Abstract
Background
Prevalence of methamphetamine (meth) injection and associated human immunodeficiency virus (HIV) risks among men who have sex with men (MSM) are unclear.
Methods
A total of 532 MSM completed 1880 mSTUDY study visits between August 2014 and June 2018 in Los Angeles, California. Assessments every 6 months included computer-assisted self-interviews and testing for sexually transmitted infections. Analyses by person and across visits adjusted for repeated measures.
Results
Of 532 participants, 51% (n = 276) reported meth use (past 6 months). Across 1880 visits, mutually exclusive substance use categories were as follows: 5% meth injection (5%), meth use without injection (33%), other substance use excluding meth (36%), and no substance use (26%). Comparisons across these categories respectively found that meth injectors reported higher prevalence of new sex partners (89%, 70%, 68%, and 51%, respectively), more were HIV positive (83%, 65%, 34%, and 50%), fewer were virally suppressed (53%, 48%, 61%, and 67%), and more had sexually transmitted infections (31%, 22%, 15%, and 15% (all P <.01).
Conclusions
Among the young MSM reporting meth injection in this Los Angeles cohort, elevated risks of acquiring or transmitting HIV suggest that they contribute significantly to sustaining the local HIV epidemic. Preventing transition to injection use has potential for HIV prevention.
Keywords: methamphetamine injection, MSM and substance use, substance use and HIV transmission
The association between methamphetamine (meth) use among men who have sex with men (MSM) in the United States and human immunodeficiency virus (HIV) acquisition is well established [1–8]. The continuation of use after HIV infection among MSM has also been clearly associated with poor continuation in care, low adherence to HIV medication and subsequently poor clinical outcomes, including high viral load (or lack of viral suppression) [9], high incidence of sexually transmitted infections (STIs), and other comorbid conditions leading to higher mortality rates [10–18]. Meth has thus been a persistent cause driving the ongoing MSM HIV epidemic.
Concurrently, the epidemic of opioid use has caused considerable morbidity, overdose, and mortality rates across the United States in the past decade, in great part due to a transition from heroin use to prescription opioids and nonprescription street opioids, such as fentanyl [19–21]. This has been associated with recent outbreaks of HIV infection among people who inject drug (PWID) in places not seen in the US epidemic in many years such as Seattle where HIV incidence among PWID had dropped 48% between 2008 and 2014 [22–24].
Although the recent outbreaks suggest a reversal of this trend, a trend among MSM toward transitions from meth use into injection meth use and then injection of a combination of meth and opioids has an alarming potential for creating high-transmission pockets that may present challenges in the progress toward ending the HIV epidemic in the US [25]. A large increase was noted in the prevalence of reporting meth as the most frequently injected drug among PWID in the National HIV Behavioral Surveillance system in Denver, Colorado, from 2.1% in 2005 to 29.6% in 2015 [26]. Moreover, when MSM from 8 large US cities who report primarily injecting meth were compared with MSM who report other drugs as primary for injection, the HIV prevalence, transmission behaviors, and self-reported STIs were all found to be higher—most notably, HIV prevalence was 50% higher [27]. The current study examined whether HIV transmission behaviors and biomarkers differed between injection and noninjection users of meth among young, diverse MSM in Los Angeles in a biobehavioral cohort.
METHODS
Participants in this study were those enrolled in the National Institutes of Health/National Institute on Drug Abuse–funded mSTUDY—a longitudinal study designed to assess the epidemiological and immunological impact of substance use and HIV on racially/ethnically diverse young MSM. The mSTUDY has been described elsewhere [28–30], but briefly, study enrollment started in August 2014 and is ongoing. Participants were recruited from a community-based organization providing a broad spectrum of services for the lesbian, gay, bisexual, and transgender community and a community-based university research clinic, both located in the Hollywood area of Los Angeles, California.
All participants in the mSTUDY between August 2014 and June 2018 were eligible and included in this analysis. Inclusion criteria for this analysis were the same as those for the mSTUDY; all participants were (1) between 18 and 45 years of age, (2) male at birth, (3) if HIV-negative, reporting condomless anal intercourse with a male partner in the past 6 months, (4) capable of providing informed consent, and (5) willing and able to return to the study every 6 months to complete study-related activities, including questionnaires, clinical assessments, and biological specimen collection. By design, participants were recruited to include half HIV-positive and half HIV-negative men. As well. half of the participants were substance users (self-report confirmed by urine drug screen) and half were not.
Study Procedures and Data Collection
After providing written informed consent, study participants completed a self-administered, questionnaire (computer-assisted survey instrument; 45–70 minutes to complete). Substance use was assessed as part of the survey, and participants were presented with a list of drugs (including cocaine powder, crack cocaine, ecstasy, heroin, marijuana, meth, poppers, and prescription medications) and asked to specify whether they had used each of the drugs in the past 6 months and method of use (ie, injection, snorting, smoking, or eating). The type of substance use, meth use in particular, was categorized by the method of use. Questions on sexual risk behaviors relevant to this analysis focused on recent behaviors (past 6 months) and included information on reports of new sex partners, concurrent partnerships (ie, sexual partnerships that overlap in time), and exchange sex, defined as having received money, drugs, shelter, or other goods for sex.
At each study visit participants completed a clinical examination (data not shown) and provided urine samples as well as rectal and pharyngeal swab samples tested for chlamydia and gonorrhea using nucleic acid amplification testing technology (Aptima Combo 2; GenProbe). In addition, blood samples were collected for syphilis and HIV testing (for HIV-negative participants), and HIV-1 RNA levels (for HIV-positive participants). Syphilis testing was conducted using the rapid plasma reagin test, with confirmatory testing done with the Treponema pallidum particle agglutination test. Syphilis disposition (ie, primary, secondary, or early latent syphilis) was obtained for each participant and based on standard-of-care health department investigation of syphilis cases, as specified by the Centers for Disease Control and Prevention treatment guidelines for sexually transmitted diseases [31]. All participants were scheduled to return every 6 months, and the study questionnaire and laboratory tests were repeated at the follow-up visits. The study was approved by the Institutional Review Board at the University of California, Los Angeles.
Analytic Approach
Descriptive statistics, including mean, range, and frequency distributions were assessed for 4 mutually exclusive categories of drug use: meth injection, meth use with no injection, other drug use (not including meth), and no drug use. Differences between visits were evaluated by drug use category, using χ 2 methods for categorical variables, adjusting for the effect of the subject (ie, repeated measures), and F statistics for type 3 tests of fixed effects (also adjusting for subject effects). Because participants could have repeated visits over the study period, we used hierarchical regression models with generalized estimating equations to account for within-subject correlations [32, 33].
We fit models with random intercepts and time effects to accommodate the repeated measures gathered from each participant and to allow participant-specific changes in responses over time. This allowed us to investigate the association between meth use pattern as reported at each visit (ie, outcome) and other fixed-effect variables, such as race/ethnicity, as well as time-varying repeated measures, such as viral load, STI incidence, and sexual behavior (ie, covariates of interest) at each visit. Variables tested for inclusion in the multivariable models were based on univariate analyses or specified a priori as risk factors based on the existing literature. All analyses were conducted using SAS software (version 9.4; SAS).
RESULTS
The 532 MSM at baseline had a mean age of 31 years (standard deviation, 6.8 years), were mostly of minority race/ethnicity (42.5% African American, 36.8% Latino), and commonly reported current unemployment, and unstable housing (see Table 1). There were differences by HIV status, in age (slightly older for HIV-positive MSM), unemployment (higher rate among the HIV positive), educational status (lower among the HIV positive), and history of incarceration (higher among the HIV positive). Drug use in general was reported frequently: 51% (n = 276) reported meth use, 9% (n = 48) reported heroin use, and 30% (n = 160) reported prescription drug misuse (eg, OxyContin, Vicodin) at least once. Across all 1880 study visits, meth use was reported at 38% of visits in the past 6 months, which included 5% of visits (n = 98) when meth injection was reported and 33% (n = 619) when noninjection meth use was reported; other drug use was reported at 38% of visits (n = 685) and no drug use at 25% of visits (n = 478). On the individual level, 90% of cohort members (480 of 532) reported any drug use at any study, 51% (276 of 532) reported any meth use at any study visit, and 17% (46 of 276) reported any meth injection at any study visit.
Table 1.
Baseline Characteristics Among mSTUDY Participants, by Human Immunodeficiency Virus Status (August 2014 to June 2018)
| Participants, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | Total (N = 532)a | HIV Positive (n = 265)a | HIV Negative (n = 267)a | P Value |
| Age, mean (SD), y | 31.3 (6.8) | 33.6 (6.4) | 28.9 (6.4) | <.01 |
| Race/ethnicity | ||||
| African American | 226 (42.5) | 108 (40.8) | 118 (44.2) | .13 |
| Hispanic/Latino | 196 (36.8) | 97 (36.6) | 99 (37.1) | |
| Other | 37 (7.0) | 15 (5.7) | 22 (8.2) | |
| White | 73 (13.7) | 45 (17.0) | 28 (10.5) | |
| Educational level | ||||
| Below high school graduate | 65 (12.3) | 40 (15.4) | 25 (9.4) | .07 |
| High school graduate | 193 (36.6) | 97 (37.3) | 96 (36.0) | |
| Beyond high school | 269 (51.1) | 123 (47.3) | 146 (54.6) | |
| Unemployed | 235 (45.6) | 143 (56.1) | 92 (35.4) | <.01 |
| Unstable housing in past 6 mob | 189 (35.5) | 94 (35.5) | 95 (35.6) | .98 |
| Ever incarcerated | 208 (39.1) | 117 (44.2) | 91 (34.1) | .02 |
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified. Sums may not equal totals owing to missing information.
bUnstable housing was defined as not having a regular place to stay in the past 6 months.
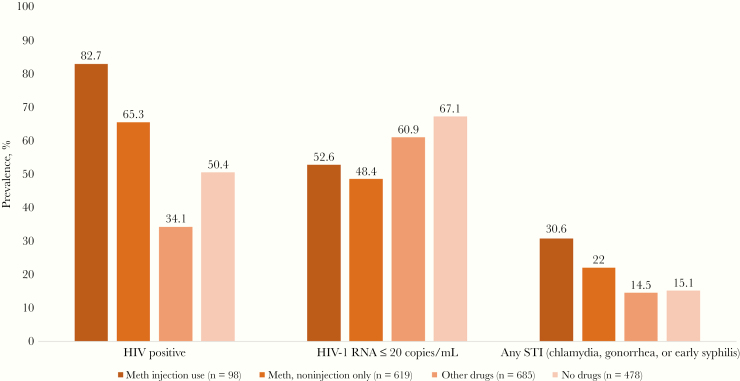
Significant differences (P ≤ .01) by type of meth consumption and other drug use were noted for HIV status, viral load, and laboratory-confirmed STI diagnosis across visits. HIV prevalence was highest among those who reported meth injection (82%) followed by noninjection meth use (65%), and other substance use not including meth (50%) and lowest among those who reported no substance use (34%) (Figure 1). Likewise, the prevalence of any STI (gonorrhea, chlamydia, or syphilis) was highest for meth injectors (30%), followed by other meth users (22%); other drug users and non–drug users had a similar prevalence, half that of the meth injectors (14% and 15%, respectively). Among HIV-positive MSM, viral suppression or RNA levels ≤20 copies/mL were seen at significantly fewer visits for meth injectors and other meth users (52.6% and 48.4%, respectively) than for other drug users and nonusers (60.9% and 67.1%, respectively).
Figure 1.
Prevalence of human immunodeficiency virus (HIV), HIV viral load (among the HIV positive), and sexually transmitted infections (STIs) across study visits among mSTUDY participants, by substance use category (August 2014 to June 2018). *P < .01. Abbreviation: Meth, Methamphetamine.
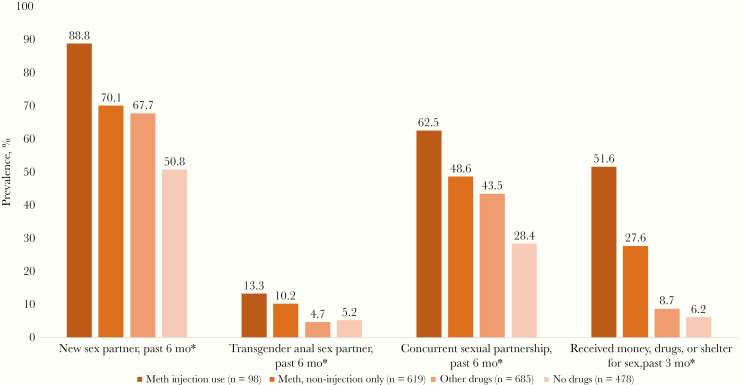
Transmission behaviors were also reported in the past 6 months at significantly more visits where meth injection was reported (P ≤ .05), all following a dose-response pattern with the highest prevalence noted among those reporting meth injection, followed by noninjection meth use, then other drug use and non–drug use (Figure 2). For reports of new sexual partners at a visit, the highest prevalence was among those reporting meth injection (88.8%), followed by those reporting other meth use (70.1%) or other drug use (67.7%), with the lowest prevalence among non–drug users (50.%). The practice of having concurrent sexual partners also followed this pattern for meth injectors, other meth users, other drug users, and nonusers (62.5%, 48.6%, 43.5%, and 28.4%, respectively). The pattern was even more pronounced for transactional sex reports (51.6%, 27.6%, 8.7%, and 6.2%, respectively). Finally, there was also a modest trend for having transgender partners, with meth injectors reporting these partners at more visits (13.3%, 10.2%, 4.7%, and 5.2%, respectively).
Figure 2.
Prevalence of sexual behavior across study visits among mSTUDY participants, by substance use category (August 2014 to June 2018). *P < .01. Abbreviation: Meth, Methamphetamine.
DISCUSSION
In this biobehavioral cohort of highly diverse young MSM, meth injection occurs more among those that are HIV positive and, most concerning, is practiced concurrently with unsuppressed viral load and active STI infection. Our study demonstrates that HIV clinical status is especially poor when meth is injected, but also when it is used in other ways. This suggests a trajectory of poorer relevant HIV outcomes for MSM who inject meth, with these men facing disorganization from more intense use patterns that interfere with their management of HIV disease.
MSM who inject meth also face a synergy of HIV transmission potential from having sex with more new, transactional, and marginalized partners while viremic and having STIs [2, 4, 15–17]. These findings ensure transmissible viremia involving sexual encounters where little communication occurs and unequal power dynamics happen during sex with a new or paid sex partner. This confirms findings noted among PWID by the National HIV Behavioral Surveillance group, of more condomless sex partners among MSM who inject meth compared with those who inject other drugs [27].
HIV transmission risks from MSM who inject meth are of concern because they may also be injecting as well as having sex with these partners. This may mean episodes with sex where individuals are exposed to HIV through multiple routes if needles are shared and condoms are not used—highly likely during these drug-charged sexual encounters.
Our findings show that men using meth in ways other than injection also represent potential transmission of HIV, as many do not sustain viral suppression and have concurrent bacterial STIs. Their risks are slightly lower than those in men who inject, demonstrating an opportunity for substance use treatment to reduce transmission potential if meth users could be prevented from escalating their use into injection. PWID who combine meth with heroin injection have been noted to be at higher risk of overdose and multiple overdoses than those who inject either drug individually [26]. Rates of overdose deaths involving meth have tripled nationally from 2011 to 2016 [34], but it is unclear whether this increase is related to the method of use. Individuals have been noted to be at high risk of overdose from injection and noninjection meth use, yet it is unclear whether injection elevates this risk [35]. Nevertheless, opportunities to reduce rates of emergency room visits, hospitalizations, and potentially long-term disability could also be achieved if harm reduction could prevent the trajectory of meth use into injection.
There are emerging patterns of unexpected deaths among MSM who use stimulants in Los Angeles, which may signal the integration of opioids, particularly fentanyl, with meth use. Once injecting meth, men may also move to injection of opioids in conjunction with meth use, a pattern we have seen in small numbers and will continue to monitor in our cohort and in the greater Los Angeles metropolitan area. It is the subject of further investigation whether combinations of fentanyl with meth are intentional or due to contamination of the drug by the supplier. Although the greatest reduction in harm would be achieved if meth use could be eliminated, prevention of injection methods among MSM would have a modest effect on the infectious disease risks and on the risk of deaths from overdose.
The findings from this study may not be generalizable to all meth-using young MSM in Los Angeles because our sample is not random. We capture the young MSM willing to participate in a cohort study with long visits, resulting in a bias, as found in most research studies, toward those that are marginally employed and have discretionary time for study participation. Moreover, our sample of those who inject meth is not large enough to allow for multivariable analysis, limiting analyses that can be currently conducted in this group. As the mSTUDY continues, we expect to have more visits from those who inject meth, and we therefore have great power to conduct more in-depth analyses. Nevertheless, ours is one of the only studies to have both behavioral and biological data from young MSM who inject meth, expanding the understanding of different forms of meth use and their clinical implications.
Our findings clearly demonstrate that if HIV is to be eliminated in the United States, greater substance use treatment for MSM will be needed to prevent and reduce the use of meth, and specifically its escalation into injection. As noted elsewhere, the challenge is that there is no medication approved for the treatment of meth, limiting the potential for medication-assisted therapy offered in substance use treatment programs to reduce use among meth injectors [27]. This suggests a need for different programming for MSM who inject meth, a further barrier to reducing risks in this group. Such patterns of drug use among MSM should be monitored in other parts of the United States, where rates of opioid injection and thus overdose and mortality rates are higher. Transitions to injection may accelerate, further obstructing opportunities to end the HIV epidemic, and increasing harm to those living with HIV and at high risk for HIV acquisition.
Notes
Financial support. The mSTUDY is funded by the National Institute on Drug Abuse (grant U01DA036267) and supported by the Center for HIV Identification, Prevention, and Treatment Services (grant P30MH58107)
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Buchacz K, McFarland W, Kellogg TA, et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS 2005; 19:1423–4. [DOI] [PubMed] [Google Scholar]
- 2. Colfax G, Coates TJ, Husnik MJ, et al. ; EXPLORE Study Team Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of San Francisco men who have sex with men. J Urban Health 2005; 82:i62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halkitis PN, Mukherjee PP, Palamar JJ. Longitudinal modeling of methamphetamine use and sexual risk behaviors in gay and bisexual men. AIDS Behav 2009; 13:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoenigl M, Chaillon A, Moore DJ, Morris SR, Smith DM, Little SJ. Clear links between starting methamphetamine and increasing sexual risk behavior: a cohort study among men who have sex with men. J Acquir Immune Defic Syndr 2016; 71:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mimiaga MJ, Fair AD, Mayer KH, et al. Experiences and sexual behaviors of HIV-infected MSM who acquired HIV in the context of crystal methamphetamine use. AIDS Educ Prev 2008; 20:30–41. [DOI] [PubMed] [Google Scholar]
- 6. Mimiaga MJ, Reisner SL, Fontaine YM, et al. Walking the line: stimulant use during sex and HIV risk behavior among black urban MSM. Drug Alcohol Depend 2010; 110:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2007; 45:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoptaw S, Reback CJ. Methamphetamine use and infectious disease-related behaviors in men who have sex with men: implications for interventions. Addiction 2007; 102(suppl 1):130–5. [DOI] [PubMed] [Google Scholar]
- 9. Fulcher J, Javanbakht M, Shover C, et al. Attributable risk of methamphetamine use on viral suppression among MSM on ART. Presented at: Conference on Retroviruses and Opportunitistic Infections (CROI); 4–7 March 2019; Seattle, WA. [Google Scholar]
- 10. Jin H, Ogunbajo A, Mimiaga MJ, et al. Over the influence: The HIV care continuum among methamphetamine-using men who have sex with men. Drug Alcohol Depend 2018; 192:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrico AW, Shoptaw S, Cox C, et al. Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams JW, Bryant KJ, Edelman EJ, et al. Association of cannabis, stimulant, and alcohol use with mortality prognosis among HIV-infected men. AIDS Behav 2018; 22:1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horvath KJ, Carrico AW, Simoni J, Boyer EW, Amico KR, Petroll AE. Engagement in HIV medical care and technology use among stimulant-using and nonstimulant-using men who have sex with men. AIDS Res Treat 2013; 2013:121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulcher JA, Hussain SK, Cook R, et al. Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J Infect Dis 2018; 218:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King WD, Larkins S, Hucks-Ortiz C, et al. Factors associated with HIV viral load in a respondent driven sample in Los Angeles. AIDS Behav 2009; 13:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feldman MB, Kepler KL, Irvine MK, Thomas JA. Associations between drug use patterns and viral load suppression among HIV-positive individuals who use support services in New York City. Drug Alcohol Depend 2019; 197:15–21. [DOI] [PubMed] [Google Scholar]
- 17. Reback CJ, Fletcher JB. Elevated HIV and STI prevalence and incidence among methamphetamine-using men who have sex with men in Los Angeles county. AIDS Educ Prev 2018; 30:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldman MB, Thomas JA, Alexy ER, Irvine MK. Crystal methamphetamine use and HIV medical outcomes among HIV-infected men who have sex with men accessing support services in New York. Drug Alcohol Depend 2015; 147:266–71. [DOI] [PubMed] [Google Scholar]
- 19. Pergolizzi JV Jr, LeQuang JA, Taylor R Jr, Raffa RB; NEMA Research Group Going beyond prescription pain relievers to understand the opioid epidemic: the role of illicit fentanyl, new psychoactive substances, and street heroin. Postgrad Med 2018; 130:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Kertesz SG. Turning the tide or riptide? the changing opioid epidemic. Subst Abus 2017; 38:3–8. [DOI] [PubMed] [Google Scholar]
- 21. Scholl L SP KM, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. Morb Mortal Wkly Rep 2018; 67:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters PJ, Pontones P, Hoover KW, et al. ; Indiana HIV Outbreak Investigation Team HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med 2016; 375:229–39. [DOI] [PubMed] [Google Scholar]
- 23. Massachusetts Department of Public Health. CDC joins Department of Public Health in investigating HIV cluster among people who inject drugs. 5 April 2018. https://www.mass.gov/news/cdc-joins-department-of-public-health-in-investigating-hiv-cluster-among-people-who-inject. Accessed 5 December 2019 . [Google Scholar]
- 24. Golden MR, Lechtenberg R, Glick SN, et al. Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs—Seattle, Washington, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glick SN, Burt R, Kummer K, Tinsley J, Banta-Green CJ, Golden MR. Increasing methamphetamine injection among non-MSM who inject drugs in King County, Washington. Drug Alcohol Depend 2018; 182:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al-Tayyib A, Koester S, Langegger S, Raville L. Heroin and methamphetamine injection: an emerging drug use pattern. Subst Use Misuse 2017; 52:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nerlander LMC, Hoots BE, Bradley H, Broz D, Thorson A, Paz-Bailey G; NHBS Group HIV infection among MSM who inject methamphetamine in 8 US cities. Drug Alcohol Depend 2018; 190:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Javanbakht M, Ragsdale A, Shoptaw S, Gorbach PM. Transactional sex among men who have sex with men: differences by substance use and HIV status. J Urban Health 2018; 96:429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fulcher JA, Shoptaw S, Makgoeng SB, et al. Brief report: recent methamphetamine use is associated with increased rectal mucosal inflammatory cytokines, regardless of HIV-1 serostatus. J Acquir Immune Defic Syndr 2018; 78:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okafor CN, Gorbach PM, Ragsdale A, Quinn B, Shoptaw S. Correlates of preexposure prophylaxis (PrEP) use among men who have sex with men (MSM) in Los Angeles, California. J Urban Health 2017; 94:710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 32. Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models Biometrika 1986;73:13–22. [Google Scholar]
- 33. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44:1049–60. [PubMed] [Google Scholar]
- 34. Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep 2018. ;67:1–14. [PubMed] [Google Scholar]
- 35. Fairbairn N, Wood E, Stoltz JA, Li K, Montaner J, Kerr T. Crystal methamphetamine use associated with non-fatal overdose among a cohort of injection drug users in Vancouver. Public Health 2008; 122:70–8. [DOI] [PubMed] [Google Scholar]