Abstract
We report the fabrication of a tubular polydimethylsiloxane (PDMS) platform containing arrays of small pores on the wall for modeling blood vessels in vitro. The thin PDMS tubes are produced following our previously reported templating approach, while the pores are subsequently generated using focused laser ablation. As such, when these perforated PDMS tube are populated with a monolayer of endothelial cells on the interior surfaces and embedded within an extracellular matrix (ECM)-like environment, the endothelial cells can sprout out from the tubes into the surrounding matrix through the open pores. When a pair of perforated PDMS tubes are placed in parallel in the matrix, formation of an interconnected network of microvasculature or larger vessels occurs, which is dependent on the flow dynamics within the PDMS tubes. Moreover, when co-cultured with tumor spheroids, the onset of tumor angiogenesis is observed. Our perforated and endothelialized PDMS tubes are believed to enable convenient vascular modeling in vitro and will likely contribute to improved biological studies as well as therapeutic screening.
Keywords: polydimethylsiloxane (PDMS), endothelial cells, vascularization, tumor spheroids, vascular model
Graphical Abstract

A tubular polydimethylsiloxane (PDMS) platform containing arrays of perforations generated using focused laser ablation, is developed for modeling blood vessels in vitro. When populated with endothelial cells on the interior surfaces and embedded within an extracellular matrix, the endothelial cells can sprout out from the tubes into the surrounding matrix through the open pores, emulating the vascularization process.
1. Introduction
Blood vessels constitute one of the most important parts of the human system by providing routes to deliver oxygen and nutrients towards all tissues while transporting wastes away, maintaining homeostasis of the body [1, 2]. Vasculogenesis is also heavily involved in many other processes such as regeneration, inflammation, and cancer progression, where chemokines are secreted by the local disease microenvironments to induce the formation of new blood vessels [3–5].
To faithfully reproduce the healthy and diseased tissues outside the human body therefore requires incorporation of the vascular system, which allows for generation of thick engineered tissues, studying vascular remodeling during disease states, and screening therapeutic agents at improved accuracy. To this end, microfluidics-based platforms possess unique advantages by providing precision in manipulating the size and pattern of microscale hollow channels that can be created through, most often, soft lithography [6]. For example, microfluidic chips containing multiple parallel microchannels have been widely utilized to produce in vitro models of the vasculature [7–12]. The endothelial cells when seeded into these microchannels, would form a confluent monolayer and could interact with the biomimetic microenvironments in the side compartments (such as those representing normal or cancerous tissues), presenting capacity in studying the angiogenesis process.
Nevertheless, the conventional microfabrication strategy using soft lithography for creating the multiscale microfluidic chips requires dedicated instrumentation that is not broadly accessible. To address this challenge, we have previously reported a simple templating method that enabled convenient production of elastomeric hollow tubes [13]. These hollow tubes made of polydimethylsiloxane (PDMS) could be readily populated with a monolayer of endothelial cells on their interior surfaces, and the oxygen permeability of PDMS would facilitate the long-time viability and functionality of the endothelia. These endothelialized PDMS tubes, when further used to link the different organ-on-a-chip components, could then accurately mimic the vascular interconnections between the various tissues with desired biological activities as they do in the human body, to facilitate drug testing on these tubular vascular models.
Built upon this work, here we have further minimized the sizes of these elastomeric PDMS tubes to the microscale at below 1 mm in diameter. We sought to demonstrate that, by perforating a tube with small pores, populating the interior surface with endothelial cells, and embedding it in a hydrogel matrix, we would be able to induce outward sprouting of the endothelial cells from the tube through the pores forming an interconnected vascular network (Figure 1). After probing the flow effects on the pattern of angiogenesis, we finally investigated the feasibility of using such perforated PDMS vessels to study breast tumor vascularization. We anticipate that our unique vascular model of hollow PDMS tubes containing small-sized perforations to serve as a class of simple yet enabling in vitro vascular models to investigate tissue angiogenesis for studying biology and screening therapeutic agents.
Fig. 1.
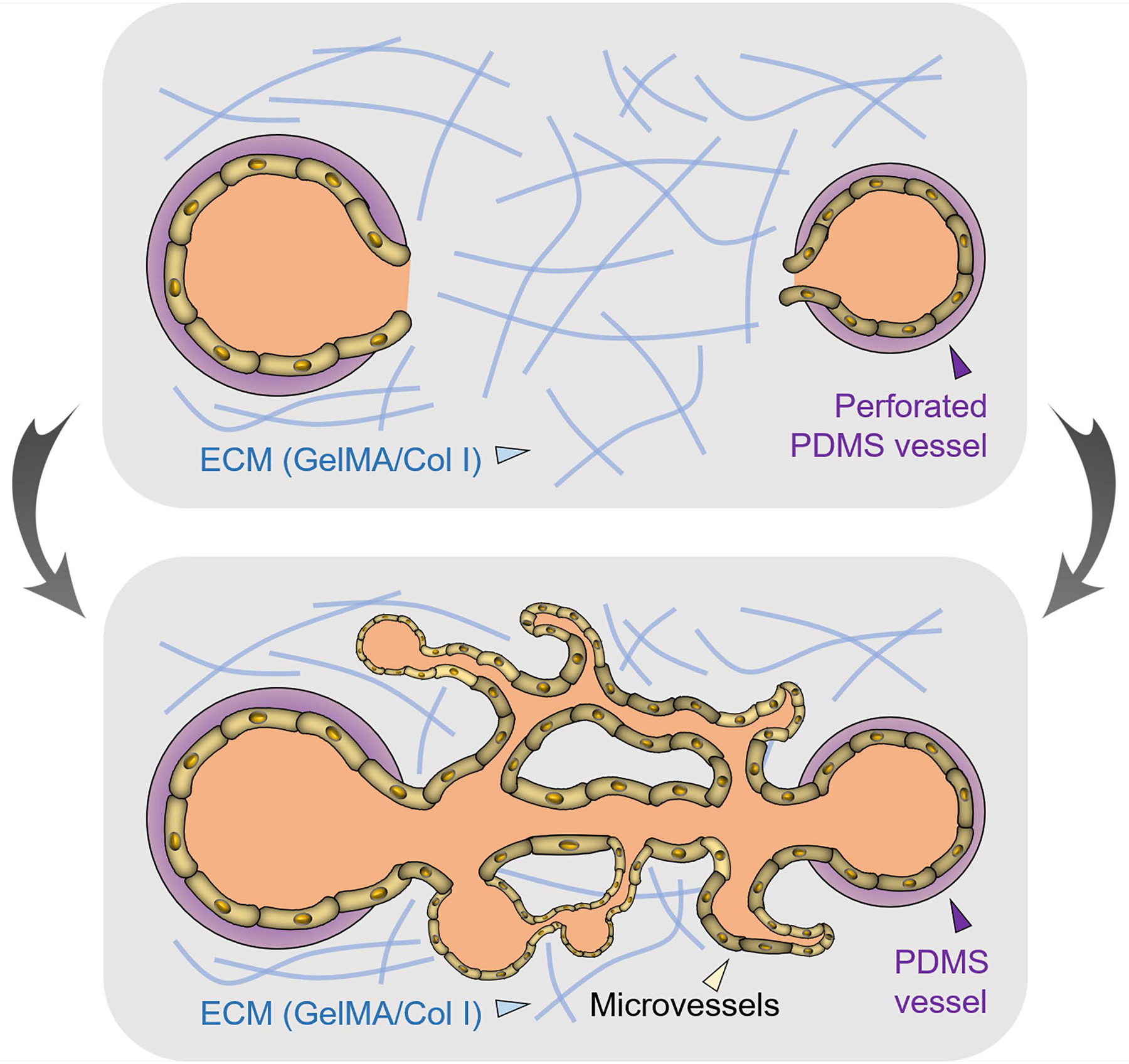
Schematic showing the use of perforated, endothelialized PDMS tubes for modeling vasculariztion in vitro.
2. Results and Discussions
The elastomeric PDMS tubes were fabricated using a templating strategy by modifying our previously reported protocol (Figure 2A). To shrink the size of the tubes as compared with those in our previous work, we used a customized tube rolled from an aluminum foil (600 μm in inner diameter (ID)) as the exterior template. Subsequently, PDMS precursor was infused into the space between the interior/exterior templates, where a 27G needle (400 μm in outer diameter (OD)) was inserted and centered at the middle of the aluminum foil tube serving as the exterior template. The setup was allowed to cure at room temperature for 24 h under constant rotation. After polymerization, the exterior template of the aluminum tube was carefully cut open through an incision, followed by careful removal of the interior template of the needle. Finally, the obtained PDMS tube was perforated by focused laser ablation using a laser cutting system, generating small-sized pores of 241.44 ± 83.22 μm in diameter (Figure 2B). Scanning electron micrographs clearly demonstrated the formation of circular pores on the wall of the PDMS tube (Figure 2C). Although the edges of the pores were not smooth due to the burning of the PDMS during laser cutting, it did not seem to affect outward endothelial sprouting from the tubes through these pores as we demonstrated below. The PDMS tube was self-standing, could be filled with medium, and the array of small pores on the surface did not affect the hydraulic tightness of the tube under static conditions (Figure 2D), making it possible to seed the perforated tubes with endothelial cells using a simple incubation method. By selecting the size of the templates and the laser parameters, it was further possible to produce perforated tubes featuring varying parameters such as the outer diameter, inner diameter, and pore size (Figure S1A).
Fig. 2.

The perforated PDMS tubes. (A) Schematics showing the procedure for fabrication of perforated PDMS tubes. (B) Quantification of size distribution of laser-ablated pore on the PDMS tubes. N = 26. (C) SEM image showing a laser-ablated pore on a PDMS tube. (D) Photograph showing a thin-walled PDMS tube with an array of perforated holes, filled with a dye solution.
Indeed, we populated the interior surface of the perforated PDMS tubes with a layer of human umbilical vein endothelial cells (HUVECs) by injecting a suspension of endothelial cells in culture medium at a density of 1 × 107 cells mL−1. The tubes were maintained under constant right-angled rotation along the circumference for up to 2 h to ensure seeding uniformity as the cells sedimented to attach to the PDMS walls during this period. The cell retention efficiency was close to 100% due to the small sizes of the pores and surface tension that prevented leakage of the cell suspension. The HUVECs homogeneously attached to the PDMS tubes (Figure 3A) and showed high viability for up to 7 days tested (Figure 3B). Moreover, the cell numbers were quantified for up to 14 days of culture and it was found that the HUVECs steadily proliferated over time (Figure 3C,D), in agreement with our observations before in PDMS tubes without the pores [13]. After 14 days of culture, it was clear from the F-actin/nuclei staining images that, the HUVECs became confluent on the interior surface of the perforated PDMS tubes, including the regions closely surrounding the pores (Figure 3D), facilitating angiogenesis through the pores when the tubes were embedded in a biomimetic tissue-like microenvironment. Confocal orthogonal views further indicated strong expression of CD31 for confluent endothelium (Figure 3E). Perforated PDMS tubes of other sizes could be conveniently populated with a layer of endothelial cells as well, using the same seeding method (Figure S1B).
Fig. 3.

Endothelialization of the perforated PDMS tubes. (A, B) Fluorescence micrographs showing the proliferation of HUVECs in the perforated PDMS tube during a 6-day culture period. (C) Quantification of numbers of HUVECs during the 14-day culture. Data are presented as mean ± standard deviation (STD); N = 6. (D) Cytoskeleton characterization of the endothelialized, perforated PDMS tubes at low and high magnifications. (E) Confocal orthogonal views showing the expressions of CD31 of the formed confluent endothelium.
As we anticipated, when we encapsulated a pair of the perforated and endothelialized PDMS tubes within a hydrogel matrix composed of gelatin methacryloyl (GelMA) and collagen type I (4:1 volume ratio) under static culture conditions, HUVECs were observed to gradually invade from the PDMS tubes through the array of pores to form an interconnected network of microvessels in between the two tubes (Figure 4A). After 7 days of angiogenesis, the microvasculature reached a high density covering approximately 55% of the area (Figure 4B), creating a multi-scale vascular network extending from the PDMS tubes at the size range of a few hundred micrometers to the spontaneously sprouted microvessels of a few tens of micrometers in sizes. This strategy is potentially useful in modeling the vascularized tissues that contain networked blood vessels across multiple size scales, where a secondary cell type may be encapsulated in the hydrogel matrices for the purpose [14, 15]. Collagen type I was used in this demonstration as a naturally derived extracellular matrix (ECM) molecule, while GelMA, a photocrosslinkable form of gelatin (degradation product of collagen containing intrinsic RGD moieties), was further adopted as another component of the matrix for on-demand gelation of the matrix [16–18] to conveniently encapsulate the perforated PDMS tubes.
Fig. 4.

Microvasculature formation under static culture. (A) Fluorescence micrographs showing microvascularization between a pair of perforated PDMS tubes through endothelial sprouting under static culture conditions. (B) Quantification of vascularity. Data are presented as mean ± standard deviation (STD); N = 3.
Interestingly, when we switched from static to dynamic culture conditions by perfusing culture medium through one of the perforated PDMS tubes in the pair during the sprouting process, we found that the angiogenesis pattern was altered. During the same 7-day culture period, instead of forming the microvascular network in between the two PDMS tubes, larger and interconnected blood vessels appeared as early as Day 3 and gradually remodeled (Figure 5A). This observation is in accordance with literature reporting that dynamic cultures featuring increased shear stress and fluid pressures enhance vascularization in vitro, and that interstitial pressure gradient might assist the endothelial sprouting and maturation in similar microfabrication setups [19].
Fig. 5.

Larger vessel formation under flow culture. (A) Fluorescence micrographs showing formation of larger vessels between a pair of perforated PDMS tubes under flow culture. (B) Fluorescence micrographs showing endothelial biomarker expressions by the formed vessels. (C) Confocal micrographs showing the hollow lumen structure of the formed vessels.
We also confirmed that these formed larger vessels connecting the two PDMS tubes could express the endothelial biomarkers including basement membrane proteins such as collagen type IV and laminin, as well as intercellular junction proteins such as CD31 and vascular endothelial (VE)-cadherin (Figure 5B). In addition, the formed vascular interconnections were proven to be hollow possessing lumen structures, as indicated in confocal projection and reconstruction images (Figure 5C). Formation of functional, lumen-containing vessels is critical in ensuring fluid transport across this vascular network interconnecting the pair of perforated PDMS tubes. Our capacity to control the pattern of vascular sprouting through altering the perfusion profiles within the PDMS tubes enables us to generate models of blood vessels of different scales to suit desired applications.
We finally investigated the feasibility of the perforated, endothelialized PDMS tubes for applications in studying tumor angiogenesis. To this end, we produced breast tumor spheroids using MCF-7 breast cancer cells using a standard hanging drop method [20]. The non-adhesive nature of the PDMS wells allowed for formation of uniform spheroids possessing diameters in the range of 500–800 μm. Both the area and the circularity of the MCF-7 spheroids (calculated from projection images) increased over the 7-day culture in the GelMA/collagen type I hydrogel matrix (Figure 6A, B), with the area centered at 0.66 ± 0.28 mm2 and the circularity at 0.77 ± 0.17 at Day 7 (Figure 6C, D). The MCF-7 breast cancer cells were also observed to migrate into the surrounding matrix away from the spheroids along with the growth in the spheroid size, indicating their invasiveness (Figure 6E).
Fig. 6.

Tumor spheroid angiogenesis from perforated PDMS tubes. (A, B) Areas and circularities of the MCF-7 breast tumor spheroids at different time points of culture in the GelMA/collagen I matrix. Data are presented as mean ± standard deviation (STD); N = 16 (Day 3), 21 (Day 4), 21 (Day 5), 20 (Day 6), and 15 (Day 7). Spheroids were not formed until Day 3. (C, D) Area and circularity distributions of the MCF-7 breast tumor spheroids at Day 7. (E) Morphologies of the MCF-7 breast tumor spheroids and outward migration of the MCF-7 cells at Day 1 and Day 7. (F) VEGF secretions by the MCF-7 breast tumor spheroids at different time points of the culture, either in the absence or in the presence of the perforated, endothelialized PDMS tube. Data are presented as mean ± standard deviation (STD); N = 3. (G) Directional endothelial cell migration from the perforated PDMS tube towards the MCF-7 breast tumor spheroid at different time points of culture in the GelMA/collagen I matrix, under static conditions. HUVECs were GFP-labeled. The autofluorescence from the MCF-7 spheroid might be attributed to its high cell density.
Furthermore, the levels of vascular endothelial growth factor (VEGF) secreted by the MCF-7 spheroids were measured using ELISA, which revealed significant elevation of concentration during the 7-day culture (Figure 6F) likely due to the size increase of the spheroids necessitating angiogenesis to occur to alleviate oxygen deficiency. As a result, when we co-encapsulated the MCF-7 spheroid with a perforated and endothelialized PDMS tube in close proximity together in the ECM-like matrix, we observed outward migration of the GFP-HUVECs from the PDMS tube through the pore nearby the spheroid, gradually reaching the body of the spheroid after 5–7 days (Figure 6G). This directional migration of endothelial cells to assume tumor angiogenesis should be attributed to the VEGF secretions by the MCF-7 spheroid, which showed a similar trend with the spheroids cultured alone (Figure 6F). Meanwhile, the outward invasion of the MCF-7 cells from the spheroid was also present during the period of culture, similar to when the spheroids were embedded in the matrices alone (Figure 6E). The tumor angiogenesis assay further proved the versatility of our perforated and endothelialized PDMS tube platform for use as in vitro vascular models under various scenarios.
3. Conclusions
In summary, we have demonstrated a unique platform for in vitro vascular modeling, based on the fabrication of elastomeric PDMS tubes containing arrays of small-sized pores on the walls. These perforated PDMS tubes could be subsequently functionalized with a monolayer of endothelium in their interior surfaces, similar to their counterparts that are smooth without pores as we previously reported. These perforated PDMS tubes are easy to produce, and may be widely used in a range of applications relating to vascular modeling in vitro, including but not limited to those demonstrated in the current work, i.e., as standalone vessels, formation of interconnected vascular networks in a tissue-like matrix, and cancer angiogenesis. We anticipate these endothelialized and perforated PDMS tubes to play important roles in modeling the morphology and function of the blood vessels that will potentially enable vascular biology studies and therapeutic screening with improved accuracy. However, systematical characterizations of the formed vascular networks still need to be performed to understand their biological and physiological relevance.
4. Experimental Section
Hydrogel matrix preparations
The composite hydrogel used as the ECM in the devices was made of 75 vol.% 1-mg mL−1 type I collagen and 25 vol.% GelMA (1% methacrylyol substitution). Gelatin, methacrylic anhydride, and 2-hydroxy-4’-(2-hydroxyethoxy)-2-methylpropiophenone (photoinitiator, PI), Dulbecco’s phosphate-buffered saline (DPBS) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. GelMA was synthesized according to our previously published protocol [21–23], and used in the presence of 0.5 w/v% PI. The 1-mg mL−1 rat tail type I collagen (Corning, Corning, NY, USA) was prepared at pH 7.0 according to the manufacturer’s instructions and maintained on ice until use. Both hydrogel solutions were mixed only shortly before being added into the devices.
Cells
HUVECs and HUVECs expressing GFP (Angio-Proteomie, Boston, MA, USA) were cultured in 75-cm3 flasks in endothelial growth medium (EGM, Lonza, Basel, Switzerland) with 10 vol.% fetal bovine serum (FBS, Thermo Fisher, Waltham, MA, USA) and 1 vol.% penicillin-streptomycin (pen/strep, Thermo Fisher). HUVECs were passaged twice a week and used until passage 8.
MCF-7 breast cancer cells were cultured in Dulbecco’s modified Eagle medium (DMEM, 4.5 g L−1 D-glucose, with L-glutamine and without sodium pyruvate, Thermo Fisher) containing 10 vol.% FBS and 1 vol.% pen/strep. Cells were passaged twice per week in 75-cm3 flasks. MCF-7 spheroids were produced following the hanging drop method [20] in 30-μL droplets at a cell concentration of 2 × 106 cells mL−1 in DMEM. The spheroids were cultured in the hanging droplets for 7 days before harvest and being encapsulated in the hydrogels in the devices.
Perforated PDMS tubes
PDMS tubes of 0.4-mm inner diameter and 0.6-mm outer diameter were produced according to a previously published protocol [13] with modification. The PDMS (Sylgard 184 Silicon Elastomer Kit, Dow Corning, Midland, MI, USA) at a ratio 10:1 (wt.%, monomer: curing agent) was injected into a custom-made aluminum foil tube (600 μm in diameter) serving as the exterior template, where a 27G needle (400 μm in outer diameter) was then inserted into the center serving as the interior template. The setup was allowed to cure at room temperature for 24 h on a rotational stand at a speed of 3 rpm. Afterwards, the aluminum foil tube was slowly cut open using a blade followed by careful removal of the interior needle template to retreive the PDMS tube. The perforations were made using a laser cutter (VLS2.30, Universal Laser Systems, Scottsdale, AZ, USA) set at a speed between 4–5% (6–8 cm s−1) and a power between 14–15% (3.5–3.75 W).
The PDMS tubes were autoclaved for sterilization. The interior surfaces of the PDMS tubes were then coated with fibronectin (fibronectin from human plasma, Sigma-Aldrich) at a concentration of 50 μg mL−1. The fibronectin solution was left to incubate for 30 min at 37 °C. The PDMS tubes were subsequently washed with EGM and HUVECs were seeded at a cell density of 1 × 107 cells mL−1. The tubes containing the suspensions of HUVECs in EGM were maintained under constant right-angled rotation along the circumference for up to 2 h as the cells sedimented to attach to the PDMS walls during this period. Alternatively, the PDMS tubes could be placed in the incubator and flipped every 30 min for 2–4 times to enable uniform cell coating. We did not find noticeable difference between the two seeding methods. Finally, the PDMS tubes were gently rinsed from the interior to remove non-adherent cells, and transferred to multi-well plates for culture.
Device assembly
A square PDMS chamber of 1-cm side length was bonded to a glass coverslip, and a pair of two 1-cm long perforated PDMS tubes were inserted in the chamber placed 0.5- or 1-mm apart at a 1-mm height. The tubes were connected by stainless steel connectors (SC27/8, Stainless steel catheter coupler, 27G × 8 mm, Instech, Plymouth Meeting, PA, USA) to 2-cm long Teflon tubing (#30 AWG thin wall tubing, Microbore PTFE tubing, 0.012” ID × 0.030” OD, Cole-Parmer, Vernon Hills, IL) when perfusion was performed. Assembled devices were autoclaved prior to experiments.
Then, 300 μL of the composite hydrogel was poured in the device. The devices were then placed in the incubator at 37 °C for 30 min for collagen polymerization and afterwards UV-exposed for 20 s at 850 mW cm−2 at a distance of 1 cm to enable photocrosslinking of GelMA. The PDMS tubes were seeded with HUVECs as described above, and 200 μL of EGM was placed in the device on top of the hydrogel for culture. The medium was changed daily. In the case of tumor angiogenesis, one MCF-7 spheroid was embedded into the hydrogel matrix prior to its gelation, while other procedures were all maintained the same.
Perfusion setup
When needed, at 1 day following HUVEC seeding one PDMS tube of each device was placed under flow to create the directional interstitial pressure gradient, which was previously reported to aid vascular sprouting and maturation [19]. Syringes pumps (PHD 2000, Infuse/withdraw PHD2000 dual syringe pump, Harvard Apparatus, Holliston, MA, USA) were used to apply a flow rate of 10 μL min−1 for the perfusion of EGM.
Staining
At desired time points, the cultures were fixed with 4 vol.% paraformaldehyde (Sigma-Aldrich), permeabilized with 0.1 vol.% Triton X-100 (Thermo Fisher), and blocked using 5 wt.% goat serum (Sigma-Aldrich). F-actin was stained with rhodamine-phalloidin (Thermo Fisher) for 20 min at room temperature and nuclei were counter-stained with 4’,6-diamidino-2-phenylindole (DAPI, Thermo Fisher) for 5 min.
Immunostaining for HUVECs were performed using antibodies against VE-Cadherin (rabbit anti-human, Abcam, Cambridge, MA, USA), CD31 (mouse anti-human, Dako, Santa Clara, CA, USA), laminin (rabbit anti-human, Abcam), and collagen type IV (rabbit anti-human, Abcam) as primary antibodies. Alexa Fluor 647-conjugated secondary antibodies were used. Primary antibodies were incubated overnight at 4 °C and secondary antibodies were incubated for 1 h at room temperature. Nuclei were counter-stained with DAPI.
ELISA assay
VEGF ELISA kit (Cat# ab100662) was obtained from Abcam and performed according to the manufacturer’s protocol. ELISA assays were performed on supernatants harvested over 7 days (one sample of 200 μL per day).
PrestoBlue assay
The PrestoBlue assays (Thermo Fisher) were performed according to the manufacturer’s protocol on six different PDMS tubes seeded with HUVECs. The absorbance values were read at 570 nm and cell numbers were calculated from the calibration curve obtained from a series of cell densities cultured in well plates.
Imaging setup
All bright-field and fluorescence images were obtained using a ZEISS Axio Observer DI microscope equipped with a ZEISS camMRm camera (Carl Zeiss, Thornwoody, NY, USA) and an X-Cite series 120-Q fluorescence lamp (Excelitas Technologies, Salem, MA, USA). The confocal images were obtained using a Leica SP5 X MP Inverted Laser Scanning Confocal Microscope (Leica, Buffalo Grove, IL, USA). The post-processing of the imaging data for the vascularization and the spheroid characterizations was conducted using the FIJI software with custom-written macros.
Supplementary Material
Acknowledgements
The authors acknowledge funding from the National Institutes of Health (K99CA201603, R00CA201603, R21EB025270, R21EB026175, R01EB028143), Brigham Research Institute, and the New England Anti-Vivisection Society (NEAVS). I.K.Y. acknowledges financial support from the National Institutes of Health Organ Design and Engineering Training program (T32EB16652). W.Z. acknowledges funding from the National Natural Science Foundation of China (31501555, 81772007, 21734003), STCSM (17JC1400200, 16391903900), and Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-07-E00027).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Margaux Duchamp, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA.
Syeda Mahwish Bakht, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA.
Jie Ju, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA.
Weijia Zhang, The Fifth People’s Hospital of Shanghai, Institutes of Biomedical Sciences, Shanghai Medical College, and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200032, P.R. China.
Yu Shrike Zhang, Division of Engineering in Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA.
References
- [1].Carmeliet P, Conway EM, Nat. Biotechnol 2001, 19, 1019. [DOI] [PubMed] [Google Scholar]
- [2].Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, Khademhosseini A, Sci. Transl. Med 2012, 4, 160ps23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baluk P, Hashizume H, Mcdonald DM, Curr. Opin. Genet. Dev 2005, 15, 102. [DOI] [PubMed] [Google Scholar]
- [4].Cao Y, Biomedecine & Pharmacotherapy 2005, 59, S340. [DOI] [PubMed] [Google Scholar]
- [5].Rouwkema J, Khademhosseini A, Trends Biotechnol. 2016, 34, 733. [DOI] [PubMed] [Google Scholar]
- [6].Hou X, Zhang YS, Trujillo-De Santiago G, Alvarez MM, Ribas J, Weiss PS, Andrews AM, Aizenberg J, Khademhosseini A, Nature Reviews Materials 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong KHK, Chan JM, Kamm RD, Tien J, Annu. Rev. Biomed. Eng 2012, 14, 205. [DOI] [PubMed] [Google Scholar]
- [8].Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD, Proct. Natl. Acad. Sci. U.S.A 2012, 109, 13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL, PLoS One 2013, 8, e56910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD, Proct. Natl. Acad. Sci. U.S.A 2015, 112, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim S, Lee H, Chung M, Jeon NL, Lab Chip 2013, 13, 1489. [DOI] [PubMed] [Google Scholar]
- [12].Park YC, Zhang C, Kim S, Mohamedi G, Beigie C, Nagy JO, Holt RG, Cleveland RO, Jeon NL, Wong JY, ACS Appl. Mater. Interfaces 2016, 8, 31541. [DOI] [PubMed] [Google Scholar]
- [13].Zhang W, Zhang YS, Bakht SM, Aleman J, Shin SR, Yue K, Sica M, Ribas J, Duchamp M, Ju J, Sadeghian RB, Kim D, Dokmeci MR, Atala A, Khademhosseini A, Lab Chip 2016, 16, 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Massa S, Seo J, Arneri A, Bersini S, Cha B-H, Antona S, Enrico A, Gao Y, Hassan S, Cox JPA, Zhang YS, Dokmeci MR, Khademhosseini A, Shin S-R, Biomicrofluidics 2017, 11, 044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Proct. Natl. Acad. Sci. U.S.A 2016, 113, 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yue K, Trujillo-De Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Biomaterials 2015, 73, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FPW, Khademhosseini A, Hutmacher DW, Nat. Protocols 2016, 11, 727. [DOI] [PubMed] [Google Scholar]
- [18].Yue K, Li X, Schrobback K, Sheikhi A, Annabi N, Leijten J, Zhang W, Zhang YS, Hutmacher DW, Klein TJ, Biomaterials 2017, 139, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD, Proct. Natl. Acad. Sci. U.S.A 2014, 111, 2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Teng Y, Bio-protocol 2015, 5, e1393. [Google Scholar]
- [21].Zhang YS, Arneri A, Bersini S, Shin S-R, Zhu K, Malekabadi ZG, Aleman J, Colosi C, Busignani F, Dell’erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Biomaterials 2016, 110, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Shaegh S. a. M., Massa S, Riahi R, Chae S-K, Hu N, Avci H, Zhang W, Silvestri A, Manbohi A, Polini A, Calzone G, Shaikh N, Sanati A, Alerasool P, Bhise NS, Budina E, Pourmand A, Skardal A, Shupe T, Bishop C, Dokmeci MR, Atala A, Khademhosseini A, Proceedings of the National Academy of Sciences USA 2017, 114, E2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ying G-L, Jiang N, Maharjan S, Yin Y-X, Chai R-R, Cao X, Yang J-Z, Miri AK, Hassan S, Zhang YS, Adv. Mater 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


