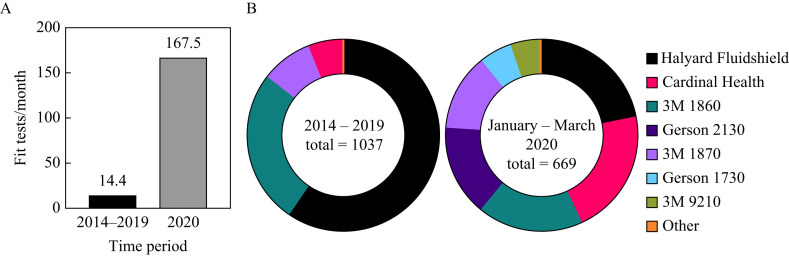Abstract
Background
Coronavirus disease 2019 has stretched the ability of many institutions to supply needed personal protective equipment, especially N95 respirators. N95 decontamination and re-use programmes provide one potential solution to this problem. Unfortunately, a comprehensive evaluation of the effects of decontamination on the fit of various N95 models using a quantitative fit test (QNFT) approach is lacking.
Aims
To investigate the effects of up to eight rounds of vaporized hydrogen peroxide (VHP) decontamination on the fit of N95 respirators currently in use in a hospital setting, and to examine if N95 respirators worn by one user can adapt to the face shape of a second user with no compromise to fit following VHP decontamination.
Methods
The PortaCount Pro+ Respirator Fit Tester Model 8038 was used to quantitatively define functional integrity, measured by fit, of N95 respirators following decontamination with VHP.
Findings
There was an observable downward trend in the functional integrity of Halyard Fluidshield 46727 N95 respirators throughout eight cycles of decontamination with VHP. Functional integrity of 3M 1870 N95 respirators was reduced significantly after the respirator was worn, decontaminated with VHP, and then quantitatively fit tested on a second user. Furthermore, inconsistencies between qualitative fit test and QNFT results were uncovered that may have strong implications on the fit testing method used by institutions.
Conclusions
The data revealed variability in the functional integrity of different N95 models after VHP decontamination, and exposed potential limitations of N95 decontamination and re-use programmes.
Keywords: COVID-19, N95, Respirator, Decontamination, Quantitative fit test
Introduction
As of October 2020, over 36.6 million people have been infected and 1 million people have died from coronavirus disease 2019 (COVID-19) worldwide [1]. Healthcare workers are at high risk of contracting COVID-19 [[2], [3], [4]], making personal protective equipment (PPE), including N95 respirators, critical for their protection. Many hospitals have universally implemented the use of N95 respirators during routine care to limit exposure to mild and asymptomatic individuals, and during aerosol-producing procedures (e.g. intubation and mechanical ventilation) [5,6]. This increase in use has left many hospitals struggling to maintain adequate stock of N95 respirators in the face of increasing supply chain shortages [[7], [8], [9], [10]].
Decontamination and re-use of N95 respirators is a potential solution to alleviate the scarcity of respirators during the COVID-19 pandemic, as per the Occupational Safety and Health Administration's (OSHA) recently updated guidelines [11]. Previous studies have validated the use of vaporized hydrogen peroxide (VHP) [12], moist heat [13], dry heat [14] or ultraviolet germicidal radiation [15] for decontamination of N95 respirators. Both structural and functional integrity, measured by visual inspection and fit, respectively, of decontaminated N95 respirators must be maintained following processing. Proper fit is defined as an intact, airtight seal against the user's face that can be measured using either a qualitative fit test (QLFT) or a quantitative fit test (QNFT), as defined by Appendix A of OSHA Standard 1920.134 [16]. A QNFT identifies proper fit more accurately than a QLFT [17,18]. However, an N95 respirator that is tested using a QNFT cannot be subsequently worn for future protection because this procedure requires puncturing a hole in the respirator to assess fit. On the other hand, N95 respirators examined via a QLFT can be kept and used by the wearer after the test is completed.
Current decontamination studies only use a mannequin head form for quantitative assessment of the fit factor, and fail to evaluate all respirator models used in hospital settings [19,20]. However, product scarcity has required the use of many different N95 models, most of which have not been evaluated after decontamination. Additionally, it can be challenging to develop and maintain systems which ensure that respirators are returned to their original user, as opposed to returning the same model but from a different user, post-decontamination. The integrity of N95 respirators worn by multiple persons has not been investigated.
This study aimed to assess the number and types of N95 respirators qualitatively fit tested and distributed by University Hospital (UH), Newark, NJ, USA before and during the COVID-19 pandemic. The goal was to provide a comprehensive evaluation of the effects of VHP decontamination on the functional integrity of all currently used N95 respirators using QNFTs. Further, this study aimed to determine if N95 respirators could be returned to new users following decontamination with no compromise to functional integrity. Finally, this study evaluated the reliability of qualitative fit testing on models that were difficult to fit quantitatively by comparing QLFT and QNFT results.
Methods
Human subjects approval
Experiments involving fit testing and decontamination of N95 respirators were part of the public health response to the COVID-19 pandemic and were thus considered exempt from institutional review board approval. All participants gave informed consent prior to participating in any experiments.
Determination of N95 respirator models
To define the scope of this targeted decontamination study, the authors examined N95 fit testing records, provided by Rutgers Environmental Health and Safety, conducted at UH between 2014 and 2020. All of the other fit tests were conducted specifically for this study in order to evaluate the effect of VHP decontamination on N95 respirator fit.
N95 decontamination
Respirators were decontaminated with a 35% aqueous hydrogen peroxide solution which was vaporized and delivered via the Steris VHP Victory system (Steris Life Sciences, Mentor, OH, USA). Respirator hanging and decontamination were similar to previously published studies [12]. Decontamination cycles were conducted in four phases: conditioning, gassing, dwell and aeration. Following initial injection, the target gaseous concentration was set to 400 ppm and maintained between 400 and 800 ppm for 3 h before overnight aeration to remove any residual VHP. Biological indicators (Spordex VHP Biological Indicator Discs; Steris Life Sciences) and chemical indicators (Steraffin VHP Type 4 Process Indicator; Steris Life Sciences) were positioned throughout the facility to validate effective VHP decontamination.
Evaluation of N95 respirator integrity
Both structural and functional integrity of N95 respirators were assessed via visual inspection and evaluation of fit, respectively. Multiple groups have defined respirator integrity using these measurements [21]. Following decontamination, respirators were visually inspected for cleanliness, and the elastic function was assessed prior to a QNFT. Any N95 respirators that were dirty, misshaped, or had damaged or snapped elastic bands were discarded. QNFT failures following decontamination with VHP resulted from a loss in respirator integrity because all users had previously passed a QNFT on the N95 respirator tested prior to decontamination.
Fit testing
To evaluate the effect of VHP decontamination on the functional integrity of N95 respirators, new unworn N95 respirators were decontaminated consecutively for up to eight cycles; after each cycle, a subset was removed for quantitative fit testing. Availability and supply limitations of N95 respirators influenced which models were evaluated at each decontamination cycle, and only cycles with at least N=3 were analysed.
Fit testing was administered following Appendix A of OSHA Standard 1920.134 with minor modifications: (1) N95 respirators tested using both QLFTs and QNFTs underwent QLFTs first; and (2) QLFT users were blinded to the type of QLFT administered (sweet or bitter). For both QNFTs and QLFTs, users underwent eight test exercises to determine fit in the following order: normal breathing, deep breathing, turning head side to side, moving head up and down, talking (rainbow passage), grimacing, bending over, and normal breathing. All users were deemed medically able to complete fit testing beforehand.
The 3M Qualitative Fit Test Apparatus FT-30, Bitter (denatonium benzoate) 1 kit and 3M Qualitative Fit Test Apparatus FT-10, Sweet (saccharine) kit (3M, Saint Paul, MN, USA) were used for QLFTs. These kits included a hood, collar and nebulizers used to aerosolize the tasting solution. All users were sensitive to tasting agents.
The QNFTs were administered using a PortaCount Pro+ Respirator Fit Tester Model 8038 with an N95-Companion (TSI, Shoreview, MN, USA). Respirators were punctured with custom grommet sampling probes to connect a sampling tube between the inside of an N95 respirator and the PortaCount Pro+ machine. Sodium chloride tablets were used for particle generation by the Particle Generator Model 8026 (TSI). All testing was conducted above the minimum recommendation of 70 ambient particles/cc and a passing test required a fit factor of ≥100. Fit factors were calculated by the apparatus from the ratio of particles outside to particles inside the respirator.
Evaluation of N95 respirator integrity on a second user
Not all N95 models will be compatible with all face types and sizes. Respirators that fail QLFTs are generally discarded, but that does not mean they will not provide protection to a different user; it simply means that the respirator and face type are incompatible. Instead of letting these respirators go to waste, they were defined as lightly used, decontaminated with VHP, and then the flexibility of their face-sealing capacity was investigated by assessing fit on a second user. These respirators were termed ‘lightly used’ because although the nosepiece had been fit and shaped to a user's face, the respirator did not undergo the extended wear that is representative of a long hospital shift. These second users had previously passed a QNFT on the model (and size where appropriate) being tested.
N95 respirator sizing
Users who passed an initial QNFT on non-decontaminated N95 respirators were considered certified to use the N95 model tested throughout this study. This preliminary QNFT was completed to account for variance and fit failure that may arise from training disparities, as reported previously [22]. Halyard Fluidshield N95 respirators were available in small (46827) and regular (46727) sizes. 3M 1860 N95 respirators were also available in small (1860S) and regular (1860) sizes. For these models, the appropriate size was determined for each participant using a QNFT before the start of the study. The correct size was then used for each subject in all experiments. All other N95 respirators assessed in this study were only available in one size.
Statistical analysis
A one-tailed Kruskal–Wallis test was used to analyse the effect of decontamination on N95 integrity. A one-tailed Mann–Whitney U-test was used to assess the differences between N95 respirators worn by one user and N95 respirators worn by a second user following decontamination. P≤0.05 was considered to indicate significance. All statistical analyses were run using Prism 8 software.
Results
Respirator models and fit testing have increased during the COVID-19 pandemic in a hospital setting
The frequency of QLFTs from January to May 2020 was approximately 10-fold higher than the average monthly frequency from 2014 to 2019 (Figure 1 A). Over 99% of fit tests conducted before 2020 were on one of four N95 models: Halyard Fluidshield 46727/46827 (59.59%), 3M 1860/3M1860S (25.75%), 3M 1870 (8.68%) and Cardinal Health (5.79%) (Figure 1B). In addition to these models, the Gerson 2130 (15.4%), Gerson 1730 (5.38%) and 3M 9210 (4.93%) N95 respirators were introduced between January and May 2020 (Figure 1B). However, the Halyard Fluidshield 46727/46827 (21.97%), 3M 1860/3M 1860S (17.64%), 3M 1870 (13%) and Cardinal Health (21.23%) models still comprised the majority of the respirators distributed (Figure 1B). Overall, an increase in the diversity, fit test frequency and use of N95 respirators was observed during the COVID-19 pandemic.
Figure 1.
Fit test frequency and distribution of N95 respirators by University Hospital, Newark, NJ, USA. (A) The frequency of qualitative fit tests increased 10-fold and (B) the diversity of N95 models expanded since the beginning of the coronavirus disease 2019 pandemic.
Variation in quantitative fit testing across different N95 models
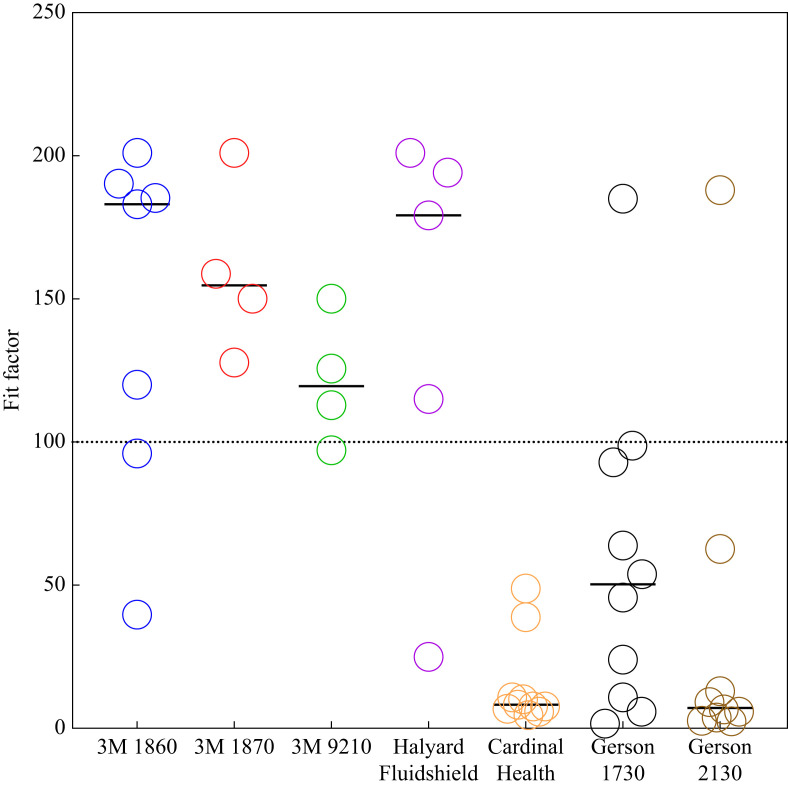
Prior to any VHP decontamination, QNFTs were conducted on the seven N95 models used at UH during the COVID-19 pandemic (Figure 2 ). Respirator models with high frequency of passing between different users were the 3M 1860/3M 1860S, 3M 1870, 3M 9210 and Halyard Fluidshield 46727 models, which had passing rates of 71%, 100%, 75% and 80%, respectively. The other models tested had much lower QNFT passing rates between different users, with the Cardinal Health, Gerson 1730 and Gerson 2130 models having passing rates of 0%, 10% and 11%, respectively. These models may work well with face types and sizes that are not representative of the volunteer population. However, for the present study, these last three models were defined as ‘difficult to fit’, and excluded from the decontamination experiments.
Figure 2.
Quantitative fit testing of N95 respirators commonly used at University Hospital, Newark, NJ, USA between January and May 2020. These data defined quantitatively difficult-to-fit N95 models: Cardinal Health, Gerson 1730 and Gerson 2130. Bars represent the median. 3M 1860/3M 1806S N=7, 3M 1870 N=4, 3M 9210 N=4, Halyard Fluidshield 46727 N=5, Cardinal Health N=10, Gerson 1730 N=10, Gerson 2130 N=9.
Decontamination with VHP does not affect the integrity of 3M 1860/3M 1860S, 3M 1870 and 3M 9210 N95 respirators, but may reduce the functional integrity of Halyard Fluidshield 46727 N95 respirators
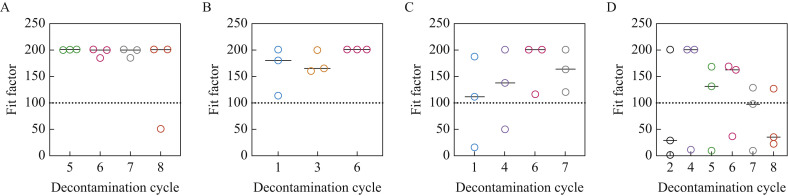
Both 3M 1860/3M 1860S and 3M 1870 N95 respirators maintained integrity following up to eight and six cycles of VHP decontamination, respectively (Figure 3 A,B). There was also no significant difference in the integrity of 3M 9210 N95 respirators following up to seven decontamination cycles (Figure 3C). A clear downward trend in the functional integrity of Halyard Fluidshield 46727 N95 respirators was observed throughout eight decontamination cycles (Figure 3D). However, due to the limited number of respirators during this critical time, the authors were unable to detect any significant differences in the data. Importantly, no defects in the elastic headbands were noted, and no corrosion was observed on the metal nosepiece and staples following eight cycles with VHP; this has also been validated by others [19].
Figure 3.
Decontamination with vaporized hydrogen peroxide does not affect the integrity of (A) 3M 1860, (B) 3M 1870 or (C) 3M 9210 N95 respirators, but potentially reduces the functional integrity of (D) Halyard Fluidshield 46727 N95 respirators. No significant differences were observed between decontamination cycles for any model. However, there was an observable downward trend in the functional integrity of Halyard Fluidshield 46727 N95 respirators throughout eight cycles of decontamination. Bars represent the median.
Decreased functional integrity of 3M 1870 N95 respirators is observed when the respirator is lightly worn and then fit tested by a second user
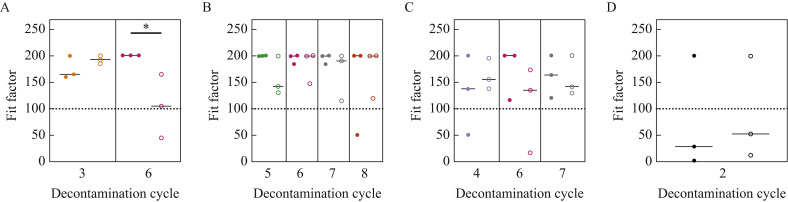
There was a significant decrease in the functional integrity of 3M 1870 N95 respirators when the respirator was lightly worn, decontaminated for six cycles with VHP, and fit tested by a second user compared with a respirator of the same model and size that had undergone the same number of decontamination cycles but had not been used previously (Figure 4 A). No significant differences were observed in the integrity of 3M 1860/3M 1860S, 3M 9210 and Halyard Fluidshield 46727 N95 respirators when fit tested by a second user following multiple rounds of decontamination (Figure 4B,C,D). Again, due to the limited supply of N95 respirators, it was not possible to assess the differences in functional integrity between first user and second user for all cycles and models (Figure S1A–D, see online supplementary material).
Figure 4.
Comparison of the functional integrity of N95 respirators that had not been worn previously with N95 respirators that had been lightly worn. (A) 3M 1870 N95 respirators that were lightly worn, decontaminated for six cycles, and fit on a new second user (open circles) had a significant decrease in functional integrity compared with 3M 1870 N95 respirators that were decontaminated for six cycles but had not been worn previously (closed circles). No significant trends were found in the functional integrity of (B) 3M 1860/3M 1860S, (C) 3M 9210 or (D) Halyard Fluidshield 46727 N95 respirators when they had been worn previously.
Discrepancies between qualitative and quantitative fit testing are apparent when evaluating Gerson 1730 N95 respirators
This study was expanded to examine the possibility of inconsistencies between QLFT and QNFT results from difficult-to-fit N95 models. Using both sweet and bitter QLFT measurements followed by a QNFT, it was determined that five of six participants who passed at least one qualitative fit test were unable to pass quantitatively when testing Gerson 1730 N95 respirators (Table I ). Unfortunately, the authors were unable to find enough participants able to qualitatively fit either Gerson 2130 or Cardinal Health N95 respirators, and therefore they were not included in this study (Table S1, see online supplementary material).
Table I.
Frequent inconsistencies between qualitative fit test and quantitative fit test results were found for Gerson 1730 N95 respirators
| Gerson 1730 |
|||
|---|---|---|---|
| Qualitative |
Quantitative |
||
| User | Sweet | Bitter | |
| 1 | P | P | F |
| 2 | P | P | F |
| 3 | F | P | F |
| 4 | P | F | F |
| 5 | F | P | F |
| 6 | P | P | P |
| 7 | P | F | F |
| 8 | F | F | F |
| 9 | F | P | F |
| 10 | P | P | P |
P, pass; F, fail.
Discussion
COVID-19 was officially declared a pandemic on 11th March 2020 and has since resulted in an overburdened healthcare system that is struggling to maintain adequate PPE, especially N95 respirators. Many clinical settings have implemented decontamination and re-use programmes in response to these shortages. However, recent evaluations of the integrity of N95 respirators following decontamination have only assessed a single model – 3M 1860/3M 1860S [[12], [13], [14], [15]] – and therefore do not account for the variety of N95 respirators currently in use. This lack of validation means that healthcare workers could be at an increased risk of receiving inadequate PPE. Thus, this study quantitatively evaluated the functional integrity of multiple N95 models currently in use at UH following decontamination with VHP.
From January to May 2020, UH increased qualitative fit testing over 10-fold and expanded the models of N95 respirators distributed compared with 6 years prior. Using unworn N95 respirators, the structural and functional integrity of these models after decontamination with VHP was examined. The 3M 1860/3M 1860S, 3M 1870 and 3M 9210 N95 respirators did not exhibit any noticeable decrease in integrity. Although not significant, a downward trend in the functional integrity of Halyard Fluidshield 46727 N95 respirators was observed over the course of five decontamination cycles, highlighting the importance of further studies.
It is crucial that decontamination and re-use programmes are able to turnaround clean N95 respirators rapidly. One potential time-saving approach is to return N95 respirators to new users instead of having to sort and return respirators to their initial user. To assess the adaptability of N95 respirators to a new face, this study quantitatively fit tested lightly worn respirators on a second user following decontamination with VHP. The results were limited to respirators that were initially only worn for a QLFT, and are thus not representative of the wear and tear associated with an extended hospital shift. Despite these limitations, the QNFT values were lower for 3M 1870 N95 respirators when they were lightly worn and fit tested by a second user compared with the other respirator models tested. It is also possible that some respirators may be less tolerant for re-use, even by the same person; however, it was not possible to assess this in the present study. Others have found a significant association between the number of shifts that N95 respirators were worn by a single user and failed QLFTs [23]. These data bring to light a significant obstacle for decontamination and re-use programmes.
A major unexpected result in this study was the inability to find many users who passed QNFTs on the Gerson 1730, Gerson 2130 and Cardinal Health N95 respirators, despite these respirators representing 42% of passing QLFTs at UH. It was wondered if differences existed between QNFT and QLFT results specifically on these difficult-to-fit models. Of the 10 participants recruited for QLFTs, six were able to pass at least one of the tasting challenges when qualitatively fit tested on Gerson 1730 N95 respirators. Interestingly, only one of these six participants was able to pass on the Gerson 1730 model using a QNFT. This discrepancy between QLFTs and QNFTs may be attributed to taste insensitivity which has been shown to increase false-positive fit testing [18,24] and could also be a symptom of COVID-19 infection [[25], [26], [27]]. Furthermore, anecdotal reports have indicated issues with competing taste profiles, such as previously eaten foods or disinfectants used to clean the hood, that may further complicate QLFT results. Together, these observations suggest that the administration of QNFTs may be warranted for fit testing these difficult-to-fit models, and should be used to assess inconsistencies between QLFTs on other N95 models.
This study has several limitations. All fit testing methods can have false pass rates of up to 11% [17]. The number of N95 respirators fit tested after each decontamination cycle was restricted by supply availability and resulted in unequal sample sizes between groups. Finally, when assessing the functional integrity of N95 respirators fit on a second user, the original N95 respirator was not worn during a long shift, and therefore may not be representative of all respirators included in decontamination and re-use programmes.
In conclusion, decontamination and re-use of 3M 1860/3M 1860S, 3M 1870 and 3M 9210 N95 respirators is a potential solution to N95 respirator supply shortages. Further studies must address the downward trends observed in the functional integrity of Halyard Fluidshield 46727 N95 respirators after decontamination with VHP. Caution should be taken when returning 3M 1870 respirators to a second user following VHP decontamination. Finally, the lack of consistency between QLFT and QNFT results may have far-reaching consequences on the type of fit test administered by institutions when determining which respirator is best for protection against aerosolized pathogens.
Acknowledgements
The authors would like to thank Dr. Jessica McCormick Ell (National Institutes of Health), Dr. Anthony Gresko (Rutgers University), Guillaume Delmas (Rutgers University), Brian Eggert (Rutgers University), and all of the other members of the Center for COVID-19 Response and Pandemic Preparedness team who helped establish the N95 decontamination and re-use programme. The authors would also like to thank Dr. Pradeep Kumar [Rutgers New Jersey Medical School (NJMS)], Dr. Padmapriya Banada (Rutgers NJMS), Dr. Sukalyani Banik (Rutgers NJMS), Heta Parmar (Rutgers NJMS), Skarleth Moran (Rutgers NJMS), Shraddha Suryavanshi (Rutgers (NJMS) and Kaheerman Saibire (Rutgers NJMS) for volunteering to participate in the fit testing studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.10.007.
Conflict of interest statement
None declared.
Funding sources
This work was supported by University Hospital, Newark, NJ, USA and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Grant No. T32AI125185).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin C., Montesinos I., Dauby N., Gilles C., Dahma H., Van Den Wijngaert S. Dynamic of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung J.C., Ho L.T., Cheng J.V., Cham E.Y.K., Lam K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8:e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19: protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Sullivan E.D. PPE guidance for covid-19: be honest about resource shortages. BMJ. 2020;369:m1507. doi: 10.1136/bmj.m1507. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO; Geneva: 2020. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance. [Google Scholar]
- 10.Kamerow D. Covid-19: the crisis of personal protective equipment in the US. BMJ. 2020;369:m1367. doi: 10.1136/bmj.m1367. [DOI] [PubMed] [Google Scholar]
- 11.Kapust P.J. Occupational Safety and Health, US Department of Labor; Washington, DC: 2020. Enforcement guidance on decontamination of filtering facepiece respirators in healthcare during the coronavirus disease 2019 (COVID-19) pandemic.https://www.osha.gov/memos/2020-04-24/enforcement-guidance-decontamination-filtering-facepiece-respirators-healthcare Available at: [last accessed November 2020] [Google Scholar]
- 12.Schwartz A., Stiegel M., Greeson N., Vogel A., Thomann W., Brown M. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl Biosaf. 2020;25:67–70. doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Donskey C.J. It's not the heat, it's the humidity: effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;48:854–855. doi: 10.1016/j.ajic.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang Y., Song Q., Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher E.M., Shaffer R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J Appl Microbiol. 2011;110:287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Labor . US Department of Labor; Washington, DC: 1998. Occupational safety and health standards personal protection equipment: respiratory protection. 1910.134. [Google Scholar]
- 17.Coffey C.C., Lawrence R.B., Zhuang Z., Campbell D.L., Jensen P.A., Myers W.R. Comparison of five methods for fit-testing N95 filtering-facepiece respirators. Appl Occup Environ Hyg. 2002;17:723–730. doi: 10.1080/10473220290107002. [DOI] [PubMed] [Google Scholar]
- 18.McKay R.T., Davies E. Capability of respirator wearers to detect aerosolized qualitative fit test agents (sweetener and Bitrex) with known fixed leaks. Appl Occup Environ Hyg. 2000;15:479–484. doi: 10.1080/104732200301269. [DOI] [PubMed] [Google Scholar]
- 19.Us Food and Drug Administration . US FDA; White Oak, MD: 2016. Final report for the Bioquell hydrogen peroxide vapor (HPV) decontamination for reuse of N95 respirators; pp. 2–46.https://www.fda.gov/media/136386/download Available at: [last accessed November 2020] [Google Scholar]
- 20.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Martinez C.E., Sossa-Briceño M.P., Cortés J.A. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H., Baek J.E., Seo H.K., Lee J.-E., Myong J.-P., Lee S.-J. Comparison of fit factor for healthcare workers before and after training with the N95 mask. J Korean Soc Occup Environ Hyg. 2014;24:528–535. [Google Scholar]
- 23.Degesys N.F., Wang R.C., Kwan E., Fahimi J., Noble J.A., Raven M.C. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020 doi: 10.1001/jama.2020.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffey C.C., Lawrence R.B., Campbell D.L., Zhuang Z., Calvert C.A., Jensen P.A. Fitting characteristics of eighteen N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1:262–271. doi: 10.1080/15459620490433799. [DOI] [PubMed] [Google Scholar]
- 25.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautier J.F., Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity. 2020;28:848. doi: 10.1002/oby.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.