in opioid use disorder (OUD) and associated injection drug use (IDU) is fueling a surge of infectious complications [1]. People who inject drugs (PWID) are at particularly high risk of infective endocarditis, epidural abscess, osteomyelitis, septic arthritis, and necrotizing soft tissue infection through nonsterile injection practices, as well as bacterial and fungal contamination of needles and drug preparation equipment [2–4]. These invasive infections generally require prolonged courses of intravenous (IV) antibiotics. However, patients who inject drugs are often ineligible to receive outpatient parenteral antibiotics (OPAT) and frequently must complete their antimicrobial therapy in an inpatient setting [5]. These admissions are challenging for patients and their healthcare teams and are frequently complicated by opioid withdrawal, leading to incomplete antimicrobial treatment [6–8]. As a result, patients with OUD-associated invasive infections have increased hospital readmissions compared with patients with non-OUD-related infections [9]. Even if patients complete their antimicrobial treatment, they are at an increased risk for additional infectious complications if their underlying OUD is not addressed during the index hospitalization.
In the absence of contraindications, medically supervised treatment is recommended for patients with OUD [10]. The 3 Food and Drug Administration (FDA)-approved medications for treatment of OUD—methadone, buprenorphine, and naltrexone—are collectively termed medications for OUD (MOUD). Data comparing the efficacy of these medications and long-term strategies in patients with invasive infections secondary to OUD are limited [6, 11]. The objective of this study was to compare MOUD treatment strategies in patients hospitalized with severe infectious complications of OUD.
METHODS
We performed a retrospective chart review of patients admitted between January 2016 and January 2019 to Barnes-Jewish Hospital, a 1400-bed, academic, tertiary care center in St. Louis, Missouri. Electronic medical records (EMR) of all patients with International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) discharge diagnosis codes corresponding with IDU or OUD (Supplemental Table 1) and ICD-10 diagnosis codes for serious infections that generally require prolonged parenteral antimicrobials (Supplemental Table 2) who received infectious diseases (ID) consultation were examined. Admissions were then individually chart reviewed by an author (L.R.M.). Patient hospitalizations were included only if all of the following criteria were met: (1) infection was attributed to IDU of opioids or OUD by the ID consultant; (2) a prolonged course of parenteral antimicrobial therapy (defined as >2 weeks) was recommended by the ID consultant; and (3) the patient was not able to receive OPAT. Patients with infections related to other substance use disorders without concomitant OUD were excluded because they would not be expected to benefit from prescription of a MOUD. Patients discharged to skilled nursing facilities, long-term care facilities, or able to receive parenteral antimicrobial therapy at dialysis centers were excluded (n = 25), because they were able to receive IV antibiotics outside of the hospital. Patients who died during the inpatient admission were excluded because the primary endpoints could not be assessed. Each admission was treated as an independent event, and therefore some patients were included in the study more than once.
Receipt of MOUD, comprising buprenorphine, methadone, or either oral or intramuscular naltrexone, was assessed by review of the medication administration records (MAR). Patients were considered to have received methadone for detoxification only if the primary provider specifically documented this intention in the chart and the patient received decreasing doses of methadone. Consultation with an addiction medicine specialist was verified via EMR review. Although addiction medicine consultation is not mandatory at our institution and is at the discretion of the treating medical service, consultation is available 7 days a week. Outcomes of interest that were analyzed were as follows: completion of parenteral antimicrobial therapy (determined by review of the ID consultation notes, MAR, and physician discharge summaries), 90-day readmission, and against medical advice (AMA) discharges. To assess “percent completion of antibiotics,” planned end dates indicated in the ID consult notes were used. Patient demographics, clinical data, and microbiology data pertaining to their acute care hospitalization for serious infection and evidence of subsequent emergency department visits and/or hospitalization were collected. This review included screening all readmissions within 90 days of discharge at any of 15 hospitals in the BJC Healthcare System, as well as 20 additional hospitals in the St. Louis Metropolitan region, together representing over 90% of healthcare facilities in the region. All hospital readmissions were reviewed and were noted to be related to the patient’s OUD or recent infectious complications, with the exception of 2 admissions for normal spontaneous delivery of an infant and an admission for new malignancy diagnosis, which were excluded from the analysis.
Data were analyzed using Statistical Analytics Software, version 9.4 (SAS Institute, Cary, NC). Descriptive statistics were performed with Prism 8 (GraphPad Software, La Jolla CA). Demographic and clinical characteristics were compared for all patients by MOUD status using Fisher’s exact tests and Mann-Whitney U test for categorical variables and continuous variables, respectively. Univariate and multivariate regression analyses were performed, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to determine the predictors for completion of antimicrobial therapy and 90-day readmissions. We built a parsimonious multivariable regression model using a forward stepwise approach (entry P value .1 and the retention P value .05). Kaplan-Meier estimates were used to describe the survival distribution for time to readmission. The log-rank statistic was used to test the difference in time to readmission. All tests for significance were 2-tailed, with P < .05 considered significant. This study was approved by the Washington University Institutional Review Board.
RESULTS
A total of 232 patient admissions meeting all inclusion criteria were reviewed; 12 patients died during the initial inpatient encounter and were subsequently excluded. Baseline patient characteristics of the 220 admissions included in this study are presented in Table 1. The MOUDs were prescribed during 46.8% (n = 103) of admissions; 19.1% (n = 42) received buprenorphine, 19.1% (n = 42) received methadone maintenance therapy, and 8.6% (n = 19) received methadone tapers for detoxification. No patients received buprenorphine tapers and no patients received naltrexone. Among all those who received either buprenorphine or methadone maintenance therapy during their inpatient stay, 75% (n = 63) had documented arrangements for postdischarge continuation of MOUD; either through a local methadone clinic, addiction medicine clinic, or another X-waivered provider (practitioners authorized to treat opioid dependency with buprenorphine). Female gender, number of prior overdoses, prior endocarditis, and hepatitis C virus (HCV) infection were more common among the MOUD group than those not prescribed MOUDs.
Table 1.
Comparison of Characteristics of 220 Patients With Infectious Complications of Opioid Use Disorder, by MOUD Treatment Status
| Characteristics | MOUD n = 103 (48.6%) | No MOUD n = 117 (53.2%) | P Value |
|---|---|---|---|
| Demographics | |||
| White | 54 (52.4%) | 66 (56.4%) | .6915 |
| Female | 66 (64.1%) | 44 (37.6%) | <.0001 |
| Median age (range) | 38 (20–61) | 41 (20–71) | .0163 |
| Comorbidities | |||
| HIV infection | 1 (1.0%) | 5 (4.3%) | .1346 |
| Hepatitis C infection | 78 (75.8%) | 53 (45.3%) | <.0001 |
| Prior endocarditis | 36 (35.0%) | 24 (20.6%) | .0163 |
| Prior valve replacement | 11 (10.7%) | 6 (5.2%) | .1250 |
| Number of prior overdoses (range) | 0.5 (0–4) | 0.2 (0–3) | .0064 |
| Number of prior SUD-related infections (range) | 2.3 (0–8) | 1.7 (0–16) | .0552 |
| Malignancy | 5 (4.9%) | 1 (0.6%) | .0697 |
| HTN | 10 (9.7%) | 16 (13.7%) | .3654 |
| Pregnant | 4 (3.9%) | 1 (0.9%) | .1505 |
| Psychiatric comorbidity | 8 (7.8%) | 14 (12.0%) | .2966 |
| None | 8 (7.8%) | 17 (14.5%) | .1097 |
| Addiction medicine consult | 81 (78.6%) | 12 (10.3%) | <.0001 |
| Admission Diagnosesa | |||
| Endocarditis | 66 (64.1%) | 59 (50.4%) | .0414 |
| Osteomyelitis | 36 (35.0%) | 33 (28.2%) | .2819 |
| Necrotizing fasciitis or myositis | 6 (5.8%) | 9 (7.7%) | .5835 |
| Isolated bacteremia/fungemia | 16 (15.5%) | 18 (15.4%) | .9756 |
| Septic arthritis | 23 (22.3%) | 36 (30.8%) | .1586 |
| Staphylococcus aureus | 69 (67.0%) | 78 (66.7%) | .9999 |
Abbreviations: HIV, human immunodeficiency virus; HTN, hypertension; MOUD, medications for opioid use disorder; SUD, substance use disorder.
aExcept for patients with isolated bacteremia or fungemia, patients may have more than 1 diagnosis, eg, endocarditis complicated by septic arthritis.
Completion of Parenteral Antimicrobial Therapy
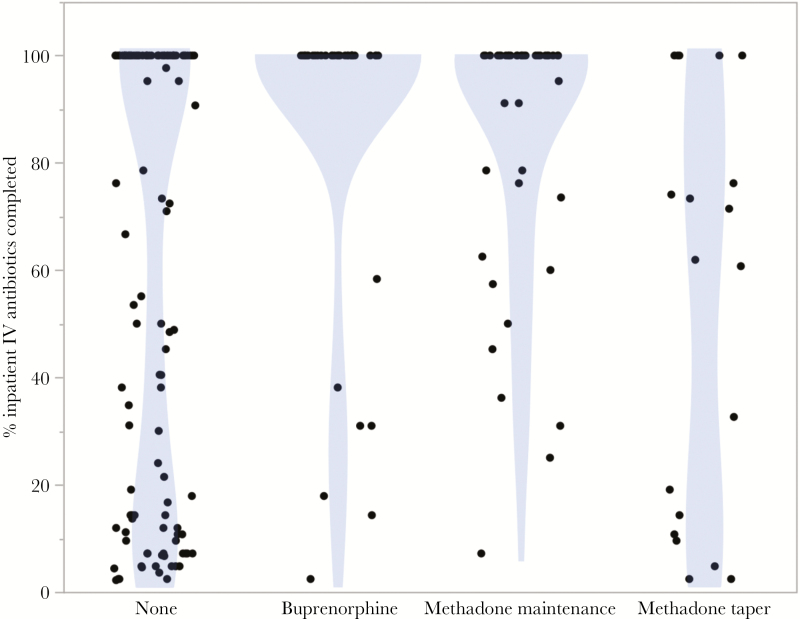
On univariate analysis, prescription of any form of MOUD (buprenorphine, methadone maintenance, or methadone taper) was associated with significantly greater retention in inpatient hospitalized care (70.87% vs 52.99%; OR = 2.159; 95% CI, 1.240–3.734; P = .0083) and a higher rate of completion of parenteral antimicrobial therapy (64.08% vs 46.15%; OR = 2.081; 95% CI, 1.228–3.610). As seen in Figure 1, among patients not prescribed any form of MOUD, 43.6% left before completing at least one half of the recommended parenteral antimicrobial course, with a bimodal distribution (mean percent of planned parenteral antimicrobial course completed 61.43%, median of 84.63%). In contrast, among those prescribed any MOUD, only 19.46% of patients left before completing one half of their course (mean percent of planned parenteral antimicrobial course completed 80.24%, median 100%). The mean length of stay for all patients regardless of MOUD strategy who completed 100% of recommended parenteral antimicrobial therapy was 6.2 weeks, with a median of 6 weeks. Discharges against medical advice and elopements were also significantly lower in the MOUD group (30 [29.13%] MOUD versus 55 [47.01%] no MOUD; OR = 2.159; 95% CI, 1.240–3.734). When further stratified by type of MOUD received, buprenorphine therapy showed the strongest association with antimicrobial completion (Table 2).
Figure 1.
Association between medications for opioid use disorder strategy during inpatient admission and percentage completion of recommended parenteral antibiotic therapy. •, Each point represents a unique patient admission.
Table 2.
Univariate Analysis of Variables Associated With 90-Day Readmission and Completion of Completion of Parenteral Antimicrobial Therapy
| 90-Day Readmission | Completion of IV Antibiotics | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Addiction medicine consult | 0.370 | .211–.648 | 4.964 | 2.734–9.011 |
| MOUD | ||||
| None | Reference | Reference | ||
| Buprenorphine maintenance | 0.426 | .202–.900 | 5.833 | 2.397–14.193 |
| Methadone maintenance | 0.528 | .255–1.092 | 1.896 | .922–3.899 |
| Methadone detoxification | 1.629 | .599–4.428 | 0.471 | .141–1.232 |
| Continuation of MOUD on DC | 0.494 | .268–.909 | 2.710 | 3.376–15.013 |
| Endocarditis | ||||
| None | Reference | Reference | ||
| Native valve | 0.864 | .495–1.507 | 2.070 | 1.818–3.626 |
| Prosthetic valve | 2.902 | .948–8.884 | 4.280 | 1.300–14.097 |
| Osteomyelitis | 0.969 | .547–1.718 | 0.367 | .204–.660 |
| Bacteremia | 0.857 | .503–1.460 | 1.136 | .922–1.263 |
| Staphylococcus aureus | 1.103 | .627–1.940 | 0.648 | .366–1.148 |
| Age ≥60 | 1.385 | .514–3.733 | 2.916 | .920–9.246 |
| Female | 1.246 | .732–2.121 | 0.692 | .406–1.180 |
| African American | 0.915 | .532–1.576 | 1.440 | .833–2.490 |
| Prior IDU infections | 2.000 | 1.118–3.578 | 1.162 | .662–2.040 |
| Hepatitis C seropositive | 1.035 | .603–1.778 | 0.711 | .412–1.226 |
Abbreviations: CI, confidence interval; DC, discharge; IDU, injection drug use; IV, intravenous; MOUD, medications for opioid use disorder; OR, odds ratio.
On multivariate logistic regression analysis, after controlling for infection type, underlying hepatitis C coinfection, and age over 60, integration of MOUD maintenance therapy with either buprenorphine (OR = 15.528; 95% CI, 5.059–47.659) or methadone maintenance (OR = 3.421; 95% CI, 1.404–8.336) was associated with increased likelihood of antimicrobial completion (Table 3). In contrast, the parenteral antimicrobial course completion rate of patients who received methadone tapers for detoxification was not significantly different from patients who did not receive MOUDs and trended toward worse outcomes than no MOUD at all (OR = 0.565; 95% CI, .169–1.884; P = .368).
Table 3.
Logistic Regression of Variables Associated With Completion of Parenteral Antimicrobial Therapy
| Variable | aOR | 95% CI | P Value |
|---|---|---|---|
| MOUD | |||
| None | Ref. | Ref. | |
| Buprenorphine maintenance | 15.528 | 5.059–47.659 | <.0001 |
| Methadone maintenance | 3.421 | 1.404–8.336 | .007 |
| Methadone detoxification | 0.565 | .169–1.884 | .353 |
| Endocarditis | |||
| None | Ref. | Ref. | |
| Native valve | 1.809 | .857–3.816 | .120 |
| Prosthetic valve | 6.739 | 1.510–30.078 | .012 |
| Osteomyelitis | 0.419 | .192–.914 | .029 |
| Bacteremia | 2.033 | 1.002–4.127 | .049 |
| Age over 60 | 5.314 | 1.399–20.186 | .014 |
| Female | 0.398 | .194–.814 | .012 |
| Hepatitis C seropositive | 0.346 | .166–.719 | .004 |
| Constant | 0.984 | .451–2.145 | .968 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; MOUD, medications for opioid use disorder; Ref., reference.
90-Day Hospital Readmission
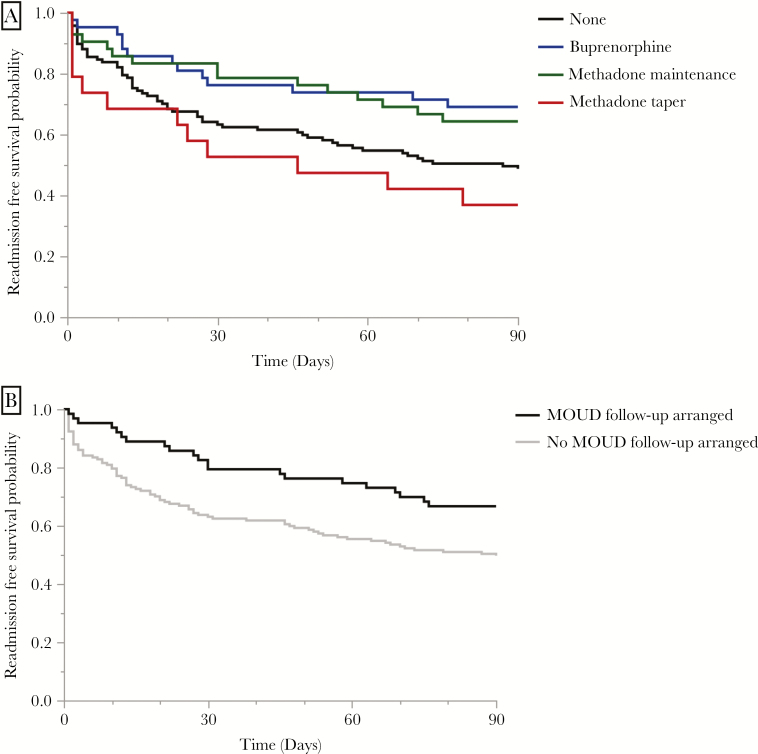
Univariate analysis identified a history of prior substance use-associated infections as a significant predictor of 90-day readmission; having multidisciplinary management with an addiction medicine specialist and being discharged on MOUD treatment were protective against readmission (Table 2). Receipt of any type of MOUD was associated with fewer readmissions (40 [38.83%] MOUD versus 60 [51.28%] no MOUD; OR = 0.203; 95% CI, .353–1.028) but did not reach statistical significance. The log-rank test for equality indicated a significant difference between the MOUD strategies observed in this cohort, and both methadone maintenance and buprenorphine maintenance were associated with increased readmission-free survival and decreased readmissions in an unadjusted Kaplan-Meier survival curve (P = .020; log-rank test) (Figure 2A). Arrangements for continuation of MOUDs after discharge was similarly associated with increased readmission-free survival and a significant decrease in readmissions (P = .013; log-rank test) (Figure 2B). Because arrangements for follow-up were made early in the hospital stay, some patients who left against medical advice did leave with appointments and resources for continuation of care after discharge.
Figure 2.
Kaplan-Meier survival curve. Readmission-free survival by (A) medication for opioid use disorder (MOUD) treatment type and (B) postdischarge MOUD access.
Based upon multivariable logistic regression analysis, adjusting for related factors, patients who had received MOUD maintenance therapy with either buprenorphine (adjusted OR [aOR] = 0.382; 95% CI, .172–.848), or methadone maintenance (aOR = 0.434; 95% CI, .200–.940), or had arrangements made for MOUD continuation after discharge (aOR = 0.350; 95% CI, .182–.675) were independently less likely to be readmitted within 90 days (Table 4).
Table 4.
Logistic Regression of Variables Associated With 90-Day Readmission
| Variable | aOR | 95% CI | P Value |
|---|---|---|---|
| MOUD | |||
| None | Ref. | Ref. | |
| Buprenorphine | 0.382 | .172–.848 | .018 |
| Methadone maintenance | 0.434 | .200–.940 | .034 |
| Methadone detoxification | 1.782 | .623–5.070 | .281 |
| Continuation of MOUD on DC | 0.350 | .182–.675 | .002 |
| Prior IDU infections | 2.698 | 1.436–5.070 | .002 |
| Constant | 0.652 | .356–1.010 | .111 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DC, discharge; IDU, injection drug use; MOUD, medications for opioid use disorder; Ref., reference.
DISCUSSION
There is wide consensus within the medical community that OUD is a relapsing, chronic illness with medical and social components, and that the best practice for management of OUD includes opioid agonist therapy with either buprenorphine or methadone [10, 12–14]. However, despite the increasing array of evidence-based interventions, pharmacotherapy with MOUDs are not widely used in hospitals [7, 11, 15]. Medically supervised opioid agonist treatment is demonstrated to prevent infectious disease complications, with both buprenorphine and methadone treatment reducing human immunodeficiency virus (HIV) and HCV transmission, increasing retention in HIV care and improving HIV viral control [16–18]. In our study, patients with a diagnosis of OUD admitted to the hospital with an invasive bacterial or fungal infection and who received either methadone or buprenorphine inpatient maintenance therapy had better outcomes. To our knowledge, this is the first study to compare different MOUD treatment strategies and outcomes among PWID with invasive bacterial and fungal infections.
This study adds to the existing body of evidence that patients with OUD should be offered pharmacotherapy and provides insights on its critical role in the treatment of OUD-related invasive infections. Hospitalizations for patients with OUD are challenging. Many patients voice fears about withdrawal, poorly controlled pain, and cravings as drivers of delayed presentations and continued substance use during hospitalizations [19, 20]. For patients with OUD admitted to the hospital with life-threatening infections related to IDU, this is often a teachable moment wherein prompt concurrent initiation of MOUDs, along with treatment of their infection, serves as a unique opportunity to engage a patient in care [19]. In addition, as the incorporation of opioid agonist therapy treats cravings and withdrawal, adverse behaviors during their inpatient evaluation are decreased. We have also anecdotally found improved nursing satisfaction with their treatment.
Our study found no statistically significant difference in outcomes between patients who received maintenance therapy with methadone versus buprenorphine, although there was a much stronger association with buprenorphine. Choice of MOUD should be tailored to a person’s use history and the availability of outpatient care postdischarge. For many, the effects of partial and full opioid agonists are indistinguishable. However, due to the ceiling effect of partial agonists, people who are dependent on higher doses of opioids may be better suited to treatment with a full agonist, such as methadone [14]. This decision of which MOUD to use should also take into account potential barriers specific to methadone treatment, including daily supervised dosing, access to specially licensed clinics (eg, waitlists or geography), and potential drug-drug interactions with many antibiotics and antiretrovirals [21]. Follow-up care for patients on buprenorphine may be obtained in the outpatient clinic setting through providers who complete a full-day educational session, meet specific criteria, and apply for a special X-waiver.
In contrast to maintenance treatment with methadone or buprenorphine, in our cohort, medically supervised rapid withdrawal tapers did not decrease readmissions. The use of medically supervised rapid withdrawal tapers has been associated with increased risk for fatal overdoses, due to loss of tolerance and low rates of short-term abstinence [22, 23]. Recent guidelines now recommend against rapid tapers in the absence of ongoing treatment for OUD [24]. Consistent with this finding, our data demonstrate that MOUD maintenance therapy, including both initiation of buprenorphine or methadone with bridging to outpatient treatment, was associated with decreased 90-day readmissions. Addiction medicine programs offer a variety of services such as nursing, counseling, social support, and education, which can be leveraged to provide a supportive environment for the integration of medical care aimed at preventing overdoses and identifying recurrent infections early [10]. More comprehensive solutions, similar to the Ryan White program for people living with HIV, aimed at providing access to these critical wraparound services and additional resources, including intensive case management and close outpatient provider follow-up along with harm reduction strategies, may be key to decreasing morbidity, mortality, and readmissions in this vulnerable population.
This study had several limitations. This was a retrospective study conducted at a single tertiary, academic medical center and may not be generalizable to other settings. However, we would argue that prospective trials of this nature are not ethical, because MOUDs are standard of care for treatment of patients with OUD. An additional limitation is the use of ICD-10 codes to identify patients; there are no specific codes for IDU or OUD-associated infections, thus codes for OUD and/or IDU were assessed separately, and it is possible that some patients were missed due to failures in documentation and coding. Selection bias could also be present; addiction medicine consultation and initiation of MOUDs were most likely nonrandom and based on patient’s clinical presentation and prognosis. In addition, it is also possible that patients who did not receive MOUDs declined treatment. However, in patients who did not receive MOUDs, there were multiple instances of chart documentation of patient requests for MOUDs (data not shown). It is unclear in these circumstances why MOUDs were not initiated; it is possible that provider bias, lack of provider education about availability, and comfort with use of these medications were barriers to initiation. Therefore, lack of appropriate MOUD initiation may represent a missed opportunity to intervene by the admitting providers.
CONCLUSIONS
Our study demonstrates substantial benefits from the use of MOUDs in hospitalized patients with invasive bacterial and fungal complications of OUD. Where available, the involvement of addiction medicine specialists should be sought for assistance in management and titration of MOUDs [25]. However, lack of addiction medicine specialist availability should not be a barrier to access [26]. There are multiple options, including the State Targeted Response Technical Assistance (STR-TA) program and through the Provider Clinical Support System (pcssnow.org), for providers without a background in addiction medicine to get assistance in treating this population. Our data suggests that when outpatient follow-up is available, there is substantial benefit to the addition of MOUDs on both completion of antimicrobial therapy, readmission rates, and survival. Access to MOUDs and other harm-reduction strategies should be prioritized in the treatment of OUD-associated infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplemental Table 1. ICD-9 and ICD 10 codes used to screen for infectious complications of opioid or injection drug use.
Supplemental Table 2. ICD-9 and ICD 10 codes used to screen for infectious complications of substance use.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financail support. This work was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Numbers KL2TR002346, CRTCUL1RR024992, and T32AI007172.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Schwetz TA, Calder T, Rosenthal E, Kattakuzhy S, Fauci AS. Opioids and infectious diseases: a converging public health crisis. J Infect Dis 2019; 220:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keeshin SW, Feinberg J. Endocarditis as a marker for new epidemics of injection drug use. Am J Med Sci 2016; 352:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewer D, Harris M, Hope V. Opiate injection-associated skin, soft tissue, and vascular infections, England, UK, 1997–2016. Emerg Infect Dis 2017; 23:1400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rapoport A, Fischer L, Santibanez S, Beekmann S, Polgreen P, Rowley C. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5:ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. [DOI] [PubMed] [Google Scholar]
- 7. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet 2009; 374:1284–93. [DOI] [PubMed] [Google Scholar]
- 8. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated Staphylococcus aureus bacteremia. Open Forum Infect Dis 2019; 6:ofz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open 2018; 1:e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunlap B, Cifu AS. Clinical management of opioid use disorder. JAMA 2016; 316:338–9. [DOI] [PubMed] [Google Scholar]
- 11. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis 2018; 18:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fullerton CA, Kim M, Thomas CP, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv 2014; 65:146–57. [DOI] [PubMed] [Google Scholar]
- 13. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; CD002209. [DOI] [PubMed] [Google Scholar]
- 14. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; CD002207. [DOI] [PubMed] [Google Scholar]
- 15. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med 2019; 13:69–74. [DOI] [PubMed] [Google Scholar]
- 16. Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011; CD004145. [DOI] [PubMed] [Google Scholar]
- 17. Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009; 105:9–15. [DOI] [PubMed] [Google Scholar]
- 18. Peles E, Schreiber S, Rados V, Adelson M. Low risk for hepatitis C seroconversion in methadone maintenance treatment. J Addict Med 2011; 5:214–20. [DOI] [PubMed] [Google Scholar]
- 19. Bearnot B, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: results from a qualitative study. J Subst Abuse Treat 2019; 102:16–22. [DOI] [PubMed] [Google Scholar]
- 20. Harris RE, Richardson J, Frasso R, Anderson ED. Experiences with skin and soft tissue infections among people who inject drugs in Philadelphia: a qualitative study. Drug Alcohol Depend 2018; 187:8–12. [DOI] [PubMed] [Google Scholar]
- 21. Kapur BM, Hutson JR, Chibber T, Luk A, Selby P. Methadone: a review of drug-drug and pathophysiological interactions. Crit Rev Clin Lab Sci 2011; 48:171–95. [DOI] [PubMed] [Google Scholar]
- 22. Wines JD Jr, Saitz R, Horton NJ, Lloyd-Travaglini C, Samet JH. Overdose after detoxification: a prospective study. Drug Alcohol Depend 2007; 89:161–9. [DOI] [PubMed] [Google Scholar]
- 23. Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend 2008; 94:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carroll CP, Triplett PT, Mondimore FM. The intensive treatment unit: a brief inpatient detoxification facility demonstrating good postdetoxification treatment entry. J Subst Abuse Treat 2009; 37:111–9. [DOI] [PubMed] [Google Scholar]
- 25. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med 2019; 13:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haffajee RL, Bohnert ASB, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med 2018; 54:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.