Abstract
Background
Persons who inject drugs (PWID) are at a disproportionately high risk of HIV infection. We aimed to determine the highest-valued combination implementation strategies to reduce the burden of HIV among PWID in 6 US cities.
Methods
Using a dynamic HIV transmission model calibrated for Atlanta, Baltimore, Los Angeles, Miami, New York City, and Seattle, we assessed the value of implementing combinations of evidence-based interventions at optimistic (drawn from best available evidence) or ideal (90% coverage) scale-up. We estimated reduction in HIV incidence among PWID, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) for each city (10-year implementation; 20-year horizon; 2018 $ US).
Results
Combinations that maximized health benefits contained between 6 (Atlanta and Seattle) and 12 (Miami) interventions with ICER values ranging from $94 069/QALY in Los Angeles to $146 256/QALY in Miami. These strategies reduced HIV incidence by 8.1% (credible interval [CI], 2.8%–13.2%) in Seattle and 54.4% (CI, 37.6%–73.9%) in Miami. Incidence reduction reached 16.1%–75.5% at ideal scale.
Conclusions
Evidence-based interventions targeted to PWID can deliver considerable value; however, ending the HIV epidemic among PWID will require innovative implementation strategies and supporting programs to reduce social and structural barriers to care.
Keywords: HIV, localized HIV microepidemics, interventions, cost-effectiveness, injection drug use, dynamic HIV transmission mode
In the United States, persons who inject drugs (PWID) continue to be disproportionately at risk of human immunodeficiency virus (HIV) infection. Successes in New York City, Vancouver, British Columbia, and countries such as Australia, the United Kingdom, and France have provided evidence that important reductions in HIV incidence among PWID are possible with the widespread provision of HIV care and services to prevent and reduce harms caused by substance use [1]. Domestically, the steady declines in HIV incidence among PWID has been a success story and several jurisdictions are now focused on preventing resurgence and getting new HIV infections attributed to drug injection to zero. Nonetheless, following the rise in prevalence of opioid injection, 2015 marked the first time in 2 decades that injection-related infections increased in the United States [2].
There is considerable evidence suggesting that broad implementation of prevention programs can be highly effective in reducing transmission of HIV and other blood-borne pathogens among PWID [1, 3, 4]. Nevertheless, the high prevalence of drug injection-related HIV infections among people living with HIV (18.1% in 2016) [5] and the lifetime prevalence of injection drug use in the United States (estimated to be 2.6%) [6] underscore how the public health response and short supply of these services have been (and remain) inadequate in many settings [1, 7, 8].
The US Centers for Disease Control and Prevention (CDC) have recommended a comprehensive approach to reduce the risk of HIV acquisition and transmission among PWID [9]. Long-standing recommendations include sterile syringe and needle distribution, and medication for opioid use disorders, both with robust evidence of effectiveness and cost-effectiveness [10–13]. In addition, the CDC’s guidance includes expanded HIV testing and the provision of antiretroviral therapy (ART) for treatment and prevention, the latter of which can have large independent effects on incidence reduction among PWID [4]. Although preexposure prophylaxis (PrEP) at current prices has not been found to be cost-effective among PWID in prior US-based modelling studies [12, 14], the US Preventive Services Task Force recently recommended that PrEP be offered to all persons at high risk of HIV acquisition, including PWID [15]. Despite a consensus that combination implementation strategies are necessary to reduce HIV incidence among PWID [1, 3], determining which combination should be expanded across cities with different underlying injection drug use epidemics is necessary to deliver maximum value and produce the greatest impact.
We hypothesized that combination implementation strategies providing the greatest value would vary across cities with different epidemiological and structural conditions. Using a dynamic compartmental HIV transmission model populated and calibrated to replicate the HIV microepidemics in 6 US cities, we aimed to determine the highest-valued combination implementation strategies to reduce the burden of HIV among PWID.
METHODS
Model Description
Our analysis builds on a previously published dynamic, compartmental HIV transmission model adapted and calibrated to replicate city-level HIV microepidemics in Atlanta, Baltimore, Los Angeles, Miami, New York City, and Seattle. We selected these 6 cities because they represent nearly one-quarter of the population of persons living with HIV in the United States and the fact that they represent diverse HIV microepidemics with extensive epidemiological and structural differences in their public health responses to HIV [16]. This computer simulation model was based on a synthesis of the best available evidence on epidemiological and structural conditions for each city and has previously been described in detail elsewhere [7, 17]. The model tracked HIV-susceptible individuals through infection, diagnosis, treatment with ART, and ART discontinuation. In each city, the adult population aged 15–64 years was partitioned by sex at birth, HIV risk group (men who have sex with men [MSM], PWID, MSM who inject drugs [MSMWID], and heterosexuals), race/ethnicity (black/African American, Hispanic/Latinx, and non-Hispanic white/others) and sexual risk behavior level (high vs low risk). We incorporated region-specific ART initiation and persistence rates stratified by race/ethnicity and HIV risk group from a prior analysis of HIV Research Network data [18].
We derived estimates of the size of the PWID population by multiplying race/ethnicity-stratified total population numbers by sex-weighted, race/ethnicity-specific prevalence estimates for each city. We assumed that gender proportions of PWID were equivalent within race/ethnicity strata and used prevalence estimates from the most recent available year [7, 19]. Given the uncertainty in population sizes for MSMWID, we derived population estimates by taking the average of 2 estimated population sizes: (1) the proportion of MSM that inject drugs and (2) the proportion of male PWID that have sex with men [7, 17, 20–22]. Finally, based on the best available evidence, we assumed that 72.7% of PWID and MSMWID had an opioid use disorder [23].
HIV transmission within the model was possible between any 2 HIV-discordant individuals. The probability of HIV transmission was determined by: (1) the probability of selecting a partner living with HIV; (2) the type of risk behavior engaged in (heterosexual or homosexual activity, or sharing injection equipment); (3) the infected individual’s HIV disease stage (acute or by CD4-based strata); (4) the infected individual’s ART status; (5) whether the uninfected individual was on PrEP; and (6) the probability of condom use. We allowed for a combination of assortative and proportional sexual partnership mixing; assortative mixing accounted for individuals being more likely to form partnerships within a common stratum (eg, race/ethnicity, risk behavior level), while proportional mixing accounted for individuals with many partners being more likely to select a partner who also had many partners. We also assumed proportional mixing among PWID (ie, individuals who share many injections were more likely to select a partner who also shares many injections). Further details on the probability of HIV transmission in the model have previously been provided elsewhere [17].
The model also captured heterogeneity in maturation (eg, rates at which individuals age out of the model) and mortality, and the disparities in accessing health, prevention, and treatment services, including HIV testing, ART, syringe service programs (SSP), medication for opioid use disorder (MOUD), and PrEP.
Model Calibration and Validation
For each city, we calibrated the model to match HIV prevalence, new diagnoses, and deaths (2012–2015), stratified by sex, race/ethnicity, and HIV risk group (17 targets total, including prevalence among PWID and MSMWID), and validated against external incidence estimates [17]. The model was used to project microepidemic trajectories over a 20-year time horizon (2020–2040), accounting for external estimates of population growth, which incorporated demographic shifts in race/ethnic composition for each city, to serve as the basis of comparison [24]. In the projections, status quo service levels of prevention, testing, and treatment services were held at their 2015 levels (Table 1) except for PrEP, which was held at 2017 levels to account for its recent rapid growth in uptake among MSM.
Table 1.
HIV Among Persons Who Inject Drugs in 2017 and Selected HIV Treatment and Prevention Service Levels in 2015 in 6 Cities
| Treatments and Services | Atlanta, GA | Baltimore, MD | Los Angeles, CA | Miami, FL | New York City, NY | Seattle, WA |
|---|---|---|---|---|---|---|
| Persons who inject drugs who are living with HIV, No. (% among all living with HIV)a | ||||||
| Prevalence | 3612 (11.3) | 4759 (21.3) | 5575 (10.8) | 2425 (9.3) | 13 037 (10.5) | 884 (12.9 |
| New diagnosesb | 67 (4.1) | 50 (11.4) | 146 (7.5) | 27 (2.3) | 64 (3.0) | 17 (10.8) |
| HIV prevention program service levels | ||||||
| Estimated annual number of syringes distributed per PWID | 2 | 20 | 19 | 6 | 24 | 196 |
| Coverage of medication for opioid use disorder among PWID, %c | 3.0 | 9.4 | 15.7 | 7.1 | 19.9 | 11.9 |
| HIV testing levels among PWID/MSMWIDd |
||||||
| Percent receiving an HIV test in the past year | 30/15 | 11/ 12 | 40/25 | 16/15 | 9/41 | 43/51 |
| HIV treatment engagement among PWID/MSMWIDd | ||||||
| Percent of diagnosed initiating ARTe | 44/38 | 55/47 | 51/44 | 48/41 | 39/42 | 51/46 |
| Percent discontinuing ARTe | 28/25 | 11/8 | 14/13 | 24/21 | 11/8 | 5/4 |
| Percent reinitiating ARTe | 42/44 | 28/29 | 23/20 | 43/46 | 31/32 | 49/50 |
Counties included in city boundaries for Atlanta, Baltimore, Los Angeles, and Miami match those included in the definition of Ryan White EMA or TGA. New York City and Seattle boundaries are restricted to a subset of counties. Counties included in cities are: Atlanta (Barrow, Bartow, Carroll, Cherokee, Clayton, Cobb, Coweta, DeKalb, Douglas, Fayette, Forsyth, Fulton, Gwinnett, Henry, Newton, Paulding, Pickens, Rockdale, Spalding, Walton); Baltimore (Anne Arundel, Baltimore City, Baltimore County, Carroll, Harford, Howard, Queen Anne’s); Los Angeles (Los Angeles county); Miami (Miami-Dade county); New York City (county and borough: New York [Manhattan], Kings [Brooklyn], Queens [Queens], Bronx [Bronx], Richmond [Staten Island]); Seattle (King county). Excluded counties for New York City compared to the Ryan White EMA definition included Westchester, Rockland, and Putnam, and excluded counties for Seattle compared to Ryan White TGA definition included Snohomish and Island.
Abbreviations: ART, antiretroviral therapy; EMA, Eligible Metropolitan Area; HIVRN, HIV Research Network; MSMWID, men who have sex with men who inject drugs; PWID, persons who inject drugs; TGA, Transitional Grant Area.
aPersons who inject drugs include men who have sex with men who inject drugs.
bNew diagnoses are from 2017 in city surveillance reports, except for Los Angeles were new diagnoses are for 2016, or from the Centers for Disease Control and Prevention’s Surveillance HIV Surveillance Supplemental Report.
cCoverage is among the 72.7% of PWID estimated to have an opioid use disorder [23].
dWhile the model runs in monthly cycles, we have converted these figures to yearly probabilities for ease of interpretation.
eART initiation rates were estimated from the HIVRN data, and ART discontinuation and reinitiation rates were estimated by a continuous-time multistate Markov model based on the same HIVRN data [18].
Interventions
We selected 14 evidence-based interventions within 4 specific domains (Table 2): HIV prevention programs (SSP, MOUD with either methadone or buprenorphine, PrEP); HIV testing; ART engagement (ART initiation and retention); and ART reengagement (reinitiation and relinkage). These interventions were selected from the US CDC Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention and from the recently published literature [25, 26].
Table 2.
Description, Effectiveness, and Scale-up Implementation Scenarios for the Evidence-Based HIV Prevention Programs and Care Interventions Included in Analysis
| Intervention | Supporting Evidence | Description and Effectivenessb | Scale-up Implementation Scenariose | |||
|---|---|---|---|---|---|---|
| Source, Evidence Levela | Study Design | Study Setting | Optimistic, % | Ideal, %c | ||
| HIV prevention programs | ||||||
| SSP | Aspinall et al 2014 [11], 2a | Meta-analysis | SSP | Clean injection equipment reduces the risk of parenteral HIV transmission by 58% | 200 syringes/PWID/yearf | 90 |
| MOUD with buprenorphine | MacArthur et al 2012 [37], 2a | Meta-analysis | Primary care and OTP | Office-based MOUD reduces the number of shared injections by 54% for PWID with OUDd | 29g | 90h |
| MOUD with methadone | MacArthur et al 2012 [37], 2a | Meta-analysis | Primary care and OTP | Opioid treatment program-based MOUD reduces the number of shared injections by 54% for PWID with OUDd | Additional scale-up of 17 | 90h |
| Full-time PrEP | Liu et al 2016 [27], 1b | RCT substudy and cohort study | Primary care | Protective level adherence to PrEP (≥4 doses/week) reduces the risk of HIV infection by 60%i | 50 | 90 |
| HIV testing | ||||||
| EMR testing offer reminder | Felsen et al 2017 [28], 2b | Pre/post | Hospital | HIV testing increases by 178% among PWID visiting the ER | 13–35 | 14–36d |
| Nurse-initiated rapid testing | Anaya et al 2008 [29], 2b | RCT | Primary care | Nurse-initiated screening and rapid testing increases HIV testing by 73% during health care visits | 34–52 | 56–87 |
| MOUD integrated rapid testing | Metsch et al 2012 [30], 1b | RCT | DTP | On-site rapid testing increases HIV testing by 352% among PWID receiving MOUD | 22 | 49 |
| ART engagement | ||||||
| Case management (ARTAS) | Gardner et al 2005 [31] 1b | RCT | HIV clinics | Contacts with a case manager increases ART initiation by 41% among PLHIV linked to care | 61 | 77 |
| Care coordination | Robertson et al 2018 [32], 2b | Pre/postj | HIV clinics | Comprehensive care coordination increases ART retention by 10% among PLHIV | 12–25 | 34–68 |
| Targeted care coordination | Robertson et al 2018 [32], 2b | Pre/postj | HIV clinics | Targeted comprehensive care coordination increases ART retention by 32% among PLHIV with CD4 < 200 cells/µL | 41–48 | 57–66 |
| EMR ART engagement reminder | Robbins et al 2012 [33], 1b | RCT | HIV clinics | Interactive EMR alerts reduces ART drop-out by 31% among PLHIV on ART | 47–84 | 60–91d |
| RAPID ART initiation | Pilcher et al 2017 [34], 3b | Cohort study | HIV clinics | Multidisciplinary care and support increases immediate ART initiation by 32% among newly diagnosed PLHIV | 38–71 | 47–90 |
| ART reengagement | ||||||
| Enhanced personal contact | Gardner et al 2014 [35], 1b | RCT | HIV clinics | Continuous contact increases ART reinitiation by 22% among PLHIV having dropped out of ART | 49 | 62 |
| Relinkage program | Bove et al 2015 [36], 2b | Cohort study | HIV clinics | Outreach using surveillance data increases ART reinitiation by 70% among PLHIV who are out of care | 10 | 22 |
Abbreviations: ARTAS, Antiretroviral Treatment Access Study; DTP, drug treatment program; EMR, electronic medical records; ER, hospital emergency room; MOUD, medication for OUD; OTP, opiate treatment program; OUD, opioid use disorder; PLHIV, people living with HIV; PrEP, preexposure prophylaxis; Pre/post, Prospective, quasi-experimental pre/post study; PWID, people who inject drugs; RAPID: rapid ART program for individuals with an HIV diagnosis; RCT, randomized control trial; SSP, syringe service program; WHO, World Health Organization.
aLevels of evidence adapted from Oxford Centre for Evidence-based Medicine Levels of Evidence: 1a, systematic review of RCTs; 1b, individual high-quality RCT; 2a, systematic review of cohort studies; 2b, individual cohort study or quasi-experimental study; 3a, systematic review of case-control studies; 3b, individual case-control study; 4, case series.
bInterventions target the PWID adult population aged 15–64 years including men who have sex with men who inject drugs.
cIdeal implementation refers to 90% adoption unless otherwise noted by d which refers to 100% adoption of EMR.
dMOUD also reduces the risk of mortality, increases quality of life, and decreases the probability of ART discontinuation.
eWhere applicable, scale-up ranges indicate evidence stratified by sex/gender and/or race/ethnicity and/or city/region.
fAs recommended by WHO [38], except Seattle (400 syringes/PWID/year) because status quo service levels were already equivalent to this level.
gAs recommended by WHO [38], 40% coverage among the 72.7% of PWID with an OUD [23] results in 29% coverage among all PWID.
hMaximum 90% coverage of both medications combined among the 72.7% of PWID with an OUD [23].
iEffectiveness defined as efficacy for 4 doses/week (96%; 95% confidence interval, 90%–99%) × protective level adherence (62.5%; associated with taking ≥ 4 doses/week), further details in Supplementary Materials.
jStudy with contemporaneous surveillance registry-based comparison group.
Although the model captured outcomes across risk groups for the entire adult population in each city, the implementation of interventions in our analysis was targeted exclusively to PWID and MSMWID (jointly referred to as PWID hereafter). Access to health services were held at status quo levels among the non-PWID population in each of the scenarios we describe below. Scale-up from status quo service levels was implemented proportionally across risk and ethnic groups over an 18-month period, entailing greater scale-up for groups receiving higher service levels at baseline, thus accounting for underlying structural barriers to health care access.
We assessed interventions individually and in all combinations (excluding any that would not practically be implemented jointly) for a total of 10 239 unique combinations. We assessed these combinations at optimistic implementation levels, where HIV testing and ART engagement and reengagement interventions were delivered at the upper bound of publicly documented evidence of scale-up [26].
Regarding the selected HIV prevention interventions, first, we defined optimistic expansion of SSP in accordance with the World Health Organization’s (WHO) definition of high coverage [38] (200 syringes per PWID per year), with the exception of Seattle (Table 2). Second, we considered scaled-up access to methadone and buprenorphine individually given the different constraints on each modality in the US [39]. We defined the optimistic expansion of MOUD as 40% coverage of treatment with buprenorphine among PWID with an opioid use disorder to reach WHO guidelines on high coverage [38]. Optimistic expansion of MOUD with methadone was derived from the highest annual growth among PWID across the 6 cities [7, 26], thus reaching 40%–55% total MOUD coverage across cities. In addition to the preventive benefit of reducing the number of injections (and therefore shared injections) [37], we incorporated evidence indicating that MOUD decreases the probability of ART discontinuation [40], improves the quality of life [41], and reduces the risk of mortality [42]. Finally, given the uncertainty about PrEP uptake among PWID [43], we assumed no coverage in the status quo and that optimistic expanded access would result in 50% coverage among PWID. The methods and data sources we used to estimate the scale of delivery and the costs of implementing, delivering, and sustaining each intervention were previously described elsewhere [7, 17, 26].
Economic Analysis
We calculated incremental costs (2018 US $) and quality-adjusted life-years (QALYs) for the entire adult population in each city associated with the implementation of evidence-based interventions targeted exclusively to PWID. The cost-effectiveness analysis conformed to best practice guidelines of the Second Panel on Cost-Effectiveness in Health and Medicine, and we used a health care sector perspective, including government, employer-paid, and out-of-pocket health care expenditures [44]. Interventions were sustained for a period of 10 years to match the goals of the Ending the HIV Epidemic initiative with outcomes evaluated over 20 years to capture long-term individual health benefits and second-order transmission effects (ie, prevented cases beyond those directly reached by the interventions). We adhered to best-practice guidelines for health economic evaluation and both costs and QALYs were reported using a 3% annual discount rate [44]. Model-projected outcomes also included new HIV infections averted and we reported reduction in incidence among PWID over a 10-year period.
In addition, we estimated health production functions, representing combination implementation strategies providing the greatest health benefits for a range of investment levels, incremental to the status quo. We followed methodological conventions [45] to estimate incremental cost-effectiveness ratios (ICERs) as the incremental cost per QALY gained for successive optimal combination implementation strategies along the health production function, compared to the next most costly strategy. We identified the strategy producing the greatest health benefits while still remaining cost-effective (highly cost-effective, ICER ≤ 1 × per capita gross domestic product; cost-effective, ICER > 1 to ≤ 3 × per capita gross domestic product) [44].
Sensitivity Analysis
We performed probabilistic sensitivity analysis (using the 2000 best-fitting calibrated parameter sets for each city) on individual interventions and the strategies producing the greatest health benefits while still remaining cost-effective to evaluate the extent of parameter uncertainty. Furthermore, using the selected combination for each city, we assessed the impact on incidence of an ideal implementation scenario, whereby each intervention reached 90% of its target population (Table 2).
We also conducted a scenario sensitivity analysis examining the impact of the changing opioid epidemic in 2 ways. First, we assumed a 40% increase in the PWID population with an opioid use disorder based on the projections of opioid injection prevalence from Chen et al [46]. Second, we accounted for increased mortality risk from the introduction of fentanyl into the illicit drug supply for PWID who were not receiving MOUD by adjusting mortality estimates for each city using state-level evidence of law enforcement encounters testing positive for fentanyl (full details are presented in the Supplementary Material) [47]. Finally, we considered in a separate scenario sensitivity analysis the impact of free PrEP provision (ie, zero PrEP medication costs) in response to recent announcements to this end [48].
RESULTS
Combination Implementation Strategies
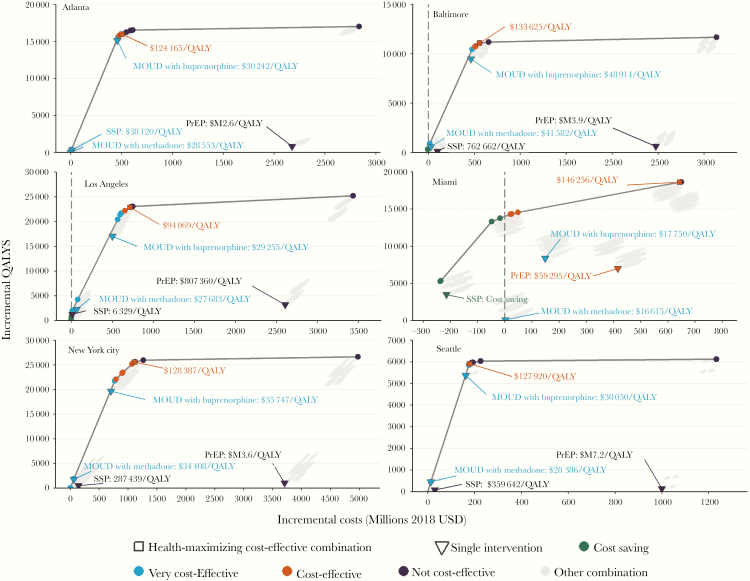
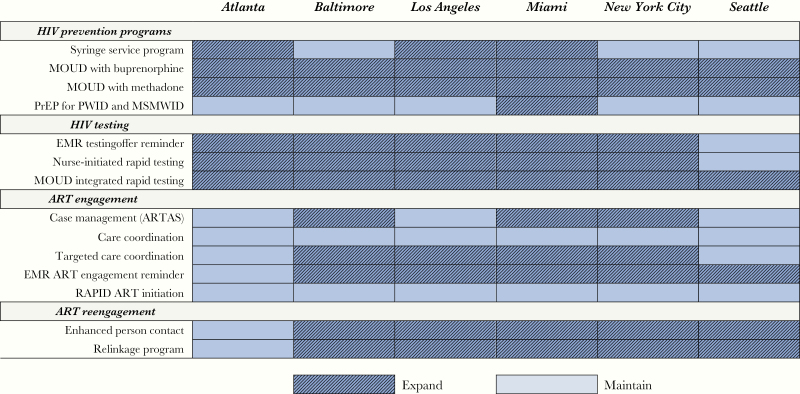
Combination implementation strategies producing the greatest health benefits while remaining cost-effective were composed of between 6 (Atlanta and Seattle) and 12 (Miami) individual interventions (Figure 1 and Figure 2). Among the 5 different combinations (Baltimore and New York City had the same set of interventions), care coordination to improve ART engagement and RAPID (Rapid ART Program for Individuals with an HIV Diagnosis) were not included in any city’s optimal strategy, while expanded access to MOUD (with buprenorphine and methadone) and rapid HIV testing integrated with MOUD were included across all cities. Additional scale-up of SSP was only recommended in cities with lower current syringe distribution levels (highly cost-effective in Atlanta and Los Angeles and cost-saving in Miami), and PrEP for PWID was only included in Miami’s optimal strategy (full results are in the Supplementary Material).
Figure 1.
City-level health production functions for evidence-based prevention and care interventions targeted to persons who inject drugs and men who have sex with men who inject drugs. Abbreviations: MOUD, medication for opioid use disorder; PrEP, preexposure prophylaxis; QALY, quality-adjusted life-year; SSP, syringe service program.
Figure 2.
Interventions composing the health-maximizing cost-effective combinations. Abbreviations: ART, antiretroviral therapy; ARTAS, Antiretroviral Treatment Access Study; EMR, electronic medical records; MOUD, medication for opioid use disorder; MSMWID, men who have sex with men who inject drugs; PrEP, preexposure prophylaxis; PWID, persons who inject drugs; RAPID, Rapid ART Program for Individuals with an HIV Diagnosis.
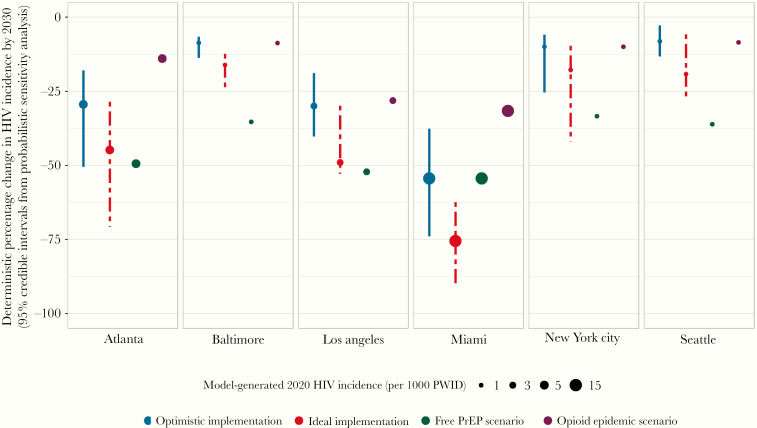
These strategies were estimated to produce QALY gains of between 5914 (95% credible interval [CI], 3791–8312) in Seattle and 25 615 (CI, 17 729–35 736) in New York City, over the 20-year study horizon. We estimated the selected strategies could reduce HIV incidence by between 8.1% (CI, 2.8%–13.2%) in Seattle to 54.4% (37.6%–73.9%) in Miami, by 2030 (Figure 3). Implementing the selected combination strategies at near-ideal levels would result in large reductions in Atlanta, Los Angeles, and Miami (44.8%, 49.0%, and 75.5%, respectively) and Baltimore, New York City, and Seattle reaching 16.1%, 17.7%, and 19.2% reductions, respectively (Figure 3).
Figure 3.
Projected reductions in HIV incidence among persons who inject drugs and men who have sex with men who inject drugs.
Effects of Individual Interventions
Expanding integrated rapid testing with receipt of MOUD was found to be cost-saving in Baltimore, Los Angeles, and Miami, and highly cost-effective in all other cities (Supplementary Table 1). Both the electronic medical records HIV testing reminder and nurse-initiated rapid HIV testing interventions were cost-saving in Baltimore and Miami, and they were either highly cost-effective or cost-effective in every other city with the exception of Seattle. Interventions designed to improve ART engagement and reengagement provided greater value within each city compared to ART initiation interventions. Among these interventions, ART relinkage provided the most value in Atlanta, Los Angeles, and Miami, targeted ART retention in Baltimore and New York City, and ART reinitiation in Seattle. Finally, the ART initiation intervention was only cost-effective in Miami and New York City.
Sensitivity Analysis
The changing opioid epidemic scenario had a profound impact on the projections and the increased mortality among PWID living with HIV resulted in 2030 status quo incidence that was now projected to be lower by 6.1% (Miami) to 19.6% (Baltimore). As a result of the lower prevalence of PWID living with HIV, strategies producing the greatest health benefits while remaining cost-effective achieved more modest incidence reductions, ranging from 8.7% in Baltimore to 31.6% in Miami. Strategies for Baltimore, Los Angeles, New York City, and Seattle included the same set of interventions, whereas expansion of SSP in Atlanta and PrEP in Miami were no longer included despite remaining cost-effective when evaluated individually. Finally, the provision of free PrEP resulted in incidence reductions that now ranged from 33.4% in New York City to 52.2% in Los Angeles, and Miami remained unchanged at 54.4% (Figure 3; full results are in the Supplementary Material).
DISCUSSION
Results from this simulation study of 6 US cities with diverse microepidemics suggest that distinct combinations of evidence-based interventions targeted to PWID were required to produce the greatest public health impact in each setting. In no city would the combination that maximized health benefits while remaining cost-effective according to international standards completely eliminate new HIV infections among PWID. Nevertheless, optimistic expansion of targeted, locally oriented strategies could achieve greater decreases in the burden of HIV in cities with relatively higher rates of new infections, with reductions in HIV incidence among PWID by 2030 ranging from 29.4% in Atlanta to 54.4% in Miami. In addition, these combinations could prevent resurgence in cities that have maintained low levels of HIV incidence among PWID and result in incidence below 1 new HIV infection per 1000 PWID in Baltimore, New York City, and Seattle.
Opioid-related harms continue to be a major public health concern in the United States and the immediate and life-long improvements in the quality of life from expanded access to MOUD has the potential to provide considerably more health benefits (measured in QALYs) to PWID than any other intervention. Whereas there are clear similarities between New York City and Baltimore (earlier epicenters of the epidemic among PWID driven by opioids) and cities like Miami, Los Angeles, and Seattle (featuring more injection of stimulants) our findings suggested that the substantial value provided by expanded access to MOUD was robust in the context of different settings with respect to injection drug use. With 1 in 4 American with an opioid use disorder receiving any care and less than a third of those in care receiving MOUD (or as low as 8% among PWID living with HIV) [49], access to evidence-based treatment has not kept pace with the increasing problems associated with the opioid epidemic in the United States [50, 51].
There has been a strong consensus among communities of injection drug users (and the scientific community) that the implementation of PrEP for PWID should only be considered together with widespread access to comprehensive, low-threshold HIV prevention and care [33, 52]. In agreement with prior US-based modelling studies [12, 14], our results indicate that the large incremental costs and modest additional health benefits of expanding PrEP among PWID across cities (eg, clusters on the right in Figure 1) did not provide sufficient value at current prices to be included in each distinct strategy. Miami offers an important counterexample. With an HIV epidemic featuring relatively higher transmission rates among MSM, PrEP provided a comparatively greater public health benefit than in other cities. Furthermore, the expansion of SSP services in Miami resulted in important cost-savings that offset a large portion of the PrEP expansion costs in the chosen health-maximizing strategy. Naturally, there is the potential to achieve greater reductions in HIV incidence when PWID have access to PrEP, as highlighted by our free PrEP sensitivity analysis. Potential price reductions from generics or following the recent approval of a new PrEP formulation by the US Food and Drug Administration [53] may offer opportunities to improve the cost-effectiveness of providing PrEP to PWID. Nevertheless, using PrEP remains an individual choice, with adherence greatly determining its efficacy. Access to this biomedical intervention needs to be considered in the context of criminalization of persons who use drugs and structural barriers to HIV prevention and care that could potentially diminish the effectiveness of PrEP among PWID. Additionally, it is important to emphasize in the context of recommendations to offer PrEP to all persons at high risk of HIV acquisition [15] that a large proportion of PWID living with HIV have yet to fully benefit from ART as treatment and prevention [49].
Recent trends in the diagnosis of PWID living with HIV have shown promise [54] yet ART engagement among those diagnosed has stalled [49, 54]. Sustained viral suppression is necessary for reducing HIV transmission risk [55], and as our analysis suggests, additional funding to improve ART engagement among PWID and to reengage those who have discontinued treatment may be well justified across most settings. These findings were consistent with previous studies noting poorer retention [56], lower probability of ART initiation [57], and reinitiation that varied across geographic regions [18] and lower rates of viral suppression for PWID relative to non-PWID [49]. There have been promising examples of reducing disparities in viral suppression rates [58]. Nonetheless, multidimensional public health strategies addressing stigma and broader social determinants of health such as the lack of fulfillment of basic needs (food, housing, and education) will be necessary to achieve and maintain undetectable viral loads among the most vulnerable communities, and ultimately stop the spread of HIV.
We have previously outlined limitations in the structure of the model and its evidence base [7, 17]. Our analysis had other limitations. First, our model was calibrated and validated using historical data and may not capture changing HIV outbreaks among PWID that are most likely indicative of emerging patterns of drug use, vulnerability, and injection behavior [59, 60]. Our sensitivity analysis on the changing opioid epidemic allowed us to assess the robustness of our results when accounting for both changing injection drug use prevalence and associated risks. Second, we did not explicitly account for the variation in injection frequency or sexual risk networks among subgroups using different substances [61]. Nonetheless, we accounted for average behavior among all PWID and conducted probabilistic sensitivity analysis on all relevant parameters, determining the value of different strategies at the population level. Lastly, we only captured HIV prevention benefits from SSP. Incorporating broader health benefits from HCV and overdose prevention would likely result in assessments of greater value even for well-resourced cities.
In conclusion, evidence-based interventions targeted to PWID can deliver considerable value; however, ending the HIV epidemic among PWID will require innovative implementation strategies and supporting programs to reduce social and structural barriers to care.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Localized HIV Modeling Study Group members. Czarina N. Behrends, Carlos Del Rio, Julia C. Dombrowski, Daniel J. Feaster, Kelly A. Gebo, Brandon D. L. Marshall, Shruti H. Mehta, Lisa R. Metsch, Bohdan Nosyk, Ankur Pandya, Bruce R. Schackman, and Steffanie A. Strathdee, (authors); Matthew Golden, Department of Medicine, Division of Allergy and Infectious Disease, University of Washington, and Director, HIV/STD Program, Public Health, Seattle and King County, WA; Gregory Kirk, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD; Julio Montaner, BC Centre for Excellence in HIV/AIDS, University of British Columbia, Canada; Steven Shoptaw, Centre for HIV Identification, Prevention and Treatment Services, School of Medicine, University of California Los Angeles, CA.
Author contributions. E. K. and B. N. conceptualized the study and wrote the first draft. E. K. and X. Z. conducted analyses. E. K., X. Z., and B. E. contributed to the evidence synthesis. B. E. contributed to manuscript development. B. N. secured funding for the study. All authors aided in the interpretation of results, provided critical revisions to the manuscript, and approved the final draft.
Disclaimer. The funders had no direct role in the conduct of the analysis or the decision to submit the manuscript.
Financial support. This work was supported by the National Institutes of Health, National Institute on Drug Abuse (grant numbers R01DA041747; P30DA040500 from the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV to B. R. S.; and R37DA019829 Method to Extend Research in Time award to S. A. S.).
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. J. C. D. has participated in research supported by grants to the University of Washington from Hologic. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Localized HIV Modeling Study Group:
Czarina N Behrends, Carlos Del Rio, Julia C Dombrowski, Daniel J Feaster, Kelly A Gebo, Brandon D L Marshall, Shruti H Mehta, Lisa R Metsch, Bohdan Nosyk, Ankur Pandya, Bruce R Schackman, Steffanie A Strathdee, Matthew Golden, Gregory Kirk, Julio Montaner, and Steven Shoptaw
References
- 1. Reddon H, Marshall BDL, Milloy MJ. Elimination of HIV transmission through novel and established prevention strategies among people who inject drugs. Lancet HIV 2019; 6:e128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 3. Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet 2010; 376:285–301. [DOI] [PubMed] [Google Scholar]
- 4. Nosyk B, Zang X, Min JE, et al. Relative effects of antiretroviral therapy and harm reduction initiatives on HIV incidence in British Columbia, Canada, 1996–2013: a modelling study. Lancet HIV 2017; 4:e303–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. HIV Surveillance Report, 2017; 29 Published 2018. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 8 January 2020. [Google Scholar]
- 6. Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 2014; 9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krebs E, Enns B, Wang L, et al. ; localized HIV modeling study group Developing a dynamic HIV transmission model for 6 U.S. cities: An evidence synthesis. PLoS One 2019; 14:e0217559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strathdee SA, Beyrer C. Threading the needle–how to stop the HIV outbreak in rural Indiana. N Engl J Med 2015; 373:397–9. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the US Department of Health and Human Services. MMWR Morb Mortal Wkly Rep 2012; 61:1–40. [PubMed] [Google Scholar]
- 10. Schackman BR, Leff JA, Polsky D, Moore BA, Fiellin DA. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med 2012; 27:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol 2014; 18:2144–55. [DOI] [PubMed] [Google Scholar]
- 12. Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med 2017; 14:e1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krebs E, Enns B, Evans E, et al. Cost-effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med 2018; 168:10–9. [DOI] [PubMed] [Google Scholar]
- 14. Bernard CL, Brandeau ML, Humphreys K, et al. Cost-effectiveness of HIV preexposure prophylaxis for people who inject drugs in the United States. Ann Intern Med 2016; 165:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owens DK, Davidson KW, Krist AH, et al. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA 2019; 321:2203–13. [DOI] [PubMed] [Google Scholar]
- 16. Panagiotoglou D, Olding M, Enns B, et al. ; Localized HIV Modeling Study Group Building the case for localized approaches to HIV: structural conditions and health system capacity to address the HIV/AIDS epidemic in six US cities. AIDS Behav 2018; 22:3071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zang X, Krebs E, Min JE, et al. ; Localized HIV Modeling Study Group Development and calibration of a dynamic HIV transmission model for 6 US cities. Med Decis Making 2020; 40:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Krebs E, Min JE, et al. ; HIV Research Network Combined estimation of disease progression and retention on antiretroviral therapy among treated individuals with HIV in the USA: a modelling study. Lancet HIV 2019; 6:e531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tempalski B, Pouget E, Cleland C, et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS One 2013; 8:e64789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. HIV infection, risk, prevention, and testing behaviors among persons who inject drugs—national HIV behavioral surveillance: injection drug use, 20 U.S. cities, 2012. HIV surveillance special report; 11. Revised ed published 2015. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 27 March 2020. [Google Scholar]
- 21. Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men—national HIV behavioral surveillance, 20 U.S. cities, 2014. HIV surveillance special report; 15. Revised ed published 2016. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 27 March 2020. [Google Scholar]
- 22. Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American community survey. JMIR Public Health Surveill 2016; 2:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nosyk B, Zang X, Krebs E, et al. Ending the epidemic in America will not happen if the status quo continues: modeled projections for HIV incidence in 6 US cities. Clin Infect Dis 2019; 69:2195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Compendium of evidence-based interventions and best practices for HIV prevention https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html. Accessed 27 March 2020.
- 26. Krebs E, Zang X, Enns B, et al. The impact of localized implementation: determining the cost-effectiveness of HIV prevention and care interventions delivered across six United States cities. AIDS 2020; 34:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felsen UR, Cunningham CO, Heo M, Futterman DC, Weiss JM, Zingman BS. Expanded HIV testing strategy leveraging the electronic medical record uncovers undiagnosed infection among hospitalized patients. J Acquir Immune Defic Syndr 2017; 75:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anaya HD, Hoang T, Golden JF, et al. Improving HIV screening and receipt of results by nurse-initiated streamlined counseling and rapid testing. J Gen Intern Med 2008; 23:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metsch LR, Feaster DJ, Gooden L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health 2012; 102:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 2005; 19:423–31. [DOI] [PubMed] [Google Scholar]
- 32. Robertson MM, Waldron L, Robbins RS, et al. Using registry data to construct a comparison group for programmatic effectiveness evaluation: the New York City HIV care coordination program. Am J Epidemiol 2018; 187:1980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robbins GK, Lester W, Johnson KL, et al. Efficacy of a clinical decision-support system in an HIV practice: a randomized trial. Ann Intern Med 2012; 157:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a us public health setting. J Acquir Immune Defic Syndr 2017; 74:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis 2014; 59:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bove JM, Golden MR, Dhanireddy S, Harrington RD, Dombrowski JC. Outcomes of a clinic-based surveillance-informed intervention to relink patients to HIV care. J Acquir Immune Defic Syndr 2015; 70:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users–2012 revision. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 39. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet 2019; 393:1760–72. [DOI] [PubMed] [Google Scholar]
- 40. Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis 2016; 63:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song DL, Altice FL, Copenhaver MM, Long EF. Cost-effectiveness analysis of brief and expanded evidence-based risk reduction interventions for HIV-infected people who inject drugs in the United States. PLoS One 2015; 10:e0116694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nosyk B, Min JE, Evans E, et al. The effects of opioid substitution treatment and highly active antiretroviral therapy on the cause-specific risk of mortality among HIV-positive people who inject drugs. Clin Infect Dis 2015; 61:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guise A, Albers ER, Strathdee SA. ‘PrEP is not ready for our community, and our community is not ready for PrEP’: pre-exposure prophylaxis for HIV for people who inject drugs and limits to the HIV prevention response. Addiction 2017; 112:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316:1093–103. [DOI] [PubMed] [Google Scholar]
- 45. Hunink MM, Weinstein MC, Wittenberg E, et al. Decision making in health and medicine: integrating evidence and values. Cambridge, UK: Cambridge University Press, 2014. [Google Scholar]
- 46. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open 2019; 2:e187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Opioid overdose: fentanyl encounters data https://www.cdc.gov/drugoverdose/data/fentanyl-le-reports.html. Accessed 16 July 2019.
- 48. Department of Health and Human Services. News release: Trump Administration secures historic donation of billions of dollars in HIV prevention drugs https://www.hiv.gov/blog/news-release-trump-administration-secures-historic-donation-billions-dollars-hiv-prevention. Accessed 9 May 2019.
- 49. Dasgupta S, Tie Y, Lemons A, Wu K, Burnett J, Shouse RL. Injection practices and sexual behaviors among persons with diagnosed HIV infection who inject drugs—United States, 2015–2017. MMWR Morb Mortal Wkly Rep 2019; 68:653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health 2015; 105:e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff (Millwood) 2019; 38:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marshall BD, Milloy MJ. Improving the effectiveness and delivery of pre-exposure prophylaxis (PrEP) to people who inject drugs. Addiction 2017; 112:580–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. US Food and Drug Administration. FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic https://www.hiv.gov/blog/fda-approves-second-drug-prevent-hiv-infection-part-ongoing-efforts-end-hiv-epidemic. Accessed 9 October 2019.
- 54. Kim N, Welty S, Reza T, Sears D, McFarland W, Raymond HF. Undiagnosed and untreated HIV infection among persons who inject drugs: results of three national HIV behavioral surveillance surveys, San Francisco, 2009–2015. AIDS Behav 2019; 23:1586–9. [DOI] [PubMed] [Google Scholar]
- 55. Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA 2019; 321:451–2. [DOI] [PubMed] [Google Scholar]
- 56. Rebeiro PF, Gange SJ, Horberg MA, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Geographic variations in retention in care among HIV-infected adults in the United States. PLoS One 2016; 11:e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 2013; 56:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doshi RK, Milberg J, Jumento T, Matthews T, Dempsey A, Cheever LW. For many served by the Ryan White HIV/AIDS Program, disparities in viral suppression decreased, 2010–14. Health Aff (Millwood) 2017; 36:116–23. [DOI] [PubMed] [Google Scholar]
- 59. Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, Arasteh K. HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS 2016; 30:815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Golden MR, Lechtenberg R, Glick SN, et al. Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs—Seattle, Washington, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chapin-Bardales J, Masciotra S, Smith A, et al. Characteristics of persons who inject drugs with recent HIV infection in the United States: national HIV behavioral surveillance, 2012. AIDS Behav 2019; 23:3277–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.