Abstract
Coronavirus disease 2019 (COVID-19) patients with pre-existing cardiovascular disease (CVD) or with cardiovascular complications have a higher risk of mortality. The main cardiovascular complications of COVID-19 include acute cardiac injury, acute myocardial infarction (AMI), myocarditis, arrhythmia, heart failure, shock, and venous thromboembolism (VTE)/pulmonary embolism (PE). COVID-19 can cause cardiovascular complications or deterioration of coexisting CVD through direct or indirect mechanisms, including viral toxicity, dysregulation of the renin–angiotensin–aldosterone system (RAAS), endothelial cell damage and thromboinflammation, cytokine storm, and oxygen supply–demand mismatch. We systematically review cardiovascular manifestations, histopathology, and mechanisms of COVID-19, to help to formulate future research goals and facilitate the development of therapeutic management strategies.
Keywords: COVID-19, cardiovascular, ACE2, endothelial cell damage, thromboinflammation, cytokine storm, type 2 myocardial infarction
COVID-19 is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has infected over 20.9 million patients and resulted in 760 633 deaths worldwide as of August 15, 2020. SARS-CoV-2 is in the same family as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses. However, compared with SARS and MERS, COVID-19 has higher transmissibility and lower mortality (although still higher than influenza) [1]. The mortality rate of COVID-19 was reported to be ~3.4% among all patients and 1.4% among patients without underlying disease, but 13.2% among patients with pre-existing CVD [2]. Among inpatients with COVID-19, the prevalence of cardiovascular comorbidities has ranged between 17.1% and 59.6%, and mortality in those with pre-existing hypertension or CVD is 1.42-fold and 3.15-fold higher than those without, respectively (Table 1 ) [2., 3., 4., 5., 6., 7.].
Table 1.
Characteristics of Respiratory Viral Infections and the Impact of Cardiovascular Comorbidities on Hospitalized Patients with Severe Viral Respiratory Infectionsa
| Viral respiratory infection | Infectious R0 | Mortality rate | Prevalence of cardiovascular comorbidities |
Influence of cardiovascular comorbidities on mortality rates |
||
|---|---|---|---|---|---|---|
| Hypertension | Cardiovascular disease | Hypertension | Cardiovascular disease | |||
| H1N1 | 1.4–1.6 [98] | 0.4% (0.3–1.5%) [99] | 20% [100] | 21% [100] | OR = 2.92, 95% CI 1.8–4.9 [96] | Mortality 2.4–6.9% [101] |
| MERS | 0.54–6.7 [102,103] | 34.3–40% [104] | 30.3%, 95% CI 18.3–42.2% [3] | 20.9%, 95% CI 10.7–31.1% [3] | OR = 2.1, 95% CI 1.1–3.7 [105] | OR = 3.5, 95% CI 3.1–4.8 [106] |
| SARS | 0.54–1.13 [103] | 9.5% [104] | 4.5%, 95% CI 2.0–7.0% [3] | 10.4–19.4% [3,107] | Mortality 35.7% [108] | OR = 7.35, 95% CI 1.8–29.5 [109] Mortality 25% |
| COVID-19 | 2.24–5.7 [110., 111., 112.] | 3.4% [2] | 17.1–59.6% [3., 4., 5., 6.] | 21.5% [6] | OR = 1.42, 95% CI 0.96–2.11 [6] Mortality 13.2% [2] |
OR = 3.15, 95% CI 2.3–4.4 [7] Mortality 8.4% [2] |
Abbreviations: CI, confidence interval; OR, odds ratio; R0, reproduction number (average rate of onward transmission).
As the deaths from COVID-19 increase, especially among patients with comorbidities, the impact of COVID-19 on the cardiovascular system is gaining widespread attention. COVID-19 could result in cardiovascular complications or deterioration of coexisting CVD through direct or indirect mechanisms. Moreover, the severity of COVID-19 correlates with cardiovascular manifestations. Given that the COVID-19 pandemic continues to expand, there has been increasing demand for deeper understanding of the interaction between the cardiovascular system and viral infection. To satisfy this demand, we provide a comprehensive state-of-the-art review of the cardiovascular manifestations and pathophysiology of COVID-19. By integrating pathological and clinical findings, this forum article aims to improve our understanding of the potential mechanisms underlying the effects of COVID-19 on the cardiovascular system, laying the foundation for improved preventative and therapeutic management strategies.
Cardiovascular Manifestations
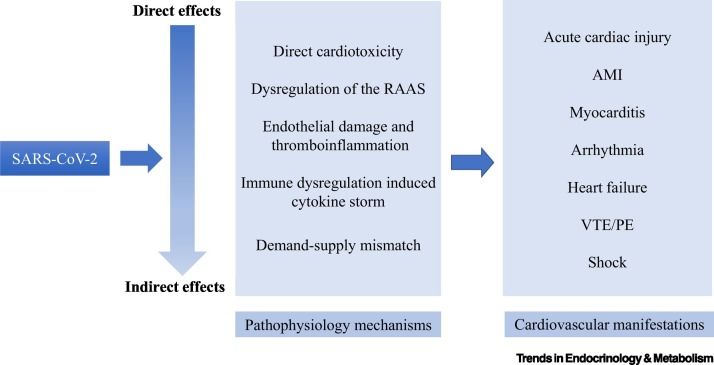
The clinical cardiovascular manifestations of COVID-19 mainly include acute cardiac injury, AMI, myocarditis, arrhythmia, heart failure, venous VTE/ PE, and shock (Figure 1 ).
Figure 1.
Mechanisms and Manifestations of Cardiovascular Implications of Coronavirus Disease 2019 (COVID-19).
Acute Cardiac Injury
Acute cardiac injury is defined as a rise of cardiac troponin values, with or without ejection fraction decline or electrocardiographic abnormalities. The prevalence of acute cardiac injury among COVID-19 patients was 10–23% [8., 9., 10., 11.], with a higher frequency in intensive care unit (ICU) patients (22.2% versus 2.0%) [9] and non-survivors (59.0% versus 1.0%) [10]. Patients with acute cardiac injury were associated with more severe illness, including higher C-reactive protein (CRP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and creatinine levels, as well as more multiple mottling and ground-glass opacity, and were more likely to receive noninvasive or invasive ventilation. Acute cardiac injury was also associated with cardiac dysfunction and malignant arrhythmias [12]. Patients with acute cardiac injury exhibited a significant higher risk of mortality both during the time from symptom onset [hazard ratio (HR) = 4.26; 95% confidence interval (CI) 1.92–9.49] and from admission to end-point (HR = 3.41; 95% CI 1.62–7.16) [13]. Greater magnitude and frequency of cardiac troponin elevation was also associated with higher mortality [13].
COVID-19 patients with previous CVD were more prone to suffer acute cardiac injury. Compared with patients without cardiac injury, there was a higher prevalence of CVD (29.3% versus 6.0%) and hypertension (59.8% versus 23.4%) in those with cardiac injury [13]. Moreover, once infected by SARS-CoV-2, patients with CVD comorbidities usually had a worse cardiac reserve and poorer tolerance to hypoxia, and were more likely to develop cardiac insufficiency (e.g., heart failure, malignant arrhythmia, or shock).
AMI
Respiratory viruses, such as SARS and influenza, are associated with AMI by increasing the risk of coronary plaque rupture [14,15]. The incidence ratio of AMI in patients during the first week after influenza diagnosis, compared with 1 year after influenza diagnosis, is 6.05 (95% CI 3.86–9.50) [16]. AMI can occur in COVID-19 patients, but the incidence of such events is unknown. Newly diagnosed AMI was reported in 5.3% of cases in an electrocardiographic study of COVID-19 [17], and in 2.9 % in another echocardiography study [18]. ST-elevation myocardial infarction (STEMI) can present as the initial clinical manifestation of COVID-19 [19], and 33.3–39.3% of patients with COVID-19 who had STEMI were diagnosed with non-obstructive coronary artery disease [19,20]. This phenomenon indicated that COVID-19 itself may be connected to endothelial dysfunction as well as to the hypercoagulable state.
COVID-19 has also profoundly reshaped the pathway of patients with AMI. The hospitalization rates for AMI fell in the early phase of COVID-19, and mildly recovered after 5 weeks with increased in-hospital mortality in the USA [21]. Many patients with AMI, perhaps owing to fear of contracting SARS-CoV-2, avoided hospitalization at the time of the COVID-19 pandemic, leading to delay and aggravation of AMI. Further studies will be necessary to elucidate the impact of COVID-19 on AMI.
Myocarditis
Myocarditis is defined as myocardial damages caused by direct viral attack on the heart. In the early period of COVID-19, five (7%) of 68 patients with myocardial damages died of circulatory failure [22], and a patient who presented with third degree atrioventricular block was reported as myocarditis [17]. A few COVID-19-related myocarditis cases have been confirmed by cardiac magnetic resonance imaging (MRI) [23., 24., 25., 26.]. However, there is only limited evidence for viral entry into cardiomyocytes. Although endomyocardial biopsy found evidence of lymphocytic inflammatory infiltrates in the myocardium, SARS-CoV-2 particles were found only in interstitial cells of the myocardium [27]. Another COVID-19 case demonstrated lymphocytic myocarditis without SARS-CoV-2 in the myocardium [28]. Thus, immune-mediated hyperinflammation may play a more significant role than viral replication or toxicity in the pathophysiology of acute myocarditis associated with COVID-19. Pericardial involvement with cardiac tamponade has also been reported [29,30].
Arrhythmia
Arrhythmias were reported in 16.7% of 138 hospitalized COVID-19 patients, with a greater proportion in ICU patients than in non-ICU patients (44.4% versus 6.9%, P = <0.001) [9]. COVID-19 presenting with various arrhythmias has a strong association with the severity of the disease. For example, compared with patients in the non-ICU group, the proportion of abnormal Q waves in electroencephalography (ECG) traces from the patients in the ICU group was significantly elevated (33.3% versus 3.9%, P = 0.006) [17]. Ventricular arrhythmias are higher among patients with acute cardiac injury than in patients without acute cardiac injury (17.3% versus 1.5%, P = <0.001) [12]. Atrial arrhythmias were more common among patients who required mechanical ventilation than among those who did not (17.7% versus 1.9%) [31]. Prolonged corrected QT (>500 ms) was found in 6% of 4250 patients with COVID-19 in a New York cohort and should not be ignored [23]. In 136 COVID-19 patients who experienced in-hospital cardiac arrest, asystole was the most common initial rhythm in 89.7% of cases, whereas shockable rhythms were found in only 5.9% of patients [32]. Sudden cardiac death has been reported in COVID-19 patients who initially had only mild symptoms but who were later discovered dead at home [33]. In contrast to the decline of patients with AMI in the early COVID-19 pandemic, out-of-hospital cardiac arrest reported an increase of 58% during the first 40 days of the COVID-19 outbreak compared with the same period in 2019. COVID-19 or other untreated diseases such as AMI combined with patient unwillingness to attend for treatment may have contributed to the phenomenon [34]. Arrhythmias in the population with COVID-19 developed secondary to hypoxemia, metabolic dysregulation, electrolyte disorder, systemic inflammation, electrical instability with adrenergic stress, AMI or myocarditis, and treatment with QT prolonging drugs.
Heart Failure
Heart failure was observed in 23% of patients with COVID-19, and the proportion of heart failure was higher in non-survivors than in survivors (52% versus 12%, P = <0.001) [10,35]. In accordance with cardiac markers such as troponin, the rise of BNP/NT-proBNP is related to poor prognosis among individuals with COVID-19 [22]. In seriously ill patients with COVID-19, 7–33% had biventricular failure [22,36]. Studies have also reported isolated right ventricular failure with or without PE [37,38]. A few case reports have revealed a novel connection between stress cardiomyopathy (Takotsubo syndrome) and COVID-19 [39., 40., 41.]. The incidence of stress cardiomyopathy (7.8%) increased significantly during the COVID-19 outbreak compared with pre-pandemic periods (1.5–1.8%) [42]. COVID-19 produced financial, social, and mental pressure that may have provoked stress cardiomyopathy. Microvascular disorder, cytokine storm, and sympathetic stress may participate in the pathophysiology of stress cardiomyopathy.
As an end-stage manifestation of CVD, heart failure may be the long-term consequence of cardiac infection by SARS-CoV-2. In a recent cohort study on 100 patients recovered from COVID-19, cardiac involvement in 78 patients and continuous myocardial inflammation in 60 patients were recognized by cardiac MRI, regardless of pre-existing CVD or the severity of the illness [43].
Shock
Cardiogenic, septic, or mixed shock is one of the criteria of critical illness in COVID-19. Shock developed in 8.7% of 138 patients with COVID-19, and was more frequent in ICU patients compared with non-ICU counterparts (30.6% versus 1.0%, P = <0.001) [8]. It is crucial to diagnose whether there is a concomitant cardiogenic factor to assist clinical decision-making, particularly when mechanical respiratory and circulatory assistance with extracorporeal membranous oxygenation (ECMO) are required because this may influence device selection (venovenous versus venoarterial) [44].
In the later phase of the COVID-19 pandemic, healthy children displaying atypical Kawasaki disease (KD) have been given much attention in the USA and Europe [45,46]. This syndrome was named pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS). It manifested as consistent fever, evidence of inflammation (neutrophilia, raised CRP, and lymphopenia), and single or multiorgan dysfunction (shock, cardiac, respiratory, kidney, gastrointestinal, or neurological disorder) in conjunction with existing or previous infection with SARS-CoV-2. Comparison of PIMS-TS with KD or KD shock syndrome showed older age and greater elevation of inflammatory markers such as CRP [47]. Untreated KD can lead to coronary aneurysms in 25% of patients. SARS-CoV-2-induced immune dysregulation may lead to PIMS-TS onset because the majority of children with PIMS-TS were positive for antibodies against SARS-CoV-2 but negative for nucleic acids [48].
VTE and PE
COVID-19 has been associated with proinflammatory and prothrombotic conditions that can result in thromboembolic events [49,50]. In fact, higher markers of thrombosis have been associated with worse clinical outcomes. In a Chinese multicenter study, elevated D-dimer levels (>1 g/l) were independent predictors of in-hospital death [10]. Furthermore, 71.4% of patients who died were diagnosed with disseminated intravascular coagulation [51]. A study reported a high incidence of VTE (69%) in ICU patients [52]. However, other centers have reported lower VTE rates (22.2%) [53]. Despite prophylactic anticoagulation, 31% of patients with COVID-19 still developed VTE [54], and 16.7% of patients were diagnosed with PE [55]. PE was more likely to occur in acute respiratory distress syndrome (ARDS) patients with COVID-19 compared with non-ARDS individuals (11.7 versus 2.1%, P = <0.008) [55]. Another study reported a cumulative incidence of PE of 20.4% (95% CI 13.1–28.7%) in critical COVID-19 patients, markedly higher than for patients in the same ICU during the same period in 2019 [56].
Mechanisms of Cardiac Manifestations in COVID-19
The pathology of COVID-19 results from both direct and indirect injuries. Direct injuries are caused by infection of target cells by the virus. Indirect injuries mainly result from immune response, inflammation reaction, circulatory dysfunction, and hypoxia. The major pathological findings of cardiac tissues and vasculature from autopsy are summarized in Table 2 . The various possible mechanisms that may contribute to the pathogenesis of cardiovascular complications of COVID-19 the gross, cellular, and molecular levels are illustrated in Figure 1.
Table 2.
Pathological Findings in the Cardiovascular System in Deaths from COVID-19a
| First author (Refs) | Study area | Sample size (age range in years) | Cardiac pathology | Vascular pathology |
|---|---|---|---|---|
| Yao [113] | China | 3 (63–79) | Manifestation of myocardial hypertrophy, multifocal necrosis and interstitial inflammatory infiltration Negative RT-PCR test of SARS-CoV-2 |
Manifestation of diffused hyaline thrombosis in microcirculation in multiple organs |
| Xu [114] | China | 1 (50) | A few interstitial inflammatory infiltrations | NA |
| Tian [115] | China | 4 (59–81) | Various degrees of focal edema, interstitial fibrosis, and myocardial hypertrophy No inflammatory infiltration Positive RT-PCR test for SARS-CoV-2 in one case (two cases with elevated troponin) |
Fibrinoid necrosis of the small vessels of lung in one case |
| Ackermann [69] | USA | 7 (66–96) | NA | Severe endothelial injury associated with intracellular virus and disrupted cell membranes in the lungs Widespread thrombosis with microangiopathy in pulmonary vessels Alveolar capillary microthrombi were ninefold more prevalent in patients with COVID-19 than in patients with influenza The amount of new vessel growth was 2.7-fold higher than in the lungs of patients with influenza |
| Barton [116] | USA | 2 (42–77) | No evidence of myocarditis One case with coronary artery disease showing microscopic evidence of acute ischemic injury |
NA |
| Bradley [117] | USA | 14 (42–84) | Lymphocytic myocarditis with viral RNA detection in one case | Five cases with focal pulmonary microthrombi |
| Buja [118] | USA | 3 (34–62) | Lymphocytic pericarditis Multifocal acute injury of cardiomyocytes without inflammatory cellular infiltrates |
One case with fibrin-rich thrombi in renal capillaries and small vessels |
| Craver [119] | USA | 1 (17) | Enlarged flabby heart with eosinophilic myocarditis | NA |
| Fox [120] | USA | 4 (44–76) | Cardiomegaly with right ventricular dilation Scattered individual myocyte necrosis without lymphocytic myocarditis |
Thrombosis and microangiopathy in the small vessels and capillaries of the lungs, with associated hemorrhage |
| Fox [121] | USA | 22 (44–79) | Nine cases with severe right ventricular dilation Scattered individual myocyte necrosis No typical lymphocytic myocarditis No SARS-CoV-2 virus in cardiac myocytes |
Scattered CD4 and CD8 lymphocytes near vascular structures SARS-CoV-2 virus in the myocardial endothelial compartment and in renal tubular epithelium |
| Stone [122] | USA | 1 (76) | Increased infiltration of the myocardium by macrophages Focal infiltration of the myocardium by lymphocytes No association with myocyte injury |
NA |
| Varga [70] | USA | 3 (58–71) | No sign of lymphocytic myocarditis | Lymphocytic endotheliitis in lung, heart, kidney, liver, and small intestine Viral inclusion structures in endothelial cells of the kidney |
| Edler [123] | Germany | 80 (52–96) | One case with small lymphocytic infiltrate in the right ventricle | 32 (40%) cases with VTE 25 (31.2%) cases with PE |
| Lindner [124] | Germany | 39 (78–89) | 24 (61.5%) cases with virus in interstitial cells of cardiac tissue Increased proinflammatory response (TNF-α, IFN-γ, CCL5, IL-6, IL-8, IL-18) in cardiac tissue with higher virus load No association between virus presence and increased infiltration of mononuclear cells in the myocardium No myocarditis |
NA |
| Nicolai [125] | Germany | 10 (69–91) | NA | Inflammatory microvascular thrombi in lung, kidney, and heart, containing NETs with platelets and fibrin |
| Puelles [126] | Germany | 27 (52–93) | Lower levels of SARS-CoV-2 copies in the heart | SARS-CoV-2 protein in the glomerular epithelial, endothelial, and tubular cells in kidney by immunofluorescence |
| Schaller [127] | Germany | 12 (64–90) | Four cases with mild lymphocytic myocarditis Two cases with signs of epicarditis |
NA |
| Wichmann [128] | Germany | 12 (52–87) | One case with mononuclear infiltrations consisting of lymphocytes in the myocardium of the right ventricle | Seven (58%) cases with VTE Microthrombi systematically observed in small lung arteries |
| Nunes [129] | Brazil | 10 (33–83) | Two cases with mild lymphomononuclear myocarditis | Two cases with endothelial changes in small vessels (cell tumefaction, vessel wall edema, and fibrinoid alteration) Two cases with fibrin microthrombi in the heart |
| Aiolfi [130] | Italy | 2 (56–70) | NA | Diffuse peripheral vessel endothelial hyperplasia and in toto muscular wall thickening in the lungs Intravascular hemorrhagic thrombosis in the lungs |
| Sala [28] | Italy | 1 (43) | Diffuse T lymphocytic inflammatory infiltrates with huge interstitial edema and limited foci of necrosis Absence of SARS-CoV-2 genome in the myocardium |
NA |
| Tavazzi [27] | Italy | 1 (69) | Viral particles in macrophages, but not in cardiomyocytes or other specific cardiac cell types | NA |
Abbreviations: CCL5, chemokine 5; IFN-γ, interferon γ; IL, Interleukin; NA, not available; NETs, neutrophil extracellular traps; PE, pulmonary embolism; RT-PCR, reverse transcription-PCR; TNF, tumor necrosis factor; VTE, venous thromboembolism.
ACE2-Mediated Viral Toxicity
Angiotensin-converting enzyme 2 (ACE2) is expressed in the vascular system (endothelial cells, vascular smooth muscle cells, and migratory angiogenic cells) and the heart (cardiofibroblasts, cardiomyocytes, endothelial cells, pericytes, and epicardial adipose cells) [57]. ACE2 functions as a virus entry receptor by binding to the spike (S) protein of SARS-CoV and SARS-CoV-2. In addition, completion of cell entry requires priming of the S subunit by the cellular serine protease TMPRSS2 (transmembrane protease serine 2) or other proteases (cathepsin L, cathepsin B, factor X, trypsin, elastase, and furin) [58]. The increased transmissibility of SARS-CoV-2 could be interpreted by the greater binding affinity of SARS-CoV-2 for ACE2 than of SARS-CoV [59]. Furthermore, patients with previous CVD were associated with more severe COVID-19 disease, possibly because they had higher plasma levels of ACE2 [60]. Nevertheless, compared with the lung, the human heart has higher expression of ACE2 and with much lower content of TMPRSS2. The susceptibility of the heart in SARS-CoV-2 infection was diminished to some extent by a lower proportion of ACE2+/TMPRSS2+ cells. Other S protein priming proteases that are prominently expressed in the human heart, cathepsin L and furin, may increase heart vulnerability to SARS-CoV-2 [61].
Dysregulation of RAAS
ACE2 converts angiotensin II (Ang II) to Ang-(1–7), and the ACE2/Ang-(1–7)/Mas axis combats the adverse impacts of RAAS, which is essential for preserving the physiological and pathophysiological equilibrium of the body. Entry of SARS-CoV-2 into cells is assisted by the interaction between S protein and ACE2 extracellular domains, leading to downregulation of surface ACE2 expression. Ang-II/angiotensin 1 receptor (AT1R) activity is then increased at the expense of the ACE2/Ang 1–7/Mas axis, leading to comprehensive negative consequences, including aldosterone secretin, fibrosis, proinflammation, hypertrophy, vasoconstriction, enhanced reactive oxygen species and vascular permeability, cardiac remolding, gut dysbiosis, and multiple organ dysfunction syndrome (MODS) in COVID-19 [57,62]. ACE2 exerts multiple protective effects in numerous organs and various diseases, and genetic ACE2 deficiency is associated with adverse results. Ace2 knockout mice display myocardial hypertrophy and interstitial fibrosis, and aggravated heart dysfunction [63]. ACE2 deficiency substantially worsened the pathogenesis in influenza virus H5N1-infected mice, and AT1R suppression relieved the severity of lung damage [64]. ACE2 can also stimulate insulin secretion and lower insulin resistance [65]. Downregulation of ACE2 expression was seen in myocardial cells in both SARS-CoV-infected mice and humans [66]. There is also a positive correlation between elevated circulating Ang II levels in COVID-19 patients and lung injury and/or viral load. In short, a direct link between tissue ACE2 downregulation and upregulation of Ang II is partially responsible for the development of cardiovascular complications or multiorgan failure following SARS-CoV-2 infection [67,68].
Endothelial Cell Damage and Thromboinflammation
Direct invasion of endothelial cells by SARS-CoV-2 infection and indirect generation of inflammation and prothrombotic conditions in vasculopathy both contribute to the pathophysiological mechanisms of COVID-19 [69., 70., 71.]. Both venous and arterial endothelia are reported to express ACE2 [69]. Furthermore, histopathological studies have provided microscopic evidence of SARS-CoV-2 viral particles in endothelial cells of the kidney, as well as obvious endotheliitis characterized by activated neutrophils and macrophages in numerous organs including the lung, intestine, and heart [70]. Von Willebrand factor (VWF), a circulating blood coagulation glycoprotein associated with endothelial dysfunction, is significantly elevated in COVID-19 patients compared with normal individuals [72]. VWF, a carrier of coagulation factor VIII, can trigger platelet aggregation and blood coagulation [73]. Subsequent platelet–neutrophil interaction and macrophage activation can further promote proinflammatory responses including cytokine storm and the formation of neutrophil extracellular traps (NETs) [74]. NETs damage the endothelium and stimulate both extrinsic and intrinsic coagulation pathways, resulting in microthrombus formation and microvascular dysfunction. High levels of NETs were reported in hospitalized patients with COVID-19, and these correlated positively with disease severity [75]. Inhibiting NETs may be a therapeutic target to reduce NET-mediated thrombotic tissue damage associated with COVID-19 [76].
Immune Dysregulation-Induced Cytokine Storm
Dysregulated immune response and subsequent cytokine storm characterize the presentation of severe COVID-19. Previous studies with human coronaviruses have reported that rapid viral replication, interference with interferon signaling, and recruitment of inflammatory cells (neutrophils and monocyte/macrophages) are mediators of hyperinflammation [77]. Immunity measurements such as white blood cells, neutrophils, lymphocyte subtypes, and inflammation parameters (CRP and procalcitonin) were independently related to acute cardiac injury in patients with COVID-19 [78]. Subsequent cytokine storm, characterized by a sharp rise in the level of multiple proinflammatory cytokines triggered by infection, has been observed following infection with H1N1 [79], SARS [77,80], or MERS [77,80] and is an important cause of death (Table 3 ). A comprehensive evaluation of the transcriptional response to SARS-CoV-2 uncovered an atypical inflammatory reaction characterized by decreased levels of type I and III interferons, increased chemokines, and high levels of interleukin (IL)-6. Reduced innate antiviral defenses and raised proinflammatory responses contribute to COVID-19 pathology [81]. Zhou and colleagues reported that, after SARS-CoV-2 infection, pathogenic T helper 1 cells and inflammatory CD14+CD16+ monocytes induced high levels of granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-6 expression [82]. Higher levels of IL-6 in the serum have also been linked to worse prognosis [10,22,83,84] and were found to correlate with fibrinogen levels in patients with COVID-19 [85]. IL-6 can activate coagulation, induce thrombosis [86], inhibit heart function [87], and cause endothelial dysfunction, leading to vascular leakage, tissue ischemia and hypoxia, and thus to a drop in blood pressure, disseminated intravascular coagulation (DIC), and MODS [88].
Table 3.
Mechanisms of Cytokine Storm in Respiratory Viral Infectionsa
| Viral respiratory infection | Cytokine storm | Refs |
|---|---|---|
| H1N1 | IL-6, IL-8, IL-9, IL-17, TNF-α, IL-15, and IL-12p70 | [79] |
| MERS | IFN-γ, TNF-α, IL-15, and IL-17 | [77,80] |
| SARS | IL-1β, IL-6, IL-12, IFN-γ, IP-10, and MCP-1 | [77,80] |
| COVID-19 | GM-CSF, IL-2, IL-6, IL-7, IL-10, IP-10, MCP-1, MIP-1A, and TNF-α | [8,131] |
Abbreviations: GM-CSF, human granulocyte-macrophage colony stimulating factor; IFN, interferon; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; MIP-1A, macrophage inflammatory protein-1α; TNF, tumor necrosis factor.
Mismatch between Oxygen Supply and Demand
Hypoxemia is the main manifestation of COVID-19, and results in an insufficiency of oxygen supply to organs with a high demand for oxygen and energy, particularly the heart [89]. The imbalance of oxygen supply and demand caused by cytokine storm as well as by endothelial dysfunction, without acute atherothrombotic plaque disruption, similar to the pathophysiology of type 2 myocardial infarction, is thought to lead to cardiac injury in COVID-19 patients [90,91]. Type 2 MI is a potential cause of cardiac damage in acute infection [90]. On the one hand, cytokine storm causes the release of IL-6 and catecholamines that increase core body temperature, heart rate, and cardiac oxygen consumption. On the other hand, endothelial dysfunction and cytokine storm affect the cardiac microenvironment, causing pathological changes such as coronary artery spasm and thrombosis, all of which lead to decreased blood supply via the coronary artery. The reflex elevation in heart rate will further decrease myocardial perfusion owing to decreased filling time. Severe hypoxemia, hypotension, and anemia in critically ill patients with COVID-19 further aggravate insufficient oxygen supply. The combination of these factors causes mismatch between oxygen supply and demand, leading to acute cardiac damage.
Indeed, compared with type 1 MI caused by plaque rupture and thrombus formation, patients with type 2 MI have higher mortality rates, and this may in part reflect a higher burden of acute and chronic multimorbidity conditions in the population with type 2 MI [92]. Given the age and comorbidity of hospitalized patients with severe COVID-19, it is reasonable to speculate that type 2 MI in these patients is likely to be an indicator of more severe COVID-19 disease and worse prognosis [13].
Concluding Remarks
The COVID-19 pandemic represents the most significant public health crisis of the century. Because of the association with increased mortality, CVD is an obvious and important comorbidity of COVID-19 and other respiratory viruses, and it is among the leading cause of mortality among COVID-19 patients [93]. The mechanisms underlying the cardiovascular manifestations of COVID-19 have not been completely elucidated and are probably multifactorial. There is currently only limited evidence for direct cardiac viral toxicity, and both indirect and direct mechanism are likely to synergistically contribute to cardiovascular damage.
Because of quarantine restrictions, the COVID-19 pandemic has been a severe challenge for both population well-being and healthcare services, particularly in the main epidemic areas of COVID-19 and low-income and middle-income countries (e.g., Brazil, India, and South Africa). This may delay medical treatment of acute cardiovascular emergencies such as stroke or AMI [94]. Therefore, it is necessary to heighten awareness and self-protection measures to reduce morbidity and mortality in individuals with CVD and related conditions. In addition, telemedicine affords a further potential means to access healthcare in rural communities and allow routine follow-up for individuals with CVD [95].
At the moment, therapeutic treatments for COVID-19 are limited to steroids and remedesivir (and maybe convalescent plasma) with unsatisfactory effects in prevention, treatment and reducing complications of COVID-19. The clinical cardiovascular presentations associated with SARS-CoV-2 infection should be closely monitored and treated to prevent mortality from cardiovascular complications. Meanwhile, vigorous efforts should be made towards vaccine development for COVID-19. Influenza vaccine can reduce the risk of cardiovascular events by 26–35% and all-cause mortality by 43% in the general adult population [96,97]. For high-risk patients with a history of recent AMI, influenza vaccination was of significant benefit because it reduced the risk of cardiovascular events by 55% [96]. Therefore, vaccination against COVID-19, if limited, should be prioritized in patients with CVD as an important secondary prevention strategy, especially in those with cardiometabolic disorders, to reduce the risk of cardiovascular events.
Acknowledgments
We thank the National Key R&D Program of China (project 2018YFC2002400; subproject 2018YFC2002405), the Project of Health Commission of Sichuan Province (19PJ093), and the International Institute of Spatial Lifecourse Epidemiology (ISLE) for research support.
References
- 1.Xiong T.Y. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World, H., Health Organization . WHO; 2020. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- 3.Liu Y. Prevalence of cardiovascular comorbidities in coronavirus disease 2019, severe acute respiratory syndrome, and middle east respiratory syndrome: pooled analysis of published data. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong T.Y. Hypertension is a risk factor for adverse outcomes in patients with coronavirus disease 2019: a cohort study. Ann. Med. 2020;52:361–366. doi: 10.1080/07853890.2020.1802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.Y. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int. J. Infect. Dis. 2020;98:462–466. doi: 10.1016/j.ijid.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins J.L. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figliozzi S. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur. J. Clin. Investig. 2020;50 doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 8.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi S. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peiris J.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzini A. Prognostic impact of high sensitive troponin T in patients with influenza virus infection: a retrospective analysis. Heart Lung. 2020;49:105–109. doi: 10.1016/j.hrtlng.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Kwong J.C. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 17.Li Y. Electrocardiograhic characteristics in patients with coronavirus infection: a single-center observational study. Ann. Noninvasive Electrocardiol. 2020 doi: 10.1111/anec.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dweck M.R. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefanini G.G. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangalore S. ST-segment elevation in patients with Covid-19 – a case series. N. Engl. J. Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman T.J. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2020;7 doi: 10.1001/jamacardio.2020.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyen D. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnecchi M. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inciardi R.M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I.C. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavazzi G. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sala S. Acute myocarditis presenting as a reverse Tako–Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua A. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur. Heart J. 2020;41:2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabbagh M.F. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2:1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal P. Clinical characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao F. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochi A.N. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldi E. Out-of-hospital cardiac arrest during the COVID-19 outbreak in Italy. N. Engl. J. Med. 2020;383:496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arentz M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullah W. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020;2:1379–1382. doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Creel-Bulos C. Acute cor pulmonale in critically ill patients with COVID-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer P. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur. Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen D. A case of Takotsubo cardiomyopathy with COVID 19. Eur. Heart J. Cardiovasc. Imaging. 2020;21:1052. doi: 10.1093/ehjci/jeaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taza F. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabri A. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puntmann V.O. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLaren G. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 45.Riphagen S. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belhadjer Z. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 47.Whittaker E. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Driggin E. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han H. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 51.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llitjos J.F. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavazzi G. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;46:1121–1123. doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klok F.A. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helms J. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poissy J. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 57.Gheblawi M. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin–angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei C. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walters T.E. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 61.Liu H. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc. Res. 2020;116:1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan W.S.D. Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018;40:9–17. doi: 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Zhong J. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 64.Zou Z. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santos R.A.S. The ACE2/angiotensin-(1–7)/MAS axis of the renin–angiotensin system: focus on angiotensin-(1–7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oudit G.Y. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang K. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ackermann M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varga Z. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teuwen L.A. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panigada M. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Löf A. A biophysical view on von Willebrand factor activation. J. Cell. Physiol. 2018;233:799–810. doi: 10.1002/jcp.25887. [DOI] [PubMed] [Google Scholar]
- 74.Tomar B. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9:1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuo Y. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Middleton E.A. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li D. SARS-CoV-2-induced immune dysregulation and myocardial injury risk in China: insights from the ERS-COVID-19 study. Circ. Res. 2020;127:397–399. doi: 10.1161/CIRCRESAHA.120.317070. [DOI] [PubMed] [Google Scholar]
- 79.Bermejo-Martin J.F. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kindler E. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blanco-Melo D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y.G. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu C. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) MedRxiv. 2020 doi: 10.1101/2020.02.26.20028589. Published online February 29, 2020. [DOI] [Google Scholar]
- 84.Cummings M.J. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ranucci M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone R.L. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pathan N. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 89.Zhou K. Towards precision management of cardiovascular patients with COVID-19 to reduce mortality. Prog. Cardiovasc. Dis. 2020;63:529–530. doi: 10.1016/j.pcad.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Musher D.M. Acute infection and myocardial infarction. N. Engl. J. Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 91.Sandoval Y., Jaffe A.S. Type 2 myocardial infarction: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;73:1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Chapman A.R. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen F. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin. Transl. Med. 2020;10 doi: 10.1002/ctm2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han Y. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation. 2020;141:e810–e816. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 95.Julien H.M. Telemedicine and the forgotten America. Circulation. 2020;142:312–314. doi: 10.1161/CIRCULATIONAHA.120.048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Udell J.A. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310:1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Y. Effects of influenza vaccination on the risk of cardiovascular and respiratory diseases and all-cause mortality. Ageing Res. Rev. 2020;62:101124. doi: 10.1016/j.arr.2020.101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coburn B.J. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1) BMC Med. 2009;7:30. doi: 10.1186/1741-7015-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson N., Baker M.G. The emerging influenza pandemic: estimating the case fatality ratio. Euro Surveill. 2009;14:19255. [PubMed] [Google Scholar]
- 100.Fajardo-Dolci G. Clinical characteristics of fatalities due to influenza A (H1N1) virus in Mexico. Thorax. 2010;65:505–509. doi: 10.1136/thx.2009.126953. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen J.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–281. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park J.E. MERS transmission and risk factors: a systematic review. BMC Public Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breban R. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382:694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zumla A. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banik G.R. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol. Sin. 2016;31:81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsuyama R. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health. 2016;16:1203. doi: 10.1186/s12889-016-3881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen C.Y. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J. Chin. Med. Assoc. 2005;68:4–10. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nie X.H. Effects of underlying disease on the prognosis in patients with severe acute respiratory syndrome. J. Capital Univ. Med. Sci. 2003;24:434–436. [Google Scholar]
- 109.Wu X. Analysis of the relative factors associated with death in adult SARS patients. Chin. Gen. Pract. 2005;8:276–278. [Google Scholar]
- 110.Zhao S. Estimating the unreported number of novel coronavirus (2019-nCoV) cases in China in the first half of January 2020: a data-driven modelling analysis of the early outbreak. J. Clin. Med. 2020;9:388. doi: 10.3390/jcm9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Q. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanche S. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao X.H. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 114.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian S. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barton L.M. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bradley B.T. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buja L.M. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Craver R. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c) Fetal Pediatr. Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fox S.E. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fox S.E. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 122.Stone J.R. Case 23-2020: a 76-year-old woman who died from COVID-19. N. Engl. J. Med. 2020;383:380–387. doi: 10.1056/NEJMcpc2004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edler C. Dying with SARS-CoV-2 infection – an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindner D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nicolai L. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puelles V.G. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schaller T. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nunes Duarte-Neto A. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020 doi: 10.1111/his.14160. Published online May 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aiolfi A. Late histological findings in symptomatic COVID-19 patients: a case report. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000021046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wan S. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 Published online February 12, 2020. [Google Scholar]