Abstract
Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and an important cause of disease. The most common species causing pulmonary disease are members of Mycobacterium avium complex (MAC). MAC pulmonary disease (MAC-PD) can be chronic, debilitating, costly, and associated with a high mortality. However, MAC diagnoses are often delayed due to the nonspecific presentation of MAC-PD and radiological findings that overlap with other pulmonary diseases. Patients with risk factors and who meet the diagnostic criteria—which include clinical, radiological, and microbiologic criteria—should be considered for treatment. Diagnosis requires 2 or more positive sputum cultures or 1 bronchoscopic specimen culture. The recommendation for those who are treated is a 3-drug regimen including macrolide, rifamycin, and ethambutol that is continued for 12 months beyond sputum culture conversion to negative. MAC-PD is difficult to treat, with frequent drug-related side effects and suboptimal treatment outcomes. Refractory and recurrent disease is common, leading to lifelong follow-up of patients. There are limited treatment options for patients with macrolide-resistant or refractory disease. Amikacin liposome inhalation suspension is recommended for treatment-refractory patients whose cultures remain positive after 6 months of guideline-based therapy. Among the research priorities to improve patient outcomes and quality of life are developing new, more rapid diagnostic tests, investigating biomarkers associated with disease progression, and identifying new drugs and routes of administration as well as new, shorter, and better-tolerated regimens.
Keywords: diagnosis, Mycobacterium avium complex, pulmonary disease, refractory disease, risk factors, treatment
PROGRAM OVERVIEW/STATEMENT OF NEED
Respiratory infections with mycobacterium avium complex (MAC), one of the most common pathogens of nontuberculous mycobacterium pulmonary disease (NTM-PD), have been steadily increasing. Five-year survival of patients with MAC pulmonary disease (MAC-PD) also remains poor. In addition, diagnoses are often delayed due to the nonspecific presentation of MAC-PD and radiological findings that overlap with other pulmonary diseases. Further precluding optimal MAC-PD treatment, the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) guideline on the diagnosis, treatment, and prevention of NTM is more than 12 years old and does not include the recent approval of liposomal amikacin for inhalation (LAI). LAI has demonstrated significant benefit in patients with MAC-PD refractory to conventional care.
The program, Mycobacterium Avium Complex: Addressing Gaps in Diagnosis and Management, will provide infectious disease and pulmonology clinicians with the most current evidence-based recommendations to support timely diagnoses and treatments of patients with MAC-PD.
Release Date: September 15, 2020
Expiration Date: September 15, 2021
TARGET AUDIENCE
This activity is intended for infectious disease and pulmonology clinicians involved in the diagnosis and management of adults with mycobacterium avium complex pulmonary disease.
EDUCATIONAL OBJECTIVES
This program is designed to address Accreditation Council for Graduate Medical Education and National Academy of Medicine competencies, including delivering patient-centered care and practicing evidence-based medicine.
Upon completion of this activity, participants will be able to:
Identify adults with MAC using the latest ATS/IDSA diagnostic criteria;
Evaluate the safety and efficacy data for the most recent additions to the MAC treatment armamentarium; and
Incorporate newer MAC agents into evidence-based treatment plans.
FACULTY
Charles L. Daley, MD
Professor of Medicine
Chief, Division of Mycobacterial and Respiratory Infections
Department of Medicine
National Jewish Health
Denver, CO
Kevin L. Winthrop, MD, MPH
Professor of Infectious Diseases, School of Medicine
Professor of Public Health, School of Public HealthOregon Health and Science University
Portland, OR
ACCREDITATION
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Penn State College of Medicine and Rockpointe. Penn State College of Medicine is accredited by the ACCME to provide continuing medical education (CME) for physicians.
CREDIT DESIGNATION
The Penn State College of Medicine designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Information about CME credit for this activity is available by contacting Penn State at 717-531-6483 or ContinuingEd@hmc.psu.edu. Reference course #G6565-20-R.
INSTRUCTIONS FOR OBTAINING CREDIT
To receive credit, learners must complete the online post-test, with a score of 70% or better, and evaluation located at www.rockpointe.com/NTMsupplement.
FEE INFORMATION
There is no fee for this educational activity.
DISCLOSURE STATEMENT
Before the activity, all faculty and anyone who is in a position to have control over the content of this activity and their spouse/life partner will disclose the existence of any financial interest and/or relationship(s) they might have with any commercial interest producing healthcare goods/services to be discussed during their presentation(s): honoraria, expenses, grants, consulting roles, speakers bureau membership, stock ownership, or other special relationships. Presenters will inform participants of any off-label discussions. All identified conflicts of interest are thoroughly vetted by Penn State College of Medicine for fair balance, scientific objectivity of studies mentioned in the materials or used as the basis for content, and appropriateness of patient care recommendations.
DISCLAIMER
The information provided at this CME/CE activity is for continuing education purposes only and is not meant to substitute for the independent medical judgment of a healthcare provider relative to diagnostic and treatment options of a specific patient’s medical condition. Recommendations for the use of particular therapeutic agents are based on the best available scientific evidence and current clinical guidelines. No bias toward or promotion for any agent discussed in this program should be inferred.
DISCLOSURES
Faculty Content Contributors
The program faculty reported the following relevant financial relationships that they or their spouse/partner have with commercial interests: C. L D. has served as a consultant for Horizon, Insmed, Johnson & Johnson, Matinas BioPharma, Meiji Seika
Pharma Co, Paratek, and Spero; and research for Insmed. K. L. W. has served as a consultant for Insmed and Paratek, and research for Insmed.
Nonfaculty Content Contributors
Penn State faculty and staff involved in the development or review of this material have nothing to disclose. The following nonfaculty content contributors and/or reviewers from Rockpointe also reported no relevant financial relationships that they or their spouse/partner have with commercial interests: Chad Williamson, MS, MBA, CMPP: Nothing to disclose
Editorial assistance was provided by P. Susan Jordan, PharmD.
US Food and Drug Administration Disclosure
The contents of some CME/CE activities might contain discussions of non-approved or off-label uses of some agents mentioned. Please consult the prescribing information for full disclosure of approved uses.
Jointly provided by Penn State College of Medicine and Rockpointe
This supplement is supported by an Educational Grant from Insmed.
Mycobacteria comprise a broad group of organisms, many of which are associated with disease. The 3 categories of mycobacteria are Mycobacterium tuberculosis complex; Mycobacterium leprae, which causes leprosy; and nontuberculous mycobacteria (NTM), which are environmental organisms. Pulmonary disease (PD) caused by NTM is becoming increasingly important as a chronic and debilitating disease primarily in older adults. It is difficult and costly to treat and associated with impaired quality of life [1, 2]. Increased awareness and a better understanding of the natural history of the disease, its diagnosis, and treatment are necessary for healthcare providers who manage patients with MAC.
NTM live in soil, lakes, and rivers, and they are ubiquitous in municipal and natural water systems. They live in biofilm, which they either make or find. They often incorporate into biofilm with other microorganisms such as amoebas and Legionella. Humans are presumed to have frequent NTM exposure. NTM are categorized as slow growers, which include Mycobacterium avium complex (MAC), Mycobacterium kansasii, and Mycobacterium xenopi, or as rapid growers, which include Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum. Slow growers take >7 days to grow in subcultures, whereas rapid growers grow in <7 days [3].
There are >190 species of NTM, and the number of species continues to rise now that current sequencing techniques can identify slight differences between organisms. As many as 80 species of NTM have caused disease in humans, although many have done so rarely and most often in patients who are immunocompromised. MAC, which consists of 12 species, accounts for most cases of disease [4–6]. Approximately 80% of NTM disease is pulmonary, and the principal disease-causing NTM species are members of MAC, the most common of which include M. avium, Mycobacterium intracellulare, and Mycobacterium chimaera [3, 7]. An Oregon population-based analysis of NTM isolates for the years 2007–2012 found that 83.6% of isolates from all disease sites were MAC and that MAC accounted for 92.8% of pulmonary isolates [7]. The second most common NTM species causing pulmonary disease was M. abscessus/M. chelonae complex (4.2%), but in other parts of the United States (US), particularly the South, M. kansasii is the second-most common isolate.
EPIDEMIOLOGY OF NTM PULMONARY DISEASE
The epidemiology of NTM disease has been difficult to determine because, unlike tuberculosis, NTM infections are not reportable in most states and countries. To obtain prevalence estimates, studies have used large claims databases and NTM isolation rates from various geographic catchments. Most such studies have identified increases in the prevalence of NTM-PD.
In the US and other countries with a low or moderate prevalence of tuberculosis, the incidence of NTM has risen dramatically since the 1990s. Data from Japan show a sharp increase in NTM-PD beginning in the mid-1990s, with a dramatic increase in incidence in 2014 that was 2.6 times that in 2007 [8]. The same study showed that, beginning in the 1990s, culture- and smear-positive cases of tuberculosis declined markedly. Similarly, the incidence of NTM-PD in Taiwan increased significantly, from 1.26 cases/100 000 patients in 2006 to 7.94 cases/100 000 patients in 2008 [9].
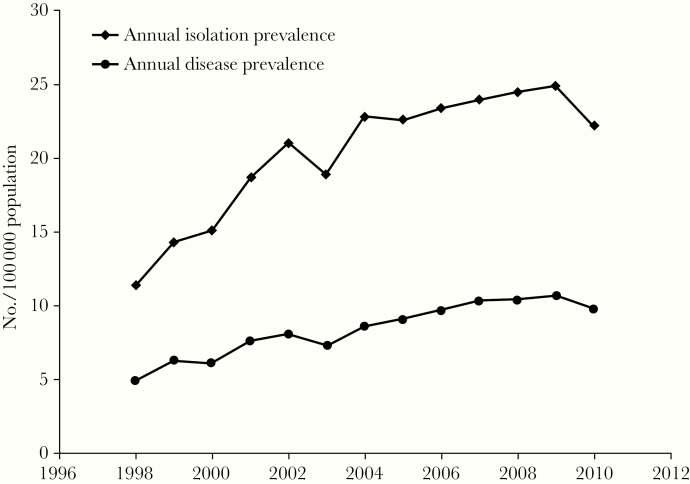
Data from Ontario, Canada, show increases in both the annual isolation and prevalence of pulmonary NTM [10]. The mean annual increase in isolation of pulmonary Mycobacterium species was 6.3% between 1998 and 2010, and the 5-year prevalence rate of NTM-PD rose significantly, from 29.3 cases/100 000 persons for 1998–2002 to 41.3/100 000 persons for 2006–2010 (Figure 1) [10]. Data from the US show a similar trend in increasing NTM incidence as well as regional differences in the incidence rates [11]. The likely reasons for the increase in incidence of NTM are multifactorial: Better detection and recognition among clinicians and radiologists, changes in environmental NTM exposure, an aging population, higher incidence of underlying chronic lung disease, and more patients with suppressed immune systems are all contributory factors.
Figure 1.
Annual isolation prevalence and disease prevalence per 100 000 persons of pulmonary nontuberculous mycobacteria, Ontario, Canada, 1998–2010 [10].
From a worldwide convenience survey of expert centers in 2008, MAC accounted for 47% of cases of NTM-PD, but its distribution varied geographically, ranging from 31% in South America to 71% in Queensland, Australia [12]. Distribution of isolates of pulmonary NTM also varied considerably by geographic area. The incidence of rapid growers ranged from a high of 31% of isolates from Asia to 20% of isolates from both North and South America to 7% in South Africa. These differences are important, as results of NTM treatment studies from 1 geographic region cannot necessarily be extrapolated to another without taking into account differential NTM species distributions between such studies.
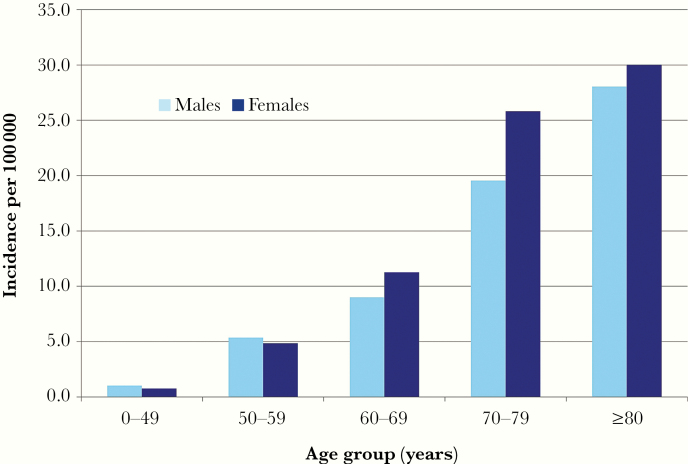
NTM-PD primarily affects people ≥60 years of age (Figure 2) [7, 13]. The incidence of NTM-PD is higher in women >60 years of age and overall. Only in those <60 years of age is the incidence of disease slightly higher in men than in women. These trends in disease occurrence are consistent worldwide. Why NTM-PD affects women to a greater extent is unclear but might be related to a longer life span, higher likelihood of visiting a doctor, or differences in risk (genetic, exposure).
Figure 2.
Average annual age- and sex-specific incidence of pulmonary nontuberculous mycobacterial disease in Oregon, 2007–2012 [7]. Adapted with permission of the American Thoracic Society. Copyright 2020 American Thoracic Society. All rights reserved.
The 5-year mortality in patients with NTM-PD is higher than that in patients who do not meet the criteria for disease. Studies from Ontario, Canada and from Oregon found that the hazard ratio for mortality in those meeting the criteria for NTM disease, compared with those who were colonized, ranged from 1.23 to 1.37 [14, 15]. In both studies, compared with the general population, the adjusted mortality was approximately 1.5 times higher in those with NTM disease.
DIAGNOSIS OF MAC PULMONARY INFECTION
The most common mycobacterial species identified in cases of NTM-PD are MAC, M. abscessus, and M. kansasii. The 2020 American Thoracic Society (ATS), European Respiratory Society (ERS), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and Infectious Diseases Society of America (IDSA) diagnostic criteria for NTM-PD are summarized in Table 1 [16]. Patients must have symptoms, radiographic evidence of disease, and at least 2 sputum cultures or 1 bronchoalveolar lavage or lung tissue culture positive for MAC. However, MAC diagnoses are often delayed due to the nonspecific presentation of MAC-PD and radiological findings that overlap with other pulmonary diseases [18].
Table 1.
2020 American Thoracic Society/European Respiratory Society/European Society of Clinical Microbiology and Infectious Disease/Infectious Diseases Society of America Diagnostic Criteria for Nontuberculous Mycobacterial Pulmonary Disease
| • Clinical criteria – Pulmonary symptoms – Radiographic evidence AND – Exclusion of other diagnoses |
| • Microbiologic criteria – Positive culture results from at least 2 sputum specimens OR – Transbronchial or other lung biopsy with histology of granulomatous inflammation and ≥1 positive culture from sputum or bronchial wash |
Source: Daley et al [16].
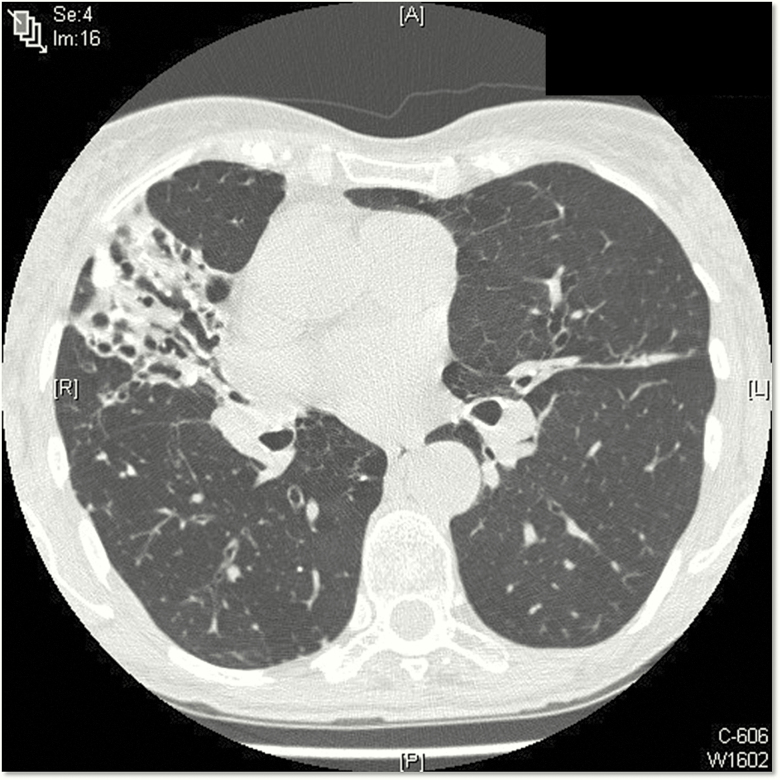
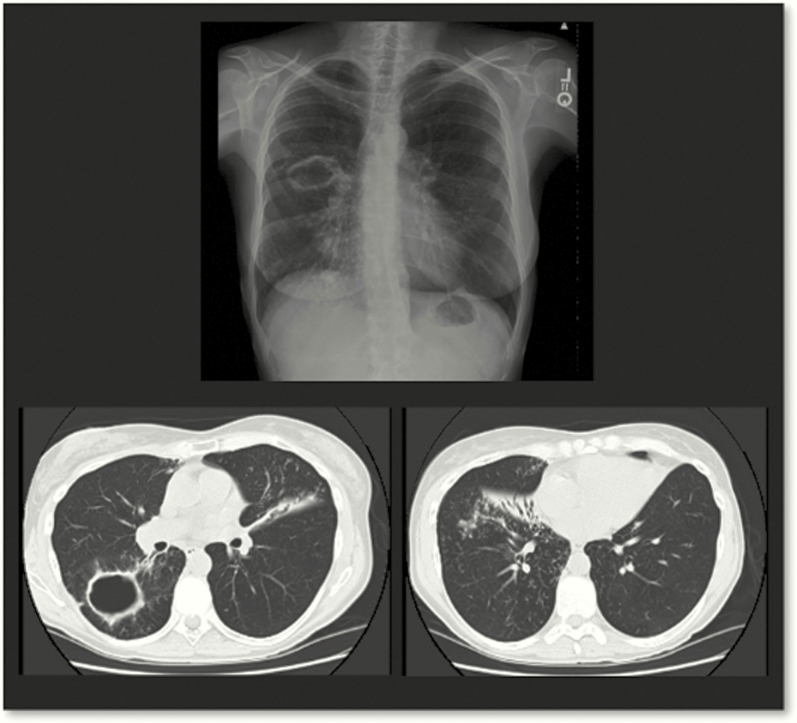
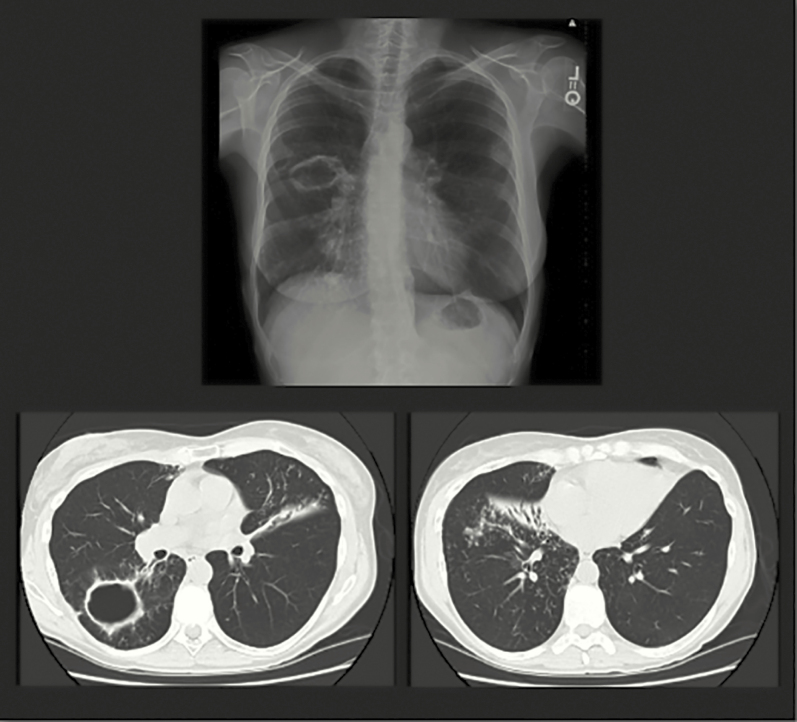
NTM-PD typically presents as 1 of 2 clinical phenotypes: (1) older men with underlying lung disease such as chronic obstructive pulmonary disease (COPD) and fibrocavitary radiographic findings and (2) older, nonsmoking women with nodular bronchiectatic disease [19]. However, it is important to note that there is a great deal of overlap between these 2 phenotypes. In older females, NTM-PD has been termed the “Lady Windermere” subtype after a character from an Oscar Wilde play. Women tend to be tall, thin, often have scoliosis or a pectus deformity, and typically have bronchiectasis in the right middle lobe or lingula (Figure 3). Computed tomography typically shows bronchiolitis (“tree in bud”) as well as nodularity and mucoid impaction. This form of NTM-PD in women usually progresses slowly compared with that in fibrocavitary disease. A Lady Windermere counterpart recently was described in older males with low body mass index and NTM-PD [20, 21].
Figure 3.
Computed tomography scan from a 74-year-old woman with recurrent Mycobacterium avium complex (MAC) pulmonary disease. This thin woman has classic features of MAC pulmonary disease, including pectus excavatum and right middle lobe and lingula bronchiectasis with infiltrate.
Risk Factors
Risk factors for NTM-PD are listed in Table 2. Some environmental risk factors might carry greater risk, such as a steam sauna compared with gardening, but the relative risk associated with environmental risk factors is unknown.
Table 2.
Risk Factors for Pulmonary Nontuberculous Mycobacterial Disease
| • Underlying lung architectural abnormalities – Bronchiectasis, cystic fibrosis – α-1 antitrypsin, emphysema – Prior infections, eg, tuberculosis – GERD with microaspiration |
| • Exposure/transmission – Gardening – Hot tubs – Others |
Abbreviation: GERD, gastroesophageal reflux disease.
Bronchiectasis is both a risk factor for and a consequence of NTM disease [22]. Bronchiectasis is associated with many conditions, including cystic fibrosis, α-1 antitrypsin and emphysema, primary ciliary dyskinesia, immunodeficiencies, autoimmune disorders, tuberculosis and other infections, and gastroesophageal reflux disease with microaspiration. The cause of NTM-PD associated with gastroesophageal disease is more likely a rapid grower species such as M. fortuitum [23]. Rheumatoid arthritis is another risk factor for NTM disease, as approximately 15%–20% of patients with rheumatoid arthritis develop chronic, underlying interstitial lung disease, and bronchitis. A population-based study with a 10-year follow-up conducted in Taiwan showed that the risk of NTM disease in patients with rheumatoid arthritis is 4.22 times greater than in patients without rheumatoid arthritis [24].
Immunosuppressive therapy is a risk factor for tuberculosis and NTM disease. A population-based study in the US found that the risk of developing NTM disease in patients with rheumatoid arthritis receiving anti–TNF-α therapy was approximately 5 times greater than that in patients with arthritis who had not received anti–TNF-α [25, 26]. The increased risk of NTM-PD in patients with chronic pulmonary disease (COPD, asthma) who take oral or inhaled corticosteroids is also well documented [9, 27–30]. The risk associated with corticosteroid use is dose and duration dependent. Oral doses of prednisone >15 mg and of inhaled corticosteroid in doses equivalent to >800 mg of fluticasone are associated with elevated risk of NTM-PD [28, 29]. Inhaled corticosteroid use for >5 years also is associated with elevated risk of NTM-PD.
TREATMENT STRATEGIES
Studies of the natural history of NTM-PD provide insight on disease progression. In 1 study of 488 patients who met the ATS/IDSA diagnostic criteria for MAC-PD and were treatment-naive, approximately 62% progressed, resulting in treatment initiation within 3 years of diagnosis, while approximately 24% remained stable and untreated [31]. Of those who remained stable, sputum cultures spontaneously converted to negative in approximately 52%. In another study of 551 patients who met the diagnostic criteria for MAC-PD, approximately 59% progressed and received treatment within 3 years of diagnosis, 41% remained stable requiring no treatment, and 52% of the stable patients had spontaneous sputum conversion [32].
Several risk factors are associated with progression of MAC-PD and initiation of treatment (Table 3) [31–33]. Patient factors include young age, male sex, low body mass index, and having comorbidities. Elevated inflammatory markers, anemia, and hypoalbuminemia are among the laboratory risk factors. Patients with fibrocavitary and/or more extensive disease are more likely to have disease progression. Not only the bacterial load but also the species of Mycobacterium are associated with progression of NTM. For example, M. intracellulare might be more pathogenic than M. avium [31].
Table 3.
Risk Factors Associated With Progression of Nontuberculous Mycobacterial Pulmonary Disease
| • Host/demographic – Male sex – Younger age – Presence of comorbidities – Low body mass index |
| • Radiographic – Fibrocavitary disease – Extent of disease |
| • Laboratory – Elevated inflammatory indices (ESR, CRP) – Anemia – Hypoalbuminemia |
| • Microbial – Bacterial load – Species |
Initiating Treatment
Before initiating treatment, antimicrobial susceptibility testing is necessary. Current guidelines recommend testing the organism’s susceptibility to clarithromycin (macrolide class representative) and to amikacin because these are the only drugs for which in vitro susceptibility correlates with treatment outcome [34]. The breakpoints for resistance are ≥32 µg/mL for clarithromycin, >64 µg/mL for intravenous amikacin, and ≥128 µg/mL for amikacin liposome inhalation suspension (ALIS). Susceptibility testing might be performed on the second-line drugs moxifloxacin and linezolid or potentially other drugs; however, their clinical effectiveness is unproven.
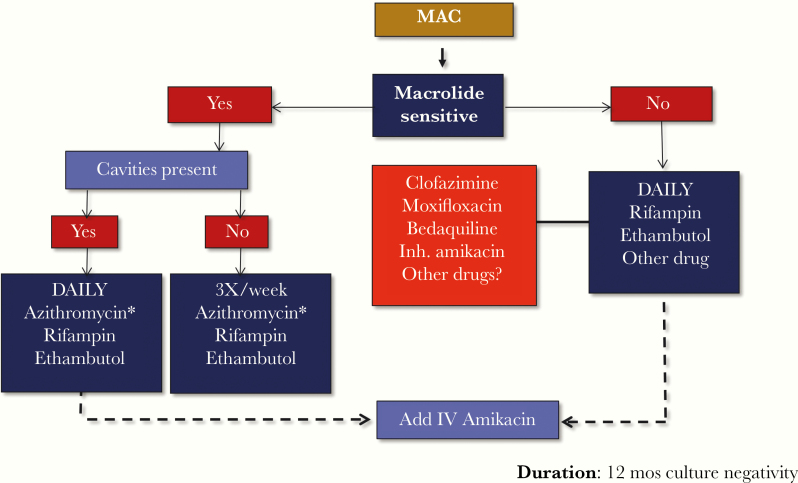
A treatment algorithm for pulmonary MAC disease is shown in Figure 4 [35]. The first consideration for initiating treatment is whether the organism is susceptible to a macrolide. A second consideration is whether there is radiographic evidence of cavitary disease. Patients with cavitary disease are prescribed daily treatment with a 3-drug regimen, and depending on the extent of the disease, patients also may receive intravenous amikacin for 2 months. The preferred macrolide is azithromycin, and it should be given with ethambutol and a rifamycin (rifampin is preferred if drug–drug interactions do not preclude its use). Patients without cavitary disease are prescribed a thrice-weekly, 3-drug regimen because studies have demonstrated similar efficacy and better tolerance when compared with daily administration [36, 37]. If the organism is not susceptible to a macrolide, a daily regimen with rifampin, ethambutol, and another drug such as clofazimine, moxifloxacin, or bedaquiline is recommended. Intravenous amikacin also may be added, as improved outcomes have been shown in patients with macrolide-resistant MAC disease. The duration of treatment is the time to achieve a 12-month period of negative cultures, necessitating collection of sputum cultures every 1–2 months.
Figure 4.
Treatment of Mycobacterium avium complex pulmonary disease. *Clarithromycin is an alternative. Abbreviations: Inh., inhaled; IV, intravenous; MAC, Mycobacterium avium complex.
There is about an 80% rate of culture conversion to negativity in patients with noncavitary macrolide-sensitive disease and a range of 50%–80% in patients with cavitary disease [36–38]. Macrolide monotherapy, or a combination regimen of a macrolide with a quinolone, is particularly associated with the development of macrolide resistance [35]. In patients with macrolide-resistant MAC-PD, the rate of sputum culture conversion is very low (5%–15%) [35, 39]. However, the rate of sputum culture conversion can be increased significantly with surgical resection plus an intravenous aminoglycoside administered for ≥6 months [35, 39].
Recurrence, including both relapse and reinfection, of MAC infection is common after a successful treatment course. In a study of 155 patients with nodular bronchiectatic disease, microbiologic recurrence (culture negative at the end of treatment that became positive) occurred in 48% of patients and a new infection in 75% determined by pulsed-field electrophoresis [37]. Another group reported a 25% recurrence rate in 190 patients, and 46% of patients developed a new infection determined by pulsed-field electrophoresis [40]. A third study of 402 patients found a 29% rate of microbiologic recurrence and a 74% rate of new infection determined by repetitive element sequence–based polymerase chain reaction [36]. In this study, patients with nodular bronchiectatic disease were twice as likely as patients with fibrocavitary disease to have a microbiologic recurrence, indicating the need for lifelong follow-up.
Refractory MAC-PD
Patients with MAC-PD should have several sputum cultures performed during their treatment course. The Nontuberculous Mycobacteria Network European Trials Group (NTM-NET) consensus panel defined treatment failure as “the re-emergence of multiple positive cultures or persistence of positive cultures with the causative species from respiratory samples after ≥12 months of antimycobacterial treatment while the patient is still on treatment” [5]. However, waiting for 12 months to consider someone to have failed treatment could result in further unnecessary disease progression if something could be done earlier in the course of therapy to improve treatment.
Several studies provide evidence to suggest that we can determine who is likely to fail treatment much earlier than 12 months into therapy. In a study of 180 patients treated with standard therapy for MAC-PD, 83% of patients had sputum conversion to culture-negative [41]. The change from baseline to month 3 in semiquantitative sputum culture score was highly predictive of subsequent sputum culture status at 12 months as well as treatment success. Others have shown that culture conversion by 6 months predicts culture status at 12 months [42]. A study of 470 treatment-naive patients with MAC-PD found that 76% achieved culture conversion to negative within 12 months of treatment. Of those patients, 94% were culture-negative within 6 months of treatment. For the 24% of patients who did not achieve culture conversion to negative at 12 months, 93% did not have negative culture conversion at 6 months. Accordingly, patients who have not converted their culture by 6 months of therapy are unlikely to do so, and such patients are considered to have “treatment-refractory” disease. Together, these studies strongly suggest that culture status or change in semiquantitative cultures in the first 6 months of therapy is predictive of subsequent response to therapy.
Optimum treatment of patients with refractory MAC-PD has yet to be determined. Several retrospective studies of different treatment strategies reported approximately 30%–50% culture conversion rates [43–47]. A study of 20 patients who switched from intermittent to daily treatment found that 30% achieved culture conversion to negative [43]. In 87 patients with refractory NTM-PD who had failed previous therapy, 50% of patients treated with clofazimine converted to culture-negative within 12 months [44]. Of 41 patients with MAC-PD and persistent positive cultures after ≥6 months of standard therapy who were then treated with a moxifloxacin-containing regimen, 29% had sputum culture conversion to negative after a median of 91 days [45]. A study of off-label use of bedaquiline in 10 patients with NTM-PD who had failed therapy found that 50% had 1 or more culture conversions to negative after 6 months of therapy [46].
One prospective, open-label, randomized study (CONVERT) evaluated the addition of ALIS to guideline-based therapy (n = 224) vs guideline-based therapy alone (n = 112) in patients with treatment-refractory MAC-PD (MAC-positive sputum cultures with ≥6 months of treatment) [48]. The primary endpoint of the study was the percentage of patients with sputum culture conversion to negative, defined as 3 consecutive monthly MAC-negative cultures by month 6. Beginning at month 1 and continuing throughout treatment, a greater percentage of patients treated with the ALIS-containing regimen achieved negative sputum cultures compared with patients treated with guideline-based therapy (Figure 5) [48]. At month 4, there was a significant difference in response favoring the ALIS-containing regimen: 29% vs 8.9% had negative sputum cultures for NTM (adjusted odds ratio, 4.22; P < .001). The microbiologic response occurred up to an amikacin minimum inhibitory concentration of ≤64 µg/mL. Patients who experienced culture conversion demonstrated greater improvement in 6-minute walk distance than nonconverters. There was no improvement in quality of life as measured by an instrument that has not been validated in patients with NTM-PD.
Figure 5.
CONVERT study results for the primary outcome of negative sputum culture results with amikacin liposome inhalation suspension plus guideline-based therapy vs guideline-based therapy alone [48]. Abbreviations: ALIS, amikacin liposome inhalation suspension; CI, confidence interval; FDA, US Food and Drug Administration; GBT, guideline-based therapy; MIC, minimum inhibitory concentration; OR, odds ratio. Reprinted with permission of the American Thoracic Society. Copyright 2020 American Thoracic Society. All rights reserved.
Respiratory treatment-emergent adverse events were much more common with ALIS therapy: dysphonia (45.7% vs 0.9%), cough (37.2% vs 15.2%), dyspnea (21.5% vs 8.9%), hemoptysis (17.5% vs 13.4%), and oropharyngeal pain (10.8% vs 1.8%). These adverse events, including dysphonia, typically occurred in the first month of therapy with declining incidence of new onset thereafter [48]. Audiological treatment-emergent adverse events also were more common with ALIS therapy: tinnitus (7.6% vs 0.9%) and dizziness (6.3% vs 2.7%). Hearing loss was not more common with ALIS therapy (4.5% vs 6.3%), and the occurrence of serious adverse events was similar for the ALIS-containing and guideline-based regimens (20.2% vs 17.9%). ALIS is currently approved by the US Food and Drug Administration for the treatment of adults who have limited or no alternative options for the treatment of MAC-PD as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy [49]. The approved dose is 590 mg/8.4 mL once daily, administered using a Lamira nebulizer system. Monitoring parameters include baseline and periodic (eg, every 3 months) audiograms and baseline spirometry, which is repeated as necessary (eg, if there is cough or shortness of breath). The labeling for ALIS includes a black box warning for an increased risk of respiratory adverse reactions. Clinically, it is important to utilize strategies that reduce the risk of dysphonia and cough, such as predosing with a bronchodilator or a break in therapy and reintroducing ALIS on a thrice-weekly regimen with an increase to daily therapy.
Adjunctive Therapy
In addition to antimicrobial treatment, the patient’s underlying lung disease as well as other comorbidities should be addressed. For patients with bronchiectasis, airway clearance is a critical component of therapy and should be individualized to the patient’s needs. Exercise and pulmonary rehabilitation should be encouraged, and nutrition should be improved.
Surgical resection of involved lung may be helpful in selected patients who have localized disease and who can tolerate surgery. Patients who may benefit from surgery include those who are failing therapy or have macrolide-resistant disease or other significant disease-related complications such as hemoptysis. In general, treatment outcomes in surgical series reported culture conversion rates of approximately 80% postoperatively with low complications rates, particularly when video-assisted thoracoscopic approaches are used [50]. Whenever possible, surgery should be performed by a surgeon with considerable experience with resectional surgery for mycobacterial disease [51].
RESEARCH PRIORITIES
An NTM Research Consortium Patient Advisory Panel met in 2015 with the goal of defining patient-centered research priorities for NTM pulmonary infections. The group’s priorities for research in the areas of treatment and clinical outcomes are summarized in Figure 6 [52]. Among the top research priorities were the prevention of NTM infection, more effective treatments with fewer adverse effects and easier administration, understanding the best chest physiotherapy methods, use of validated tools to measure quality of life, and development of a disease-specific activity and severity assessment tool.
Figure 6.
Research priorities for nontuberculous mycobacterial pulmonary infections. Abbreviations: IV, intravenous; NTM, nontuberculous mycobacteria.
Diagnosis of NTM-PD requires collection of respiratory specimens for culture and susceptibility testing, which can take weeks to months to obtain a result in many clinical settings. More rapid identification, speciation, and assessment for molecular markers of resistance would improve our diagnostic capabilities. Biomarkers that can predict which patients are more likely to develop progressive disease would improve the ability of providers to determine which patients would benefit most from antimicrobial therapy.
New treatments being studied include repurposed antimicrobials, new antimicrobials or new formulations, and nonantimicrobial drugs, among others (Table 4). Antimicrobial agents that are being used but require additional study, including alternate routes of administration, are bedaquiline, clofazimine, and tedizolid. Several nonantimicrobial drugs administered by inhalation, including nitric oxide, granulocyte-macrophage colony-stimulating factor, and gallium, currently are in clinical trials [53]. Mycobacteriophages are in early stages of development. Research continues to identify a scientific approach to prevent recurrence of MAC-PD.
Table 4.
Potential New Treatment Approaches Currently Under Study for Mycobacterium avium Complex Pulmonary Disease
| • Repurposed antimicrobials – Bedaquiline – Clofazimine – Tedizolid – Omadacycline |
| • Nonantimicrobial drugs – Inhaled nitric oxide – Inhaled GM-CSF – Inhaled gallium |
| • New antimicrobials or formulations – Gyrase inhibitor – Oral amikacin – Inhaled clofazimine |
| • Other – Bacteriophage |
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor.
CONCLUSIONS
NTM includes many species that are ubiquitous in the environment. The principal causative species for NTM-PD are members of MAC. Several groups in multiple countries have documented the increasing incidence of MAC-PD. Not all patients with risk factors and who meet the criteria for MAC-PD require treatment initially, but most will at some point during the course of their disease. Those with bronchiectasis require pulmonary hygiene and treatment of other comorbidities while the decision whether to treat the MAC infection is being made. Antimicrobial susceptibility testing for macrolides (clarithromycin or azithromycin) and amikacin is recommended for MAC disease. A 3-drug macrolide-containing regimen is recommended as the initial regimen, which is administered for 12 months beyond culture conversion to negative. A thrice-weekly regimen is recommended for patients with noncavitary disease, and a daily regimen for those with cavitary disease or in those undergoing retreatment. Intravenous amikacin is recommended for patients with macrolide-resistant or cavitary disease. Patients with refractory disease should be considered for ALIS if their sputum cultures have not converted to negative by 6 months. Patient education, active monitoring, and management strategies are important for improving patient adherence and tolerance to ALIS treatment. Surgery might be helpful in selected patients. Ongoing research for additional treatment options is critical, as outcomes in patients with macrolide-resistant MAC-PD are poor.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Note
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplement sponsorship. This supplement is supported by an Educational Grant from Insmed.
Correspondence: Chad Williamson, MS, MBA, Rockpointe, 8335 Guilford Rt, Ste. A Columbia, MD 21046 (cwilliamson@rockpointe.com).
POSTTEST
To receive credit, please complete the online CME posttest, with a score of 70% or better, and evaluation form at: www.rockpointe.com/NTMsupplement. The posttest questions are listed below for your reference and convenience. They are identical to the posttest you will find online.
However, to receive credit, you must complete the online form at: www.rockpointe.com/NTMsupplement. If you are experiencing problems or have any questions, please email cme@rockpointe.com.
-
Jane is a 66-year-old Hispanic female, US-born citizen with 40 pack-year smoking history. She has rheumatoid arthritis (RA), chronic obstructive pulmonary disease (COPD), a 10-week history of productive cough, fever, malaise, and night sweats. She is on infliximab and prednisone. She spent 1 year in Southeast Asia 30 years ago as a Peace Corps volunteer. She was screened for tuberculosis prior to starting infliximab 1 year ago. That tuberculin skin test was negative, and she does not remember having a tuberculin skin test prior to 1 year ago. What factors in Jane’s history suggest she may be at risk for having tuberculosis?
Having lived in Southeast Asia
Immunosuppression
Both a and b are correct
Neither a nor b is correct
-
What is the recommended initial treatment for noncavitary nodular bronchiectatic macrolide-susceptible nontuberculous mycobacterial pulmonary disease?
Azithromycin, rifampin, and ethambutol 3 times a week
Levofloxacin, rifampin, and ethambutol daily
Azithromycin, rifampin, and ethambutol daily
Intravenous amikacin, azithromycin, rifampin, and ethambutol 3 times a week
-
Which of the following are included in current diagnostic criteria for pulmonary NTM?
Radiographic evidence of disease, pulmonary symptoms, and at least 1 positive sputum culture
Radiographic evidence of disease, pulmonary symptom, and at least 2 positive sputum cultures
Radiographic evidence of disease, pulmonary symptoms, and 1 broncho-alveolar lavage or tissue specimen with positive culture
Both b and c are correct
None are correct
-
Ellen is a 65-year-old white woman treated for Mycobacterium avium complex (MAC) on 2 previous occasions with macrolide, rifampin, and ethambutol. She now has an acid-fast bacilli smear-positive sputum specimen and culture positive for Mycobacterium intracellulare.
What would be your next step in managing this patient?
Refer for bronchoscopy
Order antimicrobial susceptibility testing
Initiate treatment with azithromycin and a fluoroquinolone
Do not initiate treatment but follow for evidence of progression
-
Which patient/demographic feature is associated with increased risk of progression in patients with MAC pulmonary disease?
Low body mass
Female sex
Adolescence
Asian descent
-
Should Ellen be restarted on a 3-drug regimen before the results of the culture and sensitivity are reported?
Yes
No
-
By 6 months, Ellen was still culture-positive on daily azithromycin, rifampin, and ethambutol. Antimicrobial susceptibility test results were as follows: clarithromycin minimum inhibitory concentration (MIC) >32 μg/mL; amikacin MIC = 32 μg/mL.
What would be your next step in managing this patient?
Add moxifloxacin to the regimen
Add amikacin liposome inhalation suspension to the regimen
Continue the current regimen
Stop the current regimen and follow for further progression
-
Azithromycin was stopped, ethambutol and rifampin were continued, intravenous amikacin was added, and clofazimine was added. After 8 weeks of intravenous amikacin, inhaled amikacin was substituted.
Which of the following testing/monitoring would you need to discuss with Ellen prior to initiating amikacin liposome inhalation suspension therapy?
Blood sugar assessment
Hearing evaluation with audiograms
Visual acuity testing
Liver function assessment
-
Because Ellen had macrolide-resistant MAC with a large cavity in the right lower lobe and lingula and bronchiectasis in her right middle lobe, Ellen underwent video-assisted thoracoscopic surgical resection of her lingula, her right middle lobe, and her superior segment done serially over several months. Ellen continued treatment with amikacin liposome inhalation suspension, rifampin, ethambutol, and clofazimine for 12 months after surgery. Her sputum cultures converted to culture-negative. Can Ellen’s treatment be discontinued at this time?
Yes
No
References
- 1. Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc 2015; 12:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med 2011; 105:1718–25. [DOI] [PubMed] [Google Scholar]
- 3. Henkle E, Hedberg K, Schafer SD, Winthrop KL. Surveillance of extrapulmonary nontuberculous mycobacteria infections, Oregon, USA, 2007–2012. Emerg Infect Dis 2017; 23:1627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tortoli E, Rindi L, Garcia MJ, et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int J Syst Evol Microbiol 2004; 54:1277–85. [DOI] [PubMed] [Google Scholar]
- 5. van Ingen J, Aksamit T, Andrejak C, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 2018; 51:1800170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev 2014; 27:727–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc 2015; 12:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis 2016; 22:1116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis 2010; 16:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis 2013; 19:1889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. Managed Care Health Plan, 2008–2015. Ann Am Thorac Soc 2020; 17:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoefsloot W, van Ingen J, Andrejak C, et al. Nontuberculous Mycobacteria Network European Trials Group The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013; 42:1604–13. [DOI] [PubMed] [Google Scholar]
- 13. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marras TK, Campitelli MA, Lu H, et al. Pulmonary nontuberculous mycobacteria-associated deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis 2017; 23:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Novosad SA, Henkle E, Schafer S, et al. Mortality after respiratory isolation of nontuberculous mycobacteria. A comparison of patients who did and did not meet disease criteria. Ann Am Thorac Soc 2017; 14:1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daley CL, Iaccarino JM, Lance C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline: executive summary [published online ahead of print July 6, 2020]. Clin Inf Dis 2020.doi: 10.1093/cid/ciaa241 [DOI] [Google Scholar]
- 17. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 18. Maiga M, Siddiqui S, Diallo S, et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS One 2012; 7:e36902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991; 144:914–6. [DOI] [PubMed] [Google Scholar]
- 20. Ku JH, Ranches G, Siegel SAR, Winthrop KL. Lady Windermere’s counterpart? Pulmonary nontuberculous mycobacteria in men with bronchiectasis. Diagn Microbiol Infect Dis 2020; 96:114916. [DOI] [PubMed] [Google Scholar]
- 21. Holt MR, Kasperbauer SH, Koelsch TL, Daley CL. Similar characteristics of nontuberculous mycobacterial pulmonary disease in men and women. Eur Respir J 2019; 54:1900252. [DOI] [PubMed] [Google Scholar]
- 22. Fujita J, Ohtsuki Y, Shigeto E, et al. Pathological findings of bronchiectases caused by Mycobacterium avium intracellulare complex. Respir Med 2003; 97:933–8. [DOI] [PubMed] [Google Scholar]
- 23. Hadjiliadis D, Adlakha A, Prakash UB. Rapidly growing mycobacterial lung infection in association with esophageal disorders. Mayo Clin Proc 1999; 74:45–51. [DOI] [PubMed] [Google Scholar]
- 24. Yeh JJ, Wang YC, Sung FC, Kao CH. Rheumatoid arthritis increases the risk of nontuberculosis mycobacterial disease and active pulmonary tuberculosis. PLoS One 2014; 9:e110922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis 2013; 72:37–42. [DOI] [PubMed] [Google Scholar]
- 26. Winthrop KL, Iseman M. Bedfellows: mycobacteria and rheumatoid arthritis in the era of biologic therapy. Nat Rev Rheumatol 2013; 9:524–31. [DOI] [PubMed] [Google Scholar]
- 27. Dirac MA, Horan KL, Doody DR, et al. Environment or host? A case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 186:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hojo M, Iikura M, Hirano S, Sugiyama H, Kobayashi N, Kudo K. Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 2012; 17:185–90. [DOI] [PubMed] [Google Scholar]
- 29. Andréjak C, Nielsen R, Thomsen VØ, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013; 68:256–62. [DOI] [PubMed] [Google Scholar]
- 30. Liu VX, Winthrop KL, Lu Y, Sharifi H, Nasiri HU, Ruoss SJ. Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann Am Thorac Soc 2018; 15:1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017; 49:1600537. [DOI] [PubMed] [Google Scholar]
- 32. Kwon BS, Lee JH, Koh Y, et al. The natural history of non-cavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med 2019; 150:45–50. [DOI] [PubMed] [Google Scholar]
- 33. Moon SM, Jhun BW, Daley CL, Koh WJ. Unresolved issues in treatment outcome definitions for nontuberculous mycobacterial pulmonary disease. Eur Respir J 2019; 53:1801636. [DOI] [PubMed] [Google Scholar]
- 34. Clinical and Laboratory Standards Institute. M100. Performance standards for antimicrobial suceptibility testing. 28th ed. Wayne, PA: CLSI, 2018. [Google Scholar]
- 35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2006; 174:928–34. [DOI] [PubMed] [Google Scholar]
- 36. Koh WJ, Moon SM, Kim SY, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 2017; 50:1602503. [DOI] [PubMed] [Google Scholar]
- 37. Wallace RJ Jr, Brown-Elliott BA, McNulty S, et al. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014; 146:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeong BH, Jeon K, Park HY, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2015; 191:96–103. [DOI] [PubMed] [Google Scholar]
- 39. Morimoto K, Namkoong H, Hasegawa N, et al. Nontuberculous Mycobacteriosis Japan Research Consortium Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc 2016; 13:1904–11. [DOI] [PubMed] [Google Scholar]
- 40. Boyle DP, Zembower TR, Qi C. Relapse versus reinfection of Mycobacterium avium complex pulmonary disease. Patient characteristics and macrolide susceptibility. Ann Am Thorac Soc 2016; 13:1956–61. [DOI] [PubMed] [Google Scholar]
- 41. Griffith DE, Adjemian J, Brown-Elliott BA, et al. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2015; 192:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moon SM, Jhun BW, Baek SY, et al. Long-term natural history of non-cavitary nodular bronchiectatic nontuberculous mycobacterial pulmonary disease. Respir Med 2019; 151:1–7. [DOI] [PubMed] [Google Scholar]
- 43. Koh WJ, Jeong BH, Jeon K, et al. Response to switch from intermittent therapy to daily therapy for refractory nodular bronchiectatic Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 2015; 59:4994–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 2017; 152:800–9. [DOI] [PubMed] [Google Scholar]
- 45. Koh WJ, Hong G, Kim SY, et al. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother 2013; 57:2281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Philley JV, Wallace RJ Jr, Benwill JL, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olivier KN, Griffith DE, Eagle G, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017; 195:814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Griffith DE, Eagle G, Thomson R, et al. CONVERT Study Group Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018; 198:1559–69. [DOI] [PubMed] [Google Scholar]
- 49. ARIKAYCE (amikacin liposome inhalation suspension) prescribing information. Bridgewater, NJ: Insmed Inc, 2018. [Google Scholar]
- 50. Yu JA, Pomerantz M, Bishop A, Weyant MJ, Mitchell JD. Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial disease. Eur J Cardiothorac Surg 2011; 40:671–5. [DOI] [PubMed] [Google Scholar]
- 51. Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008; 85:1887–92; discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 52. Henkle E, Aksamit T, Barker A, et al. NTMRC Patient Advisory Panel Patient-centered research priorities for pulmonary nontuberculous mycobacteria (NTM) infection. An NTM Research Consortium Workshop Report. Ann Am Thorac Soc 2016; 13:S379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. ClinicalTrials.gov. Nontuberculosis Mycobacterium infection studies. Available at: https://clinicaltrials.gov/ct2/results?cond=Nontuberculous+Mycobacterium+Infection&term=&cntry=&state=&city=&dist=. Accessed 15 March 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.