Abstract
Background
Hepatitis C virus (HCV) remains endemic among people who use drugs (PWUD). Measures of HCV community viral load (CVL) and HCV care continuum outcomes may be valuable for ascertaining unmet treatment need and for HCV surveillance and control.
Methods
Data from patients in an opioid treatment program during 2013–2016 were used to (1) identify proportions of antibody and viral load (VL) tested, linked-to-care, and treated, in 2013–2014 and 2015–2016, and pre- and postimplementation of qualitative reflex VL testing; (2) calculate engaged-in-care HCV CVL and “documented” and “estimated” unmet treatment need; and (3) examine factors associated with linkage-to-HCV-care.
Results
Among 11 267 patients, proportions of HCV antibody tested (52.5% in 2013–2014 vs 73.3% in 2015–2016), linked-to-HCV-care (15.7% vs 51.8%), and treated (12.0% vs 44.7%) all increased significantly. Hispanic ethnicity was associated with less linkage-to-care, and Manhattan residence was associated with improved linkage-to-care. The overall engaged-in-care HCV CVL was 4 351 079 copies/mL (standard deviation = 7 149 888); local HCV CVLs varied by subgroup and geography. Documented and estimated unmet treatment need decreased but remained high.
Conclusions
After qualitative reflex VL testing was implemented, care continuum outcomes improved, but gaps remained. High rates of unmet treatment need suggest that control of the HCV epidemic among PWUD will require expansion of HCV treatment coverage.
Keywords: community viral load, hepatitis C virus, injection drug use, opioid use disorder, people who use drugs
Highly efficacious and well tolerated direct-acting antivirals (DAAs) can cure hepatitis C virus (HCV) infection [1]. However, an estimated 28%–75% of chronically infected people are not aware of their HCV infection [2]. It is estimated that in New York City (NYC), fewer than 30% of those with chronic HCV infection were treated in 2015–2017 [2]; the national estimate is 17% [3], reflecting significant gaps in the HCV care continuum [4].
People who use drugs (PWUD) can be successfully treated and cured of HCV infection yielding individual-level benefits and serving as cure as prevention (CasP) [1, 5]. With HCV incidence rates of 10–40/100 person-years and chronic infection prevalence ranging from 43% to 85%, HCV contributes significantly to preventable morbidity and mortality among PWUD [6–8]. Endemic HCV among PWUD is a function of the incomplete effectiveness and implementation of (1) medications for opioid use disorder (MOUD) and (2) syringe service programs (SSPs) and also of (3) low levels of screening, diagnosis, and treatment of HCV among PWUD [9–18].
Community viral load (CVL)—a group-, program-, or area-level aggregate measure of individual quantitative viral loads (VLs)—is a construct that has been applied to human immunodeficiency virus (HIV) epidemiology to assess the program and population-level impact of prevention and treatment interventions [19–24]. Studies of HIV CVL have examined a range of CVL measures of lesser or greater inclusiveness; these measures either rely entirely on directly measured data among those engaged-in-care [19, 22], (and which may serve as useful program or intervention evaluation measures or as routine surveillance measures), or may be broader measures that endeavor to reflect infection prevalence, transmission potential and VL burdens among those not yet diagnosed or linked-to-care, and which may have more generalizable population-level utility [23, 24].
The magnitude of HCV VL increases the risk of onward transmission [25–27], and quantitative HCV VLs are a standard component of treatment evaluation [20, 25]. However, HCV quantitative VL tests are consistently more expensive than qualitative HCV VL tests [28, 29]. The common screening strategy of performing HCV antibody testing only, and referring those with positive tests to other settings for VL testing, has contributed to gaps in the HCV care continuum. Some settings have addressed this by incorporating HCV VL reflex testing where qualitative VLs, which provide a dichotomous measure validly detecting active viremia and chronic infection, are reflexively performed on antibody-positive specimens [30–35].
Despite similarities in modes of transmission and prevention between HIV and HCV, CVL measures have scarcely been applied to HCV [36]. Analogous to HIV epidemiology, a family of HCV CVL measures could be constructed ranging from broad measures, which would require imputation for missing VL values for those undiagnosed, to measures that rely entirely on directly measured data, such as an engaged-in-care HCV CVL. An engaged-in-care CVL measure is a practical measure for a program or jurisdiction to calculate, because it can be calculated without additional data manipulation [19]. The Houston Health Department recently calculated an engaged-in-care HCV CVL, and it identified geographic CVL variations that could geographically focus future HCV prevention efforts [36].
Opioid treatment programs (OTPs) are important settings for engaging PWUD in the HCV care continuum and linking them to HCV care [3, 19, 37–40]. Identifying factors associated with linkage from OTPs to HCV care could inform strategies to improve linkage rates. The objectives of this paper are to (1) utilize data from patients enrolled in an OTP in NYC to examine HCV care continuum outcomes for 2 time periods (2013–2014 and 2015–2016, respectively) that reflect periods of different program testing strategies (antibody testing then off-site referral, or antibody then reflex qualitative VL testing and off-site referral) and (2) examine factors associated with linkage to HCV care [22]. We also use directly measured HCV VL data to construct engaged-in-care HCV CVLs and to examine variations among demographics subgroups of OTP patients and geographic variation based on OTP patient area of residence.
METHODS
The OTP, part of a large not-for-profit healthcare system in NYC, provides MOUD for approximately 6000 patients at any given time in separate clinics distributed throughout Manhattan and Brooklyn.
Dataset
Data from patients seen in the OTP from January 1, 2013 to December 31, 2016 were collected from the electronic medical record (EMR) and included HCV antibody, qualitative and quantitative HCV VL tests, HCV care, HCV treatment, and demographic information.
Data were examined as 2 sequential cross-sectional time periods for patients in the OTP during 2013–2014 (off-site referral of those with a positive HCV antibody test for quantitative HCV VL testing) and during 2015–2016 (reflex qualitative HCV VL testing for those with positive HCV antibody tests and subsequent off-site referral for quantitative HCV VL testing of those with reactive qualitative HCV VL tests).
Hepatitis C Virus Testing
Opioid treatment program patients were routinely offered opt-out HCV antibody testing at program entry and annual evaluations (patients were tested unless they declined testing or were already known to be antibody positive). Before 2015, HCV testing for OTP patients was a 2-step process: (1) on-site HCV antibody testing and (2) passive referral of those with positive HCV antibody tests from the OTP to other affiliated clinical sites for clinical evaluation and diagnostic tests including HCV quantitative VL tests. Beginning in January 2015, the HCV testing strategy was changed to a 3-step process: (1) on-site HCV antibody testing with (2) reflex qualitative HCV VL testing and (3) passive referral for off-site HCV VL quantitative testing and HCV care. Throughout the 4-year study period (2013–2016), all HCV antibody tests were done on-site and quantitative HCV VLs were performed at non-OTP HCV care sites.
Hepatitis C Virus Care Continuum
We compared the following HCV care continuum outcomes for 2013–2014 and 2015–2016: (1) HCV antibody tested and number positive, (2) HCV qualitative and/or quantitative HCV VL tested and number with documented viremia, (3) HCV linkage-to-care, defined as having attended a clinical evaluation for diagnostic tests including having had a quantitative HCV VL test, (4) HCV treatment, defined as evidence of HCV treatment in the EMR, and (5) achieving sustained virologic response (SVR), defined as having both an undetectable HCV VL at some time after the completion of treatment and a clinician note stating that the patient achieved SVR.
These data were used to calculate documented unmet treatment need (defined as the proportion with documented viremia not known to have been treated) and estimated unmet treatment need (defined as the proportion estimated to have active infection in the whole study population assuming a 25% rate of spontaneous clearance) [41, 42].
Hepatitis C Virus Community Viral Load Calculations
The engaged-in-care CVL includes only directly measured data from patients in the OTP who have documented antibody, qualitative, and quantitative HCV testing data, representing those successfully linked-to-HCV-care from the OTP. We calculated the HCV CVL by using the most recent HCV VL test in 2015–2016; that is, patients who were linked-to-care after a positive HCV qualitative VL test. Engaged-in-care HCV CVLs were constructed by adding each individual’s most recent HCV VL test and dividing by the number of persons contributing to each measure, and they are reported as means with standard deviations (SDs). We report the overall engaged-in-care HCV CVL, and then report engaged-in-care HCV CVL for demographic subgroups and geographic areas (using United Hospital Fund (UHF) areas) in 2015–2016.
Patients were coded by their zip code of residence in 2013 or at OTP entry if that occurred after 2013. The 179 zip codes in NYC were converted to UHF zip code areas [43] for analysis. Some UHF areas had <10 residents; therefore, we collapsed the 34 UHF zip code areas into 30 areas for analyses.
Linkage From Opioid Treatment Program to Hepatitis C Virus Care and Treatment Settings
We examined univariate and multivariate factors associated with receipt of quantitative VL testing (ie, successful linkage-to-care) among those in 2015–2016 who were found to have active HCV infection by reflex qualitative VL testing. Age was examined as a categorical variable. Race/ethnicity was examined as a categorical variable: non-Hispanic white, non-Hispanic black, Hispanic, and other race/ethnicity. Both NYC borough and UHF zip code area of patient residence were examined as geographic units.
Data Analysis
Categorical variables are presented as frequencies and proportions; continuous variables are presented as medians with interquartile ranges. Comparisons among overall OTP program care continuum outcomes between 2013–2014 and 2015–2016 were made using χ 2 tests.
First, we examined differences in demographic and geographic characteristics of patients who were linked-to-care in 2015–2016. We used binary logistic regression for univariate analyses and present unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) and P values. The multivariate logistic regression model was constructed using variables that were significant in univariate analysis along with those previously shown in the literature to be associated with the outcome (such as gender) [44] and report adjusted ORs (AORs) with 95% CIs.
Second, we examined differences in the engaged-in-care HCV CVL among demographic and geographic subgroups in 2015–2016. Hepatitis C virus CVLs were transformed to the log10 scale for analyses [19] and are presented untransformed in the tables and text. Engaged-in-care HCV CVLs among demographic subgroups were compared, and UHF zip code areas were examined using linear regression. Data were analyzed using R and SPSS (version 25.0) [45, 46]. Significance was determined at the P ≤ .05 level. This research received Institutional Board Approval at respective institutions.
RESULTS
Study Population
There were a total of 11 267 unique OTP patients during the 4-year study period. This includes 2743 patients seen in the OTP only in 2013–2014; 2631 patients seen only in 2015–2016; and 6296 patients seen at the OTP from 2013 to 2016. The total number of patients contributing to 2013–2014 and 2015–2016 data was 9039 and 8927, respectively. The distribution of the patient characteristics did not vary significantly from 2013–2014 to 2015–2016 (Table 1).
Table 1.
Individual Characteristics of OTP Patients for the Study Period, 2013–2016
| 2013–2014 Time Period 1, N = 9039 | 2015–2016 Time Period 2, N = 8927 | |||||
|---|---|---|---|---|---|---|
| Characteristics | N | n/N for Total Time Period | % | N | n/N for Total Time Period | % |
| Time Period | ||||||
| Patients in time period 1 only (2013–2014) | 2743 | 2743/9039 | 30.3% | n/a | n/a | n/a |
| Patients in time period 2 only (2015–2016) | n/a | n/a | n/a | 2631 | 2631/8927 | 29.5% |
| Patients in both time periods | 6296 | 6296/9039 | 69.7% | 6296 | 6296/8927 | 70.5% |
| Total patients contributing to time period | 9039 | 9039/9039 | 100.0% | 8927 | 8927/8927 | 100.0% |
| Sex | ||||||
| Female | 2449 | 2449/9039 | 27.1% | 2451 | 2451/9039 | 27.1% |
| Male | 6590 | 6590/9039 | 72.9% | 6476 | 6476/9039 | 71.6% |
| Race/Ethnicity | ||||||
| Black, non-Hispanic | 1988 | 1988/9039 | 22.0% | 1927 | 1927/8927 | 14.8% |
| Hispanic | 4012 | 4012/9039 | 44.4% | 3892 | 3892/8927 | 43.6% |
| White, non-Hispanic | 2419 | 2419/9039 | 26.8% | 2537 | 2537/8927 | 28.4% |
| Other race/ethnicity | 9014 | 581/9039 | 6.4% | 571 | 571/8927 | 6.2% |
| Age (in years)a | ||||||
| 18–24 | 280 | 280/9039 | 3.1% | 432 | 432/8927 | 4.8% |
| 25–34 | 1254 | 1254/9039 | 13.9% | 1457 | 1457/8927 | 16.3% |
| 35–44 | 1768 | 1768/9039 | 19.6% | 1866 | 1866/8927 | 20.9% |
| 45–54 | 3030 | 3030/9039 | 33.5% | 2904 | 2904/8927 | 32.5% |
| 55–64 | 2264 | 2264/9039 | 25.0% | 1956 | 1956/8927 | 21.9% |
| 65+ | 416 | 416/9039 | 4.6% | 301 | 301/8927 | 3.4% |
| Borough of Residence | ||||||
| Bronx | 1918 | 1918/9039 | 21.2% | 1876 | 1876/8927 | 21.0% |
| Manhattan | 3695 | 3695/9039 | 40.9% | 3471 | 3471/8927 | 38.9% |
| Queens | 778 | 778/9039 | 8.6% | 847 | 847/8927 | 9.5% |
| Brooklyn | 2180 | 2180/9039 | 24.1% | 2202 | 2202/8927 | 24.7% |
| Staten Island | 156 | 156/9039 | 1.7% | 171 | 171/8927 | 1.9% |
| Insurance Payor | ||||||
| Medicaid | 4668 | 4668/9039 | 51.6% | 4790 | 4790/8927 | 53.7% |
| Medicare | 16 | 16/9039 | 0.2% | 17 | 17/8927 | 0.2% |
| Self-payb | 923 | 923/9039 | 10.2% | 681 | 681/8927 | 7.6% |
| Other | 3448 | 3448/9039 | 38.1% | 3439 | 3439/8927 | 38.5% |
| Employment Status | ||||||
| Employed total | 1632 | 1632/8988 | 18.1% | 1627 | 1627/8927 | 18.2% |
| Unemployed total | 2766 | 2766/8988 | 30.6% | 2769 | 2769/8927 | 31.0% |
| Disabled | 4590 | 4590/8988 | 50.8% | 4484 | 4484/8927 | 50.2% |
| Methadone Dose | ||||||
| Mean, SD, range | 87.3 | 44.5 (SD) | (0–355) | 88.5 | 34.8 (SD) | (1–355) |
| ≥60 mg | 7249 | 7249/9039 | 80.2% | 7278 | 7278/8927 | 81.5% |
| <60 mg | 1790 | 1790/9039 | 19.8% | 1649 | 1649/8927 | 18.5% |
Abbreviations: HCV, hepatitis C virus infection; MG, milligrams; n/a, not applicable; OTP, opioid treatment program; SD, standard deviation.
aAge was calculated at start of the study period: January 1, 2013. The OTP enrolls individuals who are 18 years of age or older.
bMost “self-pay” patients are those who have health insurance that included time or visit limited drug treatment benefits that have been exhausted and who are therefore required to now be self-pay (sliding scale).
Hepatitis C Virus Testing at the Opioid Treatment Programs
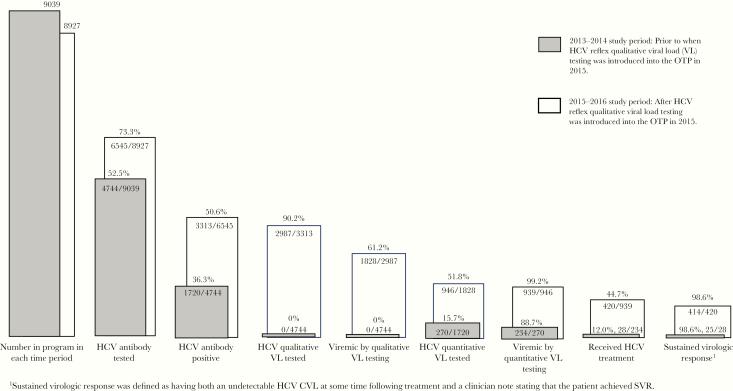
In 2013–2014, 4744 of the 9039 (52.6%) patients were HCV antibody tested (Table 2, Figure 1). Patients who did and did not receive antibody testing did not differ significantly with respect to the factors Table 1 (data not shown). There were 15.7% (270 of 1720) linked-to-care among those found to be HCV antibody positive. In 2015–2016, 6545 of the 8927 (73.3%) patients were HCV antibody tested, 3313 (50.6%) of whom were antibody positive; 90.2% (2987 of 3313) of those found to be HCV antibody positive had HCV qualitative VL tests performed; of these, 61.2% (1828 of 2987) were found to be viremic, and 946 were linked-to-care (51.8%); of these, 939 (99.3%) were viremic (Table 2, Figure 1).
Table 2.
Hepatitis C Virus Testing and Treatment Data Table for OTP Patients Enrolled in 2013–2016
| 2013–2014 (n = 9039) | 2015–2016 (n = 8927) | |||||
|---|---|---|---|---|---|---|
| Characteristics | n | n/n | % | n | n/n | % |
| No. with at least one HCV antibody test performed | 4744 | 4744/9039 | 52.5% | 6545 | 6545/8927 | 73.3% |
| HCV antibody positive | 1720 | 1720/4744 | 36.26% | 3313 | 3313/6545 | 50.6% |
| No. with at least 1 HCV qualitative viral load test performeda | n/a | n/a | n/a | 2987 | 2987/3313 | 90.2% |
| HCV qualitative VL reactive | n/a | n/a | n/a | 1828 | 1828/2987 | 61.2% |
| No. with at least 1 quantitative VL test performed | 270 | 270/1720 | 15.7% | 946b | 946/1828 | 51.8% |
| HCV quantitative VL detectable | 234 | 234/1720 | 13.6% | 939 b | 939/946 | 99.3% |
| No. with active viremia (by at least 1 qualitative or 1 quantitative VL test) | 234 | 234/1720 | 13.6% | 2085c | 2085/3313 | 62.9% |
| No. who received HCV treatment among all those with documented viremia | 28 | 28/234 | 12.0% | 420 | 420/2085 | 20.1% |
| No. who received HCV treatment after successful linkage to HCV care | 28 | 28/234 | 12.0% | 420 | 420/939 | 44.7% |
| No. who achieved SVR | 25 | 25/28 | 89.3% | 414 | 414/420 | 98.6% |
Abbreviations: HCV, hepatitis C virus infection; OTP, opioid treatment program; SVR, sustained virologic response; VL, viral load.
aApplies only to those HCV tested in the 2015–2016 time period as qualitative HCV VL testing wasn’t introduced into the OTP until 2015.
bAs reflex VL testing was rolled out in a staggered fashion in the first months of 2015, numbers tested for quantitative VL include both 761 who first had on-site reflex qualitative VL testing as well as 185 who were referred for quantitative VL testing after antibody testing only either in late 2014 or early 2015.
cAlthough 1828 individuals had reactive HCV qualitative VL tests, and 939 individuals had reactive HCV quantitative VL tests, 680 individuals were found to have viremia on both tests (data not shown); hence, (1828 + 939) – 680 = 2085 individuals with active infection.
Figure 1.
The hepatitis C virus (HCV) care continuum for patients in the opioid treatment program (OTP) in 2013–2014 and 2015–2016.
There was a significant increase in the proportion who had HCV antibody testing performed in 2015–2016 compared with 2013–2014 (73.3% vs 52.5%, P < .001). Likewise, there was a significant increase in the proportion linked to HCV care within this health system (15.7% vs 51.8%, P < .001), albeit at different stages in the testing process.
Linkage to Hepatitis C Virus Care Among Those With Active HCV Infection, 2015–2016
In univariate analysis, those of Hispanic ethnicity were significantly less likely to be linked-to-care (OR = 0.71; P < .01). Those living in the Bronx (OR = 0.58), Queens (OR = 0.66), and Brooklyn (OR = 0.70) were significantly less likely to be linked-to-care compared with those living in Manhattan (all P < .03) (Table 3). Because county of residence and UHF zip code of residence represent comparable constructs and were highly correlated (P < .01), borough of residence was retained for multivariate analysis. The multivariate model also included sex, race/ethnicity, and age. Hispanic ethnicity remained significant in the model; those who were Hispanic were less likely to be linked-to-care than non-Hispanic whites (AOR = 0.68; P < .01). Those residing in the Manhattan were more likely to be linked-to-care than those residing in other boroughs (AOR = 1.7; P < .01) (Table 3).
Table 3.
Univariate and Multivariate Associations With Linkage to Care Among OTP Patients With Active HCV Infection, 2015–2016
| Univariatea | Multivariatea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n (N = 1976) | % | n Linked to Care | % | P b | OR | 95% CI | P b | AOR | 95% CI | ||
| Sex | ||||||||||||
| Male (referent category) | 1476 | 74.7 | 628 | 42.5 | . | . | . | . | . | . | . | . |
| Female | 500 | 25.3 | 207 | 41.1 | .65 | 0.95 | 0.78 | 1.2 | .25 | 0.88 | 0.71 | 1.09 |
| Age (in years) | ||||||||||||
| 18–24 (referent category) | 50 | 2.5 | 34 | 1.7 | . | . | . | . | . | . | . | . |
| 25–34 | 255 | 12.9 | 170 | 8.6 | .27 | 0.71 | 0.39 | 1.31 | .35 | 0.73 | 0.38 | 1.42 |
| 35–44 | 378 | 19.1 | 256 | 13.0 | .34 | 0.75 | 0.41 | 1.35 | .52 | 0.81 | 0.42 | 1.54 |
| 45–54 | 602 | 30.5 | 473 | 23.9 | .96 | 1.01 | 0.57 | 1.81 | .97 | 1.0 | 0.54 | 1.90 |
| 55–64 | 603 | 30.5 | 450 | 22.8 | .76 | 0.91 | 0.51 | 1.63 | .63 | 0.85 | 0.45 | 1.61 |
| 65+ | 87 | 4.4 | 48 | 2.4 | .06 | 0.50 | 0.24 | 1.03 | .03 | 0.4 | 0.19 | 0.93 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White (referent category) | 480 | 24.3 | 225 | 46.7 | . | . | . | . | . | . | . | . |
| Hispanic | 923 | 46.7 | 354 | 38.3 | .002 | 0.71 | 0.56 | 0.88 | .002 | 0.68 | 0.53 | 0.87 |
| Non-Hispanic Black | 443 | 22.4 | 196 | 44.3 | .42 | 0.90 | 0.69 | 1.17 | .36 | 0.88 | 0.66 | 1.16 |
| Other race/ethnicity | 127 | 6.4 | 60 | 47.2 | .94 | 1.02 | 0.69 | 1.50 | .95 | 1.0 | 0.7 | 1.50 |
| Borough of Residencec | ||||||||||||
| Manhattan (referent category) | 824 | 41.7 | 399 | 48.4 | . | . | . | . | . | . | . | . |
| Bronx | 438 | 22.2 | 155 | 35.4 | .001 | 0.58 | 0.46 | 0.74 | .004 | 0.60 | 0.47 | 0.77 |
| Queens | 141 | 7.1 | 54 | 38.3 | .03 | 0.66 | 0.46 | 0.95 | .02 | 0.64 | 0.44 | 0.93 |
| Brooklyn | 483 | 24.4 | 192 | 39.8 | .002 | 0.70 | 0.56 | 0.88 | .002 | 0.70 | 0.55 | 0.88 |
| Staten Island | 34 | 1.7 | 13 | 38.2 | .25 | 0.66 | 0.33 | 1.34 | .24 | 0.65 | 0.32 | 1.33 |
| Insurance payor | ||||||||||||
| Other insurance (referent category) | 753 | 38.1 | 84 | 11.2 | . | . | . | . | . | . | . | . |
| Medicaid | 1223 | 61.2 | 521 | 42.6 | .23 | 0.89 | 0.73 | 1.08 | . | . | . | . |
| UHF zip code aread (no referent category) | 1976 | 100.0 | 835 | 42.2 | .002 | 1.02 | 1.00 | 1.18 | . | . | . | . |
| Methadone dose | ||||||||||||
| Greater than or equal to 60mg (referent category) | 1532 | 77.5 | 633 | 41.3 | . | . | . | . | . | . | . | . |
| Less than 60 mg | 444 | 22.5 | 202 | 45.5 | .12 | 0.84 | 0.68 | 1.00 | . | . | . | . |
| Employment status | ||||||||||||
| Employed (referent category) | 298 | 15.1 | 119 | 39.9 | . | . | . | . | . | . | . | . |
| Unemployed | 1672 | 84.6 | 712 | 42.6 | .39 | 1.12 | 0.87 | 1.43 | . | . | . | . |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; HCV, hepatitis C virus infection; MG, milligrams; OR, odds ratio; OTP, opioid treatment program; UHF, United Hospital Fund.
aLogistic regression was used to assess univariate associations; the multivariate model used logistic regression.
bBold font indicates statistical significance at the ≤0.05 level.
cBecause county of residence and UHF zip code area were highly correlated, only UHF zip code area was entered in the multivariate model.
dThe 34 UHF zip codes areas were collapsed into 30 zip code areas due to small cell sizes in 3 UHF zip code areas.
Hepatitis C Virus Treatment
In 2013–2014, 88.7% (234 of 270) of those having quantitative VL tests were found to be viremic; of these, 12% (28 of 234) received DAA HCV treatment within this health system (Table 2, Figure 1). Of those treated, 98.6% (25 of 28) achieved an SVR. In 2015–2016, 99.2% (939 of 946) of those having quantitative VL testing had confirmed viremia; of these, 44.7% (420 of 939) received HCV treatment, 98.6% (414 of 420) of whom achieved SVR (Table 2, Figure 1). The proportion of those found to be viremic and engaged in HCV care who received HCV treatment increased significantly (12% vs 44.7%, P < .001) (Table 2).
Engaged-in-Care Hepatitis C Virus Community Viral Load Measures
The overall engaged-in-care HCV CVL was 4 351 079 copies/mL (SD = 7 149 888; n = 1071) and did not decrease significantly from 2013–2014 to 2015–2016 (P = .13). Table 4 depicts HCV engaged-in-care CVL measures for demographic and geographic subgroups at the OTP for 2015–2016. In regression analyses, there were significant variations in mean engaged-in-care CVL by sex, race/ethnicity, age, and employment status (Table 4). Engaged-in-care HCV CVLs varied significantly among the 30 UHF zip code areas (P = .02) (Table 4) with a range of 74–77 656 774 copies/mL (lower and upper bounds of the SDs for the lowest and highest UHF area CVLs were 123 945–20 844 208 copies/mL, respectively).
Table 4.
Hepatitis C Virus Engaged in Care Community Viral Load Among Patients in an Opioid Treatment Program, New York City
| 2015–2016: HCV CVL | ||||
|---|---|---|---|---|
| Characteristics | N | CVL (copies/mL) a | CVL SDb | P Valuec |
| Sex | .023 | |||
| Female | 797 | 3 965 663 | 6 242 008 | |
| Male | 245 | 3 883 479 | 6 937 227 | |
| Race/Ethnicity | .029 | |||
| Black, non-Hispanic | 270 | 3 976 651 | 6 508 311 | |
| Hispanic | 448 | 3 733 424 | 5 794 450 | |
| White, non-Hispanic | 253 | 3 978 311 | 7 141 367 | |
| Other race/ethnicity | 71 | 5 060 616 | 6 963 862 | |
| Age (in years)d | .004 | |||
| 18–24 | 23 | 2 138 809 | 4 003 131 | |
| 25–34 | 115 | 3 068 157 | 4 800 581 | |
| 35–44 | 188 | 3 893 843 | 6 076 249 | |
| 45–54 | 349 | 4 278 028 | 7 681 138 | |
| 55–64 | 331 | 4 116 975 | 5 925 130 | |
| 65+ | 36 | 3 396 174 | 3 904 330 | |
| Borough of Residence | .351 | |||
| Bronx | 495 | 3 597 421 | 5 287 413 | |
| Manhattan | 190 | 4 753 923 | 7 778 317 | |
| Queens | 71 | 3 204 971 | 4 309 483 | |
| Brooklyn | 246 | 4 330 097 | 7 958 986 | |
| Staten Island | 15 | 2 008 577 | 3 728 379 | |
| Employment Status | .020 | |||
| Employed total | 140 | 4 815 964 | 7 176 873 | |
| Unemployed total | 707 | 3 725 917 | 6 276 418 | |
| Disabled | 191 | 4 138 561 | 6 322 261 | |
| Insurance Payor | .113 | |||
| Medicaid | 628 | 4 214 393 | 7 079 400 | |
| Medicare | 5 | 2 065 428 | 2 672 635 | |
| Self-paye | 85 | 4 763 063 | 7 133 472 | |
| Other | 10 | 7 402 553 | 7 608 470 | |
| Methadone Dose (mg) | .428 | |||
| ≥60 mg | 249 | 4 047 689 | 6 462 006 | |
| <60 mg | 793 | 3 914 516 | 6 395 908 | |
| UHF zip code area | 30 | (74–77 656 774) | (123 945–20 844 208) | .020 |
Abbreviations: CVL, community viral load; HCV, hepatitis C virus infection; MG, milligrams; SD, standard deviation; UHF, United Hospital Fund.
aIncluding viral loads excluding undetectable viral load tests.
bStandard deviations presented reflect SD for the lowest UHF area CVL and for the highest UHF area CVL, respectively.
c P value reflects univariate analyses of log-base 10 transformed CVL measures examined using linear regression; bold font indicates statistical significance at the ≤.05 level.
dAge was calculated at start of the study period: January 1, 2013. The Opioid Treatment Program enrolls individuals who are 18 years of age or older.
eMost “self-pay” patients are those who have health insurance that included time or visit limited drug treatment benefits that have been exhausted and who are therefore required to now be self-pay (sliding scale).
Overall Hepatitis C Virus Care Continuum Outcomes
There were a total of 1173 individuals with documented HCV infection in 2013–2016; 448 (38%) were linked-to-care and initiated HCV treatment, 439 (98.0%) of whom achieved SVR. The proportion receiving HCV antibody testing was significantly higher in 2015–2016 than in 2013–2014 (73.3% vs 52.5%, P < .001). If the HCV antibody prevalence identified in 2015–2016 (50.6%) is assumed to reflect the OTP cohort prevalence, an additional 2173 (ie, 50.6% of the 4295 not antibody tested) and 1205 (ie, 50.6% of the 2382 not antibody tested) patients in 2013–2014 and 2015–2016, respectively, may have been HCV antibody positive but not identified.
With the implementation of reflex testing in 2015–2016, the proportion of those HCV antibody positive whose viremic status was ascertained increased significantly from 5% to 63.1% (P < .001). In 2013–2014, 84.3% (1450 of 1720) of those found to be HCV antibody positive did not have VL testing. Assuming a 25% spontaneous clearance rate, 1088 would be expected to have active viremia. If in addition we assume that 2173 of those not antibody tested may have been antibody positive, assuming a 25% clearance rate, there would be an additional 1630 who were likely viremic, which, when combined with the 234 with documented viremia, suggests a total of 2952 viremic patients (32.7% of 9039).
In 2015–2016, 9.8% (326 of 3313 HCV antibody positive) did not have VL testing. Assuming a 25% spontaneous clearance rate, 245 would have had active viremia. Assuming that 1205 of those not antibody tested were antibody positive, with a 25% clearance rate, there would be an additional 904 who were likely viremic, which when combined with the 939 with documented viremia suggests a total of 1843 viremic patients (20.6% of 8927).
The documented unmet treatment need was 88.0% (206 of 234) and 55.3% (519 of 939) in 2013–2014 and 2015–2016, respectively. Again assuming 25% spontaneous clearance and a population prevalence of 50.6%, the estimated unmet treatment need was 99.1% (2924 of 2952) and 86.0% (2551 of 2971) in 2013–2014 and 2015–2016, respectively. Both the documented and the estimated unmet treatment need decreased significantly from 2013–2014 to 2015–2016 (P < .001) but remained high.
DISCUSSION
In this study of PWUD in an OTP, HCV care continuum outcomes were significantly improved in 2015–2016 when reflex qualitative VL testing was used; however, large numbers of patients remained undiagnosed or incompletely evaluated, and unmet treatment need and engaged-in-care CVLs remained high. Although combined prevention programs have resulted in dramatic declines in HIV infection among PWUD, the effect of combined prevention on the incidence and prevalence of HCV infection has been more modest [8, 10]. Although the observed HCV antibody prevalence in this cohort was only 30.6% in 2013–2014 and 50.6% in 2015–2016, it is not likely that this represents a real increase in prevalence but rather that the opt-out antibody testing conducted in 2013–2014 led to undertesting of those known or suspected to be previously positive; it is plausible that the combination of the availability of DAA treatment and reflex qualitative VL testing either motivated patients to more readily accept testing or that staff more effectively encouraged testing in 2015–2016. Before the implementation of combined prevention (2000–2001), HCV infection prevalence in this OTP was approximately 90% [8, 47], whereas in recent cross-sectional surveys reflecting either 2006–2013, 2011–2015, or 2007–2017 it has been 60%–69% [20, 47, 48]. The inference that the OTP cohort prevalence in 2013–2014 was 50.6% may therefore be a conservative estimate.
There was also a significant increase in the proportion linked-to-care in 2015–2016. The 2 different testing strategies reflected in 2013–2014 and 2015–2016 represent 2 of the testing strategies that are commonly used in OTPs and HCV testing sites nationally [30, 31]. Care continuum analyses included some participants who appear in both time periods and hence are not independent samples. However, their inclusion reflects recommendations for annual testing and reflects program-level outcomes in each time period. Hence, although not a controlled study, and although DAA availability may have been motivating, the data are consistent with the inference that implementation of reflex VL testing may contribute both to greater testing rates and improved to linkage-to-care.
Among those found to have active HCV infection by reflex testing in 2015–2016, Hispanics were less likely than non-Hispanic whites to be successfully linked-to-care. Furthermore, there was significant geographic variation in the proportion successfully linked-to-care, suggesting that geographically focused efforts are needed to enhance access and linkage to treatment. There was also a significant increase in treatment rates among those linked-to-care. Although reasons for this are not certain, it is likely that both the wider availability of oral DAA therapy and wider acceptance that HCV treatment can be effective among PWUD contributed to these increases [1, 5, 44]. Among patients treated for HCV with DAAs, there were high treatment completion and SVR rates. These data contribute to the growing evidence base that PWUD and people receiving methadone can successfully be engaged in HCV treatment and be cured of HCV, serving as CasP [5, 38].
A range of CVL measures have been developed and applied to the surveillance and study of HIV epidemiology; these include measures relying only on directly measured data that may be practically used by diverse programs and jurisdictions, as well as broader measures that attempt to account for undiagnosed people and for area-level prevalence that may require both more complete area-level surveillance, statistical methods such as adjustments for duplicate case reporting, and multiple imputation [20–24, 49, 50]. The application of the CVL construct to HCV epidemiology is novel; to our knowledge, there is only 1 publication, by Arnold et al [36] from the Houston Department of Health, reporting an engaged-in-care HCV CVL derived from directly measured data. The engaged-in-care CVL provides a practical measure for programs and jurisdictions [19, 22, 36]. The engaged-in-care HCV CVLs in our study of OTP patients varied significantly among demographic subgroups and by geographic area; Arnold et al [36] also identified geographic variation in their HCV CVL study. Further research of various HCV CVL measures and of their geographic variations and potential relationship to incidence is warranted [23, 24, 36].
Although rates of HCV testing, linkage-to-care, and HCV treatment all increased significantly from 2013–2014 to 2015–2016, engaged-in-care HCV CVLs did not decrease over time. This is likely due to the fact that despite significant increases in testing, linkage, and treatment, there still remained a very high unmet treatment need. Although those treated in 2013–2014 contributed a VL of zero to the engaged-in-care CVL for 2015–2016, we found there were too few patients treated to have a significant impact on HCV CVL. These findings are consistent with the observation that current combined HCV prevention strategies, which in NYC include ready availability of sterile syringes through SSPs and pharmacies, has not included a sufficient expansion of HCV treatment [3, 4]. These data strongly suggest that further improvements in testing and linkage-to-care are needed to address the large unmet treatment need, both among those with documented chronic infection and among those who remain incompletely diagnosed, to control the HCV epidemic among PWUD.
Limitations
Several important limitations should be noted. As the data were extracted from EMRs, detailed individual-level HCV risk data, such as frequencies of nonsterile injection or use of SSPs and HIV status, were not available. Furthermore, because data were of OTP patients, the HCV prevalence identified is neither a general population prevalence nor one of all PWUD; the age of the OTP cohort might contribute to overestimation, and the opt-out testing strategy may contribute to underestimation of HCV prevalence among PWUD in general. Nonetheless, the increases in the proportion of antibody tested, the proportion whose virologic status was fully ascertained, and the proportion linked-to-care did significantly increase in 2015–2016. Analyses of factors associated with linkage to HCV care were limited by the inability to adjust to factors not measured in the EMR. Although treatment response rates were excellent, not all posttreatment VL assessments were performed at ≥12 weeks posttreatment; nonetheless, 98.6% of PWUD treated were deemed by their treating clinicians to have been cured.
In addition, engaged-in-care HCV CVLs only reflect data for those successfully linked-to-care, and in settings such as this, with high proportions not fully tested or linked-to-care, they underestimate a true population-level CVL. Although the numbers and proportions not antibody or VL tested rely on directly measured data, the estimates of undiagnosed, HCV-infected patients and of unmet treatment need relied on assumptions of prevalence and of spontaneous clearance rate. Furthermore, data examined are from patients in a single large OTP receiving care in 1 large healthcare system, and they are not from a population-based representative cohort, potentially limiting generalizability and potentially contributing to outcome misclassification because some patients may have received care elsewhere.
CONCLUSIONS
In 2015–2016, in which reflex qualitative VL testing was implemented and DAAs were available, care continuum outcomes improved significantly; broader implementation of reflex testing may be valuable. However, very relevant care continuum gaps remained. Both rates of linkage-to-care and engaged-in-care HCV CVLs among the OTP patients studied varied significantly geographically, suggesting that geographically focused efforts to improve linkage-to-care and expanded treatment coverage of OTP patients are needed. Our data add further evidence that when PWUD are treated for HCV, high treatment completion and SVR rates can be achieved. Although the proportion treated increased significantly, there were still very high rates of unmet treatment need. These data suggest that control of the HCV epidemic among PWUD will require both the implementation of strategies for more complete testing and linkage-to-care of OTP patients, and this will require a significant expansion of HCV treatment.
Notes
Disclaimer. The views expressed are the authors’ own and do not necessarily represent the views of the National Institutes of Health, the United States Government, or the authors’ institutions.
Financial support. The research was supported in part by the National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA)-funded T32 training Grant 5T32DA007233-35 and the NIH/NIDA-funded P30 Center Grants P30DA011041 and P30DA040500
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017; 47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. New York City Department of Health and Mental Hygiene. Working toward a hep free NYC: HAV, HBV, and HCV in NYC: 2017 annual report. 2018. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/cd/hepatitis-abc-annual-report-2017.pdf. Accessed 8 January 2020. [Google Scholar]
- 3. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linas BP, Barter DM, Leff JA, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckhardt BJ, Scherer M, Winkelstein E, Marks K, Edlin BR. Hepatitis C treatment outcomes for people who inject drugs treated in an accessible care program located at a syringe service program. Open Forum Infect Dis 2018; 5:ofy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018; 108:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend 2015; 152:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet 2010; 376:285–301. [DOI] [PubMed] [Google Scholar]
- 10. Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS 2005; 19(Suppl 3):S20–5. [DOI] [PubMed] [Google Scholar]
- 11. de Vos AS, van der Helm JJ, Matser A, Prins M, Kretzschmar ME. Decline in incidence of HIV and hepatitis C virus infection among injecting drug users in Amsterdam; evidence for harm reduction? Addiction 2013; 108:1070–81. [DOI] [PubMed] [Google Scholar]
- 12. Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106:1978–88. [DOI] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control. European Monitoring Centre for Drugs and Drug Addiction Prevention and control of infectious diseases among people who inject drugs ECDC and EMCDDA guidance. Stockholm: ECDC; 2011. [Google Scholar]
- 15. MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy 2014; 25:34–52. [DOI] [PubMed] [Google Scholar]
- 16. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiessing L, Ferri M, Grady B, et al. ; EMCDDA DRID group Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014; 9:e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010; 105:844–59. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. Guidance on Community Viral Load: A Family of Measures, Definitions, and Method for Calculation 2011. Available at: https://stacks.cdc.gov/view/cdc/28147. Accessed 31 October 2019.
- 20. Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Das M, Raymond HF, Chu P, et al. Measuring the unknown: calculating community viral load among HIV-infected MSM unaware of their HIV status in San Francisco from National HIV Behavioral Surveillance, 2004–2011. J Acquir Immune Defic Syndr 2013; 63:e84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laraque F, Mavronicolas HA, Robertson MM, Gortakowski HW, Terzian AS. Disparities in community viral load among HIV-infected persons in New York City. AIDS 2013; 27:2129–39. [DOI] [PubMed] [Google Scholar]
- 23. Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. Lancet HIV 2016; 3:e183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herbeck J, Tanser F. Community viral load as an index of HIV transmission potential. Lancet HIV 2016; 3:e152–4. [DOI] [PubMed] [Google Scholar]
- 25. Yazdanpanah Y, De Carli G, Migueres B, et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 2005; 41:1423–30. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol 2015; 194:173–7. [DOI] [PubMed] [Google Scholar]
- 27. Syriopoulou V, Nikolopoulou G, Daikos GL, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis 2005; 37:350–3. [DOI] [PubMed] [Google Scholar]
- 28. Chapko MK, Dufour DR, Hatia RI, Drobeniuc J, Ward JW, Teo CG. Cost-effectiveness of strategies for testing current hepatitis C virus infection. Hepatology 2015; 62:1396–404. [DOI] [PubMed] [Google Scholar]
- 29. Jülicher P, Galli C. Identifying cost-effective screening algorithms for active hepatitis C virus infections in a high prevalence setting. J Med Econ 2018; 21:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Jordan AE, Perlman DC, Reed J, Smith DJ, Hagan H. Patterns and gaps identified in a systematic review of the hepatitis C virus care continuum in studies among people who use drugs. Front Public Health 2017; 5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frimpong JA, D’Aunno T, Jiang L. Determinants of the availability of hepatitis C testing services in opioid treatment programs: results from a national study. Am J Public Health 2014; 104:e75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirsch AA, Lawrence RH, Kern E, Falck-Ytter Y, Shumaker DT, Watts B. Implementation and evaluation of a multicomponent quality improvement intervention to improve efficiency of hepatitis C screening and diagnosis. Jt Comm J Qual Patient Saf 2014; 40:351–7. [DOI] [PubMed] [Google Scholar]
- 33. AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018; 67:1477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med 2013; 126:718–22. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013; 62:362. [PMC free article] [PubMed] [Google Scholar]
- 36. Arnold RM, Yang B, Yu Q, Arafat RR. Refocusing hepatitis C prevention through geographic viral load analysis. Online J Public Health Inform. 2018. Available at: http://ojphi.org/ojs/index.php/ojphi/article/view/6501. Accessed 30 August 2019.
- 37. Frimpong JA. Missed opportunities for hepatitis C testing in opioid treatment programs. Am J Public Health 2013; 103:1028–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin SA, Bosse J, Wilson A, Losikoff P, Chiodo L. Under one roof: identification, evaluation, and treatment of chronic hepatitis C in addiction care. Addict Sci Clin Pract 2018; 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlman DC, Jordan AE, Nash D. Conceptualizing care continua: lessons from HIV, hepatitis C virus, tuberculosis and implications for the development of improved care and prevention continua. Front Public Health 2016; 4:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacCarthy S, Hoffmann M, Ferguson L, et al. The HIV care cascade: models, measures and moving forward. J Int AIDS Soc 2015; 18:19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith DJ, Jordan AE, Frank M, Hagan H. Spontaneous viral clearance of hepatitis C virus (HCV) infection among people who inject drugs (PWID) and HIV-positive men who have sex with men (HIV+ MSM): a systematic review and meta-analysis. BMC Infect Dis 2016; 16:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006; 13:34–41. [DOI] [PubMed] [Google Scholar]
- 43. New York City Department of Health and Mental Hygiene. New York City United Hospital Fund (UHF) neighborhoods and NYC ZIP code areas 2006. Available at: https://a816-healthpsi.nyc.gov/epiquery/CHS/uhf-zip-information.pdf. Accessed 1 May 2019.
- 44. Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 2018; 13:e0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- 46. R Core Team. R: a language and environment for statistical computing. Austria: R Foundation for Statistical Computing V; 2019. Available at: https://www.R-project.org/. Accessed 15 December 2019. [Google Scholar]
- 47. Des Jarlais DC, Cooper HL, Arasteh K, Feelemyer J, McKnight C, Ross Z. Potential geographic “hotspots” for drug-injection related transmission of HIV and HCV and for initiation into injecting drug use in New York City, 2011–2015, with implications for the current opioid epidemic in the US. PLoS One 2018; 13:e0194799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Des Jarlais DC, Arasteh K, Feelemyer J, et al. Hepatitis C virus prevalence and estimated incidence among new injectors during the opioid epidemic in New York City, 2000–2017: protective effects of non-injecting drug use. Drug Alcohol Depend 2018; 92:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edelman EJ, Tate JP, Fiellin DA, et al. Impact of defined clinical population and missing data on temporal trends in HIV viral load estimation within a health care system. HIV Med 2015; 16:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krentz HB, Gill MJ. The effect of churn on “community viral load” in a well-defined regional population. J Acquir Immune Defic Syndr 2013; 64:190–6. [DOI] [PubMed] [Google Scholar]