Abstract
Objectives
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the pulmonary disease coronavirus disease 2019 (COVID-19, which has challenged health care facilities worldwide. The sustainability of health care systems is largely reliant on the health status of their health care workers (HCW).
This study aimed to detect the SARS-CoV-2 virus and specific antibodies among HCWs in a German hospital as a model system for the potential spread of the pandemic.
Methods
Between March and June 2020, we used a combination of RT-PCR testing to detect SARS-CoV-2 RNA and an enzyme-linked immunosorbent assay to detect the presence of anti-SARS-CoV-2 immunoglobulin G (IgG) antibodies among HCWs in a German hospital based on repetitive oropharyngeal swabs (OPSs) and blood samples.
Results
In total, 871/1081 employees participated in this prospective longitudinal study. During the study period of 9 weeks, 5329 OPSs and 2136 blood samples were analyzed. SARS-CoV-2 RNA was detected in three participants (0.34%). Anti-SARS-CoV-2 IgG antibodies were detected in 38 (4.36%) participants.
Conclusion
Our study determined a low prevalence of COVID-19 in HCW, which may reflect the effectiveness of hygiene protocols. However, it could also indicate a low prevalence of SARS CoV-2 in hospital employees. Our study protocol may serve as an instructive example for future pandemic containment protocols in hospitals.
Keywords: SARS-CoV-2, COVID-19, epidemiology, anti-SARS-CoV-2 IgG antibodies, RT-PCR, health care
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the pulmonary disorder coronavirus disease 2019 (COVID-19), which has spread throughout China and the rest of the world since late 2019. SARS-CoV-2 has caused thousands of deaths around the world, and the numbers continue to increase. Most recent reports suggest that the number of cases increased by up to 291,825 per day to a total of more than 25 million cases by the end of August 2020 (World Health Organization, 2020). This pandemic has created challenges for global health care systems and forced rapid increases in total hospital capacities, where intensive care units (ICUs) and ventilation capacities have been under particular pressure (Phua et al., 2020). This situation has also demonstrated the importance of health care workers (HCWs) for handling the increased number of patients (The Lancet, 2020).
The transmission of SARS-CoV-2 is mainly considered to occur via person-to-person contact by droplet infection (Hoehl et al., 2020). As a consequence, HCWs belong to a high risk group because they have many close person-to-person contacts, including contacts with COVID-19 patients. In addition, many SARS-CoV-2-positive individuals have no or very few symptoms, particularly in previously healthy individuals (Epstude and Harsch, 2020, Mizumoto et al., 2020a, Rothe et al., 2020; The Lancet, 2020; U.S. Food and Drug Administration, 2020). These individuals are suspected to contribute to the rapidly increasing case numbers (Li et al., 2020a, Mizumoto et al., 2020b). Strict hygiene protocols are enforced in most hospitals to prevent so-called “patient-to-staff transmission” (Korth et al., 2020). In addition, rapid societal protective measures such as social distancing, the wearing of face masks, and lockdown were rapidly established within Germany and kept in place until March 22, 2020, before they were subsequently slowly relaxed due to a decreased infection rate. However, there was some interregional variability in terms of the specific societal protective measures implemented, where the restrictions and protection plans differed among regions and even hospitals.
This high-risk work environment has led to a feeling of vulnerability for many HCWs (Canova et al., 2020). The perception that hospitals are high risk areas has also resulted in delays in patients seeking treatment in emergency situations (such as heart attack and stroke) (Bersano et al., 2020, De Rosa et al., 2020). Due to these perceptions, an unknown number of deaths may have resulted from the fear of SARS-CoV-2 infection.
Moreover, according to previous international studies, insufficient clinical data are available about the dissemination of SARS-CoV-2 in the normal population compared with HCWs. The existing data only highlight the prevalence of SARS-CoV-2 in small or medium size groups of people (Wölfel et al., 2020). More representative longitudinal studies are needed to address this problem and the first results of longitudinal studies were published recently (Behrens et al., 2020b, Behrens et al., 2020a).
Thus, we initiated a prospective trial to evaluate the longitudinal spread of SARS-CoV-2 in a secondary care medium-sized hospital during different stages of restrictions. Doctors and nurses were overrepresented in this trial but it included all employees, such as cleaning staff, and housekeeping and administration staff. In addition, all inhabitants of an affiliated convent were included in the study because of their close patient interactions, such as pastoral care. Our trial also covered a wide socioeconomic range and a good cross-section in terms of gender, age, and risk groups. The study population was considered to be representative of high risk individuals working in the health care system.
A short-term evaluation of the prospective data provided an overview of the evidence regarding the effectiveness of local hygiene protocols.
2. Materials and methods
2.1. Study design
All hospital employees and nuns aged between 18 and 90 years at the study center were given the opportunity to participate in this longitudinal monocentric trial. No pretesting was performed and the only exclusion criteria were individuals feeling too unwell to participate at the outset of the study or lacking the capacity to understand informed consent.
The study center is a secondary care hospital located in the province of Schleswig-Holstein close to the border of the city of Hamburg in Germany. The hospital care for patients from three different regions: Schleswig-Holstein, Hamburg, and Lower Saxony. Hamburg was one of the hotspot regions during the SARS-CoV-2 pandemic in Germany. The hospital has 370 inpatient beds. During the study period, two wards were dedicated solely as SARS-CoV-2 isolation wards, with a total capacity of 50 patients. The hospital has a usual critical care capacity of 20 patients who can be treated in the ICU (including mechanical ventilation). During the study period, 18 confirmed COVID-19 patients were treated in the isolation wards.
A hospital-wide local hygiene standard was established at the beginning of the pandemic situation prior to treating the first COVID-19 patient (Table 1 ).
Table 1.
Hygiene protocol for COVID-19 at the study center.
| Hygiene protocol at the study center for COVID-19 |
|---|
| All patients with suspected COVID-19 infection or compatible symptoms admitted directly to designated isolation wards. |
| All employees with direct patient care must wear surgical face masks and hospital issued clothing during the whole shift. |
| All employees with no direct patient care must wear face masks during the whole shift. |
| Hand hygiene must be performed according to the guidelines provided by the Robert Koch Institute and the World Health Organization |
| Access to the hospital is strictly regulated. |
| No training courses are held in the hospital. Tumor boards or other meetings are held with a limited number of members. |
| Access to the ICU is only allowed for registered staff members. |
From March 25, 2020, all staff members involved with direct patient care (PC) were required to adhere to basic hygiene standards (such as wearing hospital clothing and surgical masks) for the duration of their shift. All non-medical staff members or staff members with no direct patient care (NPC) responsibilities, such as administrative, pastoral care, logistics, or facility management staff, were instructed to only wear a face mask. In addition, all employees who worked with suspected or confirmed COVID-19 patients were required to wear personal protective equipment (PPE), including filtering face piece masks type 2 or 3 (FFP-2/FFP-3).
All study participants provided written and informed consent prior to enrolment.
2.2. Study activities
All participants completed an initial questionnaire containing items regarding demographics, general health and medication, working area, and risk of potential SARS-CoV-2 exposure.
All study participants were asked for a weekly oropharyngeal swab (OPS) according to the recommendations of the Centers of Disease Control and Prevention (CDC)(Centers of Disease Control and Prevention, 2020b) . In addition, the participants could choose to provide either a weekly or monthly blood specimen as part of the requirements for their participation. However, participants were not excluded if they were unable to provide the requested regular specimens. The sampling period began on April 14, 2020.
Medical employees were requested to provide their own OPS, and blood specimens were collected by other trained medical colleagues. OPS and blood specimens were collected from all non-medical employees by study staff wearing PPE (Corman et al., 2020).
Study participants who tested positive for SARS-CoV-2 RNA by PCR were informed immediately about their positive PCR result by the hospital's occupational health provider, and a list of contact patients and contact personal was generated. Participants who tested positive for anti-SARS-CoV-2 immunoglobulin G (IgG) antibodies were also informed by the occupational health provider.
2.3. PCR and serological SARS-CoV-2-analysis
In the initial phase of the study period, antibody testing was performed for anti-SARS-CoV-2 IgG using a recomWell SARS-CoV-2 IgG immunoassay test (Mikrogen Diagnostik, Germany) according to the manufacturer's instructions for blood serum samples. This enzyme-linked immunosorbent assay (ELISA) targets the SARS-CoV-2 nucleocapsid.
OPS samples were transported at room temperature within 24 h after providing the sample. Initial testing was performed using the cobas SARS-CoV-2 test (Roche Diagnostics) for screening and control. However, due to capacity limitations in the initial phase, ampliCube Coronavirus Panel (Mikrogen Diagnostics, Germany) for screening and RealStar SARS-CoV-2 RT-PCR Kit (Altona Diagnostics) as a control were used as alternative test kits.
In the second week of the study period, a change in the laboratory was necessary due to the local capacity. In the second laboratory, OPS samples were tested using the same PCR test kits.
Antibody testing was performed using an anti-SARS-CoV-2 ELISA (IgG) test kit from Euroimmun (Lübeck, Germany), which detects the S1 domain of the SARS-CoV-2 spike protein.
The follow-up after the study period employed the Architect anti-SARS-CoV-2-IgG antibody assay from Abbott (Illinois, USA),targeting the viral nucleocapsid.
2.4. Statistical analysis
The analyses were mainly descriptive because this was a prospective observational study.
All variables were calculated as means or medians with standard deviations. Categorical variables were expressed as numbers with percentages. The chi-square test was used to assess the relationships between categorical variables, and the t-test was used to assess the significance of differences between two sets of data. p < 0.05 was considered to indicate a statistically significant difference. The relationships between antibody status and selected covariables were analyzed by logistic regression. Odds ratios and 95% confidence Intervals were calculated.
Statistical analyses were performed using IBM SPSS Statistics Version 25 (IBM Co., Armonk, NY, USA).
2.5. Ethical approval
The study was approved by the Ethics Committee 038/20 I of the medical association Schleswig-Holstein, Germany and it was registered on the German Clinical Trial Register (DRKS00021270). The study was conducted in accordance with the Declaration of Helsinki.
3. Results
In total, 871/1081 (80.57%) employees at the study center were recruited for this study. No participants met the exclusion criteria. All samples were provided voluntarily. The study period was 9 weeks and it began on April 14, 2020.
Among the participants, 654 (75.09%) were women and 217 (24.91%) were men. The mean age was 40.0 (± 14.2) years. The largest professional groups represented in the study were nurses (n = 299), doctors (n = 149), and students or trainees (n = 71). Table 2 provides an overview of the characteristics of the participants according to the two subgroups comprising PC or NPC.
Table 2.
Participant demographics, medical history, and SARS-CoV-2 positive swab within the study period. Children: Participants living together with children < 16 years. Other: All other diseases.
| Patient care (n = 611) | No patient care (n = 260) | p-value | |
|---|---|---|---|
| Sex | |||
| Male (%) | 152 (24.92) | 65 (24.90) | |
| Female (%) | 458 (75.08) | 196 (75.10) | |
| Age (years) | 38.22 | 48.48 | <0.0001 |
| 18–35 (%) | 300 (49.10) | 52 (20.00) | |
| 35–50 (%) | 170 (27.82) | 78 (30.00) | |
| >50 (%) | 141 (23.08) | 130 (50.00) | |
| Body mass unit | 25.28 | 26.76 | 0.001 |
| Size of household | 2.63 | 4.18 | <0.0001 |
| Children (%) | 204 (33.39) | 76 (29.23) | 0.229 |
| Current smoker (%) | 159 (26.02) | 68 (26.15) | 0.968 |
| Medical history | |||
| Cardiac (%) | 70 (11.46) | 51 (19.62) | 0.001 |
| Pulmonary (%) | 62 (10.15) | 24 (9.23) | 0.678 |
| Metabolic (%) | 73 (11.95) | 36 (13.85) | 0.438 |
| Immunology (%) | 15 (2.45) | 19 (7.31) | 0.001 |
| Other (%) | 113 (18.49) | 46 (17.69) | 0.779 |
| SARS-CoV-2 positive (%) | 2 (0.33) | 1 (0.38) | 0.895 |
3.1. Detection of SARS-CoV-2-RNA by PCR
Throughout the duration of the study period, 5329 OPS samples were collected and analyzed. SARS-CoV-2 RNA was detected by PCR in three participants (0.34%) during the course of the study period, i.e., one physician (PC), one nurse (PC), and one nun working in pastoral care (NPC). Two of the study participants with positive OPS samples reported mild symptoms such as coughing or sneezing, and one reported being completely asymptomatic at the time of diagnosis but this participant developed delayed symptoms after diagnosis. No participants had suspected SARS-CoV-2. One of the three participants was hospitalized due to respiratory symptoms. None of the three individuals required intensive care. The transmission path could not be evaluated in any case. All detected infections were followed by an intensive screening program for possible contact patients or staff in order to prevent an outbreak in the hospital and its departments. Following contact screening, none of the patients or staff members were subsequently tested positive for SARS-CoV-2.
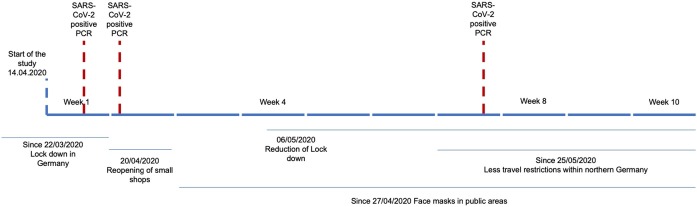
Figure 1 shows the timeline of the study period, including the local restrictions and positive PCR test results.
Figure 1.
Timeline of the study period including positive PCR tests for SARS-CoV-2.
3.2. Anti-SARS-CoV-2 IgG antibodies
In total, 2136 blood specimens were collected and analyzed.
Following both tests, SARS-CoV-2 IgG antibodies were detected in 23/871 patients (positive results: Mikrogen n = 20, Euroimmun n = 3), with a seroprevalence of 2.64%. One participant lost their antibody positivity within 84 days according to the Euroimmun assay. In addition, 15 further patients (1.61%; Mikrogen n = 8, Euroimmun n = 7) were identified with equivocal seropositive IgG antibody ratios.
Among the equivocal results determined by the Mikrogen assay, 7/8 contained no anti-SARS-CoV-2 antibodies in the follow-up samples (time after initial equivocal result: 23 to 77 days) and one individual did not submit a follow-up sample.
The three seropositive individuals according to the Euroimmun assay submitted a follow-up sample that confirmed the initial positive results (Table 3 ). In further statistical analyses, the equivocal positive results were treated as positive results.
Table 3.
Individuals with positive or equivocal SARS-CoV-2 antibody test results during the trial. NA: not available
| Individual | SCO Mikrogen | Euroimmun IgG ratio | Days to first follow-up by Euroimmun assay | Euroimmun IgG-ratio | Days to second follow-up by Euroimmun assay |
|---|---|---|---|---|---|
| Individuals tested POSITIVE by the Mikrogen assay with NEGATIVE follow-up by Euroimmun assay | |||||
| 1 | 40.9 | 0.2 | 41 | 0.2 | 57 |
| 2 | 47.6 | 0.1 | 29 | NA | NA |
| 3 | 67.1 | 0.2 | 8 | 0.1 | 40 |
| 4 | 44.5 | 0.2 | 7 | 0.2 | 39 |
| 5 | 31.0 | 0.1 | 33 | 0.2 | 84 |
| 6 | 36.0 | 0.3 | 55 | 0.2 | 84 |
| 7 | 26.8 | 0.4 | 84 | NA | NA |
| 8 | 30.5 | 0.2 | 57 | NA | NA |
| 9 | 30.4 | 0.2 | 84 | NA | NA |
| 10 | 25.2 | 0.2 | 84 | NA | NA |
| 11 | 36.8 | 0.1 | 27 | 0.2 | 78 |
| 12 | 31.2 | 0.1 | 10 | 0.1 | 25 |
| 13 | 81.1 | 0.2 | 8 | 0.1 | 29 |
| 14 | 24.7 | 0.2 | 79 | NA | NA |
| 15 | 26.5 | 0.2 | 33 | 0.1 | 56 |
| 16 | 31.3 | 0.1 | 30 | 0.2 | 78 |
| 17 | 26.7 | NA | NA | NA | NA |
| 18 | 44.8 | NA | NA | NA | NA |
| 19 | 24.2 | 0.2 | 8 | 0.2 | 28 |
| 20 | 24.2 | 0.2 | 84 | NA | NA |
| Individuals tested POSITIVE by the Euroimmun assay without previous test | |||||
| 21 | NA | 3.7 | NA | 1.38 | 22 |
| 22 | NA | 1.3 | NA | 1.4 | 26 |
| 23 | NA | 2.6 | NA | 2.8 | 65 |
| Individuals tested EQUIVOCAL by the Mikrogen assay and NEGATIVE follow-up | |||||
| 24 | 23.4 | 0.2 | 23 | 0.2 | 46 |
| 25 | 20.7 | 0.2 | 56 | NA | NA |
| 26 | 20.4 | 0.3 | 41 | 0.3 | 64 |
| 27 | 23.3 | 0.3 | 64 | 0.4 | 68 |
| 28 | 20.2 | 0.3 | 77 | NA | NA |
| 29 | 21 | 0.2 | 36 | 0.3 | 79 |
| 30 | 20.6 | 0.2 | 36 | 0.2 | 57 |
| 31 | 22.3 | NA | NA | NA | NA |
| Individuals tested EQUIVOCAL by the Euroimmun assay without previous test | |||||
| 32 | NA | 0.8 | NA | NA | NA |
| 33 | NA | 0.9 | NA | NA | NA |
| 34 | NA | 0.9 | NA | NA | NA |
| 35 | NA | 0.8 | NA | NA | NA |
| 36 | NA | 0.9 | NA | NA | NA |
| 37 | NA | 0.8 | NA | NA | NA |
| 38 | NA | 1.0 | NA | 0.6 | 84 |
* Mikrogen assay (equivocal: ratio ≥ 20 to >24; seropositive: ratio ≥ 24)
* Euroimmun assay (equivocal: ratio ≥ 0.8 to < 1.1; seropositive: ratio ≥ 1.1)
Logistic regression analysis (Table 4 ) detected significant relationships between antibody status with age and body mass index, but not with sex and employment group. The likelihood of a positive antibody test increased with increasing age (odds ratio for a 10 year difference = 1.5) but decreased with increasing body mass index (odds ratio for an increase of 5 = 0.72).
Table 4.
Logistic regression analysis. Significant relationships were found between antibody status with age and body mass index, but not with sex and employment group. Patient Care 1,00: Yes, 2.00: No
| Variable | Odds ratio | 95% confidence interval | |
|---|---|---|---|
| Body mass index (increase of 5 kg/m2) | 0.722 | (0.56, | 0.94) |
| Sex male vs. female | 1.098 | (0.50, | 2.39) |
| Age (difference 10 years) | 1.503 | (1.19, | 1.90) |
| Patient care 1.00 vs 2.00 | 0.847 | (0.41, | 1.74) |
An additional follow-up was performed to evaluate the antibody assays, which detected no positivity following the Abbott and Euroimmun assays in all individuals with a positive or equivocal initial Mikrogen assay who provided a follow-up blood specimen (Table 5 ). All individuals who tested positive following the Euroimmun assay also tested positive using all three assays in the follow-up.
Table 5.
Follow-up comparison of different anti-SARS-CoV-2-antibody assays.
| Individual | SCO Mikrogen | Euroimmun IgG ratio |
Abbott Architect |
|---|---|---|---|
| Individuals tested initially POSITIVE by the Mikrogen assay | |||
| 1 | 32.00 | 0.1 | 0.09 |
| 3 | 61.60 | 0.1 | 0.05 |
| 4 | 23.00 | 0.1 | 0.21 |
| 5 | 38.90 | 0.1 | NA |
| 6 | 39.20 | 0.1 | 0.05 |
| 7 | 18.80 | 0.1 | NA |
| 8 | 34.40 | 0.1 | 0.02 |
| 10 | 30.60 | 0.1 | 0.02 |
| 11 | 36.30 | 0.1 | 0.05 |
| 12 | 37.10 | 0.1 | 0.07 |
| 14 | 26.60 | 0.1 | 0.05 |
| 15 | 24.00 | 0.1 | 0.02 |
| 17 | 21.80 | 0.1 | 0.02 |
| Individuals tested initially POSITIVE by the Euroimmun assay | |||
| 21 | 73.90 | 2.8 | 1.6 |
| 22 | 52.70 | 1.3 | 4.87 |
| 23 | 24.10 | 0.9 | 1.59 |
| Individuals tested initially EQUIVOCAL by the Mikrogen assay | |||
| 25 | 21.80 | 0.1 | 0.07 |
| 26 | 21.00 | 0.1 | 0.02 |
| 27 | 14.40 | 0.2 | 0.21 |
| 28 | 11.70 | 0.1 | 0.02 |
| 30 | 19.60 | 0.1 | 0.31 |
Individual refers to the individuals presented in Table 3, NA: not available.
Mikrogen assay (equivocal: ratio ≥ 20 to >24; seropositive: ratio ≥ 24)
Euroimmun assay (equivocal: ratio ≥ 0.8 to < 1.1; seropositive: ratio ≥ 1.1)
Abbott assay (seronegative: ratio: <1.4; seropositive: ratio ≥ 1.4)
4. Discussion
Several studies have reported a higher transmission risk of SARS-CoV-2 in HCWs (Barrett et al., 2020, Gao et al., 2020, Li et al., 2020b, Liu et al., 2020, Reusken et al., 2020). The aim of the present study was to examine the longitudinal prevalence of SARS-CoV-2 detected in employees of a health care facility affected by the COVID-19 outbreak from the beginning of the highly active pandemic phase in a country with an overall low prevalence of SARS-CoV-2 and a highly developed health care system. During the study period of 9 weeks, 3/871 (0.34%) participants had a positive OPS for SARS-CoV-2 and they were placed in quarantine. No one who worked on an isolation ward or who treated COVID-19 patients tested positive within the period of this trial, thereby highlighting the importance of adhering to local and national hygiene guidelines to prevent patient-to-staff infections (Korth et al., 2020). A higher transmission rate could lead to a higher rate of SARS-CoV-2-positive OPS in HCWs, as shown in different parts of the world (Keeley et al., 2020, Kluytmans et al., 2020).
However, the results obtained in the present study do not support this conclusion. Similarly, Barrett et al. did not confirm this conclusion based on their study in the United States (Barrett et al., 2020).
Thus, regional differences have been reported in the spread and resulting responses of hospitals around the world. Lessels et al. reported data from an outbreak in a South African hospital that demonstrated the importance of local hygiene protocols for preventing rapid transmission to NPC employees (Lessells and Moosa, 2020). Data from German hospitals are limited regarding the rate of positive PCR tests for SARS-CoV-2 among employees and these data mostly provide a snapshot (Korth et al., 2020). The low rate of positive PCR tests supports the suggestion by Kabesch et al. that wearing face masks in hospitals might reduce the infection rate (Kabesch et al., 2020).
The cumulative incidence of SARS-CoV-2 infections during the study period in the region where the hospital is located was 0.04% (Kreis Stormarn, 2020). According to data obtained in the present study, the cumulative incidence in the hospital was almost 10 times higher than that outside the hospital. However, it is important to note that the employees lived in many different regions where the community incidence of SARS-CoV-2 could also have been variable. This factor was not measurable and it may have influenced the results obtained in this study. Furthermore, asymptomatic and untested individuals were not represented in the cumulative regional incidence.
4.1. Detection of SARS-CoV-2-RNA by PCR
In this study, OPS samples were used to detect SARS-CoV-2-RNA by PCR, as recommended by the Robert Koch Institute at the start of the study period Robert-Koch-Institut Internet, 2020, and this is still the recommendation of the CDC (Centers of Disease Control and Prevention, 2020a). OPS samples were collected by trained medical participants according to the instructions provided by the Robert Koch Institute (Robert-Koch-Institut Internet, 2020), or by specially trained staff members. Wehrhahn et al. showed that taking your own swab is an acceptable alternative for obtaining OPS (Wehrhahn et al., 2020). In the present study, it was considered appropriate to use both forms of specimen collection to avoid the risk of larger groups gathering.
4.2. Prevalence of anti-SARS-CoV-2 IgG antibodies
In this study, the overall seroprevalence of IgG antibodies against SARS-CoV-2 was 4.36%.
The seroprevalence was 4.13% using the Mikrogen assay, whereas the prevalence was 1.52% with the Euroimmun assay. Excluding equivocal results, the overall seroprevalence in this trial was 2.64%. A conclusive comparison was not possible because longitudinal testing was conducted with different assays. According to the additional Abbott assay, there might have been a large number of false positive results with the Mikrogen assay and the overall seroprevalence was 1.52% after their exclusion.
Korth et al. obtained antibody-positive results for 1.6% of their study population in another German hospital using the Euroimmun assay (Korth et al., 2020).
Another study that tested blood donors in Germany determined an overall seroprevalence of 0.91% using different antibody assays (Fischer et al., 2020).
Behrens et al. recently published the first results of their prospective longitudinal serological study from a large German hospital, where the seroprevalence was 1.86% for SARS-CoV-2 IgG (Euroimmun assay) with 0.93% additional equivocal test results (Behrens et al., 2020a).
Table 6 provides an overview of the serological evaluations reported for German HCWs, including ongoing studies, longitudinal studies, and single time-point evaluations.
Table 6.
Seroprevalence detected for anti-SARS-CoV-2 antibodies in German health care workers.
| Period | N | SARS-CoV-2 antibody rate | SARS-CoV-2 infection rate | Assay used | Additional information | |
|---|---|---|---|---|---|---|
| Krankenhaus Reinbek | 9 weeks | 871 | 4.36% | 0.344% | Mikrogen/Euroimmun | Ongoing |
| Behrens et al., 2020a | 6 weeks | 217 | 1.86% | Not performed | Euroimmun | Questionnaire used, ongoing trial |
| Schmidt et al., 2020 | 10 days | 406 | 2.9% | Not performed | Euroimmun | Questionnaire used |
| Korth et al., 2020 | 4 weeks | 317 | 1.6% | Not performed | Euroimmun | |
| Fill Malfertheiner et al., 2020 | 12 weeks | 166 | 12.65% | 16.27% | Euroimmun/Elecsys | |
| Epstude and Harsch, 2020 | 15 days | 65 | 1.54% | Not performed | Euroimmun | |
| Harsch et al., 2020 | 5 days | 18 | 0% | Not performed | Euroimmun | Single ward |
At present, the rate of seroconversion and potential related factors remain unclear. Antibodies against SARS-CoV-2 play a key role in the development of herd immunity(Kwok et al., 2020). Korth et al. concluded that HCWs with antibodies against SARS-CoV-2 could have a lower risk of COVID-19 (Korth et al., 2020). However, a positive antibody test currently gives no guarantee of immunity (Perera et al., 2020).
In this study, only two out of three SARS-CoV-2 PCR-positive participants provided a follow-up blood specimen. One participant tested positive for SARS-CoV-2 IgG antibodies according to the Euroimmun assay. The other participant initially tested equivocal positive after infection and negative in the follow-up. This initially equivocal positive result in an already confirmed infection led to the inclusion of equivocal positive results in the group of positive tested participants.
In this trial, a considerable number of the 28 participants who tested positive or equivocal for SARS-CoV-2 IgG had a negative test result in the follow-up. This discrepancy was also described by Behrens et al. who suggested that a positive serological test result should be confirmed using an alternative test (Behrens et al., 2020a). Various possible reasons might explain this seroconversion to negative results.
In particular, the longitudinal study design may explain the loss of antibodies over time. The data indicated different results after comparing both assays within a time period ranging from one week to 84 days. The loss of antibodies over time was also shown by Long et al. in previous PCR-positive individuals in China (Long et al., 2020). In addition, Ibarrondo et al. recently reported an early decrease in the antibody titers of people with mild symptoms of SARS-CoV-2 (Ibarrondo et al., 2020).
Another possible explanation is variability in the performance characteristics of the different assays used during the study period.
To evaluate the potential causes, we conducted another follow-up after the study period.
In this reevaluation, we included the Abbott assay in addition to the Mikrogen and Euroimmun assays (Bryan et al., 2020). This follow-up allowed us to interpret the higher rate of false positive results in the Mikrogen assay and it confirmed the suggestion by Behrens et al. that at least two different assays should be performed to determine a positive antibody status (Behrens et al., 2020a). All individuals who tested positive following the Euroimmun assay tested positive in the follow-up with all three assays.
Only three participants had a persistent antibody status according to two different assays, thereby resulting in an antibody-positive rate of 0.34%.
A large number of participants were considered to have undergone seroconversion to a negative antibody status according to the Euroimmun assay, which may be explained by the different antigens used (n-capsid by Mikrogen versus spike protein by Euroimmun). In addition, the discrepancies in the results according to different tests may have been related to their specificity and sensitivity. The Euroimmun assay approved by the Food and Drug Administration has a sensitivity of 90.0% and specificity close to 100.0% according to tests in less than 100 individuals U.S. Food and Drug Administration (2020). Recent studies have shown that this serological ELISA has a high specificity of 99–100% and a sensitivity up to 65% (U.S. Food and Drug Administration, 2020; Lassaunière et al., 2020, Meyer et al., 2020, Montesinos et al., 2020). By contrast, the Mikrogen assay is not listed by the FDA, and it has a sensitivity of 98.0% and specificity of 98.7% (Mikrogen Diagnostik, 2020). Krüttgen et al. compared both assays and found that the specificity and sensitivity were similar in their cohort (Krüttgen et al., 2020). The differences in the specificity may appear very low but they would have considerable consequences if the pre-test probability is very low. For example, the overall seroprevalence in the general German population is approximately 1% (Fischer et al., 2020). With a seroprevalence of 1% and specificity of 99%, the positive predictive value would only be 50%, and thus half of the positive results would be false positives. This amount can be reduced by using at least two different antibody assays. Further evaluations including additional serological assays and mid- and long-term follow-up samples for all positive tested participants are required to finally determine the actual antibody status of these individuals. Unfortunately, not all employees could be tested using both assays because of the restricted testing capacities and the need to change the laboratory during the trial.
Logistic regression determined a positive correlation between age and antibody status. Filho et al. found a negative correlation between age and antibody status in Brazil and suggested that this might have been caused by younger people gathering together in groups(Amorim Filho et al., 2020). The positive correlation found in our study may be explained by the fact that the nuns lived, worked, and communed together, or it could have been due to overestimation because of the small number of cases. This correlation should be reevaluated in further seroprevalence studies.
4.3. Limitations of the study
Due to the low incidence of SARS-CoV-2 infections during the study period, evaluations were not feasible of potential risk factors for hospitalization such as medical history, smoking, or obesity. Further limitations of this study include the problems associated with collecting samples and potential delays in their transportation. Medical staff were asked to take their own OPS samples and they handed them directly to the study center, so a clear evaluation of the sample collection time points was not possible. It was also impossible to obtain daily reports from study participants regarding possible COVID-19-symptoms. For all of the positive tested participants (either by PCR or antibodies), the source of exposure leading to the infection or to seroconversion could not be identified. This problem was also discussed by Barrett et al. (Barrett et al., 2020). Contact tracing was more complicated because the present study did not consider possible contacts outside the hospital work environment.
Due to capacity limitations, it was necessary to change the laboratory operation during the study period, which led to changes in the test protocols and this limited the longitudinal comparability.
Females and young people (in the group aged 18–30 years) were highly overrepresented in the data, especially in the PC group. Therefore, the study population in this secondary care hospital was probably representative of other health care centers (Brandstetter et al., 2020). The significant differences between both groups in terms of their previous medical history might have been attributable to the older median age in the NPC group.
The small number of SARS-CoV-2 positive PCR results limited the statistical analyses. However, it may have reflected the fact that HCWs are used to hygiene protocols and routinely equipped with adequate PPE. Alternatively, it could have indicated a lower prevalence of SARS-CoV-2 in the area of the study.
In summary, despite the limitations of this study, we obtained longitudinal epidemiological observations regarding the infection rate among high risk HCWs.
5. Conclusion
This study provides the first longitudinal data regarding the spread of SARS-CoV-2 in a hospital caring for COVID-19 patients. The low PCR-positive incidence and seroprevalence during the study period highlight the potential effectiveness of the local, regional, and national protection plans, as well as reinforcing the idea that PPE measures play important roles in preventing the spread of SARS-CoV-2.
The results contradict the suggestion that there is a higher transmission risk for employees in hospitals. The longitudinal analysis conducted in this study covered different phases of lockdown and the slow reopening.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
The study was approved by Ethics Committee 038/20 I of the medical association Schleswig-Holstein, Germany and it was registered in the German Clinical Trial Register (DRKS00021270). The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank the entire team at Krankenhaus Reinbek St. Adolf-Stift and all our staff members, as well as all employees in the participating laboratories that analyzed the samples in addition to their current immense workload. Prof. Dr. Antonio Krüger (German Research Center for Artificial Intelligence) supported this study with important inputs during the conception period.
Special thanks are also given to Alexander Ulmer (technical support, conception), Malin Wendt (graphic design), and Rebecca Zimmer (linguistic enrichment) for their great help and input throughout the entire study process.
References
- Amorim Filho L., Szwarcwald C.L., Mateos S., de O.G., Leon ACMP D.E., Medronho R de A., Veloso V.G. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica. 2020;54:69. doi: 10.11606/s1518-8787.2020054002643. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32638883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E.S., Horton D.B., Roy J., Gennaro M.L., Brooks A., Tischfield J. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. medRxiv. 2020 doi: 10.1186/s12879-020-05587-2. https://www.medrxiv.org/content/10.1101/2020.04.20.v1 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens G.M.N., Cossmann A., Stankov M.V., Schulte B., Streeck H., Förster R. Strategic Anti-SARS-CoV-2 Serology Testing in a Low Prevalence Setting: The COVID-19 Contact (CoCo) Study in Healthcare Professionals. Infect Dis Ther. 2020 doi: 10.1007/s40121-020-00334-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32886335 [cited 2020 Sep 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens G.M.N., Cossmann A., Stankov M.V., Witte T., Ernst D., Happle C. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection [Internet]. 2020;48(4):631–634. doi: 10.1007/s15010-020-01461-0. Aug [cited 2020 Sep 22]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32524515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersano A., Kraemer M., Touzé E., Weber R., Alamowitch S., Sibon I. Stroke Care During the Covid-19 Pandemic: Experience From Three Large European Countries. Eur J Neurol [Internet]. 2020 doi: 10.1111/ene.14375. https://pubmed.ncbi.nlm.nih.gov/32492764/?from_term=covid-19+reduction+of+care&from_page=2&from_pos=8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter S., Roth S., Harner S., Buntrock-Döpke H., Toncheva A., Borchers N. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020;May doi: 10.1111/pai.13278. [DOI] [PubMed] [Google Scholar]
- Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol [Internet] 2020;58(8) doi: 10.1128/JCM.00941-20. Jul 23 [cited 2020 Sep 29]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32381641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canova V., Lederer Schlpfer H., Piso R.J., Droll A., Fenner L., Hoffmann T. Transmission risk of SARS-CoV-2 to healthcare workers–observational results of a primary care hospital contact tracing. Swiss Med Wkly [Internet] 2020;150(1718) doi: 10.4414/smw.2020.20257. Apr 25 [cited 2020 Jun 4]; Available from 2020.20257. [DOI] [PubMed] [Google Scholar]
- Centers of Disease Control Prevention. CDC . 2020. Coronavirus Disease 2019 (COVID-19) [Internet] [cited 2020a Jun 22]. Available from https://www.cdc.gov/coronavirus/2019-ncov/index.html. [Google Scholar]
- Centers of Disease Control Prevention . 2019. Interim Guidelines for Clinical Specimens for COVID-19 | CDC [Internet] [cited 2020b Jun 22]. Available from https://www.cdc.gov/coronavirus/2019--nCoV/lab/guidelines-clinical-specimens.html. [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance [Internet] 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Jan 23 [cited 2020 Apr 19]; Available from https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstude J., Harsch I.A. Seroprevalence of COVID-19 antibodies in the cleaning and oncological staff of a municipal clinic. GMS Hyg Infect Control [Internet] 2020;15:Doc18. doi: 10.3205/dgkh000353. [cited 2020 Sep 22]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32733782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill Malfertheiner S., Brandstetter S., Roth S., Harner S., Buntrock-Döpke H., Toncheva A.A. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: A prospective longitudinal study. J Clin Virol [Internet] 2020;130:104575. doi: 10.1016/j.jcv.2020.104575. Sep [cited 2020 Sep 22]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32805631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Knabbe C., Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Eurosurveillance [Internet] 2020;25(28):2001285. doi: 10.2807/1560-7917.ES.2020.25.28.2001285. Jul 16 [cited 2020 Jul 17]; Available from https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.28.2001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Sanna M., Tsai M.K., Wen C.P. Geo-temporal distribution of 1,688 Chinese healthcare workers infected with COVID-19 in severe conditions-A secondary data analysis. PLoS One [Internet] 2020;15(5):e0233255. doi: 10.1371/journal.pone.0233255. [cited 2020 Jun 22]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32407411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsch I.A., Skiba M., Konturek P.C., Epstude J. Prevalence of antibodies against COVID-19 in the staff of a COVID-19 regular ward. GMS Hyg Infect Control [Internet] 2020;15:Doc09. doi: 10.3205/dgkh000344. [cited 2020 Sep 22]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan China. The New England journal of medicine. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med [Internet] 2020 doi: 10.1056/NEJMc2025179. Jul 21 [cited 2020 Aug 7];NEJMc2025179. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M., Roth S., Brandstetter S., Häusler S., Juraschko E., Weigl M. Successful containment of Covid-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr Allergy Immunol [Internet] 2020;00:1–5. doi: 10.1111/pai.13265. [cited 2020 Jun 22]; Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7264500/pdf/PAI-9999-na.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley A.J., Evans C., Colton H., Ankcorn M., Cope A., State A. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill [Internet] 2020;25(14) doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [cited 2020 Jun 23]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32290904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans M., Buiting A., Pas S., Bentvelsen R., van den Bijllaardt W., van Oudheusden A. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv [Internet] 2020 Jan 1;2020.03.23.20041913. Available from http://medrxiv.org/content/early/2020/03/31/2020.03.23.20041913.Abstract. [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis Stormarn . 2020. Zahl der bestätigten Corona-Fälle in Stormarn–Kreis Stormarn [Internet] [cited 2020 Jun 23]. Available from https://www.kreis-stormarn.de/aktuelles/pressemeldungen/2020/zahl-der-bestaetigten-corona-faelle-in-stormarn.html. [Google Scholar]
- Krüttgen A., Cornelissen C.G., Dreher M., Hornef M., Imöhl M., Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J Clin Virol [Internet]. 2020;128:104394. doi: 10.1016/j.jcv.2020.104394. [cited 2020 Jul 27]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32416599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., Tang J.W.T. Herd immunity - estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect [Internet] 2020;80(6):e32–e33. doi: 10.1016/j.jinf.2020.03.027. [cited 2020 Jun 23]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32209383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C., Fomsgaard A., Krogfelt K.A. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv [Internet] 2020 Apr 10 [cited 2020 Aug 7];2020.04.09.20056325. Available from https://www.medrxiv.org/content/10.1101/2020.04.09.20056325v1. [Google Scholar]
- Lessells R., Moosa Y., De Oliveira T. 2020. Report into a nosocomial outbreak of coronavirus disease 2019 (COVID-19) at Netcare St. Augustine's Hospital [Internet]. Available from https://www.krisp.org.za/manuscripts/StAugustinesHospitalOutbreakInvestigation_FinalReport_15may2020_comp.pdf. [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science [Internet] 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [cited 2020 Jun 22]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32179701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science [Internet] 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [cited 2020 Jun 9]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32179701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ouyang L., Guo P., Wu H., sheng, Fu P., Chen Y., liang Epidemiological, Clinical Characteristics and Outcome of Medical Staff Infected with COVID-19 in Wuhan, China: A Retrospective Case Series Analysis. medRxiv [Internet] 2020 Mar 13 [cited 2020 Jun 23];2020.03.09.20033118. Available from https://www.medrxiv.org/content/10.1101/2020.03.09.20033118v1. [Google Scholar]
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med [Internet] 2020:1–5. doi: 10.1038/s41591-020-0965-6. Jun 18 [cited 2020 Jul 13]. Available from http://www.nature.com/articles/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Meyer B., Torriani G., Yerly S., Mazza L., Calame A., Arm-Vernez I. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin Microbiol Infect [Internet] 2020;0(0) doi: 10.1016/j.cmi.2020.06.024. Jun 27 [cited 2020 Aug 7]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikrogen Diagnostik . 2020. Mikrogen - recomWell SARS-CoV-2 IgG/IgA [Internet] [cited 2020 Jul 2]. Available from https://www.mikrogen.de/produkte/produktuebersicht/testsystem/sars-cov-2-igg.html. [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill [Internet]. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [cited 2020 Jun 23]; Available from http://www.ncbi.nlm.nih.gov/pubmed/32183930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance [Internet] 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. Mar 12 [cited 2020 Jun 9]. Available from https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos I., Gruson D., Kabamba B., Dahma H., Van den Wijngaert S., Reza S. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol [Internet] 2020;128:104413. doi: 10.1016/j.jcv.2020.104413. [cited 2020 Aug 7]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32403010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A., Mok C.K., Tsang O.T., Lv H., Ko R.L., Wu N.C. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill [Internet] 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [cited 2020 Jun 23]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32347204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua J., Weng L., Ling L., Egi M., Lim C.-M., Divatia J.V. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med [Internet] 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [cited 2020 Jul 27]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32272080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Buiting A., Bleeker-Rovers C., Diederen B., Hooiveld M., Friesema I. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, the Netherlands, March 2020. Euro Surveill [Internet] 2020;25(12) doi: 10.2807/1560-7917.ES.2020.25.12.2000334. [cited 2020 Jun 22]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32234115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S., Spaccarotella C., Basso C., Calabrò M.P., Curcio A., Filardi P.P. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J [Internet] 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. Jun 7 [cited 2020 Jun 10]. Available from http://www.ncbi.nlm.nih.gov/pubmed/32412631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med [Internet] 2020;382(10):970. doi: 10.1056/NEJMc2001468. [cited 2020 Apr 19]. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7120970/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.B., Grüter L., Boltzmann M., Rollnik J.D. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS One [Internet] 2020;15(6):e0235417. doi: 10.1371/journal.pone.0235417. [cited 2020 Sep 22]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32584894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Koch-Institut . Robert-Koch-Institut; 2020. Robert-Koch-Institut [Internet] [cited 2020 Jun 24]. Available from https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Vorl_Testung_nCoV.html. [Google Scholar]
- The Lancet TL COVID-19: protecting health-care workers. Lancet (London, England) [Internet] 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [cited 2020 Jun 4]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32199474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrhahn M.C., Robson J., Brown S., Bursle E., Byrne S., New D. Self-collection: An appropriate alternative during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128:104417. doi: 10.1016/j.jcv.2020.104417. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. 2020. Virological assessment of hospitalized patients with COVID-2019. [cited 2020 Apr 19] [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration . U.S. Food & Drug Administration; 2020. EUA Authorized Serology Test Performance | FDA [Internet] [cited Jul 27]. Available from https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance. [Google Scholar]
- World Health Organization . 2020. WHO Coronavirus Disease (COVID-19) Dashboard [Internet] [cited 2020 Aug 31]. Available from: https://covid19.who.int. [Google Scholar]