Abstract
Several brain disorders exhibit sex differences in onset, presentation, and prevalence. Increased understanding of the neurobiology of sex-based differences in variability across the lifespan can provide insight into both disease vulnerability and resilience. In n = 3069 participants, from 8 to 95 years of age, we found widespread greater variability in males compared with females in cortical surface area and global and subcortical volumes for discrete brain regions. In contrast, variance in cortical thickness was similar for males and females. These findings were supported by multivariate analysis accounting for structural covariance, and present and stable across the lifespan. Additionally, we examined variability among brain regions by sex. We found significant age-by-sex interactions across neuroimaging metrics, whereby in very early life males had reduced among-region variability compared with females, while in very late life this was reversed. Overall, our findings of greater regional variability, but less among-region variability in males in early life may aid our understanding of sex-based risk for neurodevelopmental disorders. In contrast, our findings in late life may provide a potential sex-based risk mechanism for dementia.
Keywords: healthy variation, sex differences, surface area, variability, vulnerability for disease
Introduction
Sex differences in onset, presentation, and prevalence are common in many brain disorders (American Psychiatric Association 2013), which may include autism spectrum disorders (ASDs, Baio et al. 2018), schizophrenia (Abel et al. 2010; Mendrek and Mancini-Marïe 2016), depression (Hasin et al. 2018; Lim et al. 2018), and Alzheimer’s dementia (AD, Podcasy and Epperson 2016). A better understanding of sex-related differences in healthy brain architecture at different phases of the lifespan may help identify risk factors and protective mechanisms for brain disorders.
To date, studies comparing the brain in males and females have focused primarily on identifying average differences between groups (e.g., DeLacoste-Utamsing and Holloway 1982; Cosgrove et al. 2007; Kaczkurkin et al. 2018). Findings from such studies have often generated controversy (Weis et al. 1989; Fine 2013) and frequently fail to fully address the complexity of the topic (Rippon et al. 2014). For instance, when taking group-averages, males tend to have larger total brain volume (TBV) than females; however, the majority of regional differences reported can be attributed to the difference in TBV (Leonard et al. 2008; Ritchie et al. 2018). Focusing on group differences between the sexes underplays the considerable heterogeneity and overlap between and among the sexes (Rippon et al. 2014).
Recent studies have shown sex-based variability in select populations, after accounting for TBV (Ritchie et al. 2018; Wierenga et al. 2018, 2019). These findings at opposing ends of the lifespan (older adults and youth) are of particular consequence when considering the differences in prevalence between the sexes in certain brain disorders (e.g., neurodevelopmental disorders), and the timing of their onset and course (Baio et al. 2018). However, brain disorders are increasingly understood as ones where relationships between brain regions are disrupted. Examining relationships among regions (Bassett and Sporns 2017) has illuminated our understanding of brain organization across the lifespan and in brain disorders (Bassett and Bullmore 2009; Vértes and Bullmore 2015; Pichet Binette et al. 2020). However, among-region variability in males and females is not yet known. The most recent of these is particularly, noteworthy, given and they find that AD is characterized by higher gray matter volume and among-region heterogeneity (Pichet Binette et al. 2020). AD is also more prevalent in women (Podcasy and Epperson 2016), leading us to hypothesize that there is increased among-region variability in (aging) females that may relate to an increased risk for AD.
Here, we analyze data in over 3000 participants from three large, high-quality, open-source datasets to provide the first investigation (to our knowledge) of structural variability across the lifespan. Notably these datasets are independent of the datasets used previously to investigate brain structure variability (Ritchie et al. 2018; Wierenga et al. 2018, 2019). Collectively, these datasets cover periods of rapid reorganization and development in the brain from childhood through adolescence (Philadelphia Neurodevelopmental Cohort [PNC]; Satterthwaite et al. 2016), a relatively stable period during young adulthood following completion of the majority of developmental processes (Crews et al. 2007; Petanjek et al. 2011; the Human Connectome Project [HCP]; Van Essen et al. 2012), and the re-emergence of dynamic change that occurs in late life (Scahill et al. 2003) as part of the aging process (Open Access Series of Imaging Studies [OASIS-3]; Marcus et al. 2007, 2010; LaMontagne et al. 2018).
Our first aim was to examine sex-based variability by region and measurement type (surface area, cortical thickness, and subcortical volume). Based on recent literature (Ritchie et al. 2018; Wierenga et al. 2018, 2019), we hypothesized that greater variability would be present in surface area and volume measures in males compared with females. Our second aim was to investigate sex-based variability in relationships among brain regions, that is, the overall pattern of relationships between all regions within an individual. We hypothesized that such variability would be age dependent and align with differential risk for complex brain disorders at either end of the lifespan, consistent with age-dependent network reorganization (Gong et al. 2009; Chen et al. 2011; Wu et al. 2012; Pichet Binette et al. 2020), and altered relationships among structures in these disorders.
Materials and Methods
Datasets
Details of T1-weighted magnetic resonance imaging (MRI) acquisition, inclusion/exclusion criteria, and quality assessment can be found in references (Marcus et al. 2007, 2010; Van Essen et al. 2012; Satterthwaite et al. 2016; LaMontagne et al. 2018) and Supplementary Material (see Supplementary Material, Table S1 for basic demographics).
Child and Youth—PNC
Data for participants (n = 1601, aged 8–23) were included from the publicly available PNC dataset (Satterthwaite et al. 2016). Following exclusion and quality assessment, n = 1347 individuals were included for analysis. On average females were slightly older than their male counterparts (Kruskal–Wallis = 6.88, P = 0.01).
Young Adult—HCP
The HCP Young Adult S1200 (age 22–37) data were used (Van Essen et al. 2012). High-quality processed data were available for n = 1113 subjects. Females were older than males on average (Kruskal–Wallis = 73.46, P < 0.0001).
Late-Life—OASIS-3
Data from cognitively normal aging adults (n = 609, 43–95 years) from the OASIS-3 database were used (Marcus et al. 2007, 2010; LaMontagne et al. 2018). OASIS-3 is a longitudinal dataset; however, only data from one time point was included per participant. In each case, the earliest acquisition of good quality data was included. Males were slightly older than females (Kruskal–Wallis = 7.51, P = 0.006).
Data Processing
FreeSurfer was used to segment subcortical structures and generate tessellated, smoothed gray white, and pial surfaces from the T1-weighted data (Dale et al. 1999; Fischl et al. 1999a, 1999b; Fischl and Dale 2000). PNC data were processed in-house with FreeSurfer (v6.0). Metrics were then extracted from regions of the Desikan–Killiany parcellation (Desikan et al. 2006), which includes 34 cortical (surface area and cortical thickness) and seven subcortical regions (volume) per hemisphere. Global volume regions considered were TBV, cerebral and cerebellar gray and white matter. Similar summary metrics were directly available for download for the HCP and OASIS-3 datasets. HCP and OASIS-3 data were processed with FreeSurfer version 5.2 (enhanced version) (Glasser et al. 2013) and 5.3, respectively.
Statistical analysis
Analyses and graph generation were completed in the R statistical environment (v3.4.3) (R Core Team 2013).
Variance Ratio Across Measures and Regions
We regressed the variance associated with age from our measures leaving residuals that were then used for analyses. We applied a generalized additive model (GAM, mgcv package) to allow age to be modeled nonlinearly, avoiding the assumption of a linear, quadratic or cubic association between age and the metrics. Residuals were then Z-scored to provide measures of a similar magnitude for comparison. To compare variance between males and females, a variance ratio (VR) was generated with an F test (var.test). Next, to investigate the role of TBV in sex differences, we conducted similar variance tests on Z-scored residuals from GAM models where TBV (linear) in addition to age (smooth) were regressed out. A linear term for TBV was deemed appropriate based on de Jong et al. (2017).
Mahalanobis Distance
To refine our analyses, we grouped our metrics (corrected for age and Z-scored) by type; global volumes (n = 4), subcortical volumes (n = 14), surface area (n = 68), and cortical thickness (n = 68) and calculated Mahalanobis distance for each subject to their group average. For each metric type, all measures of that type were included per subject. Mahalanobis distance was calculated as the distance from each subject to their group centroid—a multidimensional center point representing the “average” male and “average” female set of metrics—while also accounting for the covariance of metrics. To account for the correlation structure, we utilized a covariance matrix computed over the full sample including both males and females. Thus a higher group average Mahalanobis distance would indicate a greater dispersion of the data in a group relative to its centroid. This was tested with a Welch two sample t-test.
Cosine Angle Dissimilarity
For the second aim, we considered the relationship of regions among each other within a subject, and the dissimilarity of these profiles across participants within one sex compared with the other. Similar to the Mahalanobis distance, analysis metrics were grouped by type (metrics corrected for age and Z-scored). We characterized the unique pattern of metrics, a structural profile, for each individual as a direction vector in multivariate space. Two individuals with similar structural profiles would have similar angles from the origin in multivariate space. Using cosine angle, we calculated the similarity between each individual and their group centroid, per metric type. A centroid was calculated by minimizing the total sum of geodesic distances from the centroid to every other point on a unit sphere using an iterative algorithm generalized to n-dimensional spheres (Buss and Fillmore 2001; Zhelezov 2017). Differences in angular deviation (cosine angle) between the sexes were tested with a t-test. Supplementary Material, Fig. S1 provides a simplified 3D example of the two multivariate approaches.
Age
Additionally to test the influence of age, we regenerated Mahalanobis distances and cosine angles from the data in which the age effect was not regressed out (data still Z-scored). We binned data according to age. Within each bin, Mahalanobis distance and cosine angle were calculated between each subject and their sex’s centroid, per metric type. Binning the data ensured that the age distribution or differences in the age distributions between males and females did not influence findings. To ensure adequate numbers were present in each bin, the OASIS-3 sample was limited to 55–80 years. Linear models with type 2 F-tests were then used to examine the age-by-sex bin interaction.
False discovery rate correction was implemented to account for multiple testing (q < 0.05) within each analysis (i.e., univariate global analyses were corrected for n = 5, subcortical analysis for n = 14, surface area and cortical thickness analyses for n = 68; multivariate analyses were corrected by 4, for the number of metric types).
Results
VR Across Measures and Regions
Volume
Across all three datasets, we found significant VR differences by sex in global (TBV [VR 1.20–1.38, q < 0.09], cerebral gray [VR 1.18–1.32, q < 0.09] and white matter [VR 1.25–1.45, q < 0.05]) and subcortical brain volumes (see Supplementary Material, Table S2 for details), with males exhibiting greater variance compared with females across most regions, while there were no regions in which females were significantly greater than males. Some differences were not consistent across datasets, see Supplementary Material, Table S2; Figs 1 and 2 (top row). For instance, VRs were not significantly different by sex in hippocampus and nucleus accumbens volumes in the PNC dataset, although we did find significance in the HCP (left hippocampus and bilateral nucleus accumbens) and OASIS-3 (bilateral hippocampus and nucleus accumbens) datasets (males > females). Putamen and pallidum volumes did show significant variance differences by sex in the PNC and OASIS-3 datasets (males > females), while there were no significant differences in the HCP data.
Figure 1.
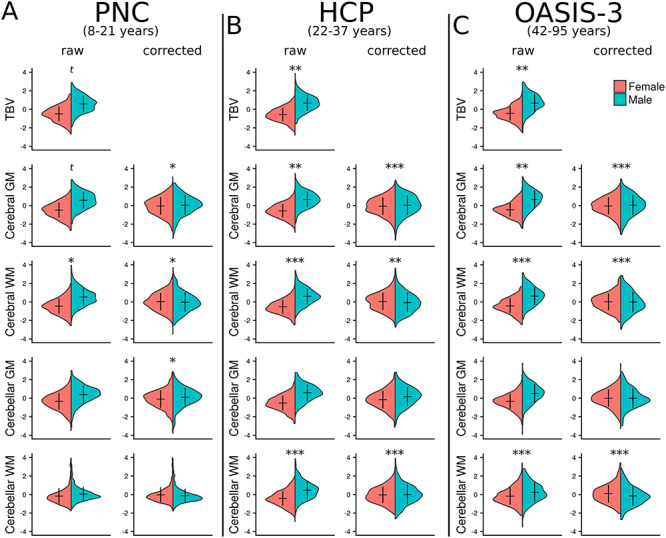
Sex differences on global volume. Global brain volume measures from three independent datasets are shown. (A) The Philadelphia Neurodevelopmental Cohort (PNC). (B) The Human Connectome Project (HCP). (C) The Open Access Series of Imaging Studies (OASIS-3). “Raw” data represent volumes corrected for age (following regression of age from the model). Corresponding “corrected” figures show volume data corrected for total brain volume (TBV) as well as age. Mean and standard deviation of the data are represented by the horizontal and vertical lines, respectively. Significance markers relate to the comparison of variances between groups (variance ratio [VR] analysis); *q < 0.05, **q < 0.01, ***q < 0.001, t—trend (q < 0.1). See Supplementary Material, Table S2 for full statistical results. GM—gray matter, WM—white matter.
Figure 2.
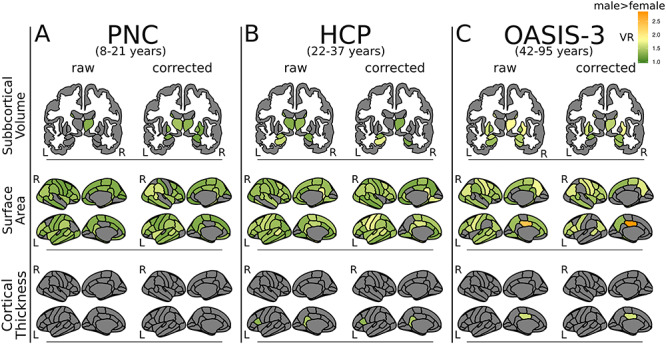
Sex differences in variance. VR results are mapped onto subcortical structures (top row) or cortical surface (middle and bottom panels) for three independent datasets. (A) The PNC. (B) The HCP. (C) The OASIS-3. VRs were generated with F-tests comparing metrics of subcortical volume, cortical surface area and cortical thickness between males and females. Only VR’s that met statistical significance are plotted (q < 0.05). VR > 1 (green–yellow–orange) indicates males > females. There were no regions where females had greater variance than males (VR < 1). “Raw” figures show results from analyses that used metrics corrected for age (following regression of age from the model). Corresponding “corrected” figures show results from analyses that used metrics corrected for TBV as well as age. Note: nucleus accumbens is not included in the figure but does show significant differences in variability between males and females (males higher) in the HCP and OASIS-3 sample (see Supplementary Material, Table S2). L—left, R—right.
Surface Area
Males demonstrated significantly greater variance in surface area compared with females across all datasets across the great majority of cortical regions (Fig. 2, middle 2 rows; PNC: 52/68 regions [VR 1.17–1.56, q < 0.05]; HCP: 59/68 regions [VR 1.19–2.14, q < 0.05]; OASIS-3: 44/68 regions [VR 1.29–2.40, q < 0.05]). There were no regions in which female variance was significantly higher than male.
Cortical Thickness
Variance in cortical thickness was similar between males and females across all datasets (Fig. 2, bottom 2 rows). However, portions of the left cingulate gyrus showed higher variance in males compared with females in the HCP dataset (VR = 1.44, q = 0.001) and the OASIS-3 dataset (VR = 1.59, q = 0.004). Additionally the left pars opercularis was significantly more variable in males compared with females in the HCP dataset (VR = 1.32, q = 0.04).
Across all metric types a comparable pattern of results was seen following correction for TBV (see Supplementary Material, Tables S2–S4).
Mahalanobis Distance
Males demonstrated greater Mahalanobis distance in global volume (F = 5.42–43.26, q < 0.05), subcortical volume (F = 11.63–46.63, q < 0.001), and surface area (F = 43.89–229.86, q < 0.001) compared with females across all datasets (see Supplementary Material, Table S3; Fig. 3). There were no significant sex differences in cortical thickness (q > 0.1). In each dataset, surface area showed the largest sex effect, followed by subcortical volumes and then global volumes (see Supplementary Material, Table S3). Analyses with age showed a significant age-by-sex interaction in surface area of the OASIS-3 sample (F = 7.18, q < 0.001), whereby male Mahalanobis distance is greater over the majority of the age distribution, but within the oldest age bin (75–80 years) females have greater Mahalanobis distance. No other age-by-sex interactions were found (q > 0.05). Significant associations between Mahalanobis distance and age were found across cortical metrics in the OASIS-3 sample; surface area (F = 16.23, q < 0.001) and cortical thickness (F = 14.88, q < 0.001). Cortical thickness Mahalanobis distance followed a nonlinear course first slightly increasing with age before declining in later years. In surface area, the younger–old participants (55–60) had a lower Mahalanobis distance, which then increased in later years.
Figure 3.
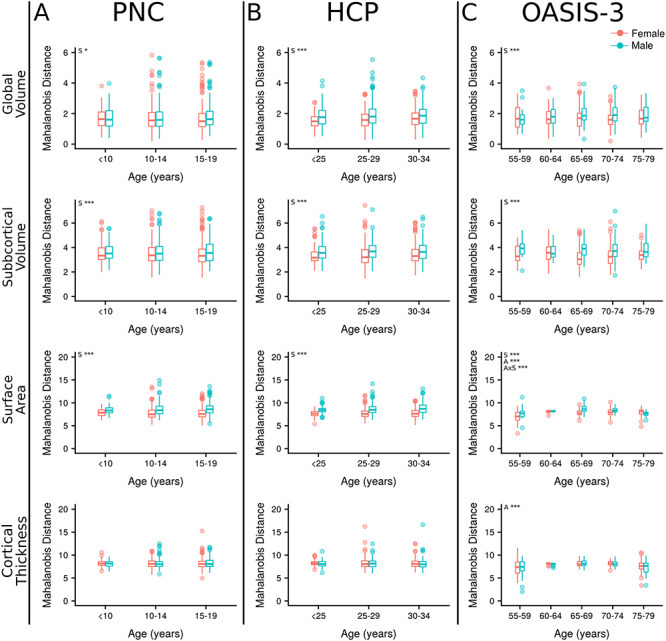
Mahalanobis distance. Mahalanobis distance plotted by age for subjects from three independent datasets. (A) The PNC. (B) The HCP. (C) The OASIS-3. Mahalanobis distance was calculated for each subject in relation to their group (male or female) average. Metrics included in each analysis were grouped by type; global volumes (n = 4), subcortical volumes (n = 14), surface area (n = 68) and cortical thickness (n = 68). S—sex, A—age, AxS—age-by-sex interaction, *q < 0.05, **q < 0.01, ***q < 0.001.
Cosine Angle Dissimilarity
Volume
Cosine angle was significantly higher in females compared with males in the PNC dataset for global volume (F = 6.83, q = 0.03). There was a significant effect of age in both the PNC (F = 4.84, q = 0.01) and HCP samples (F = 6.14, q = 0.007). There was also an age-by-sex interaction with global volume cosine angle in the OASIS-3 dataset (F = 13.18, q < 0.001), such that male cosine angle was higher than female in the latest-life group within this sample (Fig. 4; see Supplementary Material, Table S3).
Figure 4.
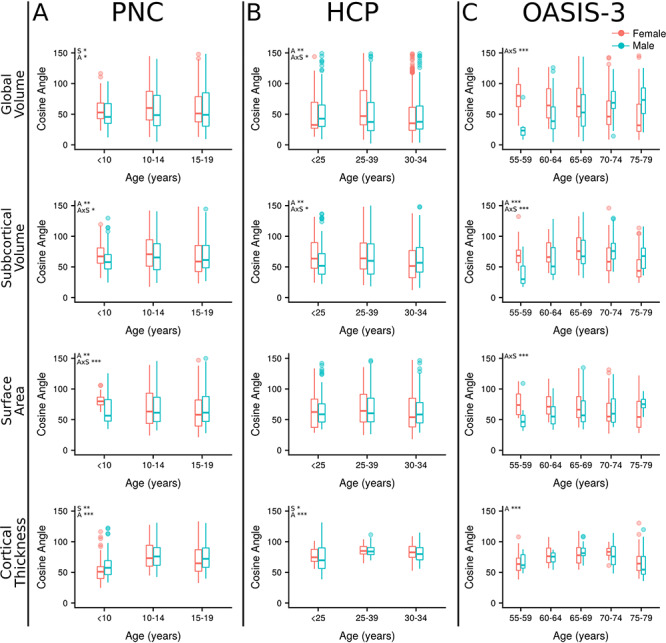
Cosine angle dissimilarity. Cosine angles are plotted by age bin for subjects from three independent datasets. (A) The PNC. (B) The HCP. (C) The OASIS-3. Cosine angle was calculated for each subject to their group (male or female) centroid within each age bin separately on the surface of an n-dimensional sphere. Metrics included in each analysis were grouped by type; global volumes (n = 4), subcortical volumes (n = 14), surface area (n = 68) and cortical thickness (n = 68). S—sex, A—age, AxS—age-by-sex interaction, *q < 0.05, **q < 0.01, ***q < 0.001.
No main effects of sex were present in cosine analysis of subcortical volume (q > 0.1). There were significant effects of age (F = 6.38–9.88, q < 0.01) and age-by-sex interactions (F = 3.64–10.94, q < 0.05) in each sample. Specifically, females had a greater cosine angle in their early life, but males had a greater cosine angle in their later life.
Surface Area
There were no significant effects of sex on surface area (q > 0.1). There was a significant effect of age (F = 6.30, q = 0.006) and an age-by-sex interaction (F = 7.88, q < 0.001) in the PNC dataset, where females displayed a greater cosine angle in their early life (<10 years) while at older ages males and females were similar. In OASIS-3, a similar age-by-sex interaction occurred (F = 7.33, q < 0.001), where males had a greater cosine angle compared with females in older groups of this late-life dataset.
Cortical Thickness
In the PNC dataset, cosine angle was greater in males compared with females (F = 9.16, q = 0.008). In the HCP sample, it was greater in females compared with males (F = 6.61, q = 0.02). Age was significantly associated with cortical thickness cosine angle across all datasets (F = 21.38–55.92, q < 0.001). In all samples, an inverted-U shape profile with age was seen with cosine angle first increasing with age before declining. No age-by-sex interactions were present in the cortical thickness analysis (q < 0.07).
Discussion
Across three large datasets and >3000 MRI scans, we examined brain structural variability in global and subcortical volumes, surface area, and cortical thickness. Using both univariate and multivariate approaches, we found that males consistently demonstrate greater variability in global and subcortical volumes, and most notably surface area compared with females across the lifespan. Our cosine angle analyses demonstrated sex-based variability in how brain regions relate to each other unveiling sex, age, and metric specific findings. When taken together, our results inform sex-based risk and protective mechanisms for neurodevelopmental and neurodegenerative disorders.
We first built on previous findings (Ritchie et al. 2018; Wierenga et al. 2018, 2019) by using a lifespan (8–95 years) approach across three datasets to show that males are more variable than females in subcortical volume and cortical surface area, a pattern which starts early and is sustained through late life. Additionally, as in Ritchie et al. 2018, we show that these variability differences are independent of variability in TBV. We then used a different approach, Mahalanobis distance, to confirm our findings for each brain phenotype. Using Mahalanobis distance allowed us to quantify the total magnitude of each individual’s dissimilarity to their group average without assuming independence of regions to each other. We speculate that the age-by-sex interactions with surface area in the aging population may relate to the rate of atrophy differing between the sexes. It has been reported previously that the rate of volume loss is higher in males compared with females (Resnick et al. 2003; Raz et al. 2004; Carne et al. 2006). Unlike surface area, we found similar regional variability of cortical thickness in both sexes across the lifespan. This is consistent with previous adult data (Ritchie et al. 2018). Findings from a former developmental study is also mostly consistent with the current study, however, the former study did indicate a small number of regions with either greater (n = 5) or smaller (n = 4) variability in males compared with females (Wierenga et al. 2019). These few regions with divergent findings may relate to methodological differences. Independent genetic factors drive cortical thickness and surface area development (Panizzon et al. 2009; Grasby et al. 2020; Hofer et al. 2019), and these properties of brain structure demonstrate divergent developmental trajectories (Raznahan et al. 2011). Additionally, surface area developmental trajectories have been found to be sexually dimorphic, while trajectories of cortical thickness are similar for males and females (Raznahan et al. 2011). Cortical thickness is also thought to vary more in relation to environmental effects than surface area (Hofer et al. 2019).
Although we focus on brain structure, the theory of greater male variability is thought to originate from Darwin (1875). This theory is supported by evidence across phenotypes (Lehre et al. 2009; Reinhold and Engqvist 2013) and species. For instance, a recent meta-analysis showed that male rodents are frequently more variable than their female counterparts across an array of behavioral, morphological, physiological, and molecular traits (Prendergast et al. 2014). Hill (2017) posits a selectivity bias resulting in greater male variability while others have suggested greater variability is a consequence of the sex chromosomes architecture, where the heterogametic (i.e., XY in human males) structure leads to higher variability (Reinhold and Engqvist 2013). More precisely, the binomial sampling of the X-chromosomes that occurs in the homogametic sex (i.e., XX in human females) results in averaging of genetic effects across the two X-chromosomes. This averaging, in principle, reduces variability within the homogametic population of any trait that is influenced by X-chromosome genes. This hypothesis (sex-chromosome hypothesis) was supported by work on body size across various species showing higher variability in the heterogametic sex (male or female [i.e., birds and butterflies]) (Reinhold and Engqvist 2013). The presence of two X chromosomes in humans may play a protective role and create a blueprint for slightly less variability in females compared with males. Recent work on sex-chromosome aneuploidy show sex-chromosomes influence both brain structure and gene-expression (Mankiw et al. 2017; Nadig et al. 2018; Raznahan et al. 2018); however, further work is required to relate the current findings with these previous works (see Supplementary Material for further discussion on the genetics and developmental processes that may relate to our results).
Although not identified by genome-wide association studies (Grasby et al. 2020; Hofer et al. 2019), there is one X-chromosome gene that can influence cortical surface area; methyl-CpG binding protein 2 (MeCP2). Mutation of this gene, which is located at Xq28, is best known as the cause of Rett syndrome, which almost exclusively affects females (Urdinguio et al. 2009). In line with the theory of the protective X-chromosome, males with similar mutations suffer severe neonatal encephalopathy, which is normally lethal within the first year of life (Villard 2007). However, not all MeCp2 variations have such severe consequences. A common variant, rs2239464, has been shown in two independent datasets to be associated with reduced cortical surface area in males only (Joyner et al. 2009). The effect was not seen in females, proposed to be due to the presence of a second copy of the gene protecting them (Joyner et al. 2009). MeCP2 is a gene expression regulator (activator or repressor) of thousands of other genes (Chahrour et al. 2008) and potentially a key player influencing sex differences in surface area variability shown here. Furthermore, animal work has shown interactions between sex hormones, MeCP2 and epigenetic regulation (Romano et al. 2016); however, further research is required to fully elucidate these.
In the context of neurodevelopmental disorders, there appears to be consensus around the concept of “windows of vulnerability,” whereby finite periods of time during development are highly sensitive to perturbation by environmental factors, as opposed to similar environmental effects at other periods of development having little effect. It is theorized that the female population is less variable and therefore less vulnerable (on average) to environmental insult during these key developmental windows. For instance, ASD—which is more prevalent in males (Baio et al. 2018)—is associated with early brain overgrowth (Courchesne 2004; Schumann et al. 2010), indexed by excessive gray and white matter volume widely across the brain. Notably the study by Schumann and colleagues showed more pronounced and more widespread overgrowth in females with ASD compared with males (Schumann et al. 2010). This can be interpreted as females with ASD requiring a greater environmental or genetic insult (indexed by the brain morphology) than males to result in a similar clinical phenotype. Our findings of greater male variability in cortical surface area support this idea. Further research will help with linking the genetic and developmental processes that underlie both higher variability in males and aberrant mechanisms in ASD. Similarly, other neurodevelopmental disorders, such as, Tourette’s disorder (Hirschtritt et al. 2015), attention-deficit/hyperactivity disorder (Grevens et al. 2018), and psychosis (Leung and Chue 2000; Aleman et al. 2003), each have a higher prevalence in males compared with females, as well as sex differences in onset and presentation—whereby males are more severely affected. Again this is in line with the current findings of higher variability in brain structure in males compared with females. On the other hand, the prevalence of anxious misery is higher in females compared with males. This may relate to different developmental processes that occur later during adolescence (Kaczkurkin et al. 2016). As mentioned, further work will be required to determine the precise link between population-based variability differences and aberrant developmental processes that relate to neurodevelopmental disorders.
Our novel approach of investigating variability between regions using cosine angle analyses revealed differences in brain structure by sex that we do not believe has been previously reported. We observed significant age-by-sex interactions across all three datasets in the variability of structural profiles of subcortical volumes. Additionally, age-by-sex interactions were found during development and during later life in surface area. These interactions in children and youth may relate to differential rates of development (and regional differentiation), which are “slower” in males compared with females (Sussman et al. 2016). This aligns with previous reports of sexually dimorphic trajectories of development, at least for surface area (Raznahan et al. 2011), and the notion that different regions are under semi-independent genetic control (Grasby et al. 2020; Seidlitz et al. 2018; Hofer et al. 2019; Strike et al. 2019). In the latest life groups of older adults (OASIS-3), variability in the relationship among regions (in global volume and surface area) was greater in males than females. We can speculate that our findings relate to a consistent pattern of atrophy across the female population drawing them closer to their group mean. In contrast, findings in males could be due to atrophy occurring in more variable regions or more variable age of onset of atrophy across the population resulting in increased variability in structural profiles with increasing age (global volume and surface area). It is possible that greater variability among regions may be a protective mechanism against brain disorders, consistent with the finding of greater variability in early life in girls compared with boys, and in late life in males compared with females. This divergent relationship between the sexes with age in surface area, global and subcortical volumes might act to increase vulnerability for brain disorders in late life such as dementia (Resnick et al. 2003), where the prevalence of AD (Podcasy and Epperson 2016) is increased in females compared with males. Recent work by Pichet Binette and colleagues (Pichet Binette et al. 2020) suggests AD is characterized by increased among-region variability. We postulated that increased among-region variability may be higher in females relating to their increased risk for AD; however, our results did not support our hypothesis, possibly due to the inclusion of only cognitively normal individuals in the current study. The former study additionally showed that people with mild cognitive impairment do not show increased among-region variability compared with healthy controls, potentially implying that such variability may emerge in later phases of AD, rather than serve as a risk factor.
Significant age effects were present in cortical thickness cosine angle across all datasets. Network approaches—using either structural covariance (gray matter volume and cortical thickness) or diffusion MRI—have shown significant age-related changes in network architecture (Gong et al. 2009; Chen et al. 2011; Wu et al. 2012), characterized by network reorganization in aging. A separate study of structural covariance of cortical thickness highlighted the similarity in network architecture between males and females in terms of small-world organization, efficiency and node vulnerability where no sex differences were seen (Lv et al. 2010). These studies are in line with the current finding of an association between cortical thickness cosine angle and age, where cosine angle may reflect variability in the occurrence of these organizational changes across the population or the increased effect of environmental influences on cortical thickness.
This study has multiple major strengths, including the use of large high-quality datasets spanning from childhood to old age and multiple complementary advanced statistical approaches. Moreover, this allowed the replication of findings across samples and across methods providing strong evidence that they are generalizable. However, there are also some limitations to consider. There was an age difference between males and females in all three datasets. All analyses were repeated with age matched groups to ensure this was not skewing findings (see Supplementary Material). The Desikan-Killiany atlas is somewhat limited in regional specificity but was chosen to minimize the number of tests required and to make analyses comparable with previous studies. Additionally, each dataset was processed with a different version of FreeSurfer and on a different platform, both of which can influence metrics (Gronenschild et al. 2012). However, considering the consistency of results across datasets we do not expect that this greatly influenced our results. By using the publicly available outputs for the HCP and OASIS data, we aimed to enhance reproducibility of our analyses. Finally, all data included in the current study were cross-sectional. It would be advantageous in future to utilize longitudinal data across the lifespan.
In conclusion, we demonstrated that males are more variable compared with females in individual brain regions with regard to surface area and volume measures. However, this increased variability in males does not extend to how brain regions relate to each other. Structural profile analyses highlighted a different aspect of variability demonstrating that the relationships between regions vary in a sex, age, and metric specific fashion. Our results—coupled with pre-print results from the ENIGMA consortium (Wierenga et al. 2020)—support the importance of future work investigating the association between sex differences in variability of brain structure and genetic factors.
Funding
The Philadelphia Neurodevelopmental Cohort was funded through National Institute of Mental Health (NIMH) RC2 grants MH089983 (Raquel E. Gur) and MH089924 (Hakon Hakonarson). Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 National Institutes of Health (NIH) Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Additional data were provided (in part) by OASIS; OASIS-3: Principal Investigators: T. Benzinger, D. Marcus, and J. Morris; NIH P50AG00561, P30NS09857781, P01AG026276, P01AG003991, R01AG043434, UL1TR000448, and R01EB009352. OASIS grants: P50AG05681, P01AG03991, R01AG021910, P50MH071616, U24RR021382, and R01MH56584.
Notes
We thank all the participants in this research and the countless researchers involved in data collection. Conflict of interest: None declared.
Supplementary Material
References
- Abel KM, Drake R, Goldstein JM. 2010. Sex differences in schizophrenia. Int Rev Psychiatry. 22:417–428. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten J-P. 2003. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 60:565–571. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC, APA. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, et al. . 2018. Prevalence of autism Spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. 2009. Human brain networks in health and disease. Curr Opin Neurol. 22:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Sporns O. 2017. Network neuroscience. Nat Neurosci. 20:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SR, Fillmore JP. 2001. Spherical averages and applications to spherical splines and interpolation. ACM Trans Graph. 20:95–126. [Google Scholar]
- Carne RP, Vogrin S, Litewka L, Cook MJ. 2006. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 13:60–72. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY. 2008. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 320:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Gong G, Evans AC. 2011. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage. 56:235–245. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 62:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. 2004. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 10:106–111. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. 2007. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 86:189–199. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- de Jong LW, Vidal J-S, Forsberg LE, Zijdenbos AP, Haight T, Alzheimer’s Disease Neuroimaging Initiative, Sigurdsson S, Gudnason V, van Buchem MA, Launer LJ. 2017. Allometric scaling of brain regions to intra-cranial volume: an epidemiological MRI study. Hum Brain Mapp. 38:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. 1875. The decent of man and selection in relation to sex [Electronic version]. New York: D Appleton and Company. [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL. 1982. Sexual dimorphism in the human corpus callosum. Science. 216:1431–1432. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. . 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Fine C. 2013. Is there neurosexism in functional neuroimaging investigations of sex differences? Neuroethics. 6:369–409. [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999a. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. 1999b. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. . 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. 2009. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 29:15684–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, CRK C, MAB MM, et al. . 2020. Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA)—Genetics working group. The genetic architecture of the human. Science 367. [Google Scholar]
- Grevens CU, Richards JS, Buitelaar JK. 2018. Sex differences in ADHD In: Banaschewski T, Zuddas A, editors. Oxford textbook of attention deficit hyperactivity disorder. Oxford, United Kingdom: Oxford University Press, pp. 154–160. [Google Scholar]
- Gronenschild EHBM, Habets P, Jacobs HIL, Mengelers R, Rozendaal N, van Os J, Marcelis M. 2012. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 7:e38234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF. 2018. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TP. 2017. An evolutionary theory for the variability hypothesis. arXiv. [q-bioPE]. [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM, Lyon GJ, et al. . 2015. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiat. 72:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E, Roshchupkin GV, Adams HHH, Knol MJ, Lin H, Li S, Zare H, Ahmad S, Armstrong NJ, Satizabal CL, et al. . 2019. Genetic determinants of cortical structure (thickness, surface area and volumes) among disease free adults in the CHARGE consortium. bioRxiv. [Google Scholar]
- Joyner AH, CR J, Bloss CS, Bakken TE, Rimol LM, Melle I, Agartz I, Djurovic S, Topol EJ, Schork NJ, et al. . 2009. A common MECP2 haplotype associates with reduced cortical surface area in humans in two independent populations. Proc Natl Acad Sci U S A. 106:15483–15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, Elliott MA, Hopson R, Roalf DR, Vandekar SN, et al. . 2016. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biol Psychiatry. 80:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD. 2018. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 44:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne PJ, Keefe S, Lauren W, Xiong C, Grant EA, Moulder KL, Morris JC, Benzinger TLS, Marcus DS. 2018. OASIS-3: longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer’s disease. Alzheimer Dement. 14:P1097. [Google Scholar]
- Lehre A-C, Lehre KP, Laake P, Danbolt NC. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev Psychobiol. 51:198–206. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. 2008. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb Cortex. 18:2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. 2000. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand. 101:3–38. [DOI] [PubMed] [Google Scholar]
- Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. 2018. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 8:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, Li J, He H, Li M, Zhao M, Ai L, Yan F, Xian J, Wang Z. 2010. Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage. 53:373–382. [DOI] [PubMed] [Google Scholar]
- Mankiw C, Park MTM, Reardon PK, Fish AM, Clasen LS, Greenstein D, Giedd JN, Blumenthal JD, Lerch JP, Chakravarty MM, et al. . 2017. Allometric analysis detects brain size-independent effects of sex and sex chromosome complement on human cerebellar organization. J Neurosci. 37:5221–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Fotenos AF, Csernansky JG, Morris JC, Buckner RL. 2010. Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J Cogn Neurosci. 22:2677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. 2007. Open access series of imaging studies (OASIS): cross-sectional MRI data in Young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 19:1498–1507. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marïe A. 2016. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 67:57–78. [DOI] [PubMed] [Google Scholar]
- Nadig A, Reardon PK, Seidlitz J, McDermott CL, Blumenthal JD, Clasen LS, Lalonde F, Lerch JP, Chakravarty MM, Raznahan A. 2018. Carriage of supernumerary sex chromosomes decreases the volume and alters the shape of limbic structures. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. . 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichet Binette A, Gonneaud J, Vogel JW, La Joie R, Rosa-Neto P, Collins DL, Poirier J, Breitner JCS, Villeneuve S, Vachon-Presseau E, et al. . 2020. Morphometric network differences in ageing versus Alzheimer’s disease dementia. Brain. 143:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 40:1–5. [DOI] [PubMed] [Google Scholar]
- Podcasy JL, Epperson CN. 2016. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 18:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Parikshak NN, Chandran V, Blumenthal JD, Clasen LS, Alexander-Bloch AF, Zinn AR, Wangsa D, Wise J, Murphy DGM, et al. . 2018. Sex-chromosome dosage effects on gene expression in humans. Proc Natl Acad Sci U S A. 115:7398–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. 2004. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 25:377–396. [DOI] [PubMed] [Google Scholar]
- R Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reinhold K, Engqvist L. 2013. The variability is in the sex chromosomes. Evolution. 67:3662–3668. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G, Jordan-Young R, Kaiser A, Fine C. 2014. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front Hum Neurosci. 8:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, et al. . 2018. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 28:2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Cosentino L, Laviola G, De Filippis B. 2016. Genes and sex hormones interaction in neurodevelopmental disorders. Neurosci Biobehav Rev. 67:9–24. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Ryan Hopsona KP, Behr M, Qiu H, et al. . 2016. The Philadelphia neurodevelopmental cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. 2003. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 60:989–994. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, et al. . 2010. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 30:4419–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlitz J, Shinn M, Raznahan A, Bullmore ET, Romero-Garcia R, Whitaker KJ, Wagstyl K, Reardon PK, Clasen L, Liu S, et al. . 2018. Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron. 97:231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike LT, Hansell NK, Couvy-Duchesne B, Thompson PM, de Zubicaray GI, McMahon KL, Wright MJ. 2019. Genetic complexity of cortical structure: differences in genetic and environmental factors influencing cortical surface area and thickness. Cereb Cortex. 29:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D, Leung RC, Chakravarty MM, Lerch JP, Taylor MJ. 2016. The developing human brain: age-related changes in cortical, subcortical, and cerebellar anatomy. Brain Behav. 6:e00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdinguio RG, Sanchez-Mut JV, Esteller M. 2009. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 8:1056–1072. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, et al. . 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage. 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértes PE, Bullmore ET. 2015. Annual research review: growth connectomics—the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry. 56:299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard L. 2007. MECP2 mutations in males. J Med Genet. 44:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Weber G, Wenger E, Kimbacher M. 1989. The controversy about a sexual dimorphism of the human corpus callosum. Int J Neurosci. 47:169–173. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, van Rossenberg F, Crone EA. 2019. Sex effects on development of brain structure and executive functions: greater variance than mean effects. J Cogn Neurosci. 31:730–753. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK, Pediatric Imaging, Neurocognition, and Genetics Study . 2018. A key characteristic of sex differences in the developing brain: greater variability in brain structure of boys than girls. Cereb Cortex. 28:2741–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Doucet GE, Dima D, Agartz I, Aghajani M, Akudjedu TN, Albajes-Eizagirre A, Alnaes D, Alpert KI, Andreassen OA, et al. . 2020. Greater male than female variability in regional brain structure across the lifespan. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. 2012. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp. 33:552–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelezov OI. 2017. N-dimensional rotation matrix generation algorithm. Am J Comput Appl Math. 7:51–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


