Abstract
In recent decades, the occurrence and distribution of arboviral diseases transmitted by Aedes aegypti mosquitoes has increased. In a new control strategy, populations of mosquitoes infected with Wolbachia are being released to replace existing populations and suppress arboviral disease transmission. The success of this strategy can be affected by high temperature exposure, but the impact of low temperatures on Wolbachia-infected Ae. aegypti is unclear, even though low temperatures restrict the abundance and distribution of this species. In this study, we considered low temperature cycles relevant to the spring season that are close to the distribution limits of Ae. aegypti, and tested the effects of these temperature cycles on Ae. aegypti, Wolbachia strains wMel and wAlbB, and Wolbachia phage WO. Low temperatures influenced Ae. aegypti life-history traits, including pupation, adult eclosion, and fertility. The Wolbachia-infected mosquitoes, especially wAlbB, performed better than uninfected mosquitoes. Temperature shift experiments revealed that low temperature effects on life history and Wolbachia density depended on the life stage of exposure. Wolbachia density was suppressed at low temperatures but densities recovered with adult age. In wMel Wolbachia there were no low temperature effects specific to Wolbachia phage WO. The findings suggest that Wolbachia-infected Ae. aegypti are not adversely affected by low temperatures, indicating that the Wolbachia replacement strategy is suitable for areas experiencing cool temperatures seasonally.
Keywords: Wolbachia, Aedes aegypti, low temperature, population replacement
Temperature is a major factor that influences the survival and development of insect species and determines their distribution (Bale et al. 2002). The occurrence of arboviral diseases that are carried by mosquitoes, such as dengue, Zika, yellow fever, and Chikungunya, depends on the temperature tolerance of their hosts (Mordecai et al. 2019). Aedes aegypti, which is more competent than Ae. albopictus (Diptera: Culicidae) at transmitting arboviral diseases (Tsai and Teng 2016, Walton 2018), is a major arboviral vector worldwide although it has a narrower distribution than Ae. albopictus (Kraemer et al. 2015, Dickens et al. 2018) due to a lower cold tolerance and lack of a diapause stage (Chang et al. 2007, Vinogradova 2007). However, there are increasing concerns that the distributions of Aedes mosquitoes, especially Ae. aegypti, will expand with climate change, making control of arboviral diseases a serious global health problem (Gould and Higgs 2009, Kraemer et al. 2015).
Because Ae. aegypti has developed resistance to chemical pesticides (Moyes et al. 2017), other ways of controlling dengue disease transmitted through this vector are being considered. One of the most promising is the use of Wolbachia, an intracellular bacterium, to reduce the transmission of dengue and other arboviruses by Ae. aegypti. In this population replacement strategy, Wolbachia is transferred from other insect species into Ae. aegypti (McMeniman et al. 2009, Walker et al. 2011, Ant et al. 2018), then released for invasion into uninfected populations. Successful releases have now been achieved with both the wMel strain (Hoffmann et al. 2011, O’Neill et al. 2018, Garcia et al. 2019) and the wAlbB strain (Nazni et al. 2019) of Wolbachia. Apart from the direct effects of these strains on inhibition of arbovirus (Walker et al. 2011, Ant et al. 2018), virus transmission may also be reduced due to fitness costs of Wolbachia infections that could reduce vector population densities (Mains et al. 2013, Rašić et al. 2014). However, fitness costs can also influence the efficiency of population replacement (Walker et al. 2011, Nguyen et al. 2015, Garcia et al. 2019) as well as potentially disease control if Wolbachia densities are reduced to low levels (Lu et al. 2012).
To date, population replacement programs have mostly been undertaken in tropical regions but there is potential for releases to take place in subtropical regions closer to the lower thermal limits of the Ae. aegypti distribution. The effects of lower temperatures on Wolbachia and Wolbachia-infected Ae. aegypti have not previously been investigated in detail but could influence the success of population replacement programs. The lower development threshold temperature (T0) for Ae. aegypti is around 10°C (Eisen et al. 2014, Tsai et al. 2018) and sustained flight is inhibited below 15°C (Rowley et al. 1968). Wolbachia may change the low temperature sensitivity of its host, given that Wolbachia strains affect temperature preference in Drosophila melanogaster (Diptera: Drosophilidae) (Truitt et al. 2019, Arnold et al. 2019). Moreover, extreme temperatures can affect Wolbachia inside mosquito hosts, thus influencing pathogen blocking efficiency (Murdock et al. 2014) or factors relevant to the invasion of Wolbachia such as Wolbachia transmission rates and cytoplasmic incompatibility (Ross et al. 2019c).
In Ae. aegypti, Wolbachia infections respond differently to heat stress, with the wAlbB infection showing a higher heat tolerance than wMel (Ross et al. 2017b). Wolbachia phages, called WO, can change Wolbachia density when they undergo lytic cycles which will lyse Wolbachia cells, a process that can be temperature-induced (Kent and Bordenstein 2010, Bordenstein and Bordenstein 2011). In D. melanogaster, Wolbachia strain wMel has complete WO particles (Wu et al. 2004), but any role of WO on Wolbachia density has not been considered in this species or in Ae. aegypti transinfected with wMel. The WO phage of wAlbB in Ae. albopictus has been investigated previously (Chauvatcharin et al. 2006, Tortosa et al. 2010, Zhang et al. 2014) but genes essential to the phage are no longer functional; thus, it is not expected to be active (Sinha et al. 2019).
In this study, we compare Wolbachia-infected and uninfected Ae. aegypti under low temperatures to better understand the relevant fitness effects associated with different Wolbachia strains. We also consider the impact of low temperatures on Wolbachia and phage WO (in the case of wMel that contains intact WO) densities. The overall aim of the study is to help predict the influence of low temperatures on Wolbachia and their infected hosts to inform releases undertaken outside tropical regions.
Materials and Methods
Mosquito Strains and Maintenance
Aedes aegypti mosquitoes were maintained at 26 ± 1°C in a controlled temperature room following methods described previously (Ross et al. 2017a). Uninfected, wMel-infected, and wAlbB-infected Ae. aegypti were tested to study the influence of Wolbachia infection on various life-history traits. The wMel-infected population was collected in May 2013 from Cairns, Australia where the local population had been invaded by releases of wMel-infected mosquitoes 2 yr earlier (Hoffmann et al. 2011). The wAlbB-infected population was generated by Xi et al. (2005). The uninfected population was established from Ae. aegypti (Wolbachia-uninfected) eggs collected in a different region of Cairns, QLD, Australia in November 2015 (Axford et al. 2016). To minimize any genetic background differences between infected and uninfected lines, the two infected lines have been repeatedly backcrossed to uninfected Cairns populations. At least three generations of backcrossing were carried out on each occasion (Yeap et al. 2011). In addition, we undertook another three generations of backcrossing (producing an expected 87.5 genetic similarity) before the experiments were initiated and maintained populations at a size of 500 adults to minimize genetic drift effects (Ross et al. 2017a). After pilot experiments to narrow down the temperature settings, eggs collected from the seventh generation after backcrossing were used in fertility experiments, while for other assays we used the fourth generation.
Low Temperature Treatments
A range of cyclical temperatures was chosen based on the spring temperatures across tropical and subtropical areas where Ae. aegypti and dengue fever have been reported (Malaysia: 23–33°C; Northern Australia: 18–29°C; South America: 11–26°C; Southern Asia: 16–23°C; https://gis.ncdc.noaa.gov). Considering that cold air flow can occur commonly in the Monsoon zone, we set up the experimental temperature cycles as 22–30 ± 1°C, 15–23 ± 1°C, 13–21 ± 1°C, and 11–19 ± 1°C with a 12:12 (L:D) h photoperiod in incubators (PG50 Plant Growth Chambers, Labec Laboratory Equipment, Marrickville, NSW, Australia), with low temperatures during the dark period and high temperatures during the light period (Supp Fig. 1 [online only]). Temperatures were checked with two data loggers (Thermochron; 1-Wire, iButton.com, Dallas Semiconductors, Sunnyvale, CA) inside each incubator.
The wMel-infected, wAlbB-infected, and uninfected Ae. aegypti lines were hatched separately in reverse osmosis (RO) water in 3-liter trays at 26 ± 1°C. Twenty-four hours after hatching, larvae were controlled to a density of 100 larvae in 400 ml RO water in a 500-ml container and then transferred to incubators set to each temperature cycle. TetraMin tropical fish food tablets (Tetra, Melle, Germany) were provided ad libitum until pupation. To test for effects of temperature shifts during the development process, half of the individuals were transferred from a high temperature cycle (22–30°C) to a low temperature cycle (11–19°C) at the fourth instar before pupation and vice versa. Four to six containers for each treatment were used to calculate the pupation and adult eclosion proportions. Pupae were counted every 2 d to estimate development time and then transferred to 19.7-liter BugDorm-1 adult cages to emerge (MegaView Science Co., Ltd., Taichung City, Xitun District, Taiwan). Fifteen adult mosquitoes from each treatment were stored in absolute ethanol every 5 d until all adults had died. Newly emerged adults from a pilot experiment with the following temperature cycles: 22–30°C, 18–26°C, 14–22°C, and 10–18°C, were stored and measured for wing length as an indication of body size (Briegel 1990).
Fertility
The wMel-infected, wAlbB-infected, and uninfected mosquito lines were reared under cycling temperatures (22–30°C or 15–23°C) with a 12:12 (L:D) h photoperiod. After emergence, mosquitoes from the high temperature cycle (22–30°C) were left for 6 d and those from the low temperature cycle (15–23°C) were left for 15 d before blood feeding to allow them to mature and mate, with the longer period at the cold temperature used to compensate for the fact that ovarian development is slowed at low temperature (Rowley et al. 1968, Sanburg and Larsen 1973). One day before blood feeding, sugar cups were removed from the cages to starve the females. Mosquitoes were fed blood by a forearm from a volunteer for 15 min in the daytime at room temperature. After blood feeding, 60 engorged females from each temperature cycle were aspirated into 70-ml specimen cups (one female in one cup) with mesh lids and a strip of sandpaper half immersed in larval rearing water. Thirty females were kept at the same temperature cycle as before blood feeding while the other 30 were transferred to the other temperature cycle to produce four treatments. Females were left for 1 wk under the high temperature cycle or 2 wk under the low temperature cycle. Females that died during this period were excluded from further analysis. Eggs on the sandpaper strips were then collected and stored at 26°C for 3 d before hatching. Fecundity was determined by counting the number of eggs on each strip and the proportion of eggs hatched was calculated by dividing the hatched eggs by the total number of eggs.
Wolbachia and Phage Density
We tested the Wolbachia density of young (0–5 d post-emergence) and old (10 d post-emergence or later) females and males of all ages when reared under each temperature cycle. We also tested adult female Wolbachia density when larvae were reared at either 11–19°C or 22–30°C and then subjected to a temperature shift before pupation. Wolbachia screening was conducted using a High Resolution Melt assay on a real-time PCR LightCycler 480 (LC480) system (Roche Applied Science, Indianapolis, IN). DNA from each sample was extracted with 200 μl of 5% Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA) and diluted 10 times in 96-well plates for Wolbachia screening (Lee et al. 2012). Each sample was pipetted three times into a 384-well plate and run with mosquito-specific (mRpS6) primers, Ae. aegypti-specific (aRpS6) primers, and Wolbachia-specific primers (w1 primers for Wolbachia wMel and wAlbB primers for Wolbachia wAlbB) (Axford et al. 2016). Three technical replicates were completed for each sample. Differences in crossing point (ΔCp) between aRpS6 and Wolbachia primers were averaged and then transformed by 2n to obtain relative Wolbachia densities.
Based on whole genomic sequencing in their natural host Wolbachia, WO elements are complete in Wolbachia wMel, but not in wAlbB. In this study, we tested the relative density of Wolbachia wMel and WO of wMel using the method of Gavotte et al. (2004) in an LC480 system. DNA from each sample was run four times with mosquito-specific (mRpS6) primers, Ae. aegypti-specific (aRpS6) primers, wMel-specific (w1) primers, and orf7 primers (WOF2). The relative WO density was obtained by calculating the differences in crossing point (ΔCp) between aRpS6 and orf2 primers and then transformed by 2n.
Data Analyses
All data analyses and visualizations were conducted using R version 3.6.0 with R studio version 1.1.453 (Team 2019). Proportional data such as egg hatch, pupation, and eclosion were analyzed with binomial logistic regression models (Hastie 2017), then the emmeans package (Lenth et al. 2018) was used to calculate main effects and interactions, and to perform pairwise comparisons between treatments. The car package (Fox et al. 2012) was used to run type III ANOVA for normally distributed data including development time, fecundity, and log-transformed Wolbachia and WO density, and Brown–Forsythe test (Brown and Forsythe 1974) for the equality of variances. The fertility data (fecundity and hatch proportions) also run with Tukey’s honest significant difference (HSD) tests (Tukey 1949) and hatch proportions were arcsine square-root transformed before calculations. Box plots were made according to McGill et al. (1978).
Results
Development
Across all mosquito lines, larval development time was approximately 8.7 d (standard error [SE] = 0.059) at 22–30°C; 15.3 d (0.075) at 15–23°C, 19.7 d (0.102) at 13–21°C, and 26.9 d (0.229) at 11–19°C (Supp Fig. 2 [online only]).
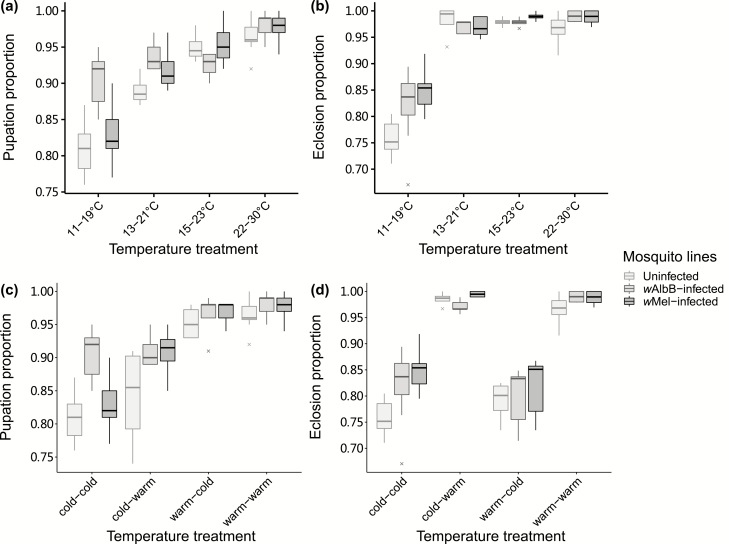
We found a decrease in pupation and eclosion proportions under low temperature cycles, although the coldest treatment still showed >75% survival (Fig. 1). For pupation success (Fig. 1a), we found effects of temperature and mosquito line as well as a significant interaction (binomial logistic regression: temperature: F3,68 = 73.932, P < 0.001; mosquito line: F2,68 = 5.018, P = 0.007; interaction: F6,68 = 3.603, P = 0.001). When temperatures were considered separately, the pupation success of wAlbB-infected Ae. aegypti was higher than the uninfected line under the two lowest temperature cycles (13–21°C: z = 2.557, P = 0.029; 11–19°C: z = 5.587, P < 0.001) but no differences were detected between uninfected and wMel-infected individuals at any temperature (all P > 0.05).
Fig. 1.
Box plots showing effect of low temperature cycles on pupation and eclosion in Wolbachia-infected Aedes aegypti. (a) Proportion of larvae that successfully pupated under different temperature cycles. (b) Proportion of pupae that successfully emerged into adults under different temperature cycles. (c) Proportion of larvae that successfully pupated when subjected to a temperature shift before pupation. (d) Proportion of pupae that successfully emerged into adults when subjected to a temperature shift before pupation. For the treatments, the first term indicates the larval rearing temperature, and the second indicates the pupal temperature. Crosses represent outliers.
For eclosion success (Fig. 1b), there was a significant effect of temperature but not of mosquito line, with no significant interaction (temperature: F3,68 = 137.233, P < 0.001; mosquito line: F2,68 = 2.305, P = 0.100; interaction: F6,68 = 1.904, P = 0.076). Compared to 22–30°C, only the lowest temperature cycle of 11–19°C significantly decreased the proportion of pupae eclosing (15–23°C: z = 0.158, P = 0.999; 13–21°C: z = −1.890, P = 0.232; 11–19°C: z = −15.961, P < 0.001). Under 11–19°C, both Wolbachia wAlbB- and wMel-infected Ae. aegypti had higher eclosion success than uninfected individuals (wAlbB: z = 2.905, P = 0.010; wMel: z = 4.000, P < 0.001).
When final instar larvae were subjected to temperature shifts, we found that pupation success depended on larval rearing temperature, pupation temperature, and mosquito line (binomial logistic regression: larval rearing temperature: F1,69 = 196.615, P < 0.001; pupation temperature: F1,69 = 11.865, P = 0.001; mosquito line: F2,69 = 10.905, P < 0.001; Fig. 1c). No interaction was significant (all P > 0.2). In contrast, eclosion success depended on pupation temperature and mosquito line instead of larval rearing temperature (larval rearing temperature: F1,69 = 0.584, P = 0.445; pupation temperature: F1,69 = 250.629, P < 0.001; mosquito line: F2,69 = 4.144, P = 0.016; Fig. 1d), with significant interactions between larval rearing temperature and mosquito line (mosquito line: larval rearing temperature: F2,69 = 4.092, P = 0.017) and a three-way interaction (F2,69 = 6.190, P = 0.002).
Fertility
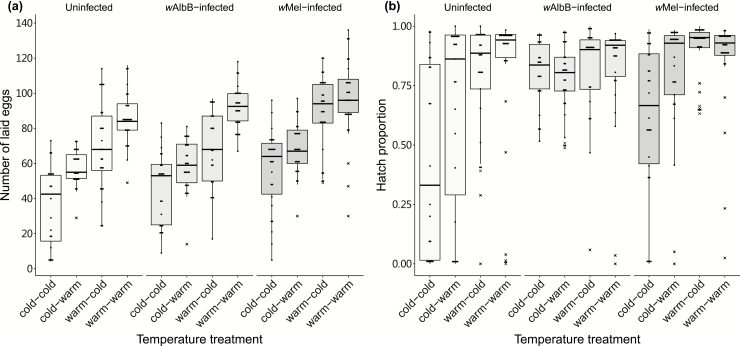
Females reared at temperature cycles of 22–30°C and 15–23°C were blood fed and interchanged to test the influence of low temperature on fertility. We observed variability in fecundity (the number of eggs laid per female) and egg viability of the next generation when females suffered from low temperature treatments (Fig. 2; Supp Table 1 [online only]). Fecundity was influenced by the temperature before blood feeding and to a small extent by temperature after blood feeding, with no significant difference between mosquito lines (three-way ANOVA: temperature before blood feeding: F1,282 = 24.140, P < 0.001; temperature after blood feeding: F1,282 = 7.537, P = 0.006; mosquito lines: F2,282 = 2.107, P = 0.124; Fig. 2a), the only notable interaction being a marginally nonsignificant interaction between temperature after blood feeding and mosquito lines (F2,282 = 2.716, P = 0.068). When mosquito lines were considered separately, the fecundity of wMel-infected females was not influenced by temperature after blood feeding (F1,103 = 1.025, P = 0.314) while wAlbB-infected and uninfected females were affected (wAlbB: F1,90 = 20.273, P < 0.001; uninfected: F1,89 = 7.550, P = 0.007).
Fig. 2.
Fertility of 6-d-old Aedes aegypti females under temperature cycles of 22–30°C (warm) or 15-d-old females under 15–23°C (cold) and with these treatments interchanged between the age groups after blood feeding. (a) Number of eggs laid per female. (b) Hatch proportions of these eggs. For the treatments, the first term indicates the temperature before blood feeding, and the second indicates the temperature after blood feeding. Points represent individuals. Crosses represent outliers.
After counting the hatch proportion of these eggs (Fig. 2b), we found that females that experienced the low temperature cycle before blood feeding had significantly decreased egg viability in the next generation (binomial logistic regression: F1,282 = 523.682, P < 0.001), but the temperature after blood feeding had no effect (F1,282 = 1.669, P = 0.196). There were also significant differences between mosquito lines (mosquito line: F2,282 = 144.736, P < 0.001) with significant interactions (all P < 0.001). Wolbachia-infected Ae. aegypti produced a higher proportion of eggs that hatched compared to uninfected females (wAlbB: z = 13.691, P < 0.001; wMel: z = 15.437, P < 0.001). The wMel-infected and uninfected females produced lower-quality eggs under low temperature before blood feeding than those under high temperature (wAlbB: z = −2.110, P = 0.348; wMel: z = −19.397, P < 0.001; uninfected: z = −19.591, P < 0.001), the Brown–Forsythe test also showed that the equality of variances for these two mosquito lines changed significantly under different temperature cycles before blood feeding (wAlbB: F1,92 = 0.583, P = 0.447; wMel: F1,105 = 14.987, P < 0.001; uninfected: F1,91 = 13.687, P < 0.001).
Wolbachia and WO Density
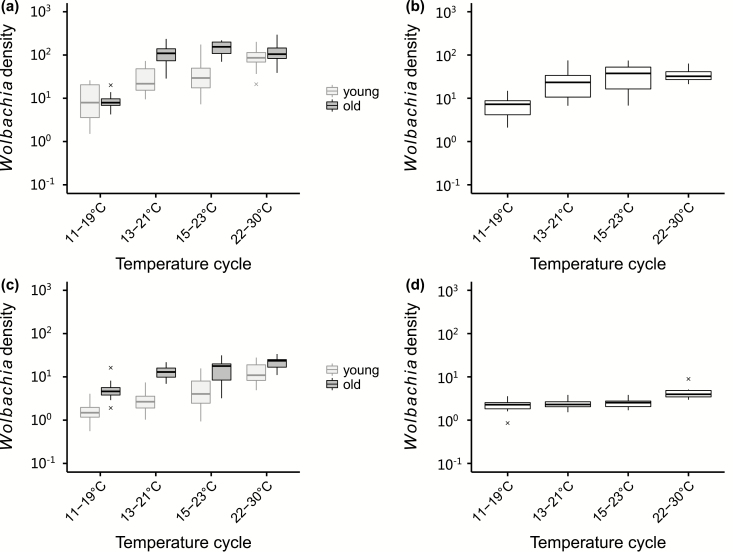
Low rearing temperatures suppressed wAlbB and wMel Wolbachia density in both male and female Ae. aegypti (ANOVA: wAlbB-infected female: F3,107 = 40.716, P < 0.001; wAlbB-infected male: F3,37 = 12.029, P < 0.001; wMel-infected female: F3,126 = 26.463, P < 0.001; wMel-infected male: F3,35 = 7.218, P < 0.001; Fig. 3). For female mosquitoes, there was a significant interaction between temperature and mosquito age (wAlbB: F3,103 = 9.288, P < 0.001; wMel: F3,122 = 4.041, P = 0.009), in that the Wolbachia density increased as females became older. For wAlbB, the influence of age was significant under 13–21°C and 15–23°C (11–19°C: F1,21 = 0.036, P = 0.852; 13–21°C: F1,28 = 37.607, P < 0.001; 15–23°C: F1,27 = 41.014, P < 0.001; 22–30°C: F1,27 = 2.938, P = 0.098) while for wMel it was significant under all temperature cycles (11–19°C: F1,23 = 23.413, P < 0.001; 13–21°C: F1,29 = 71.268, P < 0.001; 15–23°C: F1,33 = 19.894, P < 0.001; 22–30°C: F1,37 = 17.396, P < 0.001).
Fig. 3.
Relative density of wAlbB and wMel in Aedes aegypti reared under rearing temperature cycles of 22–30°C, 15–23°C, 13–21°C, or 11–19°C. (a) Wolbachia wAlbB density in young (0–5 d) and old (≥10 d) female adults; (b) Wolbachia wAlbB density in male adults; (c) Wolbachia wMel density in young (0–5 d) and old (≥10 d) female adults; (d) Wolbachia wMel density in male adults.
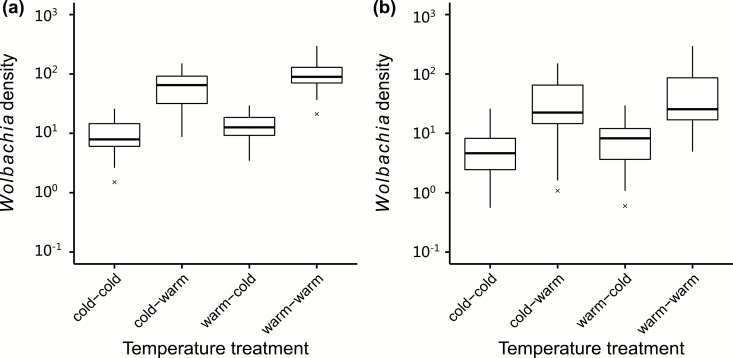
When wAlbB-infected females were subjected to a temperature shift during the final larval instar before pupation (Fig. 4a), the larval rearing temperature had only a marginally significant effect on Wolbachia density in adults while the temperature after the larval stage had a larger effect (ANOVA: temperature during the larval stage: F1,109 = 5.550, P = 0.020; temperature after the larval stage: F1,109 = 112.996, P < 0.001). For wMel-infected females (Fig. 4b), Wolbachia density in adults was only significantly influenced by the temperature after the larval stage (temperature during the larval stage: F1,120 = 2.767, P = 0.099; temperature after the larval stage: F1,120 = 44.927, P < 0.001).
Fig. 4.
Relative Wolbachia density in Aedes aegypti females infected with (a) wAlbB or (b) wMel when final instar larvae were subjected to a temperature shift before pupation. For the treatments, the first term indicates the temperature during the larval stage, and the second indicates the temperaure after the larval stage.
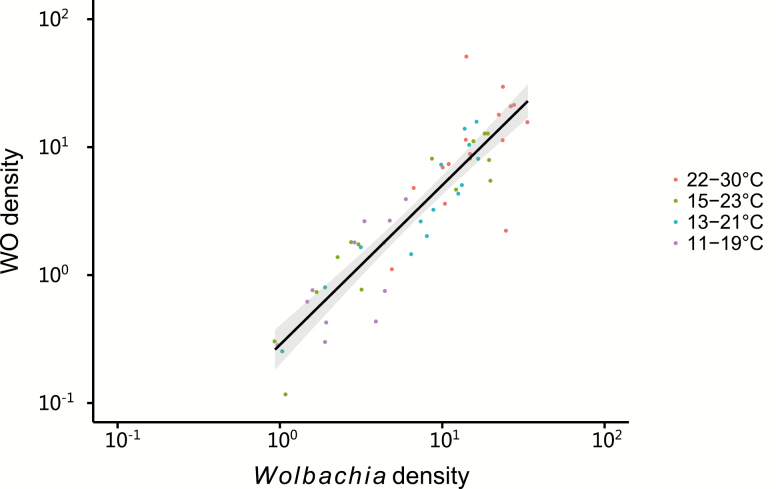
We tested the relative density of Wolbachia wMel and phage WO in Ae. aegypti adults (Fig. 5) and found a strong positive relationship between Wolbachia density and WO density that fitted a linear model (log2 (WO density) = 1.248 * log2 (wMel density) − 1.672; R2 = 0.817, F1,78 = 348.2, P < 0.001). There were no significant differences in slopes between the lines of different temperature cycles (all P > 0.05), indicating that the relationship between phage density and Wolbachia density does not depend on temperature. Thus, any changes in phage density track those in Wolbachia density.
Fig. 5.
The relationships between Wolbachia density and phage density in wMel-infected Aedes aegypti. Points represent individuals. A linear regression line and SE around the line are shown.
Discussion
Our study tested for effects of low temperature cycles on Ae. aegypti life history. The effects we detected depended to some extent on Wolbachia infection status and were influenced by conditions experienced previously. In particular, pupation success tended to depend on larval temperature instead of pupal temperature. On the other hand, pupal eclosion success mostly depended on pupal temperature cycles, with a sharp threshold in success at 11–19°C. The results suggest that the pupae of Ae. aegypti have higher cold tolerance than the larvae, given that eclosion was only affected by the lowest temperature cycle, whereas pupation success was also affected by the intermediate conditions. Experiments on other Diptera species have also highlighted the higher susceptibility of larvae to temperature extremes compared to pupae (Jensen et al. 2007, Bowler and Terblanche 2008).
In the fertility assays, we found that both the low temperature before and after female blood feeding influenced the numbers of eggs laid. This could be an issue related to the nutritional status of female mosquitoes. No more sugar was provided to female mosquitoes after blood feeding to match previous studies (Foster 1995, Tsunoda et al. 2010, Ross et al. 2017a), whereas it was provided prior to feeding. Sugar availability may interact with temperature to affect mosquito fecundity. Larval rearing temperature influences mosquito body size (Supp Fig. 3 [online only]) and smaller mosquitoes may have an increased need for sugar in order to lay eggs. The low temperature treatments after blood feeding may have similar effects in that it increases nutrition requirements (Carrington et al. 2013).
Another factor that may be important is female age, since fecundity can decline with female age (Maciel-de-Freitas et al. 2011); however, it is worth noting that the physiological age of females experiencing low temperatures that slow maturation will be less than the physiological age of females of an equivalent chronological age held at warmer temperatures. One the other hand, the hatch proportion of these eggs depended on the temperature before blood feeding, not after blood feeding, which may reflect incomplete gonadal development (Kindlmann and Dixon 1992).
Variability in fertility between individual females sharply increased under low temperatures before blood feeding, highlighting effects on phenotypic variances as well as trait means. We note that some uninfected and wMel-infected females produced low-quality eggs with poor hatch rates. The reason for this increase in variation is unclear. However, it is worth noting that the physiological development of individuals under low temperatures can be variable (Bowler and Terblanche 2008, Mitchell et al. 2013), which may cause these females to have mated insufficiently to allow for fertilization (and hatching) of all their eggs. Low temperatures may also suppress mating behavior and therefore lead to more variable egg hatch rates.
Low temperatures can influence Wolbachia by suppressing infection densities. In the case of wMel that contains WO, any change in density was not influenced by WO, indicating that WO is not induced by temperature change; the phage may be inactive after being transferred with wMel into Ae. aegypti. Beyond any phage-mediated effects, temperature after the larval stage influenced adult Wolbachia density to a greater extent than temperature during the larval stage. Consistent with previous findings (Duron et al. 2007, Ulrich et al. 2016), the density of Wolbachia in adults increased over time, particularly under low temperatures. This phenomenon may be associated with slower development at low temperatures; however, an impact of low temperature independent of age was apparent for wAlbB in that at 11–19°C the density did not recover (Fig. 3). Any reduction in density may influence maternal transmission and cytoplasmic incompatibility associated with Wolbachia, although we did not test for these effects directly. Overall, Wolbachia appears relatively stable at low temperatures, in contrast to high temperatures where effects on density are dramatic, resulting in cytoplasmic incompatibility loss and maternal transmission failure for wMel and wMelPop-CLA (Ross et al. 2017b).
In the experiments carried out here under low temperatures, the Wolbachia infections showed no negative effects on Ae. aegypti life history. Some results instead point to positive effects on host fitness (Figs. 1 and 2), although issues arising from laboratory rearing such as genetic drift can potentially affect fitness estimates (Ross et al. 2019a). The wAlbB-infected Ae. aegypti had a higher pupation success and egg hatch proportions under cold conditions than wMel-infected and uninfected lines, and the wMel-infected Ae. aegypti also performed relatively better than the uninfected line in terms of eclosion success. This was unexpected given that previous studies usually point to transinfected Wolbachia generating fitness costs on its novel host (Ross et al. 2019b), which may limit its spread after release (Schmidt et al. 2018). The positive effects of wAlbB may link to its original host, Ae. albopictus, given that Ae. albopictus has higher cold tolerance than Ae. aegypti (Chang et al. 2007, Dickens et al. 2018). Fitness effects associated with Wolbachia may be expressed regardless of the host; a good example is the life-shortening effect of wMel-Pop in Drosophila and mosquitoes (Reynolds et al. 2003, McMeniman et al. 2009). Perhaps cold resistance is partly regulated by Wolbachia, which may also contribute to the somewhat higher cold tolerance of wMel (Truitt et al. 2019); however, this infection is at a relatively lower frequency in colder areas of the range of its native host, D. melanogaster, which appears to be partly related to costs triggered by Wolbachia in overwintering adults (Kriesner et al. 2016). In this case, when using Wolbachia to control arboviral diseases transmitted by Ae. aegypti in temperate and subtropical regions, fitness benefits associated with some Wolbachia strains may assist in its invasion and spread in populations, but there is then a risk that the endosymbiont might increase mosquito populations overall.
In conclusion, we show that low temperatures reduce Ae. aegypti fitness but found no negative effects of Wolbachia infections, with beneficial effects identified for some traits. Temperature shifts at different life stages show that the effects of temperature on life history strongly depend on the life stage of exposure. Although Wolbachia infections in Ae. aegypti are suppressed at low temperatures, this reduction in density is not induced by WO (at least for wMel) and density can recover as mosquito age increases. Our findings demonstrate the feasibility to use Wolbachia to control arboviral diseases where Ae. aegypti occur in temperate and subtropical regions, though more research is required.
Supplementary Material
Acknowledgments
This research was supported by National Health and Medical Research Council (1132412, 1118640, www.nhmrc.gov.au) and the Wellcome Trust (108508, wellcome.ac.uk) for financial support. We also thank Cameron Patrick from Statistical Consulting Centre and Melbourne Statistical Consulting Platform, the University of Melbourne, for his statistical advice.
References Cited
- Ant T. H., Herd C. S., Geoghegan V., Hoffmann A. A., and Sinkins S. P.. . 2018. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 14: e1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold P. A., Levin S. C., Stevanovic A. L., and Johnson K. N.. . 2019. Drosophila melanogaster infected with Wolbachia strain wMelCS prefer cooler temperatures. Ecol. Entomol. 44: 287–290. [Google Scholar]
- Axford J. K., Ross P. A., Yeap H. L., Callahan A. G., and Hoffmann A. A.. . 2016. Fitness of wAlbB Wolbachia Infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am. J. Trop. Med. Hyg. 94: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale J. S., Masters G. J., Hodkinson I. D., Awmack C., Bezemer T. M., Brown V. K., Butterfield J., Buse A., Coulson J. C., and Farrar J.. . 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8: 1–16. [Google Scholar]
- Bordenstein S. R., and Bordenstein S. R.. . 2011. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS One. 6: e29106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler K., and Terblanche J. S.. . 2008. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol. Rev. Camb. Philos. Soc. 83: 339–355. [DOI] [PubMed] [Google Scholar]
- Briegel H. 1990. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 36: 165–172. [Google Scholar]
- Brown M. B., and Forsythe A. B.. . 1974. Robust tests for the equality of variances. J. Am. Stat. Assoc. 69: 364–367. [Google Scholar]
- Carrington L. B., Armijos M. V., Lambrechts L., Barker C. M., and Scott T. W.. . 2013. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One. 8: e58824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. H., Hsu E. L., Teng H. J., and Ho C. M.. . 2007. Differential survival of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) larvae exposed to low temperatures in Taiwan. J. Med. Entomol. 44: 205–210. [DOI] [PubMed] [Google Scholar]
- Chauvatcharin N., Ahantarig A., Baimai V., and Kittayapong P.. . 2006. Bacteriophage WO-B and Wolbachia in natural mosquito hosts: infection incidence, transmission mode and relative density. Mol. Ecol. 15: 2451–2461. [DOI] [PubMed] [Google Scholar]
- Dickens B. L., Sun H., Jit M., Cook A. R., and Carrasco L. R.. . 2018. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob. Health. 3: e000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O., Fort P., and Weill M.. . 2007. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity (Edinb). 98: 368–374. [DOI] [PubMed] [Google Scholar]
- Eisen L., Monaghan A. J., Lozano-Fuentes S., Steinhoff D. F., Hayden M. H., and Bieringer P. E.. . 2014. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J. Med. Entomol. 51: 496–516. [DOI] [PubMed] [Google Scholar]
- Foster W. A. 1995. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40: 443–474. [DOI] [PubMed] [Google Scholar]
- Fox J., Weisberg S., Adler D., Bates D., Baud-Bovy G., Ellison S., Firth D., Friendly M., Gorjanc G., and Graves S.. . 2012. Package ‘car’. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Garcia G. A., Sylvestre G., Aguiar R., da Costa G. B., Martins A. J., Lima J. B. P., Petersen M. T., Lourenço-de-Oliveira R., Shadbolt M. F., Rašić G., . et al. 2019. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 13: e0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavotte L., Vavre F., Henri H., Ravallec M., Stouthamer R., and Boulétreau M.. . 2004. Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol. Biol. 13: 147–153. [DOI] [PubMed] [Google Scholar]
- Gould E. A., and Higgs S.. . 2009. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 103: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T. J. 2017. Generalized additive models, pp. 249–307. In J. M. Chambers and T. J. Hastie (eds.) Statistical models in S. Routledge. Chapman and Hall/CRC, London, UK. [Google Scholar]
- Hoffmann A. A., Montgomery B. L., Popovici J., Iturbe-Ormaetxe I., Johnson P. H., Muzzi F., Greenfield M., Durkan M., Leong Y. S., Dong Y., . et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 476: 454–457. [DOI] [PubMed] [Google Scholar]
- Jensen D., Overgaard J., and Sørensen J. G.. . 2007. The influence of developmental stage on cold shock resistance and ability to cold-harden in Drosophila melanogaster. J. Insect Physiol. 53: 179–186. [DOI] [PubMed] [Google Scholar]
- Kent B. N., and Bordenstein S. R.. . 2010. Phage WO of Wolbachia: lambda of the endosymbiont world. Trends Microbiol. 18: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindlmann P., and Dixon A. F.. . 1992. Optimum body size: effects of food quality and temperature, when reproductive growth rate is restricted, with examples from aphids. J. Evol. Biol. 5: 677–690. [Google Scholar]
- Kraemer M. U., Sinka M. E., Duda K. A., Mylne A. Q., Shearer F. M., Barker C. M., Moore C. G., Carvalho R. G., Coelho G. E., Van Bortel W., . et al. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner P., Conner W. R., Weeks A. R., Turelli M., and Hoffmann A. A.. . 2016. Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution. 70: 979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., White V. L., Weeks A. R., Hoffmann A. A., and Endersby N. M.. . 2012. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl. Environ. Microbiol. 78: 4740–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R., Singmann H., and Love J.. . 2018. Emmeans: estimated marginal means, aka least-squares means computer program, R package version 1.1. [Google Scholar]
- Lu P., Bian G., Pan X., and Xi Z.. . 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 6: e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R., Koella J. C., and Lourenco-de-Oliveira R.. . 2011. Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans. R. Soc. Trop. Med. Hyg. 105: 452–458. [DOI] [PubMed] [Google Scholar]
- Mains J. W., Brelsfoard C. L., Crain P. R., Huang Y., and Dobson S. L.. . 2013. Population impacts of Wolbachia on Aedes albopictus. Ecol. Appl. 23: 493–501. [DOI] [PubMed] [Google Scholar]
- McGill R., Tukey J. W., and Larsen W. A.. . 1978. Variations of box plots. Am. Stat. 32: 12–16. [Google Scholar]
- McMeniman C. J., Lane R. V., Cass B. N., Fong A. W., Sidhu M., Wang Y. F., and O’Neill S. L.. . 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 323: 141–144. [DOI] [PubMed] [Google Scholar]
- Mitchell K. A., Sinclair B. J., and Terblanche J. S.. . 2013. Ontogenetic variation in cold tolerance plasticity in Drosophila: is the Bogert effect bogus? Naturwissenschaften. 100: 281–284. [DOI] [PubMed] [Google Scholar]
- Mordecai E. A., Caldwell J. M., Grossman M. K., Lippi C. A., Johnson L. R., Neira M., Rohr J. R., Ryan S. J., Savage V., Shocket M. S., . et al. 2019. Thermal biology of mosquito-borne disease. Ecol. Lett. 22: 1690–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C. L., Vontas J., Martins A. J., Ng L. C., Koou S. Y., Dusfour I., Raghavendra K., Pinto J., Corbel V., David J. P., . et al. 2017. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 11: e0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock C. C., Blanford S., Hughes G. L., Rasgon J. L., and Thomas M. B.. . 2014. Temperature alters Plasmodium blocking by Wolbachia. Sci. Rep. 4: 3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazni W. A., Hoffmann A. A., NoorAfizah A., Cheong Y. L., Mancini M. V., Golding N., Kamarul G. M. R., Arif M. A. K., Thohir H., NurSyamimi H., . et al. 2019. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 29: 4241–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. H., Nguyen H. L., Nguyen T. Y., Vu S. N., Tran N. D., Le T. N., Vien Q. M., Bui T. C., Le H. T., Kutcher S., . et al. 2015. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors. 8: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill S. L., Ryan P. A., Turley A. P., Wilson G., Retzki K., Iturbe-Ormaetxe I., Dong Y., Kenny N., Paton C. J., Ritchie S. A., . et al. 2018. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rašić G., Endersby N. M., Williams C., and Hoffmann A. A.. . 2014. Using Wolbachia-based release for suppression of Aedes mosquitoes: insights from genetic data and population simulations. Ecol. Appl. 24: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Reynolds K. T., Thomson L. J., and Hoffmann A. A.. . 2003. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 164: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. A., Axford J. K., Richardson K. M., Endersby-Harshman N. M., and Hoffmann A. A.. . 2017a. Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J. Vis. Exp. 14: e56124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. A., Wiwatanaratanabutr I., Axford J. K., White V. L., Endersby-Harshman N. M., and Hoffmann A. A.. . 2017b. Wolbachia Infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 13: e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. A., Endersby-Harshman N. M., and Hoffmann A. A.. . 2019a. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol. Appl. 12: 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. A., Turelli M., and Hoffmann A. A.. . 2019b. Evolutionary ecology of Wolbachia releases for disease control. Annu. Rev. Genet. 53: 93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. A., Ritchie S. A., Axford J. K., and Hoffmann A. A.. . 2019c. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl. Trop. Dis. 13: e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley W. A., Graham C. L., and Williams R. E.. . 1968. A flight mill system for the laboratory study of mosquito flight. Ann. Entomol. Soc. Am. 61: 1507–1514. [Google Scholar]
- Sanburg L. L., and Larsen J. R.. . 1973. Effect of photoperiod and temperature on ovarian development in Culex pipiens pipiens. J. Insect Physiol. 19: 1173–1190. [DOI] [PubMed] [Google Scholar]
- Schmidt T. L., Filipović I., Hoffmann A. A., and Rašić G.. . 2018. Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity (Edinb). 120: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A., Li Z., Sun L., and Carlow C. K. S.. . 2019. Complete genome sequence of the Wolbachia wAlbB endosymbiont of Aedes albopictus. Genome Biol. Evol. 11: 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R. C. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Tortosa P., Charlat S., Labbé P., Dehecq J. S., Barré H., and Weill M.. . 2010. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One. 5: e9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt A. M., Kapun M., Kaur R., and Miller W. J.. . 2019. Wolbachia modifies thermal preference in Drosophila melanogaster. Environ Microbiol. 21: 3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. J., and Teng H. J.. . 2016. Role of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse) in local dengue epidemics in Taiwan. BMC Infect. Dis. 16: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. J., Lin T. H., Teng H. J., and Yeh H. C.. . 2018. Critical low temperature for the survival of Aedes aegypti in Taiwan. Parasit. Vectors. 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda T., Fukuchi A., Nanbara S., and Takagi M.. . 2010. Effect of body size and sugar meals on oviposition of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 35: 56–60. [DOI] [PubMed] [Google Scholar]
- Tukey J. W. 1949. Comparing individual means in the analysis of variance. Biometrics. 5: 99–114. [PubMed] [Google Scholar]
- Ulrich J. N., Beier J. C., Devine G. J., and Hugo L. E.. . 2016. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl. Trop. Dis. 10: e0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova E. B. 2007. Diapause in aquatic insects, with emphasis on mosquitoes, pp. 83–113. In V. R. Alekseev, B. De Stasio, and J. J. Gilbert (eds.), Diapause in aquatic invertebrates: theory and human use. Springer, Dordrecht, Netherlands. [Google Scholar]
- Walker T., Johnson P. H., Moreira L. A., Iturbe-Ormaetxe I., Frentiu F. D., McMeniman C. J., Leong Y. S., Dong Y., Axford J., Kriesner P., . et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 476: 450–453. [DOI] [PubMed] [Google Scholar]
- Walton E. L. 2018. Dengue in Taiwan: pointing the finger at Aedes aegypti. Biomed. J. 41: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Sun L. V., Vamathevan J., Riegler M., Deboy R., Brownlie J. C., McGraw E. A., Martin W., Esser C., Ahmadinejad N., . et al. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z., Dean J. L., Khoo C., and Dobson S. L.. . 2005. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 35: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap H. L., Mee P., Walker T., Weeks A. R., O’Neill S. L., Johnson P., Ritchie S. A., Richardson K. M., Doig C., Endersby N. M., . et al. 2011. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 187: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhan X., Wu X., Yang X., Liang G., Zheng Z., Li Z., Wu Y., and Zheng X.. . 2014. A field survey for Wolbchia and phage WO infections of Aedes albopictus in Guangzhou City, China. Parasitol. Res. 113: 399–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.