Abstract
The clinical application of lung ultrasound (LUS) in the assessment of coronavirus disease 2019 (COVID-19) pneumonia severity remains limited. Herein, we investigated the role of LUS imaging in COVID-19 pneumonia patients and the relationship between LUS findings and disease severity. This was a retrospective, observational study at Tongji Hospital in Wuhan, on 48 recruited patients with COVID-19 pneumonia, including 32 non-critically ill patients and 16 critically ill patients. LUS was performed and the respiratory rate oxygenation (ROX) index, disease severity, and confusion, blood urea nitrogen, respiratory rate, blood pressure, and age (CURB-65) score were recorded on days 0–7, 8–14, and 15–21 after symptom onset. Lung images were divided into 12 regions, and the LUS score (0–36 points) was calculated. Chest computed tomography (CT) scores (0–20 points) were also recorded on days 0–7. Correlations between the LUS score, ROX index, and CURB-65 scores were examined. LUS detected COVID-19 pneumonia in 38 patients. LUS signs included B lines (34/38, 89.5%), consolidations (6/38, 15.8%), and pleural effusions (2/38, 5.3%). Most cases showed more than one lesion (32/38, 84.2%) and involved both lungs (28/38, 73.7%). Compared with non-critically ill patients, the LUS scores of critically ill patients were higher (12 (10–18) vs 2 (0–5), p < 0.001). The LUS score showed significant negative correlations with the ROX index on days 0–7 (r = −0.85, p < 0.001), days 8–14 (r = −0.71, p < 0.001), and days 15–21 (r = −0.76, p < 0.001) after symptom onset. However, the LUS score was positively correlated with the CT score (r = 0.82, p < 0.001). The number of patients with LUS-detected lesions decreased from 27 cases (81.8%) to 20 cases (46.5%), and the LUS scores significantly decreased from 4 (2–10) to 0 (0–5) (p < 0.001) from days 0–7 to 17–21. We conclude that LUS can detect lung lesions in COVID-19 pneumonia patients in a portable, real-time, and safe manner. Thus, LUS is helpful in assessing COVID-19 pneumonia severity in critically ill patients.
Keywords: Coronavirus disease 2019, Lung ultrasound, Pneumonia
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak a pandemic [1]. Patients with SARS-CoV-2 infection can develop coronavirus disease 2019 (COVID-19). As of 14 Aug 2020, more than 20 730 000 COVID-19 cases have been confirmed and more than 751 000 deaths have occurred worldwide [2]. More than 89 600 COVID-19 cases have been confirmed in China; approximately 20% of these were severe cases, with a mortality rate of approximately 5.4% (as of 14 Aug 2020) [2]. COVID-19 can lead to respiratory failure and secondary multiple organ failure, ultimately increasing mortality [3]. At the same time, COVID-19 pneumonia can complicate and change the clinical situation rapidly. Therefore, it is important to diagnose, accurately assess the extent of the disease, and monitor lung lesions in a timely manner in real time without increasing the risk of contagion.
Lung ultrasound (LUS) is an effective imaging method for diagnosing pleural and pulmonary lesions; it can be performed continuously, in real time, and with no radiation exposure, to evaluate disease severity [4]. The LUS score is a semi-quantitative scoring system that has been applied for diagnosing, progression monitoring, and prognosis predicting, in conditions such as acute respiratory distress syndrome (ARDS), pneumonia, hydrothorax, and pulmonary edema [5], [6], [7], [8].
Some studies have evaluated the efficacy of LUS in the assessment of COVID-19 pneumonia severity. Sofia et al. [9] described ultrasonic and clinical findings at different clinical stages in COVID-19. Two articles reported that the LUS features of COVID-19 pneumonia/ARDS can be used to assess disease severity in 20 patients with COVID-19 pneumonia [10], [11]. Two narrative reviews suggested that the LUS score could objectively grade COVID-19 associated lung injury [12], [13]. However, the relationship between the LUS score and the disease severity of COVID-19 pneumonia is not clearly defined. This study investigated the LUS features of COVID-19 pneumonia and the relationship between LUS findings and COVID-19 pneumonia severity.
2. Materials and methods
2.1. Study design and participants
This retrospective, observational study was conducted at the Sino-French Xincheng Branch of Tongji Hospital of the Huazhong University of Science and Technology in Wuhan, China. The institution is a tertiary teaching hospital responsible for the treatment of government-referred patients with COVID-19. The Aid Hubei Medical Team of Peking University People’s Hospital took over one of the wards at the Sino-French Xincheng Branch of Tongji Hospital on 7 February 2020 to care for patients with severe COVID-19. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. [14].
Patients with COVID-19 pneumonia were consecutively recruited between 7 February 2020 and 8 March 2020. All patients were over 18 years old and met the WHO interim guidance diagnostic criteria for COVID-19 pneumonia [15]. SARS-CoV-2 infection was confirmed by a nucleic acid test with real-time polymerase chain reaction (RT-PCR) tests of a nasopharyngeal swab sample, in accordance with a previously protocol [16]. The exclusion criteria were as follows: pulmonary edema related to heart failure; chronic obstructive pulmonary diseases (COPDs); severe hemodynamic instability and inability to change body position; severe thoracic deformity, extensive subcutaneous emphysema, and inability to undergo a LUS examination; and patients without LUS data of all 12 lung regions according to the LUS protocol.
Based on the time interval between onset of symptoms and the LUS examination, we designated three groups of patients in our study: group 1 (LUS examination done 0–7 d after symptom onset); group 2 (LUS examination done 8–14 d after symptom onset); and group 3 (LUS examination done 15–21 d after symptom onset).
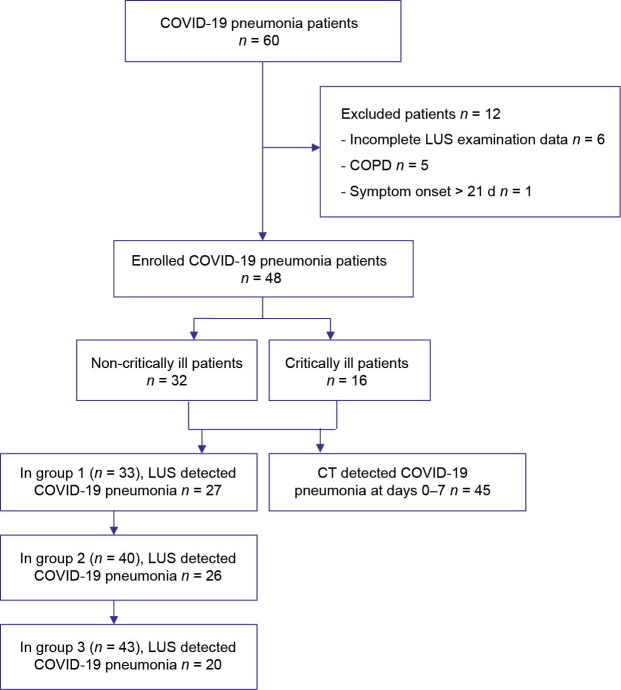
Between 7 February 2020 and 8 March 2020, 60 patients with COVID-19 pneumonia were recruited. Twelve patients were excluded because of incomplete LUS examination data (n = 6), the presence of COPDs (n = 5), or an interval between symptom onset and LUS examination greater than 21 d (n = 1). Finally, the data of 48 patients with COVID-19 pneumonia were included. Based on the time interval between symptom onset and LUS examination, 33 patients were assigned to group 1, 40 to group 2, and 43 to group 3 (Fig. 1 ).
Fig. 1.
Flowchart showing the process of enrolment, exclusion, and assessment of recruited patients with COVID-19 pneumonia.
This study was approved by the Medical Ethics Committee of Peking University People’s Hospital (2020PHB048-01). Informed consent was orally obtained from all patients.
2.2. Data collection
2.2.1. Clinical data
The severity of COVID-19 pneumonia was defined according to the Chinese specific management guidelines (version 7.0) [17]. Patients with COVID-19 pneumonia were divided into two groups—critically ill and non-critically ill—according to the disease severity. Critically ill patients met one of the following conditions: ① Respiratory failure had occurred and mechanical ventilation was required; ② shock had occurred; or ③ other organ failure requiring intensive care unit (ICU) treatment had occurred. Of the 48 cases of COVID-19 pneumonia, 32 were classified as non-critically ill and 16 as critically ill. Patient characteristics were retrieved from the electronic patient data management system. The collected baseline data included sex, age, comorbidities (hypertension, diabetes mellitus, coronary heart disease, and chronic renal failure), clinical symptoms (temperature > 37.3 °C, cough, purulent expectoration, dyspnea, and thoracic pain), respiratory data (pulse oxygen saturation, respiratory rate, fraction of inspired oxygen (FiO2), the respiratory rate oxygenation (ROX) index, and invasive or noninvasive mechanical ventilation), the confusion, blood urea nitrogen, respiratory rate, blood pressure, and age (CURB-65) score, and laboratory indicators (SARS-CoV-2 nucleic acid test results, leukocyte and lymphocyte counts, C-reactive protein (CRP) levels, and interleukin-6 (IL-6) levels). Changes in disease severity, CURB-65 score, and ROX index were recorded for groups 1, 2, and 3 at the same time as the LUS examinations.
The FiO2 in nasal catheter patients was calculated according to the following formula: FiO2 (%) = 21 + 4 × oxygen flow rate (in L∙min−1) [18]. A FiO2 of 5–8 L∙min−1 by face mask was approximated as 50%, a FiO2 of 8–10 L∙min−1 was approximated as 60%, and a FiO2 above 10 L∙min−1 was approximated as 80%. The actual FiO2 monitored by the ventilator was considered to be the FiO2 of mechanical ventilation. The ROX index, defined as the ratio of oxygen saturation as measured by pulse oximetry/FiO2 to respiratory rate, was assessed as a clinical indicator of oxygenation [19]. The objective was to maintain the pulse oxygen saturation above 93%. If the oxygen administered through a nasal catheter or face mask could not achieve the target pulse oxygen saturation, patients were provided with noninvasive or invasive mechanical ventilation. The CURB-65 score was calculated using confusion (C), blood urea nitrogen (U) > 7 mmol∙L−1, respiratory rate (R) > 30 breaths per minute, blood pressure (B) (systolic pressure < 90 mmHg (1 mmHg ≈ 133.322 Pa) or diastolic pressure < 60 mmHg), and age > 65 years old [20]. One point was allocated to each positive item, for a total score of up to 5 points.
2.2.2. Lung ultrasound
Three well-trained critical care physicians from the research team performed LUS on days 0–7, 8–14, and 15–21. LUS was performed on the same day as the computed tomography (CT) scan. The ultrasonographers were trained according to the Chinese Critical Ultrasound Study Group (CCUSG) training course [21]. Their experience ranged from one to three years. They were blinded to the patients’ clinical details and CT findings, and did not contribute to the patients’ diagnostic or treatment strategies. We primarily used an Esaote MyLab Alpha ultrasound machine (Esaote Europe B.V., the Netherlands) with a 3.5–5.0 MHz convex ultrasound probe. A 7.5 MHz linear probe was occasionally used. LUS-related images included A lines, B lines, consolidations, pleural line changes, and pleural effusions.
2.2.3. Lung ultrasound score
The scanning protocol included the assessment of six lung regions in each lung (upper anterior, lower anterior, upper lateral, lower lateral, upper posterior, and lower posterior) [22]. Each region was assigned a score as follows [22]: 0 point for normal aeration; 1 point for moderate loss of aeration (multiple spaced B lines, or coalescent B lines in less than 50% of the intercostal space examined in the transversal plane, or subpleural consolidations); 2 points for severe loss of aeration (diffused coalescent B lines occupying the whole intercostal space); and 3 points for complete loss of lung aeration (lung consolidation). The most severe ultrasound pattern observed in one or several intercostal spaces was considered to be characteristic for the region of interest. All patients underwent LUS and had 12 lung regions examined. The final LUS score was the sum of the scores of the 12 regions (0–36 points).
Inter-observer agreement was assessed in a random subset of the study population. We calculated that a sample size of 70 measurements would be required, according to a difference in overall agreement and a change probability of 0.6, with a 20% accepted relative error. Seventy LUS points were assessed to determine the inter-observer agreement. LUS images were analyzed independently by two researchers. Any disputes were resolved by consulting with a third researcher until an agreement was reached. LUS image readers were blinded to the patients’ clinical details and CT findings.
2.2.4. Chest computed tomography
Chest CTs were performed on days 0–7 using a Siemens Sensation 64-row spiral CT machine (Siemens, Germany). The scanning parameters were as follows: tube voltage, 120 kV; tube current, 100–200 mA; slice thickness, 5, 1.0, or 1.25 mm for thin scanning; and high-resolution image reconstruction with soft-tissue and bone reconstruction algorithms. The acquired images were transferred to a workstation. The CT semi-quantitative analysis method was used to evaluate the pulmonary lesions, including ground-glass opacities, consolidation shadows, reticulation/interlobular septal thickening, irregular solid nodules, fibrous stripes, and no related lesions [23]. Each of the five lung lobes was assessed for degree of involvement to obtain the five-grade score [24]: 0 point, no involvement; 1 point, < 25%; 2 points, 26%–50%; 3 points, 51%–75%; and 4 points, 76%–100%. The total score was reached by summing the five lobes scores (ranging from 0 to 20). Chest-CT findings were analyzed by an experienced radiologist and a critical respiratory specialist, both of whom were blinded to the sonographic and clinical details. The consensus of both specialists was required for a CT-based diagnosis.
2.3. Statistical analyses
Continuous variables were tested for normality; the normally distributed variables were expressed as mean ± standard deviation and the non-normally distributed continuous variables were expressed as median (interquartile range). Categorical variables were expressed as percentages. For between-group comparisons, a Student’s t-test was used for continuous variables, a nonparametric Mann–Whitney U test for non-normally distributed continuous variables, and Pearson χ 2 test for categorical variables. The Pearson correlation test was used to compare the correlations of continuous variables, and the Spearman correlation test was used to compare the correlations of categorical variables. A receiver operating characteristic (ROC) curve was used to analyze the LUS score in order to determine the optimal threshold for predicting COVID-19 pneumonia severity. The area under the ROC curve (AUROC) was then calculated. The optimal cut-off value was defined as the point with the highest Youden’s index (sensitivity + specificity − 1). The 95% confidence interval (CI) values were calculated. LUS data and clinical characteristics of different disease courses were compared with a generalized estimating equation for normally distributed variables and categorical variables, and a Friedman test for non-normally distributed continuous variables. A p value < 0.05 was considered to be statistically significant. Cohen’s kappa (κ) statistics were used to assess LUS inter-observer agreement. Statistical analyses were performed using SPSS statistical software package version 17.0 (IBM, USA) and MedCalc version 19.0.7 (MedCalc Software bvba, Belgium).
3. Results
The baseline characteristics of the two patient groups (16 critically ill vs 32 non-critically ill patients) were not significantly different (p > 0.05), including gender (8 men (50.0%) vs 18 men (56.3%)), age ((64.8 ± 11.6) years vs (62.0 ± 14.3) years), or comorbidities, such as coronary heart disease (four cases (25.0%) vs nine cases (28.1%)), diabetes mellitus (four cases (25.0%) vs seven cases (21.9%)). Symptoms such as cough (16 cases (100%) vs 25 cases (78.1%)), purulent expectoration (16 cases (100%) vs 21 cases (65.6%)), dyspnea (11 cases (68.8%) vs 6 cases (18.8%)), and thoracic pain (eight cases (50.0%) vs four cases (12.5%)) were more frequent in critically ill patients than in non-critically ill patients (p < 0.05). All six patients who required mechanical ventilation and all four patients who died were critically ill patients. Baseline patient characteristics are summarized in Table 1 .
Table 1.
Baseline characteristics of patients with COVID-19 pneumonia.
| Characteristic | Non-critical n = 32 |
Critical n = 16 |
p value |
|---|---|---|---|
| Men, n (%) | 18 (56.3) | 8 (50.0) | 0.682 |
| Age (year) | 62.0 ± 14.3 | 64.8 ± 11.6 | 0.513 |
| Comorbidities | |||
| Coronary heart disease, n (%) | 9 (28.1) | 4 (25.0) | 0.818 |
| Hypertension, n (%) | 13 (40.6) | 5(31.3) | 0.527 |
| Diabetes mellitus, n (%) | 7 (21.9) | 4 (25.0) | 0.808 |
| Chronic renal failure, n (%) | 6 (18.8) | 2 (12.5) | 0.584 |
| Symptoms | |||
| Fever, n (%) | 12 (37.5) | 8 (50.0) | 0.408 |
| Cough, n (%) | 25 (78.1) | 16 (100) | 0.043 |
| Purulent expectoration, n (%) | 21 (65.6) | 16(100) | 0.008 |
| Dyspnea, n (%) | 6 (18.8) | 11 (68.8) | 0.001 |
| Thoracic pain, n (%) | 4 (12.5) | 8 (50.0) | 0.005 |
| Laboratory findings | |||
| SARSCoV2 nucleic acid positive, n (%) | 30 (93.8) | 12 (75.0) | 0.064 |
| Leukocyte count, mean ± SD, (109 L−1) | 5.6 ± 2.0 | 7.9 ± 3.7 | 0.007 |
| Lymphocyte count, mean ± SD, (109 L−1) | 1.1 ± 0.5 | 0.9 ± 0.4 | 0.110 |
| CRP, median (IQR) (mg∙L−1) | 25.7 (2.4–81.7) | 81.4 (27.0–185.6) | 0.016 |
| IL-6, median (IQR) (pg∙mL−1) | 12.7 (2.6 – 39.8) | 46.2 (9.2–117.0) | 0.017 |
| LUS score (IQR) | 2 (0–5) | 12 (10–18) | < 0.001 |
| CT score (IQR) | 6 (2–8) | 12 (9–18) | < 0.001 |
| ROX index, mean ± SD | 10.5 ± 2.6 | 5.2 ± 2.4 | < 0.001 |
| CURB-65 (IQR) | 1(0–2) | 3 (2–3) | < 0.001 |
| Mechanical ventilation, n (%) | 0 | 6 (37.5) | |
| Hospital mortality, n (%) | 0 | 4 (25.0) | |
SD: standard deviation; IQR: interquartile range.
3.1. LUS features of COVID-19 pneumonia
Among the 48 patients who underwent LUS, 38 patients were detected with COVID-19 pneumonia based on the following features: B lines, 34 patients (89.5%); pleural line changes, ten patients (26.3%); consolidations, six patients (15.8%); and pleural effusions, two patients (5.3%). Among the ten patients with negative LUS, three patients were also negative on CT, six of the seven patients with positive CT had a CT score ≤ 2, and one patient with a CT score of 6 had lung lesions far away from the pleura. The lesions were most frequently bilateral (28 cases, 73.7%), and 32 patients (84.2%) had more than one lesion. The most common lesions were located at the bilateral lower lateral lung (55 regions, 72.4%) and the bilateral lower posterior lung (62 regions, 81.6%). Overall inter-observer agreement for LUS showed excellent agreement (κ = 0.864, p < 0.001).
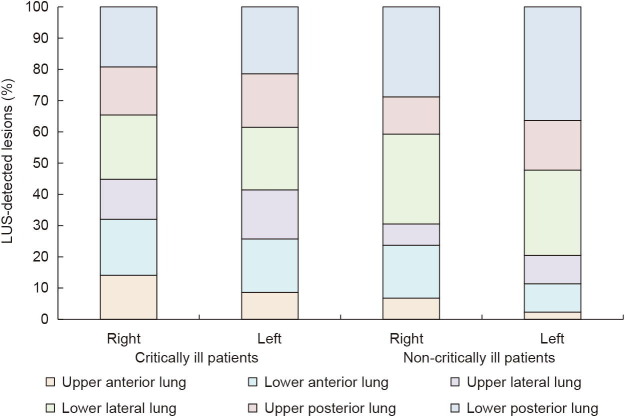
The LUS characteristics of 16 critically ill patients and 32 non-critically ill patients were analyzed. In critically ill patients, B lines were observed in 16 cases (100%), and consolidation in two cases (12.5%). In non-critically ill patients, B lines were observed in 18 cases (56.3%) and consolidation in four cases (12.5%). There were significantly more LUS-detected lesions in critically ill patients than in non-critically ill patients, both proportional in the two patient groups (16 cases (100%) vs 22 cases (68.8%), p = 0.012) and region-wise (148 regions (77.1%) vs 103 regions (26.8%), p < 0.001). Critically ill patients were more likely than non-critically ill patients to have bilateral LUS-detected lesions (15 cases (93.8%) vs 13 cases (40.6%), p < 0.001) and more than one lesion (16 cases (100%) vs 16 cases (50.0%), p = 0.001). LUS-detected lesions were more frequently located in the bilateral lower lateral lung (30 regions (93.8%) vs 25 regions (39.1%), p < 0.001) and lower posterior lung (32 regions (100%) vs 30 regions (46.9%), p < 0.001) in critically ill patients than in non-critically ill patients. These results are shown in Fig. 2 .
Fig. 2.
LUS characteristics according to disease severity. Stacked bars show the proportion of patients with LUS-detected lesions in six regions of unilateral lung according to disease severity.
3.2. LUS score and disease severity
3.2.1. Correlation between LUS score and indicators of disease severity
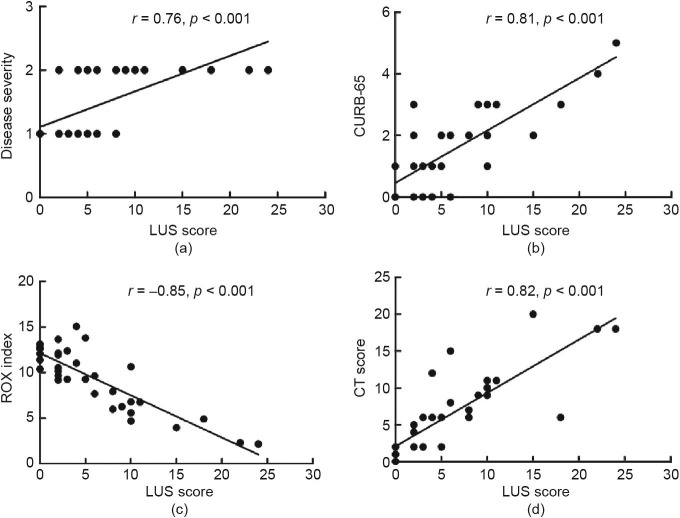
The LUS score was significantly higher in critically ill patients than in non-critically ill patients (12 (10–18) vs 2 (0–5), p < 0.001). In critically ill patients, the ROX index was significant lower ((5.2 ± 2.4) vs (10.5 ± 2.6), p < 0.001), and the CURB-65 score (3 (2–3) vs 1 (0–2), p < 0.001) was significantly higher than those in non-critically ill patients (Table 1). From group 1 to group 3, the LUS score significantly decreased (4 (2–10) vs 0 (0–5), p < 0.001), while the ROX index ((9.3 ± 3.4) vs (14.9 ± 6.8), p < 0.001) significantly increased. The LUS score showed significant negative correlations with the ROX index (r = −0.85, 95% CI: –0.92 to –0.72, p < 0.001), and significant positive correlations with disease severity (r = 0.76, 95% CI: 0.56–0.88, p < 0.001) and CURB-65 score (r = 0.81, 95% CI: 0.64–0.90, p < 0.001), in group 1 (Fig. 3 ). The LUS score showed significant negative correlations with the ROX index (r = −0.71, 95% CI: –0.83 to –0.50, p < 0.001), and significant positive correlations with disease severity (r = 0.63, 95% CI: 0.39–0.79, p < 0.001) and CURB-65 score (r = 0.70, 95% CI: 0.50–0.83, p < 0.001), in group 2. The LUS score showed significant negative correlations with the ROX index (r = −0.76, 95% CI: –0.86 to –0.59, p < 0.001), and significant positive correlations with disease severity (r = 0.56, 95% CI: 0.31–0.74, p < 0.001) and CURB-65 score (r = 0.71, 95% CI: 0.53–0.84, p < 0.001), in group 3.
Fig. 3.
Correlation of LUS score with disease severity on days 0–7. (a) LUS score and disease severity; (b) LUS score and CURB-65; (c) LUS score and ROX index; (d) LUS score and CT score.
3.2.2. LUS score versus CT score in assessing disease severity
All 48 patients underwent CT on days 0–7, and 45 patients were CT-positive. The CT scores of critically ill patients were significantly higher than those of non-critically ill patients (12 (9–18) vs 6 (2–8), p < 0.001). The CT score was negatively correlated with ROX index (r = −0.70, 95% CI: –0.84 to –0.47, p < 0.001), and positively correlated with CURB-65 score (r = 0.62, 95% CI: 0.35–0.79, p < 0.001) and disease severity (r = 0.60, 95% CI: 0.32–0.79, p < 0.001).
The LUS score was positively correlated with the CT score (r = 0.82, 95% CI: 0.66–0.91, p < 0.001) (Fig. 3). The correlation coefficient between the LUS score and the ROX index was compared with the correlation coefficient between the CT score and the ROX index (r = −0.85 vs –0.70), with no statistical difference (p > 0.05). This indicates that LUS may be an alternative to CT in evaluating disease severity.
3.2.3. LUS cut-off value in assessing critically ill patients
The AUROC of the LUS score assessing critically ill patients was 0.96 (95% CI: 0.88–0.99), with a cut-off value of 7, a sensitivity of 80.8% (95% CI: 60.6%–93.4%), and a specificity of 95.8% (95% CI: 85.7%–99.5%) (Fig. 4 ).
Fig. 4.
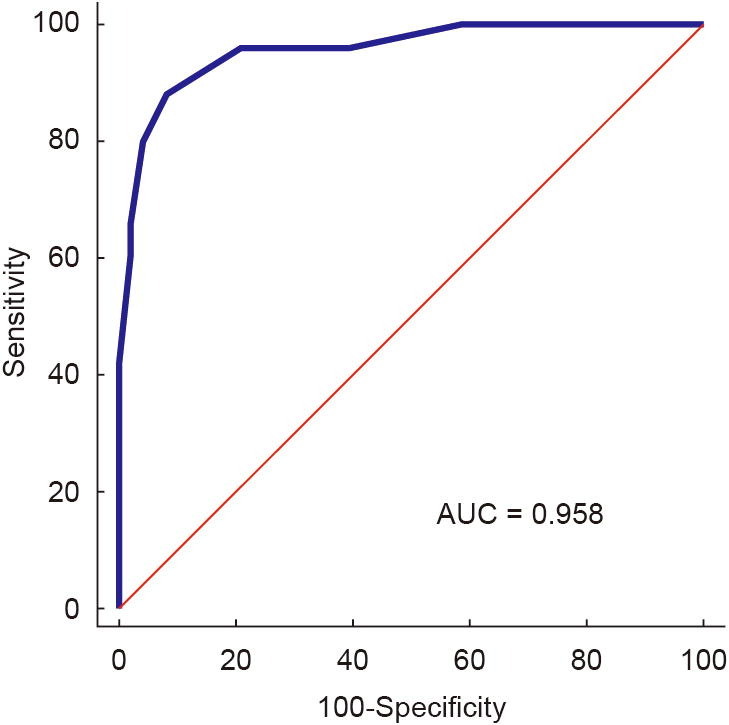
ROCs for LUS score predicting the severity of COVID-19 pneumonia. AUC: area under curve.
3.3. LUS and different disease courses
In group 1, 27 out of 33 patients with COVID-19 pneumonia were detected by LUS; in group 2, 26 out of 40 patients were detected by LUS; and in group 3, 20 out of 43 patients were detected by LUS. From group 1 to group 3, disease remission was demonstrated by LUS. From group 1 to group 3, the number of patients with LUS-detected lesions decreased from 27 (81.8%) to 20 (46.5%) (p = 0.001). The number of patients with B lines decreased from 27 (81.8%) to 20 (46.5%) (p = 0.001). The number of patients with more than one lesion decreased from 27 (81.8%) to 19 (44.2%) (p < 0.001). The number of patients with bilateral lesions declined from 20 (60.6%) to 17 (39.5%) (p = 0.001) (Table 2 and Fig. 5 ).
Table 2.
Ultrasonic features and clinical and laboratory findings at days 0–7, 8–14, and 15–21 after symptom onset in patients with COVID-19 pneumonia.
| Sonographic, clinical, and laboratory finding | Group 1 n = 33 |
Group 2 n = 40 |
Group 3 n = 43 |
p valuea |
|---|---|---|---|---|
| Patients with LUS-detected lesions, n (%) | 27 (81.8) | 26 (65.0) | 20 (46.5) | 0.001 |
| Features of LUS-detected lesions | ||||
| B lines, n (%) | 27 (81.8) | 26 (65.0) | 20 (46.5) | 0.001 |
| Consolidation, n (%) | 3 (9.1) | 5 (12.5) | 3 (7.0) | 0.214 |
| Pleural line changes, n (%) | 7 (21.2) | 5 (12.5) | 6 (14.0) | 0.284 |
| Pleural effusion, n (%) | 2 (6.1) | 2 (5.0) | 1 (2.3) | 0.426 |
| Locations of lung lesions | ||||
| Patients with > 1 lesion, n (%) | 27 (81.8) | 26 (65.0) | 19 (44.2) | < 0.001 |
| On the right side, n (%) | 4 (12.1) | 5 (12.5) | 2 (4.7) | 0.082 |
| On the left side, n (%) | 3 (9.1) | 0 | 1 (2.3) | 0.231 |
| On both sides, n (%) | 20 (60.6) | 21 (52.5) | 17 (39.5) | 0.001 |
| LUS-detected lesions per region | ||||
| Bilateral upper anterior lung, n (%) | 14 (21.2) | 13 (16.3) | 16 (18.6) | 0.084 |
| Bilateral lower anterior lung, n (%) | 28 (42.4) | 21 (26.3) | 21 (24.4) | 0.001 |
| Bilateral upper lateral lung, n (%) | 20 (30.3) | 16 (20.0) | 14 (16.3) | 0.037 |
| Bilateral lower lateral lung, n (%) | 37 (56.1) | 41 (51.3) | 30 (34.9) | < 0.001 |
| Bilateral upper posterior lung, n (%) | 25 (37.9) | 21 (26.3) | 17 (19.8) | 0.003 |
| Bilateral lower posterior lung, n (%) | 40 (60.6) | 46 (57.5) | 35 (40.7) | 0.003 |
| LUS score (IQR) | 4 (2–10) | 3 (0–5) | 0 (0–5) | < 0.001 |
| CT score (IQR) | 6 (2–11) | |||
| ROX index, mean ± SD | 9.3 ± 3.4 | 11.6 ± 4.8 | 14.9 ± 6.8 | < 0.001 |
| CURB-65 (IQR) | 1 (0–3) | 1 (0–2) | 1 (0–1) | 0.016 |
| Critically ill patients, n (%) | 15 (45.5) | 10 (25.0) | 6 (14.0) | < 0.001 |
| Laboratory findings | ||||
| SARS-CoV-2 nucleic acid positive, n (%) | 33 (100) | 14 (35.0%) | 4 (9.3%) | < 0.001 |
| Leukocyte count, median (IQR) (109 L−1) | 5.1 (4.5–7.2) | 5.8 (5.0–8.4) | 5.5 (4.5–6.6) | 0.840 |
| Lymphocyte count, mean ± SD (109 L−1) | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.4 | 0.015 |
| CRP, median (IQR) (mg∙L−1) | 46.2 (3.3–145.7) | 19.5 (4.1–75.2) | 7.2 (2.5–25.6) | 0.012 |
| IL-6, median (IQR) (pg∙mL−1) | 15.6 (3.5–46.6) | 6.3 (3.2–30.4) | 3.7 (1.7–7.0) | 0.001 |
Data were compared between days 0–7 and 15–21. Patients were grouped by time from symptom onset: group 1 (LUS at days 0–7 after symptom onset); group 2 (LUS at days 8–14 after symptom onset); and group 3 (LUS at days 15–21 after symptom onset).
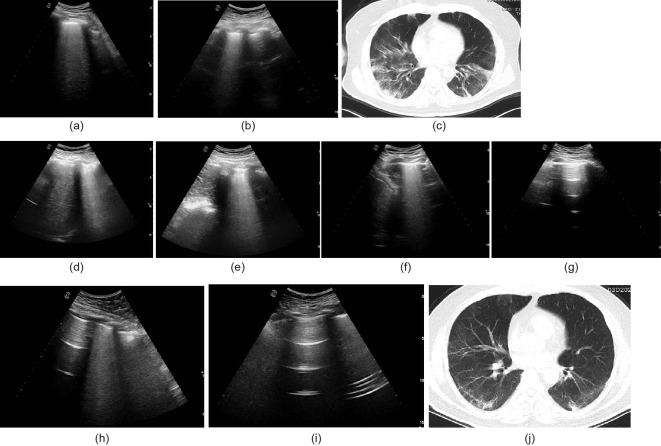
Fig. 5.
A typical case of a COVID-19 pneumonia patient. (a) B lines at the right lower lateral lung reflecting pneumonia on day 6; (b) B lines at the left lower lateral lung reflecting pneumonia on day 6; (c) chest CT showing multiple infiltrations in both lungs on day 6; (d) B lines at the right lower lateral lung reflecting pneumonia on day 13; (e) B lines at the left lower lateral lung reflecting pneumonia on day 13; (f) B lines at the right lower lateral lung reflecting pneumonia on day 17; (g) LUS showing no lesion at the left lower lateral lung on day 17; (h) B lines decreased at the right lower lateral lung on day 24; (i) LUS showing no lesion at the left lower lateral lung on day 24; (j) chest CT showing that the infiltration in both lungs was significantly reduced on day 21.
4. Discussion
After the outbreak of COVID-19, there has been a global pandemic, and the number of COVID-19 patients continues to rise [1]. In patients with comorbidities or complications [25], the hospitalization and intensive care unit admission rates increase significantly [26]. The development of quick and convenient methods to assess COVID-19 pneumonia severity will improve triage decision-making and diagnosis, as well as the choice for subsequent therapy. Currently, studies on COVID-19 severity and prognosis have mainly focused on clinical features and risk factors analysis. Zhou et al. [3] found that older age, a high sequential organ failure assessment (SOFA) score, and D-dimer levels greater than 1 μg∙mL−1 were associated with a higher rate of in-hospital deaths. Thus far, other methods of examination for pneumonia have shortcomings. X-ray cannot predict the mortality and prognosis of ARDS [27]. Furthermore, a chest CT requires patients to be moved to the CT room, which increases the risk of virus exposure and nosocomial outbreaks, and a CT cannot be repeated and performed at any moment. Point-of-care LUS can reduce the risks associated with moving patients and virus spreading in critically ill patients. LUS is a fast, accurate, and portable examination that can be performed in real time with no radiation exposure, to evaluate lung lesions. However, only a few studies have reported employing LUS to assess COVID-19 pneumonia severity [28], [29], [30], and these were mainly descriptive studies or narrative reviews. A study found that LUS was performed to assess the severity of respiratory failure in six patients with COVID-19 [28]. Another study that recruited ten patients with COVID-19 suggested that LUS score monitoring seemed to reflect disease progression [29]. A narrative review indicated that LUS may be used to identify areas of poor lung aeration and to monitor recruitment maneuvers on lung aeration in the ICU [30]. Whereas the present study focused on assessing the value of LUS in patients with critically ill COVID-19 pneumonia and monitoring the disease changes. This study demonstrated a good correlation between LUS score and disease severity, which indicated that the LUS score could be used to distinguish disease severity in COVID-19 pneumonia.
The main findings of our study are as follows: ① The LUS signs of COVID-19 pneumonia are mainly B lines, most lesions on bilateral lesions, more than one lesion, and most lesions located in the bilateral lower lateral lung and the bilateral lower posterior lung, and critically ill patients have more LUS-detected lesions and a higher LUS score than non-critically ill patients; ② the LUS score can assess the severity of COVID-19 pneumonia, and LUS is an alternative modality to a CT scan to evaluate disease severity; and ③ the LUS score significantly decreased with improvement of the disease condition. These findings indicate that during outbreaks of COVID-19 pneumonia, LUS can be an effective method to assess disease severity.
The LUS signs of COVID-19 pneumonia were pulmonary edema (B lines in 89.5% of the patients) and consolidation (15.8%). Most cases showed multiple lesions (84.2%) and were bilateral (73.7%). From days 0–7 to days 15–21 after symptom onset, there was a significant reduction in the percentage of patients with LUS-detected lesions (81.8% vs 46.5%), and the percentage of patients with more than one lesion decreased from 81.8% to 44.2%.
Shi et al. [31] demonstrated chest-CT findings of bilateral, subpleural, and ground-glass opacities, as well as ill-defined boundary lesions, in patients with COVID-19 pneumonia, similar to the lung lesions distribution in our study. An autopsy pathology of COVID-19 showed that the exfoliation reaction was more evident than that in severe acute respiratory syndrome (SARS), while pulmonary fibrosis and consolidation were not as serious as those observed in SARS [32]. Moreover, common radiologic findings of SARS are ground-glass opacities and mixed lesions of ground-glass opacities with irregular consolidation [33]. This is consistent with the findings of this study, in that there were more B lines and less consolidations (15.8% vs 45.5%) [33]. The results of our study indicate that, although there is a wide lesion range in COVID-19 pneumonia, the incidence of interstitial fibrosis may be lower in the later stages of the disease, and the subsequent prognosis may be relatively good.
The LUS score has been widely used in recent years to quantitatively evaluate the severity and prognosis of lung lesions [5], [6], [7], [8]. The number of lung regions and the number of ultrasound scores are mainly used to calculate the total score and to quantify the aeration area of the lungs: the smaller the aeration area, the higher the LUS score. In our study, lung lesions were assessed using the universally acknowledged 12-region LUS scoring method.
One study has found that the LUS score can assess the disease severity of ARDS [5], and two other studies have found that the LUS score can assess the prognosis of ARDS [6], [7]. A study indicated that the LUS score seemed to reflect disease progression in ten patients with COVID-19 [29]. The CURB-65 score has been found to assess the severity of pneumonia [20]. In our study, the LUS score was negatively correlated with the ROX index and positively correlated with the CURB-65 score. The ROX index was used to predict the risk of intubation in patients with high-flow nasal oxygen therapy [19]. In this study, we adopted the ROX index to assess the degree of hypoxia of patients with COVID-19 pneumonia. Thus, the LUS score could assess the severity of COVID-19 pneumonia.
We also analyzed the correlation between the LUS score and the CT score, and found that the correlation coefficient between the LUS score and the CT score was 0.82, indicating that the LUS could be an alternative modality to the CT scan for evaluating disease severity. Similar findings were previously reported in ARDS patients. Chiumello et al. [34] reported that the average global agreement between LUS and CT scan was 0.775 in ARDS patients. Recently, another study on ARDS patients found that the overall agreement of LUS findings with the correlating lobe on CT was 87% [35]. However, LUS is fast, portable, and noninvasive, and can be conducted in real time, which can reduce the risk of infection spreading during outbreaks of COVID-19. Thus, LUS is more suitable for the assessment of COVID-19 pneumonia severity than the CT scan.
The LUS score can also be used to differentiate between various degrees of COVID-19 pneumonia severity. Our results showed that the cut-off value of LUS in the assessment of critically ill patients was 7 points. Li et al. [36] found that, in patients with ARDS, the 12-region LUS score can predict the severity of the disease, with 7 being mild, 11 moderate, and 18 severe. During the global pandemic of COVID-19, a large number of patients may present in a short period of time, leading to a shortage of medical supplies and assessment equipment. LUS could be used for the quick assessment of disease severity and to recognize critically ill patients in early stages, thereby providing a reference for triage, diagnosis, and treatment.
Our study has several limitations. First, this study took place in a single ward with critically ill patients and a small patient sample size. A larger sample size and prospective studies are needed to further evaluate the clinical value of LUS in COVID-19 pneumonia. Second, in our study, patients were treated in the infectious disease ward, but the partial pressure of oxygen (pO2) in the majority of patients was not detected due to limited conditions; thus, the correlation between LUS score and pO2/FiO2 ratio could not be verified. Finally, all patients recruited in our study were inpatients with COVID-19 pneumonia. However, LUS may also be used in the screening of suspected COVID-19 pneumonia cases.
5. Conclusions
LUS can assess lung lesions in COVID-19 pneumonia in a portable, real-time, and safe manner. Furthermore, LUS can conveniently and safely assess the disease severity in critically ill COVID-19 patients. Thus, LUS can be an alternative to CT scans in the assessment of COVID-19 pneumonia severity. Prospective cohort studies or randomized controlled trials are needed to confirm the role of LUS in severity assessment and treatment guidance in COVID-19.
Acknowledgments
Acknowledgements
We acknowledge all healthcare workers involved in the diagnosis and treatment of COVID-19 patients in Wuhan. This work was supported by the Michigan Medicine-Peking University Health Science Center Joint Institute for Translational and Clinical Research (BMU20160527), Peking University Clinical Scientist Program supported by “the Fundamental Research Funds for the Central Universities” (BMU2019LCKXJ005), and the National Natural Science Foundation of China (81971808).
Compliance with ethics guidelines
Fengxue Zhu, Xiujuan Zhao, Tianbing Wang, Zhenzhou Wang, Fuzheng Guo, Haiyan Xue, Panpan Chang, Hansheng Liang, Wentao Ni, Yaxin Wang, Lei Chen, and Baoguo Jiang declare that they have no conflict of interest or financial conflicts to disclose.
References
- 1.WHO director—General’s opening remarks at the media briefing on COVID-19—11 March 2020 [Internet]. Geneva: World Health Organization; 2020 Mar 11 [cited 2020 Aug 14]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 2.Coronavirus disease (COVID-19) situation report—207 [Internet]. Geneva: World Health Organization; 2020 Aug 14 [cited 2020 Aug 14]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200814-covid-19-sitrep-207.pdf?sfvrsn=2f2154e6_2.
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touw H.R., Tuinman P.R., Gelissen H.P., Lust E., Elbers P.W. Lung ultrasound: routine practice for the next generation of internists. Neth J Med. 2015;73(3):100–107. [PubMed] [Google Scholar]
- 5.Pisani L., Vercesi V., van Tongeren P.S.I., Lagrand W.K., Leopold S.J., Huson M.A.M. The diagnostic accuracy for ARDS of global versus regional lung ultrasound scores—a post hoc analysis of an observational study in invasively ventilated ICU patients. Intensive Care Med Exp. 2019;7(Suppl 1):44. doi: 10.1186/s40635-019-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X.T., Ding X., Zhang H.M., Chen H., Su L.X., Liu D.W. Chinese Critical Ultrasound Study Group (CCUSG). Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care. 2016;20(1):385. doi: 10.1186/s13054-016-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin W., Zou T., Qin Y., Yang J., Li Y., Zeng X. Chinese Critical Ultrasound Study Group (CCUSG). Poor lung ultrasound score in shock patients admitted to the ICU is associated with worse outcome. BMC Pulm Med. 2019;19(1):1. doi: 10.1186/s12890-018-0755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z., Jiang L., Xi X., Jiang Q., Zhu B., Wang M. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15(1):98. doi: 10.1186/s12890-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sofia S., Boccatonda A., Montanari M., Spampinato M., D’ardes D., Cocco G. Thoracic ultrasound and SARS–COVID-19: a pictorial essay. J Ultrasound. 2020;23(2):217–221. doi: 10.1007/s40477-020-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Q.Y., Wang X.T., Zhang L.N., Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing C., Li Q., Du H., Kang W., Lian J., Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):174. doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID-19—a narrative review. Anaesthesia. 2020;75(8):1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetrugno L., Bove T., Orso D., Barbariol F., Bassi F., Boero E. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37(4):625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Clinical management of COVID-19—interim guidance [Internet]. Geneva: World Health Organization; 2020 May 27 [cited 2020 Aug 14]. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health Commission of the People’s Republic of China; State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Beijing: National Health Commission of the People’s Republic of China; 2020 Mar 3 [cited 2020 Aug 14]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Chinese.
- 18.Branson R.D., Hess D.R., Chatburn R.L. J.B. Lippincott Company; Philadelphia: 1995. Respiratory care equipment. [Google Scholar]
- 19.Roca O., Caralt B., Messika J., Samper M., Sztrymf B., Hernández G. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 20.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.cc-e-is.org [Internet]. Beijing: Chinese Critical Ultrasound Study Group; c2020 [cited 2020 Aug 14]. Available from: http://www.cc-e-is.org.
- 22.Rouby J.J., Arbelot C., Gao Y., Zhang M., Lv J., An Y. APECHO Study Group. Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med. 2018;198(3):398–401. doi: 10.1164/rccm.201802-0227LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Fang Y., Liao J., Yu W., Yao L., Cui H. Clinical and CT features of the COVID-19 infection: comparison among four different age groups. Eur Geriatr Med. 2020 doi: 10.1007/s41999-020-00356-5. Epub 2020 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K., Fang Y., Li W., Pan C., Qin P., Zhong Y. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228) doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 27.Brown L.M., Calfee C.S., Howard J.P., Craig T.R., Matthay M.A., McAuley D.F. Comparison of thermodilution measured extravascular lung water with chest radiographic assessment of pulmonary oedema in patients with acute lung injury. Ann Intensive Care. 2013;3(1):25. doi: 10.1186/2110-5820-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho Y.J., Song K.H., Lee Y., Yoon J.H., Park J.Y., Jung J. Lung ultrasound for early diagnosis and severity assessment of pneumonia in patients with coronavirus disease 2019. Korean J Intern Med. 2020;35(4):771–781. doi: 10.3904/kjim.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dargent A., Chatelain E., Kreitmann L., Quenot J.P., Cour M., Argaud L. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpicelli G., Lamorte A., Villén T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020;46(7):1445–1448. doi: 10.1007/s00134-020-06048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z. Gross examination report of a COVID-19 death autopsy. J Forensic Med. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang R., Sun H., Song L., Song W., Cui H., Li B. Plain radiograph and CT features of 112 patients with SARS in acute stage. J Peking Univ (Health Sci) 2003;35(Suppl):29–33. Chinese. [PubMed] [Google Scholar]
- 34.Chiumello D., Umbrello M., Sferrazza Papa G.F., Angileri A., Gurgitano M., Formenti P. Global and regional diagnostic accuracy of lung ultrasound compared to CT in patients with acute respiratory distress syndrome. Crit Care Med. 2019;47(11):1599–1606. doi: 10.1097/CCM.0000000000003971. [DOI] [PubMed] [Google Scholar]
- 35.Tierney D.M., Huelster J.S., Overgaard J.D., Plunkett M.B., Boland L.L., St Hill C.A. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48(2):151–157. doi: 10.1097/CCM.0000000000004124. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Yang Q., Li L., Guan J., Liu Z., Han J. The value of lung ultrasound score on evaluating clinical severity and prognosis in patients with acute respiratory distress syndrome. Chin Crit Care Med. 2015;27(7):579–584. doi: 10.3760/cma.j.issn.2095-4352.2015.07.008. Chinese. [DOI] [PubMed] [Google Scholar]